Abstract
Background
Interleukin-2 (IL-2) antagonist has been used as an induction therapy in many centres in calcineurin inhibitor-sparing regimens. Tacrolimus has overwhelmingly replaced cyclosporine in the maintenance immunosuppressive protocols in many transplant centres. The aim of our study and meta-analysis is to explore the effect of IL-2 induction therapy on the rate of rejection and patient and graft survival in standard-risk renal transplant patients with tacrolimus-based maintenance immunotherapy. Secondary aims included assessment of the effect of IL-2 induction therapy on creatinine change and the risk of cytomegalovirus (CMV) infection.
Methods
We conducted a systematic review in different databases to identify studies and research work that assessed the effect of IL-2 antibody induction therapy on renal transplant outcomes. Inclusion criteria for our meta-analysis were all studies that compared IL-2 induction therapy with placebo or no induction therapy in standard-risk renal transplant recipients on tacrolimus-based maintenance immunosuppressive therapy. Data collected were the name of the first author, journal title, year of publication, country where the study was conducted, number of patients in the IL-2 induction therapy arm and in the placebo arm, number of patients who had biopsy-proven rejection and graft survival in each arm. A random effects model was used for the meta-analysis.
Results
Of the 470 articles found in different databases, 7 were included in the meta-analysis. Forest plot analysis for rate of rejection during the follow-up period post-transplant showed no significant difference between the groups. There was no evidence of heterogenicity between included studies (I2 = 21.8%, P = 0.27). The overall risk difference was −0.02 [95% confidence interval (CI) −0.05–0.01]. A random effects meta-analysis for patient and graft survival was performed using forest plot analysis and showed no significant effect of IL-2 receptor (IL-2R) antibody induction on patient or graft survival compared with placebo. The overall risk difference was −0.01 (95% CI −0.04–0.01) and 0.00 (95% CI −0.00–0.01), respectively. Three of the included studies showed no effect of basiliximab on creatinine change, two showed no effect on risk of CMV infection and two showed less risk of post-transplant diabetes in the basiliximab group.
Conclusion
IL-2R antibody induction therapy has no significant effect on the rate of rejection or patient or graft survival in standard-risk renal transplant recipients on tacrolimus-based maintenance immunotherapy. More randomized controlled studies are needed.
Keywords: basiliximab, daclizumab, rejection, renal transplantation, tacrolimus
INTRODUCTION
Outcomes of renal transplantation have improved significantly over the last decade [1]. One of the main obstacles to better transplant outcomes is the recurrent episodes of acute rejection, thus decreasing graft survival [2]. Many immunosuppressive protocols have been designed aiming to overcome this challenge [3]. Interleukin-2 receptor (IL-2R) antibody, a non-depleting immunosuppressive agent, has been authorized for induction therapy since 2000, with the aim of reducing the risk of acute rejection and improving graft survival [4]. It is a monoclonal antibody agent that inhibits T-cell proliferation through binding to IL-2 receptors on the T-helper cell surface [4]. Compared with T-cell depletive immunotherapy, IL-2 induction therapy does not increase the risk of infection or risk of cancer [5].
Many studies have proved that the use of IL-2R antibody as induction therapy reduces the risk of acute rejection episodes in renal transplant patients on cyclosporine-based maintenance immunotherapy [6–8]. Furthermore, it has been used as an induction therapy in many centres in calcineurin inhibitor–sparing regimens [9–11]. In 2010, a meta-analysis included all randomized controlled studies that assessed the outcome of IL-2 induction therapy against placebo and those on T-cell depletive induction therapy [12]. In this cohort, the renal transplant recipients were principally at low immunological risk, with more than one-third of the patients having no previous transplants. This meta-analysis showed a lower risk of rejection in IL-2R antibody induction compared with placebo. It also showed no privilege of T-cell-depleting agents over IL-2R antibody induction therapy. These results were one of the main reasons that led the 2009 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines to recommend IL-2R antibody induction therapy as part of routine immunosuppressive protocols [13]. In most of the studies included in this meta-analysis, cyclosporine was the main maintenance immunotherapy.
