Abstract
In the adult brain, chloride (Cl−) influx through GABAA receptors is an important mechanism of synaptic inhibition. However, under a variety of circumstances, including acquired epilepsy, neuropathic pain, after trains of action potentials or trauma, and during normal early brain development, GABAA receptor activation excites neurons by gating Cl− efflux because the intracellular Cl− concentration (Cli) is elevated. These findings require an inducible, active mechanism of chloride accumulation. We used gramicidin-perforated patch recordings to characterize Cl− transport via NKCC1, the principal neuronal Cl− accumulator, in neonatal CA1 pyramidal neurons. NKCC1 activity was required to maintain elevated Cli such that GABAA receptor activation was depolarizing. Kinetic analysis of NKCC1 revealed reversible transmembrane Cl− transport characterized by a large maximum velocity (v max) and high affinity (K m), so that NKCC1 transport was limited only by the net electrochemical driving force for Na+, K+, and Cl−. At the steady-state Cli, NKCC1 was at thermodynamic equilibrium, and there was no evidence of net Cl− transport. Trains of action potentials that have been previously shown to induce persistent changes in neuronal E Cl (reversal potential for Cl−) did not alter v max or K m of NKCC1. Rather, action potentials shifted the thermodynamic set point, the steady-state Cli at which there was no net NKCC1-mediated Cl− transport. The persistent increase in Cli required intact α2/α3 Na+-K+-ATPase activity, indicating that trains of action potentials reset the thermodynamic equilibrium for NKCC1 transport by lowering Nai. Activity-induced changes in Na+-K+-ATPase activity comprise a novel mechanism for persistent alterations in synaptic signaling mediated by GABA.
Keywords: seizure, sodium pump, development, long-term potentiation, dendrite, action potential, gramicidin-perforated patch
Introduction
Mature neurons are typically hyperpolarized and inhibited by the net chloride (Cl−) influx mediated by GABAA receptor activation. In contrast, in the developing brain, GABA depolarizes and excites neurons by gating Cl− efflux. Whether Cl− flows into or out of a cell through GABAA receptor-operated channels depends on the transmembrane Cl− gradient. Cation chloride cotransporters expressed at different stages of development are thought to regulate intracellular Cl− concentration (Cli), and therefore, the response to GABA (Lu et al., 1999; Marty et al., 2002; Stein et al., 2004; Wang et al., 2005). NKCC1, expressed early in development, transports Na+, K+, and Cl− into the cell in various electroneutral stoichiometries (Geck et al., 1980; Russell, 2000). In the mature brain, KCC2 moves 1 K+ and 1 Cl− across the membrane. Normally transport is outward, but transport by KCC2 is reversible and the direction of transport is dictated by the free energy gradients for K+ and Cl− (Staley and Proctor, 1999).
In response to a variety of conditions, the polarity of the GABA response can change from hyperpolarizing and inhibitory to depolarizing and excitatory. Examples of such conditions include trauma to postsynaptic (van den Pol et al., 1996) and presynaptic (Coull et al., 2003) neurons, animal models of neuropathic pain (Coull et al., 2003), seizures (Khalilov et al., 2003), and human epilepsy (Cohen et al., 2002). It is clear from developmental studies that reduced expression of NKCC1 (Vardi et al., 2000; Marty et al., 2002; Wang et al., 2002; Ikeda et al., 2003) and increased expression of KCC2 (Clayton et al., 1998; Rivera et al., 1999; Wang et al., 2002) can reverse the polarity of GABA responses.
However, persistent changes in the response to GABA can also occur immediately after trains of action potentials (APs) (Fiumelli et al., 2005). The mechanism by which this rapid and persistent change in the direction of Cl− flux occurs is not well understood. The purpose of the present work was first to understand the regulation of Cl− in the developing brain, and then to determine the mechanism by which Cl− regulation changes after trains of action potentials.
NKCC1-mediated Cl− transport has been demonstrated and characterized in multiple systems, but not in neonatal neurons, where it contributes to normal development and also to seizure activity (Russell, 2000; Dzhala et al., 2005). Here, we show that NKCC1-mediated Cl− transport is the principle mechanism by which neonatal hippocampal pyramidal neurons accumulate Cli to make GABA depolarizing. We describe NKCC1 Cl− cotransport with a model analogous to Ohm's law, in which the combined electrochemical gradients of the transported ions provide the driving force for Cl− transport and transporter kinetics represent conductance. This thermodynamic model of NKCC1-mediated Cl− transport is supported by evidence that transport is reversible and the direction is sensitive to changes in intracellular Na+ and Cl−. Finally, we show that the α2/α3 isoform of Na+-K+-ATPase is required for the rapid and persistent increase in Cli that occurs after a train of action potentials. Activity-induced changes in Na+ and K+ gradients represent a novel form of plasticity in GABAergic signaling.
Materials and Methods
Slice preparation.
All animals were housed and cared for according to institutional guidelines. We prepared acute hippocampal slices from male Sprague Dawley rats, age postnatal day 4 (P4) through P6, when NKCC1 expression is high and KCC2 expression is low (Dzhala et al., 2005). The brain was removed and submerged in ice-cold solution containing (in mm) 87 NaCl, 75 sucrose, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2·2 H2O, 7 MgCl2·6 H2O, 2.25 NaH2PO4·H2O, and 25 glucose. The solution was continuously bubbled with 95% O2/5% CO2. The cerebellum was removed with a razor blade, and the brain was cut sagittally into two hemispheres. For each hemisphere, the diencephalon, midbrain, and remaining hindbrain were bluntly dissected away and discarded. Hemispheres were affixed to an agar block (4% agar) attached to the stage of a vibrating blade microtome (Leica Microsystems, Wetzlar, Germany) and submerged in the above ice-cold solution. Horizontal hippocampal slices (400 μm) were cut and placed in a slice chamber at room temperature in a nominally bicarbonate-free incubation solution containing (in mm) 77 NaCl, 73.5 sucrose, 2.5 KCl, 26 HEPES, 1.25 CaCl2·2 H2O, 4.5 MgCl2·6 H2O, 1.75 NaH2PO4·H2O, and 17.5 glucose. pH was titrated to 7.4 with NaOH. The solution was continuously bubbled with 100% O2. Slices recovered at room temperature for at least 1 h before experiments.
The GABAA channel conducts anions, mainly Cl− and bicarbonate (Bormann et al., 1987). In the present work, we estimate the Cli by measuring the reversal potential of GABA-evoked currents (E GABA). E GABA approximates the reversal potential for Cl− (E Cl) when the bicarbonate flux through the GABAA channel is minimized. This is achieved by buffering extracellular solutions with HEPES instead of bicarbonate and CO2 (Staley et al., 1995; Staley and Proctor, 1999). Therefore, in the present work, the incubation solution and artificial CSF (ACSF) in which cells were recorded were buffered with HEPES instead of bicarbonate/CO2.
Slices were transferred to a submerged slice chamber (Warner Instruments, Hamden, CT) and perfused at 2–3 ml/min at 32°C with nominally CO2/bicarbonate-free ACSF containing (in mm) 131.5 NaCl, 2.5 KCl, 26 HEPES, 2 CaCl2·2 H2O, 2 MgCl2·6H2O, 1.25 NaH2PO4·H2O, 10 glucose, and the GABAB receptor antagonist CGP 55845A (1 μm). pH was titrated to 7.3 at 32°C with NaOH. The solution was continuously bubbled with 100% O2. Slices were stabilized with a custom-made harp of platinum wire and nylon strings. CA1 pyramidal neurons were easily visualized with a 40× water-immersion objective using a Zeiss (Thornwood, NY) Axioskop with differential interference contrast optics.
Gramicidin-perforated patch-clamp electrophysiology.
Data were collected with a Multiclamp 700A amplifier and digitized at 10 kHz using pClamp 8.2 software (Molecular Devices, Union City, CA). To prevent the Cl− concentration of the recording electrode from setting the Cli, recordings were performed using gramicidin-perforated patch (Abe et al., 1994; Ebihara et al., 1995; Kyrozis and Reichling, 1995). Gramicidin stock (40 mg/ml in DMSO) was prepared daily and diluted to 80 μg/ml in the recording pipette solution containing (in mm) 10 HEPES and 150 KCl (“0 mm Napipette”) or 10 HEPES, 141.1 KCl, and 8.9 NaCl (“9 mm Napipette”). pH was titrated to 7.2 with KOH. Osmolarity was titrated to 290 mOsm/L. Recordings used 0 mm Napipette unless otherwise noted. All cells were recorded with gramicidin-perforated patch except for one. For this single whole-cell recording, the intracellular solution was comprised of (in mm) 121 potassium gluconate, 4 Na2ATP, 0.3 Na3GTP, 1 QX-314, 1 EGTA, 10 HEPES, 6 KCl, and 2 MgCl2; pH 7.2 with KOH; 290 mOsm. This solution had 10 mm Cl− and 8.9 mm Na+ (same as the 9 mm Napipette gramicidin recordings). This cell was one of the three cells recorded for the control action potential experiment (see Fig. 6 D). We included this cell in the data set because it used the same recording pipette Na+ concentration as the gramicidin-recorded cells, and because the important aspect of this experiment was the change in Cli after action potentials, rather than the absolute Cli.
