Abstract
The δ2 glutamate receptor (GluRδ2) is predominantly expressed in Purkinje cells and plays crucial roles in cerebellar functions: GluRδ2−/− mice display ataxia and impaired motor learning. In addition, long-term depression (LTD) at parallel fiber (PF)–Purkinje cell synapses is abrogated, and synapse formation with PFs and climbing fibers (CFs) is severely disturbed in GluRδ2−/− Purkinje cells. Recently, we demonstrated that abrogated LTD was restored in GluRδ2−/− Purkinje cells by the virus-mediated expression of the wild-type GluRδ2 transgene (Tgwt) but not by that of mutant GluRδ2 lacking the C-terminal seven residues to which several PDZ proteins bind (TgΔCT7). These results indicated that the C terminus of GluRδ2 conveys the signal(s) necessary for LTD. In contrast, other phenotypes of GluRδ2−/− cerebellum, especially morphological abnormalities at PF and CF synapses, could not be rescued by virus-mediated transient expression. Thus, whether these phenotypes are mediated by the same signaling pathway remains unclear. To address these issues and to further delineate the function of GluRδ2 in vivo, we generated transgenic mice that expressed TgΔCT7 on a GluRδ2−/− background. Interestingly, although TgΔCT7 restored abnormal PF and CF synapse formation almost completely, it could not rescue abrogated LTD in GluRδ2−/− Purkinje cells. Furthermore, although the gross motor discoordination of GluRδ2−/− mice was restored, the cerebellar motor learning underlying delayed eyeblink conditioning remained impaired. These results indicate that LTD induction and motor learning are regulated by signaling via the C-terminal end of GluRδ2, whereas other functions may be differentially regulated by other regions of GluRδ2.
Keywords: cerebellum, LTD, glutamate receptor, PDZ domains, Purkinje cell, eyeblink
Introduction
The δ2 glutamate receptor (GluRδ2), a member of the ionotropic glutamate receptor (iGluR) family (Araki et al., 1993; Lomeli et al., 1993), is predominantly expressed on the postsynaptic sites of parallel fiber (PF)–Purkinje cell synapses (Landsend et al., 1997; Zhao et al., 1997) and plays indispensable roles in cerebellar functions: GluRδ2-null mice display ataxia and impaired motor-related learning tasks such as eyeblink conditioning (Yuzaki, 2004). Detailed analyses of GluRδ2-null mice have revealed two crucial roles of GluRδ2 at PF synapses: the formation or stabilization of synaptic contacts and the control of long-term depression (LTD), an elemental process underlying cerebellar motor learning. Furthermore, although wild-type Purkinje cells become innervated by single climbing fibers (CFs) by the end of the third postnatal week, adult GluRδ2-null Purkinje cells remain innervated by supernumerary CFs. Despite these essential roles played by GluRδ2 in the cerebellum, the underlying mechanisms are not well understood, mainly because of the lack of specific agonists for GluRδ2.
To obtain clues to the functioning of GluRδ2 without relying on pharmacological tools, we recently exploited the “transgenic rescue” approach. We generated lines of mice that expressed mutant GluRδ2 transgenes in a GluRδ2-null background. Because a mutant GluRδ2 transgene, in which the glutamate-binding motif conserved in iGluRs was mutated, rescued all abnormal phenotypes of GluRδ2-null mice, we concluded that GluRδ2 was unlikely to be activated by glutamate analogs in vivo (Hirai et al., 2005). Similarly, because a mutant GluRδ2 transgene, in which the ion selectively filter was mutated, rescued the phenotypes of GluRδ2-null mice, GluRδ2 was considered unlikely to function as a Ca2+-permeable channel (Kakegawa et al., 2007). We therefore hypothesize that although GluRδ2 belongs to the iGluR family, it may function as a nonionotropic receptor. Indeed, using a virus-mediated “rescue” approach, we recently demonstrated that abrogated LTD was restored in GluRδ2-null Purkinje cells by the transduction of the wild-type GluRδ2 transgene (Tgwt) but not by the transduction of a mutant GluRδ2 lacking the C-terminal seven residues (TgΔCT7) (Kohda et al., 2007); this region binds to several PDZ [postsynaptic density-95 (PSD-95)/Discs large/zona occludens-1) proteins such as PSD-93, synaptic scaffolding molecule (S-SCAM), protein tyrosine phosphatase PTPMEG, and delphilin (Yuzaki, 2004). These results indicate that PDZ proteins that bind to the C terminus of GluRδ2 may convey intracellular signals necessary for the induction of LTD. Nevertheless, whether the other abnormal phenotypes of GluRδ2-null Purkinje cells, especially the morphological abnormalities at PF and CF synapses, are also mediated by the same signaling pathway remains unclear, because these phenotypes could not be rescued by the virus-mediated transient expression of the wild-type GluRδ2 transgene (Kohda et al., 2007). To address these issues and to further delineate the function of GluRδ2 in vivo, we generated transgenic mice that expressed TgΔCT7 on a GluRδ2-null background. We demonstrated, for the first time, that signaling via the C-terminal end of GluRδ2 was dispensable for morphological integrity at PF and CF synapses, whereas signaling was absolutely required for the induction of LTD and motor learning, indicating that GluRδ2 may function via at least two distinct mechanisms.
Materials and Methods
All the experimental procedures involving the use of animals were approved by the Animal Resource Committee of the Keio University School of Medicine.
Animals.
Mutant mice carrying the GluRδ2 transgene were generated as described previously (Hirai et al., 2005). Briefly, a mutant GluRδ2 transgene lacking the last seven amino acids at the C terminus (TgΔCT7) was inserted into the BamHI site of pL7ΔAUG. The resulting plasmids were digested with KpnI and EcoRI, and the linearized L7-GluRδ2 constructs were injected into fertilized eggs (Genome Information Research Center, Osaka University, Osaka, Japan). Twelve TgΔCT7 founders were then bred onto a GluRδ2−/− background (termed GluRδ2−/−/TgΔCT7). Homozygous transgenic lines were established and confirmed by backcrossing with wild-type mice.
Antibodies.
To detect the GluRδ2 protein, we used two kinds of rabbit polyclonal anti-GluRδ2 antibodies with distinct epitopes (see Fig. 1A): an anti-GluRδ2(837–888) antibody (Takayama et al., 1995) that recognizes the 52 amino acids of the C-terminal juxtamembrane segment (837–888 amino acids); and an anti-GluRδ2(973–992) antibody (Chemicon, Temecula, CA) that recognizes the 20 amino acids of the most C-terminal region (973–992 amino acids).
Figure 1.
