Abstract
Background
The diagnosis of myocarditis is challenging, and the treatment is generally delayed due to misdiagnosis or missed diagnosis. Endomyocardial biopsy (EMB) is not a specific or sensitive method. A case-controlled observational study was conducted to evaluate early gadolinium enhancement (EGE) and left ventricular functional parameters on Artificial Intelligence in cine-MRI in patients with acute myocarditis.
Material/Methods
We selected 21 patients with pathologically proven acute myocarditis. We analyzed the EGE findings (total/serial number and location of positive-segments using the 17-segment model according to the American Heart Association) and clinical characteristics (symptoms, arrhythmias in ECG, coronary angiography, and EMB). All patients were divided into positive EGE and negative EGE groups to analyze left ventricular functional parameters (LVEF, FS, LVEDD, LVEDV, LVESV, LVMM, LVSV, CO, and CI) on Artificial Intelligence.
Results
We enrolled 21 patients (11 males) with a mean age of 32.6±9.8 years (range, 16 to 51 years). Abnormalities on EGE were found in 2/3 of patients, involving 41 segments among multiple locations on the myocardium. The differences in LVEF (40.2±10.2% vs. 51.3±3.6%), LVESV (69.0±16.1ml vs. 52.5±10.6ml) and LVSV (42.6±11.4 vs. 52.8±2.8 ml) on Artificial Intelligence was statistically significant between the positive EGE and negative EGE groups (p<0.05).
Conclusions
Our results suggest a significant role of EGE on the basis of Lake Louise criteria in evaluating patients with clinical suspicion of acute myocarditis. Parameters, including LVEF, LVESV, and LVSV, on Artificial Intelligence, may be useful independent predictors for capillary leakage and microcirculatory disturbance in myocarditis.
MeSH Keywords: Artificial Intelligence, Heart Diseases, Heart Function Tests, Magnetic Resonance Imaging
Background
Myocarditis is a potentially fatal heart disease characterized by myocardial inflammation, cardiac muscle cellular edema, necrosis, and fibrosis in myocardial interstitium [1,2]. Viruses are currently considered the most frequent trigger of myocarditis in Europe and America. Initially, coxsackieviruses were deemed to be the most common shared trigger for myocarditis due to the high antibody titers detected in patients during acute and subacute myocarditis. Parvovirus B19 and human herpesvirus 6 are also increasingly recognized and taken into account when considering fulminant or nonfulminant myocarditis. Adenoviruses are also found in endomyocardial biopsies of patients with clinically suspected myocarditis [3]. Prospective autopsy research has recently implicated myocarditis as a trigger for sudden cardiac death in up to 12% of young adults and as the proegumenal cause in nearly 9% of dilated cardiomyopathy in adverse outcomes [4,5]. Endomyocardial biopsy (EMB) is an important technique for the diagnosis of acute myocarditis, but there remain numerous limitations. Acute myocarditis generally presents not as a systemic, but as a patchy or focal inflammation, calling into question the value of EMB with a rather high misdiagnosis rate, showing the need for more accurate and non-ionizing methods for to diagnosing myocarditis [6,7]. With all its limitations, EMB is still considered the criterion standard for diagnosis of myocarditis. Myocardial tissue characterization techniques in MRI can assess inflammatory changes such as edema, hyperemia, capillary leakage, microcirculatory disturbance, and myocyte necrosis [8]. Early gadolinium enhancement (EGE) MRI, recognized as the standard of reference for assessment of capillary leakage and microcirculatory disturbance, is performed 3 to 5 min after injecting gadolinium-based MRI contrast media, which can provide crucial prognostic information on myocarditis and nonischemic cardiomyopathy [9]. Local myocardial systolic and diastolic dysfunction can be calculated by left ventricular wall motion, segmental wall thickening rate, or elasticity of myocardial fibers. Left ventricular functional parameters on Artificial Intelligence represent the overall level of cardiac function, and directly or indirectly reflect cardiac volume, ejection function, and myocardial oxygen consumption [10]. Repeatable assessment of left ventricular myocardial contractility using non-invasive and non-ionizing techniques is the current trend of development [11]. Cardiac cine-MRI obtains left ventricular functional parameters on Artificial Intelligence such as Left Ventricular Ejection Fraction (LVEF), Fraction Shortening (FS), Left Ventricular End-diastolic Dimension (LVEDD), Left Ventricular End-diastolic Volume (LVEDV), Left Ventricular End-systolic Volume (LVESV), Left Ventricular Myocardial Mass (LVMM), Left Ventricular Stroke Volume (LVSV), Cardiac Output (CO), and Cardiac Index (CI). We performed the present case-controlled observational study to assess the MRI findings, including EGE and left ventricular functional parameters, on Artificial Intelligence, and relate them to clinical findings in patients with acute myocarditis.