Figure 6.
Activity-dependent, long-term increases in Cli require Na+-K+-ATPase and NKCC1. All cells recorded with 9 mm Napipette. A, After a train of action potentials in the recorded cell (20 Hz, 2.5 min) (Fiumelli et al., 2005), Cli reaches a persistently higher steady state. Data from a single cell are shown as mean ± SD. B, Cl− transport rate as a function of the magnitude of Cl− depletion before (♦) and after postsynaptic action potentials (◇) for the cell in A. As in Figure 5 B, in control conditions, there is no relationship between the size of the Cli depletion and Cl− transport rate. After the train of action potentials, Cl− transport with 9 mm Napipette more closely resembles Cl− transport with 0 mm Napipette (Fig. 5 B) in that larger Cli depletions correlate with slower NKCC1 Cl− transport. C, The selective α2/α3 Na+-K+-ATPase antagonist DHO (10 μm) prevents the persistent increase in Cli after the train of action potentials. Data from a single cell are presented as mean ± SD. D, Inhibition of α2/α3 Na+-K+-ATPase (n = 4) or NKCC1 (n = 2) prevents the persistent increase in Cli after a train of action potentials. For each cell, Cli was normalized to its value before action potentials.
Recording pipettes were pulled from nonfilamented thin wall borosilicate glass (Warner Instruments) to resistances of 1.5–5 MΩ. Pipettes were tip-filled with gramicidin-free pipette solution by immersing the tip in gramicidin-free solution while applying negative pressure to the back end of the pipette for 3–5 s. The pipette was then back-filled with gramicidin-containing pipette solution. The gramicidin-free solution at the tip allowed us to apply positive pressure to the electrode without blowing gramicidin solution onto the slice. Pipettes to picospritz GABA were pulled to 3–7 MΩ resistances and back-filled with GABA-containing ACSF.
The recording electrode and GABA puffer pipette were visually guided to the hippocampal CA1 pyramidal cell layer. In current clamp, a gigaohm seal was obtained at the soma. After several minutes, the recording was switched to voltage clamp; the cell was held at or near the measured resting membrane potential. Experiments began once the access resistance fell below 40 MΩ and was stable (usually after 25–60 min). The Cl− transport kinetics experiments we present here rely on changing Cli with an inward or outward Cl− current. Dendrites have a higher surface-to-volume ratio than the soma, so a Cl− charge transfer that has almost no effect on somatic Cli can significantly change dendritic Cli (Staley and Proctor, 1999). Therefore, Cl− currents were recorded at the soma in response to pressure application of GABA (100 μm in ACSF) to the apical dendrites ∼100 μm from the soma (5–15 ms, 5 psi; Picospritzer II, Parker, Fairfield, NJ) (Staley and Proctor, 1999). The optimal location for the GABA puffer pipette was determined by monitoring the size of GABA-evoked currents in the voltage-clamped neuron while advancing the pipette into the slice.
Dendritic currents can be underestimated if the dendrites are inadequately voltage clamped; this is often improved by using Cs+ in the recording electrode. We chose not to use Cs+ in these experiments, because we did not want to alter K+ gradients or NKCC1 transport. To minimize the error, we integrated the GABA-evoked responses over time to obtain the Cl− charge transfer (Staley and Mody, 1992). Space-clamp error should not affect the accuracy of the estimated Cl− transport rates because each test current should be affected to the same degree.
Quantification of GABA-evoked currents and calculation of Cli.
To determine the steady-state E Cl, GABA-evoked currents were recorded during a series of 1 s voltage steps. Nominally bicarbonate-free ACSF was used in all experiments to minimize bicarbonate flux through the GABA channel so that the E GABA would approximate E Cl. Cl− currents were evoked at five to seven different membrane potentials, in 5 mV increments with the middle step approximately where the currents reversed (E Cl). We usually limited the membrane potential (V m) range to within ±15 mV of E Cl to minimize the possibility of altering Cli with large Cl− influxes or effluxes. Ten seconds elapsed between each voltage step to prevent receptor desensitization or changes in Cli from confounding our results.
We calculated the charge transfer of GABAA receptor-mediated currents by calculating the integral of the GABA-evoked current waveform after subtracting the baseline current. Charge transfer/voltage plots were obtained by fitting the data to the Goldman–Hodgkin–Katz constant field equation:
 |
where I is current; Clo is extracellular Cl− concentration; G = GABAA channel Cl− permeability; F is Faraday's constant (96,487 C · mol−1); R is gas constant (8.315 J · mol−1 · K−1); and T is temperature (in Kelvins). E Cl is the value of V m that corresponds to the zero-current condition for the constant field equation (Eq. 1):
 |
We calculated the Cli based on E Cl using Equation 2.
Estimating Cl− transport.
To estimate Cl− transport, we transiently changed Cli and measured the rate at which Cli returned to baseline. The experimental protocol is illustrated in Figure 1 B (“Cli depletion”) and Figure 2(“Cli load”). To transiently change Cli, we created a large driving force for Cl− to flow into or out of the cell by stepping the V m to potentials negative (Fig. 1 B) or positive (Fig. 2) to E Cl for 1 s. Two hundred milliseconds into the voltage step, GABA was puffed onto the apical dendrites. When V m was hyperpolarized to E Cl, Cl− efflux through GABAA channels transiently lowered Cli (“Cli depletion”). Conversely, when GABA was applied as V m was depolarized to E Cl, Cl− influx transiently increased Cli (“Cli load”). We coupled each Cli load or depletion current with a single “test” Cl− current evoked 1–6 s later. The test current was evoked at a V m near E Cl, so that small changes in Cli caused by the Cli load or depletion would be easily seen as a change the direction and/or size of the test current. By measuring the charge transfer of the test currents at 1 s intervals after the Cli load or depletion, we estimated the rate at which Cli recovered to baseline. We assumed that any voltage-gated currents activated during the voltage steps did not significantly alter our results for two reasons. First, Cli consistently returned to baseline within seconds of the Cli load or depletion; and second, the test potential had no effect on the time constant of Cli recovery.
Figure 1.

NKCC1 activity is required to maintain elevated Cli. In the presence and absence of 10 μm bumetanide, a selective inhibitor of NKCC1, GABAA receptor-mediated Cl− conductances were evoked by pressure application of 100 μm GABA to the apical dendrites of a gramicidin-perforated patch-clamped CA1 pyramidal neuron in an acute hippocampal slice from a P5 rat. All cells recorded with 0 mm Napipette. A, Selective NKCC1 inhibition (○) hyperpolarizes E Cl compared with control (●). Fit lines are calculated with the constant field equation (Eq. 1). B–D, “Cli depletion”: quantification of inward Cl− transport after dendritic Cl− efflux lowers Cli. B, To lower dendritic Cli, V m was stepped to −123 mV for 1 s, and GABA was applied to the dendrites to elicit a large outward Cl− flux. V m was then stepped to −63 mV (3 mV negative to steady-state E Cl), and a second GABA puff evoked a “test” current. Six trials of paired GABA puffs were performed, each with a different delay between Cli depletion and the test current. Calibration: 100 pA refers to Cli depletion current; 10 pA is for all other currents. C, As the interval between the Cli depletion and test current increases, the charge transfer of the test currents returns to steady state. D, Cli is calculated from the shift in E Cl that accounts for the direction and charge transfer of each test current. Fit lines in C and D are single exponentials (Eq. 4).
Figure 2.
“Cli load”: quantification of outward Cl− transport after dendritic Cl− influx raises Cli. The cell was recorded with 9 mm Napipette. A, To raise dendritic Cli, V m was stepped to −5.5 mV for 1 s; 200 ms into the depolarizing voltage step, GABA was applied to the dendrites to elicit a large inward Cl− flux. V m was then stepped to −68.5 mV (2.6 mV negative to steady-state E Cl), and a second GABA puff evoked a “test” current. As in Figure 1 B, six trials of paired GABA puffs were performed, each with a different delay between Cli load and the test current. B, As the interval between the Cli load and test current increases, the charge transfer of the test currents returns to steady state. C, Cli is calculated from the shift in E Cl that accounts for the direction and charge transfer of each test current. Fit lines in B and C are single exponentials (Eq. 4).
