Abstract
Background
The recombinant avirulent Newcastle disease virus (NDV) LaSota strain expressing the rabies virus glycoprotein (rL-RVG) can induce much greater apoptosis than can NDV in gastric carcinoma cells, but the mechanisms involved remains unclear.
Material/Methods
The 2 gastric carcinoma cell lines were divided into the rL-RVG group, the NDV group, and the PBS group. MTT assay was used to detect and analyze cell viability. siRNA for α7-nAChR, α7-nAChR antagonist, or α7-nAChR agonist, AKT antagonist, and p-AKT agonist were used for pretreatment. The protein expressions of RVG, NDV, α7-nAChR, cleaved caspase-3, p-AKT, PI3K, Bcl-2, and Bax proteins were detected by Western blot assay. Immunofluorescence was used to detect expressions of α7-nAChR proteins. Light microscopy, flow cytometry, and TUNEL assay were used to assess apoptosis.
Results
The results showed that 2 virus concentrations over 103 dilution caused greater cell proliferation inhibition. rL-RVG treatment increased the expression of α7-nAChR, cleaved caspase-3, and Bax protein but decreased the expression of p-AKT, PI3K, and Bcl-2 protein. When the groups were pretreated with α7-nAChR antagonist, the α7-nAChR, cleaved caspase-3, and Bax protein expression increased, but the expression of p-AKT, PI3K, and Bcl-2 protein was clearly decreased. However, the results in the α7-nAChR agonist group were the opposite. When treated with the AKT antagonist, the result was the same as in the rL-RVG treatment group. The result in the AKT agonist group was the opposite of that in the AKT antagonist group. Compared with the NDV group, the results of light microscopy, FCM, and TUNEL assay showed that α7-nAChR antagonist significantly affected the apoptosis of gastric cancer cells in the rL-RVG group.
Conclusions
rL-RVG leads to much greater apoptosis through the α7-nAChR/PI3K/AKT pathway.
MeSH Keywords: alpha7 Nicotinic Acetylcholine Receptor, Apoptosis, Newcastle Disease Virus, Oncogene Protein v-akt, Rabies Virus, Stomach Neoplasms
Background
Cancer is the second leading cause of human mortality worldwide [1,2] and stomach cancer is one of the main causes of cancer-related mortality [1]. Many studies have demonstrated that biological treatment, including oncolytic virotherapy, has great potential for the treatment of cancer [3,4]. Newcastle disease virus (NDV) is an avian paramyxovirus that can spread from birds to humans, but the pathogenicity in humans is minimal, limited to mild flulike symptoms and conjunctivitis [5]. A lentogenic strain of this virus has demonstrated selective cytopathic efficacy against cancer cells [6]. NDV has been explored as an oncolytic agent [7,8]. Acetylcholine receptors (AChR) are highly expressed in non-small cell lung cancer, gastric cancer, bladder cancer, and colorectal cancer [9]. Many studies have found that α7-nAChR affects expression and activation of cancer cell proliferation, invasion, metastasis, angiogenesis, and the inflammatory response [10–12]. The rabies virus glycoprotein has a nicotine receptor blocking function similar to that of coral snake venom [13]. We have previously reported that rL-RVG [14] can induce greater autophagy and apoptosis in gastric carcinoma cells than can NDV [15]. In this investigation, we focused on the molecular mechanisms of apoptosis in gastric carcinoma HGC or SGC cells treated with rL-RVG through inhibition of the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathway.
Material and Methods
Materials
EDTA-2Na, 1640, and trypsin were from Gibco (NY, USA). Fetal bovine serum (FBS) was supplied by Hyclone (UT, USA). The human cancer cell lines SGC-7901 and HGC were purchased from the Cancer Cell Repository (Shanghai Cell Bank, 2010-02-20). rL-RVG and NDV were kindly provided by Harbin Veterinary Research Institute (Harbin, Heilongjiang, China). α7-nAChR antagonist methyl lycaconitine citrate hydrate (MLA): sc-253043 was obtained from Santa Cruz (CA, USA), α7-nAChR agonist acetylcholine bromide (ACB) was from Sigma (USA), AKT antagonist MK-2206 was purchased from Merck (Darmstadt, Germany), AKT agonist IGF-1 was from Abcam (USA), and Hoechst 33342 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-Bax and anti-Bcl-2 were from Boster (Wuhan, Hubei, China). Rabbit monoclonal anti-p-AKT antibody and anti-PI3K antibody were from Cell Signaling Technology (Beverly, MA, USA), Anti-cleaved-caspase-3 was from ImmunoWay (Newark, DE, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit, HRP-conjugated goat anti-mouse, and FITC-conjugated goat anti-mouse antibodies were purchased from CWBio (Shanghai, China). Cy3-conjugated goat anti-rabbit antibody was purchased from KPL (Washington, DC, USA), and TUNEL assay kits were from Nanjin (China). A short interfering (si) RNA specific for human α7-nAChR was obtained from GenePharma (Shanghai, China).
Cell culture
SGC and HGC cells were cultured in RPMI 1640 medium with 10% (v/v) fetal bovine serum at 37°C with 5% CO2 and 100% humidity. After growing to 50–70% confluence in 6-well plates, the SGC and HGC cells were treated with rL-RVG, NDV, or phosphate-buffered saline (PBS).