Material and Methods
All subjects gave informed consent for this study, and all medical examinations were performed according to the guidelines of the Medical Ethics Committee of the First Affiliated Hospital of Chengdu Medical College. The authors obtained consent from parents or guardians of minors included in the study, or the Medical Ethics Committee approved the lack of parent or guardian consent. The authors also affirm that all ongoing and related trials are registered (Registration No. ChiCTR1800020447). This case-controlled observational study was conducted at the First Affiliated Hospital of Chengdu Medical College in China, from April 1st to September 31st 2018. Inclusion criteria were: (a) Patients with presumptive clinical diagnosis of myocarditis in accordance with a combination of clinical signs and symptoms, including chest pain, exhaustion, and palpitations, as well as 24-h dynamic electrocardiographic abnormities; and (b) Patients with negative CT coronary angiography or negative X-ray coronary angiography. Exclusion criteria were: (a) Patients without pathologically proven myocarditis; (b) Patients with contraindication for MRI examination; (c) Patients with a serious arrhythmia or cannot trigger ECG gating; (d) Patients with claustrophobia or allergy to Gd-DTPA. We enrolled 21 patients (11 males) with a mean age of 32.6±9.8 years (range, 16 to 51 years). The sample size was determined according to the trial study protocol, which also explains some specific statistical methods in this research. On admission, patients underwent endomyocardial biopsy, and patients with acute coronary-like syndrome were assessed with coronary angiography performed urgently. The biopsies (3 to 4 for each ventricle) were obtained in the apical-septal region. Two of the 3 samples were frozen for molecular studies, and the remaining samples were fixed in formalin and paraffin. All selected patients with a positive-EMB, which suggested pathologically proven acute myocarditis, underwent cardiac MRI with gadolinium and were seen for follow-up visits after 3 months. Double-blind method was used to minimize potential detection bias. The aim of this study was to evaluate and compare left ventricular functional parameters on Artificial Intelligence in cine-MRI between positive and negative EGE in patients with acute myocarditis.
Early gadolinium enhancement (EGE)
All MRI studies were performed with a whole-body clinical 3.0T MRI system (Philips Achieva, TX) equipped with high-performance gradients. Images were acquired in the four-chamber and two-chamber long-axis views with cine-MRI steady-state free precession sequences and in the short-axis plane with black-blood fast spin echo sequences, with double inversion recovery for suppression of the blood signal and an additional inversion recovery pulse for suppression of the fat signal to demonstrate the presence of feasible areas of high signal intensity caused by edema. Then, a stack of continuous short-axis sections was acquired with cine-MRI sequences to assess regional and global biventricular function kinetics. Subsequent to perfusion imaging, an additional dose of 0.1 mmol/kg of gadolinium-DPTA was administered at a rate of 2.0 ml/s. EGE-MRI images were acquired after 3 to 5 min with breath-holding for each stack in the utilization of an IR-prepared and segmented GRE sequence. These quintessential settings were: a stack of 8 contiguous slices in the short-axis view with the orientations identical to perfusion imaging, a slice thickness of 10 mm in the absence of an intersection gap, a TR/TE of 6.1/3.0 ms, an FOV of 320 mm, a matrix of 192×160, and a flip angle of 25°. Qualitative myocardial regional wall motion was assessed, and cardiac function analysis was performed using the segmental model for 17 segments in short-axis orientation on the Target Map of the American Heart Association (AHA). The approach of the 17-segment model was used to define abnormal myocardial enhancement on late gadolinium enhancement, while presence of myocardial edema on T2-STIR and abnormalities on early gadolinium enhancement were also segmentally described using the 17-segment model. Total number and serial number of positive-segments on EGE were observed. The location of enhancement within the myocardium was described as „subendocardial”, „midwall”, or „subepicardial”, involving a variety of sections consisting of „basal”, „middle”, and „apex”.