Cli was calculated for each time point by estimating the change in E Cl that would account for the direction and size of each GABA-evoked test current. For the experiments shown in Figure 1 B–D, Cli was calculated at each time interval according to the steady-state permeability (estimated from the data in Fig. 1 A and Eq. 1) and the direction and charge transfer of the GABA-evoked Cl− current at each time interval after Cli depletion (Staley and Proctor, 1999). By combining Equations 1 and 2, we estimate Cli for each test current:
 |
Figures 1 D and 2 C show Cli as a function of seconds after Cli depletion. These data were fit with a monoexponential curve (Eq. 4) using a least-squares algorithm where t = 0 is the time at which the membrane potential is stepped to a value near E Cl (Fig. 1 B). We could not use an earlier time point for t = 0 because the Cl− transport measurements would be confounded by the ongoing Cli load or depletion current.
To determine the NKCC1-specific component of Cli transport, this protocol was repeated in the same cell after selectively blocking NKCC1 with 10 μm bumetanide (Russell, 2000). In control conditions, Cli returns to baseline via NKCC1 and diffusion; in the presence of 10 μm bumetanide, there is only diffusion. To estimate Cl− transport by NKCC1 alone, we performed a “bumetanide subtraction.” In the presence and absence of bumetanide, Cli recovered to baseline with monoexponential kinetics (Eq. 4, Fig. 3 B). Monoexponential rate constants are additive; therefore, the rate constants for NKCC1-mediated Cl− transport and diffusion were additive:
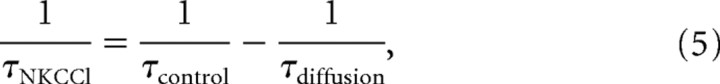 |
where τcontrol represents the time constant of Cli recovery in the absence of bumetanide and τdiffusion represents the time constant of Cli recovery in the presence of 10 μm bumetanide (Fig. 3). Repetitive GABA application can cause GABAA receptor desensitization. Because receptor desensitization is insensitive to 10 μm bumetanide, it was subtracted out as part of the bumetanide-insensitive change in Cli.
Figure 3.
Quantification of NKCC1-mediated Cl− transport. A, After an acute Cli depletion, Cli returns to steady state via NKCC1 transport and dendritic diffusion. When NKCC1 is blocked with 10 μm bumetanide, the increase in Cli back to steady state is well described by Cl− diffusion alone (see supplemental material, available at www.jneurosci.org). B, Cli as a function of time after Cli depletion for control (●) and with NKCC1 blocked (○) for a single neuron recorded with 0 mm Napipette; each is fit by a single exponential. C, The NKCC1 Cl− transport time constant (τNKCC1) is determined by subtracting monoexponential rate constants (k = 1/τ). D, After dendritic Cl− efflux, NKCC1 returns Cli to steady state by inward Cl− transport (n = 5, all recorded with 0 mm Napipette). After dendritic Cl− influx, NKCC1 returns Cli to steady state via outward Cl− transport (n = 3, all recorded with 9 mm Napipette). Both inward and outward Cl− transport recover to the same steady state Cli (p = 0.71). Inward and outward Cl− transport data are corrected for non-NKCC1 transport as in C and are presented as mean ± SEM.
Estimating the regulation of Cl− transport by NKCC1.
The rate of Cl− transport by NKCC1 can be expressed in analogy to Ohm's law:
where the NKCC1-mediated Cl− transport rate is analogous to current flow, the combined Na+, K+, and Cl− electrochemical gradient (Eq. 7) produces driving force, and transport capacity (Eq. 9) represents the conductance.
The driving force, or change in free energy (ΔG), associated with NKCC1 Cl− transport is a function of the transmembrane concentration gradients of the transported ions:
 |
where Nao, Ko, and Clo are the extracellular concentrations and Nai, Ki, and Cli are the intracellular concentration of the transported ions; x:y:z is the ratio of Na+:K+:Cl− transported by NKCC1 (1:1:2, 1:2:3, or 1:4:5). To create a dimensionless term, free energy (driving force) can be normalized to ΔG max, its maximum value at t = 0. NKCC1 reaches thermodynamic equilibrium (Cl− transport rate = 0) when ΔG = 0. The Cli at which NKCC1 is at thermodynamic equilibrium (ΔG = 0) is obtained when the bracketed expression in Equation 7 equals 1, or
 |
This equation predicts the Cli at which there is no net transport via NKCC1 because there is no net electrochemical driving force to drive transport. The only free variable in the driving force calculation is steady-state Nai.
The transport capacity (v MM, analogous to the conductance term in Ohm's law) can be modeled using Michaelis–Menten kinetics for enzymatic activity (Läuger, 1987; Alvarez-Leefmans and Russell, 1990; Russell, 2000):
 |
where [Cl−] is the substrate Cl− concentration (Clo for inward transport; Cli for outward transport), v max is the maximum velocity of transport, and K m is the Cl− affinity of the transporter (substrate Cl− concentration at which Cl− transport is half-maximal). When Cli is expressed as a function of seconds after a Cli load or depletion, extrapolating the monoexponential fit line (Eq. 4) back to t = 0 gives an estimate of v max (the velocity at t = 0). Therefore, K m was the only free variable in calculating transport capacity.
In analogy to Ohm's law (Eq. 6), the velocity of NKCC1-mediated Cl− transport (v NKCC1) is predicted by multiplying the normalized driving force ΔG/ΔG max by the conductance term v MM (Läuger, 1987):
 |
Activity-induced changes in Cli.
For the experiments presented in Figure 6, neurons were switched from voltage clamp to current clamp; action potentials were evoked by injecting depolarizing current pulses (1.5 ms, 2 nA) at 20 Hz for 2.5 min as described previously (Fiumelli et al., 2005). Recordings were switched back to voltage-clamp mode after the train of action potentials.
Statistical analyses.
Student's t test was used to test for significant differences between control and experimental conditions. SigmaPlot (SyStat Software, Point Richmond, CA) was used to test whether data were normally distributed. ANOVA with correction for the mean of the observations was used to test the validity of correlations between two variables. In all analyses, p < 0.05 was considered significant. All data are presented as mean ± SEM unless noted otherwise.
Drugs.
The NKCC1 inhibitor bumetanide was stored as a 50 mm stock solution in ethanol at 4°C (protected from light) and then diluted to 10 μm in ACSF daily. The α2/α3 Na+-K+-ATPase inhibitor dihydroouabain (DHO) was diluted directly into ACSF before each experiment. Bumetanide and DHO were bath applied. All chemicals were purchased from Sigma (St. Louis, MO) except QX-314 and CGP 55845A (Tocris, Ellisville, MO).
Junction potentials.
Junction potentials were calculated using pClamp 9.2 (Molecular Devices) and subtracted during data analysis: 0 mm Napipette, −3.7 mV; 9 mm Napipette, −3.5 mV; whole cell, −15.2 mV.
Results
NKCC1 is required for depolarizing GABA
The Na+:K+:Cl− transporter NKCC1 is highly expressed in neonatal neurons when GABA is depolarizing and excitatory (Dzhala et al., 2005). To test whether NKCC1 is required for neonatal hippocampal neurons to maintain elevated an elevated Cli and therefore depolarize in response to GABA, we measured the resting membrane potential (RMP) and E Cl of voltage-clamped hippocampal CA1 pyramidal cells from postnatal day 4–6 rats. Neurons were recorded in the absence and presence of 10 μm bumetanide, a selective inhibitor of NKCC1 (Gillen et al., 1996; Russell, 2000). We used the gramicidin-perforated patch technique to maintain the native Cli. Gramicidin channels are impermeable to anions such as Cl−, but allow cations (such as Na+ and K+) and small uncharged molecules to pass through (Akaike, 1996). The GABAA channel conducts anions, mainly Cl− and bicarbonate. To minimize the bicarbonate flux through the GABAA channel so that E GABA ≈ E Cl, we used HEPES-buffered (nominally CO2- and bicarbonate-free) ACSF in all experiments.
