Abstract
Background
Chronic kidney disease (CKD) is associated with multiple comorbidities, hospitalizations and mortality. In older adults, social isolation and poor mobility contribute to these outcomes. We tested the hypothesis that a glomerular filtration rate (GFR) <45 mL/min/1.73 m2 (CKD Stages 3b–5) is associated with social isolation and that mobility limitation is a key driver of social isolation in patients with CKD.
Methods
Data from 9119 participants, ages 57–107 years, from the 2016 wave of the Health and Retirement Study’s Venous Blood Study were used for this cross-sectional analysis. Kidney function measured by estimated GFR (eGFR) was the predictor and patients were classified as CKD Stages 3b–5 or non-CKD Stages 3b–5 (eGFR ≤45 or >45 mL/min/1.73 m2). The outcomes tested were mobility limitation assessed by self-report and social contact and participation measures assessed by the Psychosocial Life Questionnaire. The associations among kidney function, mobility and social isolation were examined with logistic and ordinary least squares regression, adjusted for covariates and testing for interaction with gender.
Results
Participants with CKD Stages 3b–5 (N = 999) compared with non-CKD Stages 3b–5 were older (74.9 versus 68.2 years, P < 0.001) and fewer were female (15% versus 58%, P < 0.001). CKD Stages 3b–5 were associated with higher odds of difficulty walking several blocks [odds ratio 1.44 (95% confidence interval 1.16–1.78)]. Participants with CKD Stages 3b–5 had reduced social contact and social participation (B = −0.23, P < 0.05; B = −0.62, P < 0.05, respectively). Women with CKD Stages 3b–5 were 2.7 times more likely to report difficulty walking several blocks than men with CKD Stages 3b–5, but social isolation in CKD Stages 3b–5 did not vary by gender. In CKD Stages 3b–5 patients, mobility limitation was a risk factor for reduced social contact and participation but did not explain the poor social contact and participation.
Conclusion
CKD Stages 3b–5 was associated with both mobility limitation and social isolation in a population-based study of older adults. In contrast to older adults without CKD Stages 3b–5, mobility limitation did not explain the lack of social contact and poor social participation, suggesting other factors are more important.
Keywords: CKD, elderly, mobility, outcomes, social isolation
INTRODUCTION
The population of American adults >65 years of age increased from 12.4% to 15.2% from 2000 to 2016 [1]. In the elderly, social and family support are associated with the preservation of functional ability. ‘Social isolation’ is an objective measure of a lack of social interactions/engagement and contact with family or friends and is well documented in older adults. Studies have demonstrated a positive effect of social engagement in the elderly, with men particularly affected [2]. Mechanisms by which social engagement improve well-being include dissemination of health-related information, provision of illness coping strategies and promoting healthy attitudes, behavior and self-worth.
A lack of social contact is associated with cardiovascular disease, cerebrovascular disease and dementia [3], problems that are also highly prevalent in chronic kidney disease (CKD). In addition, social isolation is temporally associated with mortality in the elderly, as shown in a meta-analysis of 70 studies (3 407 134 participants) with a mean follow-up of 7 years, where social isolation was associated with a 29% increased relative risk for death [3]. Social isolation has been reported in patients undergoing dialysis [4] and in transplant recipients [5], but in pre-dialysis CKD, the prevalence of social isolation and its risk factors has not been quantified.
Between 21 and 25% of older adults have CKD Stages 3–5 [6]. CKD is often called premature aging, with a high risk of hospitalizations, decreased functional ability and overt disability. In the elderly without CKD, a risk factor for social isolation is mobility limitation. In 1321 participants in the National Survey of American Life, older age, male gender, those requiring less self-care and higher mobility impairment were associated with social isolation from friends [7]. Another study showed that the Short Physical Performance Battery, a validated test for physical function in the elderly, strongly correlates with social isolation (r = 0.42, P < 0.01).
In CKD, multiple studies have shown poor mobility [8]. In the Health Aging and Body Composition Study, older adults with CKD were more likely to develop difficulty walking or climbing stairs compared with those without CKD, after adjustment for demographics [9]. Similarly, in the Framingham Offspring Cohort, those with CKD had 91% increased age- and gender-adjusted odds of developing mobility–disability over time compared with those without CKD [10]. The etiology of musculoskeletal disease and mobility impairment in CKD is multifactorial [11]. However, the contribution of mobility limitation to social isolation is unknown in CKD. We hypothesized that mobility limitation is a key driver of social isolation in males and females with CKD. To test this hypothesis, we determined whether mobility limitation explains differences in social isolation between those with and without CKD, when accounting for gender, using data from the Health and Retirement Study (HRS).