Left ventricular functional parameters on Artificial Intelligence
The outline of endocardial and epicardial contours was delineated in the short-axis planes of the cine-MRI images, for calculating regional contractile function and biventricular global systolic function of left ventricle, including LVEF, FS, LVEDD, LVEDV, LVESV, LVMM, LVSV, CO, and CI. All patients were divided into positive EGE and negative EGE groups to analyze left ventricular functional parameters on Artificial Intelligence – Workstation EWS Cardiac Analysis Software, Philips Achieva 3.0T TX. For evaluation of regional and global left ventricular function, the slice-summation calculation method was utilized. The endocardial lineaments of all short-axis cine-MRI images circumvoluting the left ventricle were delineated at end-diastole and end-systole, enabling calculation of end-diastolic volume, end-systolic volume, ejection fraction, end-diastolic dimension, fraction shortening, myocardial mass, stroke volume, cardiac output, and cardiac index. Systolic wall motion was described as “normokinetic”, “slightly”, “moderate”, “severely hypokinetic”, “akinetic”, or “dyskinetic”. All the results were reviewed by 2 human operators, and the outline of endocardial and epicardial contours was corrected by Semi-Automatic Correction Software.
Lake louise consensus criteria
All diagnoses were strictly in accordance with Lake Louise Consensus Criteria proposed by the American Journal of Cardiology in 2009 [12]:
In the setting of clinically suspected myocarditis, CMR findings are consistent with myocardial inflammation if at least 2 of the following criteria are present: (a) Regional or global myocardial SI increase in T2-weighted images; (b) Increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images; (c) There is at least 1 focal lesion with nonischemic regional distribution in inversion recovery-prepared gadolinium-enhanced T1-weighted images.
A CMR study is consistent with myocyte injury and/or scar caused by myocardial inflammation if Criterion (c) above is present.
A repeat CMR study at 1 to 2 weeks after the initial CMR study is recommended if: (a) None of the criteria are present, but the onset of symptoms has been very recent and there is strong clinical evidence for myocardial inflammation; and (b) One of the criteria is present.
Statistical analysis
Statistical analysis was done with the SPSS 17.0 software package for Windows. All quantitative results were expressed in the text, figures, and tables. ANOVA was used to analyze differences in the left ventricular function parameters between positive EGE and negative EGE groups, and results are shown as mean ±SD. A p value of < 0.05 was considered to be significant.
Results
Clinical presentations
We assessed 67 patients for eligibility in this study: 33 patients were excluded for not meeting inclusion criteria and 13 patients were excluded for undergoing endomyocardial biopsy. We enrolled 21 patients with active lymphocytic myocarditis confirmed by the histological and immunohistochemical examination (Figure 1). Chest pain was the most common clinical symptom (20/21 patients), resembling generally ischemia-like chest pain. Exhaustion and palpitations were present in 11/21 and 9/21 of patients, respectively. Arrhythmias in 24-h dynamic ECG findings were tachycardia sinusal (n=12), atrial fibrillation (n=1), ventricular tachycardia (n=7), ventricular premature contraction (n=7), and supraventricular premature beat (n=10). Five patients with acute coronary-like syndrome were assessed with coronary angiography performed as an urgent procedure, and then we excluded coronary artery disease. All results are shown in Table 1.
Figure 1.