MTT assay
SGC or HGC cell suspensions (1×104) were transferred into 96-well plates and continued to culture in 10% FBS at 37°C with 5% CO2 for 24 h. The next day, the cells were infected with rL-RVG (109.8 EID50/ml) or NDV (109.8 EID50/ml) [14] diluted 10, 102, 103, 104, or 105 times. After 1 h, the cells were cultured as described previously and divided into an rL-RVG group, an NDV group, or a PBS blank control group and then each well was treated with 20 μl MTT (5 mg/ml, PBS) and continued to culture for 4 h on the third day. After that, we added 150 μl of DMSO into each well for 10 min. Lastly, a standard spectrophotometer was used to measure the absorbance in triplicate. The morphological changes of infected cells were assessed under a light microscope.
Interference test with agonist or antagonist or siRNA
α7-nAChR antagonist MLA or agonist ACB was also applied. When the SGC and HGC cells had grown to 50–70% confluence in the 6-well plates, the cells were pretreated with 10−3 mmol/L MLA or ACB [16]. Furthermore, the AKT antagonist MK-2206 or agonist IGF-1 was pretreated as described above, and 12 h later, the cells were treated with rL-RVG, NDV, or PBS for 24 h [2]. Finally, the cells were extracted and analyzed by immunofluorescence and Western blot analysis. Moreover, the siRNA for α7-nAChR was also applied, and the HGC cells were pretreated with si-α7-nAChR (50 nM) following the manufacturer’s instructions. The next day, the cells were infected with rL-RVG or NDV for 24 h and then analyzed by Western blot.
Immunofluorescence assay
Cells in logarithmic growth phase were infected with rL-RVG, NDV, or PBS for 24 h and then fixed in 4% paraformaldehyde at 4°C overnight, followed by immunofluorescence staining with antibodies against α7-nAChR and Hoechst 33342 staining. lmmunofluorescence microscopy was used to observed the stained SGC and HGC cells.
Western blot assay
RIPA lysis buffer with a protease antagonist cocktail was used to lyse SGC and HGC cells with or without treatment. BCA kits were used to assess protein concentrations. After separating by electrophoresis, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. Next, the membrane was blocked with 5% BSA for 1 h. Then, the membrane was incubated with primary antibodies against specific proteins (NDV and RVG for infection; cleaved caspases 3, Bcl-2, and Bax; α7-nAChR, PI3K, and p-AKT for α7-nAChR/PI3K/p-AKT pathway), and then continued to be treated by HRP-conjugated secondary rabbit or mouse antibodies. Finally, the proteins were detected and analyzed by using Pierce ECL Plus Substrate under a Typhoon9400 Variable Mode Imager.
Flow cytometry assay
Apoptotic and viable cells were analyzed with flow cytometry (FCM) and annexin V/propidium iodide (PI) double-staining to detect membrane events. FCM analysis of the labeled cells was performed by FACSort flow cytometry, and the data were analyzed with CellQuest software. The cytograms of the 4 quadrants were used to differentiate normal (annexin V2−/PI−), early apoptotic (annexin V+/PI−), late apoptotic (annexin V+/PI+), and necrotic (annexin V2−/PI+) cells. Apoptosis was calculated as the total numbers of early and late apoptotic cells. All experiments were carried out in triplicate.
TUNEL assay
In interference testing, the HGC cells were pretreated with α7-nAChR inhibitor MLA (10−3 mol/L) or α7-nAChR agonist ACB (10−3 mol/L) 4 h before infection with NDV or rL-RVG (10 MOI). Annexin V/PI staining was performed to detect apoptosis/necrosis, and 12 h later, treated by the diluted rL-RVG or wild-type NDV at an MOI of 10, with PBS as negative control. TUNEL assay kits were used according to the manufacturer’s instructions. At 24 h after infection, ice-cold 4% paraformaldehyde (Solar Biotech, Beijing, China) was used to fix the cells for 30 min at room temperature, then, the cells were washed with PBS and continued to incubated with 3% hydrogen peroxide in methanol at room temperature for 10 min. After being rinsed with PBS, the samples were incubated with 0.1% Triton X-100 and 0.1% sodium citrate in water at room temperature for 30 min. The pretreated specimens were treated with 50 μL of TdT-labeled reaction buffer at 4°C overnight in the dark and then in a humidified atmosphere at 37°C for an additional 2–3 h. Subsequently, the slides were incubated with 50 μL of streptavidin-HRP for 60 min, followed by detection with 50 μL of diaminobenzidine reagent for 10 min. Finally, the cells were observed and imaged under an optical microscope (ECLIPSE TS100, Nikon, Japan). This process was repeated 3 times.
Statistical analysis
All data and results were presented as the mean ± standard deviation and were analyzed by SPSS 19.0 software (IBM Corp., Armonk, NY). One-way analysis of variance (ANOVA) was used to analyze the statistical significance, and then post hoc analysis was performed to compare the differences between multiple groups. P<0.05 was a statistically significant difference. All experiments were repeated at least 3 times.