MATERIALS AND METHODS
Dataset
The HRS is a longitudinal study, supported by the National Institute on Aging and the Social Security Administration, of a representative sample of community-dwelling, noninstitutionalized adults in the USA [12, 13]. Data collection started in 1992 and a new cohort of individuals 51–56 years of age was added every 6 years, with a total of 13 waves of data collected on 43 478 individuals. At each time point, core surveys were collected about retirement, disability, occupation, industry, work environment, income, demographics, health conditions and healthcare utilization, cognition, physical functioning, family characteristics and insurance. For this analysis, we used data from the HRS 2016 core survey [14] as well as two datasets collected in 2016, the Venous Blood Study (VBS) with home blood collections [15] and the Psychosocial and Lifestyle Questionnaire (PLQ). In 2016, all HRS respondents were asked whether they would consent to a venous blood draw. The VBS cohort comprises respondents with valid data who consented to a blood draw and met with phlebotomists to provide a blood sample. The PLQ cohort is a ‘half-sample’ of the 2016 HRS respondents. The PLQ cohort comprises respondents who completed the PLQ and who provided venous blood samples in 2016. Therefore all participants in the PLQ cohort were part of the VBS cohort.
The entire HRS is under current institutional review board approval by the relevant committees at the University of Michigan and the National Institute on Aging, the primary sponsor of the HRS. This analysis is approved by the institutional review board at the Indiana University School of Medicine.
Predictors
Kidney function was determined based on estimated glomerular filtration rate (eGFR). Serum creatinine was measured by the enzymatic method (Roche Diagnostics, Indianapolis, IN, USA) with calibration semi-annually to isotope dilution mass spectrometry standards [16]. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [17] and CKD Stages 3b–5 was defined as an eGFR ≤45/mL/min/1.73 m2 and non-CKD Stages 3b–5 as an eGFR >45/mL/min/1.73 m2. A measure of albuminuria was not available, so CKD cannot be excluded in those with an eGFR >60 mL/min/1.73 m2. Also, given the single creatinine measure, the absence of data on albuminuria and the potential for ‘normal’ age reduction in eGFR [18], we chose a lower eGFR threshold (45 mL/min/1.73 m2 for comparisons).
Outcomes
Our outcomes of interest were self-reported mobility limitation and standardized social interaction-related outcomes called ‘social contact’ and ‘participation in social activities’.
Mobility limitation was measured by self-report of the difficulty in completing four different tasks: walking several blocks, walking one block, climbing several flights of stairs and climbing one flight of stairs. A mobility limitation index was created by summing all four tasks (0 = no difficulty, 4 = difficulty with all tasks).
Social contact was quantified by a self-administered questionnaire, the Participant Lifestyle Questionnaire (PLQ), collected from a random half-sample of the population every 4 years. Questions included participation in general activities, relationships with others and views on life. Based on this survey, the outcome ‘social contact’ is a measure of nonspouse contact with friends and family. Respondents were asked to indicate how often they met in person with their adult children, extended family and friends. The response categories ranged from 1 (less than once a year/never) to 6 (three or more times per week). A total ‘frequency of contact’ measure was calculated by averaging the three types of social contact. Higher values represented more social contact, whereas lower values represented more social isolation from friends and family. ‘Social participation’ was assessed by quantifying how often the subject participated in six social activities: taking care of grandchildren, volunteering for youth organizations, charity work, educational activities, social clubs and civic organizations. Responses ranged from 0 (never/not relevant) to 6 (daily). A global social participation measure was created by summing all six categories.
Covariates
Sociodemographic covariates included age, gender, race/ethnicity, formal education in years and marital status. Comorbidities were obtained by self-report: yes/no for hypertension, diabetes, heart condition, stroke and trouble with pain. Body mass index (BMI) was calculated from self-reported height and weight and ‘obese’ was defined as a BMI >30 kg/m2.
Statistical analysis
Sociodemographic characteristics, self-reported mobility limitation and self-reported health conditions (comorbidities) were compared across CKD Stages 3b–5 versus non-CKD Stages 3b–5 using t-tests for continuous variables and chi-squared tests for categorical measures.
Logistic regression with self-reported mobility limitation as the dependent variable and CKD Stages 3b–5 as the independent variable was performed. Two models were created for the relationship of components of mobility and the mobility index with CKD Stages 3b–5. Model 1 was adjusted for sociodemographic characteristics (age, gender, race/ethnicity, formal education in years and marital status) and Model 2 for sociodemographic characteristics and comorbidities (yes/no for hypertension, diabetes, heart condition, stroke and trouble with pain). For the mobility limitation index as the outcome, proportional odds ratios (ORs) were created due to the ordinal nature of the variable and cumulative probabilities were calculated. Interactions for gender differences in the effect of CKD Stages 3b–5 on self-reported mobility limitation were tested formally with differences in social contact in aging [19, 20]. An interaction variable was created (i.e. female × difficulty walking several blocks) and predicted probabilities were calculated and graphed. Model fit was assessed by likelihood ratios (LRs).
For ‘social contact’ and ‘social participation’, ordinary least squares were used to test each of their relationships with CKD Stages 3b–5. Two models were built to test this association. Model 1 studied the relationship of CKD Stages 3b–5 and gender on social contact and participation adjusted for sociodemographic factors and comorbidities. In Model 2, self-reported mobility limitation was entered into Model 1 to evaluate whether mobility limitation explained any association between CKD Stages 3b–5 and social contact/participation. Model fit was assessed by R2. A third set of models was created to test whether the effect of CKD Stages 3b–5 on social isolation varied by gender. In Model 3, an interaction term (i.e. female × CKD Stages 3b–5) was added to fully adjusted models.