Figure 1 A illustrates the Cl− currents from a single neuron with the corresponding charge transfer–voltage relationship in the absence and presence of 10 μm bumetanide. V m was stepped to a test potential for 1 s. Two hundred milliseconds into the voltage step, 100 μm GABA was pressure applied to the apical dendrites ∼100 μm from the soma. Cl− currents were evoked at a new test potential every 10 seconds. At relatively hyperpolarized membrane potentials, the current is inward, which corresponds to Cl− efflux. At depolarized potentials, GABA evokes outward currents (Cl− influx). Cl− charge transfer was calculated as the area under the current curve after baseline subtraction. The charge transfer–voltage relationships and E Cl were calculated with the Goldman–Hodgkin–Katz constant field equation (Eq. 1). On average, Cl− currents reversed at −64.4 ± 4.4 mV, which corresponds to a Cli of 13.1 ± 2.0 mm. Mean RMP was −67.0 ± 1.6 mV. After the addition of 10 μm bumetanide, E Cl hyperpolarized to −77.6 ± 4.5 mV, corresponding to a Cli that was lowered by −37 ± 9% to 8.0 ± 1.5 mm (n = 5; p = 0.02). Bumetanide had no effect on the GABAA conductance (control: 20.3 ± 5.7 μS; +Bum: 25.0 ± 6.7 μS; n = 5; p = 0.62) or holding current (control: −24.7 ± 8.5 pA; +Bum: −31.6 ± 13.8 pA; n = 6; p = 0.47). All cells were recorded with 0 mm Na+ in the recording pipette (Napipette). These results indicate that NKCC1 is necessary for neonatal CA1 pyramidal cells to maintain elevated Cli and thus enable depolarizing responses to GABAA receptor activation.
Cl− transport quantified
NKCC1 is required for neonatal neurons to maintain elevated Cli and depolarize in response to GABA. However, NKCC1-mediated Cl− transport has not been demonstrated or characterized in the developing hippocampus, where it contributes to both normal development and seizures (Dzhala et al., 2005). We measured inward Cl− transport by transiently lowering Cli and monitoring Cli at 1 s intervals as it returned to baseline. The experimental protocol is illustrated in Figure 1 B. First, we measure steady-state (i.e., baseline) E Cl, as in Figure 1 A. Then, to measure Cl− transport, we applied a 1 s hyperpolarizing voltage step (usually 60 mV negative to E Cl). Two hundred milliseconds into the voltage step, GABA was puffed onto the apical dendrites. The rationale was that the hyperpolarized V m created a large driving force for Cl− efflux; in the small compartment of the dendrite, Cl− efflux will decrease Cli. For the cell in Figure 1 B, the Cli depletion current evokes a Cl− efflux (shown by the large inward current). V m was then stepped to a test potential near E Cl, and a second GABA puff was applied. We performed six trials of paired GABA puffs, each trial having a different time interval (range 1–6 s) between the Cli depleting current (first GABA puff) and the test current (second GABA puff). Immediately after Cli depletion when Cli is low, the first test current reflects Cl− influx. Over a few seconds, as Cli increases back to its steady-state level, the test currents reverse and return to baseline. Fifteen seconds elapsed between the each trial. We set t = 0 s to be the time at which the hyperpolarizing voltage step ends and V m is stepped to a potential near E Cl.
The charge transfer of each test current was calculated by measuring the area under the current waveform after baseline subtraction. Figure 1 C shows charge transfer as a function of time after Cli depletion for the cell illustrated in Figure 1 B. The first test current is positive, indicating a negative shift in E Cl immediately after Cli depletion. Within a few seconds, charge transfer returns to its negative baseline value. To estimate the Cli at each time interval, we calculated the change in E Cl that would account for the size and direction of the charge transfer when compared with baseline (please see Materials and Methods for further details). Figure 1 D shows that Cli rises back to its baseline value within a few seconds after Cli depletion for the cell in Figure 1, B and C.
NKCC1-mediated ion transport is reversible, meaning Cl− can be transported in or out of the cell (Russell, 2000). We estimated the rate of outward Cl− transport by calculating Cli as a function of time after an increase in Cli (“Cli load”). Figure 2 A illustrates the experimental protocol. To measure outward Cl− transport, we performed a similar experiment to that shown in Figure 1 B–D. The protocol was the same as in Figure 1 B, except that V m was stepped to a depolarizing potential (60 mV positive to E Cl) to provide a large driving force for Cl− influx to transiently raise Cli (“Cli load”). Then, at 1 s intervals, we measured the direction and charge transfer of GABA-evoked currents as described above. For the cell in Figure 2, when V m was depolarized to E Cl, GABA evoked a large outward current (inward Cl− flux). One second later, GABA evokes a large inward test current, which indicates that the Cli load induced a positive shift in E Cl. The size of the test currents decreases back to steady state over the next few seconds. Figure 2 B shows the charge transfer of the test currents recovering to baseline following the Cli load. Figure 2 C shows the Cli at each time interval after the Cli load, calculated based on the estimated change in E Cl compared with baseline. After a transient increase, Cli decreases back to its steady-state value within several seconds.
NKCC1 Cl− transport quantified
The techniques described above allow us to measure the rate at which Cli returns to baseline after a Cli depletion (Fig. 1 B–D) or load (Fig. 2). In these control conditions, Cli most likely returns to steady state by the combination of NKCC1-mediated transport plus non-NKCC1 mechanisms (such as diffusion from neighboring sections of dendrite). This is illustrated in Figure 3 A. To estimate the NKCC1-specific component of Cl− transport, we repeated the Cli load and depletion experiments in each cell after bath applying 10 μm bumetanide, a selective inhibitor of NKCC1. In the presence of 10 μm bumetanide, Cli recovers to baseline by non-NKCC1 mechanisms (Fig. 3 A). The method used to calculate NKCC1-mediated Cl− transport is illustrated in Figure 3 and described further in Materials and Methods. Figure 3 B shows that in control and bumetanide, Cli returns to baseline via a monoexponential kinetics (Eq. 4). Monoexponential rate constants (k = 1/τ) are additive, so the rate of NKCC1-specific transport (1/τNKCC1) was calculated by subtracting non-NKCC1 transport (1/τbumetanide) from the total (1/τcontrol). This calculation is shown in Figure 3 C. Figure 3 D shows averaged NKCC1-mediated Cl− transport. After an average decrease in dendritic Cli of −1.0 ± 0.3 mm, NKCC1 transports Cl− into the cell with the time constant (τNKCC1) of 0.6 ± 0.1 s and a v max of 1.6 ± 0.3 mm /s (n = 5; each recorded with 0 mm Napipette). When Cli was transiently increased by evoking the first GABA-gated current at a membrane potential positive to E Cl, monoexponential NKCC1-mediated transport kinetics were also demonstrated for outward Cl− transport when 9 mm Na+ was included in the perforated patch electrode solution. After an increase in dendritic Cli of +1.3 ± 0.4 mm, NKCC1 transported Cl− out of the cell with τNKCC1 = 0.9 ± 0.3 s and v max = 1.6 ± 0.5 mm/s (n = 3). For both inward and outward Cl− transport, NKCC1 activity caused Cli to relax to the same steady-state value of 12.9 ± 1.3 mm (n = 8). NKCC1 transport requires all three transported ions to be present on the side of the membrane from which ions are being transported (Russell, 2000). Consistent with this, no outward NKCC1-mediated Cl− transport took place in neurons recorded with perforated patch-pipette solution containing 0 mm Na+ (τ NKCC1 = 0.1 ± 2.6 s; n = 5). Data presented in Figures 1, 2, and 3 A–C are from single cells.
Thermodynamic regulation of NKCC1 and Cli
The mechanism by which NKCC1-mediated Cl− transport sets a particular Cli is unknown (Alvarez-Leefmans and Russell, 1990; Russell, 2000). Michaelis–Menten enzyme kinetics models describe the affinity of an enzyme for its substrate (K m) based on the substrate concentration (Clo) and the observed initial/maximum velocity of transport. These models are often used to model Cl− transport (Michaelis and Menten, 1913; Tas et al., 1987; Gasbjerg and Brahm, 1991; Lauf et al., 1992; Staley and Proctor, 1999). Therefore, we attempted to fit NKCC1-mediated Cl− transport using Michaelis–Menten kinetic models. Figure 4 A shows the average data of NKCC1-mediated inward Cl− transport from Figure 3 D fit with various transport models. We found that the initial/maximum velocity of Cl− transport was well described by the Michaelis–Menten model (Eq. 9). However, this model did not fit with the relaxation back to steady-state Cli that we observed (Fig. 4 A, inset), even when the model was modified to include noncompetitive or uncompetitive antagonism by intracellular chloride (data not shown).
