Abstract
Classical benzodiazepine sensitive GABAA receptor subtypes, the major mediators of fast synaptic inhibition in the brain are heteropentamers that can be assembled from α1–3/5, β1–3, and γ2 subunits, but how neurons orchestrate their selective accumulation at synapses remains obscure. We have identified a 10 amino acid hydrophobic motif within the intracellular domain of the α2 subunit that regulates the accumulation of GABAA receptors at inhibitory synaptic sites on both axon initial segments and dendrites in a mechanism dependent on the inhibitory scaffold protein gephyrin. This motif was sufficient to target CD4 (cluster of differentiation molecule 4) molecules to inhibitory synapses, and was also critical in regulating the direct binding of α2 subunits to gephyrin in vitro. Our results thus reveal that the specific accumulation of GABAA receptor subtypes containing α2 subunits at inhibitory synapses is dependent on their ability to bind gephyrin.
Keywords: GABAA receptor, gephyrin, synaptogenesis, CD4, clustering, GABAA receptor trafficking
Introduction
GABAA receptors are Cl− selective ligand-gated ion channels that mediate the majority of fast synaptic inhibition in the brain and are the sites of action for both benzodiazepines and barbiturates. These receptors are heteropentamers that can be assembled from six families of homologous subunits: α1–6, β1–3, γ1–3, δ, ε, and π, generating the basis for extensive GABAA receptor heterogeneity (Rudolph and Mohler, 2006). Biochemical, cell biological and genetic methodologies all suggest that the majority of benzodiazepine receptor subtypes assembled in the brain are composed of α, β, and γ2 subunits (Rudolph and Mohler, 2006).
It is emerging that neurons can target individual GABAA receptor subtypes to distinct types of inhibitory synapses (Kittler et al., 2002; Luscher and Keller, 2004). This is best exemplified in hippocampal pyramidal neurons, which express up to 11 differing GABAA receptor subunits (Pirker et al., 2000). These neurons are able to cluster receptors containing α2 subunits at synapses on the axon initial segment (AIS) and dendrites, whereas receptors containing α1 subunits are more uniformly expressed on both the AIS and dendrites (Nusser et al., 1996; Kittler et al., 2002; Luscher and Keller, 2004). In addition it is also evident that receptors containing α2 subunits are enriched on the cell bodies of pyramidal neurons opposed to CCK-positive terminals (Nyiri et al., 2001). Although it is widely accepted that interactions with the cytoskeleton are critical in regulating the synaptic accumulation of other ligand-gated ion channels such as ionotropic glutamate or glycine receptors (GlyR) (Craig et al., 2006), less is understood about the mechanism neurons use to control the accumulation of GABAA receptors at synaptic sites. One protein that has received considerable attention is gephyrin, initially described as a regulator of GlyR receptor synaptic clustering via its ability to cross-link these proteins to the actin cytoskeleton, and microtubules (Kneussel and Betz, 2000). In addition gephyrin colocalizes with GABAA receptors in many brain regions suggesting a role in regulating the subcellular distribution of these proteins (Luscher and Keller, 2004). Although gene knock-out of gephyrin was initially reported to abolish GABAA receptor clustering in cultured neurons (Kneussel et al., 1999a), specific effects on receptor subtypes incorporating α2 subunits have also been described in spinal cord and hippocampal neurons (Kneussel et al., 2001; Levi et al., 2004). However, whether gephyrin mediates its effects on GABAA receptor synaptic accumulation via a direct interaction or is mediated by unknown intermediate effectors remains to be determined.
In this study, we have addressed how hippocampal neurons regulate the clustering of GABAA receptor subtypes at inhibitory synapses. Collectively our experiments have identified a 10 amino acid sequence within the major intracellular domain of the α2 subunit that regulates the accumulation of GABAA receptors at postsynaptic specializations, in a process dependent on gephyrin. Finally we have established that this motif enables the α2 subunit to interact with gephyrin. Thus gephyrin dependent clustering of GABAA receptors at inhibitory synapses is facilitated by the direct binding of this protein to a hydrophobic motif within the intracellular domain of the receptor α2 subunit.
Materials and Methods
Antibodies.
Endogenous α2-containing receptors were detected with a guinea pig α2(1–12) (Fritschy and Mohler, 1995) antiserum. Recombinant receptors were tagged on their N terminus with the 9E10 (α1; N terminus) or hemagglutinin (HA) tag (α2; N terminus) and visualized with rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The AIS was identified with a monoclonal antibody against the Na channel type II (Santa Cruz Biotechnology). A rabbit polyclonal antibody against vesicular inhibitory amino acid transporter (VIAAT) was a generous gift from Dr. Bruno Gasnier (Centre National de la Recherche Scientifique, Paris, France). The monoclonal anti-gephyrin antibody was from Connex (Hamburg, Germany).
Affinity purification assays.
Glutathione-S-transferase (GST) fusion proteins were purified from Escherichia coli (Bedford et al., 2001) and extensively dialyzed against PBS and centrifuged at 100,000 × g to remove any precipitated protein. Fusion proteins were then bound to glutathione immobilized on agarose and exposed to ∼100 ng of gephyrin synthesized by in vitro translation in the absence of radiolabel. After extensive washing in 250 mm NaCl/PBS bound material was eluted with 1% SDS at 95°C for 3 min (Bedford et al., 2001) subjected to SDS-PAGE and immunoblotted with anti-gephyrin antibodies coupled to HRP-conjugated secondary antibodies.
Confocal microscopy.
Cells grown on glass coverslips were fixed with 4% paraformaldehyde and 4% sucrose in PBS. Unspecific binding was blocked by incubation with 5% BSA in PBS (Kittler et al., 2000b, 2004). Primary and secondary antibodies were diluted in 2% BSA/PBS. Secondary antibodies included FITC-, Texas Red-, and Cy5-coupled anti-rabbit, mouse and guinea pig IgG (Jackson ImmunoResearch, West Grove, PA). Where appropriate, cells were permeabilized with 0.05% Triton X-100 (TX-100) for 5 min. In some experiments, cell surface proteins were selectively labeled by incubation with primary and secondary antibodies after fixation without permeabilization (Connolly et al., 1996; Kittler et al., 2000a,b). Intracellular epitopes were then measured (e.g., VIAAT or type II Na+ channels) using the respective primary and secondary antibodies after membrane permeablization.
Images were taken on a Bio-Rad (Hercules, CA) Radiance 2100MP confocal microscope. To quantify receptor clustering on the AIS images were taken with a 60× objective on the confocal microscope and zoomed in with an additional threefold magnification. Recognizable clusters >0.5 but less that 2 μm2 after correction for background were counted and their number per 30 μm of AIS or dendrite was then calculated. A receptor cluster was defined as being ∼0.5–2 μm in length, and approximately twofold to threefold more intense than background diffuse fluorescence. Synaptic clusters were colocalized with or directly apposed to presynaptic marker staining. Clusters further than 1 μm from presynaptic marker staining were considered extrasynaptic. Statistical significance was assessed using the students-t test. All data analysis was performed blinded.
Gephyrin RNAi.
To block gephyrin expression in neurons we used a plasmid vector that expresses active shRNAi against gephyrin together with green fluorescent protein as detailed by (Jacob et al., 2005). Embryonic day 18 (E18) hippocampal neurons were nucleofected with this plasmid (Kittler et al., 2004) and cultured for an additional 14–24 d in vitro (DIV), a procedure that leads to almost total ablation of gephyrin expression in transfected neurons (Jacob et al., 2005).
Hippocampal cell culture.