Results
Protein expression of NDV and RVG proteins after infection with rL-RVG and NDV
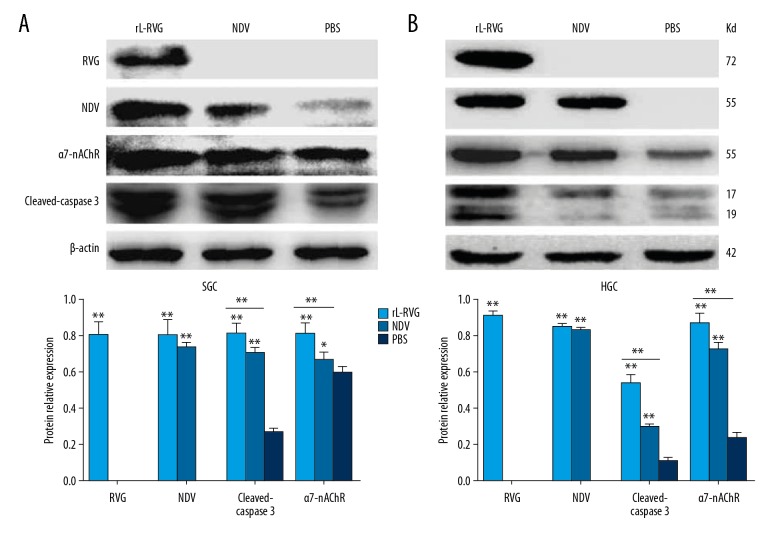
Western blot analysis revealed that HGC and SGC cells expressed the NDV HN protein after infection with NDV, and they also expressed NDV protein and RVG protein after infection with rL-RVG, while the NDV HN and RVG proteins were expressed at very low levels in the PBS group. Compared with the NDV group, RVG and NDV protein expression was higher in the rL-RVG group (P<0.05), as shown in Figure 1.
Figure 1.
Protein expression of RVG, NDV, α7-nAChR, and cleaved caspase 3 was assessed by Western blot. (A) for SGC, (B) for HGC. Protein expression of RVG was only detected in the rL-RVG group (** P<0.01), while protein expression of NDV was higher in the rL-RVG and NDV groups compared with the PBS groups (** P<0.01). The expression level of cleaved caspase 3 in the rL-RVG group was higher than that in the NDV group or PBS group (** P<0.01). The expression level of α7-nAChR in the rL-RVG group was higher than in the other groups (** p<0.01).
The changes in SGC and HGC cell proliferation after infection with rL-RVG and NDV
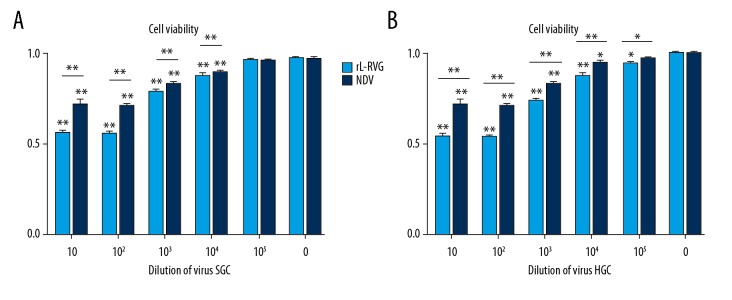
After infection with rL-RVG and NDV, cell viability of SGC and HGC were detected and analyzed by MTT assay. Cell viability of SGC and HGC cells increased with decreased virus concentration, and, compared with the control group, the dose-response curve reached a plateau at the 103 dilution. The OD of MTT in the rL-RVG group was weaker than in the NDV-infected group, which suggested that rL-RVG had a stronger inhibitory ability. The results are shown in Figure 2.
Figure 2.
Proliferation changes of gastric cancer cells after infecting with rL-RVG or NDV were monitored by MTT analysis. (A) Dose-response curve of SGC cells after infection with rL-RVG or NDV for 24 h. The cells infected with rL-RVG for 103 dilution showed significantly decreasing absorbance when compared with the NDV group (* p<0.05). (B) Dose-response curve of HGC cells after infecting with rL-RVG or NDV for 24 h. The cells infected with rL-RVG for 103 dilution showed significantly decreased absorbance when compared with cell viability of the NDV group (** p<0.01).
Effects of rL-RVG on the protein expression of α7-nAChR
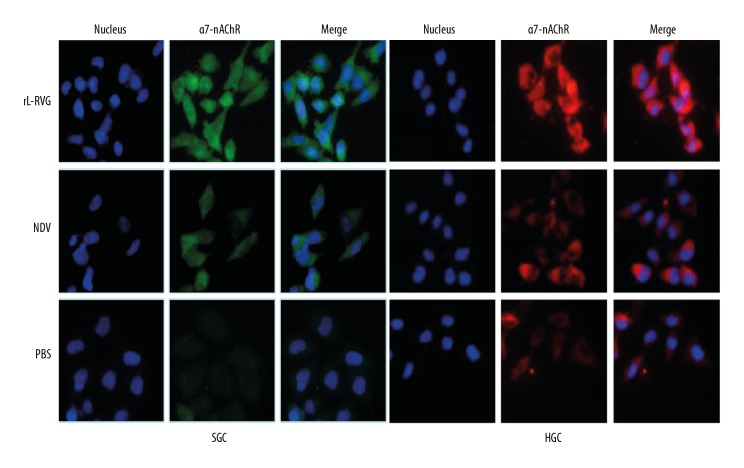
Western blot analysis showed that when the SGC and HGC cells were infected with rL-RVG or NDV, the protein expression of α7-nAChR in the rL-RVG group was obviously higher than in the NDV group and PBS group (P<0.01). Moreover, the α7-nAChR protein expression in immunofluorescence had the same trend, as shown in Figures 1 and 3.
Figure 3.
α7-nAChR expression was detected by immunofluorescence (×200 magnification). (A) for SGC, (B) for HGC. The α7-nAChR protein (green) was expressed at highest levels in the rL-RVG group, while the highest expression of α7-nAChR protein (red) was in the rL-RVG group.