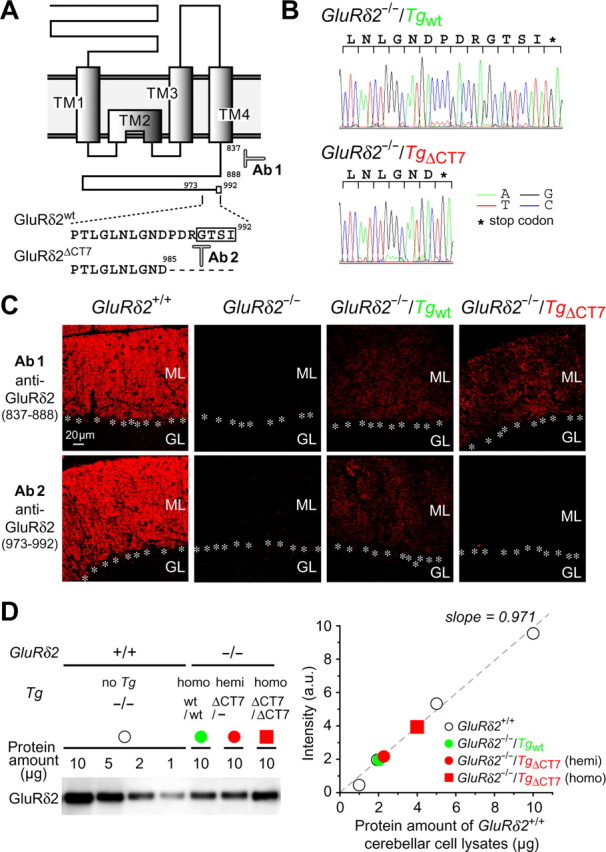
Characterization of GluRδ2−/−/TgΔCT7 mice. A, Putative membrane topology of GluRδ2 and amino acid sequences of the extreme C-terminal regions. The open box surrounds the GluRδ2 PDZ-binding motif. The seven amino acids (PDRGTSI) at the end of the C terminus are deleted in GluRδ2ΔCT7. The 52 amino acids of the C-terminal juxtamembrane segment (837–888 amino acids) and the 20 amino acids at the end of the C-terminal region (973–992 amino acids) are epitopes against anti-GluRδ2(837–888; Ab1)-specific and anti-GluRδ2(973–992; Ab2)-specific antibodies, respectively. B, Representative sequence traces of genomic PCR products from GluRδ2−/−/Tgwt and GluRδ2−/−/TgΔCT7 mice. C, Immunofluorescence images showing GluRδ2 expression using anti-GluRδ2(837–888) (top) and anti-GluRδ2(973–992) (bottom) antibodies in GluRδ2+/+, GluRδ2−/−, GluRδ2−/−/Tgwt, and GluRδ2−/−/TgΔCT7 mice. GluRδ2 staining by anti-GluRδ2(973–992) antibodies was not detectable in GluRδ2−/−/TgΔCT7 cerebella. ML, Molecular layer; GL, granular layer; asterisks, Purkinje cell soma. D, Quantitative immunoblot analysis of cerebellar lysates from each mouse. The band intensities of the GluRδ2 protein (using anti-GluRδ2(837–888) antibodies) in 10 μg of GluRδ2−/−/Tgwt (green circle) and GluRδ2−/−/TgΔCT7 [homozygotes (homo), red square; hemizygotes (hemi), red circle] cerebellar lysates were compared with those in various amounts (10, 5, 2, and 1 μg) of GluRδ2+/+ (open circles) cerebellar cell lysates (slope = 0.971 using the linear least-squares fitting method). The x-axis shows the protein amount of GluRδ2+/+ cerebellar cell lysates.
Immunohistochemistry.
Under deep anesthesia with an intraperitoneal injection of pentobarbital, adult mice (over 4 weeks of age) were fixed by cardiac perfusion with 4% paraformaldehyde in 0.1 m sodium phosphate buffer (PBS, pH 7.4), and the cerebellum was removed and postfixed by 4% paraformaldehyde in PBS overnight. After rinsing the specimens with PBS, parasagittal slices (100 μm) were prepared using a microslicer (DTK-2000; D.S.K., Kyoto, Japan) and were permeabilized using 0.4% Triton X-100 (Sigma, St. Louis, MO) in PBS containing 0.2% normal goat serum and 0.2% bovine serum albumin for 6 h at 4°C. Immunohistochemical staining was performed using anti-GluRδ2 antibodies (1:500) at 4°C followed by incubation with Alexa546-conjugated secondary antibodies (diluted 1:1000; Invitrogen, Eugene, OR). The stained slices were viewed using a confocal laser-scanning microscope (Fluoview; Olympus Optical, Tokyo, Japan).
Immunoblotting.
To examine the total expression level of GluRδ2, whole cerebella were homogenized in lysis buffer (50 mm NaF, 1% NP-40, 20 mm EDTA, 0.1% SDS, 50 mm Tris-HCl, pH 8.0) containing a protease inhibitor mixture (Calbiochem, La Jolla, CA). To prepare the postsynaptic density (PSD) fraction, whole cerebella were homogenized in buffer containing 0.32 m sucrose and 5 mm HEPES, pH 7.5, and the nuclear fraction was removed by centrifugation at 800 × g for 5 min. The supernatant was centrifuged at 12,000 × g for 15 min at 4°C, and the pellet was resuspended in 1% Triton X-100/PBS so that the protein concentration became 1 mg/ml. After incubation for 1 h at 4°C, the insoluble “PSD fraction” was precipitated by centrifugation at 100,000 × g for 30 min at 4°C. After washing with 1% Triton X-100/PBS for 30 min, the PSD fraction was solubilized in the SDS-PAGE sample buffer. The total homogenates and the PSD fractions were analyzed by immunoblotting with anti-GluRδ2(837–888) antibodies. The specificity of the anti-GluRδ2 antibodies was established using Western blots, and the bound immunoglobulin was detected using an enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ). The fluorescence was quantified using an image analyzer (LAS-3000; Fujifilm, Tokyo, Japan).
Electron microscopy.
Conventional electron microscopy (EM) analysis and postembedding immunogold EM analysis were performed as described previously (Fukaya et al., 2003; Hirai et al., 2005). The number of gold particles, synapses, and lengths of the PSD and active zone were measured on scanned electron micrographs using MetaMorph software (Molecular Devices, Sunnyvale, CA).
Behavioral analysis.
Walking footprint pattern analysis and the rotor-rod test were performed as described previously (Hirai et al., 2005; Kakegawa et al., 2007). The eyeblink conditioning test was also performed as described previously (Kishimoto et al., 2001). Briefly, male mice (over 8–10 weeks of age) were surgically implanted with four Teflon-coated stainless steel wires (A-M Systems, Carlsborg, WA) under the left eyelid to record electromyographic activity and to deliver an electric shock. A periorbital shock (100 Hz square pulses for 100 ms) was applied as an unconditioned stimulus (US), and a tone (1.0 kHz, 85 dB for 350 ms) was used as a conditioning stimulus (CS). In the delay paradigm, the US overlapped the CS in time such that the two stimuli terminated simultaneously (see diagram in Fig. 7C). To calculate the conditioned response (CR), the electromyogram amplitude was analyzed 200 ms before the US onset in the CS–US paired trials. The spontaneous eyeblink frequency, which was recorded for 2 d before the acquisition phase, was measured in 100 nonstimulus trials.
Figure 7.
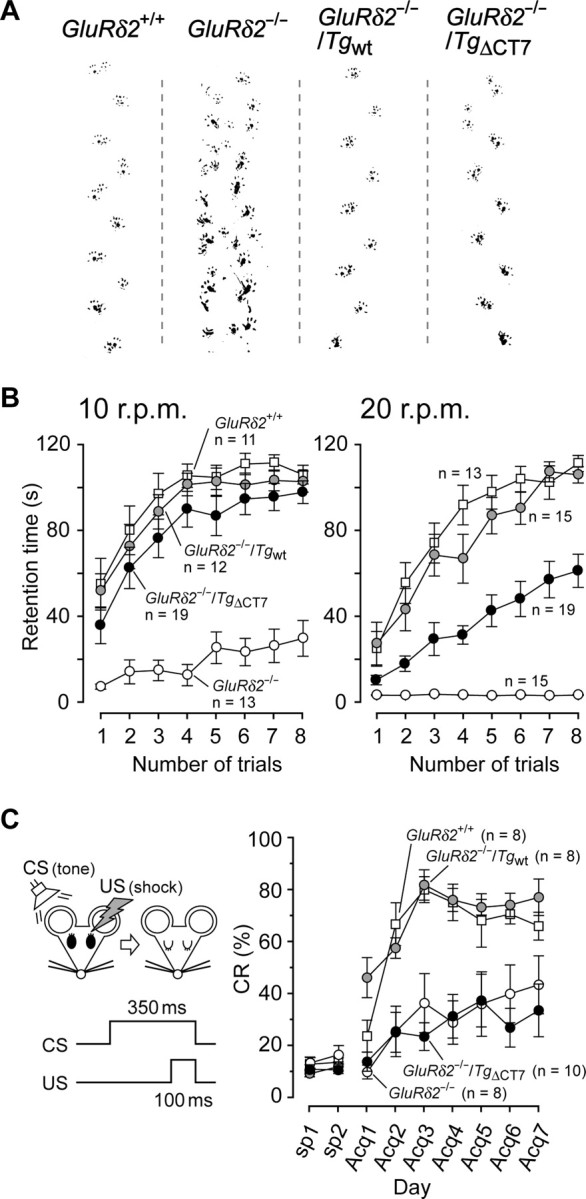
GluRδ2−/−/TgΔCT7 mice do not exhibit an ataxic gait but do exhibit impaired motor performance and cerebellar motor learning. A, Representative walking footprint patterns in each mouse. B, Results of rotor-rod test at speeds of 10 r.p.m. (left) and 20 r.p.m. (right) in GluRδ2+/+ (open squares), GluRδ2−/− (open circles), GluRδ2−/−/Tgwt (gray circles), and homozygous GluRδ2−/−/TgΔCT7 (filled circles) mice. The mice were allowed a maximum retention time of 120 s per trial. C, Results of a delay eyeblink conditioning test. The left diagram shows the temporal relationship between CS (tone, 350 ms) and US (electric shock, 100 ms). In the acquisition (Acq) phase from days 1 to 7 (Acq1 to Acq7), the daily sessions consisted of 10 blocks of trials in which each block consisted of nine CS–US paired trials and one CS-only trial. The y-axis shows the percentage of CR against CS; the CR values at days before training (sp1 and sp2) indicate the spontaneous eyeblink responses.