Neuronal cultures were prepared essentially as detailed previously (Kittler et al., 2000b, 2004). Briefly, hippocampi were isolated from E18 rat brains and dissociated after trypsinization. Cells were plated on poly-l-lysine-coated glass coverslips in serum containing attachment medium, which was replaced after 6 h by Neurobasal medium containing B27. Cultures were grown at a density of 300,000 cells per 6 cm dish without additional glial feeder layer and were used after 18–21 DIV. Human embryonic kidney (HEK)-293 cells were transfected using electroporation with equimolar ratios of expression constructs and receptor expression was then measured 48 h after transfection (Connolly et al., 1996a).
Neuronal transfection.
Dissociated E18 hippocampal neurons were transfected by nucleofection according to the protocol of the manufacturer (Amaxa, Cologne, Germany) with 3 μg of single subunit plasmid DNA, plated and maintained in culture for 18–21 d (Couve et al., 2004; Kittler et al., 2004; Jacob et al., 2005).
Recombinant receptors and chimeras.
The α1 (GenBank accession number, P62812) and α2 (accession number, P26048) GABAA receptor cDNAs in PRK5 have been described previously (Bedford et al., 2001; Kittler et al., 2005). The p1 isoform of gephyrin in PRK5 (Kirsch et al., 1995) was a gift from Dr. R. Harvey. Subunit chimeras were constructed by PCR (Kittler et al., 2005) and verified by full-length sequencing. For the cluster of differentiation molecule 4 (CD4) chimeras the intracellular loop and transmembrane domain 4 (TM4) of α1, α2, chimeras 10 and 11, β3 (accession number, 2108275C) and γ2 (accession number, P22723) were cloned into CD4, substituting for the intracellular tail of the native protein including transmembrane region 4. The tailless CD4 was a generous gift from Dr. Volker Hauke (Freie University, Berlin, Germany) and was modified at its N terminus with the HA epitope. HA neurofascin was a gift from Dr. V. Bennett (Duke University, Durham, NC). HEK-293 cells were transfected using electroporation as detailed previously and used for experimentation 48 h later (Connolly et al., 1996b).
Protein overlay assays.
GST fusion proteins of the intracellular loop of GABAA receptor subunits were expressed in E. coli BL21 as detailed previously (Bedford et al., 2001; Kittler et al., 2005). SDS-soluble lysates were then transferred to nitrocellulose and either subject to immunoblotting with GST antibodies, or slowly renatured with a gradient of 7M-0M of guanidine hydrochloride in buffer [(in mm) 10 HEPES, pH 7.5, 70 KCl, 80 NaCl, 5 EDTA, and 1 mercaptoethanol]. Membranes were then blocked in 5% BSA in buffer with 0.03% Triton X-100 and 1% BSA in buffer without detergent, each for 1 h. The p1 isoform of gephyrin was labeled to in excess of 106 cpm/μg of protein with 35S-methionine using the TNT T7quick coupled transcription/translation system kit (Promega, Madison, WI), diluted with 1% BSA in buffer and incubated with the membrane overnight at 4°C. Thereafter the blot was washed with 5% BSA in buffer, then buffer only, air dried and exposed to a phosphoimager screen.
Results
The synaptic clustering of GABAA receptors containing α2 subunits
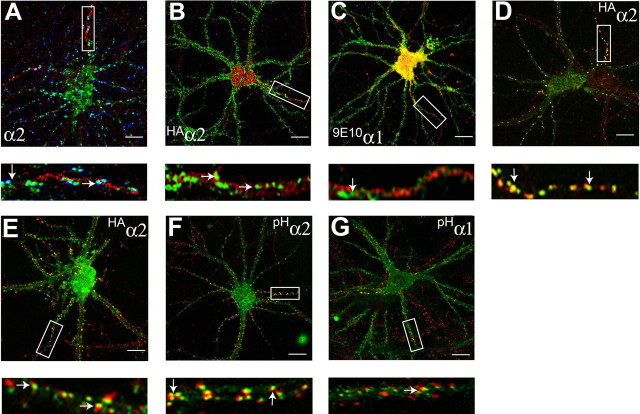
In the rodent brain, immunofluorescence and electron microscopy have revealed that hippocampal pyramidal neurons can cluster GABAA receptor subtypes containing α2 at synaptic sites on the AIS and cell bodies of these neurons, whereas, in contrast, α1 subunit-containing receptors exhibit a more diffuse distribution (Nusser et al., 1996; Nyiri et al., 2001). To assess whether this phenomenon also operates for GABAA receptors in cultured hippocampal neurons, 18–21 DIV hippocampal neurons were fixed and stained with antibodies against an extracellular epitope on the α2 subunit, then subsequently permeabilized and labeled with antibodies against the type II Na+ channel (an accepted marker for the AIS) (Pan et al., 2006), and the VIAAT, a specific marker for presynaptic inhibitory terminals. α2 subunit clusters of >0.5 but <2.0 μm on the first 30 μm of the AIS were judged to be synaptic if they overlapped, or were directly opposed to VIAAT-positive presynaptic terminals. Based on these criteria 78.6 ± 10.2% of α2 clusters on the AIS were deemed to be synaptic (Fig. 1A). Likewise, the majority of α2 receptor clusters on other neuronal processes (72.9 ± 10.6%) were also found to be synaptic (Fig. 1A). Our results thus demonstrate that cultured hippocampal neurons maintain the ability to cluster GABAA receptor subtypes containing α2 subunits at inhibitory synapses on the AIS (Nusser et al., 1996).
Figure 1.
Analyzing GABAA receptor accumulation on the AIS in hippocampal neurons. A, Endogenous GABAA receptors containing α2 subunits cluster on the AIS. Neurons 18–21 DIV were stained under nonpermeabilized) conditions (without TX-100) with antibodies against an extracellular epitope in the α2 subunit (green) and after permeabilization with 0.05% TX-100 and then with antibodies against VIAAT (blue) and type II Na channels (red). Images were acquired by confocal microscopy. The arrows represent synaptic clusters containing α2 subunits on the AIS. B, C, Differential clustering of recombinant GABAA receptor α2 subunits on the AIS and dendritic processes. 18–21 DIV neurons expressing HAα2 (B) or 9E10α1 (C) subunits were stained with HA or 9E10 antibodies under nonpermeabilized conditions (without TX-100; green), permeabilized with 0.05% TX-100, and then stained with antibodies against type II Na channel (red). The arrows represent clusters of α2 or α1 subunits on the AIS. D, E, Neurons expressing HAα2 were also stained with HA antibody (without TX-100; green) and antibodies against an extracellular epitope on the endogenous α2 subunit (without TX-100; red; D), or permeabilized with 0.05% TX-100 and stained with anti-VIAAT antibodies (red; E). The arrows represent puncta containing HAα2/endogenous α2 subunits and HA/VIAAT in D and E, respectively. F, G, To confirm our results with immunohistochemistry, neurons expressing pHα2 (green) (F) or pHα1 (green) (G) were stained with antibodies against VIAAT (red). In A–G, the bottom panels represent enlargements of the boxed areas in the top panels. Scale bars: A–C, E, 7 μm; D, 10 μm; F, G, 5 μm.
To explore the mechanisms underlying the clustering of GABAA receptors we compared the distribution of individual recombinant α subunit isoforms modified with N-terminal reporter epitopes on the cell surface of hippocampal neurons (Connolly et al., 1996a; Taylor et al., 2000; Kittler et al., 2002; Christie et al., 2006). Critically, the addition of these reporters does not modify the assembly or functional properties of GABAA receptors (Kittler et al., 2000a; Wulff et al., 2007). To reduce variability and to control for possible differences in subcellular clustering mechanisms we limited our initial measurements to the AIS. Thus, 18–21 DIV neurons expressing α2 subunits modified with N-terminal HA epitopes (HAα2) were first stained with HA antibodies, then permeabilized and stained with antibodies against type II Na+ channels. Robust HA staining was evident in nonpermeabilized neurons, suggesting efficient targeting of GABAA receptors containing HAα2 subunits to the plasma membrane (Fig. 1B). Similar experiments were performed on neurons expressing α1 subunits modified with N-terminal myc reporters (9E10α1). 9E10α1 subunits were able to access the plasma membrane as measured by staining with 9E10 antibody in nonpermeabilized neurons, but, in contrast to HAα2, 9E10α1 subunits had a more diffuse staining pattern (Fig. 1C). Quantitative analysis revealed that large 9E10α1 clusters were present on the AIS at a density of 3.8 ± 0.5/30 μm, a value significantly lower than that for HAα2 (p < 0.01; n = 10).
