Abstract
It is well accepted that fear extinction does not cause erasure of the original conditioned stimulus (CS)–unconditioned stimulus association in the adult rat because the extinguished fear often returns (e.g., renewal and reinstatement). Furthermore, extinction is NMDA and GABA dependent, showing that extinction involves new inhibitory learning. We have recently observed each of these extinction-related phenomena in 24-d-old but not in 17-d-old rats. These results suggest that different neural processes mediate extinction early in development. However, the neural processes underlying extinction in the developing rat are unknown. Therefore, the present study investigated amygdala involvement in extinction and reextinction during development. In experiment 1, temporary inactivation of the amygdala (using bupivacaine, a sodium channel modulator) during extinction training impaired extinction of conditioned fear in 17- and 24-d-old rats. In experiment 2, 17- and 24-d-old rats were conditioned, extinguished, and then reconditioned to the same CS. After reconditioning, the CS was reextinguished; at this time, some rats at each age had their amygdala temporarily inactivated. Reextinction was amygdala independent in 24-d-old rats, as previously shown in adult rats. However, reextinction was still amygdala dependent in 17-d-old rats. In Experiment 3, the age at conditioning, reconditioning, reextinction, and test was held constant, but the age of initial extinction varied across groups; reextinction was found to be amygdala independent if initial extinction occurred at 24 d of age but amygdala dependent if it occurred at 17 d of age. Consistent with previous findings, these results show that there are fundamental differences in the neural mechanisms of fear extinction across development.
Keywords: extinction, developing rat, amygdala, unlearning, reextinction, fear
Introduction
Extinction refers to the decrease in conditioned response (CR) to a previously trained conditioned stimulus (CS) after nonreinforced presentations of the CS. Fear extinction, in particular, has received considerable attention over the past decade, because of its theoretical importance and its obvious clinical implications for the treatment of various anxiety disorders (Davis and Myers, 2002). Compared with fear acquisition, however, we have a relatively poor understanding of the mechanisms mediating fear extinction. Early theoretical models suggested that extinction was caused by the “unlearning” or “erasure” of the original fear memory (Rescorla and Wagner, 1972). However, it is now more widely accepted that the reduced CR after extinction reflects learning of a second, competing association that inhibits the expression of the original association (Bouton, 2002). The primary evidence for this view comes from behavioral studies showing that performance to an extinguished CS recovers without any retraining (e.g., spontaneous recovery, renewal, and reinstatement).
Interestingly, recent studies suggest that extinction occurring early in development is not caused by new learning. These studies report that 17-d-old rats fail to show renewal or reinstatement of extinguished fear, whereas 24-d-old rats do (all age labels in the present study refer to the age at extinction training) (Kim and Richardson, 2007a,b). Also, reducing GABA-inhibitory activity by pretest injections of the GABA receptor inverse agonist FG7142 has no effects on extinguished fear in 17-d-old rats, but reverses extinction in 24-d-old rats (Kim and Richardson, 2007a) and in adult rats (Harris and Westbrook, 1998). Further, preextinction injection of the NMDA antagonist MK-801 has no effect on long-term extinction in 17-d-old rats, whereas long-term extinction in 24-d-old rats is impaired (Langton et al., 2007), as in adult rats (Baker and Azorlosa, 1996).
In the studies described above, the levels of fear learning and the rate of extinction did not differ between age groups. Hence, the developmental dissociations observed in extinction are not attributable to potential quantitative differences in fear learning or extinction in these ages. Rather, it appears that qualitatively different neural mechanisms underlie extinction across development. To date, however, no one has explicitly examined whether the neural structures underlying fear extinction in adult rats are also crucial for extinction in the developing rat. In adult rats, the amygdala is an important neural structure for extinction. Studies show that blocking various neurotransmitter systems in the amygdala during extinction training disrupts long-term extinction [for review, see Barad et al. (2006) and Quirk and Mueller (2007)]. For example, intra-amygdala infusions of the NMDA receptor antagonist d,l-2-amino-5-phosphonopentanoic acid (AP5) before extinction training disrupts extinction (Falls et al., 1992). This result, and many others, indicates that extinction requires an intact amygdala in the adult rat. Therefore, the present study used a temporary inactivation technique to disrupt amygdala function during extinction training to investigate the neural mechanisms of extinction in the developing rat.
Materials and Methods
Experiments 1A and 1B
Subjects.
Experimentally naive Sprague Dawley-derived rats, bred and housed in the School of Psychology, The University of New South Wales, were used. Rats were either 23 ± 1 or 16 ± 1 d of age at the start of experiment 1A and 1B, respectively. All rats were male, and no more than one rat per litter was used per group. Rats were housed with their littermates and mother in plastic boxes (24.5 cm long × 37 cm wide × 27 cm high) covered by a wire lid. Animals were maintained on a 12 h light/dark cycle (lights on at 6 A.M.) with food and water available ad libitum. Animals were treated according to the principles of animal use outlined in The Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (Seventh Edition), and all procedures were approved by the Animal Care and Ethics Committee at The University of New South Wales.
Surgery.
Rats were anesthetized with isoflurane (Laser Animal Health, Queensland, Australia) mixed with oxygen and nitrous oxide. The gas was delivered via a single tube attached to a nosepiece (Stoelting, Wood Dale, IL) that fit onto a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Twenty-six-gauge guide cannulas (Bioscientific, Sydney, Australia) were bilaterally implanted between the central and the basolateral nucleus of the amygdala. The stereotaxic coordinates used for experiment 1A (surgery age: 22 d) were 2.1 mm posterior, ±4.3 mm lateral, and 7.4 mm ventral to bregma; and for experiment 1B (surgery age: 15 d), 1.9 mm posterior, ±4.1 mm lateral, and 7.0 mm ventral to bregma. The guide cannulas were fixed directly onto the skull with cyanoacrylate glue and were surrounded with dental cement. Cannula stylets were inserted to prevent clogging of the guide cannulas. After surgery, rats were injected subcutaneously with 0.03 ml of a 100 mg/ml solution of cephazolin and of a 300 mg/ml solution of benacillin. While the rats were undergoing surgery, the dam was removed from the home cage. After surgery, the pups were placed back into the home cage and given ∼30 min for temperature and skin odors to return to normal before the dam was returned [for additional details, see Hofer (1991)]. It has previously been shown that rats these ages recover from surgery well within 24 h (Weber and Richardson, 2001). Rats were decapitated <1 h after the final test, and tissue sections were collected and stained for Nissl substance for verification of cannula placement.
Drug infusion.