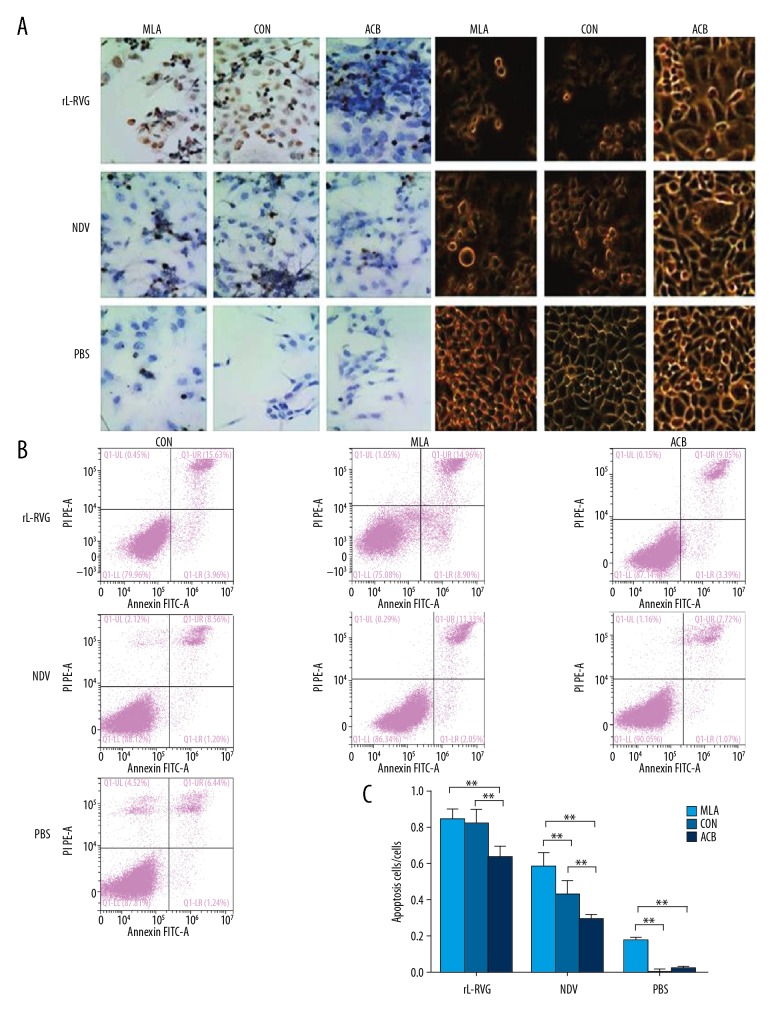
Effects of rL-RVG, α7-nAChR agonist, or antagonist pretreatment on morphological changes and the apoptosis in HGC cells
After being infected with rL-RVG or NDV, the HGC cell morphology changed gradually from adherent growth state to a state of suspended growth, with shriveled cell membranes detected under an inverted microscope, especially in the rL-RVG-infected groups. When groups were treated by α7-nAChR antagonist MLA, the HGC cell morphological changes were much more obvious, including cell membrane shriveling and suspended growth, but the rL-RVG+MLA group cells showed little change. While the NDV group and PBS group were treated by MLA, the state of suspended growth and cell membrane shrivel appeared much more obvious compared with the rL-RVG group. When groups were treated by α7-nAChR agonist ACB, the HGC cell morphological changes trended to survive, but the morphological changes had no difference between the PBS +ACB group and the control group, as shown in Figure 4A.
Figure 4.
Effect of rL-RVG, α7-nAChR antagonist, or agonist pretreatment on morphological changes and apoptosis in HGC cells. (A) Morphological changes of infected or uninfected HGC cells. When groups were treated by α7-nAChR antagonist MLA, the morphological changes in HGC cells were clearly more pronounced, including cell membrane shriveling and suspended growth, but the rL-RVG+MLA group showed little change. When groups were treated with α7-nAChR agonist ACB, the HGC cell morphological changes trended to persist, but there was no significant difference in morphological changes between the PBS+ACB group and the control group in comparison with the rL-RVG group. (B) TUNEL assay was used to assess apoptosis of infected HGC cells. (C) There were more apoptotic cells in the rL-RVG group than in the NDV group or PBS group (** P<0.01). When groups were treated with the α7-nAChR antagonist MLA, the apoptotic cells were more abundant than in the groups without pretreatment (P<0.01), except for the rL-RVG+MLA group and the rL-RVG group (P>0.05). When the α7-nAChR agonist ACB cells were pretreated before virus infection, the number of apoptotic cells decreased (** P<0.01), including early apoptotic (annexin V+/PI−) and late apoptotic (annexin V+/PI+) cells. * P<0.05.
TUNEL assay indicated that the apoptosis level of rL-RVG-infected HGC cells was higher than in the NDV group or PBS group, and there were more apoptotic cells in the rL-RVG group than in the NDV-infected group. When the rL-RVG groups were pretreated by α7-nAChR antagonist MLA or α7-nAChR agonist ACB, we found little difference between the rL-RVG groups and the rL-RVG+MLA group, but there were fewer apoptotic cells in the rL-RVG+ACB group than in the rL-RVG group. On the other hand, the NDV and PBS pretreatment groups with MLA had more apoptotic cells than in the NDV and PBS groups. The numbers of apoptotic cells in the NDV group and the PBS group decreased after being pretreated with ACB, as shown in Figure 4B. When FCM analysis was used to detected the apoptosis of cells with interference, Annexin V staining showed that pretreatment with α7-nAChR agonist ACB led to a significant decrease in apoptosis, but pretreatment with α7-nAChR inhibitor MLA led to an obvious increase in apoptosis, except in the rL-RVG+MLA group and the rL-RVG group. The results were in agreement with the Western blot results, as shown in Figure 4C.