We used immunohistochemistry to compare the distribution of endogenous α2 and HAα2 subunits on the neuronal cell surface using an antibody against the first 12 amino acids in the α2 subunit (Fritschy and Mohler, 1995). This antibody does not recognize HAα2 because of the insertion of the HA epitope between amino acids 4 and 5 in this protein (data not shown). Significantly 92 ± 5.5% of endogenous GABAA receptor clusters containing α2 subunits also contained HA immunoreactivity (Fig. 1D). In addition, 78.6 ± 7.4% of HA α2 clusters were synaptic as measured by colocalization with VIAAT (Fig. 1E). Together these observations suggest that the trafficking itineraries and synaptic targeting of GABAA receptors containing endogenous or recombinant α2 subunits are very similar in cultured hippocampal neurons. Unfortunately, because of low expression levels of endogenous α1 subunits that have been previously documented in cultured hippocampal pyramidal neurons we were unable to perform similar analysis for recombinant α1 subunits.
To verify our observations using immunohistochemistry, we expressed pHluorin-tagged (Miesenbock et al., 1998) GABAA receptor α1 and α2 subunits in hippocampal neurons (pHα2 and pH α1, respectively). Previous studies have revealed that addition of this reporter to the N terminus of receptor subunits does not compromise their ability to assemble with β and γ2 subunits to form benzodiazepine-sensitive heteromeric GABAA receptors (Kittler et al., 2000a; Jacob et al., 2005; Bogdanov et al., 2006; Wulff et al., 2007). As measured by live imaging, both pHα2 and pH α1 could access the plasma membrane but pHα2 appeared to exhibit more extensive clustering (data not shown). To verify synaptic clustering, expressing neurons were fixed, permeabilized and stained with anti-VIAAT antibodies and the number of VIAAT-positive clusters were calculated for both constructs (Fig. 1F,G). Receptors incorporating pHα2 subunits showed significantly higher levels of synaptic clustering compared with those incorporating pHα1 (35.7 ± 3.4 vs 10.9 ± 4.2/30 μm, respectively).
Together these experiments demonstrate that GABAA receptors containing α2 subunits are enriched at VIAAT-positive synapses on both the AIS and dendrites of cultured neurons compared with those incorporating α1 subunits.
Identifying the amino acid residues that regulate the clustering of GABAA receptors containing α2 subunits
To establish the molecular basis underlying the preferential clustering of GABAA receptors containing α2 subunits we created subunit chimeric (CH) α2/α1 subunits. Our initial constructs centered on exchanging the major intracellular domains (ICDs) and TM4, as subunit ICDs are generally accepted as playing a critical role in regulating GABAA receptor membrane trafficking (Luscher and Keller, 2004). To control for variability arising from potential differences in subcellular specific clustering mechanisms we initially compared chimera clustering only on AIS.
In nonpermeabilized 19–21 DIV neurons, CH1 and CH2 were able to access the cell surface (Fig. 2A,B), but their clustering on the AIS differed. CH1, which contains the ICD/TM4 of α2, accumulated on this structure at levels similar to those seen with HAα2 subunits (Figs. 2A, 3) (13.2 ± 0.9 clusters/30 μm; n = 15) whereas CH2, which contains the ICD/TM4 of α1, exhibited significantly lower levels of clustering (Figs. 2B, 3) (4.5 ± 0.6/30 μm; p < 0.01; n = 10). These results are consistent with a role for the ICD/TM4 domain of the α2 subunit in regulating GABAA receptor clustering at least on the AIS, an issue that was analyzed using CH3, CH4 and CH5. Although these chimeras were all able to access the plasma membrane, as illustrated by robust fluorescence in nonpermeabilized neurons, their accumulation on the AIS differed. CH3 (Fig. 2C) and CH5 showed levels of clustering very similar to those observed for HAα2 (Fig. 3) (12.6 ± 0.7 and 13.2 ± 0.6/30 μm, respectively), but, in contrast, clusters of CH4 on the AIS were not detected (Fig. 2D). These experiments suggest that the amino acids 307–347 play a critical role in regulating the clustering of GABAA receptors containing α2 subunits on the AIS.
Figure 2.
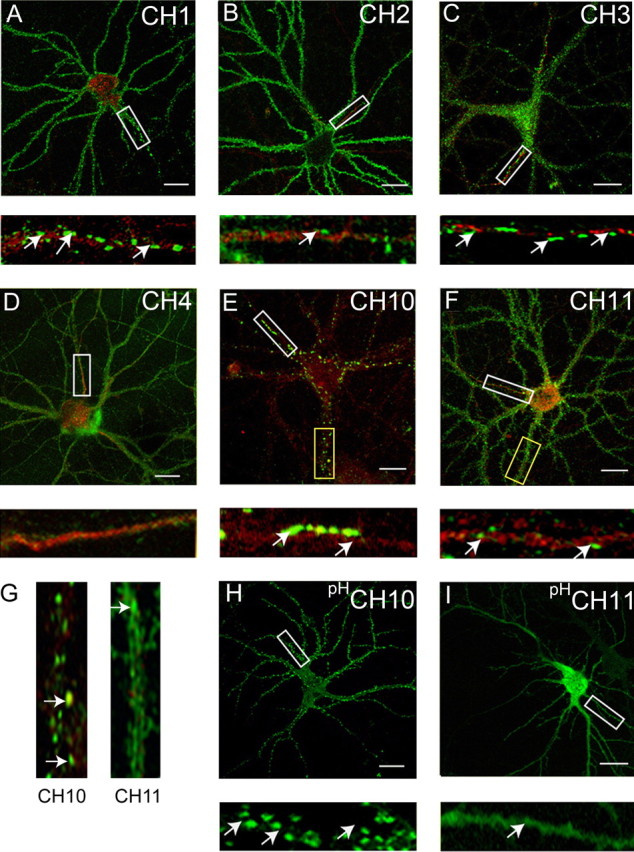
Analyzing the clustering of GABAA receptor α1/α2 subunit chimeras in hippocampal neurons. A–F, Differential clustering of GABAA receptor chimeras. 18–21 DIV neurons expressing CH1 (A), CH2 (B), CH3 (C), CH4 (D), CH10 (E), or CH11 (F) were stained with HA (CH2, CH11) or 9E10 (CH1, CH3, CH4, and CH10) antibodies under nonpermeabilized conditions (without TX-100; green) and after permeabilization with 0.05% TX-100 with antibodies against the type II Na+ channel (red). Scale bars: 10 μm. Bottom panels represent enlargements of the white boxes in the top panels and the AIS. G, Enlargements of the respective dendrites in yellow boxed areas in E and F are shown. H, I, Live imaging of hippocampal neurons expressing pHluorin-tagged GABAA receptor α1/2 subunit chimeras. 18–21 DIV neurons expressing pHCH10 (H) and pHCH11 (I) were subject to live confocal imaging at 37°C. Bottom panels represent enlargements of the boxed areas in the top panels. Scale bars: 7 μm.
Figure 3.