For infusion of bupivacaine hydrochloride (5% w/v; Delta West, Perth, Australia) or saline, the cannula stylets were removed and 33-gauge injection cannulas, extending 1 mm below the tip of the guide cannula, were inserted. The injection cannulas were connected to a microsyringe driven by a microinfusion pump (syringe pump SP101IZ; World Precision Instruments, Sarasota, FL) via PE50 tubing. Rats were placed in individual plastic buckets, and solution (0.5 μl per hemisphere) was delivered over 2 min. The injection cannulas were left in position for an additional 2 min before withdrawal to minimize dragging of the solution along the injection track.
Bupivacaine, like lidocaine, is an amide-linked local anesthetic that binds to the intracellular portion of sodium channels and blocks sodium influx into nerve cells, which prevents depolarization. However, it is more lipophilic than lidocaine and, hence, has a longer duration of action (Caterall and Mackie, 1996).
Apparatus.
A set of two identical chambers that were rectangular (30 cm long × 30 cm wide × 23 cm high) and wholly constructed of Plexiglas, with the exception of the grid floor (3 mm stainless steel rods set 1 cm apart), were used. A custom-built constant-current shock generator could deliver electric shock to the floor of each chamber as required. Two side walls consisted of vertical black and white stripes (5 cm each), and the other two walls and the ceiling were transparent. Two high-frequency speakers were mounted on the ceiling of each of these chambers. Each chamber was housed within a separate wood cabinet so that external noise and visual stimulation were minimized. White and red light-emitting diodes located on the cabinet door were the sole sources of illumination in these chambers. A low, constant background noise (48 dB; measured by Bruel Kjaer sound level meter; type 2235, A scale) was produced by ventilation fans located within the cabinets.
The CS was a white noise; noise level in the chambers was increased by 8 dB when the CS was presented. A computer controlled all presentations of the CS and the footshock unconditioned stimulus (US) (0.6 mA, 1 s). The software and hardware used were developed at The University of New South Wales.
Procedure.
All rats received surgery 1 d before training. For training on day 1, rats were placed in an experimental chamber, and after a 2 min adaptation period, the CS was presented for 10 s. The shock US was administered during the last second of the CS. Rats at 23 d of age received three pairings of the CS and US, whereas 16-d-old rats received six pairings (to equate initial levels of freezing at extinction). The intertrial interval (ITI) ranged from 85 to 135 s with a mean of 110 s.
On day 2, rats received extinction training. Six minutes before extinction, rats received bilateral amygdala infusion of either saline or bupivacaine. Extinction training consisted of a 2 min adaptation period and then 30 presentations of the 10 s CS in the absence of shock (ITI = 10 s).
For test on day 3, rats were placed in an experimental chamber, and their baseline level of freezing in the absence of the CS was recorded for 1 min. The CS was then presented for 2 min.
For extinction training and test, freezing was scored by a time-sampling procedure whereby each rat was scored every 3 s as freezing or not freezing. The percentage of observations scored as freezing was then determined. Freezing was defined as the absence of all movement other than that required for respiration (Fanselow, 1980). A second scorer unaware of the experimental condition of each rat scored a random sample (25%) of all rats tested in this study. The interrater reliability was high (r = 0.93).
Experiments 2A and 2B
Subjects.
Subjects were male Sprague Dawley-derived rats obtained and housed as described in experiment 1. Rats were 23 ± 1 and 16 ± 1 d of age at the start of experiment 2A and 2B, respectively.
Surgery and behavioral apparatus.
Surgical procedures and behavioral apparatus were as described in experiment 1, except that slightly different stereotaxic coordinates were used because rats received surgery at different ages in this experiment. Stereotaxic coordinates for experiment 2A (surgery age, 25 d) remained the same as in experiment 1A, whereas for experiment 2B (surgery age, 18 d), the coordinates were −2.0 mm posterior, ±4.3 mm lateral, and 7.4 mm ventral to bregma.
Procedure and intracranial drug infusions.
Rats were fear conditioned (day 1) and extinguished (day 2) as described in experiment 1. On day 3, all rats had cannulas surgically implanted. For retraining on day 4, rats received two pairings of the CS and US. On day 5, two groups at each age received reextinction training (parameters were identical to the initial extinction training); 6 min before reextinction, rats in one group received bilateral amygdala infusions of saline, whereas rats in the other group were infused with bupivacaine. Rats in the nonreextinguished control group received infusions (half with saline and half with bupivacaine) and then were exposed to the experimental chamber for an equal amount of time as the other groups, but without any CS presentations. On day 6, all rats were tested as described in experiment 1.
Experiment 3
Subjects.
Subjects were male Sprague Dawley-derived rats obtained and housed as described in experiment 1. All rats were 16 ± 1 d of age at the start of the experiment.
Surgery and behavioral apparatus.
Surgical procedures and behavioral apparatus were as described in experiment 1, and the stereotaxic coordinates used were the same as in experiments 1A and 2A.
Procedure and intracranial drug infusions.
Experiment 3 was a 2 (drug: saline vs bupivacaine at reextinction) × 2 (initial extinction age: 17 vs 24) factorial design. At 16 d of age, four groups of rats were fear conditioned as described in experiment 1. Then two groups (17-saline and 17-bupivacaine) were given extinction training at 17 d of age, whereas the other two groups (24-saline and 24-bupivacaine) received extinction training at 24 d of age. Extinction training, whenever it occurred, was as described in experiment 1. All rats received cannula placement surgery at 25 d of age. On the day after surgery, all rats were retrained as described in experiment 2. At 27 d of age, rats received bilateral amygdala infusions of either saline or bupivacaine 6 min before reextinction as described in experiment 2. All rats were tested 1 d after the reextinction session as described in experiment 2.
Results
Baseline freezing and histology
No significant differences in baseline freezing levels at test were detected in any experiment (Table 1). A total of 130 rats were used in this study. Data from 24 rats were excluded from analysis either for sustaining amygdala damage or misplacement of cannula(s). Cannula placements for experiment 1 can be seen in Figure 1. The placements were comparable across all experiments.
Table 1.
Mean ± SEM levels of baseline freezing at test for all groups across all experiments
| Experiment | Groups | Mean percentage freezing at baseline |
|---|---|---|
| 1A | Saline | 15 ± 5 |
| Bupivacaine | 28 ± 7 | |
| 1B | Saline | 6 ± 4 |
| Bupivacaine | 19 ± 6 | |
| 2A | Control | 36 ± 6 |
| Saline | 24 ± 6 | |
| Bupivacaine | 24 ± 9 | |
| 2B | Control | 19 ± 11 |
| Saline | 19 ± 5 | |
| Bupivacaine | 30 ± 5 | |
| 3 | 17-Saline | 32 ± 7 |
| 17-Bupivacaine | 32 ± 7 | |
| 24-Saline | 30 ± 7 | |
| 24-Bupivacaine | 30 ± 8 |
No significant differences were found between any groups.
