Abstract
Early in development, the depolarizing GABAAergic signaling is needed for normal neuronal differentiation. It is shown here that hyperpolarizing reversal potentials of GABAAergic postsynaptic currents (EGABA) appear earlier in female than in male rat CA1 pyramidal neurons because of increased potassium chloride cotransporter 2 (KCC2) expression and decreased bumetanide-sensitive chloride transport in females. Three episodes of neonatal kainic acid-induced status epilepticus (3KA-SE), each elicited at postnatal days 4 (P4)–P6, reverse the direction of GABAAergic responses in both sexes. In males, 3KA-SE trigger a premature appearance of hyperpolarizing GABAAergic signaling at P9, instead of P14. This is driven by an increase in KCC2 expression and decrease in bumetanide-sensitive chloride cotransport. In 3KA-SE females, EGABA transiently becomes depolarizing at P8–P13 because of increase in the activity of a bumetanide-sensitive NKCC1 (sodium potassium chloride cotransporter 1)-like chloride cotransporter. However, females regain their hyperpolarizing GABAAergic signaling at P14 and do not manifest spontaneous seizures in adulthood. In maternally separated stressed controls, a hyperpolarizing shift in EGABA was observed in both sexes, associated with decreased bumetanide-sensitive chloride cotransport, whereas KCC2 immunoreactivity was increased in males only. GABAA receptor blockade at the time of 3KA-SE or maternal separation reversed their effects on EGABA. These data suggest that the direction of GABAA-receptor signaling may be a determining factor for the age and sex-specific effects of prolonged seizures in the hippocampus, because they relate to normal brain development and possibly epileptogenesis. These effects differ from the consequences of severe stress.
Keywords: seizure, patch clamp, GABAA receptor, development, hippocampus, histochemistry
Introduction
Neonatal rats are more susceptible to seizures, but more resilient to seizure-induced acute injury or epileptogenesis, compared with adults (Moshé, 1993; Jensen, 1999; Galanopoulou et al., 2002; Holmes et al., 2002). Early life seizures or status epilepticus (SE) do not necessarily result in epilepsy (Moshé, 1993; de Rogalski Landrot et al., 2001; Holmes et al., 2002; Roch et al., 2002; Raol et al., 2006; Xiu-Yu et al., 2007). They may have, however, long-lasting consequences, potentially impacting on the level of functioning and behavior (Holmes, 1991; Sogawa et al., 2001; Hsu et al., 2003; Swann, 2004; Akers et al., 2006; Lai et al., 2006).
Among the signaling pathways with prominent role in brain development is the GABAA receptor system. In the embryonic and early postnatal brain, GABAAergic signaling is depolarizing and activates calcium signaling processes important for neuronal survival, migration, proliferation, and differentiation (Ben-Ari, 2002; Galanopoulou, 2005a). It gradually switches to its classical hyperpolarizing mode, after region- and cell type-specific tempos (Rivera et al., 1999). In sexually dimorphic structures like the substantia nigra, the timing of the switch is also gender specific (Galanopoulou et al., 2003a; Galanopoulou, 2005a, 2006; Kyrozis et al., 2006). The different developmental windows during which each neuronal structure is exposed to the neurotrophic and differentiating effects of depolarizing GABA further amplify the functional diversity across brain regions, ages, and sex. In the hippocampus of male rats or rats of unspecified sex, the timing of the functional switch of GABAA receptors has been placed around postnatal day 13.5 (P13.5) (Rivera et al., 1999; Khazipov et al., 2004; Banke and McBain, 2006). The direction of GABAA receptor signaling is determined by the activity of chloride cotransporters, which either decrease [like potassium chloride cotransporter 2 (KCC2)] or increase intracellular chloride [like sodium potassium chloride cotransporter 1 (NKCC1)] (Rivera et al., 1999; Farrant and Kaila, 2007). The gradual shift from an NKCC1-dominant state in immature neurons to a KCC2-dominant state in more mature neurons triggers this functional switch (Plotkin et al., 1997; Rivera et al., 1999; Farrant and Kaila, 2007). In human epileptic temporal lobe tissue and adult rats subjected to seizures, an aberrant shift toward an NKCC1-dominant state and re-emergence of depolarizing GABAAergic signaling have been demonstrated and proposed to underlie certain epileptic discharges (Cohen et al., 2002; Rivera et al., 2002; Huberfeld et al., 2007).
To determine the effects of SE on the reversal potential of postsynaptic GABAAergic currents (EGABA) in neonatal rat CA1 pyramidal neurons, the ontogeny and factors governing GABAAergic signaling were determined in naive neonatal male and female rats and in rats subjected to three episodes of neonatal kainic acid-induces SE (3KA-SE). The seizure effects were further differentiated from the effects of maternal separation. This report demonstrates sex-specific patterns of GABAAergic signaling in CA1 pyramidal neurons, identifies sex-specific effects of 3KA-SE on EGABA and correlates them with the expression of KCC2 and NKCC1 chloride cotransporters, indicates that GABAA receptors mediate the 3KA-SE effects, and dissociates the effects of 3KA-SE and maternal separation.
Parts of this paper have been published previously in abstract form (Galanopoulou and Moshé, 2004; Galanopoulou, 2005b, 2007a,b,c).
Materials and Methods
Animals
The offspring of timed pregnant Sprague Dawley pups (Taconic Farms, Germantown, NY) that were used in this study were born in our animal housing facility and day of birth was considered as P0. Rats were maintained in our facility under standard procedures, in accordance with the Association for Assessment of Laboratory Animal Care guidelines. All procedures included in this study have been approved by the Animal Institute review committee. Rats were maintained in a 12 h alternating light/dark cycle and pregnant females were observed two to three times daily to monitor the time of delivery. Seizure induction and maternal separation were performed at P4–P6. Rats subjected to SE or maternal separation were kept at room temperature in cages together with five to six other pups, but without the dam, for 6 h daily with no access to food or water during P4–P6. They were subsequently returned to the dam. Selected female rats were weaned from the dam at P21, and each one was maintained in cages with one to two other female rats until they became 2–3 months old, with food ad libitum. After the electrode implantation for electroencephalographic (EEG) monitoring, rats were housed individually.
Experimental procedures
Gramicidin-perforated patch clamp.
Male and female rats were deeply anesthetized with ketamine (100 mg/kg, i.p.), decapitated, and 330-μm-thick coronal sections through the anterior dorsal hippocampus were obtained (VT1000S vibratome; Leica, Nussloch, Germany). Procedures for harvesting and maintaining acute slices for electrophysiology have been described previously (Galanopoulou, 2006). To maintain chloride equilibrium, gramicidin-perforated patch clamp was used (Akaike, 1994; Kyrozis and Reichling, 1995; Galanopoulou, 2006; Kyrozis et al., 2006). Gramicidin-perforated patch clamp was performed at room temperature, in 95% O2/5% CO2 bubbled artificial CSF (ACSF) supplemented with the glutamate receptor inhibitors 6-cyano-7-nitroquinoxalene-2, 3-dione (CNQX; 10 μm) and 2-amino-5-phosphonopentanoic acid (AP5; 50 μm). Borosilicate glass electrodes were used, filled with electrode solution [containing (in mm) 77 K2SO4, 2 MgCl2 × 6 H2O, 0.5 CaCl2, 5 EGTA, 10 HEPES, 305 mOsm, pH 7.3] that contained the cation-permeable ionophore gramicidin. Initial experiments were done using 1–5 μg/ml gramicidin (Sigma, St Louis MO). However, because of change in the gramicidin lot, its concentration was subsequently adapted to 30 μg/ml, so as to maintain good access. Preparation of gramicidin stock and final working solutions for gramicidin were otherwise as described previously (Galanopoulou, 2006). A stainless-steel bipolar stimulating electrode (FHC, Bowdoinham, ME) was placed within the stratum radiatum, along the visual centripetal pathway of pyramidal neuronal axonal processes, and at 100–200 μm approximate distance from the neurons of the CA1 pyramidal layer. To obtain postsynaptic currents (PSCs), electrical stimulation with 100 μs pulses was done on command. Neurons were approached under visual guidance, and were patched to obtain an initial seal >1 GΩ. Subsequently they were maintained under voltage clamp with a holding voltage of −70 mV, until access resistance improved. Slices were then incubated in ACSF with glutamate inhibitors (AP5, 50 μm and CNQX, 10 μm), to isolate the GABAA receptor-mediated PSCs. Analysis of the reversal potential for GABAA receptor-mediated PSCs (EGABA) was done when access resistance was <100 MΩ (usually 35–55 MΩ) and EGABA measurements were stable. To this end, 5–10 mV voltage steps were applied under voltage-clamp mode (range, −110 to −30 mV) around the estimated EGABA, and neurons were stimulated at each voltage. Current–voltage (I–V) curves were then constructed by plotting the peak current after stimulation (15–25 ms after stimulation) versus the holding voltage at the same step; EGABA was determined as the voltage at which no current was passing through. The average EGABA, as estimated from three to five such attempts, was accepted, provided that the results were stable within 2 mV range. In addition, neurons were studied under current clamp recordings (holding current, 0 pA) to study firing rates and resting membrane potential (Vr). To determine whether the isolated PSCs were GABAAergic, at the end of the experiment, bicuculline 60 μm was bath applied. In the neurons included in this study, bicuculline abolished the observed PSCs. To indicate the direction of observed GABAAergic PSCs, measurements are presented as EGABA − Vr. One and, rarely, two neurons per rat were studied. In the bumetanide experiments, a low dose of bumetanide (20 μm) was bath applied and EGABA − Vr was determined ∼10 min later, when EGABA had stabilized.