Electrophysiology.
Parasagittal cerebellar slices (200 μm) were prepared from GluRδ2−/− and each transgenic mouse (postnatal day 24–38), as described previously (Hirai et al., 2005; Kakegawa et al., 2007). Whole-cell recordings were made from visually identified Purkinje cells using a 60× water-immersion objective attached to an upright microscope (BX51WI; Olympus Optical) at room temperature. The resistance of patch pipettes was 3–5 MΩ when filled with an intracellular solution of the following composition (in mm): 65 Cs-methanesulfonate, 65 K-gluconate, 20 HEPES, 10 KCl, 1 MgCl2, 4 Na2ATP, 1 Na2GTP, 5 sucrose, and 0.4 EGTA, pH 7.25 (295 mOsm/kg). The solution used for slice storage and recording consisted of the following (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 d-glucose, bubbled continuously with a mixture of 95% O2 and 5% CO2. Picrotoxin (100 μm; Sigma) was always added to the saline to block inhibitory synaptic transmission. The EPSCs were recorded using an Axopatch 200B amplifier (Molecular Devices), and the pClamp system (version 9.2; Molecular Devices) was used for data acquisition and analysis. The signals were filtered at 1 kHz and digitized at 4 kHz.
To evoke EPSCs derived from CF (CF–EPSCs) and PF (PF–EPSCs) inputs onto Purkinje cells, square pulses were applied through a stimulating electrode placed on the granular layer and the molecular layer (∼50 μm away from the pial surface), respectively. The selective stimulation of CFs and PFs was confirmed by the paired-pulse depression and paired-pulse facilitation (PPF) of EPSC amplitudes at a 50 ms interstimulus interval (ISI), respectively. To search for multiple CFs innervating the recorded Purkinje cell, the stimulating electrode was moved systematically in the granular layer, and the stimulus intensity was gradually increased at each stimulation site (pulse width, 50 μs; strength, 0–200 μA). For the LTD sessions, PF–EPSCs were recorded successively at a frequency of 0.1 Hz from Purkinje cells clamped at −80 mV. After a stable PF–EPSC amplitude had been observed for at least 10 min, a conjunctive stimulation that consisted of 30 single PF stimuli together with a 200 ms depolarizing pulse from a holding potential of −60 to +20 mV was applied for LTD induction. Access resistances were monitored every 10 s by measuring the peak currents in response to 2 mV, 50 ms hyperpolarizing steps throughout the experiments; the measurements were discarded if the resistance changed by >20% of its original value. The normalized EPSC amplitude on the ordinate represents the EPSC amplitude for the average of six traces for 1 min divided by that of the average of six traces for 1 min immediately before the conjunctive stimulation.
Data analysis and statistics.
The results are presented as the means ± SEM, and the statistical significance was defined as p < 0.05, by using the unpaired Student's t test, the one-way ANOVA followed by Bonferroni's multiple comparison test, and multiple t test with Bonferroni's correction.
Results
Generation of GluRδ2−/−/TgΔCT7 rescue mice
Previously, we used the Purkinje cell-specific L7 promoter (Hirai et al., 2005) to drive the expression of a wild-type GluRδ2 transgene (Tgwt) and obtained a transgenic rescue line called GluRδ2−/−/Tgwt by breeding transgenic mice onto a GluRδ2−/− background (Hirai et al., 2005; Kakegawa et al., 2007). Similarly, to examine the function regulated by the C terminus of GluRδ2, we generated a GluRδ2−/−/TgΔCT7 line that expressed a mutant GluRδ2 transgene lacking the C-terminal seven amino acids (TgΔCT7); this region is known to bind to PSD-93, S-SCAM, PTPMEG, and delphilin (Yuzaki, 2004). Both GluRδ2−/−/Tgwt and GluRδ2−/−/TgΔCT7 lines were bred to homozygosity with regard to each transgene. We confirmed that TgΔCT7 was properly inserted into the genome by sequencing the genomic DNA (Fig. 1B). In addition, we confirmed the expression of the transgenic protein products using an immunohistochemical analysis with anti-GluRδ2(837–888) antibody, which recognizes the C-terminal juxtamembrane segment (Fig. 1A), a region common to both Tgwt and TgΔCT7 proteins (Fig. 1C). Both Tgwt and TgΔCT7 were selectively expressed in the cerebellum (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). In contrast, the anti-GluRδ2(973–992) antibody, which recognizes the C-terminal end of GluRδ2 (Fig. 1A), failed to reveal immunoreactivity in the GluRδ2−/−/TgΔCT7 cerebellum (Fig. 1C). To quantify the expression level of transgenes, band intensities of the GluRδ2 protein in 10 μg of GluRδ2−/−/Tgwt or GluRδ2−/−/TgΔCT7 cerebellar cell lysates were compared with those in various amounts of wild-type cerebellar cell lysates by immunoblot analysis using anti-GluRδ2(837–888) antibody. We found that the expression levels of Tgwt and TgΔCT7 proteins were ∼20 and 40% of those of endogenous GluRδ2 protein in wild-type (GluRδ2+/+) cerebellum, respectively (Fig. 1D). Indeed, hemizygous GluRδ2−/−/TgΔCT7 mice, which contained half the number of TgΔCT7 copies, and homozygous GluRδ2−/−/Tgwt expressed nearly equivalent amount of transgenic GluRδ2 proteins (Fig. 1D).
Postsynaptic expression of TgΔCT7 protein
One of the functions of many PDZ proteins interacting with the C termini of membrane receptors is to anchor them at postsynaptic sites in neurons (Sheng and Sala, 2001). Thus, to examine the role of the C terminus in localizing GluRδ2 at synapses, we performed an immunogold EM analysis using anti-GluRδ2(837–888) antibody. Although hemizygous GluRδ2−/−/TgΔCT7 mice and homozygous GluRδ2−/−/Tgwt mice had similar transgene protein expression levels (Fig. 1D), the number of immunogold particles observed at the PSD of the PF–Purkinje cell synapses were lower in the hemizygous GluRδ2−/−/TgΔCT7 mice (6 ± 3 particles/μm) than in the homozygous GluRδ2−/−/Tgwt mice (15 ± 4 particles/μm; one-way ANOVA followed by Bonferroni's test, F(3,8) = 18.58; p = 0.094) (Fig. 2A,B). Nevertheless, the homozygous GluRδ2−/−/TgΔCT7 mice, which expressed twice as much transgenic protein as the homozygous GluRδ2−/−/Tgwt mice, exhibited a similar number of immunogold particles at the PSD (14 ± 3 particles/μm; p = 1.00), compared with the homozygous GluRδ2−/−/Tgwt (Fig. 2A,B). Indeed, at extrasynaptic cell surfaces and CF–Purkinje cell synapses, immunogold particles were rarely observed in either homozygous GluRδ2−/−/Tgwt (0.5 ± 0.3 particles/μm at extrasynaptic cell surfaces, 0.9 ± 0.2 particles/μm at CF–Purkinje cell synapses) or homozygous GluRδ2−/−/TgΔCT7 mice (0.3 ± 0.2 particles/μm at extrasynaptic cell surfaces; 0.5 ± 0.1 particles/μm at CF–Purkinje cell synapses; both p < 0.001 vs PF–Purkinje cell synapses) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material), indicating that transgene proteins were selectively expressed at PF–Purkinje cell synapses. Furthermore, the immunoblot analysis revealed that the homozygous GluRδ2−/−/Tgwt and homozygous GluRδ2−/−/TgΔCT7 lines expressed comparable levels of GluRδ2 transgene proteins in the PSD fraction of cerebellar lysates (n = 3; t test, p = 0.813) (Fig. 2C). Therefore, GluRδ2 lacking its C terminus could be normally localized at the PSD of PF–Purkinje cell synapses, although the efficiency of this localization was approximately half of that of wild-type GluRδ2, indicating a facilitative but nonessential role of the C terminus in the synaptic localization of GluRδ2.