Figure 1.
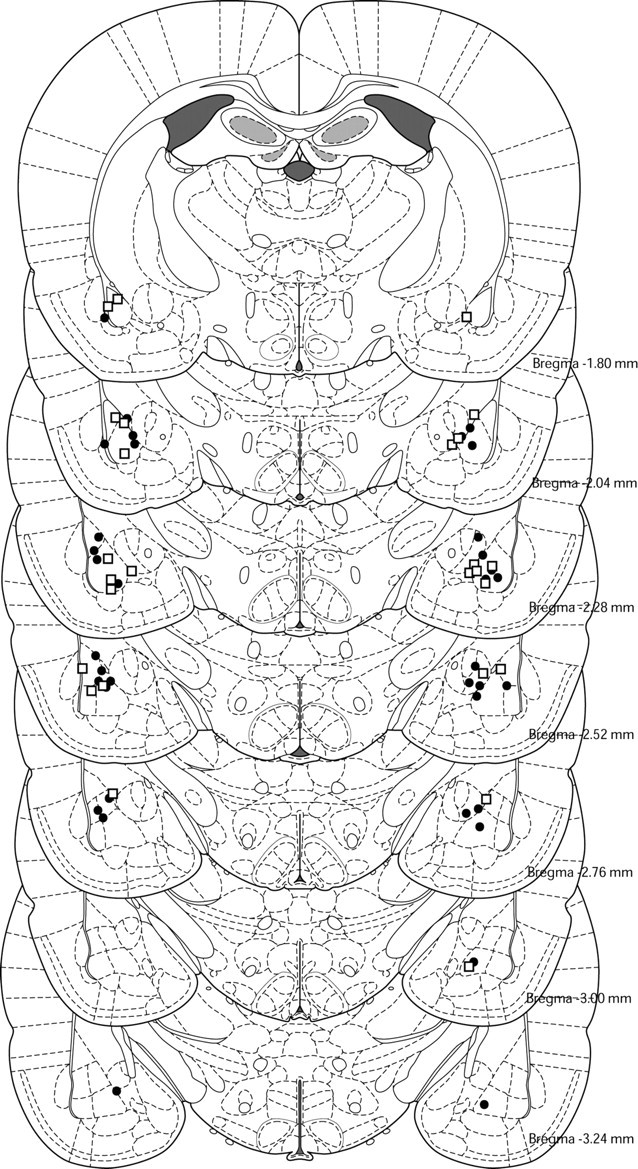
Histological reconstruction of coronal sections showing cannula placements in the amygdala for experiments 1A (●) and 1B (□).
Experiments 1A and 1B: extinction is amygdala dependent in both 17- and 24-d-old rats
Recent findings show that 17-d-old rats, in contrast to 24-d-old rats, fail to show extinction-related phenomena such as reinstatement or renewal (Kim and Richardson, 2007a,b; Yap and Richardson, 2007). Further, drugs that disrupt long-term extinction (i.e., MK-801) or extinction expression (i.e., FG7142) in the adult rat have no observed effects on extinction in 17-d-old rats (Kim and Richardson, 2007a, Langton et al., 2007). These results suggest that different neural processes mediate extinction in younger compared with older rats. Previous research using adult rats has consistently found that fear extinction is dependent on the amygdala (Falls et al., 1992); therefore, experiment 1 examined whether the amygdala is also involved in extinction in the developing rat by using a temporary inactivation technique. Rats at 16 and 23 d of age (in experiments 1A and 1B, respectively) were first trained to fear an auditory CS by pairing it with a shock US. Twenty-four hours later, rats at each age received intra-amygdala infusions of either saline or bupivacaine before extinction training. They were then tested for extinction retention 24 h later, drug-free. Previous studies have shown that with these parameters, the level of initial learning and the rate of extinction are very comparable across these two ages (Kim and Richardson, 2007a,b; Langton et al., 2007).
It is worth noting that in this study we did not aim at a particular nucleus in the amygdala because virtually nothing is known about the neural structures involved in fear extinction in the developing rat. For the same reason, we used a local anesthetic bupivacaine, rather than a drug that acts on a specific neurotransmitter system (e.g., NMDA antagonist AP5). Bupivacaine, and other sodium-channel modulators such as tetrodotoxin, has previously been used to reversibly inactivate brain areas such as the nucleus accumbens, the amygdala, and the ventral medial prefrontal cortex (vmPFC) during fear-conditioning tasks in rats (Haralambous and Westbrook, 1999; Weber and Richardson, 2004; Corcoran and Quirk, 2007).
Experiment 1A results
Group saline exhibited substantial levels of freezing to the CS in the first minute of extinction (a block of three trials) that decreased substantially by the last minute, whereas group bupivacaine exhibited very low levels of freezing throughout extinction, indicating that the amygdala was inactivated in this group (Fig. 2 A, left). A mixed-design ANOVA of the extinction data yielded significant main effects of block (F (1,16) = 21.32; p < 0.0001) and group (F (1,16) = 37.51; p < 0.0001), as well as a block × group interaction (F (1,16) = 9.10; p < 0.01).
Figure 2.