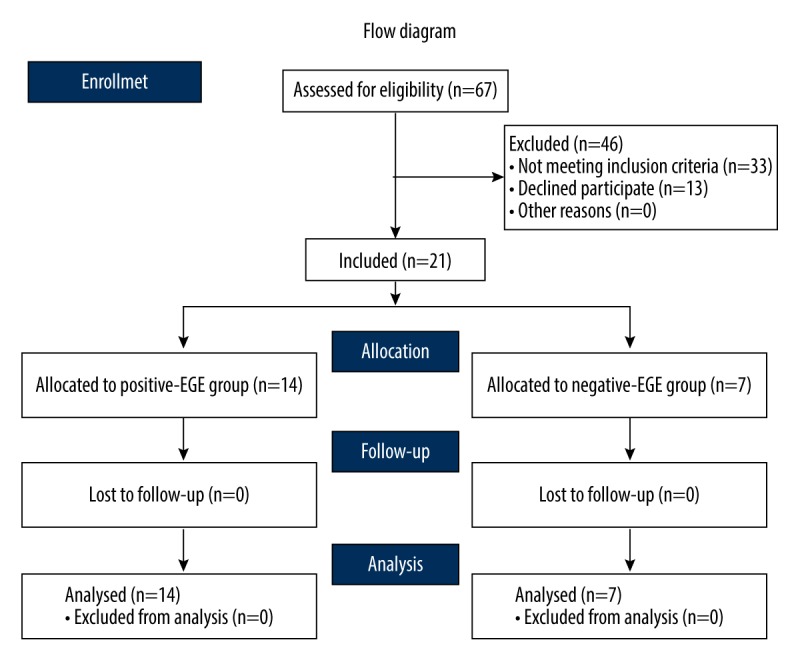
We assessed 67 patients for eligibility in this study: 33 patients were excluded by not meeting inclusion criteria and13 patients were excluded due to not undergoing endomyocardial biopsy. We selected 21 patients with active lymphocytic myocarditis confirmed by histological and immunohistochemical examination.
Table 1.
Clinical characteristics of patients with pathologically proven acute myocarditis.
| No. | Sex | Age | Symptoms | Arrhythmias | ECG | Coronary angiography |
|---|---|---|---|---|---|---|
| 1 | F | 33 | C, E, P | TS | NA | NA |
| 2 | F | 36 | C, E, P | TS, SPB | STE, STD | N |
| 3 | M | 48 | E | NA | N | NA |
| 4 | M | 19 | C | NA | STE | NA |
| 5 | F | 32 | C, P | TS, VPC, SPB | STE | NA |
| 6 | M | 23 | C, E | VT | RBBB | NA |
| 7 | F | 33 | C, E, P | TS, VPC, SPB | STD, NT | N |
| 8 | M | 21 | C, E | AF | LAFB | NA |
| 9 | F | 38 | C, E | TS | STD | NA |
| 10 | F | 25 | C | TS | NA | NA |
| 11 | M | 37 | C, E, P | TS, SPB | STE, NT | NA |
| 12 | M | 36 | C, P | TS | STE | NA |
| 13 | F | 41 | C | VT | LBBB | NA |
| 14 | F | 47 | C, E, P | TS, VT, VPC, SPB | STE, STD | N |
| 15 | F | 24 | C | NA | N | NA |
| 16 | M | 31 | C | NA | STE | NA |
| 17 | M | 32 | C, E | VT, VPC, SPB | NT | NA |
| 18 | M | 51 | C, P | TS, VT, VPC, SPB | STD, NT | N |
| 19 | M | 22 | C, P | TS, VT, VPC, SPB | NA | N |
| 20 | F | 16 | C | VT, VPC, SPB | NA | NA |
| 21 | M | 40 | C, E | TS, SPB | NT | NA |
C – chest pain; E – exhaustion; P – palpitation; TS – sinus tachycardia; AF – atrial fibrillation; VT – ventricular tachycardia; VPC – ventricular premature contraction; SPB – supraventricular premature beat; NA – not available.