A sensitivity analysis was performed for the relationship of CKD Stages 3b–5 with demographics, mobility limitation and social isolation using a more traditional cut-off for CKD Stages 3b–5 with eGFR > and <60 mL/min/1.73 m2.
All analyses were weighted with the 2016 VBS scaled sample weights. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Subject characteristics for the analysed population are shown in Table 1, with the first section for the VBS respondents (N = 8806) and the lower rows showing variables from the PLQ subset (N = 3421). The mean age of the VBS sample was 69 ± 8.9 years, with 13.2 ± 2.9 years of education and 59% of the sample was married. On average, respondents reported having difficulty with one mobility limitation task, most commonly climbing stairs. Thirty-one percent of the sample reported difficulty walking several blocks. The mean eGFR was 75.5 ± 22.3 mL/min/1.73 m2. Ten percent of the study population met our definition of CKD Stages 3b–5 (eGFR ≤45 mL/min/1.73 m2), whereas 29% had an eGFR <60 mL/min/1.73 m2. The most common self-reported comorbidity was hypertension, and 40% of the population reported pain. BMI was available on 8683 respondents and 33% of them were obese (BMI >30 kg/m2).
Table 1.
Descriptive statistics of the overall study population
| Predictors and covariates | Value | Standard deviation | Range |
|---|---|---|---|
| Sociodemographic characteristics (N = 8806) | |||
| Age (years), mean | 68.9 | 8.96 | 57–107 |
| Female, % | 54 | 0–1 | |
| Race/ethnicity, % | |||
| Non-Hispanic White | 78 | 0–1 | |
| Non-Hispanic Black | 10 | 0–1 | |
| Hispanic/Latino | 9 | 0–1 | |
| Other race | 3 | 0–1 | |
| Education (years), mean | 13.2 | 2.92 | 0–17 |
| Married, % | 59 | 0–1 | |
| Self-reported mobility limitation (N = 8806), % | |||
| Difficulty walking several blocks | 31 | 0–1 | |
| Difficulty walking one block | 15 | 0–1 | |
| Difficulty climbing several flights of stairs | 46 | 0–1 | |
| Difficulty climbing one flight of stairs | 19 | 0–1 | |
| Mobility Limitation Index, mean | 1.11 | 0–4 | |
| CKD biomarkers (N = 8806) | |||
| Serum creatinine (mg/dL), mean | 0.96 | 0.46 | 0.28–14.1 |
| eGFR (mL/min/1.73 m2), mean | 75.5 | 22.33 | 2.69–140.25 |
| CKD Stages 3b–5 (eGFR ≤45 mL/min/1.73 m2), % | 10 | 0–1 | |
| CKD Stages 3–5 (eGFR ≤60 mL/min/1.73 m2), % | 29 | 0–1 | |
| Self-reported health conditions (N = 8806), % | |||
| Hypertension | 59 | 0–1 | |
| Diabetes | 25 | 0–1 | |
| Heart condition | 25 | 0–1 | |
| Stroke | 7 | 0–1 | |
| Trouble with pain | 40 | 0–1 | |
| BMI (N = 8683), mean | 28.51 | 6.01 | 10.30–70.69 |
| Obese (BMI >30 kg/m2), % | 33 | 0–1 | |
| Social contact and participation* (N = 3420), mean | |||
| Frequency of contact with family and friends | 3.68 | 1.07 | 1–6 |
| Adult children | 3.84 | 1.42 | 1–6 |
| Extended family | 3.19 | 1.47 | 1–6 |
| Friends | 4.07 | 1.30 | 1–6 |
| Participation in social activities | 6.41 | 4.95 | 0–31 |
| Taking care of grandchildren | 2.08 | 1.88 | 0–6 |
| Volunteering for youth organizations | 0.59 | 1.14 | 0–6 |
| Charity work | 1.10 | 1.51 | 0–6 |
| Educational activities | 0.62 | 1.14 | 0–6 |
| Social clubs | 1.35 | 1.61 | 0–6 |
| Civic organizations | 0.71 | 1.09 | 0–6 |
Significant differences (P < 0.05) between the full and half-sample include race/ethnicity (e.g. White: 78% versus 82%); no other variables are significantly different among the two samples.
The sample size of the PLQ half-sample was 3421, and this is a subset of the VBS respondents. The average frequency of social contact score was 3.68, which corresponded to meeting up about once or twice a month. The average social participation score was 6.41 out of an observed range of 0–31 and thus respondents participated in social activities infrequently. The most common activity was taking care of grandchildren. Comparison of the VBS with the PLQ cohorts showed that there were differences in race and ethnicity, but no other measures (see Supplementary data, Table S1).
Tables 2 and 3 show results from the VBS respondents. Table 2 shows differences between those with and without CKD Stages 3b–5. Subjects with CKD Stages 3b–5 were significantly older, more likely to be male, reported significantly more mobility limitation for each indicator and had a higher overall mobility index compared with those with higher eGFR (all P < 0.001). Those with CKD Stages 3b–5 also had a significantly higher prevalence of comorbidities and health conditions compared with the non-CKD Stages 3b–5 group, with the notable exception of pain. BMI as well as the percentage of obese subjects were not significantly different between those with or without CKD Stages 3b–5.