Tacrolimus has overwhelmingly replaced cyclosporine in the maintenance immunosuppressive protocols in many transplant centres [14]. This was based on many studies that showed lower rejection rates in tacrolimus-based immunotherapy compared with cyclosporine [15, 16]. The change to tacrolimus as a maintenance immunotherapy may explain the significant decline in the rate of rejection episodes from ∼ 50% 30 years ago to ∼ 10% today [3, 16]. This raises the question of the significance of IL-2R antibody induction therapy in the tacrolimus era and whether it provides any benefits in decreasing the risk of rejection, especially in standard-risk renal transplant patients. The primary aim of our study and meta-analysis is to explore the effect of IL-2R antibody induction therapy on the rate of rejection and graft and patient survival in standard-risk renal transplant patients on tacrolimus-based maintenance immunotherapy. Secondary aims included assessment of the effects of IL-2 induction therapy on creatinine change, new-onset diabetes post-transplant (NODAT) and risk of cytomegalovirus (CMV) infection.
MATERIALS AND METHODS
Search strategy and inclusion criteria
We conducted a systematic review of the PubMed, MEDLINE, Embase and Cochrane databases to identify studies and research work that assessed the effect of IL-2 induction therapy on renal transplant outcomes. The keywords used for this search were basiliximab, daclizumab, interleukin-2, outcome, rejection and graft survival. The literature search was performed by two independent librarians and selected papers for the meta-analysis were reviewed and accepted by all authors. Manual searches were also conducted through references of relevant and selected papers. Any conflicts among the results of the literature search were reviewed and settled with a consensus of all authors. Inclusion criteria for our meta-analysis were all studies that compared IL-2 induction therapy with placebo or no induction therapy in standard-risk renal transplant recipients. Studies excluded were studies with no control group, studies that compared IL-2 induction therapy with other depleting or non-depleting antibodies, cyclosporine-based maintenance immunotherapy, high-risk renal transplant recipients, follow-up period <1 year, studies performed on animals, dual organ transplant, organ transplant other than the kidneys, language other than English, review articles and case reports and studies that had similar cohorts to other larger studies.
Definitions and data extraction
Standard risk for renal transplant was defined as less than two human leucocytic antigen DR (HLA-DR) mismatches, panel reactive antibody (PRA) <20% and recipients with no more than one previous transplant. This was based on the patient characteristics in most of the papers included in this meta-analysis. Data collected were name of the first author, journal title, year of publication, country where the study was conducted, number of patients in the IL-2R antibody induction arm and in the placebo arm, number of patients who had biopsy-proven or clinical rejection and graft survival in each arm. Primary outcomes were the number of acute rejection episodes and patient and graft survival 1-year post-transplant. Secondary outcomes were creatinine changes, new onset diabetes after transplant (NODAT) and CMV infection. Ethical approval was not required for the meta-analysis, as all studies included were already published and available in different search engines.
Statistical analysis and publication bias
Statistical analyses were done using Stata version 13 (StataCorp, College Station, TX, USA). The risk difference between both arms in the included studies was estimated from the number of events in each arm. The risk difference was estimated for the number of rejection episodes and for graft survival. A random effects model was used for the meta-analysis. Heterogenicity among included studies for the effect sizes was assessed using the DerSimonian method. P-values <0.1 were the cut-off for heterogenicity. Forest plots were used to display the results of the meta-analysis and the 95% confidence interval (CI) was calculated for each study and displayed on the same graph. Funnel and Galbraith plots were used to assess publication bias by estimating the effect size against standard error. Two independent proportions analyses were used to compare baseline categorical variables between the IL-2R antibody induction and no-induction group, while independent t-test analysis was used for continuous variables.
RESULTS
A flow chart for the literature search is shown in Figure 1. Of the 470 articles found in different databases, 81 were repetitions; 389 were screened, of which 379 were excluded. Ten articles were fully explored. Three of these were excluded due to the short follow-up period. Seven articles were included in the meta-analysis. These are shown in Tables 1 and 2 [17–23].