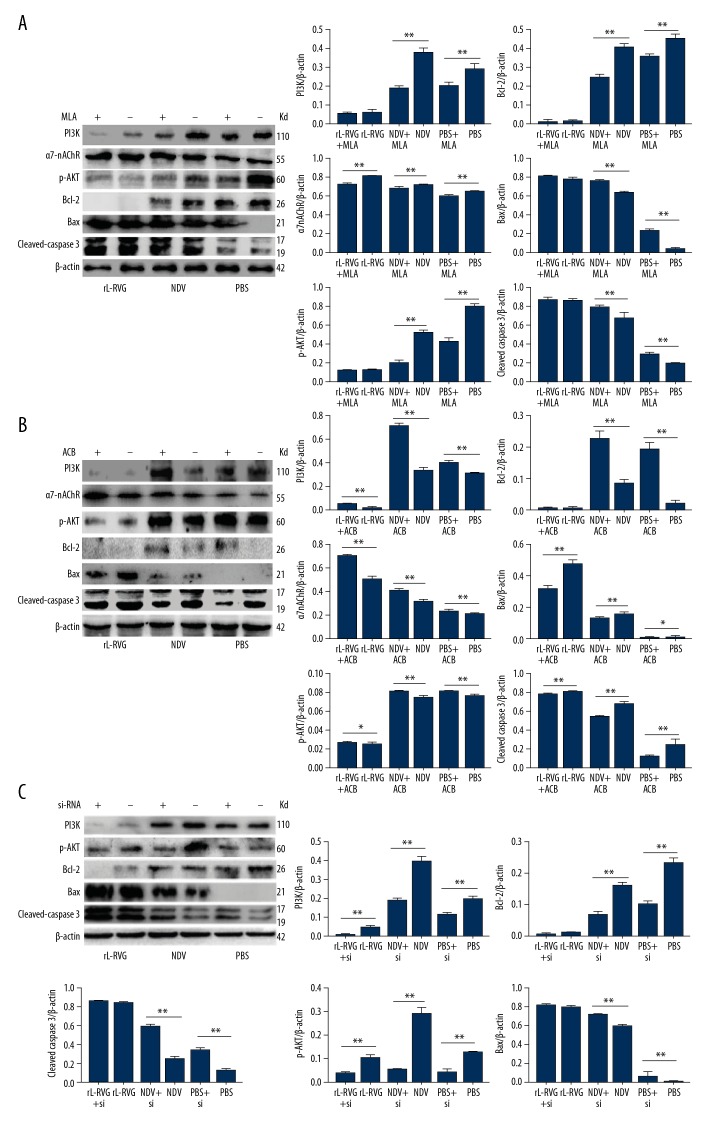
Effects of α7-nAChR agonist or antagonist and siRNA pretreatment on the expression of α7-nAChR and apoptosis-related proteins
We chose the HGC cell line for antagonist and agonist treatment study. The α7-nAChR antagonist MLA was used to pretreat the gastric carcinoma HGC cells before cells were infected with rL-RVG or NDV. α7-nAChR protein expression decreased significantly (P<0.01) in NDV and PBS groups pretreated with MLA, but the difference between the rL-RVG group and rL-RVG+MLA group was not significant (P>0.05), as shown in Figure 5A. When the α7-nAChR agonist ACB was used to pretreat HGC cells before being infected with rL-RVG or NDV, the α7-nAChR protein expression increased significantly (P<0.05) in ACB-pretreated groups, as shown in Figure 5B. Furthermore, when α7-nAChR siRNA was used to pretreat the gastric carcinoma HGC cells before being infected with virus rL-RVG or NDV, the α7-nAChR protein expression decreased significantly (P<0.01) in the α7-nAChR siRNA-pretreated groups, as shown in Figure 5C.
Figure 5.
The contribution of rL-RVG, α7-nAChR antagonist MLA, agonist ACB, or siRNA downregulation to the expression of α7-nAChR, p-AKT, PI3K, and apoptosis-associated proteins in HGC cells after infection. (A) For α7-nAChR antagonist. (B) For α7-nAChR agonist. (C) For α7-nAChR siRNA. When the groups were pretreated with α7-nAChR antagonist MLA, the expression of Bax/Bcl-2 and cleaved caspase 3 proteins increased in HGC cells compared to cells without MLA or α7-nAChR siRNA treatment (** P<0.01), except for the rL-RVG +MLA group and the rL-RVG group. The expression of the Bax/Bcl-2 and cleaved caspase 3 proteins decreased significantly (** P<0.01) in cells with α7-nAChR agonist ACB treatment compared with cells without pretreatment. The expression of α7-nAChR proteins decreased in MLA-pretreated groups (** P<0.01), but increased in ACB-pretreated groups (** P<0.01). The expression of p-AKT and PI3K proteins decreased in MLA and α7-nAChR siRNA-pretreated groups (** P<0.01), except for the rL-RVG group and the pretreated groups, but increased in the ACB-pretreated groups (** P<0.01). * P<0.05.