Figure 2.
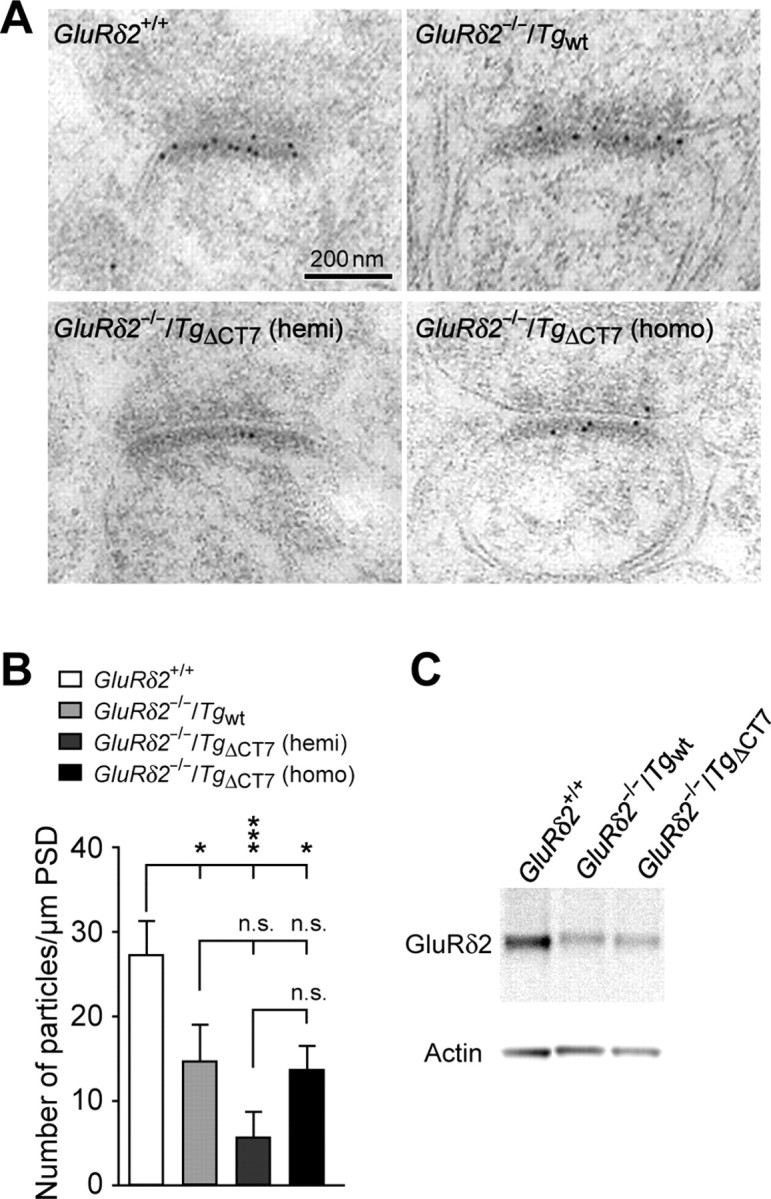
Localization of transgenic GluRδ2 proteins at the PSD of the PF–Purkinje cell synapses. A, Representative images of immunogold electron microscopic analysis using anti-GluRδ2(837–888) antibodies in GluRδ2+/+, homozygous GluRδ2−/−/Tgwt, hemizygous GluRδ2−/−/TgΔCT7 (hemi), and homozygous GluRδ2−/−/TgΔCT7 (homo) cerebella. The number of gold particles is correlated with the amount of GluRδ2 protein. B, Histogram showing the averaged number of gold particles per 1 μm PSD length. ***p < 0.001; *p < 0.05. n.s., No significance. C, Representative immunoblotting data from the isolated PSD fraction of homozygous GluRδ2−/−/Tgwt and homozygous GluRδ2−/−/TgΔCT7 cerebellar lysates using anti-GluRδ2(837–888) antibodies.
To investigate other functions mediated by the C terminus of GluRδ2, we compared the phenotypes of homozygous GluRδ2−/−/Tgwt and homozygous GluRδ2−/−/TgΔCT7 mice in the following analyses, because these lines expressed similar amounts of transgene products at their PF–Purkinje cell synapses.
Impaired cerebellar LTD in GluRδ2−/−/TgΔCT7 mice
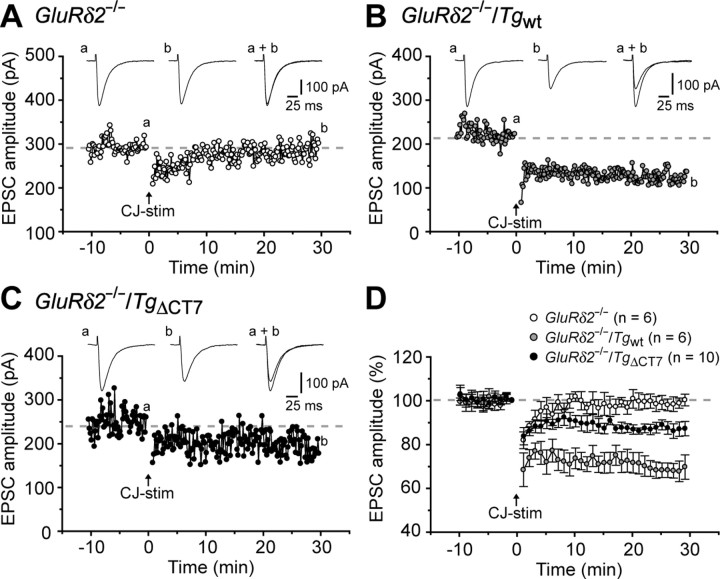
First, we examined whether abrogated LTD in GluRδ2-null Purkinje cells was rescued by TgΔCT7. No difference in PF–EPSC kinetics was observed between GluRδ2−/−/Tgwt (10–90% rise time, 3.3 ± 0.2 ms, n = 20; decay time constant, 14.7 ± 0.7 ms; n = 20) and GluRδ2−/−/TgΔCT7 mice (10–90% rise time, 3.1 ± 0.2 ms, n = 12, t test, p = 0.290; decay time constant, 16 ± 0.9 ms, n = 12; t test, p = 0.250), indicating that the basal properties of AMPA receptors were unaffected by the deletion of the PDZ-binding motif of GluRδ2. As reported previously (Hirai et al., 2005), the conjunctive stimulation of PFs and the depolarization of Purkinje cells induced no LTD in GluRδ2−/−Purkinje cells (Fig. 3A,D) (100 ± 3% at t = 30 min; n = 6 from four mice), whereas it induced robust LTD in GluRδ2−/−/Tgwt Purkinje cells (Fig. 3B,D) (69 ± 6% at t = 30 min, n = 6 from four mice; one-way ANOVA followed by Bonferroni's test, F(2,19) = 18.33, p < 0.001 vs GluRδ2−/− mice). In contrast, LTD was not fully restored in GluRδ2−/−/TgΔCT7 Purkinje cells (Fig. 3C,D) (87 ± 3% at t = 30 min, n = 10 from six mice; p = 0.002 vs GluRδ2−/−/Tgwt mice, and p = 0.062 vs GluRδ2−/− mice). These results indicate that the C terminus of GluRδ2 mediates intracellular signals that are crucial for LTD induction, a finding consistent with our previous study using the virus-mediated expression of TgΔCT7 (Kohda et al., 2007). Interestingly, in both studies, the expression of TgΔCT7 produced a slight but significant reduction in the EPSC amplitude after conjunctive stimulation (Fig. 3D). Thus, there might be a minor component of cerebellar LTD that is induced independently of the PDZ protein-based signaling by GluRδ2.