FIGURE 1:
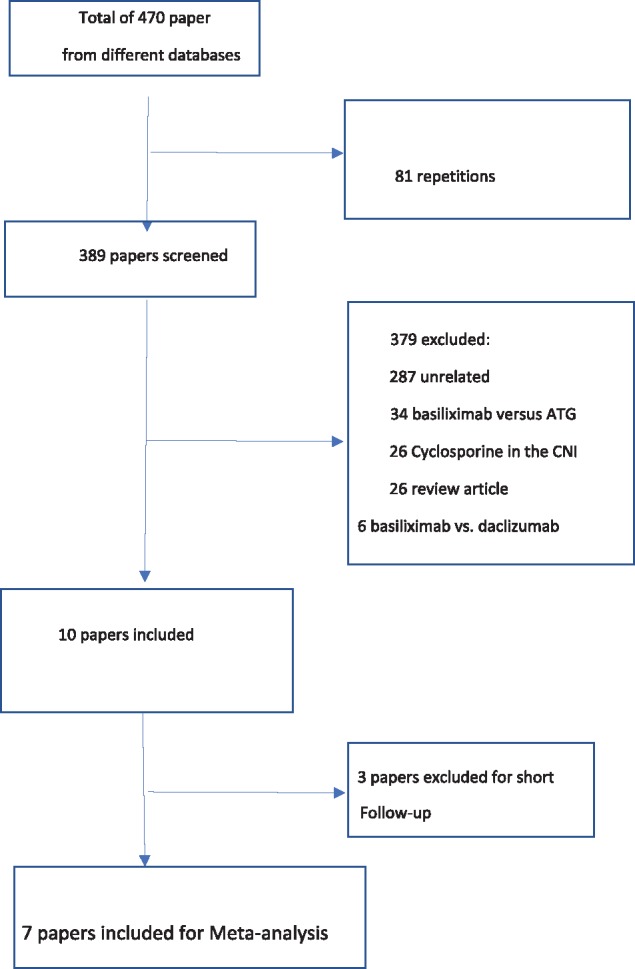
Flow chart for the literature search.
Table 1.
Papers included in the meta-analysis
| Reference | Type of study | Country | Journal | Year |
|---|---|---|---|---|
| Wiland et al. [17] | Retrospective observational study | USA | Transplantation | 2004 |
| Gralla and Wiseman [20] | Retrospective observational study | USA | Transplantation | 2010 |
| de Sandes-Freitas et al. [19] | Retrospective observational study | Brazil | International Urology and Nephrology | 2013 |
| Schwarz et al. [20] | Retrospective observational study | Austria | Transplantation Proceedings | 2015 |
| Umber et al. [21] | Retrospective observational study | Italy | Journal of Nephrology | 2017 |
| Gavela Martinez et al. [22] | Retrospective observational study | Spain | Transplantation Proceedings | 2009 |
| Baek et al. [23] | Prospective study | Korea | Experimental and Clinical Transplantation | 2016 |
Table 2.
Baseline characteristics for papers included in the meta-analysis
| References | Follow-up period, years | Patients on IL-2R antibody induction, n | Rejections, n | Graft survival (no. of patients) | Deaths, n | Patients not on induction, n | Rejections, n | Graft survival (no. of patients) | Deaths, n |
|---|---|---|---|---|---|---|---|---|---|
| Wiland et al. [17] | 1 | 126 | 5 | 119 | 136 | 29 | 129 | ||
| Gralla and Wiseman [18] | 1 | 14 482 | 1676 | 13 859 | 377 | 14 204 | 1843 | 13 607 | 355 |
| de Sandes-Freitas et al. [19] | 1 | 134 | 23 | 118 | 8 | 132 | 21 | 113 | 7 |
| Schwarz et al. [20] | 1 | 83 | 20 | 65 | 27 | ||||
| Umber et al. [21] | 1 | 56 | 11 | 54 | 2 | 58 | 16 | 52 | 1 |
| Gavela Martinez et al. [22] | 1 | 21 | 2 | 19 | 1 | 36 | 6 | 35 | 1 |
| Baek et al. [23] | 1 | 72 | 3 | 72 | 0 | 36 | 1 | 36 | 0 |
A total of 29 426 patients were included in the meta-analysis (male 62.3% in the IL-2R antibody induction group and 62.9% in the no-induction group). Baseline characteristics are shown in Table 3. Diagnosis of acute rejection episodes was confirmed by renal biopsy in all studies included in the meta-analysis except for Wiland et al. [17], where this was not clearly mentioned. There were no significant differences between baseline characteristics of patients included in the IL-2R antibody induction group and the no-induction group, as shown in Table 3. Maintenance therapy in all studies included in the meta-analysis was triple therapy [steroids, calcineurin inhibitor (CNI) and mycophenolate or azathioprine].
Table 3.