The SGC and HGC cells was assessed by Western blot analysis at 24 h after being infected with NDV or rL-RVG. Western blot assay showed the expression levels of cleaved caspase 3 proteins in the rL-RVG-infected groups were higher than in the NDV and PBS groups, as shown in Figure 1. We chose HGC cells for further study. When the groups were treated with α7-nAChR antagonist MLA or α7-nAChR siRNA, the expression level of apoptosis-related proteins cleaved caspases 3 and Bax/Bcl-2 increased significantly (P<0.05) in the pretreated NDV and pretreated PBS groups, but the difference between the rL-RVG and pretreated groups was insignificant (P>0.05), as shown in Figure 5A and 5C. When the virus induced apoptosis in HGC cells after pretreating with α7-nAChR agonist ACB, the levels of the apoptosis-related proteins cleaved caspases 3 and Bax/Bcl-2 decreased significantly (P<0.05), as shown in Figure 5B.
Effects of rL-RVG, α7-nAChR agonist, and antagonist and siRNA pretreatment on the expression of PI3K/p-AKT pathway proteins in HGC cells
HGC cells was assessed routinely by Western blot analysis at 24 h after being infected with NDV or rL-RVG. The expression of PI3K and p-AKT proteins in the rL-RVG-infected groups were higher than in the NDV or PBS groups, as shown in Figure 5A–5C. When the groups were treated with α7-nAChR antagonist or α7-nAChR siRNA, the expression of PI3K and p-AKT proteins decreased significantly (P<0.05), but the differences between the rL-RVG group and the pretreatment-rL-RVG group were not obvious, as shown in Figure 5A and 5C. However, in the HGC cells infected with rL-RVG or NDV after pretreatment with α7-nAChR agonist, the levels of PI3K and p-AKT increased significantly (P<0.05), as shown in Figure 5B.
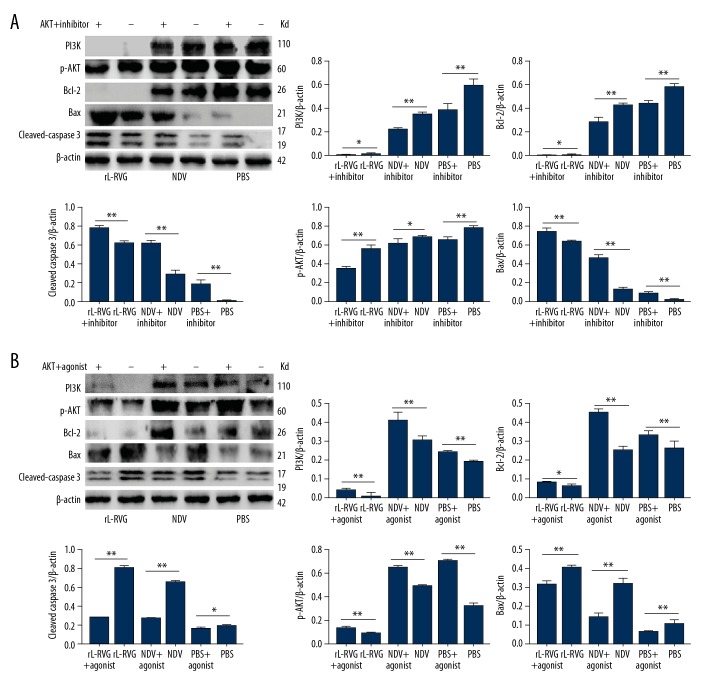
Effects of AKT antagonist or agonist on the expression of PI3K or p-AKT proteins and the apoptosis-associated proteins in gastric cancer HCG cells
When HGC cells were pretreated with AKT antagonist MK-2206, the expression of PI3K and p-AKT proteins and apoptosis-related proteins cleaved caspases 3 and Bax/Bcl-2 decreased significantly (P<0.05) in the NDV or PBS groups, but there was no insignificant difference between the rL-RVG and pretreated groups (P>0.05), as shown in Figure 6A. When the HGC cells were pretreated with AKT agonist IGF-1 before infection with NDV or rL-RVG, the expression levels of the apoptosis-related proteins cleaved caspases 3 and Bax/Bcl-2 increased significantly (P<0.05), as shown in Figure 6B.
Figure 6.
AKT antagonist and AKT agonist contributed to upregulating p-AKT, PI3K, and apoptosis-associated proteins expression in HGC cells after infection. (A) For AKT inhibitor. (B) For AKT agonist. When the groups were pretreated with AKT antagonist MK-2206, the expression of the Bax/Bcl-2 and cleaved caspase 3 proteins increased in HGC cells compared with cells without AKT antagonist (** P<0.01). The expression of the Bax/Bcl-2 and cleaved caspase 3 proteins decreased significantly (** P<0.01) in cells with AKT agonist IGF-1 treatment compared with cells without pretreatment. On the other hand, the expression of PI3K and p-AKT proteins decreased in AKT antagonist-pretreated groups (** P<0.01), but increased in AKT agonist-pretreated groups (** P<0.01).
Discussion
NDV is an oncolytic agent with obvious efficacy extensively demonstrated in both preclinical and clinical studies [17,18]. It has been reported that rL-RVG spreads more easily and has much higher antitumor potency [2]. Multiple mechanisms underly NDV-induced cytotoxicity, such as immunomodulatory mechanisms [19–21], autophagy pathways [22,23], and apoptotic pathways [24]. But the mechanisms by which rL-RVG exerts greater apoptosis than NDV are not completely clear. Our study demonstrates that the α7-nAChR is antagonized and the PI3K/p-AKT pathway is inhibited, and then promotes apoptosis in gastric carcinoma cells infected with rL-RVG.