Cardiac MRI findings on artificial intelligence
We found that 2/3 of patients displayed abnormal myocardial enhancement on EGE, involving 41 segments among multiple locations on the myocardium (Table 2). The subepicardial myocardial region was the most frequent site of positive EGE (93%), and midwall enhancement was less common (Figure 2). In certain patients, the pattern of positive EGE differed according to the position within the left ventricle, i.e., subepicardial involvement in the left ventricular septum and mid-wall involvement in inferior or anterior left ventricular wall. Involvement of the basal, middle, and apex (serial number of segments for 2, 3, 4, 5, 7, 12, and 17) was found in a female patient, presenting as a transmural (including subendocardial, mid-wall, and subepicardial) positive EGE, with patchy shape, yet there was never any isolated subendocardial positive EGE. According to the 17-segment model, the septum in the left ventricular wall (serial number of segments for 2, 3, 8, 9, and 14) was most frequently involved, i.e., 16 of 41 segments with positive EGE. In no patients had right-ventricular positive EGE. Clinical follow-up at 6 months, available for 17 patients, was uneventful for 13 patients, while 4 patients showed a relapse of myocarditis at 1, 3, 4, and 6 months, respectively. Regional wall motion abnormalities (Figure 3) were found on cardiac cine-MRI in 11 patients (9 in the positive group and 2 in the negative EGE group). In some patients, there was a match between global left ventricular functional abnormalities and positive EGE. The difference in LVEF (40.2±10.2% vs. 51.3±3.6%), LVESV (69.0±16.1 ml vs. 52.5±10.6 ml), and LVSV (42.6±11.4 vs. 52.8±2.8ml) on Artificial Intelligence was statistically significant (p<0.05) between the positive EGE and negative EGE groups (Table 3).
Table 2.
Characteristics of EGE in patients with pathologically proven acute myocarditis.
| No. | EGE | Total number of segments | Serial number of segments | Location of segments | |||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | Middle | Apex | Subepicardial | Mid-wall | Subendocardial | ||||
| 1 | + | 3 | 2, 8, 9 | + | + | − | + | − | − |
| 2 | + | 4 | 3, 4, 6, 17 | + | − | + | + | + | − |
| 3 | − | NA | NA | − | − | − | − | − | − |
| 4 | + | 1 | 1 | + | − | − | + | − | − |
| 5 | − | NA | NA | − | − | − | − | − | − |
| 6 | − | NA | NA | − | − | − | − | − | − |
| 7 | − | NA | NA | − | − | − | − | − | − |
| 8 | + | 2 | 4,5 | + | − | − | + | − | − |
| 9 | + | 3 | 7, 9, 17 | − | + | + | + | + | − |
| 10 | + | 1 | 5 | + | − | − | + | − | − |
| 11 | + | 6 | 1, 2, 4, 7, 8, 11 | + | + | − | + | + | + |
| 12 | + | 2 | 3, 4 | + | − | − | − | − | − |
| 13 | − | NA | NA | − | − | − | − | − | − |
| 14 | + | 7 | 2, 3, 4, 5, 7, 12, 17 | + | + | + | + | + | + |
| 15 | + | 2 | 10, 11 | − | + | − | + | − | − |
| 16 | − | NA | NA | − | − | − | − | − | − |
| 17 | + | 1 | 6 | + | − | − | + | − | − |
| 18 | + | 4 | 1, 2, 3, 9 | + | + | − | + | − | − |
| 19 | − | NA | NA | − | − | − | − | − | − |
| 20 | + | 3 | 3, 9, 17 | + | + | + | + | + | − |
| 21 | + | 2 | 8, 10 | − | + | − | + | − | − |
‘+’ – positive; ‘−’ – negative; NA – not available.
Figure 2.
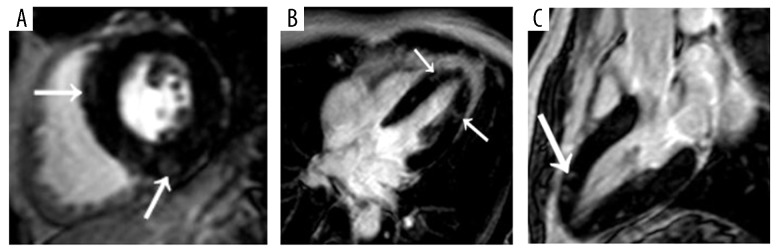
(A) Short-axis, (B) four-chamber, and (C) long-axis three-dimensional delayed-enhancement T1-weighted multishot gradient-echo IR MR images (6.1/3.0, 25° flip angle) of a diffuse form of acute myocarditis in a 36-year-old man. Nodular subepicardial EGE (arrows in A and C) of the left ventricular septum, lateral and inferior wall associated with band-like or nodular centro-myocardial EGE (arrow in B) predominating in the lateral wall of the left ventricle.