Induction of SE.
Pups were weighed on P4, P5, and P6. Seizure induction was typically done between 2:00 P.M. and 8:00 P.M. to adjust for circadian cycle related differences in the responses. Seizures were induced with intraperitoneal injections of kainic acid (KA): 1.25 mg/kg at P4, 1.5 mg/kg at P5, and 2 mg/kg at P6 (KA456 groups). The incremental doses were necessary to ensure that rats would develop SE each day of seizure induction. Behavioral monitoring was done frequently to ensure that rats had continuous seizures: continuous until the onset of SE and every 30–60 min thereafter to monitor whether SE was maintained. Seizure progression included an initial stage with immobility followed by bouts of scratching behavior, hyperactivity and ataxia, focal limb myoclonus, and tonic or tonic-clonic seizures without interictal recovery. Behavioral seizures lasted for at least 5–6 h, starting to abate toward the end of the monitoring and separation period (6 h). Typically, most pups, especially on days P4 and P6, had seizures at the time of return to the cage. The morning after, the level of activity was similar to controls (CONs) and no behavioral seizures were observed at that time.
Maternal separation.
To account for the potential effects of maternal separation, a different set of pups (SS456 groups) was injected with saline at the same time points and volumes as those used for the KA injections (P4–P6), and was kept separate from the dams for 6 h daily.
Effects of GABAA blockade.
To test the role of GABAA receptors in the effects of 3KA-SE, separate groups of pups were injected with two bicuculline doses (BKB groups; 2 mg/kg, i.p.; 10 min before and 6 h after each KA injection). For comparison, respective controls and maternally separated pups received two daily bicuculline doses, as described in Table 1. Administration of the selected bicuculline doses in maternally separated rats typically did not produce overt behavioral seizure activity and only rarely (8% of tested rats) infrequent and brief myoclonic or tonic seizure-like behaviors were observed, which did not evolve into SE. In contrast, in KA-injected rats, bicuculline (BKB groups) exacerbated the severity of KA-induced behavioral seizures, as rats manifested more intense clonic or tonic-clonic seizure activity.
Table 1.
Nomenclature of experimental groups used in this study
| Group | Treatment |
|---|---|
| CON | Naïve controls, saline injected at P4, P5, and P6, but not separated from the dam |
| KA456 | Rats subjected to KA-induced SE and undergone 6 h daily separation from the dam at P4, P5, and P6 |
| SS456 | Rats saline-injected at P4, P5, and P6, at the onset of their 6 h daily maternal separation period |
| BB | Rats injected with bicuculline 2 mg/kg (i.p.) 10 min prior to each KA injection and 6 h later (P4, P5, P6), but did not undergo maternal separation |
| BKB | Rats injected with bicuculline 2 mg/kg (i.p.) 10 min prior to each KA injection and 6 h later (P4, P5, P6); otherwise similar to KA456 |
| BSB | Rats injected with bicuculline 2 mg/kg (i.p.) 10 min prior to each saline injection and 6 h later (P4, P5, P6); otherwise similar to SS456 |
Fluoro-Jade B staining and immunochemistry.
Rats were deeply sedated (pentobarbital 100 mg/kg, i.p.), transcardially perfused with formalin (Sigma), brains were dissected, fixed overnight in formalin and preserved in 30% sucrose in PBS until sunk (4°C), fast frozen at −35°C, and kept at −80°C until further use (Galanopoulou, 2006). Rats intended for Fluoro-Jade B studies were killed at P7. Rats intended for immunochemical analysis were killed at P10. Fluoro-Jade B staining (Schmued et al., 1997) was done on 40-μm-thick coronal sections, cut on a Microm cryostat (Microm International, Walldorf, Germany). The following primary antibodies were used for the chloride cotransporters: rabbit polyclonal anti-KCC2 against amino acid sequence 932–1043 of rat KCC2 (Millipore, Billerica MA) (Williams et al., 1999) and rabbit polyclonal NKCC1 antibody against a 22 amino acid sequence at the C terminus of rat NKCC1 (Millipore) (Moore-Hoon and Turner, 1998); both were obtained from Millipore. The specificity of these antibodies has been reported in previous studies (Moore-Hoon et al., 1998; Williams et al., 1999; Aronica et al., 2007). In brief, immunochemistry was performed on free-floating 40 μm sagittal sections containing the anterior-dorsal hippocampi. Sections were incubated in 1% hydrogen peroxide in PBS (30 min, room temperature), and blocking solution for 1 h. Blocking solution consisted of PBS with 10% normal goat serum (NGS), 0.1% Triton X-100, and 0.1% bovine serum albumin (BSA) in KCC2 assays. In NKCC1 experiments, sections were blocked with PBS with 10% NGS, 0.3% Triton X-100, and 0.1% BSA. Incubation with primary antibodies was carried at 4°C in a shaker incubator for 3 d (1:250 dilution of anti-KCC2 antibody in PBS with 1.5% NGS, 0.1% Triton X-100, and 0.1% BSA; 1:500 dilution of anti-NKCC1 antibody in PBS with 1.5% NGS, 0.3% Triton X-100, and 0.1% BSA). Finally, sections were incubated with biotinylated anti-rabbit IgG (heavy and light chains) secondary antibodies made in goat (1:200; Vector Laboratories, Burlingame CA) and development of the colorigenic peroxidase-based reaction was performed as per manufacturer's instructions, using the Vectastain Elite and the NovaRed substrate kit (Vector Laboratories) (Galanopoulou, 2006).
Densitometry.
To minimize bias in the intergroup comparisons caused by interassay variability, each assay included sections from one brain of each group (male and female CONs, SS456, and KA456). Microphotographs, magnified 400×, of optical fields corresponding to the CA1 pyramidal region were captured via a Nikon (Melville, NY) Ellipse 1000M microscope, transferred via a Eikonix (Burlington, MA) 1400 Series digital imaging camera to a G4 computer (Apple Computer, Cupertino, CA). Microphotographs were labeled with codes to allow unbiased and blind to investigator assessment during densitometry. Codes were revealed at the end of the study. Signal densitometry of KCC2- and NKCC1-immunoreactive pyramidal neurons was done with the Scion (Frederick, MD) Imager 1.62c (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) software, after conversion to grayscale images. A maximum of four optical fields per CA1 section were selected per brain. Because CA1 pyramidal neurons are densely packed in the CA1 pyramidal layer, and because, at times, the borders of neuronal bodies were not clearly visible, as in weakly stained P10 male control KCC2-immunoreactive CA1 layers, regions of interests over the CA1 pyramidal layers were selected for densitometry. Background measurements were also obtained from the same optical field, derived from regions outside the CA1 layer that did not have any visible specific staining. The subtracted value of the “mean CA1 pyramidal layer density” minus “mean background density” of the same optical field was used in the final analysis. An average of three to four sections per rat were used. To minimize interassay variability, all values were referred to as the “percentage of mean KCC2-immunoreactivity (-ir) or NKCC1-ir in the P10 male control group of the same assay.” The obtained values were normally distributed and with comparable variance.
Statistics.
Statistical comparison of the electrophysiological data were done with ANOVA (one-way or multifactorial with Dunnett's post hoc) or Student's t test as indicated (Statview and JMP7 softwares; SAS Institute, Cary, NC). The immunochemical densitometric measurements were analyzed with repeated measures ANOVA with Tukey post hoc. Statistical significance was set at 0.05.
EEG monitoring at P7.
To characterize the electrographic correlates of KA-induced seizures, five male and four female P6 rats were anesthetized with isoflurane and epidural EEG electrodes were placed at P6 at the right frontal and bilateral occipital regions, using the EEG headmounts of the EEG/EMG system (Pinnacle Technology, Lawrence, KS). P6 was chosen for the surgery, as epidural EEG headmounts were better tolerated at this age compared with younger pups. Pups were allowed to recover overnight and they were injected with kainic acid (2 mg/kg, i.p.) at P7. EEG and video monitoring were done for 30–45 min before KA injection (baseline), 6 h post-KA injection, and for 4 h daily at P8–P10. Pups were returned to the dam after each monitoring period. Acquisition and analysis of the EEG was performed using the Sirenia software (Pinnacle Technology). Electrographic seizures were defined as EEG patterns of high-amplitude rhythmic activity or repetitive spikes with evolution in frequency or amplitude, that were at least three times higher than the baseline activities and lasted for at least 3 s.
EEG monitoring in adulthood.