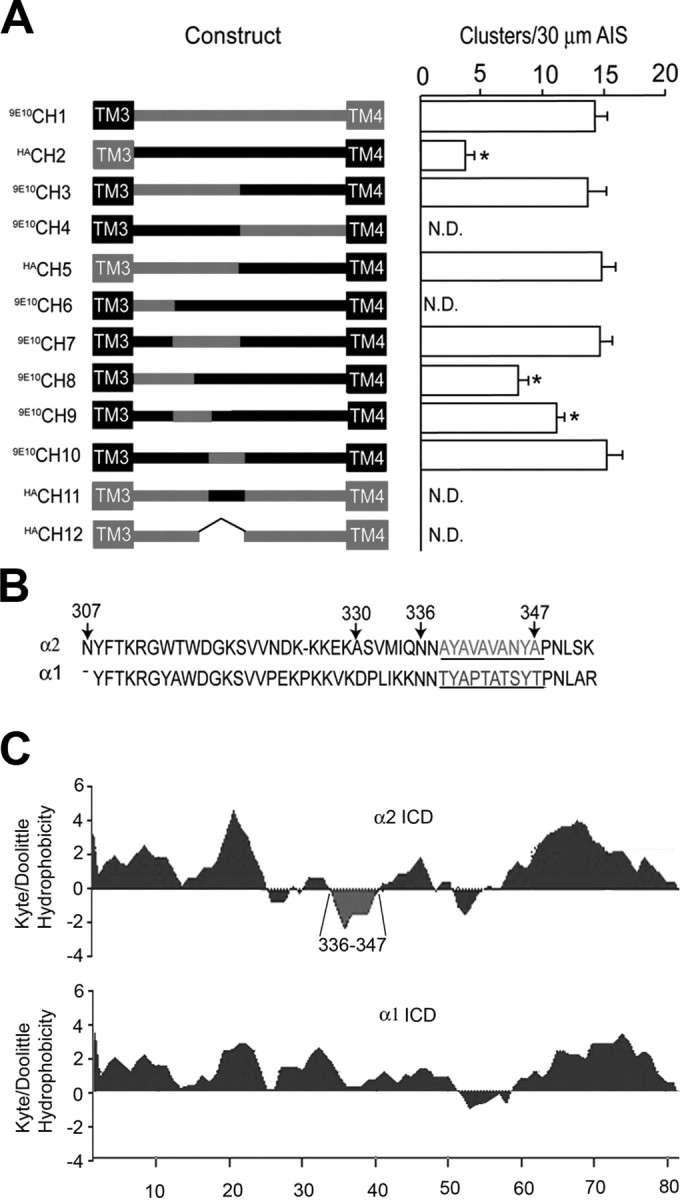
Analyzing the clustering of α1/2 subunit chimeras on the AIS. A, The structures of CH1-CH12 are indicated in the line diagram. The positions of transmembrane domains are indicated by boxes with sequences derived from the α1 and α2 subunits indicated in black and red, respectively. Chimeras are encoded as follows: CH1 residues 1–306 of the α1 subunit and residues 307–423 of α2; CH2 residues 1–306 of the α2 subunit and residues 306–428 of α1; CH3 residues 307–347 of the α2 subunit substituted for the equivalent amino acids in the α1 subunit; CH4 residues 1–347 of α1 and 348–423 of α2; CH5 residues 1–347 of α2 and 348–428 of α1; CH6 residues 307–329 of the α2 subunit exchanged for the corresponding domain in the α1; CH7 residues 330–347 of the α2 subunit exchanged for the corresponding region in α1; CH8 residues 307–335 of the α2 subunit exchanged for the corresponding domain of α1; CH9 residues 330–335 of the α2 subunit exchanged for the corresponding domain of α1; CH10 residues 336–347 of the α2 subunit exchanged for the corresponding domain of the α1 subunit; CH11 residues 336–348 of the α1 subunit exchanged for the corresponding domain of α2 subunit; and CH12 residues 336–347 deleted from the α2 subunit. Chimeras were modified with either the 9E10 or HA epitopes as indicated. To measure the clustering of these constructs on the AIS, images were recorded from transfected neurons and the AIS was identified by Na+ channel fluorescence and its characteristic morphology. After background subtraction, the number of receptor clusters >0.5 μm2 were counted per 30 μm AIS. Data were then compared with the number of clusters seen with wild-type α2 subunits (control). An asterisk indicates significantly different from control (p < 0.01; n = 11–25 in at least 5 independent transfections). B, An alignment of residues 307–352 of the GABA receptor α1 and α2 subunits. The domain of α2 critical for receptor clustering is shown in red and the corresponding domain of α1 is indicated in blue. The positions of residues 307, 330, 336, and 347 in the α2 subunit are also shown. C, The Kyte and Doolittle hydrophobicity of the major intracellular domains of the α2 (residues 306–390) and α1 (307–391) subunits were determined as indicated. Residues 336–347 of the α2 subunit are highlighted in red.
To further delineate the amino acids between residues 307–347 of the α2 subunit that regulate clustering, we used CH6 and CH7 which contain residues 307–329 and 330–347, respectively, from the α2 subunit were exchanged for the corresponding regions in the α1 subunit. Whereas CH7 exhibited levels of clustering on the AIS (Fig. 3) (13.2 ± 1.2; 30 μm) similar to those seen for HAα2 subunits, clusters of CH6 were not detected on this structure (Fig. 3) (n = 75 neurons). The role of residues 330–347 was further probed using CH8, CH9, and CH10, in which amino acids 307–335, 330–335, and 336–347 of the α2 subunit were exchanged for the corresponding residues in α1. Whereas CH8 (Fig. 3) (8.4 ± 0.9) and CH9 (Fig. 3) (10.2 ± 0.8; 30 μm) were able to form clusters on the AIS, their levels were significantly lower than those for HAα2 subunits (p < 0.01). In contrast CH10 was able to form clusters on the AIS (Fig. 2E) at levels similar to those of wild-type α2 subunits (Fig. 3) (13.1 ± 0.6; 30 μm; n = 18), strongly suggesting that residues 336–347 are critical in regulating the clustering of GABAA receptors containing α2 subunits on the AIS. To further confirm this, two additional constructs were produced: CH11 in which amino acids 336–347 in the α2 subunit were replaced with the corresponding domain of the α1 subunit and CH12 in which they were deleted. In agreement with the results with CH10, no large clusters containing either CH11 (Fig. 2F) or CH12 (Fig. 3) were evident on the AIS (n = 85 neurons).
In addition to analyzing clustering on the AIS, the role that residues 336–347 play in regulating GABAA receptor clustering on dendrites was examined. This was achieved by counting the number of clusters between 0.5 and 2.0 μm on Na+ type II negative processes. For CH10, 25.5 ± 5.6 clusters were found per/30 μm (n = 4), whereas the clustering of CH11 was significantly (p < 0.01) reduced to 5.4 ± 3.3/30 μm (n = 4) (Fig. 2G).
To confirm our observations using immunohistochemistry we modified CH10 and CH11 with N-terminal pHluorin reporters. At 18–21 DIV, expressing neurons were subjected to live confocal imaging at 37°C and, consistent with our observations with fixed neurons, both pHCH10 and pHCH11 were able to access the cell surface as measured by robust fluorescence (Fig. 2H,I). However whereas CH10 (Fig. 2H) formed large clusters on the plasma membrane on neuronal processes, CH11 exhibited a largely diffuse distribution (Fig. 2I).
To control for possible alterations in the assembly of the chimeric α subunits, their capacity to access the plasma membrane on coexpression with the β3 subunit was assessed in HEK-293 cells. First, we compared the ability of CH10 and CH11 to access the plasma membrane expressed alone or with the β3 subunit. Using immunofluorescence under permeabilized and nonpermeabilized conditions it was evident that both CH10 and CH11 were retained within a compartment resembling the endoplasmic reticulum (ER) when expressed alone, but were able to access the plasma membrane after coexpression with the β3 subunit (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). In addition to this, CH1–9 and CH12 were also able to translocate to the cell surface on coexpression with β3 (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), but were retained within the ER on homomeric expression (data not shown). These results suggest that CH1–12 are capable of assembling with β3 subunits and robustly access the cell surface consistent with wild-type α1 and α2 subunits (Kittler et al., 2002).