Table 2.
Descriptive characteristics of the study population by CKD status (N = 8806)
| Predictors and covariates | CKD status |
P-value | |
|---|---|---|---|
| CKD Stages 3b–5 (n = 999) | Non-CKD Stages 3b–5 (n = 7807) | ||
| Sociodemographic characteristics | |||
| Age (years), mean ± SD | 74.92 ± 8.9 | 68.20 ± 9.0 | <0.001 |
| Female, % | 15 | 58 | <0.001 |
| Race/ethnicity, % | |||
| Non-Hispanic White | 78 | 78 | 0.53 |
| Non-Hispanic Black | 11 | 10 | 0.35 |
| Hispanic/Latino | 8 | 9 | 0.22 |
| Other race | 3 | 3 | 0.21 |
| Education (years), mean ± SD | 12.78 ± 3.3 | 13.26 ± 3.2 | <0.001 |
| Married, % | 61 | 59 | 0.13 |
| Self-reported mobility limitation, % | |||
| Difficulty walking several blocks | 45 | 29 | <0.001 |
| Difficulty walking one block | 24 | 14 | <0.001 |
| Difficulty climbing several flights of stairs | 55 | 45 | <0.001 |
| Difficulty climbing one flight of stairs | 28 | 18 | <0.001 |
| Mobility Limitation Index, mean ± SD | 1.52 ± 1.5 | 1.06 ± 1.4 | <0.001 |
| Self-reported health conditions, % | |||
| Hypertension | 78 | 57 | <0.001 |
| Diabetes | 39 | 23 | <0.001 |
| Heart condition | 47 | 22 | <0.001 |
| Stroke | 14 | 6 | <0.001 |
| Trouble with pain | 39 | 40 | 0.50 |
| Obese (BMI >30 kg/m2), % | 31 | 34 | 0.13 |
| BMI, mean ± SD | 28.4 ± 5.2 | 28.5 ± 6.1 | 0.45 |
SD, standard deviation.
Table 3.
OR estimates (95% confidence intervals) of mobility limitation by CKD status (N = 8806)
| Predictors and covariates | Mobility Limitation Index |
Difficulty walking several blocks |
Difficulty walking one block |
Difficulty climbing several flights |
Difficulty climbing one flight |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| eGFR ≤45 mL/min/1.73 m2 CKD Stages 3b–5 | 1.68*** (1.42–2.00) | 1.32** (1.10–1.58) | 1.80*** (1.49–2.18) | 1.44*** (1.16–1.78) | 1.78*** (1.41–2.25) | 1.43** (1.11–1.82) | 1.44*** (1.19–1.74) | 1.09 (0.89–1.34) | 1.87*** (1.49–2.36) | 1.47** (1.16–1.87) |
| Female | 1.79*** (1.60–2.01) | 1.76*** (1.57–1.99) | 1.49*** (1.30–1.71) | 1.39*** (1.20–1.62) | 1.39*** (1.16–1.66) | 1.23* (1.02–1.49) | 1.96*** (1.74–2.22) | 2.02*** (1.77–2.31) | 2.08*** (1.75–2.47) | 2.01*** (1.68–2.40) |
| Intercept 1 | −1.83*** | −3.18*** | −2.44*** | −4.37*** | −2.86*** | −4.60*** | −2.15*** | −3.46*** | −2.77*** | −4.13*** |
| Intercept 2 | −2.71*** | −4.24*** | – | – | – | – | – | – | – | – |
| Intercept 3 | −3.46*** | −5.13*** | – | – | – | – | – | – | – | – |
| Intercept 4 | −4.17*** | −5.95*** | – | – | – | – | – | – | – | – |
| DF | 8 | 13 | 8 | 13 | 8 | 13 | 8 | 13 | 8 | 13 |
| LR | 1478.42*** | 3346.14*** | 948.27*** | 2484.20*** | 527.907*** | 1374.39*** | 1185.30*** | 2376.76*** | 807.52*** | 1629.68*** |
DF: degrees of freedom; LR, likelihood ratio. Model 1: adjusted for sociodemographic characteristics; Model 2: Model 1 + self-reported health conditions.
P < 0.05;
P < 0.01;
P < 0.001.