Figure 3.
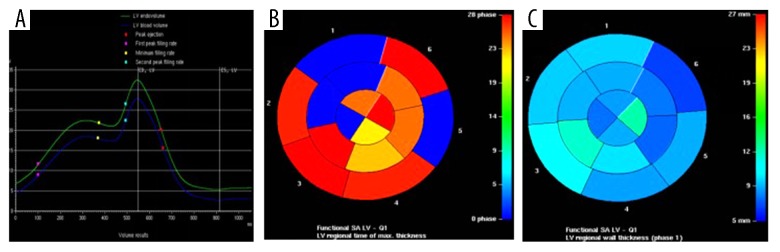
(A) Left ventricular global volume-time curve, (B) color-gradation map for left ventricular regional time of thicknessmax, and (C) color-gradation map for left ventricular regional wall thickness of myocarditis in a 41-year-old woman. Arrhythmia of left ventricle is indicated in A. Longer time (B) or thicker wall (C) are displayed in the warmer color, while the shorter and thinner wall are presented in the colder color.
Table 3.
Comparison of left ventricular functional parameters in positive/negative EGE groups.
| Positive EGE | Negative EGE | p Value | |
|---|---|---|---|
| LVEF (%) | 40.2±10.2 | 51.3±3.6 | 0.01 |
| FS (%) | 24.4±2.4 | 25.9±1.1 | 0.12 |
| LVEDD (mm) | 45.9±4.1 | 44.2±5.9 | 0.45 |
| LVEDV (ml) | 114.5±11.0 | 110.5±9.6 | 0.43 |
| LVESV (ml) | 69.0±16.1 | 52.5±10.6 | 0.03 |
| LVMM (g) | 58.3±5.9 | 54.8±6.0 | 0.22 |
| LVSV (ml) | 42.6±11.4 | 52.8±2.8 | 0.03 |
| CO (L/min) | 3.1±0.8 | 3.6±0.4 | 0.13 |
| CI (L/min*m2) | 2.7±0.3 | 3.0±0.6 | 0.07 |
LVEF – left ventricular ejection fraction; FS – fraction shortening; LVEDD – left ventricular end-diastolic dimension; LVEDV – left ventricular end-diastolic volume; LVESV – left ventricular end-systolic volume; LVMM – left ventricular myocardial mass; LVSV – left ventricular stroke volume; CO – cardiac output; CI – cardiac index.
Discussion
Cardiac MRI, a multi-directional approach in patients with clinically suspected acute myocarditis, is reported to be quite sensitive and specific in differentiating it from other myocardial diseases, especially for ischemic coronary disease [13]. EGE is useful in evaluating capillary leakage and microcirculatory disturbance, as well as for establishing etiology, quantification, identifying prognostic factors, regular follow-up, and assessment of treatment response in myocarditis [14]. The comprehensive diagnosis of myocarditis was based on clinical symptoms, coronary angiography, ECG, laboratory examination, and clinical follow-up [15]. The latest insights regarding assessment of MRI patterns of acute myocarditis have led to more comprehensive imaging acquisition protocols, including first-pass and EGE examinations, as well as cardiac cine-MRI, for evaluation of regional myocardial contractile function. The first point was represented by symptoms of acute infarction-like presentation in the absence of coronary stenoses in this study. It is interesting that patients with this presentation did not reveal severely dilated ventricles and had moderate left ventricular dysfunction. The most typical location of enhancement was in the subepicardial site of the left ventricular myocardial wall. The etiological agent did not affect cardiac myocytes, but the vascular endothelial cells were involved in endothelial dysfunction and exudation of inflammatory cells to the myocardial interstitium, with consequent damage to myocytes. Focal distribution of the inflammatory process probably accounts for the fact that left ventricular function is not severely damaged. We found that septal enhancement was commonly shown as patchy or linear subepicardial enhancement with normal appearance of the subendocardial layers. Presence of subepicardial and mid-wall enhancement is reasonably suspicious for myocarditis and enables exclusion of ischemic cardiomyopathy, which begins in the subendocardium [16]. By utilizing CMRI as a guideline to endomyocardial biopsy, the enhancement in 88% of patients with clinically diagnosed myocarditis was reported, with the most frequently involved site being the lateral