To determine whether spontaneous seizures occurred in adulthood as a result of the 3KA-SE, seven KA456 and three CON female rats underwent stereotactic surgery for implantation of left hippocampal electrodes at 2–3 months of age. Before surgery, rats were anesthetized with a mixture of ketamine (70 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.). A wire electrode (E363/3; PlasticsOne, Roanoke, VA) was placed at the left dorsal hippocampus, at the following coordinates: −4.2 mm anteroposterior, 2.6 mm left, −3.6 mm depth. Bilateral cortical screws were implanted at 2 mm anteroposterior, 3 mm lateral. A reference screw electrode was placed at the right frontal bone and a ground electrode in the midline anterior to the occipital suture. The electrodes were fixed to the skull with dental acrylic cement. EEG and video monitoring was started at least 2–3 d after surgery. EEG was recorded 7 h a day (9:00 A.M. until 4:00 P.M.), 7–10 d per rat. Video monitoring was done for an average of 2–3 h daily per rat (Lado et al., 2001). EEG recording and analysis was done with the Stellate Harmonie software (Stellate Systems, Montreal, Quebec, Canada). At the end of the monitoring period, rats were killed with lethal doses of pentobarbital, and brains were collected and Nissl stained to confirm that the placement of the electrode was indeed in dorsal hippocampus.
Results
Ontogeny of EGABA changes in male and female naive rats
To investigate the effects of 3KA-SE on EGABA, the ontogenetic patterns of EGABA changes were first determined in P4–P18 male and female CON pups. The following age groups were compared: P4–P7, P8–P13, and P14–P18. The Vr was similar between sexes (F(1,55) = 0.85) and across ages (F(2,54) = 2.3). In contrast, significant sex-specific developmental changes were noted in EGABA and EGABA − Vr, as presented in Table 2 and Figure 1.
Table 2.
Ontogeny of EGABA changes in male and female rat CA1 pyramidal neurons
| Groups | n | EGABA(mean ± SEM; mV) | p | EGABA − Vr (mean ± SEM; mV) | p |
|---|---|---|---|---|---|
| Male P4–P7 | 7 | −58.7 ± 3 | 8.3 ± 2.8 | ||
| Male P8–P13 | 7 | −62.4 ± 3.1 | 5.6 ± 3 | ||
| Male P14–P18 | 8 | −74.2 ± 2.9 | < 0.05** | −3.3 ± 2.8 | < 0.05** |
| Female P4–P7 | 12 | −68 ± 2.4 | < 0.05* | −3 ± 2.4 | < 0.05* |
| Female P8–P13 | 17 | −71.3 ± 2 | < 0.05* | −2.5 ± 1.2 | < 0.05* |
| Female P14–P18 | 6 | −81.2 ± 3.3 | < 0.05** | −10.6 ± 3.2 | < 0.05** |
EGABA and EGABA − Vr were determined in P4–P18 male and female CA1 pyramidal neurons from CON rats, using gramicidin-perforated patch clamp. Significant sex- and age-related differences were observed.
*Significant difference versus same age male group; **significant difference versus either of the P4–P7 or P8–P13 same-sex groups.
Figure 1.
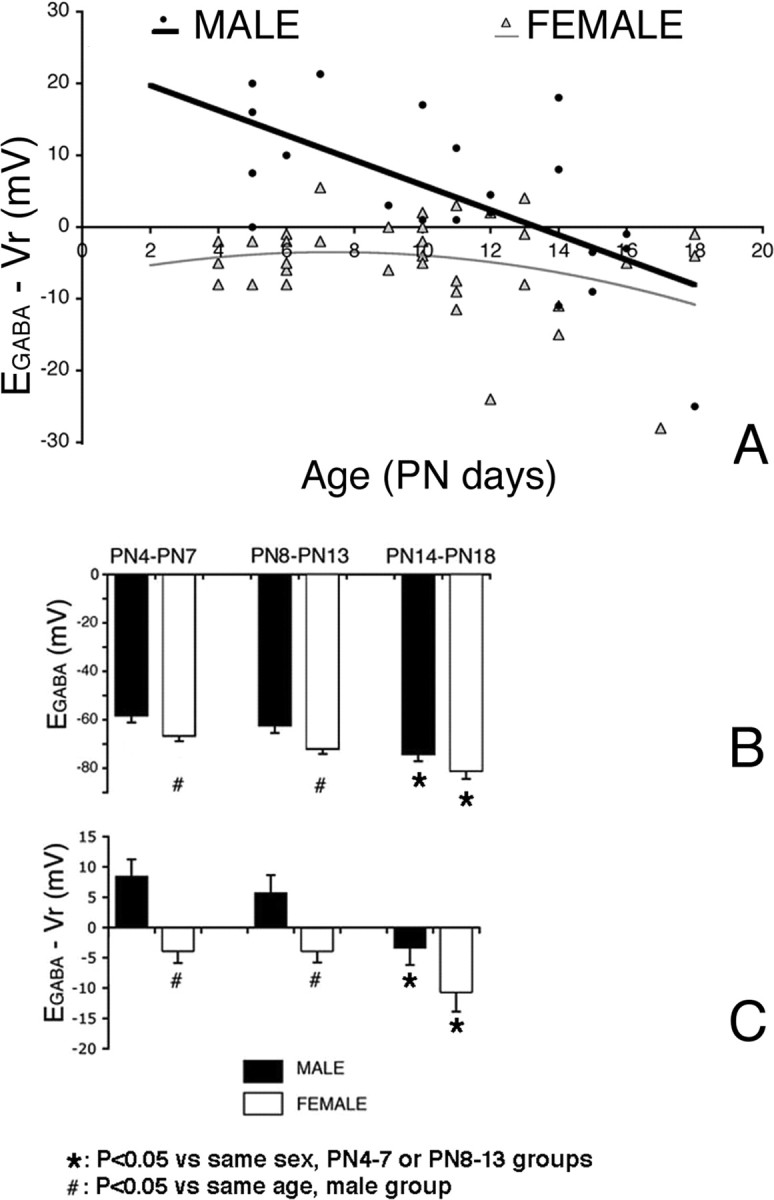
Sex differences in EGABA in neonatal rat CA1 pyramidal neurons. A, A scattergram of the EGABA − Vr values in control pups is depicted. Ninety-three percent of males (blue dots) had depolarizing EGABA − Vr values until P14 and subsequently switched to hyperpolarizing. Eighty-three percent of P4–P13 females (pink dots), however, already manifested hyperpolarizing or isoelectric responses by P4. B, C, Significant differences in mean EGABA (B) and EGABA − Vr (C) between P4–P7, P8–P13, and P9–P14 male and female rat CA1 pyramidal neurons are noted. Results in B and C represent the mean ± SEM. PN, Postnatal day.
EGABA was significantly more negative in females than in age-matched males (F(1,55) = 11, p < 0.01), and this difference was more pronounced in the P4–P14 groups (i.e., before the time of EGABA switch in males) (Table 2, Fig. 1, supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The sex difference in EGABA was observed for both P4–P7 and P8–P13 age groups, but not at P14–P18. In addition, in both sexes, EGABA was significantly more negative in P14–P18 rats than in P4–P7 or P8–P13 rat CA1 pyramidal neurons of the same sex (F(2,54) = 11.2, p < 0.001). No significant differences were noted in EGABA between P4–P7 and P8–P13 same-sex rats.
Similar to EGABA, sex and age differences were noted in EGABA − Vr (sex, F(1,55) = 16, p < 0.001; age, F(2,54) = 6.6; p < 0.01). In males, depolarizing EGABA − Vr values were observed before P14 in 13 of 14 neurons (Fig. 1). Using best-fit analysis, the age of EGABA switch to hyperpolarizing values was estimated at P13.7. Between P14 and P18, six of eight studied male CA1 neurons had hyperpolarizing EGABA values (Fig. 1). In both P4–P7 and P8–P13 age groups, females had typically negative or isoelectric EGABA − Vr, except for five neurons that exhibited mildly depolarizing potentials. As shown in Figure 1A, these were scattered between P7–P13. EGABA − Vr retained hyperpolarizing values in females at least until P18. These show that the functional maturation of GABAA receptor signaling to hyperpolarizing in females occurs earlier than in male rat CA1 pyramidal neurons.
Characterization and effects of 3KA-SE
Characterization of KA-SE induced at P4–P6
Based on the above findings, it was decided to induce KA-SE at a time when the direction of GABAA receptor signaling was different in male and female CA1 pyramidal neurons, i.e., before P14. Because no significant EGABA differences were found between P4–P7 and P8–P13 age groups, seizure induction at P4–P6 gave the additional advantage of allowing for an interim week, before the age when normal EGABA switch occurs in males. Behavioral monitoring of pups injected with KA at P4 did not reveal any sex differences in the latency to onset of seizures. Latencies to onset of first tonic seizure were 24.8 ± 1.9 min in males (n = 30 rats) and 23.2 ± 2.2 min in females (n = 29 rats). Mortality was 5% in KA456 rats, occurring usually at P4–P7. No episodes of rejection by the dam were observed in the experimental groups, which also included SS456 pups. Growth curves were similar in CON, KA456, and SS456 groups (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). To determine whether neuronal injury occurred as a result of 3KA-SE, Fluoro-Jade B staining was done at P7 (Fig. 2). No significant presence of Fluoro-Jade B-stained cells in the CA1 pyramidal region or other hippocampal regions was noted in these groups (n = 5 male and female rats per group) (Fig. 2).