It is found that nicotine promotes gastric cancer cell proliferation and metastasis related to α7-nAChR [25]. α7-nAChR antagonists of α-coral snake toxins (α-BTX) and α-cyclophosphamide can reduce the effect on promoting proliferation of nicotine [26]. α7-nAChR pathway activation promotes tumor cell proliferation and inhibits apoptosis via PI3K/AKT signaling pathways [27]. Recent studies suggest that some chemotherapeutic drugs, including 5-Fluorouracil and Ixabepilone, can downregulated α7-nAChR and promote gastric cancer cell apoptosis through the AKT pathway [28,29]. The rabies virus glycoprotein has nicotine receptor blocking function similar to that of coral snake venom [10].
When gastric cancer cells were infected by the rL-RVG, the stable expression of RVG protein indicated that rL-RVG infected the SGC and HGC cells successfully. The expression of the α7-nAChR protein was upregulated in the rL-RVG group and α7-nAChR is beneficial to cell survival [25,28,29]. In our study, we found the expression of cleaved caspase 3 in the rL-RVG group was higher than in the NDV or PBS groups, and the rL-RVG group had many more apoptotic cells than in the NDV group. In contrast, there was a higher level α7-nAChR expression in the rL-RVG group, suggesting that the rabies virus glucoprotein (RVG) produced in the rL-RVG group could combine and inhibit α7-nAChR competitively in the rL-RVG-infected gastric cancer cells, which led to increased α7-nAChR expression via negative feedback. When the α7-nAChR antagonist MAL was pretreated, the expression level of the α7-nAChR apoptosis-related proteins significantly increased. In contrast, when the α7-nAChR agonist ACB was pretreated, the level expression of the α7-nAChR protein increased and the level of apoptosis-related proteins decreased, which suggested the α7-nAChR agonist ACB can activate nAChR and inhibit apoptosis [30]. It appears that the mechanism by which rL-RVG promotes gastric cancer cell apoptosis involves antagonizing α7-nAChR.
α7-nAChR agonist can induce Bcl-2 phosphorylation to inhibit apoptosis [31]. In the present study, α7-nAChR antagonist MLA and rL-RVG downregulated the expression of the Bcl-2, p-AKT, and PI3K, and there was a stronger decrease in the rL-RVG group. However, when HGC cells were pretreated with α7-nAChR agonist ACB, the levels of Bcl-2, p-AKT, and PI3K expression increased significantly, which suggested rL-RVG antagonizes α7-nAChR and inhibits the PI3K/AKT pathway and then inhibits the bcl-2 pathway. Activation of the PI3K/AKT pathway can lead to Bax Ser184 residue phosphorylation and inactivation, thus inhibiting apoptosis [32]. Activation of AKT can inhibit mitochondrial release cytochrome and apoptosis factor to inhibit cell apoptosis [33]. In our study, rL-RVG promoted HGC cell apoptosis by inhibiting expression of the PI3K/AKT pathway-related proteins. When HGC cells was pretreated with the AKT antagonist MK-2206, the expression of Bcl-2, p-AKT, and PI3K were strongly decreased. In contrast, when the AKT agonist IGF-1 was used in advance, the expression of the Bcl-2, p-AKT, and PI3K strongly increased and the expression of Bax and cleaved caspase 3 strongly decreased. These results indicate rL-RVG inhibits PI3K/p-AKT by the α7-nAChR pathway. In addition, ERK and AKT pathways are overactive in NDV-infected RPMI-7951 cells [34], and NDV transiently activates the PI3K/AKT pathway in chicken cells at an early phase of infection [35]. In the present study, we also found that the expression of PI3K and AKT in the NDV group was sometimes increased compared with the PBS group.
Conclusions
In conclusion, this study shows that the α7-nAChR/PI3K/AKT signaling pathway participates in the effect of rL-RVG on SGC and HGC cells. Furthermore, rL-RVG can promote apoptosis through activating Bax and Caspase 3 signaling pathways. In addition, rL-RVG antagonized α7-nAChR, which promoted the PI3K/p-AKT pathway [27], and inhibited the survival of gastric cancer cells, suggesting that the α7-nAChR/PI3K/AKT pathway played an important role in rL-RVG virus-induced apoptosis. Our research demonstrates that rL-RVG promotes apoptosis by antagonizing α7-nAChR and the inhibiting PI3K/AKT pathway in gastric carcinoma cells. Our results support the great advantages of rL-RVG in biological treatment of gastric cancer.
Acknowledgements
The authors thank Ai-hua Gong and Zhi-jian Zhang from Jiangsu University (Jiangsu, China) for suggestions regarding experiments. The authors would also like to thank Professor Zhigao Bu and Dr Jinying Ge from the State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, China for supplying the recombinant Newcastle disease virus.