Figure 4.
NKCC1 Cl− transport is thermodynamically regulated and analogous to Ohm's law, current flow = conductance × driving force. A, NKCC1-mediated inward Cl− transport is best fit by a modified Ohm's law model (Eq. 10) where Cl− transport velocity (analogous to current flow) equals Michaelis–Menten conductance multiplied by the driving force (ΔG/ΔG max; B). Data points are the same as presented in Figure 3 D (●), now fit with the thermodynamic model of transport (1 Na+:4 K+:5 Cl−; Eq. 10) rather than with a simple monoexponential function (Eq. 4). All cells were recorded with 0 mm Napipette. Inset, The driving force depends on the Na+:K+:Cl− transport stoichiometry (1:4:5, solid line). Other ratios (1:1:2, 1:2:3, and 1:3:4; dotted lines) and Michaelis–Menten kinetics alone (dashed line) do not describe NKCC1-mediated Cl− transport. B, For each transport ratio, the Cl− transport velocity (right ordinate) parallels the normalized driving force (left ordinate). As Cli returns to its steady-state value, the driving force for transport and the velocity of transport go to zero with the same time constant.
Another cation-chloride cotransporter, the neuronal K+-Cl− transporter KCC2 (Payne, 1997), is at or near thermodynamic equilibrium at the steady-state K+ and Cl− concentrations (Staley and Proctor, 1999). This means that no net transport takes place until a change in the electrochemical gradient of one of the transported ions creates a driving force for ion transport. This is illustrated by the fact that KCC2 Cl− transport follows a monoexponential relaxation back to the steady-state Cli after a Cli load or depletion (Staley and Proctor, 1999). We have observed this same behavior following Cli load or depletion with NKCC1-mediated Cl− transport (Fig. 3 D). This implies that NKCC1 transport may be thermodynamically regulated as well, meaning that ion transport only takes place when there is an electrochemical driving force to do so (Alvarez-Leefmans and Russell, 1990; Russell, 2000).
We tested the hypothesis that Cl− transport by NKCC1 is thermodynamically regulated by modeling transport as an analogy to Ohm's law [current = conductance × driving force (Eqs. 6, 10)] (Läuger, 1987). Current represents the rate of NKCC1-mediated Cl− transport; conductance represents the affinity of the transporter for substrate [calculated by the Michaelis–Menten equation (Eq. 9)], and driving force is provided by the combined electrochemical gradient of the transported ions as a function of the transport ratio (Eq. 7). The only free variables are the steady-state intracellular Na+ concentration (Nai) and K m, NKCC1's affinity for Cl−, which was consistently high in all experiments (<1 mm). Figure 4 A shows the NKCC1-mediated Cl− transport data modeled with various transport ratios. These are the same data points as those shown in Figure 3 D, now fit with the thermodynamic model (Eq. 10). The data are best described by a thermodynamic model incorporating a transport stoichiometry of 1 Na+:4 K+:5 Cl−. This model predicts thermodynamic equilibrium at a physiologically reasonable Nai of 3–5 mm (Rose et al., 1999) and steady-state Cli equal to the experimentally observed steady state. The inset to Figure 4 A shows that other transport ratios (1:1:2 and 1:2:3) predict Cli to be much higher than the steady-state value we observed. Figure 4 B shows that the driving force (ΔG) for Cl− transport and the Cl− transport velocity are maximal immediately following a Cli depletion. As NKCC1 transports Cl− into the cell, the driving force for transport, and therefore the velocity of transport, decrease to zero as Cli returns to steady state. In summary, the results presented thus far show that NKCC1 transports Cl− in the direction that allows the free energy of the combined Na+:K+:Cl− gradient to return to zero after a transient change in Cli.
Our results suggest that NKCC1 transport is regulated by the combined driving force created by the electrochemical gradients of the transported ions. We reasoned that if this were true, changing the transmembrane Na+ or K+ gradients should change the driving force for coupled Na+-K+-Cl− transport, and therefore, should change steady-state Cli (Eq. 8). Changes in Nai and Ko would have the most influence on E Na and E K, and thus the most influence on NKCC1-mediated transport and Cli; of the two, Nai is more labile in single-cell experiments performed in perfused slices. The inward Cl− transport data presented thus far have been from recordings using 0 mm Na+ in the recording pipette. To test the hypothesis that altering steady-state Nai changes steady-state Cli, we repeated our gramicidin-perforated patch measurements of NKCC1-mediated inward Cl− transport using a recording pipette solution containing Na+ (9 mm Napipette) (Rose and Ransom, 1997). We predicted that compared with recordings with 0 mm Napipette, increased Nai would decrease steady-state Cli by altering the combined electrochemical driving force for NKCC1 transport.
In contrast to our hypothesis, the steady-state Cli was not significantly changed (9 mm Napipette: Cli = 13.9 ± 1.1 mm; n = 6; 0 mm Napipette: Cli = 13.0 ± 1.5 mm; n = 8; p = 0.65). We had assumed that Nai would equal Napipette, but that is probably not the case. It has been shown that dialysis from the recording pipette solution is progressively less effective at changing ionic equilibria in dendrites because of transmembrane transport along the length of the dendrite (Rose and Ransom, 1997; Yu and Salter, 1998; Jarolimek et al., 1999). Consistent with this, RMP was significantly more negative with 9 mm Napipette (−74.0 ± 1.2 mV; n = 6) than with 0 mm Napipette (-66.1 ± 1.5 mV; n = 8; p = 0.002), indicating increased activity of an electrogenic transporter such as Na+-K+-ATPase in the cells recorded with 9 mm Napipette (Jarolimek et al., 1999). Values for Cli and RMP here differ slightly from those presented earlier in Results because this data set includes all cells in which we measured Cli and RMP, not just those cells in which we measured Cl− transport kinetics.
Although the steady-state Cli was unaffected by 9 mm Napipette, NKCC1-mediated inward Cl− transport was significantly slower (τNKCC1 = 2.2 ± 0.03 s; n = 3) than transport with 0 mm Napipette (τNKCC1 = 0.6 ± 0.1 s; n = 5; p = 0.000006). These data are shown in Figure 5 A.
Figure 5.
Intracellular sodium thermodynamically limits Cl− transport by NKCC1. A, NKCC1-mediated Cl− transport with recording pipette Na+ concentration (Napipette) of 0 mm (●, n = 5; same data as presented in Figs. 3 D, 4 A) or 9 mm (■, n = 3); fit lines were calculated as in Figure 4 A with a 1 Na+:4 K+:5 Cl− transport ratio (Eq. 10). Inset, The slower initial velocity and τ of NKCC1 Cl− transport with 9 mm Napipette can be accounted for by a transient decrease in the free energy available for transport (Fig. 4 B) arising from a transient increase in Nai that resolves with a time constant of 1.85 s (solid line). Ninety-five percent confidence interval is shown as dotted lines. B, With 9 mm Napipette, NKCC1 Cl− transport rate is independent of the size of the Cli depletion (■, R 2 = 0.067; n = 3; p = 0.83). With 0 mm Napipette, larger Cli depletions correlate with slower NKCC1 Cl− transport (●, R 2 = 0.99; n = 5; p = 0.0002). Each data point represents NKCC1 transport from one cell. There are two overlapping data points (●) at ∼35 pC.
The major regulator of Nai is Na+-K+-ATPase. Therefore, Na+ imported by NKCC1 during coupled Na+:K+:Cl− transport will be cleared from the dendrite via Na+-K+-ATPase activity. We hypothesized that the additional 9 mm Na+ in the recording pipette saturated the cell's Na+-K+-ATPase activity, which prevented the Na+ imported by NKCC1 from being rapidly cleared from the dendrite. We reasoned that the reduced ability to clear imported Na+ from the dendrite led to a transient Nai accumulation that reduced the driving force for further NKCC1-mediated transport. According to our thermodynamic model, further Na+-K+-Cl− transport would take place only as quickly as Na+-K+-ATPase and diffusion remove the excess Nai.
To test this hypothesis, we modeled the 9 mm Napipette Cl− transport data shown in Figure 5 A according to a modified version of our thermodynamic model. In previous experiments, Nai was a free variable with a single steady-state value. To test whether a transient increase in Nai could account for the difference in Cl− transport rate between 0 mm Napipette and 9 mm Napipette, we modified the model to allow Nai to change over time. We found that the slower transport rate with 9 mm Napipette was well described when the 0 mm Napipette Cl− transport data were combined with a transient increase in Nai that returns to baseline with a τ of 1.85 s. The predicted Nai transient is shown in the inset to Figure 5 A. The transient increase in Nai that predicts the observed transport rates is physiologically reasonable, which supports the idea that Nai and Na+-K+-ATPase are important regulators of NKCC1 transport, and therefore, Cli.