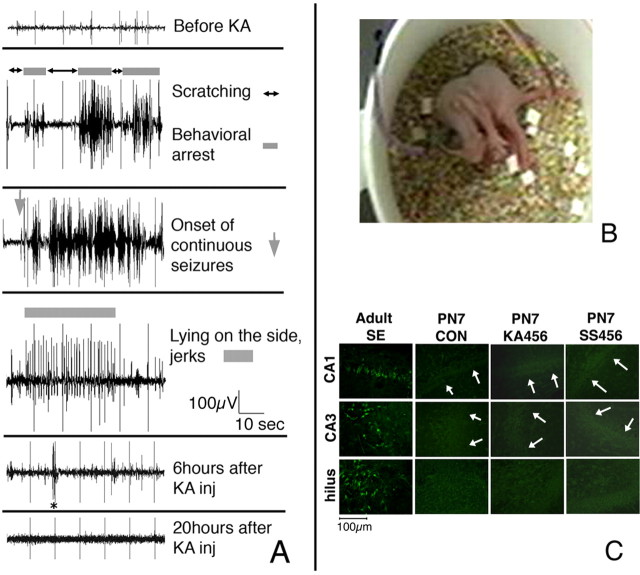
Figure 2.
KA-induced seizures in neonatal rats evolve into SE but do not increase the number of Fluoro-Jade B-stained cells in the hippocampus. A, B, P7 male and female pups (n = 9) with epidural recordings were injected with kainic acid (2 mg/kg, i.p.) and monitored continuously for 6 h as well as for 4 h daily at P8–P10. The EEG derivations in A represent bipolar recordings from the right frontal and left occipital electrodes. Initially, bouts of scratching (without any change in EEG activity) interrupted by frequent periods of behavioral arrest that correlated with evolving high-amplitude EEG activity were noted. After the onset of continuous seizure activity (SE), tonic seizures (B) were also observed. Toward the end of SE, episodes of repetitive clonic jerks while the pup was lying on the side were also associated with EEG seizure patterns, as indicated by the gray bar. The ictal events became less frequent and interictal bursts of epileptiform activity were noted (asterisk). In eight of nine pups, no electrographic seizures were noted at P8–P10. C, To determine whether the three episodes of KA-SE at P4–P6 resulted in neurodegeneration in the hippocampus, Fluoro-Jade B staining was performed. There were no Fluoro-Jade B-stained cells seen in the hippocampus. The figures are representative of n = 5 rats per group. As a positive control, the hippocampal sections from an adult rat subjected to a single episode of lithium-pilocarpine SE [methods described by Galanopoulou et al. (2003b)] 48 h before being killed are shown, which demonstrate many Fluoro-Jade B-stained (green) neurons in the hilus, CA1, and CA3 pyramidal layers. Magnifications are 400×. Scale bar, 100 μm. The white arrows indicate the borders of the CA1 and CA3 pyramidal regions. PN, Postnatal day.
EEG characterization of KA-SE
To characterize the electrographic correlates of the observed seizures, EEG monitoring using epidural electrodes was done in nine pups at P7 (five male and four female pups). Pups were injected with KA and monitored with video-EEG. Electrographic seizure activity appeared early in the course (10–15 min after KA injection) as frequent bursts of high-amplitude rhythmic activity (Fig. 2), coinciding with periods of immobility or behavioral arrest. These evolved into a continuous pattern of high-amplitude rhythmic activity intermixed with spikes or polyspikes that persisted for ∼4.5–5.5 h. During this time, pups exhibited tonic or tonic-clonic seizures or episodes of ataxic movements and swimming-like behavior. Electroclinical seizures were still observed sporadically but less frequently by the end of the 6 h monitoring period. Follow-up EEG monitoring until P10 revealed electrographic seizures in only one female pup at P8 and P9. This pup had electroclinical seizures characterized by bursts of high-amplitude rhythmic activity associated with behavioral arrest followed by ataxic hindlimb alternating extensions at P8. Rare brief bursts of epileptiform activity, shorter than 10 s, were seen at P9 but not at P10, with no clear behavioral correlate.
Effects of KA-SE at P4–P6 on the direction of GABAA responses
To test whether early life 3KA-SE alter the direction of GABAAergic signaling, EGABA − Vr was determined in CA1 pyramidal neurons after P7 in pups subjected to 3KA-SE and their respective controls. Given the sex differences in the direction of the GABAAergic signaling in control neonatal CA1 pyramidal neurons, further assessment of the effects of neonatal seizures on EGABA − Vr was done separately for males and females. The effect of treatment (control, 3KA-SE, maternal separation) was significant in both male (F(2,30) = 9.6, p < 0.001) and female pups (F(2,39) = 25.44, p < 0.0001). Results are summarized in Table 3 and Figure 3.
Table 3.
Effects of treatment on EGABA − Vr of rat CA1 pyramidal neurons
| Group | Saline injection at P4–P6 |
Bicuculline administration at P4–P6 (two daily doses) |
|||
|---|---|---|---|---|---|
| n | EGABA−Vr (mean ± SEM; mV) | n | EGABA − Vr (mean ± SEM; mV) | ||
| Male P9–P14 | |||||
| CON | 10 | 5.5 ± 2.7 | BB | 9 | 1.0 ± 1.4 |
| KA456 | 10 | −2.2 ± 1.0** | BKB | 6 | 4.2 ± 1.8* |
| SS456 | 12 | −7.7 ± 2.2** | BSB | 9 | 4.2 ± 2.0* |
| Female P8–P13 | |||||
| CON | 16 | −2.5 ± 1.2 | BB | 9 | 2.2 ± 1.8 |
| KA456 | 14 | 7.2 ± 1.5** | BKB | 5 | −4.8 ± 2.6* |
| SS456 | 13 | −8.6 ± 1.8** | BSB | 5 | 4.8 ± 1.6* |
EGABA and EGABA − Vr were determined in P9–P14 male and P8–P13 female CA1 pyramidal neurons of the tested experimental groups, using gramicidin-perforated patch clamp.
*Significant difference versus the respective saline-injected, same-sex group that had the same treatment otherwise (i.e. CON, KA456, or SS456); **significant difference versus CON same-sex group.
Figure 3.
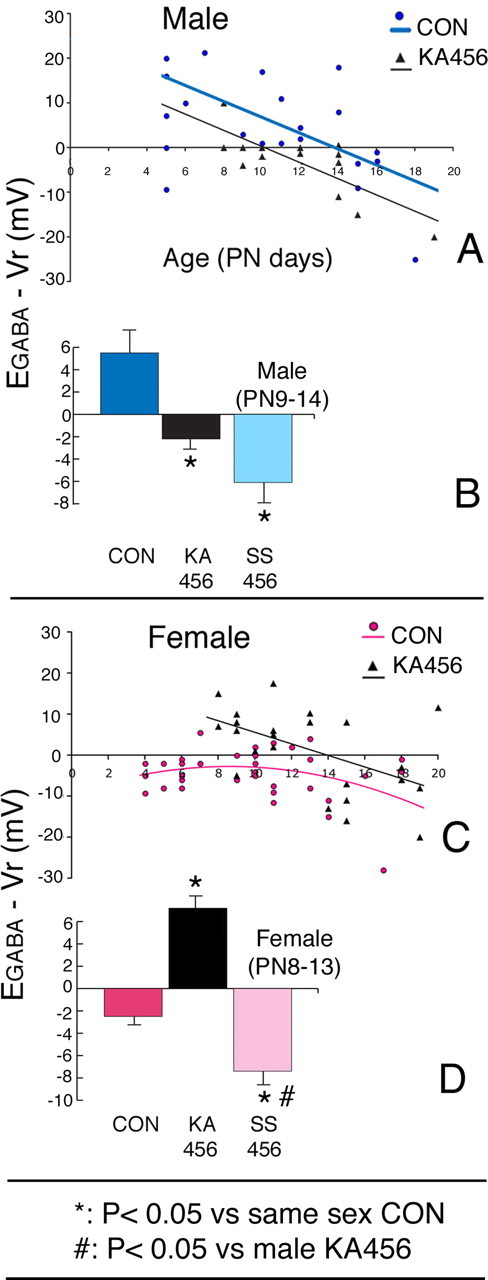
Effects of three episodes of neonatal KA-SE (KA456) and maternal separation on EGABA − Vr of male and female rat CA1 pyramidal neurons. A, Male rats; scattergram of EGABA − Vr values according to age shows a negative shift in KA456 (black triangles) versus CON (blue circles) male rats. B, Male rats; comparisons of EGABA − Vr among the three groups show that both KA456 and SS456 have hyperpolarizing EGABA − Vr. C, Female rats; scattergram of EGABA − Vr values according to age shows a transient reappearance of depolarizing EGABA − Vr in the KA456 group, between P8–13. D, Female rats; comparisons of EGABA − Vr among the three groups show more depolarizing EGABA − Vr in KA456 pups and more hyperpolarizing EGABA − Vr in SS456 pups at P8–13. Results in B and D are shown as mean ± SEM. PN, Postnatal day.