The results of our immunohistochemical and live imaging experiments suggest a critical role for residues 336–347 (Fig. 3B) within the intracellular domain of the α2 subunit in regulating the accumulation and/or clustering of GABAA receptors on the AIS, and dendrites of neurons. It is interesting to note that these amino acids are highly hydrophobic in nature compared with the corresponding domain of the α1 subunit and are predicted to form an α-helical structure (Fig. 3C).
Amino acids 336–347 in the α2 subunit are sufficient to target reporter molecules to inhibitory synapses
To further examine the differential clustering of GABAA receptor α1 and α2 subunits we examined the ability of the ICDs of these proteins to modify the subcellular targeting of CD4 modified at its extracellular N terminus with an HA epitope. To do so, the cytoplasmic tail of CD4 was removed and replaced by the ICD/TM4 and putative extracellular tail of individual GABAA receptor subunits and chimeras (Fig. 4A). As a control, we first examined the subcellular distribution of wild type CD4 molecules. In 18–21 DIV hippocampal neurons this protein exhibited a diffuse staining pattern on the plasma membrane with immunoreactivity being evident on the cell body and neuronal processes as measured by immunofluorescence with HA antibodies (Fig. 4B), with no accumulation at inhibitory synapses being evident (data not shown). In contrast CD4α2 formed clusters on the surfaces of both neuronal processes and the cell body (Fig. 4C). When amino acids 336–347 of the α2 subunit were replaced with the corresponding amino acids of α1 in CD4CH11 (Fig. 4D), the distribution was mostly diffuse. CD4α2 showed significantly higher levels of clustering on the membrane compared with CD4CH11 (19.4 ± 4.5 vs 5.4 ± 3.2/30 μm, respectively). We also examined the subcellular distribution of CD4 constructs modified with the ICD/TM4 of the GABAA receptor β3 or γ2 subunits, and these proteins exhibited largely diffuse distributions on the plasma membrane (Fig. 4E).
Figure 4.
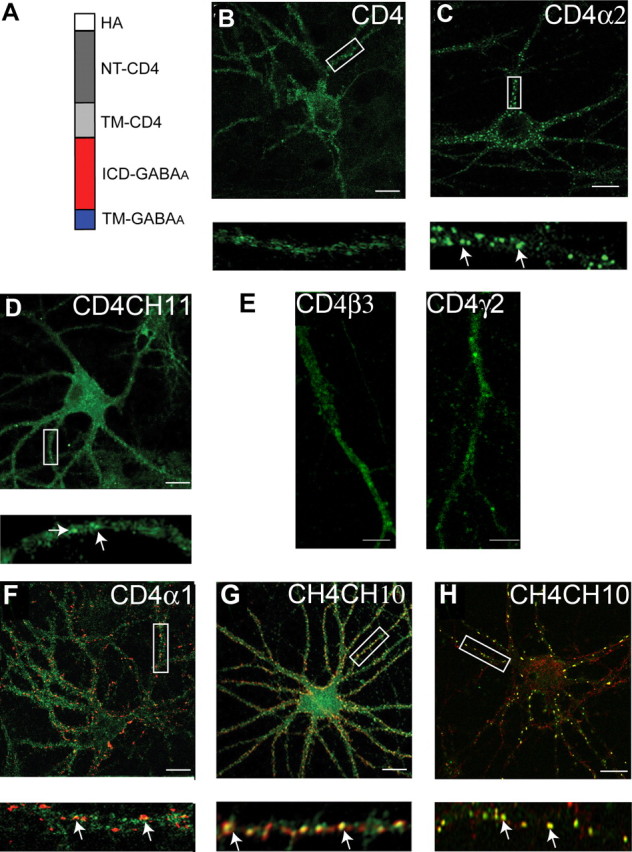
Clustering of CD4/GABAA receptor chimeras in neurons. A, The structure of GABAA receptor/CD4 chimeras. White box, Extracellular HA epitope; dark gray box, the N terminus of CD4; light gray box, transmembrane domain of the CD4; red box, major intracellular domain of GABAA receptor subunit; blue box, transmembrane 4 of the GABAA receptor subunit. B–F, Images recorded from neurons expressing CD4 (B) CD4α2 (C) and CD4CH11 (D) constructs stained with HA antibody under nonpermeabilized conditions. The arrows represent clusters of CD4 immunoreactivity. E, Processes from neurons expressing CD4β3 and CD4γ2 stained with HA antibody. F, G, Images of neurons expressing CD4α1 (F) and CD4CH10 (G) stained with 9E10 antibody (without membrane permeabilization; green) and after treatment with 0.05% TX-100 with antibodies against VIAAT (red). The arrows represent puncta that contain both VIAAT and CD4 staining. H, Neurons expressing CD4CH10 were stained with HA antibody (without membrane permeabilization; green) and an antibody against endogenous α2 subunits (without membrane permeabilization; red). The arrows represent puncta that contain both HA and endogenous α2 immunoreactivity. In B–H, the bottom panels represent enlargements of the boxed areas in the top panels and the arrows indicate GABAA receptor clusters. Scale bars: B–D, F–H, 10 μm; E, 5 μm.
To address the role of residues 336–347 in the targeting of CD4 to synaptic sites, we compared CD4α1 and CD4CH10 (containing amino acids 336–347 from α2) (Fig. 4F,G). CD4CH10 produced large clusters on the plasma membrane. It was evident that 80 ± 8.5% CD4CH10 clusters colocalized with VIAAT compared with 12.5 ± 5.4% of CD4α1. We further compared the distribution of CD4CH10 molecules to endogenous α2 subunits and in excess of 85% of CD4CH10 clusters also contained endogenous α2 subunits (Fig. 4H). However, the total number of clusters of endogenous GABAA receptors containing α2 subunits was not significantly different in neurons expressing CD4CH10 and controls (data not shown). Together these experiments revealed that amino acids 336–347 are sufficient to target CD4 to inhibitory synapses that contain endogenous α2 subunits.
The accumulation of GABAA receptor chimeras and CD4 constructs at synaptic sites is dependent on gephyrin expression
Data derived from gephyrin knock-out mice and knockdown experiments using antisense oligonucleotides or shRNAi have illustrated that reducing gephyrin expression leads to a highly significant reduction in the clustering of GABAA receptor subtypes containing α2 subunits at postsynaptic inhibitory specializations (Essrich et al., 1998; Kneussel et al., 1999a; Levi et al., 2004; Jacob et al., 2005). Therefore, given the differential pattern of CD4CH10 and CD4CH11, we compared their subcellular localization with that for gephyrin using immunohistochemistry. CD4CH10 exhibited a clustered distribution on the cell body and processes of expressing neurons and some of these puncta contained gephyrin immunoreactivity (Fig. 5A). In contrast, CD4CH11 exhibited diffuse staining in neuronal processes and cell bodies with little overlap with gephyrin staining (Fig. 5B). Significantly (p < 0.01; n = 6) higher colocalization of CD4CH10 clusters with gephyrin was evident compared with those containing CD4CH11 (59.4 ± 5.4 vs 6.6 ± 3.2%, respectively).
Figure 5.