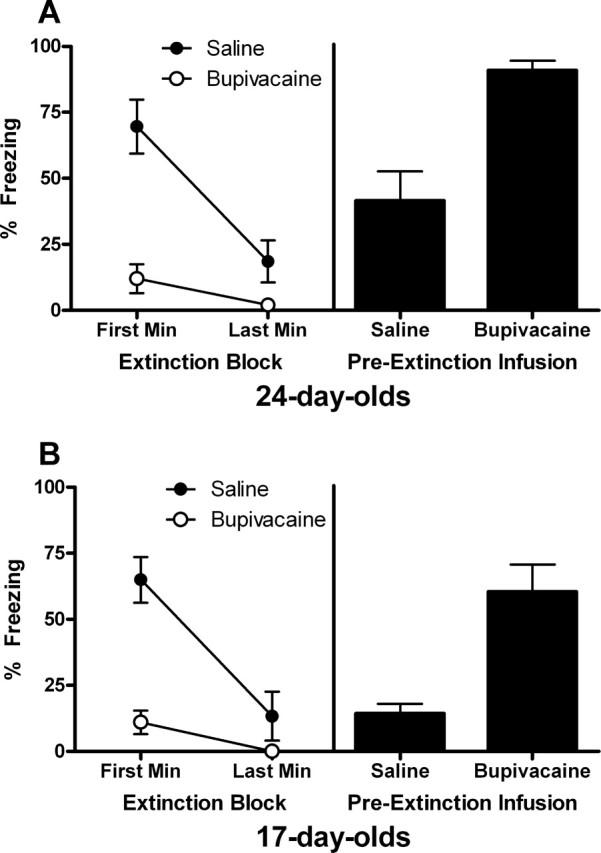
A, Extinction (left) and test (right) data for experiment 1A. Twenty-four-day-old rats exhibited substantial levels of freezing in the first minute of extinction (a block of 3 CS trials) that decreased substantially by the last minute when infused with saline, whereas group bupivacaine exhibited very low levels of freezing throughout extinction. At test, 24-d-old rats that received preextinction infusion of bupivacaine exhibited substantially higher levels of freezing (drug-free) compared with rats that received infusion of saline. This result indicates that the amygdala is necessary for extinction in 24-d-old rats. B, Extinction (left) and test (right) data for experiment 1B. Seventeen-day-old rats exhibited a substantial level of freezing in the first block of extinction that decreased substantially by the last minute when infused with saline, whereas infusion of bupivacaine blocked freezing throughout extinction. Temporary inactivation of the amygdala at extinction impaired extinction at test in 17-d-old rats, as shown by higher levels of freezing in rats that received infusion of bupivacaine compared with saline (right). This result indicates that the amygdala is necessary for extinction in 17-d-old rats.
The mean levels of CS-elicited freezing at test are shown in Figure 2 A (right). Rats that received a preextinction infusion of bupivacaine exhibited substantially higher levels of freezing at test (drug-free) compared with rats that had received saline (t (16) = 4.46; p < 0.005). This result shows that temporary inactivation of the amygdala during extinction training disrupts long-term extinction in 24-d-old rats.
Experiment 1B results
As in experiment 1A, group saline exhibited a substantial level of freezing in the first block of extinction that decreased substantially by the last minute, whereas group bupivacaine exhibited very low levels of freezing throughout extinction (Fig. 2 B, left). A mixed-design ANOVA of the extinction data yielded significant main effects of block (F (1,11) = 28.24; p < 0.0001) and group (F (1,11) = 35.36; p < 0.0001), as well as a block × group interaction (F (1,11) = 11.89; p < 0.005).
The mean levels of CS-elicited freezing at test are shown in Figure 2 B (right). Temporary inactivation of the amygdala during extinction training impaired long-term extinction at test, as shown by higher levels of freezing in rats that had received an infusion of bupivacaine compared with those given saline (t (11) = 4.23; p < 0.005). This result shows that the amygdala is necessary for extinction in 17-d-old rats.
Experiments 2A and 2B: reextinction is amygdala independent in 24-d-old rats but amygdala dependent in 17-d-old rats
Experiments 1A and 1B demonstrated that extinction in the developing rat is dependent on the amygdala, as it is with adult rats. Contrary to recent behavioral studies illustrating developmental differences in extinction, this result suggests that the role of the amygdala in extinction is the same, or at least very similar, across development. To further examine the role of the amygdala in extinction in the developing rat, in the next set of experiments we examined the effect of temporary inactivation of the amygdala in reextinction. In reextinction, rats are reconditioned to the previously extinguished CS and then reextinguished. This preparation has been used in previous studies to elucidate the neural mechanisms behind the initial extinction training (Morgan et al., 2003) (M. Weber, R. F. Westbrook, P. Carrive, and R. Richardson, unpublished observations). For example, in Morgan et al. (2003), adult rats were trained to fear an auditory CS by pairing it with shock. Some rats then received electrolytic lesions of the vmPFC. Rats were then subjected to extinction trials over multiple days, and it was shown that posttraining vmPFC lesions had little effect on extinction rate. However, when rats were reconditioned to the same CS and then reextinguished, lesioned rats showed resistance to extinction during reextinction trials.
Interestingly, in the adult rat, the amygdala is particularly important only if extinction happens for the first time but appears to be less critical for reextinction (Weber, Westbrook, Carrive, and Richardson, unpublished observations). That is, when a fear-eliciting CS (which was a context) in adult rats was extinguished, reconditioned, and then reextinguished, rats exhibited low levels of freezing (i.e., good extinction) the next day regardless of whether the amygdala was inactivated or not at the time of reextinction. From this, it appears that the amygdala is involved in the initial learning of the CS–no-US memory acquired in extinction training, but once that memory has been acquired, then the amygdala is no longer needed for subsequent extinction training episodes, at least in the adult rat. In experiment 2 we examined whether a similar transition from amygdala dependence to amygdala independence also occurs in reextinction in the developing rat.
In experiments 2A and 2B, 24- and 17-d-old rats (respectively) were conditioned, extinguished, reconditioned to the same CS, and then reextinguished; some rats had their amygdala inactivated at the time of reextinction. According to previous research on extinction in the developing rat, 24-d-old rats are essentially adult-like (e.g., they show renewal and reinstatement). Therefore, we hypothesized that reextinction in 24-d-old rats would be amygdala independent. The primary question in this experiment was whether reextinction would be amygdala independent in 17-d-old rats.
Experiment 2A results
For the initial extinction session (drug-free) at 24 d of age, all groups exhibited substantial levels of freezing in the first minute that decreased substantially by the last minute (Fig. 3 A). A mixed-design ANOVA of these data yielded a significant main effect of block (F (1,22) = 81.53; p < 0.0001), but no effect of group (F (2,22) = 1.25; p = 0.31) or block × group interaction (F < 1). For reextinction, group saline exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute, whereas group bupivacaine exhibited very low levels of freezing throughout (Fig. 3 B). A mixed-design ANOVA of these data yielded significant main effects of block (F (1,15) = 19.85; p < 0.0001) and group (F (1,15) = 33.31; p < 0.0001), as well as a block × group interaction (F (1,15) = 9.28; p < 0.01).
Figure 3.