These results suggest that Na+ imported by NKCC1 itself can decrease the driving force for further NKCC1 transport if Na+ is not cleared from the dendrite quickly enough. According to our thermodynamic model, large Cli depletions create a large driving force for combined inward Na+:K+:Cl− transport. In the small compartment of the dendrite, a large Cli depletion could therefore increase Nai to the extent that further inward NKCC1 transport is inhibited.
To test the hypothesis that large Cli depletions correlate with slow transport, we examined the relationship between τNKCC1 and the size of the Cli depletion in recordings made with 0 or 9 mm Na+ in the recording pipette (Napipette). These data are shown in Figure 5 B. In recordings with 0 mm Napipette, reduced NKCC1 transport rate (increased τNKCC1) correlated with increased size of the Cli depletion (R 2 = 0.99; n = 5; p = 0.0002). This suggests that transient increases in Nai by NKCC1-mediated Na+:K+:Cl− transport limit the velocity of further NKCC1 transport. In contrast, no relationship between Cli depletion size and NKCC1 transport rate was seen in recordings with 9 mm Napipette (R 2 = 0.067; n = 3; p = 0.83). This is consistent with Na+ export (presumably by Na+-K+-ATPase) that was already saturated by the combination of pipette Na+ and the NKCC1-mediated Na+ influx triggered by even the smallest of Cli depletions. Together, these results support the thermodynamic model of NKCC1 Cl− transport and provide further evidence that Nai is a key regulator of NKCC1, and therefore, Cli.
Action potentials increase steady-state Cli and improve Na+ handling
Thus far, we have examined the thermodynamic regulation of NKCC1 transport, the major Cli accumulation mechanism in neonatal neurons. Our thermodynamic model of Cl− transport provides the tools to test specific hypotheses about activity-dependent changes in Cli. Consider the following: trains of action potentials have been shown to cause persistent increases in neuronal Cli (Woodin et al., 2003; Fiumelli et al., 2005). Trains of action potentials are also known to cause long-lasting increases in Na+-K+-ATPase activity, which lowers Nai (Ritchie and Straub, 1957; McDougal and Osborn, 1976; Thompson and Prince, 1986; Morita et al., 1993; Parker et al., 1996). The results we have presented thus far have shown that NKCC1 transport is thermodynamically regulated and sensitive to changes in Nai. If Nai can limit inward Cl− transport as our data suggest, then Na+-K+-ATPase activity, which sets Nai, must be integral to controlling the concentration of Cl− in the cell. Because trains of action potentials increase Na+-K+-ATPase activity, the changes in Cli observed following action potential trains could be caused by altered Na+ and K+ transmembrane gradients from increased Na+-K+-ATPase activity. A decreased steady-state Nai would shift NKCC1's thermodynamic equilibrium such that net Cl− transport would come to rest at a higher steady-state Cli (Eq. 8; see Fig. 7).
Figure 7.
Action potentials increase Cli by increasing Na+ export by Na+-K+-ATPase. Left, In control conditions, Na+-K+-ATPase activity determines the baseline Nai (gray liquid), which determines the rate of NKCC1 transport and therefore, Cli. Right, After a train of action potentials, increased α2/α3 Na+-K+-ATPase activity leads to a decrease in Nai. Low steady-state Nai increases steady-state Cli by shifting the driving force for NKCC1-mediated Cl− transport (Eq. 8).
We hypothesized that if NKCC1-mediated transport is thermodynamically limited, as our results suggest, changes in the Na+ and K+ gradients mediated by increased Na+-K+-ATPase activity should result in persistent changes in Cli. To test this hypothesis, steady-state E Cl and Cl− transport kinetics were measured before and after a 20 Hz, 2.5 min train of action potentials in the recorded cell (Fiumelli et al., 2005). Data from a representative cell are shown in Figure 6, A and B. The action potential train caused a persistent increase in Cli of 3.7 ± 0.5 mm following the action potentials (Cli before APs, 13.6 ± 3.1 mm; Cli after APs, 17.3 ± 3.4 mm; n = 3; p = 0.02). Based on steady-state Cli, the calculated steady-state Nai decreased from 2.7 mm before APs to 0.8 mm after APs for NKCC1 at thermodynamic equilibrium (Eq. 8). We monitored Cli in these cells for 27, 33, and 42 min following the train of action potentials. Therefore, in reference to these results, “persistent” means up to 42 min after action potentials. There was no change in holding current during that time (before APs: −22 ± 15 pA; after APs: −23 ± 13 pA; n = 3; p = 0.69). In control conditions, as in previous experiments with 9 mm Na+ in the patch pipette (Fig. 5 B), there was no correlation between the Cl− transport rate and the size of the Cli depletion (R 2 = 0.02; p = 0.87). However, in the same cells after the train of action potentials, slower Cl− transport correlated with larger Cli transients, as it had in previous experiments with 0 mm Na+ in the recording pipette. (R 2 = 0.97; p = 0.02). In other words, action potentials caused Cl− transport in neurons recorded with 9 mm Napipette to behave as if they were being recorded with 0 mm Napipette (compare with Fig. 5 B). These results imply that in control conditions, the Na+ load from the recording pipette had saturated the mechanisms by which Na+ is cleared from the dendrite. Therefore, the Na+ imported by NKCC1 after even the smallest Cli depletion was sufficient to slow down further inward Cl− transport. In contrast, after the action potentials, larger Cli depletions correlated with slower Cl− transport (as we saw in recordings with 0 mm Napipette). This implies a persistently lower Nai and/or an improved ability to rapidly clear Na+ from the dendrite. Either mechanism could be explained by an increase in Na+-K+-ATPase activity (Fig. 7).
α2/α3 Na+-K+-ATPase is required for activity-induced increase in Cli
We proposed that an activity-dependent increase in Na+-K+-ATPase activity was responsible for altering Nai and thus the Cli at which NKCC1 comes to electrochemical equilibrium. We reasoned that if this were true, then blocking Na+-K+-ATPase should prevent the persistent increase in Cli observed following trains of action potentials. We hypothesized that the “auxiliary” α2 and/or α3 Na+-K+-ATPase isoforms were responsible for the activity-induced change in Na+ handling by the cell and the change in Cl− transport. To test this hypothesis, we bath applied the selective α2/α3 Na+-K+-ATPase inhibitor DHO (10 μm), and then measured steady-state E Cl and Cl− transport kinetics before and after a 20 Hz, 2.5 min train of action potentials in the recorded cell. Representative data are illustrated in Figure 6 C.
In control conditions, DHO had no effect on Cli compared with control cells (control: 13.6 ± 3.1 mm; n = 3; DHO: 15.4 ± 3.1 mm; n = 4; p = 0.71). However, in the presence of DHO, action potential trains decreased steady-state Cli by 0.9 ± 0.02 mm (n = 4; p = 0.01), corresponding to an increase in Nai of ∼0.6 mm (Eq. 8). These results contrast with those from previous experiments performed without DHO in which action potentials increased Cli with a corresponding decrease in Nai. This implies that without functional α2/α3 Na+-K+-ATPase, the Na+ imported into the dendrite during the action potentials increased Nai to the point that the thermodynamic equilibrium for NKCC1 reset to a lower steady-state Cli (Fig. 7). Furthermore, in the presence of DHO, there was no relationship between the time constant of Cl− transport (τ) and the size of the Cli depletion, neither before nor after the train of action potentials [for the cell in Fig. 6 C, τ versus Cli depletion (pC): +DHO before APs: R 2 = 0.36 ± 0.97; p = 0.59; +DHO after APs: R 2 = 0 ± 0.68; p = 0.99]. In other words, selectively blocking the α2/α3 Na+-K+-ATPase with DHO prevented the train of action potentials from making transport with 9 mm Napipette more closely resemble transport with 0 mm Napipette. These results were in direct contrast to previous experiments showing that with intact α2/α3 Na+-K+-ATPase activity, the train action potentials induced a direct relationship between the rate of Cl− transport and the size of the Cli depletion (Fig. 6 B). These results indicate that α2/α3 Na+-K+-ATPase isoforms are required for trains of action potentials to bring about persistent increases in the steady-state Cli. These data also support the conclusion that NKCC1-mediated Cl− transport is controlled by the combined electrochemical gradient of the transported ions such that Cli is sensitive to changes in Nai.