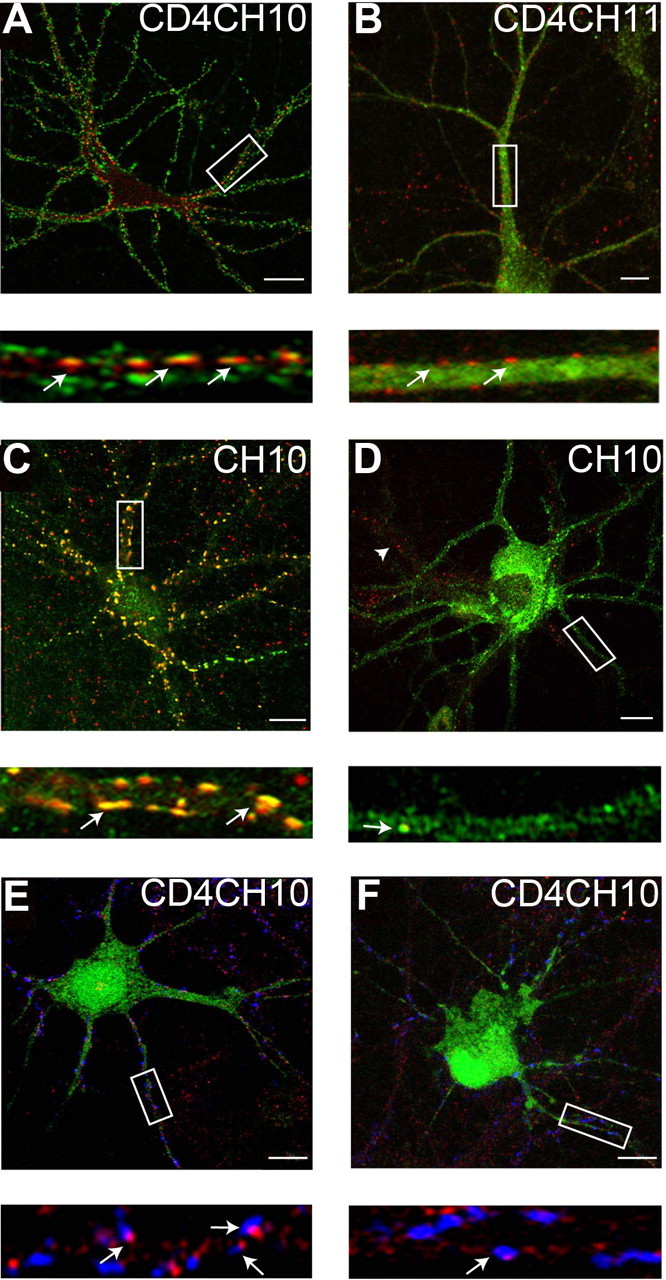
Gephyrin mediates the clustering of GABAA receptor chimeras and CD4 expression constructs at synaptic sites. A, B, Selective colocalization of CD4-GABAA receptor clusters with gephyrin. 18–21 DIV hippocampal neurons expressing CD4CH10 (A) or CD4CH11 (B) were stained with HA antibodies (without membrane permeabilization green) against the reporters in these proteins and after membrane permeabilization with antibodies against gephyrin (red) and images then collected by confocal microscopy; arrows indicate puncta that contain both HA and gephyrin immunoreactivity. C, D, Gephyrin expression is critical for the clustering of CH10 in hippocampal neurons. At 18–21 DIV, hippocampal neurons expressing CH10 and a fourfold higher level of a control plasmid (C) or pGEPH1 (D) were stained with 9E10 antibodies (without membrane permeabilization green) and after permeabilization with gephyrin (red) antibodies and subject to confocal microscopy. Colocalizing puncta are indicated by arrows, and the arrowhead represents an untransfected neuron. F, G, The accumulation of CD4CH10 at synaptic sites is dependent on gephyrin expression. At 18–21 DIV, hippocampal neurons expressing control (E) or pGEPH1 (F) were stained with antibodies against the HA epitope (without membrane permeabilization; red) and after membrane permeabilization with antibodies against VIAAT (blue), and endogenous GFP fluorescence is in green. GFP fluorescence has been subtracted from the bottom panels in E and F for clarity. The number of CD4 puncta that were apposed to VIAAT staining on neuronal dendrites were then calculated under control conditions and with pGEPH1 with arrows indicating puncta containing both VIAAT and CD4 immunoreactivity. In A–F, the bottom panels show enlargements of the boxed areas in the top panels arrows. Scale bars: A, C, D, 10 μm; B, E, F, 7 μm.
We further examined the role of gephyrin in regulating synaptic clustering of constructs containing residues 336–347 of the α2 subunit using plasmid pGEPH1 which expresses active shRNAi against gephyrin (Jacob et al., 2005). Previous studies have illustrated that, in neurons transfected with this plasmid, gephyrin expression is largely abolished as is the clustering of endogenous GABAA receptors containing α2 subunits or recombinant β3 or γ2 subunits (Jacob et al., 2005). To initiate these experiments we examined the role of gephyrin in regulating the clustering of CH10 (Figs. 2, 3) by expressing the respective plasmid with a fivefold molar excess of pGEPH1 or a control plasmid in hippocampal neurons. In 18–21 DIV cultures large clusters of CH10 were evident on neuronal processes and cell bodies and in excess of 80% of these contained gephyrin immunoreactivity (Fig. 5C). In contrast the level of gephyrin immunoreactivity was greatly reduced in neurons expressing pGEPH1 and the levels of CH10 clustering also appeared to be lower in these cells (Fig. 5D). Decreasing gephyrin expression significantly reduced the number of CH10 clusters from 14.2 ± 3.2/30 μm in control to 2.5 ± 2.1/30 μm with pGEPH1 (p < 0.01, n = 6).
We also tested the role of gephyrin in regulating the accumulation of CD4CH10 at synaptic sites. For these experiments CD4CH10 was coexpressed in neurons with a plasmid that expresses GFP and active RNAi against gephyrin (pGEPH1/GFP) or a control sequence (Jacob et al., 2005). At 18–21 DIV neurons were stained with HA and VIAAT antibodies and visualized using confocal microscopy. In neurons expressing GFP and control RNAi, puncta of CD4CH10 immunoreactivity that colocalized with VIAAT immunoreactivity were evident on both neuronal processes and cell bodies (Fig. 5E). In contrast in neurons expressing GFP and an active RNAi against gephyrin, the level of gephyrin immunoreactivity was largely abolished which disrupted CD4CH10 clustering (Fig. 5F). Quantifying these results, it was evident that blocking gephyrin expression significantly reduced (p < 0.01) the number of CD4CH10/VIAAT-positive clusters from 8.2 ± 2.3/30 μm dendrite under control conditions to 2.1 ± 0.8/30 μm in the absence of gephyrin (p < 0.01; n = 6).
As a control for specificity we examined the effects of gephyrin shRNAi on the clustering of AMPA receptors incorporating GluR1 subunits. The number of GluR1 clusters colocalizing with the presynaptic marker synapsin was very similar in neurons expressing GFP (12.4 ± 2.4/50 μm) and those expressing GFP and gephyrin shRNAi (10.5 ± 3.2/50 μm) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). These results are consistent with data accrued from gephyrin knock-out mice and knockdown experiments, which have shown that AMPA receptor clustering is not modified on ablating gephyrin expression (Kneussel et al., 1999a; Levi et al., 2004; Jacob et al., 2005).
Together, these results suggest a critical role for gephyrin in regulating the accumulation of GABAA receptors at inhibitory synapses dependent on amino acids 336–347 within the intracellular domain of the α2 subunit.