VBS respondents
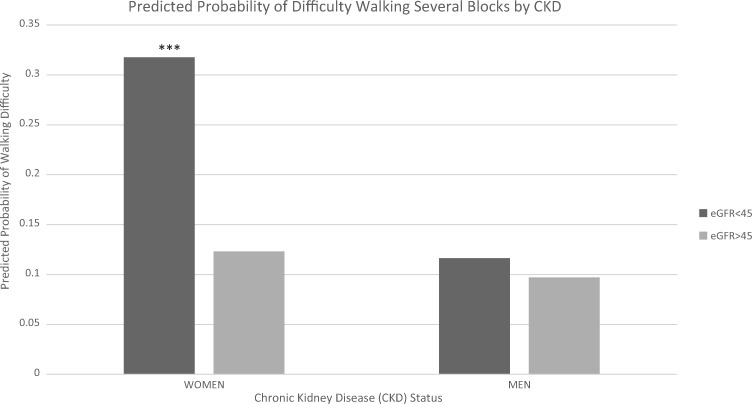
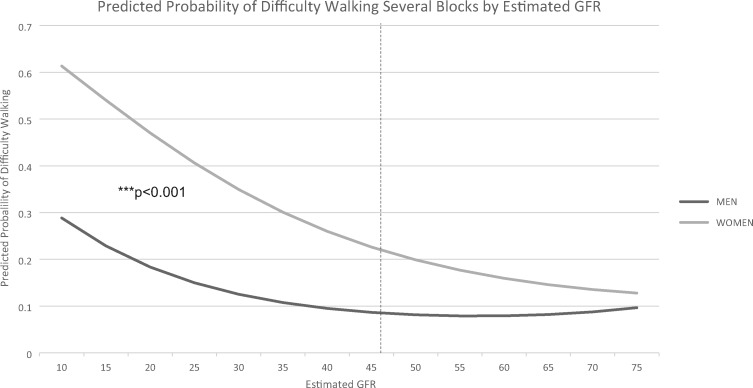
Table 3 lists the OR estimates of mobility limitation in subjects with compared with without CKD Stages 3b–5. In the fully adjusted model (Model 2), the cumulative probability that a participant with CKD Stages 3b–5 had difficulty with one or more mobility tasks was 0.41 compared with 0.35 for a participant without CKD Stages 3b–5. For each of the individual mobility assessments, CKD Stages 3b–5 was associated with a 1.44–1.87 times increase in Model 1 versus a 1.09–1.47 increase in Model 2 after fully adjusting for comorbidities. In each model (with the exception of the fully adjusted one for difficulty climbing several flights of stairs), CKD Stages 3b–5 was significantly associated with increased odds of mobility limitation. Women had greater odds of mobility limitation across all measures in both models. We therefore examined an interaction between difficulty walking several blocks and gender. Women with CKD Stages 3b–5 had higher predicted probabilities of reporting difficulty walking several blocks compared with men (Figure 1). Figure 2 shows the predicted probability of reporting difficulty walking several blocks for women and men using a continuous, nonlinear measure (i.e. quadratic) of eGFR. An eGFR of 45 mL/min/1.73 m2 is associated with a visual decline in the predicted probability of difficulty walking several blocks in males and females.
FIGURE 1.
Predicted probability of difficulty walking several blocks by CKD status and gender. Predicted probabilities are calculated from binary logistic regression and are fully adjusted (i.e. sociodemographic characteristics and self-reported health conditions). All covariates are set at the sample mean. ***P < 0.001.
FIGURE 2.
Predicted probability of difficulty walking several blocks by eGFR values and gender. Predicted probabilities are calculated from gender-stratified models, which are fully adjusted (i.e. sociodemographic characteristics and self-reported health conditions). All covariates are set at the sample mean.
PLQ respondents
Table 4 presents social contact and social participation scores for those with and without CKD Stages 3b–5 and were part of the PLQ cohort. Compared with those without CKD Stages 3b–5, those with CKD Stages 3b–5 participated significantly less in all activities (all P < 0.01), with the exception of civic organizations, whereas those with CKD Stages 3b–5 and non-CKD Stages 3b–5 participated equally infrequently.
Table 4.
Bivariate statistics by CKD status (N = 3420)
| Social contact and participation | CKD Stages 3b–5 |
P-value | |
|---|---|---|---|
| Present (n = 399) | Absent (n = 3022) | ||
| Frequency of contact with family and friends | 3.37 ± 1.2 | 3.72 ± 1.1 | <0.001 |
| Adult children | 3.56 ± 1.5 | 3.87 ± 1.4 | <0.001 |
| Extended family | 2.90 ± 1.5 | 3.22 ± 1.5 | <0.001 |
| Friends | 3.76 ± 1.5 | 4.11 ± 1.3 | <0.001 |
| Participation in social activities | 5.13 ± 4.6 | 6.56 ± 5.0 | <0.001 |
| Taking care of grandchildren | 1.82 ± 1.8 | 2.11 ± 1.9 | 0.01 |
| Volunteering for youth organizations | 0.37 ± 0.9 | 0.62 ± 1.2 | <0.001 |
| Charity work | 0.82 ± 1.4 | 1.14 ± 1.6 | <0.001 |
| Educational activities | 0.38 ± 1.0 | 0.64 ± 1.0 | <0.001 |
| Social clubs | 1.17 ± 1.6 | 1.37 ± 1.6 | 0.02 |
| Civic organizations | 0.61 ± 1.2 | 0.72 ± 1.1 | 0.05 |
Values presented as mean ± standard deviation. Frequency of contact (6 = three or more times per week); higher values equal more contact. Participation in social activities (6 = more than once a week); higher values equal more participation.