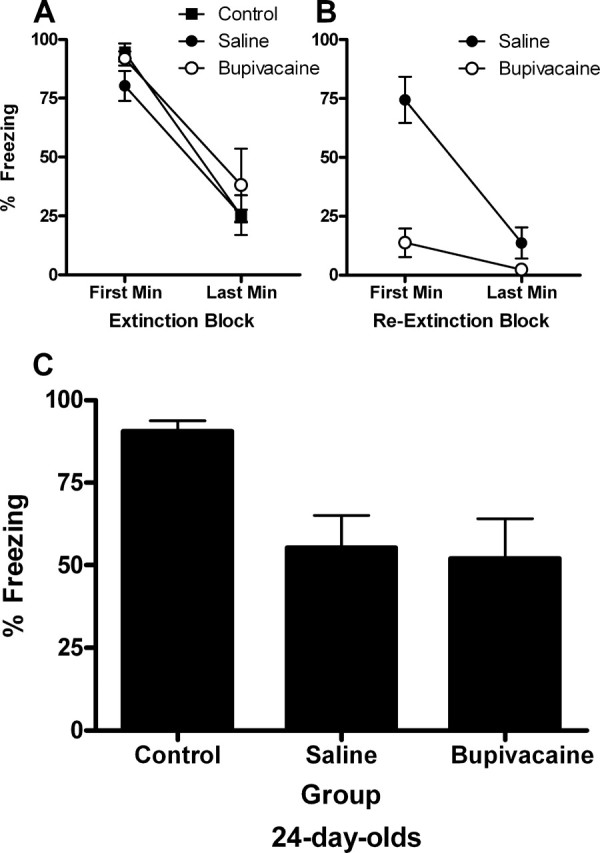
Extinction, reextinction, and test data for experiment 2A. A, For the initial extinction session (drug-free) at 24 d of age, all groups exhibited significant levels of freezing in the first minute that decreased substantially by the last minute. There were no group differences. B, For reextinction, group saline exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute, whereas group bupivacaine exhibited very low levels of freezing throughout. Group control did not receive any CS presentations and were exposed to the context only. C, Temporary inactivation of the amygdala at reextinction had no effects on reextinction, as shown by low levels of freezing in groups saline and bupivacaine compared with the nonreextinguished control group. As found previously in the adult rat, this result indicates that the amygdala is no longer involved in reextinction in 24-d-old rats.
The mean levels of CS-elicited freezing at test are shown in Figure 3 C. Temporary inactivation of the amygdala at the time of reextinction had no effects on subsequent performance at test, as shown by comparable, low levels of freezing in groups saline and bupivacaine and the high levels of freezing in the nonreextinguished control group. A one-way ANOVA of the test data revealed a significant effect of group (F (2,22) = 5.94; p < 0.01). Subsequent post hoc comparisons, with Tukey's honestly significant difference (HSD) test, showed that group control froze significantly more than the other two groups (p < 0.05), which did not differ. As found previously in the adult rat (Weber, Westbrook, Carrive, and Richardson, unpublished observations), this result indicates that the amygdala is not involved in extinction when extinction is occurring for the second time at 27 d of age.
Experiment 2B results
For the initial extinction session (drug-free) at 17 d of age, all groups exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute (Fig. 4 A). A mixed-design ANOVA of these data yielded a significant main effect of block (F (2,18) = 82.59; p < 0.0001) but no effect of group (F < 1) or block × group interaction (F < 1). For reextinction, group saline exhibited substantial levels of freezing in the first minute of reextinction that decreased substantially by the last minute, whereas group bupivacaine exhibited very low levels of freezing throughout (Fig. 4 B). A mixed-design ANOVA of these data yielded significant main effects of block (F (1,14) = 8.00; p < 0.01) and group (F (1,14) = 20.49; p < 0.0001), but a nonsignificant block × group interaction (F (1,14) = 1.92; p = 0.19).
Figure 4.
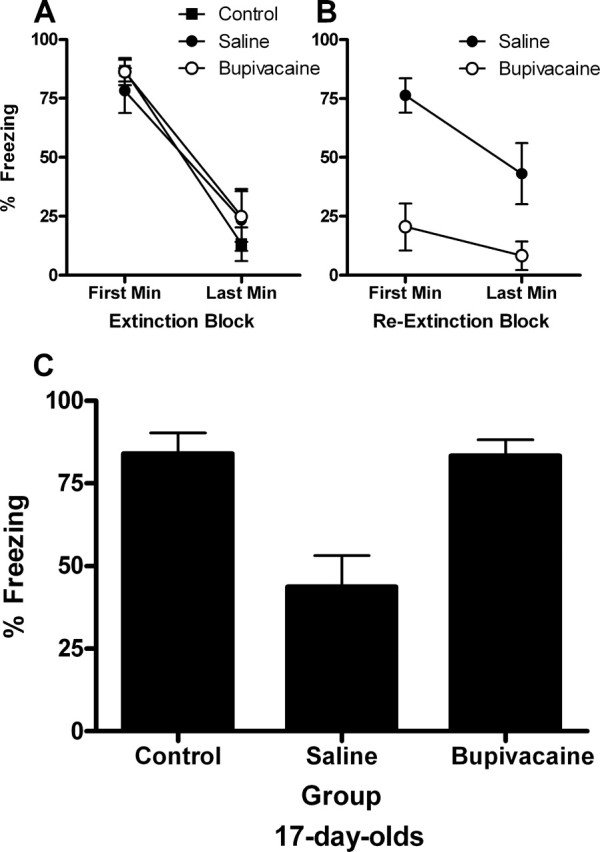
Extinction, reextinction, and test data for experiment 2A. A, For the initial extinction session (drug-free) at 17 d of age, all groups exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute. B, For reextinction, group saline exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute, whereas group bupivacaine exhibited very low levels of freezing throughout. Group control did not receive any CS presentations and were exposed to the context only. C, Temporary inactivation of the amygdala disrupted reextinction in rats that were extinguished at 17 d of age and then reextinguished at 20 d of age, as shown by high levels of freezing in groups bupivacaine and control compared with group saline. This result indicates that reextinction is amygdala dependent in the 17-d-old rat.
The mean levels of CS-elicited freezing at test are shown in Figure 4 C. Temporary inactivation of the amygdala disrupted reextinction in rats reextinguished at 20 d of age, as shown by comparable, high levels of freezing in groups bupivacaine and control and low levels of freezing in group saline. A one-way ANOVA of the test data revealed a significant effect of group (F (2,18) = 11.97; p < 0.0001). Subsequent post hoc comparisons, with Tukey's HSD test, showed that group saline froze significantly less than either of the other two groups (p < 0.005), which did not differ. This result indicates that reextinction is amygdala dependent even when it happens for the second time at 20 d of age.