Footnotes
Source of support: This research was supported by the National Natural Science Foundation of China (grant no. 81672999) and the Natural Science Foundation of Jiangsu Province (grant no. BK20151333)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bu XF, Wang MB, Zhang ZJ, et al. Autophagy is involved in recombinant Newcastle disease virus (rL-RVG)-induced cell death of stomach adenocarcinoma cells in vitro. Int J Oncol. 2015;47:679–89. doi: 10.3892/ijo.2015.3039. [DOI] [PubMed] [Google Scholar]
- 3.Cuddington BP, Mossman KL. Oncolytic bovine herpesvirus type 1 as a broad-spectrum cancer therapeutic. Curr Opin Virol. 2015;13:11–16. doi: 10.1016/j.coviro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Vähä-Koskela MJ, Heikkilä JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CB, Pomeroy BS, Schrall K, et al. An outbreak of conjunctivitis due to Newcastle Disease Virus (NDV) occurring in poultry workers. Am J Public Health Nations Health. 1952;42:672–78. doi: 10.2105/ajph.42.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaacov B, Elihaoo E, Lazar I, et al. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008;15:795–807. doi: 10.1038/cgt.2008.31. [DOI] [PubMed] [Google Scholar]
- 7.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012;7:347–67. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganar K, Das M, Sinha S, et al. Newcastle disease virus: Current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu CC, Huang CY, Cheng WL, et al. Silencing A7-nAChR levels increases the sensitivity of gastric cancer cells to ixabepilone treatment. Tumour Biol. 2016;37(7):9493–501. doi: 10.1007/s13277-015-4751-x. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta P, Rizwani W, Pillai S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeschen C, Weis M, Aicher A, et al. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–36. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlov VA, Wang H, Czura CJ, et al. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 2003;9:125–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Gastka M, Horvath J, Lentz TL. Rabies virus binding to the nicotinic acetylcholine receptor alpha subunit demonstrated by virus overlay protein binding assay. J Gen Virol. 1996;77:2437–40. doi: 10.1099/0022-1317-77-10-2437. [DOI] [PubMed] [Google Scholar]
- 14.Ge J, Wang X, Tao L, et al. Newcastle disease virus-vectored rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats. J Virol. 2011;85(16):8241–52. doi: 10.1128/JVI.00519-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Su C, Hang M, et al. Recombinant Newcastle disease virus rL-RVG enhances the apoptosis and inhibits the migration of A549 lung adenocarcinoma cells via regulating alpha 7 nicotinic acetylcholine receptors in vitro. Virol J. 2017;14:190. doi: 10.1186/s12985-017-0852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorence RM, Roberts MS, O’Neil JD, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle dis-ease virus. Curr Cancer Drug Targets. 2007;7:157–67. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- 18.Burke J, Nieva J, Borad MJ, et al. Oncolytic viruses: Perspectives on clinical development. Curr Opin Virol. 2015;13:55–60. doi: 10.1016/j.coviro.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Zeng J, Fournier P, Schirrmacher V. Induction of interferon-α and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology. 2002;297:19–30. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]
- 20.Jestin V, Cherbonnel M. Interferon-induction in mouse spleen cells by the Newcastle disease virus (NDV) HN protein. Ann Rech Vet. 1991;22:365–72. [PubMed] [Google Scholar]
- 21.Park MS, Shaw ML, Munoz-Jordan J, et al. Newcastle disease virus (NDV)-based assay demonstrates interferon antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J Virol. 2003;77:1501–11. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata M, Hino S, Saito A, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koks CA, Garg AD, Ehrhardt M, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in ortho-topic glioma through the induction of immuno-genic cell death. Int J Cancer. 2015;136:E313–25. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 24.Meng S, Zhou Z, Chen F, et al. Newcastle disease virus induces apoptosis in cisplatin-resistant human lung adenocarcinoma A549 cells in vitro and in vivo. Cancer Lett. 2012;317:56–64. doi: 10.1016/j.canlet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Lien YC, Wang W, Kuo LJ, et al. Nicotine promotes cell migration through alpha7 nicotinic acetylcholine receptor in gastric cancer cells. Ann Surg Oncol. 2011;18:2671–79. doi: 10.1245/s10434-011-1598-2. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta P, Rastogi S, Pillai S, et al. Nicotine induces cell proliferation by β-arrestin – mediated activation of Src and Rb – Raf-1 pathways. J Clin Invest. 2006;116:2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen WY, Huang CY, Cheng WL, et al. Alpha 7-nicotinic acetylcholine receptor mediates the sensitivity of gastric cancer cells to 5-fluorouracil. Tumor Biol. 2015;36:9537–44. doi: 10.1007/s13277-015-3668-8. [DOI] [PubMed] [Google Scholar]
- 29.Tu CC, Huang CY, Cheng WL, et al. Silencing A7-nAChR levels increases the sensitivity of gastric cancer cells to ixabepilone treatment. Tumor Biol. 2016;37:9493–501. doi: 10.1007/s13277-015-4751-x. [DOI] [PubMed] [Google Scholar]
- 30.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–58. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Cui W, Hu S, Chan HH, et al. Bis(12)-hupyridone, a novel acetylcholinesterase antagonist, protects against glutamate-induced neuronal excitotoxicity via activating α7 nicotinic acetylcholine receptor/phosphoinositide 3-kinase/Akt cascade. Chem Biol Interact. 2013;203(1):365–70. doi: 10.1016/j.cbi.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Xin M, Deng X. Nicotine inactivation of Bax through phosphorylation. J Biol Chem. 2005;280:10781–89. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 33.Gibson EM, Henson ES, Haney N, et al. Epidermal growth factor protects epithelial-derived cells from tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by inhibiting cytochrome c release. Cancer Res. 2002;62:488–96. [PubMed] [Google Scholar]
- 34.Pap M, Bátor J, Szeberényi J. Sensitivity of human malignant melanoma cell lines to Newcastle disease virus. Anticancer Res. 2015;35:5401–6. [PubMed] [Google Scholar]
- 35.Kang Y, Yuan R, Zhao X, et al. Transient activation of the PI3K/Akt pathway promotes Newcastle disease virus replication and enhances anti-apoptotic signaling responses. Oncotarget. 2017;8:23551–63. doi: 10.18632/oncotarget.15796. [DOI] [PMC free article] [PubMed] [Google Scholar]