Table 5 shows the models of estimates for both social contact and social participation (PLQ cohort). Model 1 is adjusted for sociodemographic characteristics and self-reported health conditions. Model 2 includes difficulty walking several blocks as an indicator of mobility limitation. CKD Stages 3b–5 was independently associated with a lower frequency of social contact (0.23 decrease on a scale of 1–6) and lower social participation (0.62 decrease on a scale of 0–6). When evaluating standardized betas of social contact (Model 2), CKD Stages 3b–5 had the second largest effect size (β = −0.07) next to female gender (β = 0.10).
Table 5.
Frequency of contact and participation in social activities by CKD status (N = 3420) OLS regression for coefficient estimates (and standard errors)
| Predictors and covariates | Frequency of contact with friends and family |
Participation in social activities |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| CKD Stages 3b–5 | −0.23** (0.09) | −0.23* (0.09) | −0.28** (0.09) | −0.67* (0.30) | −0.62* (0.29) | −0.60 (0.33) |
| Female | 0.20*** (0.05) | 0.21*** (0.05) | 0.20*** (0.05) | 0.04 (0.22) | 0.15 (0.22) | 0.15 (0.23) |
| Difficulty walking several blocks | – | −0.15* (0.06) | −0.15** (0.06) | – | −1.36*** (0.23) | −1.36*** (0.23) |
| Female × CKD Stages 3b–5 | – | – | 0.31 (0.25) | – | – | −0.11 (0.62) |
| Intercept | 3.42*** | 3.38*** | 3.39*** | 3.72*** | 3.37*** | 3.37*** |
| DF | 3419 | 3419 | 3419 | 3419 | 3419 | 3419 |
| R 2 | 0.030 | 0.036 | 0.035 | 0.121 | 0.133 | 0.133 |
DF: degrees of freedom. Models adjusted for sociodemographic characteristics and self-reported health conditions.
P < 0.05;
P < 0.01;
P < 0.001.
Model 1: CKD Stages 3b–5 and sociodemographic factors. Model 2: Model 1 + difficulty walking several blocks + self-reported health conditions. Model 3: Model 2 with an interaction term of gender and CKD Stages 3b–5.
The introduction of difficulty walking several blocks in Model 2 did not explain the association between CKD Stages 3b–5 and our social isolation measures. For example, the estimated coefficient for CKD Stages 3b–5 in the social contact models was identical in Models 1 and 2 (see Table 5). The effect of CKD Stages 3b–5 on social contact and participation was not explained by mobility limitation; both measures were independently associated with social isolation, net of all other covariates. Women had higher social contact scores, but there was no difference in gender with social participation (Table 5). Gender did not moderate the effect of CKD Stages 3b–5 and mobility limitation on social contact/participation. The interaction term was not significant in either the social contact or participation models.
In sensitivity analyses, we altered the definition of CKD to include all respondents with an eGFR ≤60 mL/min/1.73 m2 compared with those with an eGFR >60 mL/min/1.73 m2 for descriptive statistics (Supplementary data, Table S2). We also compared those with an eGFR divided at 60, as well as between CKD Stages 3b–5 (N = 998, 13% female), CKD Stage 3a (n = 1512, 15% female) and non-CKD (n = 6176, 70% female) for mobility limitation and social isolation (data not shown). The magnitude of differences in mobility limitation and social isolation between CKD Stages 3b–5, 3a and non-CKD did not attain statistical significance in some cases. The size of the comparison groups, as well as gender distributions within these smaller groups, may influence the levels of significance.
DISCUSSION
In the present analysis from the HRS, we examined mobility and social interaction assessed by social contact and social participation. Our results demonstrated that the coexistence of CKD Stages 3b–5 in this older adult cohort is associated with a reduction in all three parameters, but the data do not suggest that the mobility impairment is primarily responsible for the reduced social interaction in CKD Stages 3b–5 group. CKD Stages 3b–5 is associated with 1.44 times increased odds of difficulty walking several blocks compared with those without CKD Stages 3b–5, a 0.23 reduction in the frequency of contact (scale 1–6) and a 0.67 reduction in participation in social activities (scale 0–6). Mobility limitation (measured by difficulty walking several blocks) did not significantly change the relationship between CKD Stages 3b–5 and the frequency of social contact or participation. CKD Stages 3b–5 continued to be associated with a 0.23 reduction in the frequency of contact and a 0.62 reduction in participation in social activities, even with the addition of mobility limitation. There was also no significant improvement in the model R2 with the addition of mobility limitation to CKD Stages 3b–5 in the models predicting social contact or participation. This is in contrast with much of the aging literature, where mobility impairment reduces social interaction [21, 22]. In the National Survey of American Life population, lower mobility (measured by ability to move in the home, stand for 30 min and walk a distance) was associated with a 21% increased risk of social isolation from friends [7]. In fact, a thematic analysis in the elderly showed that they participated in an intervention to increase physical activity because it improved their social interaction [23]. A possible explanation is that rather than mobility driving a lack of social interaction, other factors resulting from the CKD, such as fatigue, perceived disease stigma or increased medical visits, reduce social interaction in patients with CKD and poor mobility. Research in adult male rodents showed that social isolation results in a significant reduction in physical activity and decreases in activity-related sympathetic nerve system output, which leads to a decrease in aggressive behavior [24]. It could be that decreased physical activity in humans with CKD is an effect of social isolation and not a cause.