Comparison of baseline characteristics of IL-2R antibody induction group and no-induction group
| Baseline characteristics | IL-2R antibody induction group (n = 14 974) | No-induction group (n = 14 667) | P-value |
|---|---|---|---|
| Male (%) | 62.3 | 62.9 | 0.28 |
| Recipient mean age (years) | 48.65 | 47.09 | P-value cannot be obtained as raw data are not available |
| Donor mean age (years) | 40.05 | 38.95 | P-value cannot be obtained as raw data are not available |
| Mean cold ischaemia time (h) | 19.3 | 16.1 | P-value cannot be obtained as raw data are not available |
| Extended criteria donors, n | 1347 | 1388 | 0.16 |
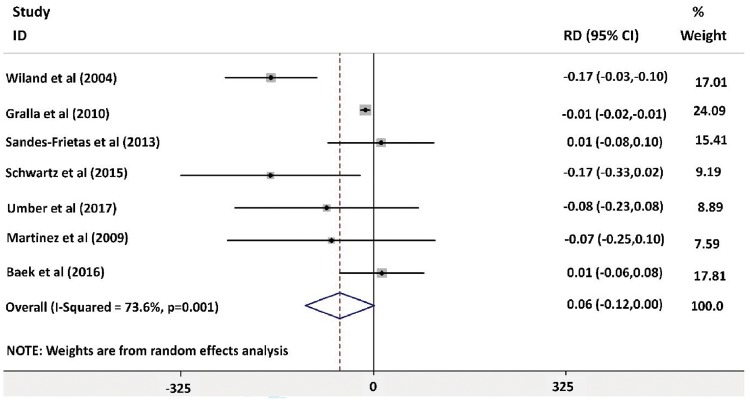
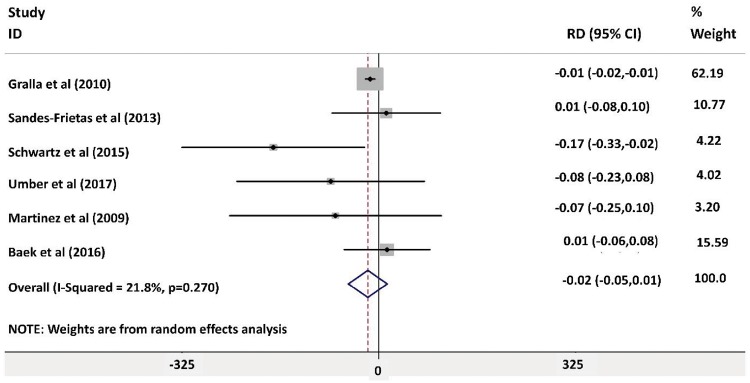
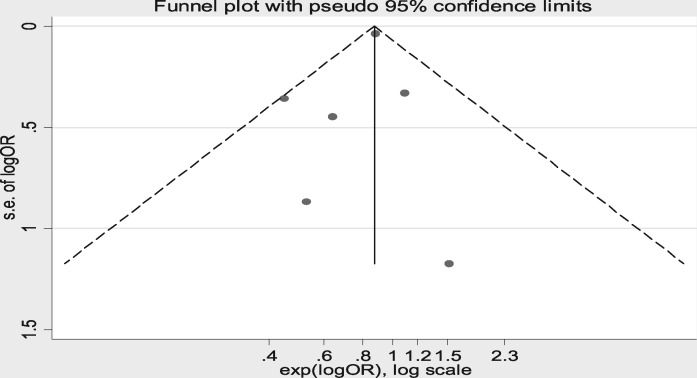
Forest plot analysis for the rate of rejection during the follow-up period post-transplant showed no significant difference between the groups, as shown in Figure 2 [risk difference −0.06 (95% CI −0.12–0.00)]. The highest weight in the forest plot analysis was for the article by Gralla and Wiseman [18] [weight 24.09% (95% CI −0.02 to −0.01)] followed by Baek et al. [23] [weight 17.81% (95% CI −0.06–0.08)]. There was evidence of heterogeneity between included studies (I2 = 73.6%, P = 0.001). The risk of bias was assessed using a Galbraith plot, as shown in Supplementary data, Figure S3. Wiland et al. [17] was removed from the forest plot analysis to eliminate the risk of heterogeneity, as shown in Figure 3 (I2 = 21.8%, P = 0.27). The overall risk difference was −0.02 (95% CI −0.05–0.01), indicating that IL-2R antagonist has no influence on rejection rates. There was no evidence of heterogeneity in the analysis (I2 = 21.8%, P = 0.27). There was no risk of bias after excluding the article by Wiland et al. [17] using funnel plot and Galbraith plot analysis as shown in Figure 4 andSupplementary data, Figure S6.