After the addition of DHO, there were no significant changes in holding current (control: −8.5 ± 6.1 pA; +DHO: −7.2 ± 7.7 pA; n = 4; p = 0.59), input resistance (control: 159.7 ± 104.3 MΩ; +DHO: 204.8 ± 134.9 MΩ; n = 4; p = 0.24), or resting membrane potential (control: −64.0 ± 2.4 mV; +DHO: −63.9 ± 2.7 mV; n = 4; p = 0.90), implying that the α2/α3 isoforms do not significantly contribute to the cell's baseline functioning. Furthermore, DHO did not change action potential peak amplitude (control: 99.8 ± 0.01 mV; +DHO: 99.8 ± 0.01 mV; n = 4; p = 0.44) or half-width (control: 4.9 ± 1.1 ms; +DHO: 4.7 ± 0.2 ms; n = 4; p = 0.83), indicating that the lack of activity-dependent changes in the presence of DHO was not an artifact of defective action potential firing.
Finally, we tested the hypothesis that NKCC1 was necessary for the activity-induced increase in Cli. When NKCC1 was inhibited by 10 μm bumetanide, action potential trains had no effect on Cli (n = 2) (Fiumelli et al., 2005). Together, these results indicate that an activity-dependent increase in Na+-K+-ATPase activity alters the transmembrane gradients of Na+ and K+ such that a new equilibrium point is reached for NKCC1-mediated Cl− transport.
Discussion
In the present work, we show that neonatal rat CA1 pyramidal neurons require NKCC1 activity to maintain elevated Cli to the extent that GABA is depolarizing. When NKCC1 is selectively blocked with 10 μm bumetanide, the resting Cli drops by more than one-third to make GABA inhibitory. We show that after a transient decrease in Cli, NKCC1 transports Cl− into the cell, and after an increase in Cli, NKCC1 transports Cl− out of the cell. This is best explained by equilibrative transport: transient changes in Cli create a driving force for coupled Na+:K+:Cl− transport that diminishes to zero as Cli returns to steady state. In neonatal hippocampal pyramidal neurons, NKCC1 transport is thermodynamically regulated by the combined Na+, K+, and Cl− electrochemical gradients and is consistent with a net Na+:K+:Cl− transport ratio of 1:4:5.
We investigated activity-dependent changes in E Cl and found that trains of action potentials persistently increased the steady-state Cli and made Cl− transport with 9 mm Napipette more closely resemble transport with 0 mm Napipette (Fig. 7). Three Na+-K+-ATPase isoforms have been described in neurons. The α1 is uniformly expressed on the plasma membrane, whereas the α2 and α3 isoforms are expressed in a pattern on the plasma membrane that parallels the underlying endoplasmic reticulum (Juhaszova and Blaustein, 1997). The α1 isoform is thought to control the bulk intracellular Na+ concentration; the α2 and α3 subunits are considered “auxiliary” isoforms that are recruited during increased cellular activity (Blanco and Mercer, 1998). The α1 isoform has a low affinity for ouabain and is therefore unaffected by 10 μm DHO (McCarren and Alger, 1987; Sweadner, 1989; Berrebi-Bertrand et al., 1990), whereas the α2/α3 isoforms are relatively ouabain sensitive and selectively inhibited by 10 μm DHO (Gao et al., 1995; Juhaszova and Blaustein, 1997). Dihydroouabain prevented the activity-induced changes in Cli and the saturating Na+ condition for NKCC1 transport, indicating that α2/α3 isoforms of Na+-K+-ATPase were necessary for the activity-dependent increase in Cli. Plasticity of Na+-K+-ATPase activity is well established (Therien and Blostein, 2000), and is a logical means of altering neuronal signaling (Ross and Soltesz, 2001), but this is the first demonstration of involvement of Na+-K+-ATPase or NKCC1 in long-term synaptic plasticity.
This is also the first quantification of NKCC1-mediated Cl− transport in the neonatal hippocampus. Our results confirm and extend previous reports on the kinetics of NKCC1 transport in a variety of experimental systems (Russell, 2000). A benefit of the electrophysiology technique is that we observed NKCC1 Cl− transport with the time resolution of seconds, rather than minutes. Most estimates of NKCC1 transport rates come from measurement of bumetanide-sensitive 86Rb flux (Rb+ substitutes for K+). Reported transport rates are on the order of pmol · cm−2 · s−1. One pmol · cm−2 · s−1 corresponds to ∼14 mm/s in a dendrite of the geometry calculated in supplemental material (available at www.jneurosci.org). These rates of transport are compatible with the rates we observed, given the differences in the time resolution of the techniques, the levels of NKCC1 expression, and the ion gradients at which transport was measured. Our results are also consistent with the change in Cl− transport as a function of developmental age and level of synaptic activity (Ben Ari, 2002; Achilles et al., 2007).
The RMP measurements we report here are slightly depolarized compared with the gramicidin-perforated patch measurements by Tyzio et al. (2003). This could be attributable to the differences between CA3 and CA1 neurons or a difference between Wistar and Sprague Dawley rats, because it has been shown that Long–Evans and Sprague Dawley rats are 2 d off from each other developmentally (Talos et al., 2006). In the absence of NKCC1 (if there are no other mechanisms to change Cli), Cl− will be passively distributed (E Cl = RMP). Here, we show that E Cl is 10 mV negative to RMP in the absence of NKCC1 activity. It has been shown in neonatal CA3 pyramidal neurons that the RMP measured with gramicidin-perforated patch is depolarized compared with RMP measured with noninvasive cell-attached techniques (Tyzio et al., 2003). Tyzio et al. (2003) reported that in cell-attached recordings of CA3 pyramidal cells RMP was stable at −77 mV throughout development, which is the value we report here for E Cl in the absence of NKCC1 activity. It is possible that gramicidin recordings distort RMP locally (at the soma), with less distortion in dendrites, such that the dendritic RMP was more negative than our measured somatic RMP (Tyzio et al., 2003).
KCC2 mediates mostly outward Cl− transport in mature neurons; low levels of expression in this age group could account for the hyperpolarized E Cl in the absence of NKCC1 activity (Dzhala et al., 2005). The kinetics we describe for NKCC1 are similar to those reported for KCC2 (Staley and Proctor, 1999). Both transporters have maximum transport rates in the range of mmol · L−1 · s−1, which has important implications for a “push–pull” model of Cl− homeostasis, where the two transporters could work in concert to maintain steady-state Cli. This hypothesis of coupled inward and outward Cl− transport is attractive from the point of view of precise Cli control. However, oppositely directed transporters operating at these velocities would waste enormous amounts of ATP so that Na+-K+-ATPase could maintain the necessary transmembrane ion gradients. We have applied 100 μm bumetanide, a concentration that inhibits KCC2, at the end of some experiments (data not shown), and have not observed any change in Cli beyond what was seen with 10 μm bumetanide, suggesting that KCC2 does not contribute significantly to Cli in the neonatal period.
Action potentials caused a persistent increase in Cli that required intact α2/α3 Na+-K+-ATPase activity. It has been shown that the increase in Na+-K+-ATPase activity that occurs following neuronal activity is transient (Thompson and Prince, 1986). Consistent with this, there was no change in holding current up to 42 min following the train of action potentials, suggesting that Na+-K+-ATPase activity was not persistently increased. This implies that once a new lower steady-state Nai has been reached, the Na+ pump does not need to continue running at an increased rate to maintain the lower steady-state Nai. However, we cannot exclude a longer-lasting change in α2/α3 Na+-K+-ATPase activity because once Nai is low, the pump current may be so small that it does not make a significant contribution to the resting membrane potential and holding current.
Inducible modifications of E GABA mediated by activity-dependent alterations in Na+-K+-ATPase activity represents a fundamental new motif of long-term synaptic plasticity. There are undoubtedly many mechanisms underlying long-term plasticity of GABA signaling as a result of persistent changes in E GABA, and these mechanisms are likely to vary depending on the age and conditions under which plasticity is studied (van den Pol et al., 1996; Cohen et al., 2002; Coull et al., 2003, 2005; Khalilov et al., 2003; Woodin et al., 2003; Fiumelli et al., 2005). For example, changes in NKCC1 and KCC2 phosphorylation (Kahle et al., 2005) and membrane trafficking (Del Castillo et al., 2005) as well as changes in KCC2 activity have been demonstrated to be important under appropriate conditions (De Koninck, 2007). Here we establish an entirely new mechanism: activity-dependent long-term changes in E GABA in the developing nervous system can be caused by activity-dependent changes in Na+-K+-ATPase activity (McDougal and Osborn, 1976; Thompson and Prince, 1986; Ross and Soltesz, 2001), which alter the Cli at which a secondary active Cl− transporter is at equilibrium (Fig. 7). Our findings emphasize the variety of mechanisms that may contribute to plasticity of E GABA and the caution that should be used in extrapolating such mechanisms from one experimental condition to another. With these cautions in mind, our findings may be relevant to the correlated circadian fluctuations in E Cl and Na+-K+-ATPase activity observed in hypothalamic suprachiasmatic nucleus neurons (Wagner et al., 1997; Wang and Huang, 2004). Finally, the finding that NKCC1 is at thermodynamic equilibrium at the steady-state Cli in neurons suggests caution in the interpretation of E GABA determined by gramicidin-perforated patch recordings, because these recordings may indirectly alter E Cl by altering Na+ and K+ gradients.