Figure 3.
LTD at PF–Purkinje cell synapses is severely reduced in GluRδ2−/−/TgΔCT7 mice. A–C, Representative LTD data recorded from Purkinje cells in GluRδ2−/− (A), GluRδ2−/−/Tgwt (B), and homozygous GluRδ2−/−/TgΔCT7 (C) mice. Inset traces show the PF–EPSCs just before (a) or 30 min after (b) the conjunctive stimulation (CJ-stim, arrow) and their superimposition (a + b). D, Summary of the LTD sessions in each mouse. The averaged amplitudes of the PF-EPSC over 1 min were normalized to the baseline value, which was the average of the 1 min responses (six traces) that occurred just before CJ-stim.
Restoration of PF release probability in GluRδ2−/−/TgΔCT7 mice
In the previous study, although the Sindbis virus-mediated transient expression of Tgwt restored LTD induction, it failed to rescue the other phenotypes of GluRδ2−/− mice (Kohda et al., 2007). One of these phenotypes is an impairment in presynaptic function observed at PF–Purkinje cell synapses (Kashiwabuchi et al., 1995); GluRδ2−/− mice exhibit increased PPF of PF–EPSCs, reflecting a lower presynaptic release probability (Zucker and Regehr, 2002). Indeed, the ratio of PPF at interpulse intervals <350 ms was significantly elevated in GluRδ2−/− Purkinje cells (Fig. 4B,E) (2.38 ± 0.07 at 50 ms ISI; n = 15) than that in GluRδ2+/+ Purkinje cells (Fig. 4A,E) (1.64 ± 0.04 at 50 ms ISI; n = 20; one-way ANOVA followed by Bonferroni's test, F(3,75) = 29.38, p < 0.001). In contrast, Purkinje cells in GluRδ2−/−/Tgwt mice (Fig. 4C) as well as in GluRδ2−/−/TgΔCT7 mice (Fig. 4D) displayed PPF ratios that were significantly smaller (Fig. 4E) (1.73 ± 0.04 at 50 ms ISI, n = 20 and 1.80 ± 0.07 at 50 ms ISI; n = 24, respectively) than that of GluRδ2−/− mice (p < 0.001 for both transgenic mice) and comparable with that of GluRδ2+/+ mice (Fig. 4E) (p = 1.000 vs GluRδ2−/−/Tgwt mice, and p = 0.235 vs GluRδ2−/−/TgΔCT7 mice). Therefore, the reduced release probability of PFs in GluRδ2−/− mice was completely restored by the expression of TgΔCT7 as well as by the expression of Tgwt. Because GluRδ2 is expressed exclusively at postsynaptic sites, these results indicate that GluRδ2 is involved in a retrograde signaling pathway that modifies the presynaptic release probability; however, its C terminus is not required for this function. In addition, successful recovery of an impaired presynaptic function in GluRδ2−/− mice by using the transgenic mice-based approach, but not by the virus-mediated approach, indicates that these phenotypes may require a longer time period of expression, which cannot be achieved by the Sindbis virus because of its cytotoxicity (Kohda et al., 2007).
Figure 4.
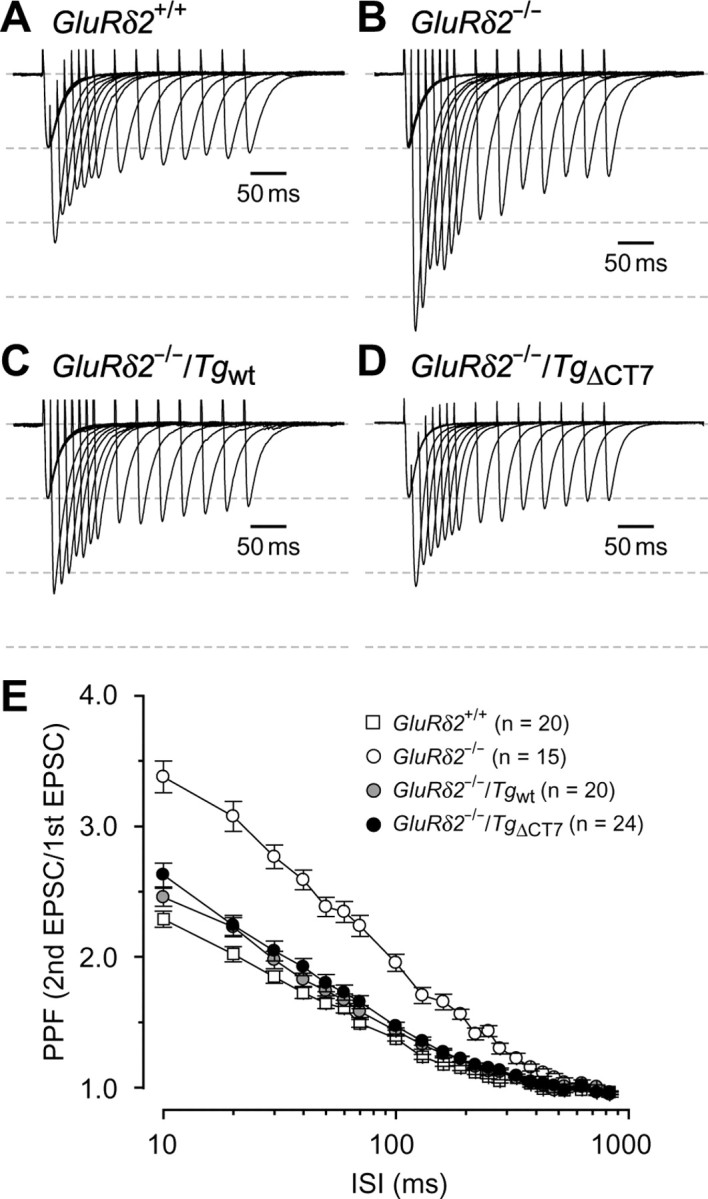
Rescue from the enhanced PPF of PF–EPSCs in GluRδ2−/− mice by TgΔCT7 expression. A–D, Representative PF–EPSCs recorded from Purkinje cells clamped at −70 mV in GluRδ2+/+ (A), GluRδ2−/− (B), GluRδ2−/−/Tgwt (C), and homozygous GluRδ2−/−/TgΔCT7 (D) mice. All traces were normalized with the first PF–EPSC amplitude. The widths between the dashed lines correspond to the amplitude of the first PF–EPSC. E, Summarized plots of the PPF values of the PF–EPSCs, which were defined as the amplitude of the second EPSC divided by that of the first EPSC, in various ISI (10–830 ms).
Restoration of PF–Purkinje cell synaptic integrity in GluRδ2−/−/TgΔCT7 mice
GluRδ2 plays crucial roles in the formation and maintenance of PF–Purkinje cell synapses (Yuzaki, 2004); the number of PF–Purkinje cell synapses was markedly reduced in GluRδ2−/− cerebella, and many spines remain uninnervated without presynaptic contact (Kurihara et al., 1997; Lalouette et al., 2001). Interestingly, serial EM analysis revealed that almost all the spines in GluRδ2−/−/TgΔCT7 cerebella as well as GluRδ2−/−/Tgwt cerebella were in contacted with PF terminals (Fig. 5C,D) (98.4 ± 1.1% in GluRδ2−/−/TgΔCT7 mice, and (Fig. 5B,D) (99.7 ± 0.3% in GluRδ2−/−/Tgwt mice; total of 300 spines counted in three mice; t test with Bonferroni's correction, p = 0.536), whereas only 59.9 ± 2.2% spines were in contacted with PFs in GluRδ2−/− cerebella (Fig. 5A,D) (total of 300 spines counted in three mice; t test with Bonferroni's correction, p < 0.01). In addition to the loss of PF synapses, the remaining PF–Purkinje cell synapses in GluRδ2−/− mice exhibit another unique phenotype: the length of the PSD does not match that of the opposing presynaptic active zone (Kurihara et al., 1997; Lalouette et al., 2001). Serial EM analysis revealed that the occurrence of mismatching between the PSD and the active zone at PF synapses was also significantly less frequent in GluRδ2−/−/TgΔCT7 cerebella (7 ± 5%) than in GluRδ2−/− cerebella (29 ± 3%; 300 spines counted in three representative mice from each line; t test with Bonferroni's correction, p = 0.019). However, it was still more frequent in GluRδ2−/−/TgΔCT7 cerebella than in GluRδ2−/−/Tgwt cerebella (0.5 ± 0.4%), although the difference was not statistically significant (t test with Bonferroni's correction, p = 0.454). These results indicate that GluRδ2 lacking the C terminus could regulate PF–Purkinje cell synaptic integrity almost completely, although we cannot rule out the possibility of a minor contribution from a C terminus-based signaling pathway.