FIGURE 2:
Forest plot analysis for risk of rejection 1-year post-transplant.
FIGURE 3:
Forest plot analysis for risk of rejection 1-year post-transplant after excluding Wiland et al. [18].
FIGURE 4:
Funnel plot for random effects meta-analysis for risk of rejection after excluding Wiland et al. [18].
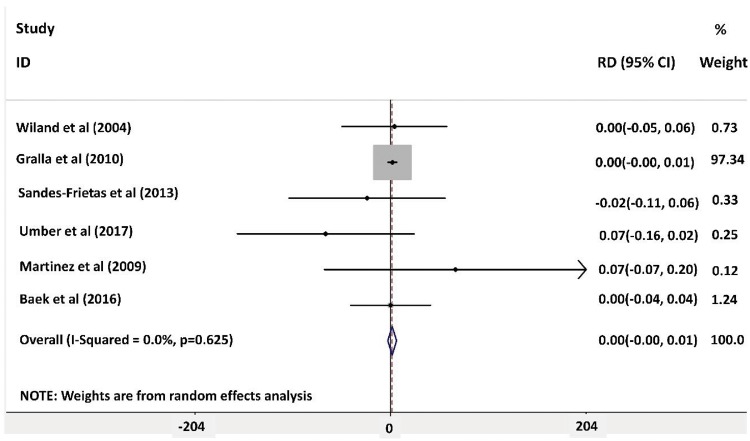
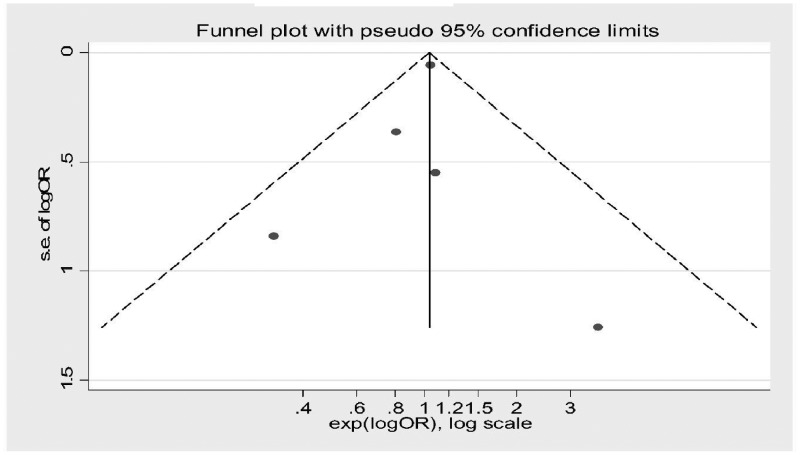
Random effects meta-analysis for graft survival was done using forest plot analysis and showed no significant effect of IL-2R antibody induction on graft survival compared with placebo. Forest plot analysis is shown in Figure 5. The greatest weight was for the article by Gralla and Wiseman [18] [weight 97.34% (95% CI 0.00–0.01). There was no evidence of heterogenicity among included studies (I2 = 0.0%, P = 0.625). The risk of bias was assessed using funnel plot analysis and a Galbraith plot as shown in Figure 6 and Supplementary data, Figure S9, respectively.
FIGURE 5:
Forest plot analysis for graft survival 1-year post-transplant.
FIGURE 6:
Funnel plot for random effects meta-analysis for graft survival post-transplant.
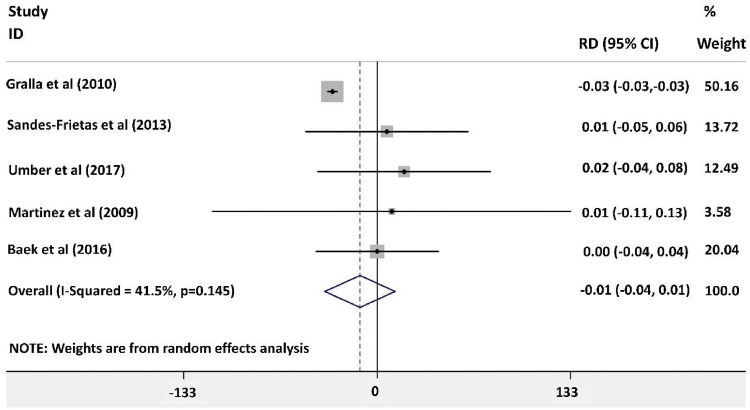
Forest plot analysis for patient survival 1-year post-transplant showed no significant different between both groups as shown in Figure 7 [risk difference −0.01 (95% CI −0.04 – 0.01)]. The greatest weight was for the article by Gralla and Wiseman [18] [weight 50.16% (95% CI −0.03)] There was no evidence of heterogenicity (I2 = 41.5%, P = 0.145).