Our data demonstrate that CKD Stages 3b–5 is associated with lower social contact (increased isolation) compared with those with a higher eGFR. To the best of our knowledge, this has not been previously documented in nondialysis-dependent CKD. However, social contact among patients undergoing dialysis has been described. A systematic analysis of seven studies from five countries in 123 young adults receiving dialysis described social isolation from others as a result of their kidney disease due to difficulties with their appearance or feeling ‘sick’ [5]. These same reasons may exist in nondialysis-dependent CKD, and dietary and fluid restriction may further augment the social isolation. The consequences of social isolation in the elderly general population include frailty, falls, disability and mortality [24–26], outcomes increased in CKD patients [8, 27, 28]. In the CKD Stages 3b–5 group, it is interesting that the highest frequency of contact was with friends and not with family. In the elderly, social contact with friends, as opposed to family, was associated with increased physical activity [29], and yet CKD Stages 3b–5 respondents had lower mobility in the present study.
We also found decreased social participation in older adults with predialysis CKD. This is consistent with the finding in the above study in young adults on dialysis where participants reported that barriers to engage in sports and education prevented them from feeling ‘normal’ [5]. Among the different avenues for participation in social activities in these adults >57 years of age, those with CKD Stages 3b–5 most often cared for grandchildren, although the frequency of caring for grandchildren was lower in those with CKD Stages 3b–5 compared with those without, presumably due to the poorer functional capacity of those with CKD. In the general population, studies have shown that caring for grandchildren helps prevent cognitive decline [30], although it is unclear if the same would be observed in the presence of CKD. Notably, social isolation has also been associated with an inflammatory state in community-dwelling individuals [31, 32]. Studies have also shown that social factors affect the human transcriptome with both up-regulation of immune function in inflammatory-related diseases such as heart disease, neurodegenerative diseases and some cancers, and down-regulation of immune response to infections [33]. Thus it is plausible that social isolation, whether social contact or social participation, may worsen the pro-inflammatory state of CKD.
We found women with CKD Stages 3b–5 were 20% more likely than men to have mobility impairment. This is consistent with other studies in the aging population, where mobility measured subjectively or objectively is poorer in women [33]. Reasons ascribed for decreased mobility in women include a higher prevalence of disabling osteoarthritis-related pain, depression [34], a higher lifetime risk of stroke [35] and differential reporting of poor mobility [36]. A study of 512 women and 967 men with eGFR <20 mL/min/1.73 m2 showed that women were more likely to report joint pain compared with men [37]. In contrast, we did not identify any gender differences in social contact or social participation in our study. This is in contrast to the general population, where older men tend to be more socially isolated [2, 38] and men’s health is more negatively impacted from social isolation [2]. Factors leading to social isolation in CKD are likely different compared with healthy older adults and the effect of the disease process may not vary by gender.
The strengths of our study include the sample size, the systematic recording of data elements, small amounts of missing data and collection of this novel measure of social isolation. Limitations include a lack of albuminuria (therefore the inability to exclude CKD in those with eGFR >60 mL/min/1.73 m2) or repeated measures of creatinine to confirm progressive CKD. However, the use of a lower GFR (Stage 3b or ≤45 mL/min/m2) implies the presence of clinically meaningful CKD. Furthermore, the prevalence of CKD Stages 3b–5 is consistent with studies in the general population for this age group, including the National Health and Nutrition Examination Survey [6]. Another limitation is that this is a cross-sectional analysis. The VBS was started in 2016 and follow-up data for our variables of interest are currently being collected, precluding longitudinal analysis. Finally, only half-samples were asked to answer the PLQ, therefore our sample size for the social isolation parameters is smaller than for mobility, possibly limiting our ability to assess the relationship.
CONCLUSION
‘Social isolation’, an objective measure of the lack of social contact and participation in activities, is well documented in older adults and is associated with poor outcomes, including increased burden of falls, frailty and mortality in the elderly [25, 26]. Poor mobility is considered one of the published etiologies for social isolation in the elderly. In this cross-sectional analysis of a large population-based study, we show that CKD Stages 3b–5 is also associated with lower social contact and participation in social activities, but mobility appears to be coexisting rather than causative, suggesting other causes of social isolation in CKD. These observations are important in the design of interventions at the community and individual level to improve mobility limitation and social isolation in CKD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr Sharon M. Moe for valuable comments that improved the manuscript.
FUNDING
The HRS is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. This analysis was also supported by the National Institutes of Health (NIDDK K23 DK102824 to R.N.M.).