wall, and it is can be used to predict and identify myocardial interstitial fibrosis and chronic myocardial remodeling [17,18]. In addition, the ventricular septum and, less frequently, the lateral wall, are most commonly involved without ventricular septum thickening, which differs from characteristics of enhancement in hypertrophic cardiomyopathy. Cardiac cine-MRI, with higher specificity for the detection of left ventricular thrombus, allows increasingly accurate measurement of chamber volumes and ventricular function compared to echocardiography [19], although echocardiography is the most widely available imaging method at present [20]. The differences in LVEF, LVESV, and LVSV on Artificial Intelligence were statistically significant, while no statistical difference was found in LVEDV between the positive EGE and negative EGE groups, suggesting that capillary leakage and microcirculatory disturbance did not affect left ventricular diastolic function but did affect diastolic systolic function. LVESV or LVSV may be used as a supplementary predictor for evaluation of systolic function in myocarditis, except for LVEF. If regional dysfunction was present, contractile abnormalities were generally mild to moderate, so akinesia or severely hypokinetic was never found. In some patients, there was a match between global left ventricular functional abnormalities and positive EGE. Dornier et al. [21] found that an overestimation by approximately 2 ml was observed for both LVEDV and LVESV with tagged MRI, and the difference between LVEF obtained by tagged MRI and standard cine-MRI was approximately 1%, which is explained by the overestimation of LVEDV. Relevant research reports demonstrated that LVESV and LVEF are not significantly affected by fluctuations in spatial resolution, but time resolution did, showing that time resolution can be considered sufficient in patients with normal cardiac rate [10,22].
This study has certain limitation. This was a small observational study, which may lack enough data (only 21 patients), and studies with larger samples are urgently needed to support our conclusions. In early acute myocarditis, myocyte injury is not present, and it might be impossible to observe positive EGE at present. Further research is needed to better understand and categorize these negative EGE patients with clinically suspected acute myocarditis.
Conclusions
Our results show that the parameters LVEF, LVESV, and LVSV on Artificial Intelligence are useful as independent predictors of capillary leakage and microcirculatory disturbance; they do not affect left ventricular diastolic function but do affect diastolic systolic function in myocarditis. LVEF is commonly used to measure cardiac function based on its clinical importance, and probably reflects a combination of cardiac dilatation and myocardial systolic function, making it more challenging to interpret than the simpler mathematical measurement of left ventricular enlargement. LVESV or LVSV, but not LVEF, can be used as a supplementary predictor for evaluation of systolic function in myocarditis. EGE, coupled with clinical presentation and cardiac cine-MRI, can, on the basis of Lake Louise criteria, be used in evaluating capillary leakage and microcirculatory disturbance in patients with clinically suspected acute myocarditis, especially in patients with normal coronary arteries and unexplained chest pain, exhaustion, or arrhythmia. It can detect the presence and extent of vascular damage to quantify the impact on left ventricular regional and global function.