FIGURE 7:
Forest plot analysis for patient survival 1-year post-transplant.
Only three articles compared changes in creatinine between both groups. de Sandes-Freitas et al. [19] showed no difference in renal function between both groups at the 1-year follow-up (creatinine 1.49 mg/dL in the IL-2R antibody induction group, creatinine 1.47 mg/dL in the no-induction group). Gavela Martinez et al. [22] and Baek et al. [23] showed similar results, with no difference between the groups (P > 0.05). de Sandes-Freitas et al. [19] and Baek et al. [23] were the only studies in our meta-analysis to assess the risk of NODAT between the groups. Of the studies included in the meta-analysis, Baek et al. [23] was the only one to address the risk of CMV infection in transplant patients who receive IL-2R antibody as induction therapy. It found no significant difference in the risk of CMV infection (2.8% in the IL-2R antibody induction group and 0% in the no-induction group).
DISCUSSION
In this meta-analysis, seven studies were included for assessment of risk of acute rejection in the standard-risk population with IL-2R antibody induction versus placebo, five of them were used in the meta-analysis for graft survival 1-year post-transplant. To our knowledge, this is the first meta-analysis to be conducted on this topic in the tacrolimus era.
Formerly, Webster et al. [12] conducted a large meta-analysis that included primarily studies with cyclosporine-based maintenance immunosuppression. Authors of this meta-analysis reported a decrease in the risk of acute rejection of 28% in those receiving IL-2R antibody induction therapies, with a relative risk of 0.72. Based on these results, they concluded that for every 100 renal transplant recipients receiving IL-2R antibody induction therapy, the risk of acute rejection episodes would be expected to decrease by 14 patients. They also concluded that the number needed to treat to prevent one rejection event was seven patients. The KDIGO guidelines, based on results of this meta-analysis, recommend IL-2R antibody induction as standard induction therapy in low-risk renal transplant patients [13]. However, since many centres today use tacrolimus as maintenance immunotherapy, questions about the validity of the results of this meta-analysis were raised.
Many studies have compared long-term outcomes of renal transplant using cyclosporine and tacrolimus as maintenance immunotherapy. The Symphony study showed better graft survival and less risk of acute rejection events at 1-year follow-up post-transplant in patients receiving tacrolimus compared with those receiving cyclosporine [16]. Furthermore, a long-term multicentre European study found similar results when they followed-up the patients for 5 years post-transplant [24]. In 2002, Vincenti et al. [25] showed similar results in a multicentre trial in the USA with a follow-up period of 5 years after transplant. The favourable long-term outcome for patients using tacrolimus as maintenance therapy compared with cyclosporine could be due to a less nephrotoxic effect of the former compared with the latter [26]. It could also be related to the more powerful immunosuppressive effect of tacrolimus compared with cyclosporine, ending in a lower rate of chronic rejection. The better outcomes of patients on tacrolimus maintenance therapy and fewer nephrotoxic effects raised concerns about the need and the benefit of induction therapy in the standard-risk population. Our meta-analysis showed no additional benefit of IL-2R antibody induction therapy in standard-risk patients in the tacrolimus era.
In previous studies there were conflicting data about the actual benefit of IL-2R antibody induction therapy in the standard-risk population. Willoughby et al. [27], using a composite endpoint of acute rejection and graft loss or death, found favourable outcomes for those having IL-2R antibody induction therapy versus placebo (tacrolimus and mycophenolate mofetil were the drugs used for maintenance immunotherapy). On the other hand, Lim et al. [28], on analysing data from the Australian registry database, did not find any significant decrease in the risk of rejection when using IL-2R antibody induction therapy in the low- or intermediate-risk population (cyclosporine and tacrolimus were the CNIs used in the study for maintenance immunotherapy). Both of these studies were not included in our meta-analysis due to the short follow-up period (6 months). Similarly, in 2015, Tanriover et al. [29] proved in a large study using data from the Organ Procurement and Transplantation Network (OPTN) registry that there is no additional benefit from using IL-2R antibody induction therapy in transplants from living donors. This study was also excluded from our meta-analysis, as almost half of the included patients were high-risk transplants with PRA > 20% and also because of overlap between data included in this study and that used in Gralla and Wiseman [18]. In 2016, Tanriover et al. [30] repeated the same study using OPTN data on transplants from deceased donors. The study showed no additional benefit from using IL-2R antibody induction therapy in transplants from deceased donors. Most of the included patients in this study did not meet our criteria defining a standard-risk population (PRA < 20%, no more than two transplants, DR mismatch <2). Subgroup analysis of low-risk transplants showed no effect of IL-2 induction therapy on rejection rates or survival; however, details of this group were not thoroughly discussed in the study. Also, there was overlap of the data in this study with that of Gralla and Wiseman [18] and therefore it was not included in our meta-analysis.