Experiment 3: amygdala dependence or independence of reextinction is dependent on the age at initial extinction
Previous findings show that disrupting amygdala function during reextinction training has no effects on reextinction in the adult rat. Experiment 2A replicated this finding in 24-d-old rats. Interestingly, experiment 2B demonstrated that temporarily inactivating the amygdala at reextinction disrupted reextinction in 17-d-old rats. It should be noted, however, that training, extinction, retraining, reextinction, and test all occurred at different ages in these two experiments (i.e., it was not merely the age at reextinction that differed). Because of this, the different outcomes in experiments 2A and 2B could be attributable to differences in the rat's age at the (1) time of fear conditioning, (2) the time of the initial extinction training, or (3) the time of reextinction. First, it could be that conditioning at 23 d of age is different from conditioning at 16 d of age, such that if conditioning occurs at 16 d of age, extinction and reextinction always requires the amygdala. A second explanation for the developmental differences observed in experiments 2A and 2B is that initial extinction at 17 d of age is fundamentally different from initial extinction at 24 d of age. For example, initial extinction at 24 d of age involves learning a CS–no-US association, and subsequent reextinction simply involves retrieval of the original CS–no-US memory. However, initial extinction at 17 d of age may involve unlearning, and therefore, subsequent reextinction still requires the amygdala. From this perspective, one would predict that if the initial extinction happens at 17 d of age and reextinction happens at 27 d of age, reextinction would still require the amygdala because the initial extinction that happened at 17 d of age involved unlearning. The final possible explanation of the differences between experiments 2A and 2B is that reextinction that happens before ∼24 d of age depends on the amygdala, whereas reextinction occurring after ∼24 d of age does not depend on the amygdala. From this perspective, one would predict that if the initial extinction happens at 17 d of age and reextinction happens at 27 d of age, reextinction would be amygdala independent because reextinction occurred after 24 d of age.
To test these alternative potential accounts, experiment 3 again examined temporary inactivation of the amygdala at reextinction but kept the training, retraining, reextinction, and test age constant across groups (16, 26, 27, and 28 d of age, respectively). The only thing that varied across groups was the age at the time of the initial extinction training session (either 17 or 24 d of age; see Table 2 for design). If the training age is the critical factor determining whether reextinction is amygdala dependent or independent, then rats in both groups should perform similarly; that is, based on the results of experiment 2B, reextinction should be amygdala dependent in both groups. In contrast, if age at the time of the initial extinction is the critical factor, then rats extinguished at 17 d of age should exhibit amygdala-dependent reextinction, whereas rats extinguished at 24 d should exhibit amygdala-independent extinction. Finally, if age at the time of reextinction is the critical factor, then reextinction should be amygdala independent in both groups, based on the results of experiment 2A.
Table 2.
Experiment 3 design
| Groups | Train | Extinction | Surgery | Retrain | Reextinction | Test |
|---|---|---|---|---|---|---|
| 17-Saline | 16 | 17 | 25 | 26 | 27 (saline) | 28 |
| 17-Bupivacaine | 16 | 17 | 25 | 26 | 27 (bupivacaine) | 28 |
| 24-Saline | 16 | 24 | 25 | 26 | 27 (saline) | 28 |
| 24-Bupivacaine | 16 | 24 | 25 | 26 | 27 (bupivacaine) | 28 |
Numbers refer to the rat's age in days.
For the initial extinction session (drug-free) that occurred either at 17- or 24-d of age, all groups exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute (Fig. 5 A, left). A mixed-design ANOVA of these data yielded a significant main effect of block (F (1,25) = 99.138; p < 0.0001), but no effect of extinction age (F (1,25) = 2.19; p = 0.152) nor block × extinction age interaction (F (1,25) = 2.38; p = 0.136); there were no other significant main effects or interactions (Fs < 1).
Figure 5.
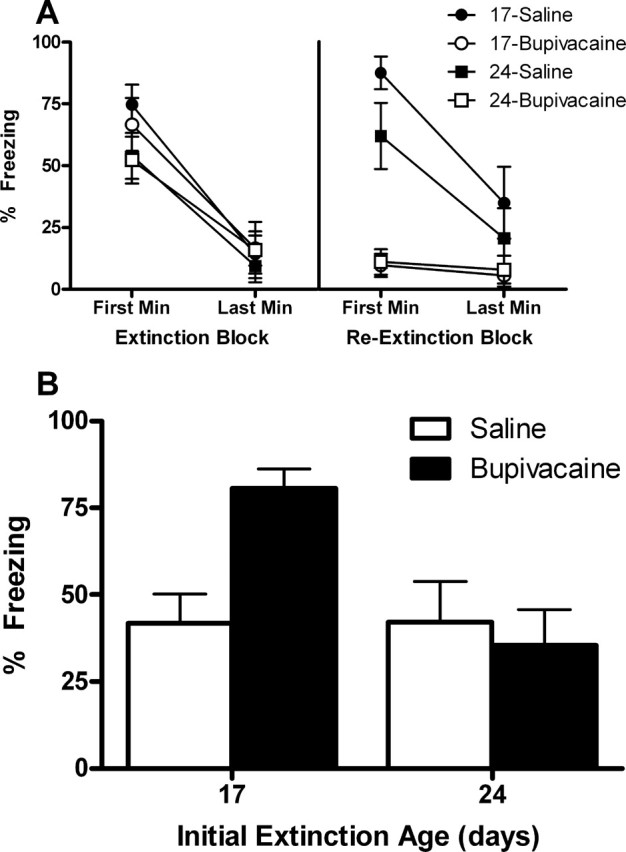
A, For the initial extinction session (drug-free), all groups exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute (left). That is, extinction rates were similar whether it was given at 17 or 24 d of age. For reextinction (right), both saline groups exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute, whereas both bupivacaine groups exhibited very low levels of freezing throughout. These results indicate that reextinction was comparable across groups regardless of when initial extinction occurred. B, Temporary inactivation of the amygdala disrupted reextinction when the initial extinction training occurred at 17 d of age, as shown by high levels of freezing in group bupivacaine compared with group saline at this age. However, when the initial extinction training session occurred at 24 d of age, inactivation of the amygdala at reextinction had no effects (i.e., the rats with the inactivated amygdala at reextinction exhibited as much extinction at test as did rats with an active amygdala). The significant interaction confirms that temporary inactivation of the amygdala at reextinction has differential effects depending on the age at the time of initial extinction.