Footnotes
Source of support: 1. The Applied Basic Research on Projects in Yunnan Province (Grant No. 2017FE468-178). 2. The Yunnan Province Medical Subject Leaders Training Project (Grant No. D-201646). 3. The Young and Middle-Aged Technical Academic Leaders Training Project in Yunnan province (Grant No. 2015HB068). 4. Natural Science Foundation of Chengdu Medical College (CYZ17-34)
References
- 1.Radunski UK, Lund GK, Stehning C, et al. CMR in patients with severe myocarditis: Diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. 2014;7(7):667–75. doi: 10.1016/j.jcmg.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Sagar S, Liu PP, Cooper LT., Jr Myocarditis. Lancet. 2012;379(9817):738–47. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466–72. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 4.Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92(3):316–20. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnani JW, Dec GW. Myocarditis: Current trends in diagnosis and treatment. Circulation. 2006;113(6):876–90. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 6.Stiermaier T, Föhrenbach F, Klingel K, et al. Biventricular endomyocardial biopsy in patients with suspected myocarditis: Feasibility, complication rate and additional diagnostic value. Int J Cardiol. 2017;230:364–70. doi: 10.1016/j.ijcard.2016.12.103. [DOI] [PubMed] [Google Scholar]
- 7.Yelgec NS, Dymarkowski S, Ganame J, et al. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007;17(9):2211–17. doi: 10.1007/s00330-007-0612-3. [DOI] [PubMed] [Google Scholar]
- 8.Meilhac A, Roubille F. Could inflammatory biomarkers be of greater interest than troponins for patients admitted for myocardial syndromes? A single-center real-life retrospective study. Arch Cardiovasc Dis Suppl. 2018;10(1):152. [Google Scholar]
- 9.Cummings KW, Bhalla S, Javidan-Nejad C, et al. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29(1):89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Schwab F, Marcus R, et al. Feasibility of free-breathing, GRAPPA-based, real-time cardiac cine assessment of left-ventricular function in cardiovascular patients at 3 T. Eur J Radiol. 2015;84(5):849–55. doi: 10.1016/j.ejrad.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Yang ZG, Xu HY, et al. Assessment of left ventricular myocardial deformation by cardiac MRI strain imaging reveals myocardial dysfunction in patients with primary cardiac tumors. Int J Cardiol. 2018;253:176–82. doi: 10.1016/j.ijcard.2017.09.194. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chertow DS, Childs RW, Arai AE, et al. Cardiac MRI findings suggest myocarditis in severe Ebola virus disease. JACC Cardiovasc Imaging. 2017;10(6):711–13. doi: 10.1016/j.jcmg.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisotzkey BL, Soriano BD, Albers EL, et al. Diagnostic role of strain imaging in atypical myocarditis by echocardiography and cardiac MRI. Pediatr Radiol. 2018;48(6):835–42. doi: 10.1007/s00247-017-4061-0. [DOI] [PubMed] [Google Scholar]
- 15.Sachdeva S, Song X, Dham N, et al. Analysis of clinical parameters and cardiac magnetic resonance imaging as predictors of outcome in pediatric myocarditis. Am J Cardiol. 2015;115(4):499–504. doi: 10.1016/j.amjcard.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Jue J, Romano S, Indorkar R, et al. Cardiac MRI and acute myocarditis. QJM. 2017;110(3):183–84. doi: 10.1093/qjmed/hcw199. [DOI] [PubMed] [Google Scholar]
- 17.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: A comparison to histology and molecular pathology. Circulation. 2004;109(10):1250–58. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KH, Li T, Afonso L. Diagnostic approach and role of MRI in the assessment of acute myocarditis. Cardiol Rev. 2009;17(1):24–30. doi: 10.1097/CRD.0b013e318184b383. [DOI] [PubMed] [Google Scholar]
- 19.Mouquet F, Lions C, de Groote P, et al. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol. 2008;18(12):2765–69. doi: 10.1007/s00330-008-1067-x. [DOI] [PubMed] [Google Scholar]
- 20.Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152(1):75–84. doi: 10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Dornier C, Somsen GA, Ivancevic MK, et al. Comparison between tagged MRI and standard cine MRI for evaluation of left ventricular ejection fraction. Eur Radiol. 2004;14(8):1348–52. doi: 10.1007/s00330-004-2311-7. [DOI] [PubMed] [Google Scholar]
- 22.Miller S, Simonetti OP, Carr J, et al. MR Imaging of the heart with cine true fast imaging with steady-state precession: Influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology. 2002;223(1):263–69. doi: 10.1148/radiol.2231010235. [DOI] [PubMed] [Google Scholar]