Our analysis showed a possible degree of heterogeneity among included studies when assessing the risk of acute rejection, while the studies were homogeneous when assessing graft survival. This could be due to variations in the definition of a standard-risk population among different studies and centres. The stratification of immunological risk depends on several factors related to the recipient and the characteristics of the donor. The risk factors for acute rejection included the age of the recipient and donor, degree of HLA mismatch, cold ischaemia time, ethnicity, PRA, presence of donor-specific antibodies, blood group incompatibility and previous failed transplants [13]. In the UK, guidelines to standardize definitions of high and low immunological risk populations are under development [31]. On repeat analysis after eliminating the risk of heterogenicity, again IL-2 induction therapy did not show any benefit in decreasing the risk of rejection.
The strengths of our study are the inclusion of several studies from different countries and thereby being less influenced by local findings compared with single-country studies and generalization of the population of studies, thus having higher statistical power to detect an effect. Limitations include several points. The follow-up period of 1 year is too short to draw definitive conclusions on graft survival. All we know is that 12 months is not always enough time to evaluate the damage of a previous acute rejection. The percentage of extended criteria donors in our analysis is low compared to the current set-up in clinical transplantation which is around 20–60% of deceased donor grafts [32]. One cannot exclude that, in particular, this type of graft may benefit from IL-2R antibody induction use in order to spare CNIs.
In conclusion, IL-2R antibody induction therapy has no significant effect on the rate of rejection and patient or graft survival in standard-risk renal transplant recipients on tacrolimus-based maintenance immunotherapy.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published elsewhere except in abstract form.
Supplementary Material
REFERENCES
- 1. Chang SH, Russ GR, Chadban SJ. et al. Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation 2007; 84: 611–618 [DOI] [PubMed] [Google Scholar]
- 2. McDonald S, Russ G, Campbell S. et al. Kidney transplant rejection in Australia and New Zealand: relationships between rejection and graft outcome. Am J Transplant 2007; 7: 1201–1208 [DOI] [PubMed] [Google Scholar]
- 3. Meier-Kriesche HU, Schold JD, Srinivas TR. et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4: 378–383 [DOI] [PubMed] [Google Scholar]
- 4. McKeage K, McCormack PL.. Basiliximab: a review of its use as induction therapy in renal transplantation. BioDrugs 2010; 24: 55–76 [DOI] [PubMed] [Google Scholar]
- 5. Andres A. Cancer incidence after immunosuppressive treatment following kidney transplantation. Crit Rev Oncol Hematol 2005; 56: 71–85 [DOI] [PubMed] [Google Scholar]
- 6. Nashan B, Moore R, Amlot P. et al. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet 1997; 350: 1193–1198 [DOI] [PubMed] [Google Scholar]
- 7. Charpentier B, Thervet E.. Placebo-controlled study of a humanized anti-TAC monoclonal antibody in dual therapy for prevention of acute rejection after renal transplantation. Transplant Proc 1998; 30: 1331–1332 [DOI] [PubMed] [Google Scholar]
- 8. Vincenti F, Kirkman R, Light S. et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med 1998; 338: 161–165 [DOI] [PubMed] [Google Scholar]
- 9. Mourad G, Rostaing L, Legendre C. et al. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004; 78: 584–590 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez E, Gutierrez E, Hernandez Y. et al. Anti-CD25 monoclonal antibody sequential immunosuppressive induction therapy in renal transplants with high risk of delayed graft function. Transplant Proc 2005; 37: 3736–3737 [DOI] [PubMed] [Google Scholar]
- 11. Sandrini S. Use of IL-2 receptor antagonists to reduce delayed graft function following renal transplantation: a review. Clin Transplant 2005; 19: 705–710 [DOI] [PubMed] [Google Scholar]