Figure 5.
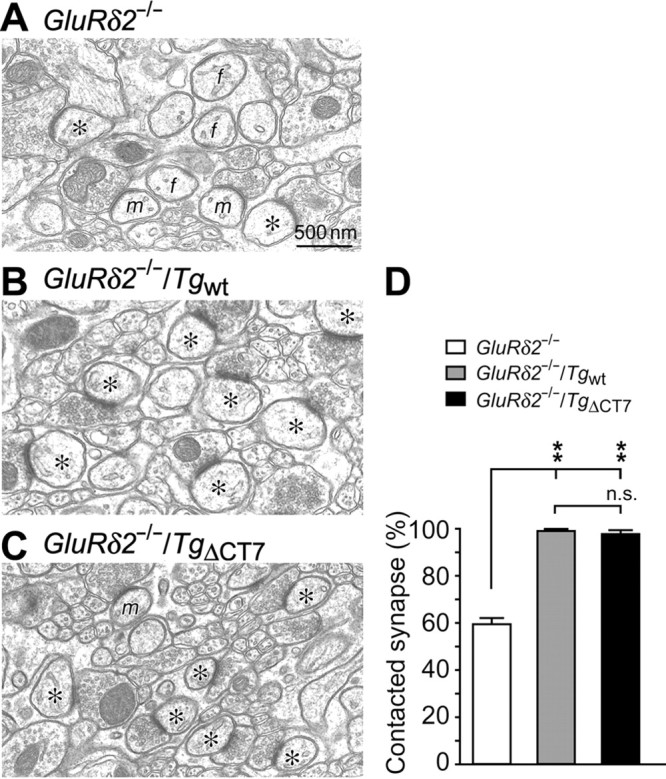
Rescue of impaired PF–Purkinje cell synaptic integrity in GluRδ2−/− mice by TgΔCT7 expression. A–C, Representative EM images showing PF–Purkinje cell synapses in GluRδ2−/− (A), GluRδ2−/−/Tgwt (B), and homozygous GluRδ2−/−/TgΔCT7 (C) cerebella. The asterisks indicate Purkinje cell spines in contact with PF terminals, and f and m indicate free/naked spine and mismatched synapses, respectively. D, Quantitative data showing the percentage of contacted synapses of the 300 total Purkinje cell spines counted in three mice. **p < 0.01. n.s., No significance.
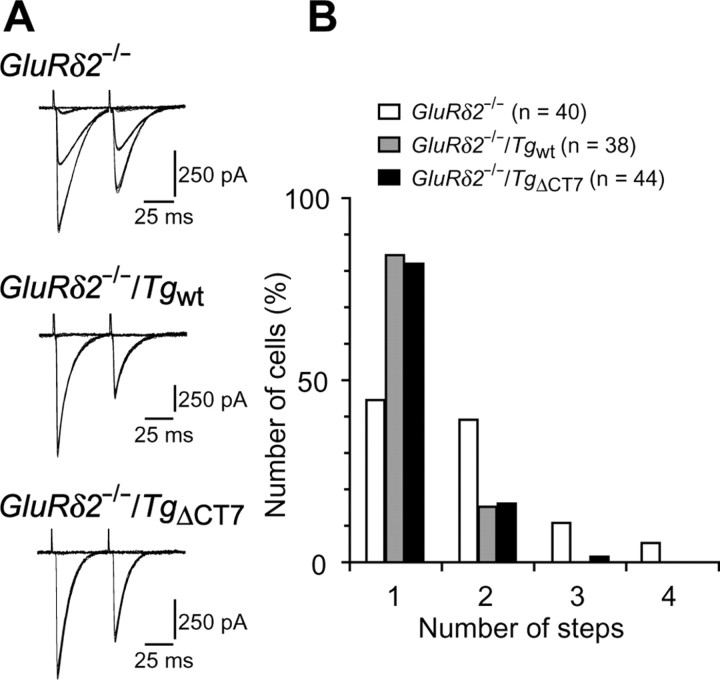
Innervation of GluRδ2−/−/TgΔCT7 Purkinje cells by single CFs
Immature Purkinje cells are innervated by several CFs originating from the inferior olives of the medulla. The redundant CFs are gradually eliminated during development, and most Purkinje cells become innervated by single CFs by the end of the third postnatal week in mice. In contrast, many GluRδ2−/− Purkinje cells remain innervated by multiple CFs even in adulthood, indicating that GluRδ2 plays a role in the developmental pruning of redundant CF inputs (Kashiwabuchi et al., 1995; Hashimoto et al., 2001). Because a single CF has a single threshold for excitation, increasing the stimulus intensity normally elicits CF–EPSCs in an all-or-none manner. Although single EPSCs were elicited in only ∼45% of the Purkinje cells from postnatal day 24 (P24) to P38 GluRδ2−/− mice (Fig. 6A,B), >80% of the Purkinje cells from GluRδ2−/−/TgΔCT7 and GluRδ2−/−/Tgwt mice attained a one-to-one relationship with CFs (Fig. 6A,B); this percentage was comparable with that of GluRδ2+/+ mice (Kashiwabuchi et al., 1995; Hashimoto et al., 2001). Some CF–EPSCs in GluRδ2−/− Purkinje cells are reportedly associated with a markedly slower rise time; this may be attributed to the abnormal CF innervation of the distal dendrites of GluRδ2−/− Purkinje cells (Ichikawa et al., 2002; Miyazaki et al., 2004). However, CF–EPSCs in GluRδ2−/−/TgΔCT7 as well as GluRδ2−/−/Tgwt cells had a rapid rise time similar to that in wild-type cells (0.5 ± 0.1 ms, n = 14 in GluRδ2−/−/TgΔCT7; 0.5 ± 0.1 ms, n = 19 in GluRδ2−/−/Tgwt) (Fig. 6A). These results indicate that although the virus-mediated transient expression of TgΔCT7 did not work (Kohda et al., 2007), the transgenic mice-based expression of TgΔCT7 restored the normal process of CF synapse elimination in GluRδ2−/− mice.
Figure 6.
Rescue of multiple CF innervation pattern in a single Purkinje cell from a GluRδ2−/− mouse by TgΔCT7 expression. A, Representative CF–EPSCs (ISI, 50 ms) recorded from Purkinje cells clamped at −10 mV in GluRδ2−/− (top), GluRδ2−/−/Tgwt (middle), and homozygous GluRδ2−/−/TgΔCT7 (bottom) mice. B, Histogram showing the number of CFs innervating single Purkinje cells in each mouse. The number of CF–EPSCs induced by different stimuli thresholds (0–200 μA) was counted.