KA456 male pups manifested hyperpolarizing EGABA − Vr at an earlier age (P9) compared with CONs (switch at P14) (Fig. 3A), resulting in significantly more negative EGABA − Vr values at the time of sex-specific GABAAergic responses (P9–P14) (p < 0.05) (Fig. 3B, Table 3) (p < 0.05). GABAA responses remained hyperpolarizing until at least P19.
In contrast, female KA456 pups showed a transient positive shift in EGABA − Vr toward depolarizing values from P8 until P13 (13 of 14 studied females; p < 0.05 vs CONs) (Fig. 3, Table 3). After P14, however, EGABA − Vr values returned to hyperpolarizing values in 8/10 neurons (−6.4 ± 3.1 mV; n = 10), which were similar to female CONs.
These data indicate that 3KA-SE at P4–P6 have sex-specific effects on the direction of GABAAergic postsynaptic responses of rat CA1 pyramidal neurons. In males, neonatal 3KA-SE accelerate the EGABA switch, whereas in females, 3KA-SE trigger a transient reappearance of depolarizing GABAAergic signaling.
KCC2 expression in CA1 pyramidal neurons as a function of sex and 3KA-SE
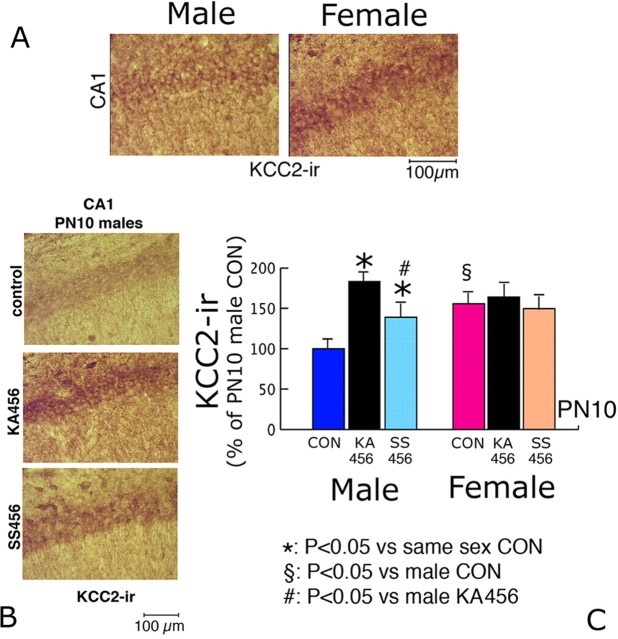
To explain the sex-specific effects of 3KA-SE on EGABA, KCC2-ir in CA1 pyramidal neurons from male and female KA456 P10 pups was studied. P10 was chosen as it falls within the period of sex-specific GABAA responses.
The expression of KCC2-ir was increased in female CON CA1 pyramidal neurons (155.7 ± 12% compared with male pups, semiquantitative method; p < 0.05; n = 5 rats per group), in agreement with the earlier appearance of hyperpolarizing GABAAergic signaling in female CON pups.
3KA-SE had sex-specific effects on KCC2-ir. In male KA456 pups, KCC2-ir was increased (183.6 ± 18.3% of CON values, semiquantitative method, n = 5 rats) (Fig. 4). In contrast, 3KA-SE had no significant effect on KCC2-ir in female rat CA1 pyramidal neurons (n = 5 rats per group) (Fig. 4). Consequently, the observed negative shift in EGABA in male CA1 pyramidal neurons after 3KA-SE can be explained on the basis of increased KCC2-ir expression. To further investigate the basis of EGABA changes in female KA456 rats, the expression and activity of NKCC1 were studied.
Figure 4.
Increased KCC2-ir underlies the negative shift in EGABA in P10 female CON and male KA456 and SS456 groups. A, Male and female rats; representative photos (400× magnification) of CA1 pyramidal neurons from P10 CON rats using a KCC2 specific antibody, counterstained with Novared substrate (brown–red color). Increased KCC2-ir is present in female CA1 pyramidal neurons. B, Male P10 CON, KA456, and SS456 CA1 pyramidal neurons stained with KCC2-specific immunochemistry, using Novared substrate. High levels of KCC2-ir is present in KA456 rats, intermediate in SS456, and low in CON. C, Male and female rats; the results of densitometric analysis of KCC2-ir in CA1 neurons of the three treatment groups are depicted as means ± SEM. Significant sex differences are documented among CON groups. In males only, both KA456 and SS456 have increased KCC2-ir compared with CON (KA456 > SS456 > CON). PN, Postnatal day. Scale bar, 100 μm.
Sex- and 3KA-SE-specific expression and activity of NKCC1 in CA1 pyramidal neurons
To determine the contribution of NKCC1 in EGABA, NKCC1 protein expression was initially compared semiquantitatively with immunochemistry (Fig. 5A). Neither gender nor treatment-specific changes in NKCC1-ir were found in P10 male or female CA1 pyramidal neurons. However, the activation of NKCC1 also depends on post-translational modifications that are not detected by the used anti-NKCC1 antibody, including protein phosphorylation. To directly assess the relative contribution of NKCC1-like chloride cotransport activity among groups, the bumetanide inhibition assay was used. Low doses of bumetanide (20 μm) were bath applied, as these are known to selectively inhibit NKCC1-mediated chloride cotransport. The bumetanide-induced shift in EGABA was determined ([EGABA]bumetanide − EGABA) (Fig. 5B, supplemental Fig. 3, available at www.jneurosci.org as supplemental material). [EGABA]bumetanide was measured at the point of maximal bumetanide effect (10 min of bumetanide bath application). These studies were performed in P9–P14 pups, during the developmental period after 3KA-SE induction, when the sex- and treatment-specific differences in EGABA were still observed.
Figure 5.
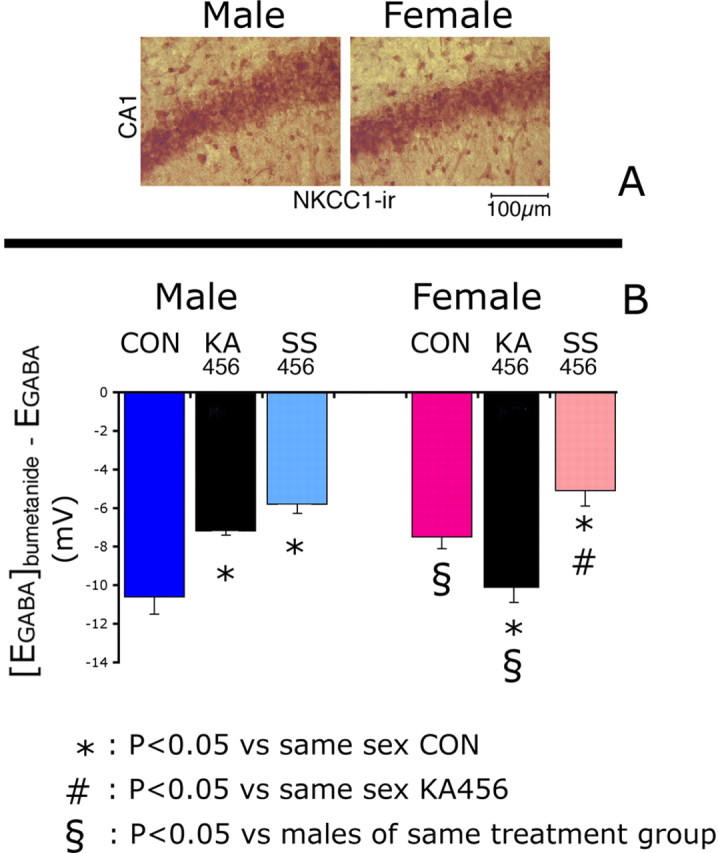
Sex- and treatment-related differences in the activity of bumetanide-sensitive NKCC1-like chloride cotransport in rat P8–14 CA1 pyramidal neurons. A, Male and female rats; representative photos (400× magnification) of the CA1 pyramidal layers of P10 CON rats, stained with NKCC1 specific antibody and counterstained with Novared substrate (brown–red color). No differences are noted in NKCC1-ir between sexes. Scale bar, 100 μm. B, Male and female rats; comparison of [EGABA]bumetanide − EGABA among CON, KA456, and SS456 groups demonstrates significant sex differences in CON and KA456 groups, but not in SS456. In males, the effect of bumetanide on EGABA is smaller in KA456 and SS456 compared with CONs. In females, the effect of bumetanide on EGABA is as follows: KA456 > CON > SS456. Results in B represent the mean ± SEM.
In CON groups, [EGABA]bumetanide − EGABA was −10.6 ± 1.4 mV in male pups (n = 4 neurons) and −7.5 ± 0.7 mV in females (n = 8 neurons; p < 0.05 vs male CONs) (Fig. 5B). The increased bumetanide-sensitive chloride cotransport activity in male CONs may therefore contribute, in conjunction with the lower KCC2 expression, to the more depolarizing GABAAergic responses observed in them.