- 12. Webster AC, Ruster LP, McGee R. et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev 2010; 1: CD003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1–S155 [DOI] [PubMed] [Google Scholar]
- 14. Muntean A, Lucan M.. Immunosuppression in kidney transplantation. Clujul Med 2013; 86: 177–180 [PMC free article] [PubMed] [Google Scholar]
- 15. Webster A, Woodroffe RC, Taylor RS. et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005; 4: CD003961. [DOI] [PubMed] [Google Scholar]
- 16. Ekberg H, Tedesco-Silva H, Demirbas A. et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562–2575 [DOI] [PubMed] [Google Scholar]
- 17. Wiland AM, Fink JC, Weir MR. et al. Should living-unrelated renal transplant recipients receive antibody induction? Results of a clinical experience trial. Transplantation 2004; 77: 422–425 [DOI] [PubMed] [Google Scholar]
- 18. Gralla J, Wiseman AC.. The impact of IL2ra induction therapy in kidney transplantation using tacrolimus- and mycophenolate-based immunosuppression. Transplantation 2010; 90: 639–644 [DOI] [PubMed] [Google Scholar]
- 19. de Sandes-Freitas TV, Felipe CR, de Franco MF. et al. Basiliximab induction in patients receiving tacrolimus-based immunosuppressive regimens. Int Urol Nephrol 2013; 45: 537–546 [DOI] [PubMed] [Google Scholar]
- 20. Schwarz C, Böhmig G, Steininger R. et al. Tacrolimus, mycophenolate mofetil, and low-dose steroids with or without interleukin-2 receptor antibody induction therapy: a retrospective cohort analysis. Transplant Proc 2015; 47: 2446–2449 [DOI] [PubMed] [Google Scholar]
- 21. Umber A, Killackey M, Paramesh A. et al. A comparison of three induction therapies on patients with delayed graft function after kidney transplantation. J Nephrol 2017; 30: 289–295 [DOI] [PubMed] [Google Scholar]
- 22. Gavela Martinez E, Avila Bernabeu AI, Sancho Calabuig A. et al. Use of basiliximab induction in low-immunological risk renal transplant recipients receiving tacrolimus-based immunosuppression. Transplant Proc 2009; 41: 2337–2338 [DOI] [PubMed] [Google Scholar]
- 23. Hee Baek C, Hyun Kim J, Yu H. et al. Usefulness of tacrolimus without basiliximab in well-matched living-donor renal transplant recipients in Korea. Exp Clin Transplant 2016; 14: 389–393 [DOI] [PubMed] [Google Scholar]
- 24. Mayer AD. Chronic rejection and graft half-life: five-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc 2002; 34: 1491–1492 [DOI] [PubMed] [Google Scholar]
- 25. Vincenti F, Jensik SC, Filo RS. et al. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation 2002; 73: 775–782 [DOI] [PubMed] [Google Scholar]
- 26.McCarthy A. Tacrolimus better than cyclosporine at preserving renal function. Medscape, 12 June 2009
- 27. Willoughby LM, Schnitzler MA, Brennan DC. et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statistical approaches to reduce bias in observational comparisons. Transplantation 2009; 87: 1520–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim WH, Chadban SJ, Campbell S. et al. Interleukin-2 receptor antibody does not reduce rejection risk in low immunological risk or tacrolimus-treated intermediate immunological risk renal transplant recipients. Nephrology (Carlton) 2010; 15: 368–376 [DOI] [PubMed] [Google Scholar]
- 29. Tanriover B, Zhang S, MacConmara M. et al. Induction therapies in live donor kidney transplantation on tacrolimus and mycophenolate with or without steroid maintenance. Clin J Am Soc Nephrol 2015; 10: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanriover B, Jaikaransingh V, MacConmara MP. et al. Acute rejection rates and graft outcomes according to induction regimen among recipients of kidneys from deceased donors treated with tacrolimus and mycophenolate. Clin J Am Soc Nephrol 2016; 11: 1650–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrews PA, Burnapp L. British Transplantation Society / Renal Association UK Guidelines for Living Donor Kidney Transplantation 2018: Summary of Updated Guidance. Transplantation 2018; 102: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messina M, Diena D, Dellepiane S. et al. Long-term outcomes and discard rate of kidneys by decade of extended criteria donor age. Clin J Am Soc Nephrol 2017; 12: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.