The GABAA receptor α2 subunit can selectively bind to gephyrin
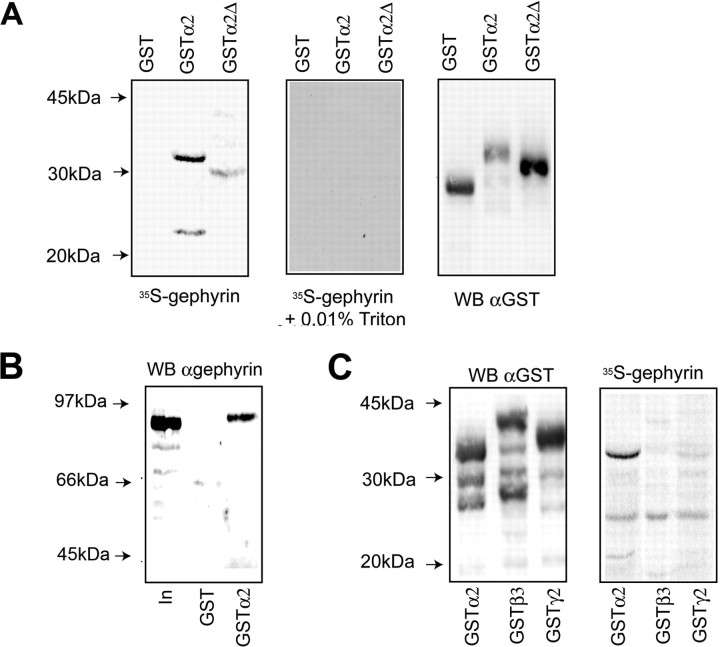
Our experiments strongly suggest a critical role for residues 336–347 in regulating the clustering of GABAA receptors at synaptic sites in a process dependent on gephyrin expression. We thus examined whether these residues play any role in mediating the binding of GABAA receptors to gephyrin using overlay assays. To do so we expressed the intracellular loop of the GABAA receptor α2 subunit as GST fusion protein (GSTα2) in E. coli (Bedford et al., 2001). SDS-soluble extracts were transferred to a nitrocellulose membrane and re-natured by exposure to 7 m guanidine HCl, followed by progressive dilution. Membranes were blocked with BSA and overlaid with the p1 isoform of gephyrin labeled with 35S-methionine using in vitro transcription/translation. Under these conditions robust binding of the p1 isoform of gephyrin (Kirsch et al., 1995) to a major band of 35 kDa corresponding to GSTα2 and several degradation products were evident, however no binding was observed to lysates expressing GST alone (Fig. 6). Interestingly, interaction was totally inhibited by low levels of detergent (0.01% Triton X-100), suggesting that hydrophobic interactions mediate the binding of gephyrin and GABAA receptors (Fig. 6A). To assess the role of residues 336–347 in mediating gephyrin binding we expressed a GSTα2 fusion protein in which residues 330–347 were deleted (GSTα2Δ). Unfortunately, deletion of residues 336–347 alone produced a highly unstable fusion protein. After controlling for the level of input by immunoblotting with anti-GST antibody it was evident that deletion of residues decreased gephyrin binding to 5% of that seen with GST-α2 (Fig. 6A) (p < 0.01).
Figure 6.
Residues of 336–347 mediate the direct binding of gephyrin to the α2 subunit. A, B, Direct binding of gephyrin to the intracellular domain of the α2 subunit. SDS-soluble extracts were prepared from E. coli expressing GST-α2, GST-α2Δ, (residues 330–347) or GST, were overlaid with 35S-methionine-labeled gephyrin in the absence and presence of 0.01% Triton X-100 or immunoblotted with anti-GST antibodies (A). The level of gephyrin binding was corrected for input levels and the level of gephyrin binding to GST-α2Δ was then compared with that seen GST-α2 (control, 100%). Deletion of these residues reduced gephyrin binding to 5.4% of control. The ability of gephyrin to bind to GSTα2 or GST when immobilized on glutathione agarose was measured. Ten micrograms of the respective fusion proteins in the absence of detergent were exposed to unlabeled gephyrin synthesized by in vitro translation and bound material was immunoblotted with anti-gephyrin antibodies. In is 10% of the input used in each assay (B). In, Twenty percent of the starting material. C, Analyzing the binding of gephyrin to the intracellular domains of the receptor β3 and γ2 subunits. SDS-soluble extracts from E. coli expressing GST-α2, β3, and γ2 subunits were overlaid with 35S-methionine gephyrin or immunoblotted with anti-GST antibodies as indicated.
To verify our results using gel overlay assays we examined the ability of gephyrin to bind to GSTα2 when immobilized on glutathione. For these experiments, purified GST proteins were dialyzed extensively against PBS to remove any traces of detergent and then bound to GST agarose and exposed to in vitro translated gephyrin. Bound material was then immunoblotted with anti-gephyrin antibodies (Fig. 6B). A band ∼90 kDa representing gephyrin could be detected binding to immobilized GSTα2, but not to GST alone, supporting our experiments using gel overlay assays.
Finally, we compared the ability of gephyrin to bind to the intracellular domains of the receptor a2, γ2, and β3 subunits. The β3 and γ2 subunits were chosen because they have been suggested previously to play roles in the gephyrin dependent clustering of GABAA receptors (Kirsch et al., 1995; Luscher and Keller, 2004; Alldred et al., 2005). Abundant expression of all three fusion proteins was evident in E. coli, but, whereas significant binding of gephyrin to GSTa2 was evident, the levels bound to GSTβ3 or GSTγ2 were greatly reduced after correction for background binding to GST (Fig. 6C).
Together, our results provide evidence that GABAA receptors can bind directly to gephyrin and suggest a predominant role for the α2 subunit in mediating this interaction.
Discussion
Differential clustering of GABAA receptors containing α1 and α2 subunits
Neurons have the capacity to assemble a large number of structurally distinct GABAA receptor subtypes with distinct physiological and pharmacological properties (Kittler and Moss, 2003; Luscher and Keller, 2004; Rudolph and Mohler, 2006). In addition, individual GABAA receptor subtypes within the same neuron can be selectively targeted to extrasynaptic sites, axo-axonic and axo-dendritic synapses. However, how neurons orchestrate these events remains to be elucidated (Kittler and Moss, 2003; Luscher and Keller, 2004).
To begin to address the cellular mechanisms responsible for the synaptic clustering of GABAA receptors we examined how receptor subtypes containing α2 subunits are enriched at inhibitory synapses on the AIS. Using triple immunofluorescence we first established that this phenomenon is also evident for endogenous GABAA receptors containing α2 subunits in cultured hippocampal neurons. We then evaluated the ability of cultured hippocampal neurons to cluster GABAA receptors containing recombinant α1 and α2 subunits on the AIS. Although recombinant α1 subunits were able to access the plasma membrane, the number of clusters they formed on the AIS was greatly reduced compared with recombinant α2 subunits.
This key observation provided us with an assay to determine the amino acids responsible for this enhanced clustering of GABAA receptors containing α2 subunits. To do so, we produced chimeric α1/2 subunits and measured their ability to form clusters on the AIS. We limited our initial studies to the AIS to avoid complications arising from possible subcellular specific clustering mechanisms for individual receptor subtypes. Under these experimental constraints it was evident that the enhanced ability of GABAA receptors containing α2 subunits to form clusters on the AIS was dependent on amino acid residues within the major intracellular domain of this subunit. The use of more refined chimeras exchanging smaller domains of the α1 and α2 subunits revealed that amino acids 336–347 of the α2 subunit were critical in mediating the enhanced synaptic clustering of GABAA receptors containing α2 subunits compared with those containing α1 subunits on both the AIS and dendrites. Critically, substitution of these amino acids also disrupted the clustering of GABAA receptors at synapses on the cell bodies and dendrites of pyramidal neurons. Therefore these results suggest a critical role for residues 336–347 of the α2 subunit in regulating the clustering of GABAA receptors at both axoaxonic and axodendritic synapses.