Impaired cerebellar motor learning in GluRδ2−/−/TgΔCT7 mice
Finally, we examined whether the motor discoordination and impaired motor learning observed in GluRδ2−/− mice in vivo (Kashiwabuchi et al., 1995; Kishimoto et al., 2001) could be rescued by the expression of TgΔCT7. Unlike GluRδ2−/− mice, GluRδ2−/−/TgΔCT7 mice as well as GluRδ2−/−/Tgwt mice exhibited no ataxia and could walk along a straight line with regular steps (Fig. 7A) (supplemental movie S1, available at www.jneurosci.org as supplemental material). Similarly, GluRδ2−/−/TgΔCT7 mice performed as well as GluRδ2−/−/Tgwt and GluRδ2+/+ mice did on the rotor-rod task at 10 rpm (Fig. 7B) (one-way ANOVA followed by Bonferroni's test, F(3,51) = 33.91, p > 0.05 for both mice). However, when the test was performed at 20 rpm, the GluRδ2−/−/TgΔCT7 mice exhibited a significantly poorer performance than the GluRδ2−/−/Tgwt and GluRδ2+/+ mice (Fig. 7B) (one-way ANOVA followed by Bonferroni's test, F(3,58) = 85.09, p < 0.001 for both mice). Furthermore, the delayed eyeblink conditioning test, which reflects cerebellum-dependent classical associative learning (Ito, 1989; Thompson and Krupa, 1994), revealed that unlike Tgwt, TgΔCT7 could not rescue impaired motor learning in GluRδ2−/− mice (Fig. 7C) (one-way ANOVA followed by Bonferroni's test, F(3,30) = 4.60, p = 0.038 vs GluRδ2+/+; p = 0.011 vs GluRδ2−/−/Tgwt mice). These results suggest that because GluRδ2 lacking the C-terminal PDZ-binding motif could restore the motor coordination required for gait and motor coordination during the less demanding rotor-rod task, it may convey indispensable signals necessary for motor coordination during complex motor tasks and motor learning in vivo.
Discussion
In the present study, we demonstrated that although a mutant GluRδ2 transgene lacking the C-terminal PDZ-binding motif successfully rescued several abnormalities of the GluRδ2−/− cerebellum, such as enhanced PPF at PF–Purkinje cell synapses (Fig. 4), the appearance of uninnervated spines (Fig. 5), and the sustained innervation of Purkinje cells by multiple CFs (Fig. 6), it could not restore abrogated LTD at PF–Purkinje cell synapses (Fig. 3). Furthermore, although the gross motor discoordination of GluRδ2−/− mice was restored (Fig. 7) (supplemental movie S1, available at www.jneurosci.org as supplemental material), the cerebellar motor learning underlying eyeblink conditioning remained impaired even after the expression of TgΔCT7 (Fig. 7). These findings indicate, for the first time, that signaling via the C-terminal end of GluRδ2 was dispensable for morphological integrity at PF and CF synapses, whereas signaling was absolutely required for the induction of LTD and motor learning.
LTD as a major mechanism underlying motor learning in the cerebellum
Although recent studies using genetically modified mice primarily support the hypothesis that LTD of PF–Purkinje cell synapses underlies certain forms of associative motor learning, some developmental abnormalities that are often observed in these mice, such as the sustained innervation of Purkinje cells by multiple CFs and the reduction in PF synapses, have precluded an unequivocal interpretation. For example, mice lacking GluRδ2 (Kashiwabuchi et al., 1995), metabotropic glutamate receptor 1 (Aiba et al., 1994; Conquet et al., 1994), phospholipase C β4 (Miyata et al., 2001), or Gαq (Hartmann et al., 2004) all show impaired LTD together with abnormal CF innervation patterns. Furthermore, because most signaling molecules involved in LTD are not specific to PF–Purkinje cell synapses, the genetic modification of these molecules may also affect other synapses. For example, although motor learning was impaired in L7-PKCI transgenic mice (De Zeeuw et al., 1998), in which protein kinase C (PKC) activities were specifically inhibited in Purkinje cells, all isoforms of PKCs may have been affected in all synapses, including PF–, CF–, and interneuron–Purkinje cell synapses. Importantly, GluRδ2−/−/TgΔCT7 mice are unique in that they showed impairment in LTD and motor learning in the absence of morphological abnormalities at CF or PF synapses and that the transgene was specifically expressed at PF–Purkinje cell synapses (Figs. 1C, 2) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). To the best of our knowledge, no neuronal circuits have ever been related to a behavioral defect at this level of synaptic specificity. Therefore, the data strongly support the notion that cerebellar LTD at PF synapses, although not essential for gross motor coordination, is a major mechanisms underlying motor learning during eyeblink conditioning.
Transgenic mice-based rescue approach
In the previous study, we showed that the virus-mediated expression of Tgwt, but not TgΔCT7, could successfully rescue impaired LTD induction in GluRδ2−/− Purkinje cells in slice preparations (Kohda et al., 2007). In contrast, LTD was normally induced in cultured GluRδ2−/− Purkinje cells transfected with a mutant GluRδ2 that lacked the PDZ-ligand domain (Yawata et al., 2006). In that study, glutamate was iontophoretically applied to Purkinje cells to induce LTD and to monitor the AMPA currents; thus, the observed phenomenon may have included changes at extrasynaptic regions. Moreover, molecular mechanisms underlying LTD in cultured Purkinje cells may not be exactly the same as those observed in slice preparations. The present data obtained from transgenic mice confirmed our previous result that the C terminus of GluRδ2 was indispensable for the induction of LTD. Furthermore, other functions of GluRδ2 in Purkinje cells such as the regulation of PF synaptic integrity, PF release probability, and motor learning in vivo could only be examined using the transgenic mice-based rescue approach, which enables stable and long-term expression of transgenes in vivo.
Another advantage of the transgenic mice-based approach is that we could obtain several lines of mice expressing various levels of transgene protein. In this study, by comparing hemizygous GluRδ2−/−/TgΔCT7 mice with homozygous GluRδ2−/−/Tgwt mice, which had similar total transgene protein expression levels (Fig. 1D), we were able to demonstrate a facilitatory role of the C terminus in the synaptic localization of GluRδ2. In contrast, the expression level of the transgenes is difficult to control precisely using a virus-based or transfection-based approach. For example, we previously reported that virally expressed TgΔCT7 and Tgwt proteins were similarly localized at the dendritic spines of Purkinje cells (Kohda et al., 2007). We suspect that the overexpression of transgenes by viral promoters may have masked the slight facilitatory role of the C terminus.
The structure–function relationship of a gene product can also be examined using a knock-in approach, in which an endogenous gene is replaced with a mutant gene by homologous recombination. This approach has an advantage in that, unlike transgenic mice-based method, the total expression levels of the mutant gene are similar to those of the endogenous gene. However, if a mutation modifies the protein expression levels at synapses, its effect on other synaptic functions should be carefully interpreted, because the total expression levels of mutant genes are fixed in knock-in mouse. In contrast, by comparing homozygous GluRδ2−/−/TgΔCT7 mice with homozygous GluRδ2−/−/Tgwt mice, which expressed similar amounts of protein at their synapses, we could elucidate the direct role of the C terminus of GluRδ2 on LTD and motor learning, separately from its role on synaptic localization.
Functions achieved by the C terminus of GluRδ2
Our present study indicated that functions of GluRδ2 could be classified into at least two categories: those that highly depend on the C-terminal PDZ-binding motif, and those that have a weaker relationship. Although the induction of LTD at PF synapses critically required intracellular signals mediated by the C terminus of GluRδ2, the morphological integrity at PF and CF synapses and presynaptic PF function did not absolutely require such signals. The failure of TgΔCT7 to rescue LTD is unlikely to reflect a difference in the sensitivity of this assay, because abrogated LTD was restored by even half the amount of GluRδ2 proteins at the synapses in the hemizygous GluRδ2−/−/Tgwt mice (Yuzaki, 2005). Thus, these two classes of functions likely reflect distinct mechanisms by which GluRδ2 operates in Purkinje cells.
Although GluRδ2 signaling mechanisms remain primarily unknown even after its cloning >14 years ago, the results of this study provide the first clue as to where to look for keys to GluRδ2 signaling: molecules that bind to the C-terminal 7 amino acids of GluRδ2 play a crucial role in LTD induction and motor learning. For example, delphilin is reported to bind Src tyrosine kinase via the FH1 domain (Miyagi et al., 2002), and PTPMEG contains a protein tyrosine phosphatase domain (Hironaka et al., 2000). Because cerebellar LTD depends on the tyrosine phosphorylation status in Purkinje cells (Boxall et al., 1996), the C terminus of GluRδ2 may regulate LTD induction through an interaction with delphilin and PTPMEG.