For reextinction, both saline groups exhibited substantial levels of freezing in the first minute of extinction that decreased substantially by the last minute, whereas both bupivacaine groups exhibited very low levels of freezing throughout (Fig. 5 A, right). A mixed-design ANOVA of these data yielded significant main effects of block (F (1,25) = 23.32; p < 0.0001) and drug (F (1,25) = 43.62; p < 0.0001), as well as a block × drug interaction (F (1,25) = 17.10; p < 0.0001). There were no effects of extinction age (F (1,25) = 1.95; p = 0.175), nor drug × extinction age interaction (F (1,25) = 2.84; p = 0.104); there were no other significant main effects or interactions (F values < 1). These results indicate that reextinction was comparable across saline groups regardless of when initial extinction occurred, and that bupivacaine groups had their amygdala inactivated as indicated by the absence of freezing.
The mean levels of CS-elicited freezing at test are shown in Figure 5 B. Temporary inactivation of the amygdala disrupted reextinction when the initial extinction training occurred at 17 d of age. However, when the initial extinction training session occurred at 24 d of age, inactivation of the amygdala at reextinction had no effects (i.e., the rats with the inactivated amygdala at reextinction exhibited as much extinction at test as did rats with an active amygdala). ANOVA revealed a significant main effect of extinction age (F (1,25) = 7.12; p < 0.05), and an extinction age × group interaction (F (1,25) = 7.30; p < 0.05). The effect of group was not significant (F (1,25) = 3.60; p = 0.069). The significant interaction confirms that temporary inactivation of the amygdala at reextinction has differential effects depending on the age at the time of initial extinction. Subsequent post hoc comparisons, with Tukey's HSD test, showed that group 17-bupivacaine froze significantly more than all the other groups (p < 0.005); no other group differences were statistically significant.
Discussion
Several recent studies have reported that 17-d-old rats fail to exhibit a variety of extinction-related phenomena, whereas 24-d-old rats do (Kim and Richardson, 2007a,b; Langton et al., 2007; Yap and Richardson, 2007). To extend these findings, we examined the effect of temporarily inactivating the amygdala on extinction and reextinction in the developing rat. Experiments 1A and 1B demonstrated that extinction retention is impaired in both 24- and 17-d-old rats if the amygdala is inactivated during extinction training. This is the first demonstration of amygdala involvement in extinction at these ages. Experiment 2A showed that the amygdala was not involved in reextinction of a CS that had been previously extinguished and then retrained in 24-d-old rats. In contrast, reextinction was still amygdala dependent in 17-d-old rats in experiment 2B. Interestingly, it was shown in experiment 3 that the amygdala dependence of reextinction was critically determined by the age of initial extinction, not the age of conditioning, reconditioning, reextinction, or test. That is, when age was kept constant across these other stages, reextinction was amygdala independent if initial extinction occurred at 24 d of age but amygdala dependent if initial extinction occurred at 17 d of age.
Considering the role of the amygdala as a site for acquisition, consolidation, and storage of fear memories in adult rats (Gale et al., 2004; Phelps and LeDoux, 2005; Davis, 2006), perhaps it is not very surprising that fear extinction also involves the amygdala in 24- and 17-d-old rats. The amygdala appears to develop very early, because various studies have shown that neurotransmitter systems that mediate fear conditioning in adult rats also affect fear conditioning in the developing rat (Kim et al., 2006; Weber et al., 2006; Langton et al., 2007). One study explicitly showed that the amygdala participates in conditioned fear in 12-d-old rats (Sullivan et al., 2000). Hence, it is mostly likely that fear memories, once acquired, are stored in the amygdala in 24- and 17-d-old rats. Therefore, extinction of conditioned fear at these ages would also likely require a functional amygdala.
Although the amygdala is involved in extinction in both 24- and 17-d-old rats, experiments 2A, 2B, and 3 suggest that the processes mediating extinction at these ages are very different. These experiments showed that if extinction occurs at 24 d of age, reextinction is amygdala independent, whereas the amygdala is still necessary for reextinction if extinction occurs at 17 d of age. The findings with the 24-d-old rats replicate those in adult rats (Weber, Westbrook, Carrive, and Richardson, unpublished observations). This result, along with previous research on other extinction-related phenomena in the developing rat (Kim and Richardson, 2007a,b; Langton et al., 2007), indicates that the processes mediating extinction in 24-d-old rats is the same, or at least very similar, to those that mediate extinction in the adult rat.
Extinction in the 17-d-old rat and current models of extinction
The present study shows that the amygdala is necessary for reextinction if extinction occurs at 17 d of age, which is contrary to what is found in older rats. This suggests that the neural mechanisms involved in extinction at this age are fundamentally different. Current models of extinction implicate the amygdala, mPFC, and hippocampus as components of the neural circuitry of extinction (Quirk et al., 2006; Sotres-Bayon et al., 2006; Myers and Davis, 2007). The amygdala has strong connections with the mPFC, and studies indicate that the infralimbic portion of the vmPFC sends a strong excitatory input to the lateral nucleus (LA) and to the GABAergic intercalated cells of the amygdala (McDonald et al., 1996; Price, 2003). Hence, current models of extinction maintain that the reduced responding observed after extinction is caused by a GABAergic inhibition of amygdala activity via the mPFC (Hobin et al., 2003; Sotres-Bayon et al., 2006). The hippocampus is thought to be a critical part of this neural circuit, responsible for the context modulation of extinction. There are both inhibitory and excitatory hippocampal projections to the mPFC (Ishikawa and Nakamura, 2003); thus, current models of extinction maintain that the hippocampus inhibits or excites the mPFC and thereby modulates the expression of extinction.
Interestingly, both the mPFC and hippocampus are late-maturing structures in the rat. For example, the cortical layers of the mPFC attain their adult proportional width at ∼24 d of age (Van Eden and Uylings, 1985). Also, the hippocampus has long been known for its delayed maturation (Wilson, 1984), and behavioral studies show that young rats (e.g., 18-d-old rats) are impaired in learning about context (Rudy, 1993; Rudy and Morledge, 1994). Hence, the observed developmental differences in extinction are likely caused by 17-d-old rats having a different neural circuitry for extinction. There are several findings that support this notion. As mentioned earlier, renewal and reinstatement are not observed in 17-d-old rats, suggesting an absence of hippocampal involvement in extinction in these rats (Kim and Richardson, 2007a,b; Yap and Richardson, 2007). Further, we have shown that reducing GABAergic inhibition by pretest injection of FG7142 failed to recover an extinguished fear response in 17-d-old rats (Kim and Richardson, 2007a). The failure to observe GABAergic modulation of extinction expression in 17-d-old rats indicates that the vmPFC-mediated inhibition of the amygdala is not involved in extinction in these rats. Finally, using immunolabeling of the long-term cell plasticity marker pMAPK, we have obtained preliminary evidence showing postextinction pMAPK activation in the vmPFC in 24-d-old rats but not in 17-d-old rats (our unpublished observations). Overall, it appears that the hippocampus and vmPFC are not involved in the neural circuit underlying extinction of conditioned fear in 17-d-old rats.