To further evaluate the significance of amino acids 336–347 in the clustering of GABAA receptors we examined their ability to regulate the subcellular distribution of the structurally unrelated membrane protein CD4. This revealed that the intracellular domain of the α2 subunit but not the corresponding regions of the β3 or γ2 subunits was able to modulate the clustering of CD4 on the plasma membrane. It was further evident that amino acids 336–347 within the ICD of the α2 subunit were sufficient to target and cluster CD4 at inhibitory synapses.
The ability of amino acids 336–347 of the α2 subunit to cluster proteins at inhibitory synapses is dependent on gephyrin
Gephyrin is a multifunctional protein that promotes the clustering of GlyR receptors at synaptic sites via direct binding to the β subunits of these receptors and is also responsible for the synthesis of molybdenum cofactors (Kneussel and Betz, 2000). In addition, gephyrin has been implicated in GABAA receptor clustering, but the underlying mechanism remains obscure (Sassoe-Pognetto and Fritschy, 2000). We thus sought to determine the possible role gephyrin plays in regulating the clustering of GABAA receptors or CD4 constructs containing residues 336–347 of the α2 subunit. Using immunohistochemistry, GABAA receptor subunits or CD4 chimeras containing residues 336–347 of the α2 subunit showed striking colocalization with gephyrin. In contrast, constructs in which these residues were absent showed minimal overlap with gephyrin. Our results are thus consistent with studies demonstrating that GABAA receptors containing the α2 subunit show high levels of colocalization with gephyrin and that in knock-out mice specific deficits in the clustering of receptor subtypes containing α2 subunits are evident (Sassoe-Pognetto et al., 1995; Essrich et al., 1998; Sassoe-Pognetto and Fritschy, 2000; Jacob et al., 2005). Given the evident colocalization between GABAA receptors containing residues 336–347 of the α2 subunit and gephyrin, we used plasmid-based RNAi (Jacob et al., 2005) to further evaluate the role that gephyrin plays in the clustering of proteins containing residues 336–347. Inhibiting gephyrin expression dramatically reduced the clustering of GABAA receptors or CD4 chimeras containing residues 336–347 of the α2 subunit at postsynaptic specializations. Together, these results demonstrate that residues 336–347 of the α2 subunit are significant in regulating gephyrin-dependent clustering of GABAA receptors.
We also explored the role of this motif in mediating the binding of gephyrin to GABAA receptors. For this facet of our study, we examined the binding of GST fusion proteins encoding the major intracellular domains of GABAA receptor subunits to the p1 isoform of gephyrin using gel overlay assays and affinity purification. This methodology revealed that gephyrin could bind directly to the intracellular domain of α2 and deletion analysis further demonstrated that binding of gephyrin to the α2 subunit intracellular domain was critically dependent on amino acids 330–347 within this protein. However, under the same conditions minimal binding of gephyrin to the intracellular domains of the γ2 and β3 subunits was evident. Previous studies in HEK-293 cells have suggested that the receptor β3 subunit may play a role in regulating the association of GABAA receptors and gephyrin. The γ2 subunit has also been strongly implicated in controlling gephyrin dependent clustering of GABAA receptors, as gene knock-out of this subunit ablates both receptor synaptic clustering and gephyrin accumulation at inhibitory synapses (Luscher and Keller, 2004; Alldred et al., 2005). However, the role of receptor β3 and γ2 subunits in regulating the synaptic clustering of GABAA receptors may be independent of the ability of these proteins to bind gephyrin as demonstrated here. In support of this argument, overexpression of the α6 subunit leads to the relocation of γ2 subunits to extrasynaptic sites suggesting that the γ2 subunit per se is not sufficient to localize GABAA receptors to synaptic sites (Wisden et al., 2002).
We also noted that binding of the GABAA receptor intracellular domains to gephyrin was inhibited by very low concentrations of detergent. This detergent sensitivity provides an explanation for the difficulty in demonstrating the interaction of these proteins in detergent-solubilized membrane extracts. In agreement with this detergent sensitivity, amino acids 336–347 are highly hydrophobic and are conserved in human, rat and mouse α2 subunits. In this context it is interesting to note that similar hydrophobic interactions have been shown to control the binding of GlyR receptor β subunits to gephyrin (Kneussel et al., 1999b). Consistent with our binding experiments, immunohistochemical studies have demonstrated that GABAA receptor subtypes containing α2 subunits are highly enriched at synaptic sites where they exhibit striking colocalization with gephyrin (Sassoe-Pognetto and Fritschy, 2000). In addition, this direct binding of gephyrin to α2 subunits provides a molecular mechanism for the selective loss of GABAA receptor synaptic clusters containing receptor α2 subunits evident in gephyrin deficient mice (Kneussel et al., 2001; Levi et al., 2004). Interestingly, individual GABAA receptors can have mixed α subunits (Sieghart and Sperk, 2002), and binding of gephyrin to α2 may facilitate the accumulation of other α subunit isoforms at inhibitory synapses (Sassoe-Pognetto and Fritschy, 2000). However, it is also evident that gephyrin is often found clustered with GABAA receptors that do not contain α2 subunits and clearly further experiments are required to analyze whether gephyrin can bind to other receptor subunits.
In summary, our studies have identified a specific amino acid motif within the α2 subunit that selectively mediates the accumulation of GABAA receptors at central inhibitory synapses via a mechanism dependent on direct interaction with the inhibitory postsynaptic scaffold protein gephyrin.
Footnotes
This work was supported by a Marie Curie European Union postdoctoral fellowship to V.T. S.J.M. was supported by National Institutes of Health Grants NS 046478, 048045, 051195, and 056359, the Medical Research Council (U.K.), and the Wellcome Trust. We thank our former colleague Dr. Cecilia Gold for her helpful comments and unfailing support of this work. We thank Margie Maronski from the Dichter laboratory for preparation of cultured hippocampal neurons, John Raper (University of Pennsylvania) for the GFP-gephyrin construct, Yolande Haydon for manuscript preparation, Vann Bennett (Duke University) for the HA neurofascin, Margot Ernst (Medical University Vienna) for help with secondary structure predictions, and Karoline Fuchs (Medical University Vienna) for advice on molecular biology.
References
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996a;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Wooltorton JR, Smart TG, Moss SJ. Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proc Natl Acad Sci USA. 1996b;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Restituito S, Brandon JM, Charles KJ, Bawagan H, Freeman KB, Pangalos MN, Calver AR, Moss SJ. Marlin-1, a novel RNA-binding protein associates with GABA receptors. J Biol Chem. 2004;279:13934–13943. doi: 10.1074/jbc.M311737200. [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Kuhse J, Betz H. Targeting of glycine receptor subunits to gephyrin-rich domains in transfected human embryonic kidney cells. Mol Cell Neurosci. 1995;6:450–461. doi: 10.1006/mcne.1995.1033. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Wang J, Connolly CN, Vicini S, Smart TG, Moss SJ. Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using gamma 2 subunit green fluorescent protein chimeras. Mol Cell Neurosci. 2000a;16:440–452. doi: 10.1006/mcne.2000.0882. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000b;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol (Lond) 2000;525:1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci. 1999a;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Hermann A, Kirsch J, Betz H. Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin. J Neurochem. 1999b;72:1323–1326. doi: 10.1046/j.1471-4159.1999.0721323.x. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABAA receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Fritschy JM. Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci. 2000;12:2205–2210. doi: 10.1046/j.1460-9568.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Taylor PM, Connolly CN, Kittler JT, Gorrie GH, Hosie A, Smart TG, Moss SJ. Identification of residues within GABAA receptor alpha subunits that mediate specific assembly with receptor beta subunits. J Neurosci. 2000;20:1297–1306. doi: 10.1523/JNEUROSCI.20-04-01297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V, Lujan R, Jones A, Korpi ER, Mody I, Sieghart W, Somogyi P. Ectopic expression of the GABA(A) receptor alpha6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppä E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, Wisden W. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]