3KA-SE had sex-specific effects. Male KA456 pups had reduced [EGABA]bumetanide − EGABA (−7.25 ± 0.25 mV, n = 4 neurons; p < 0.05 compared with male CONs). In female KA456, [EGABA]bumetanide − EGABA was increased compared with female CONs (−10.1 ± 0.3 mV; n = 5 neurons; p < 0.05).
These data indicate that 3KA-SE decrease bumetanide-sensitive NKCC1-like chloride cotransport in males but increase it in females.
Role of GABAA receptors on the sex-specific 3KA-SE-induced changes in GABAA receptor signaling
Because 3KA-SE have different effects on EGABA in neurons with predominantly depolarizing (i.e., male) versus hyperpolarizing/isoelectric EGABA values (i.e., female), the possibility that GABAA receptors mediate these sex-specific effects on EGABA was examined. The effects of neonatal 3KA-SE were tested in male and female pups in which GABAA receptor signaling had been inhibited at the time of 3KA-SE. To achieve this, rats were injected with two bicuculline injections (2 mg/kg, i.p. before and 6 h after each KA injection; BKB groups) and EGABA was determined during the period of divergent GABAA responses (i.e., P9–P14 in male, P8–P13 in female rats). The experimental groups, including respective controls, are listed in Table 1. Detailed descriptions of the preliminary studies leading to this protocol and of the intergroup comparisons are included in the supplemental text (available at www.jneurosci.org as supplemental material). None of these treatments resulted in significant presence of Fluoro-Jade B-stained degenerating cells in the hippocampus (data not shown).
The effects of two daily doses of bicuculline at P4–P6 were significant in both male (F(1,55) = 7.2; p < 0.01) and female (F(1,61) = 7.7, p < 0.05) pups (Table 3, Fig. 6). Bicuculline treatment (BKB group) (Table 3, Fig. 6) reversed the effects of 3KA-SE on EGABA − Vr in both male and female pups, suggesting that in both sexes, GABAA receptors mediate the effects of 3KA-SE on EGABA. Interestingly, in otherwise naive pups, blockade of GABAA receptors with bicuculline (BB groups) (Table 3 and Fig. 6) abolishes the sex differences in EGABA, shifting EGABA−Vr toward more depolarizing potentials (i.e., more similar to males).
Figure 6.
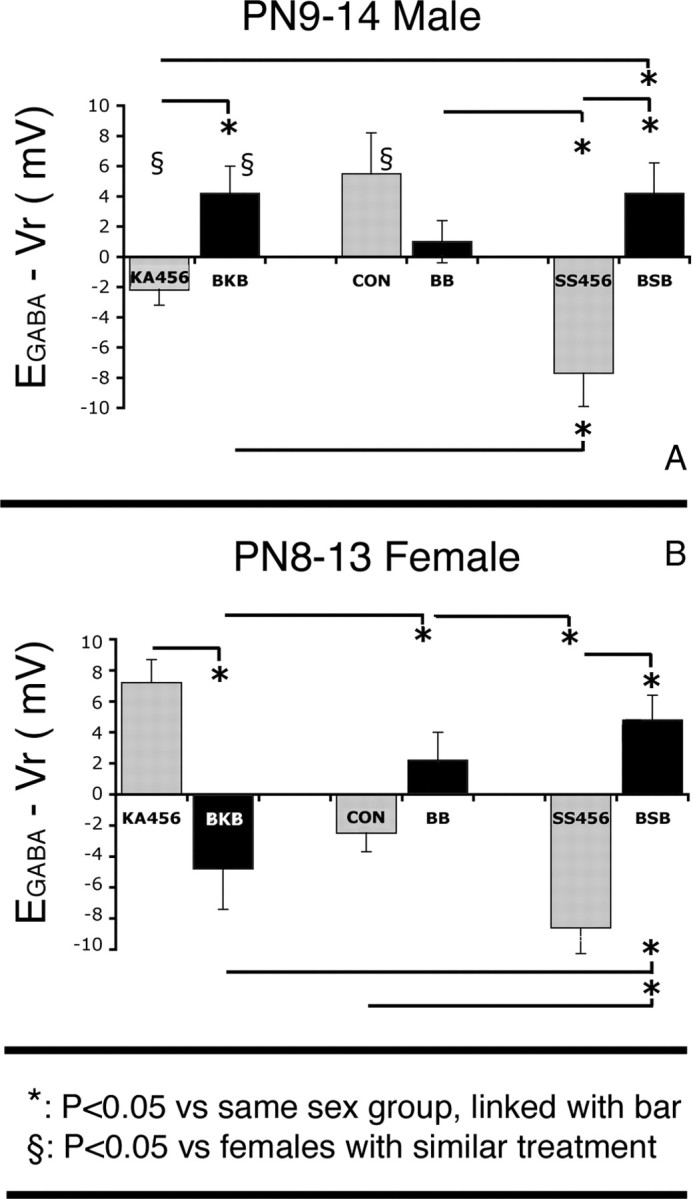
A, B, Effects of bicuculline treatment on EGABA − Vr of P9–P14 male (A) and P8–P13 female (B) rat CA1 pyramidal neurons. Bicuculline was given 10 min before kainic acid or saline injections and 6 h later at P4, P5, and P6, in both males and females. The bicuculline treatments reversed the directions of EGABA − Vr shifts in the CA1 pyramidal neurons of KA456 and SS456 groups, but their effects were not statistically significant in CON. No sex differences in EGABA − Vr were seen in BB-treated pups. Results represent the mean ± SEM. PN, Postnatal day.
Effects of maternal separation
Neonatal maternal separation causes a negative shift in EGABA
In both sexes, maternal separation resulted in hyperpolarized EGABA−Vr compared with same-sex CON pups (Fig. 3, Table 3). The effects of maternal separation were clearly separated from those of 3KA-SE in female but not in male pups (Fig. 3).
Effects of maternal separation on KCC2 and NKCC1
To identify the molecular correlates of the negative EGABA shifts in SS456 pups, the expression of KCC2-ir, NKCC1-ir, and bumetanide-sensitive chloride cotransport activity were determined.
KCC2-ir was increased in male SS456 CA1 pyramidal neurons compared with CONs, but was less than in KA456 pups (139.14 ± 15% of CON values; semiquantitative scale; n = 5 rats; p < 0.05 vs male CON and vs KA456) (Fig. 4). No changes in KCC2-ir were observed in female SS456 pups.
NKCC1-ir in CA1 pyramidal neurons of male and female SS456 pups showed no significant differences from either CON or KA456 groups. In contrast, [EGABA]bumetanide − EGABA values were reduced compared with same-sex groups in both males (−5.9 ± 0.7 mV, n = 4 neurons; p < 0.05 compared with male CON; no significant difference compared with male KA456) and females (−5.1 ± 0.8 mV; n = 5 neurons; p < 0.05 compared with female CON or KA456 groups) (Fig. 5).
These data indicate that prolonged maternal separation augments the hyperpolarizing GABAA responses in both sexes, yet through different molecular pathways. In male SS456 pups, this is effected by increase in KCC2-ir and less active bumetanide-sensitive chloride cotransport. In female SS456 pups, this is attributed only to lower bumetanide-sensitive chloride cotransport.
Role of GABAA receptors in the effects of maternal separation on EGABA
To determine whether the effects of maternal separation on EGABA were also mediated through GABAA receptors, the effects of two daily doses of bicuculline (BSB groups) (Table 1) were studied. Bicuculline treatment reversed the effects of maternal separation in both sexes (BSB groups) (Table 3, Fig. 6). These indicate that, similar to 3KA-SE, GABAA receptor inhibition at the time of maternal separation can reverse its effects on EGABA in both sexes, yet females are more sensitive, as they require only a single daily dose of bicuculline.
The transient aberrant switch of GABAA receptor signaling in female CA1 pyramidal neurons is not sufficient to trigger epileptogenesis
EEG and video monitoring was done in seven KA456 and three CON adult female rats to detect spontaneous seizures. No electrographic or behavioral seizures were observed, suggesting that the transient 3KA-SE induced reappearance of depolarizing GABAA signaling does not suffice to trigger epilepsy in adulthood.
Discussion
This is the first direct electrophysiological evidence in acute slices that the direction of GABAA receptor signaling in rat CA1 pyramidal neurons is sex specific in normal development and as a result of neonatal KA-SE. Maternal separation also influences EGABA. Specifically, hyperpolarizing GABAA responses occur earlier in female neonatal rat CA1 pyramidal neurons than in male. Neonatal 3KA-SE reverse the direction of GABAA responses, via GABAA receptor-mediated pathways. EGABA becomes hyperpolarizing in male and transiently depolarizing in female pups, after 3KA-SE, because of sex-specific changes in the expression or activity of chloride cotransporters. Maternal separation produces a negative shift in EGABA in both sexes, but with sex-specific regulatory effects on chloride cotransporters.