A different neural circuit for extinction at 17 d of age can explain the current findings on the amygdala dependence of reextinction in two ways. The first explanation is that the CS–no-US extinction memory is stored in the amygdala in 17-d-old rats. In the adult models of extinction, the storage of the extinction memory is not localized in one neural structure, but is posited to be dependent on the entire amygdala-mPFC-hippocampus circuit (Barad et al., 2006; Farinelli et al., 2006; Quirk and Mueller, 2007). In the present study, inactivation of the amygdala may have disrupted reextinction because at 17 d of age, the initial extinction memory was stored solely in the amygdala because of vmPFC and hippocampus immaturity.
The second explanation is that extinction involves unlearning of the original CS–US association in 17-d-old rats because of the different neural circuit involved in extinction at this age; thus, subsequent reextinction still requires the amygdala. This explanation is supported by a finding that shows that preextinction systemic injection of the NMDA antagonist MK-801 does not disrupt extinction in 17-d-old rats (Langton et al., 2007). NMDA dependence of extinction learning in the adult rat is taken as evidence for the new learning account of extinction (Lattal et al., 2006). Hence, NMDA-independent extinction in 17-d-old rats suggests that extinction is not new learning in these rats. Further, extinction expression is not mediated by GABAergic inhibitory activity in 17-d-old rats, showing that extinction is not inhibition in rats this age (Kim and Richardson, 2007a).
Many recent reviews on extinction now suggest both “new learning” and “unlearning” as mechanisms for extinction in the adult rat (Delamater, 2004; Barad et al., 2006; Lattal et al., 2006; Myers and Davis, 2007). It may be the case that when extinction occurs early in development, the balance between unlearning and new learning processes of extinction is simply shifted compared with the adult rat, in that extinction relies more on unlearning rather than new learning. A potential way of testing whether unlearning occurs in extinction at 17 d of age is to record neural activity in LA neurons after extinction. After CS–US pairings, CS-elicited neural activity in LA neurons increases in the adult rat; during extinction, the responses of many of these cells return to pretraining levels (Quirk et al., 1995; Repa et al., 2001). However, Repa et al. (2001) found a population of LA cells in which CS-elicited activity remained elevated throughout extinction training. Repa et al. (2001) inferred that these cells represent the original CS–US memory, demonstrating that extinction does not erase the original CS–US association in the adult rat. Investigating whether this population of cells show a different response after extinction in 17-d-old rats would be helpful in determining whether unlearning is a mechanism for extinction in the developing rat.
It is clear that more work needs to be done in examining extinction during development. Such research not only provides a unique way of assessing extinction processes, but is important also because of the long-held belief that early learning experiences have a profound impact on later behavior (Mineka and Zinbarg, 2006). Jacobs and Nadel (1985, 1999), for example, suggested that fear acquired early in development is particularly resistant to the effects of extinction, and forms the basis of anxiety disorders emerging later in life. However, recent evidence and the current findings show that fear acquired early in development not only can be extinguished, but may even be erased.
Footnotes
This work was supported by an Australian Postgraduate Award (J.H.K.) and Discovery Grants DP0346139 and DP0666953 (R.R.) from the Australian Research Council.
References
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–328. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Caterall WA, Mackie K. Local anaesthetics. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill; 1996. pp. 331–347. [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers K. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambous T, Westbrook RF. An infusion of bupivacaine into the nucleus accumbens disrupts the acquisition but not the expression of contextual fear conditioning. Behav Neurosci. 1999;113:925–940. doi: 10.1037//0735-7044.113.5.925. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. Microsurgery: window on hidden developmental processes. In: Shair H, Barr G, Hofer M, editors. Developmental psychobiology: new methods and changing concepts. New York: Oxford UP; 1991. pp. 19–31. [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdala projections within the prefrontal cortex in the rat. J Neurosci. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WJ, Nadel L. Stress-induced recovery of fears and phobias. Psychol Rev. 1985;92:512–531. [PubMed] [Google Scholar]
- Jacobs WJ, Nadel L. The first panic attack: a neurobiological theory. Can J Exp Psychol. 1999;53:92–107. doi: 10.1037/h0087302. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007a;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007b;88:48–57. doi: 10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, McNally GP, Richardson R. Recovery of fear memories in rats: role of GABA in infantile amnesia. Behav Neurosci. 2006;120:40–48. doi: 10.1037/0735-7044.120.1.40. [DOI] [PubMed] [Google Scholar]
- Langton JL, Kim JH, Nicholas J, Richardson R. The effect of NMDA-receptor antagonist MK801 on the acquisition and extinction of learned fear in the developing rat. Learn Mem. 2007;14:665–668. doi: 10.1101/lm.692407. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Radulovic J, Lukowiak K. Extinction: does it or doesn't it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry. 2006;60:344–351. doi: 10.1016/j.biopsych.2006.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann NY Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology Rev. 2007:1–17. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of pavlovian conditioning: variations in the effectiveness of reinforcement and non-reinforcement. In: Prokasy AH, editor. Classical conditioning II: current research and theory. New York: Appleton-Century-Croft; 1972. pp. 64–99. [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;197:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: ontogeny of conditioned fear and the amygdala. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Weber M, Richardson R. Centrally administered corticotropin-releasing factor and peripheral injections of strychnine hydrochloride potentiate the acoustic startle response in preweanling rats. Behav Neurosci. 2001;115:1273–1282. doi: 10.1037//0735-7044.115.6.1273. [DOI] [PubMed] [Google Scholar]
- Weber M, Richardson R. Pretraining inactivation of the caudal pontine reticular nucleus impairs the acquisition of conditioned fear-potentiated startle to an odor, but not a light. Behav Neurosci. 2004;118:965–974. doi: 10.1037/0735-7044.118.5.965. [DOI] [PubMed] [Google Scholar]
- Weber M, McNally GP, Richardson R. Opioid receptors regulate retrieval of infant fear memories: effects of naloxone on infantile amnesia. Behav Neurosci. 2006;120:702–709. doi: 10.1037/0735-7044.120.3.702. [DOI] [PubMed] [Google Scholar]
- Wilson DA. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Dev Brain Res. 1984;16:61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Yap CSL, Richardson R. Extinction in the developing rat: an examination of renewal effects. Dev Psychobiol. 2007;49:565–575. doi: 10.1002/dev.20244. [DOI] [PubMed] [Google Scholar]