AUTHORS’ CONTRIBUTIONS
R.N.M. and K.M.L. were involved in the conception and design of the study, in data analysis and interpretation and in drafting the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1.Population Research Bureau. https://www.prb.org (1 August 2018, date last accessed)
- 2. Yang YC, McClintock MK, Kozloski M. et al. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J Health Soc Behav 2013; 54: 183–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valtorta NK, Kanaan M, Gilbody S. et al. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart 2016; 102: 1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melzer SM, Leadbeater B, Reisman L. et al. Characteristics of social networks in adolescents with end-stage renal disease treated with renal transplantation. J Adolesc Health Care 1989; 10: 308–312 [DOI] [PubMed] [Google Scholar]
- 5. Bailey PK, Hamilton AJ, Clissold RL. et al. Young adults’ perspectives on living with kidney failure: a systematic review and thematic synthesis of qualitative studies. BMJ Open 2018; 8: e019926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy D, McCulloch CE, Lin F. et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016; 165: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatters LM, Taylor HO, Nicklett EJ. et al. Correlates of objective social isolation from family and friends among older adults. Healthcare 2018; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roshanravan B, Robinson-Cohen C, Patel KV. et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fried LF, Lee JS, Shlipak M. et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc 2006; 54: 750–756 [DOI] [PubMed] [Google Scholar]
- 10. Liu CK, Lyass A, Massaro JM. et al. Chronic kidney disease defined by cystatin C predicts mobility disability and changes in gait speed: the Framingham Offspring Study. J Gerontol A Biol Sci Med Sci 2014; 69: 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moorthi RN, Avin KG.. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017; 26: 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fisher GG, Ryan LH.. Overview of the Health and Retirement Study and introduction to the special issue. Work Aging Retire 2018; 4: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sonnega A, Faul JD, Ofstedal MB. et al. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol 2014; 43: 576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health and Retirement Study (HRS 2016. Core) public use dataset. Ann Arbor, MI: University of Michigan
- 15.Health and Retirement Study (Venous Blood Study (VBS) 2016) public use dataset. Ann Arbor, MI: University of Michigan
- 16. Latham K, Williams MM.. Does neighborhood disorder predict recovery from mobility limitation? Findings from the Health and Retirement Study. J Aging Health 2015; 27: 1415–1442 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hommos MS, Glassock RJ, Rule AD.. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol 2017; 28: 2838–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avlund K, Due P, Holstein BE. et al. Changes in social relations in old age. Are they influenced by functional ability? Aging Clin Exp Res 2002; 14: 56–64 [PubMed] [Google Scholar]
- 20. Mendes de Leon CF, Glass TA, Berkman LF.. Social engagement and disability in a community population of older adults: the New Haven EPESE. Am J Epidemiol 2003; 157: 633–642 [DOI] [PubMed] [Google Scholar]
- 21. Rosso AL, Taylor JA, Tabb LP. et al. Mobility, disability, and social engagement in older adults. J Aging Health 2013; 25: 617–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke PJ, Ailshire JA, Nieuwenhuijsen ER. et al. Participation among adults with disability: the role of the urban environment. Soc Sci Med 2011; 72: 1674–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Capalb DJ, O'Halloran P, Liamputtong P.. Why older people engage in physical activity: an exploratory study of participants in a community-based walking program. Aust J Prim Health 2014; 20: 74–78 [DOI] [PubMed] [Google Scholar]
- 24. Cacioppo JT, Cacioppo S, Capitanio JP. et al. The neuroendocrinology of social isolation. Annu Rev Psychol 2015; 66: 733–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pohl JS, Cochrane BB, Schepp KG. et al. Falls and social isolation of older adults in the National Health and Aging Trends Study. Res Gerontol Nurs 2018; 11: 61–70 [DOI] [PubMed] [Google Scholar]
- 26. Pantell M, Rehkopf D, Jutte D. et al. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am J Public Health 2013; 103: 2056–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roshanravan B, Khatri M, Robinson-Cohen C. et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 2012; 60: 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker SR, Gill K, Macdonald K. et al. Association of frailty and physical function in patients with non-dialysis CKD: a systematic review. BMC Nephrol 2013; 14: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsen BA, Strong D, Linke SE.. The association between family and friend integration and physical activity: results from the NHIS. Int J Behav Med 2014; 21: 529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burn K, Szoeke C.. Grandparenting predicts late-life cognition: results from the Women's Healthy Ageing Project. Maturitas 2015; 81: 317–322 [DOI] [PubMed] [Google Scholar]
- 31. Hafner S, Emeny RT, Lacruz ME. et al. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: results from the MONICA/KORA study. Brain Behav Immun 2011; 25: 1701–1707 [DOI] [PubMed] [Google Scholar]
- 32. Eisenberger NI, Moieni M, Inagaki TK. et al. In sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology 2017; 42: 242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cole SW. Human social genomics. PLoS Genet 2014; 10: e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boerma T, Hosseinpoor AR, Verdes E. et al. A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC Public Health 2016; 16: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petrea RE, Beiser AS, Seshadri S. et al. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 2009; 40: 1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mechakra-Tahiri SD, Freeman EE, Haddad S. et al. The gender gap in mobility: a global cross-sectional study. BMC Public Health 2012; 12: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van de Luijtgaarden MWM, Caskey FJ, Wanner C. et al. Uraemic symptom burden and clinical condition in women and men of ≥65 years of age with advanced chronic kidney disease: results from the EQUAL study. Nephrol Dial Transplant 2018; doi: 10.1093/ndt/gfy155 [DOI] [PubMed] [Google Scholar]
- 38. Kobayashi LC, Steptoe A.. Social isolation, loneliness, and health behaviors at older ages: longitudinal cohort study. Ann Behav Med 2018; 52: 582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.