The estimated age of EGABA switch in male rats (P13.7) agrees with published studies using male or rats of undetermined sex (Khazipov et al., 2004; Banke and McBain, 2006). In most female CA1 pyramidal neurons, the switch has already occurred before P4. The rare depolarizing GABAA responses in females at P7–P13 may be random or attributed to the increase in carbonic anhydrase VII that favors depolarizing GABAA signaling (Rivera et al., 2005). These sex differences may explain the significant variability in EGABA − Vr measurements in previous studies, before EGABA switch (Khazipov et al., 2004; Banke and McBain, 2006). As is the case in the substantia nigra (Galanopoulou et al., 2003a), the earlier switch is driven by the higher KCC2 expression in females. Additionally, there is less active bumetanide-sensitive chloride transport in female neurons. GABAA receptor-mediated neuronal depolarizations can activate L-type voltage sensitive calcium channels (Reichling et al., 1994; Owens et al., 1996; Galanopoulou et al., 2003a), initiating a cascade of calcium-sensitive signaling processes important for differentiation, plasticity, migration, proliferation, and neuronal communication (Ben-Ari, 2002). Therefore, in P4–P14 rat CA1 pyramidal neurons, GABAA receptor activation, under physiologic conditions, can promote calcium-sensitive differentiation only in males, amplifying the already pre-existing sexually dimorphic phenotype of the neonatal hippocampus, as has been proposed for the substantia nigra neurons (Galanopoulou et al., 2003a; Galanopoulou, 2005a). Such events may contribute to the sexual dimorphism of the hippocampus, because it relates to morphology, connectivity, protein expression, and function (Cahill, 2006). Previously, sex-specific in vivo effects of high doses of the GABAAergic agonist muscimol on CREB (cAMP response element-binding protein) phosphorylation were reported in CA1 neurons (Auger et al., 2001). After the current manuscript was initially submitted, muscimol was reported to increase calcium in male but not in female neurons from mixed populations of hippocampal cells after 7 d in culture (Nunez and McCarthy, 2007).
3KA-SE at P4–P6 reverses the direction of GABAA signaling in both sexes by altering the expression of KCC2 and NKCC1. In males, 3KA-SE accelerated the EGABA switch to hyperpolarizing values, whereas in females it caused a transient reappearance of depolarizing GABAA responses. This is not caused by deafferentation (Nabekura et al., 2002), because no neuronal injury was noted in P7 hippocampi, regardless of treatment. The female data are in accordance with studies on adult or immature neurons with hyperpolarizing GABAAergic signaling at the time of seizures; under these circumstances, seizures decrease the relative expression of KCCs over NKCC1, favoring a switch to depolarizing GABAAergic signaling (Rivera et al., 2002, 2004; Khalilov et al., 2003; Okabe et al., 2003; Wake et al., 2007). A determining factor for the effects of seizures on EGABA is the direction of GABAA signaling at the time of SE. This is supported by the observation that bicuculline reverses the effects of 3KA-SE on EGABA in both sexes. It has been reported that multiple daily, brief flurothyl-induced seizures in neonatal rats do not change the timing of GABAA receptor switch (Isaeva et al., 2006). This may indicate that prolonged but not brief seizures influence EGABA switch. However, in that study, sex was not specified, and this may contribute to the lack of seizure effect on EGABA. Indeed, if sex is not accounted for in the EGABA − Vr scattergrams presented herein (Fig. 3), EGABA switch will occur around P14 in both CON and KA456 pups. Finally, the difference may be model specific. Flurothyl has GABAA receptor antagonistic effects (Krasowski, 2000). Consequently, flurothyl-induced seizures may be similar to our BKB groups, which counteract the effects of 3KA-SE on EGABA.
At the molecular level, the 3KA-SE-induced appearance of precocious hyperpolarizing GABAA responses in males is caused by an increase in KCC2-ir and decrease in bumetanide-sensitive chloride transport. Indeed, activation of GABAA receptors during SE can depolarize CA1 neurons and upregulate KCC2 (Galanopoulou et al., 2003a). The transient depolarizing GABAA responses in females, after 3KA-SE, are secondary to increased activity of bumetanide-sensitive cotransporters. Alternatively, other factors that relate to seizures, like the brain-derived neurotrophic factor (BDNF) may have cell type-specific regulatory effects on KCC2 expression (Rivera et al., 2004). Because BDNF is released during seizures (Rivera et al., 2002), it would be interesting to test whether sex differences in BDNF signaling exist in P4–P6 CA1 pyramidal neurons.
The aberrant re-emergence of depolarizing GABAA receptor signaling in epileptic tissues is proposed as an important feature of the epileptic state, involved in the generation of interictal and possibly ictal epileptic discharges (Cohen et al., 2002; Khalilov et al., 2003; Sipila et al., 2006). Interestingly, neither male nor female KA456 rats were able to maintain depolarizing GABAA receptor signaling beyond P14, suggesting that the developmental factors governing EGABA switch were stronger than the effects of 3KA-SE. Moreover, none of the female KA456 rats developed spontaneous seizures in young adulthood. Although more extended monitoring periods may be necessary, if depolarizing GABAA receptor signaling is a critical component of the epileptic state, the resilience of the immature brain to maintain depolarizing GABAAergic signaling after 3KA-SE may prevent epilepsy. Indeed, in the majority of in vivo studies in naive rodents, the risk of developing epilepsy is rare if SE is induced in the first postnatal week and increases with age thereafter (Stafstrom et al., 1992; Moshé, 1993; de Rogalski Landrot et al., 2001; Holmes et al., 2002; Roch et al., 2002; Raol et al., 2006; Suchomelova et al., 2006; Xiu-Yu et al., 2007).
GABAA receptors are activated by neurosteroids and mediate stress effects (Bianchi et al., 2002; Spigelman et al., 2002). Neonatal stress, including maternal separation, has long-term consequences on brain development, which can be sex specific (Nunez et al., 2000; Hsu et al., 2003; Zimmerberg and Kajunski, 2004; Stamatakis et al., 2006). It is shown here that prolonged maternal separation causes a negative EGABA shift in both sexes, although brief maternal separation did not influence EGABA (Isaeva et al., 2006). The precocious termination of the age-appropriate depolarizing GABAA signaling may disrupt normal brain development in male, but not in female, CA1 pyramidal neurons, which have already switched at the time of experimentation, leading to sex-specific long-term sequelae.
Maternal separation decreases bumetanide-sensitive chloride transport in both sexes. Low bumetanide concentrations preferentially inhibit NKCC1, decreasing EGABA (Russell, 2000; Dzhala et al., 2005; Banke and McBain, 2006; Reynolds et al., 2007). NKCC1-ir was similar among the groups, suggesting that bumetanide either inhibited other chloride transporters or led to post-translational inactivation of NKCC1. Stress signaling can modulate the activity of NKCC1 by altering its phosphorylation (Darman et al., 2001; Piechotta et al., 2002; Moriguchi et al., 2005; Anselmo et al., 2006; Delpire and Gagnon, 2006; Strange et al., 2006; Vitari et al., 2006). In males only, neonatal separation also increases KCC2-ir, contributing to the negative EGABA shift. This could result from stress-induced GABAA receptor-mediated depolarization of male CA1 pyramidal neurons (Galanopoulou et al., 2003a) or other sex-specific stress-signaling pathways.
The separation stress-induced decrease in EGABA requires active GABAA receptors, but is not directed by the depolarizing or hyperpolarizing GABAA PSCs. One possibility is that chloride gradient around stress-responsive, δ-subunit-containing extrasynaptic GABAA receptors (Smith et al., 2007) may not differ between male and female rat P4–P6 CA1 pyramidal neurons. In contrast, the observed sex differences in GABAA PSCs may pertain to synaptically located GABAA receptors, which are strongly activated during seizures. Intracellular differences in chloride concentration and KCC2 expression, both across the axonodendritic processes and around extrasynaptic GABAA receptors, have been described previously (Gulyas et al., 2001; Duebel et al., 2006; Gavrikov et al., 2006).
Prolonged bicuculline administration abolished the 3KA-SE, stress, and sex-related differences in EGABA − Vr, rendering it depolarizing, with single exception the female BKB group. GABAA receptors may therefore act as sensors and broadcasters of epigenetic (seizures, stress) and sex-related signals implicated in the regulation of EGABA. The negative EGABA shift in BKB female neurons may stem from GABAA-independent pathways activated by 3KA-SE in females. Alternatively, excessive GABA release during seizures may reverse the bicuculline inhibition of GABAA receptors in females.
In summary, the direction of GABAA receptor signaling in rat neonatal CA1 pyramidal neurons is sex specific, potentially contributing to their sexual differentiation. Consequently, seizures have sex-specific effects on hippocampal GABAA-related function, distinct from separation stress, with important repercussions for brain development and possibly epileptogenesis.
Footnotes
This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke Grants NS 45243 and 20253, and grants from the Rett Syndrome Research Foundation and the Heffer Family Medical Foundation. I am grateful to Drs. Solomon Moshé and Dominick Purpura for helpful discussions, feedback, and support related to this work. I would also like to acknowledge the excellent technical assistance of Ms. Qianyun Li with the immunochemistry assays and Dr. Lenka Chudomelova and Ms. Hong Wang with electrode placement surgeries, as well as Dr. Andreas Kyrozis for introduction to the gramicidin-perforated patch clamp.