In contrast, the morphological integrity at PF synapses may not require the C terminus. Similarly, reduced PF presynaptic release probability in GluRδ2−/− mice was completely restored by the expression of TgΔCT7 (Fig. 4). Recently, many synaptic adhesion molecules have been shown not only to participate in the formation of synapses but also to regulate presynaptic functions (Dalva et al., 2007). For example, presynaptic release probabilities were regulated by postsynaptic adhesion molecules such as EphB (Contractor et al., 2002), N-cadherin (Jungling et al., 2006), and neuroligin (Futai et al., 2007) by interacting with their presynaptic counterparts. Although many adhesion molecules are anchored at postsynaptic sites by multiple intracellular protein–protein interactions involving C-terminal domains of AMPA and NMDA receptors (Contractor et al., 2002; Futai et al., 2007; Silverman et al., 2007), the N-terminal extracellular domain of AMPA receptors has been shown recently to bind to N-cadherin (Nuriya and Huganir, 2006; Saglietti et al., 2007). Therefore, we speculate that the N-terminal domain of GluRδ2 may also bind directly or indirectly to some adhesion molecules located at the presynaptic sites, thereby regulating morphological synaptic integrity and presynaptic functions.
To what extent the second class of functions depends on the C terminus of GluRδ2, however, remains unclear. For example, although not statistically significant, the occurrence of mismatching between the PSD and the active zone at PF synapses was higher in GluRδ2−/−/TgΔCT7 than in GluRδ2−/−/Tgwt cerebella. In addition, although CF-EPSCs in GluRδ2−/−/TgΔCT7 Purkinje cells showed normal kinetics, the puncta that were immunopositive for vesicular glutamate transporter 2, which is predominantly expressed at CF terminals, tended to reach the distal region of Purkinje cell dendrites (data not shown). Thus, although the uninnervated PF synapses (Fig. 5) and sustained innervation by multiple CFs (Fig. 6) were completely rescued in GluRδ2−/−/TgΔCT7 Purkinje cells, minor abnormalities that may require additional signaling via the C terminus of GluRδ2 remained. To address this issue more clearly, new rescue lines that express higher levels of TgΔCT7 are necessary. Nevertheless, the C-terminal PDZ-binding motif is not crucial for most of the functions of GluRδ2 that are related to morphological synaptic integrity in the cerebellum.
Footnotes
This work was supported by the Grant-in-Aid for the Ministry of Education, Culture, Sports, Science and Technology of Japan (W.K., M.Y.), the Takeda Science Foundation, the Keio Gijuku Academic Development Funds, and the Keio University Grant-in-Aid for the Encouragement of Young Medical Scientists (W.K.). We thank members of the Genome Information Research Center (Osaka University) for the generation of transgenic mice.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- Boxall AR, Lancaster B, Garthwaite J. Tyrosine kinase is required for long-term depression in the cerebellum. Neuron. 1996;16:805–813. doi: 10.1016/s0896-6273(00)80100-2. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci USA. 2003;100:4855–4860. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Blum R, Kovalchuk Y, Adelsberger H, Kuner R, Durand GM, Miyata M, Kano M, Offermanns S, Konnerth A. Distinct roles of Gα(q) and Gα11 for Purkinje cell signaling and motor behavior. J Neurosci. 2004;24:5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, Sakimura K, Mishina M, Hashikawa T, Konnerth A, Watanabe M, Kano M. Roles of glutamate receptor δ2 subunit (GluRδ2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21:9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Miyazaki T, Kakegawa W, Matsuda S, Mishina M, Watanabe M, Yuzaki M. Rescue of abnormal phenotypes of the δ2 glutamate receptor-null mice by mutant δ2 transgenes. EMBO Rep. 2005;6:90–95. doi: 10.1038/sj.embor.7400312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka K, Umemori H, Tezuka T, Mishina M, Yamamoto T. The protein-tyrosine phosphatase PTPMEG interacts with glutamate receptor δ2 and ε subunits. J Biol Chem. 2000;275:16167–16173. doi: 10.1074/jbc.M909302199. [DOI] [PubMed] [Google Scholar]
- Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, Mishina M, Inoue Y, Watanabe M. Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor δ2. J Neurosci. 2002;22:8487–8503. doi: 10.1523/JNEUROSCI.22-19-08487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Jungling K, Eulenburg V, Moore R, Kemler R, Lessmann V, Gottmann K. N-cadherin transsynaptically regulates short-term plasticity at glutamatergic synapses in embryonic stem cell-derived neurons. J Neurosci. 2006;26:6968–6978. doi: 10.1523/JNEUROSCI.1013-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Miyazaki T, Hirai H, Motohashi J, Mishina M, Watanabe M, Yuzaki M. Ca2+ permeability of the channel pore is not essential for the δ2 glutamate receptor to regulate synaptic plasticity and motor coordination. J Physiol (Lond) 2007;579:729–735. doi: 10.1113/jphysiol.2006.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, Aizawa S, Mishina M. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluRδ2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical eyeblink conditioning in glutamate receptor subunit δ2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci. 2001;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- Kohda K, Kakegawa W, Matsuda S, Nakagami R, Kakiya N, Yuzaki M. The extreme C-terminus of GluRδ2 is essential for induction of long-term depression in cerebellar slices. Eur J Neurosci. 2007;25:1357–1362. doi: 10.1111/j.1460-9568.2007.05412.x. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. Impaired parallel fiber–Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor δ2 subunit. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J. Neurobiological effects of a null mutation depend on genetic context: comparison between two hotfoot alleles of the delta-2 ionotropic glutamate receptor. Neuroscience. 2001;105:443–455. doi: 10.1016/s0306-4522(01)00193-2. [DOI] [PubMed] [Google Scholar]
- Landsend AS, Amiry-Moghaddam M, Matsubara A, Bergersen L, Usami S, Wenthold RJ, Ottersen OP. Differential localization of δ glutamate receptors in the rat cerebellum: coexpression with AMPA receptors in parallel fiber-spine synapses and absence from climbing fiber-spine synapses. J Neurosci. 1997;17:834–842. doi: 10.1523/JNEUROSCI.17-02-00834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, Wisden W. The rat δ1 and δ2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Yamashita T, Fukaya M, Sonoda T, Okuno T, Yamada K, Watanabe M, Nagashima Y, Aoki I, Okuda K, Mishina M, Kawamoto S. Delphilin: a novel PDZ and formin homology domain-containing protein that synaptically colocalizes and interacts with glutamate receptor δ2 subunit. J Neurosci. 2002;22:803–814. doi: 10.1523/JNEUROSCI.22-03-00803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Kim HT, Hashimoto K, Lee TK, Cho SY, Jiang H, Wu Y, Jun K, Wu D, Kano M, Shin HS. Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C beta4 mutant mice. Eur J Neurosci. 2001;13:1945–1954. doi: 10.1046/j.0953-816x.2001.01570.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto K, Shin HS, Kano M, Watanabe M. P/Q-type Ca2+ channel α1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24:1734–1743. doi: 10.1523/JNEUROSCI.4208-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Huganir RL. Regulation of AMPA receptor trafficking by N-cadherin. J Neurochem. 2006;97:652–661. doi: 10.1111/j.1471-4159.2006.03740.x. [DOI] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, Passafaro M. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–8516. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama C, Nakagawa S, Watanabe M, Mishina M, Inoue Y. Light- and electron-microscopic localization of the glutamate receptor channel δ2 subunit in the mouse Purkinje cell. Neurosci Lett. 1995;188:89–92. doi: 10.1016/0304-3940(95)11403-j. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Krupa DJ. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- Yawata S, Tsuchida H, Kengaku M, Hirano T. Membrane-proximal region of glutamate receptor δ2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. J Neurosci. 2006;26:3626–3633. doi: 10.1523/JNEUROSCI.4183-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzaki M. The δ2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cells. Cerebellum. 2004;3:89–93. doi: 10.1080/14734220410028921. [DOI] [PubMed] [Google Scholar]
- Yuzaki M. Transgenic rescue for characterizing orphan receptors: a review of delta2 glutamate receptor. Transgenic Res. 2005;14:117–121. doi: 10.1007/s11248-005-2685-6. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Wang YX, Petralia RS. δ-Glutamate receptors are differentially distributed at parallel and climbing fiber synapses on Purkinje cells. J Neurochem. 1997;68:1041–1052. doi: 10.1046/j.1471-4159.1997.68031041.x. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]