Footnotes
We thank Javier Alvarez-Leefmans for his helpful feedback.
References
- Abe Y, Furukawa K, Itoyama Y, Akaike N. Glycine response in acutely dissociated ventromedial hypothalamic neuron of the rat: new approach with gramicidin perforated patch-clamp technique. J Neurophysiol. 1994;72:1530–1537. doi: 10.1152/jn.1994.72.4.1530. [DOI] [PubMed] [Google Scholar]
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Yamada J, Fukuda A, Luhmann HJ, Kilb W. Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons. J Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. doi: 10.1016/s0079-6107(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Russell JM. New York: Plenum; 1990. Chloride channels and carriers in nerve, muscle, and glial cells. [Google Scholar]
- Ben Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berrebi-Bertrand I, Maixent JM, Christe G, Lelievre LG. Two active Na+/K+-ATPases of high affinity for ouabain in adult rat brain membranes. Biochim Biophys Acta. 1990;1021:148–156. doi: 10.1016/0005-2736(90)90027-l. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol (Lond) 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl− cotransporter expression in rat neocortex. Brain Res Dev Brain Res. 1998;109:281–292. doi: 10.1016/s0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Coull JAM, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7:93–99. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Del Castillo IC, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, Matthews JB. Dynamic regulation of Na+-K+-2Cl− cotransporter surface expression by PKC-ε in Cl−-secretory epithelia. Am J Physiol Cell Physiol. 2005;289:C1332–C1342. doi: 10.1152/ajpcell.00580.2004. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol (Lond) 1995;484:77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumelli H, Cancedda L, Poo Mm. Modulation of GABAergic transmission by activity via postsynaptic Ca2+-dependent regulation of KCC2 function. Neuron. 2005;48:773–786. doi: 10.1016/j.neuron.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Gao J, Mathias RT, Cohen IS, Baldo GJ. Two functionally different Na/K pumps in cardiac ventricular myocytes. J Gen Physiol. 1995;106:995–1030. doi: 10.1085/jgp.106.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbjerg PK, Brahm J. Kinetics of bicarbonate and chloride transport in human red cell membranes. J Gen Physiol. 1991;97:321–349. doi: 10.1085/jgp.97.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geck P, Pietrzyk C, Burckhardt BC, Pfeiffer B, Heinz E. Electrically silent cotransport on Na+, K+ and Cl− in Ehrlich cells. Biochim Biophys Acta. 1980;600:432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Gillen CM, Brill S, Payne JA, Forbush B., III Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J Biol Chem. 1996;271:16237–16244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Toyoda H, Yamada J, Okabe A, Sato K, Hotta Y, Fukuda A. Differential development of cation-chloride cotransporters and Cl− homeostasis contributes to differential GABAergic actions between developing rat visual cortex and dorsal lateral geniculate nucleus. Brain Res. 2003;984:149–159. doi: 10.1016/s0006-8993(03)03126-3. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Lewen A, Misgeld U. A furosemide-sensitive K+-Cl− cotransporter counteracts intracellular Cl− accumulation and depletion in cultured rat midbrain neurons. J Neurosci. 1999;19:4695–4704. doi: 10.1523/JNEUROSCI.19-12-04695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Rinehart J, los Heros P, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl− in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci USA. 2005;102:16783–16788. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Lauf PK, Bauer J, Adragna NC, Fujise H, Zade-Oppen AM, Ryu KH, Delpire E. Erythrocyte K+-Cl− cotransport: properties and regulation. Am J Physiol. 1992;263:C917–C932. doi: 10.1152/ajpcell.1992.263.5.C917. [DOI] [PubMed] [Google Scholar]
- Läuger P. Dynamics of ion transport systems in membranes. Physiol Rev. 1987;67:1296–1331. doi: 10.1152/physrev.1987.67.4.1296. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Marty S, Wehrle R, Alvarez-Leefmans FJ, Gasnier B, Sotelo C. Postnatal maturation of Na+, K+, 2Cl− cotransporter expression and inhibitory synaptogenesis in the rat hippocampus: an immunocytochemical analysis. Eur J Neurosci. 2002;15:233–245. doi: 10.1046/j.0953-816x.2001.01854.x. [DOI] [PubMed] [Google Scholar]
- McCarren M, Alger BE. Sodium-potassium pump inhibitors increase neuronal excitability in the rat hippocampal slice: role of a Ca2+-dependent conductance. J Neurophysiol. 1987;57:496–509. doi: 10.1152/jn.1987.57.2.496. [DOI] [PubMed] [Google Scholar]
- McDougal DB, Osborn LA. Post-tetanic hyperpolarization, sodium-potassium-activated adenosine triphosphatase and high energy phosphate levels in garfish olfactory nerve. J Physiol (Lond) 1976;256:41–60. doi: 10.1113/jphysiol.1976.sp011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis L, Menten M. Die kinetik der invertinwirkung. Biochem Zeitschrift. 1913;49:333–369. [Google Scholar]
- Morita K, David G, Barrett JN, Barrett EF. Posttetanic hyperpolarization produced by electrogenic Na+-K+ pump in lizard axons impaled near their motor terminals. J Neurophysiol. 1993;70:1874–1884. doi: 10.1152/jn.1993.70.5.1874. [DOI] [PubMed] [Google Scholar]
- Parker D, Hill R, Grillner S. Electrogenic pump and a Ca2+- dependent K+ conductance contribute to a posttetanic hyperpolarization in lamprey sensory neurons. J Neurophysiol. 1996;76:540–553. doi: 10.1152/jn.1996.76.1.540. [DOI] [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Ritchie JM, Straub RW. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol (Lond) 1957;136:80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Regulation of intracellular sodium in cultured rat hippocampal neurones. J Physiol. 1997;499:573–587. doi: 10.1113/jphysiol.1997.sp021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Kovalchuk Y, Eilers J, Konnerth A. Two-photon Na+ imaging in spines and fine dendrites of central neurons. Pflugers Arch. 1999;439:201–207. doi: 10.1007/s004249900123. [DOI] [PubMed] [Google Scholar]
- Ross ST, Soltesz I. Long-term plasticity in interneurons of the dentate gyrus. Proc Natl Acad Sci USA. 2001;98:8874–8879. doi: 10.1073/pnas.141042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Proctor WR. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl− and HCO3 − . J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol. 2006;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas PW, Massa PT, Kress HG, Koschel K. Characterization of an Na+/K+/Cl− co-transport in primary cultures of rat astrocytes. Biochim Biophys Acta. 1987;903:411–416. doi: 10.1016/0005-2736(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Prince DA. Activation of electrogenic sodium pump in hippocampal CA1 neurons following glutamate-induced depolarization. J Neurophysiol. 1986;56:507–522. doi: 10.1152/jn.1986.56.2.507. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J Neurophysiol. 2003;90:2964–2972. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Obrietan K, Chen G. Excitatory actions of GABA after neuronal trauma. J Neurosci. 1996;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Ohno K, Furukawa T, Ueki T, Ikeda M, Fukuda A, Sato K. Differential expression of KCC2 accounts for the differential GABA responses between relay and intrinsic neurons in the early postnatal rat olfactory bulb. Eur J Neurosci. 2005;21:1449–1455. doi: 10.1111/j.1460-9568.2005.03975.x. [DOI] [PubMed] [Google Scholar]
- Wang HY, Huang RC. Diurnal modulation of the Na+/K+-ATPase and spontaneous firing in the rat retinorecipient clock neurons. J Neurophysiol. 2004;92:2295–2301. doi: 10.1152/jn.00061.2004. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Yu XM, Salter MW. Gain control of NMDA-receptor currents by intracellular sodium. Nature. 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]