References
- Akaike N. Glycine responses in rat CNS neurons studied with gramicidin perforated patch recording. Jpn J Physiol. 1994;44(Suppl 2):S113–S118. [PubMed] [Google Scholar]
- Akers KG, Nakazawa M, Romeo RD, Connor JA, McEwen BS, Tang AC. Early life modulators and predictors of adult synaptic plasticity. Eur J Neurosci. 2006;24:547–554. doi: 10.1111/j.1460-9568.2006.04921.x. [DOI] [PubMed] [Google Scholar]
- Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl- cotransporter in HeLa cells. Proc Natl Acad Sci USA. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, Gorter JA. Differential expression patterns of chloride transporters, Na+-K+-2Cl-cotransporter and K+-Cl-cotransporter, in epilepsy-associated malformations of cortical development. Neuroscience. 2007;145:185–196. doi: 10.1016/j.neuroscience.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci. 2006;26:11720–11725. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the delta subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Darman RB, Flemmer A, Forbush B. Modulation of ion transport by direct targeting of protein phosphatase type 1 to the Na-K-Cl cotransporter. J Biol Chem. 2001;276:34359–34362. doi: 10.1074/jbc.C100368200. [DOI] [PubMed] [Google Scholar]
- Delpire E, Gagnon KB. SPAK and OSR1, key kinases involved in the regulation of chloride transport. Acta Physiol (Oxf) 2006;187:103–113. doi: 10.1111/j.1748-1716.2006.01565.x. [DOI] [PubMed] [Google Scholar]
- de Rogalski Landrot I, Minokoshi M, Silveira DC, Cha BH, Holmes GL. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Brain Res Dev Brain Res. 2001;129:27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- Duebel J, Haverkamp S, Schleich W, Feng G, Augustine GJ, Kuner T, Euler T. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron. 2006;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Vidaurre J, Moshé SL. Under what circumstances can seizures produce hippocampal injury: evidence for age-specific effects. Dev Neurosci. 2002;24:355–363. doi: 10.1159/000069047. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. GABA receptors as broadcasters of sexually differentiating signals in the brain. Epilepsia. 2005a;46(Suppl 5):107–112. doi: 10.1111/j.1528-1167.2005.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sex-specific effects of kainate-induced neonatal seizures on KCC2 mRNA expression in the hippocampus of infant rats. Neurology. 2005b;64(Suppl 1):A135. [Google Scholar]
- Galanopoulou AS. Sex- and cell-type-specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur J Neurosci. 2006;23:2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Effects of neonatal status epilepticus and stress on GABAA receptor signaling in the hippocampus: importance of gender. Epilepsia [Erratum (2007) 48:2379] 2007a;48(Suppl 6):119. [Google Scholar]
- Galanopoulou AS. Severe neonatal status epilepticus and stress prematurely terminate the depolarizing GABAA-ergic signaling in male hippocampus: implications for an altered brain development, but not necessarily epileptic. Ann Neurol. 2007b;62(Suppl 11):S29. [Google Scholar]
- Galanopoulou AS. Developmental patterns in the regulation of chloride homeostasis and GABA(A) receptor signaling by seizures. Epilepsia. 2007c;48(Suppl 5):14–18. doi: 10.1111/j.1528-1167.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Moshé SL. Kainate-induced status epilepticus increases KCC2 mRNA expression in the hippocampus of immature rats. Soc Neurosci Abstr. 2004;30:946.20. [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABAA receptor function in rat substantia nigra. Exp Neurol. 2003a;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Alm EM, Veliskova J. Estradiol reduces seizure-induced hippocampal injury in ovariectomized female but not in male rats. Neurosci Lett. 2003b;342:201–205. doi: 10.1016/s0304-3940(03)00282-9. [DOI] [PubMed] [Google Scholar]
- Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci USA. 2006;103:18793–18798. doi: 10.1073/pnas.0604551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Sik A, Payne JA, Kaila K, Freund TF. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur J Neurosci. 2001;13:2205–2217. doi: 10.1046/j.0953-816x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- Holmes GL. The long-term effects of seizures on the developing brain: clinical and laboratory issues. Brain Dev. 1991;13:393–409. doi: 10.1016/s0387-7604(12)80037-4. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Khazipov R, Ben-Ari Y. New concepts in neonatal seizures. NeuroReport. 2002;13:A3–A8. doi: 10.1097/00001756-200201210-00002. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci USA. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Khazipov R, Holmes GL. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur J Neurosci. 2006;23:1559–1566. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Acute and chronic effects of seizures in the developing brain: experimental models. Epilepsia. 1999;40(Suppl 1):S51–S58. doi: 10.1111/j.1528-1157.1999.tb00879.x. discussion S64–S56. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Krasowski MD. Differential modulatory actions of the volatile convulsant flurothyl and its anesthetic isomer at inhibitory ligand-gated ion channels. Neuropharmacology. 2000;39:1168–1183. doi: 10.1016/s0028-3908(99)00221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Chudomel O, Moshé SL, Galanopoulou AS. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci Lett. 2006;398:1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Lado FA, Sperber EF, Moshé SL. Anticonvulsant efficacy of gabapentin on kindling in the immature brain. Epilepsia. 2001;42:458–463. doi: 10.1046/j.1528-1157.2001.30900.x. [DOI] [PubMed] [Google Scholar]
- Lai MC, Holmes GL, Lee KH, Yang SN, Wang CA, Wu CL, Tiao MM, Hsieh CS, Lee CH, Huang LT. Effect of neonatal isolation on outcome following neonatal seizures in rats—the role of corticosterone. Epilepsy Res. 2006;68:123–136. doi: 10.1016/j.eplepsyres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Moore-Hoon ML, Turner RJ. Molecular and topological characterization of the rat parotid Na+-K+-2Cl- cotransporter1. Biochim Biophys Acta. 1998;1373:261–269. doi: 10.1016/s0005-2736(98)00112-6. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- Moshé SL. Seizures in the developing brain. Neurology. 1993;43:S3–7. [PubMed] [Google Scholar]
- Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, Fukuda A, Akaike N. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J Neurosci. 2002;22:4412–4417. doi: 10.1523/JNEUROSCI.22-11-04412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Koss WA, Juraska JM. Hippocampal anatomy and water maze performance are affected by neonatal cryoanesthesia in rats of both sexes. Horm Behav. 2000;37:169–178. doi: 10.1006/hbeh.2000.1572. [DOI] [PubMed] [Google Scholar]
- Okabe A, Yokokura M, Toyoda H, Shimizu-Okabe C, Ohno K, Sato K, Fukuda A. Changes in chloride homeostasis-regulating gene expressions in the rat hippocampus following amygdala kindling. Brain Res. 2003;990:221–226. doi: 10.1016/s0006-8993(03)03528-5. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABAA-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006;47:1665–1673. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol (Lond) 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol (Lond) 2007;582:507–524. doi: 10.1113/jphysiol.2007.129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol (Lond) 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch C, Leroy C, Nehlig A, Namer IJ. Predictive value of cortical injury for the development of temporal lobe epilepsy in 21-day-old rats: an MRI approach using the lithium-pilocarpine model. Epilepsia. 2002;43:1129–1136. doi: 10.1046/j.1528-1157.2002.17802.x. [DOI] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Schuchmann S, Voipio J, Yamada J, Kaila K. The cation-chloride cotransporter NKCC1 promotes sharp waves in the neonatal rat hippocampus. J Physiol (Lond) 2006;573:765–773. doi: 10.1113/jphysiol.2006.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa Y, Monokoshi M, Silveira DC, Cha BH, Cilio MR, McCabe BK, Liu X, Hu Y, Holmes GL. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Brain Res Dev Brain Res. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behav and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res Dev Brain Res. 1992;65:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Mantelas A, Papaioannou A, Pondiki S, Fameli M, Stylianopoulou F. Effect of neonatal handling on serotonin 1A sub-type receptors in the rat hippocampus. Neuroscience. 2006;140:1–11. doi: 10.1016/j.neuroscience.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Strange K, Denton J, Nehrke K. Ste20-type kinases: evolutionarily conserved regulators of ion transport and cell volume. Physiology (Bethesda) 2006;21:61–68. doi: 10.1152/physiol.00139.2005. [DOI] [PubMed] [Google Scholar]
- Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–243. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]
- Swann JW. The effects of seizures on the connectivity and circuitry of the developing brain. Ment Retard Dev Disabil Res Rev. 2004;10:96–100. doi: 10.1002/mrdd.20018. [DOI] [PubMed] [Google Scholar]
- Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J Biol Chem. 1999;274:12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- Xiu-Yu S, Ruo-Peng S, Ji-Wen W. Consequences of pilocarpine-induced recurrent seizures in neonatal rats. Brain Dev. 2007;29:157–163. doi: 10.1016/j.braindev.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Kajunski EW. Sexually dimorphic effects of postnatal allopregnanolone on the development of anxiety behavior after early deprivation. Pharmacol Biochem Behav. 2004;78:465–471. doi: 10.1016/j.pbb.2004.03.021. [DOI] [PubMed] [Google Scholar]