Abstract
Two firing modes of thalamocortical (TC) neurons, tonic and burst firings, are thought to reflect the divergent states of sensory signal transmission from the thalamus to the cortex. However, the behavioral consequences of changes in the thalamic firing between the two modes have not been well demonstrated. Moreover, although the firing modes of TC neurons are known to be affected by corticothalamic inputs via thalamic metabotropic glutamate receptor type 1 (mGluR1)–phospholipase C β4 (PLCβ4) pathway, its molecular mechanisms have not been well elucidated. We addressed these questions using PLCβ4-deficient mice, which show decreased visceral pain responses. We demonstrate that burst and tonic firings of TC neurons are concomitantly regulated by PLCβ4 pathway. Blocking of this pathway by the mutation simultaneously increases bursting and decreases tonic firing of TC neurons through concurrent upregulation of T- and L-type Ca2+ currents. The mice with increased bursting and decreased tonic firing of TC neurons showed reduced visceral pain responses. Furthermore, we show that modulation of the Ca2+ channels or protein kinase C (PKC), a downstream molecule of PLCβ4, altered the firing modes of TC neurons and pain responses in the predicted ways. Our data demonstrate the molecular mechanism and behavioral consequences of altered firing modes of TC neurons in relaying the visceral pain signals. Our study also highlights the thalamic PLCβ4–PKC pathway as a “molecular switch” for the firing modes of TC neurons and thus for pain sensory gating.
Keywords: thalamus, Ca2+ channels, phospholipase Cβ4, visceral pain, sensory gating, knock-out mice
Introduction
The thalamus plays a pivotal role in sensory signal processing, integrating sensory inputs from the periphery as well as inputs from other brain regions and then sending the integrated information to the cortex (McCormick and von Krosigk, 1992; Steriade et al., 1993; Sherman and Guillery, 1996; Llinás et al., 1998; Jones, 2000; Le Masson et al., 2002). Thalamocortical (TC) neurons produce two distinct firing patterns that are thought to reflect the status of signal transmission from the thalamus to the cortex. Tonic firing is generally believed to represent a relay mode of afferent sensory signals to the cortex (McCormick and von Krosigk, 1992). In contrast, low-threshold burst firing has been associated previously with drowsy/sleep states or loss of consciousness in several studies (von Krosigk et al., 1993; Kim et al., 2001; Crunelli and Leresche, 2002). However, controversy remains regarding the exact role of low-threshold burst firing in the sensory gating function of the thalamus (Sherman, 2001; Swadlow and Gusev, 2001; Kim et al., 2003).
The transition between these two firing modes primarily depends on the balance between excitatory and inhibitory inputs onto TC neurons. Inhibitory inputs from reticular nucleus (von Krosigk et al., 1993; Huntsman et al., 1999) and inputs from the ascending reticular activation system (Steriade, 1996) to TC neurons have been thought to modulate the activity of TC neurons. In addition, activation via descending corticothalamic pathways was found previously to increase the excitability of TC neurons via metabotropic glutamate receptor type 1 (mGluR1) pathway (McCormick and von Krosigk, 1992; Pedroarena and Llinás, 2001). The importance of mGluRs in modulating thalamic activity was corroborated by reports showing high expression of mGluR1 in TC neurons (Shigemoto et al., 1992), exclusively at postsynaptic membranes of corticothalamic inputs (Vidnyanszky et al., 1996). In the brain (Kim et al., 1997), especially in the thalamus (Miyata et al., 2003), mGluRs are linked to phospholipase C (PLC) activity. PLCβ4 is the major subtype in the thalamic relay region (Watanabe et al., 1998) and is extensively linked to mGluR1 in thalamic neurons (Miyata et al., 2003). Mice lacking PLCβ4 or wild-type mice treated with an mGluR1 antagonist or a PLC inhibitor display a reduced Formalin-induced inflammatory pain response (Miyata et al., 2003) but maintain normal pain responses involving spinal reflexes.
Despite a number of studies investigating thalamic sensory gating, the actual behavioral consequences of changes in the thalamic firing modes have not been well demonstrated. Moreover, although the firing modes of TC neurons are known to be affected by corticothalamic inputs via the mGluR1 pathway, its molecular mechanisms have not been well elucidated. Here, using PLCβ4−/− mice, which show a diminished visceral pain response, we demonstrate that the thalamic mGluR1–PLCβ4 pathway concomitantly controls both burst and tonic firing modes by simultaneously modulating T- and L-type Ca2+ channels. These results highlight a critical role of the thalamic mGluR1–PLCβ4 pathway in setting the firing modes of thalamocortical neurons and provide new insight into the mechanisms by which corticothalamic inputs contribute to the control of thalamic sensory gating.
Materials and Methods
Animals.
The PLCβ4 knock-out mouse line was maintained in two inbred strains, C57BL/6J and 129S4/SvJae. Male homozygous mutant PLCβ4−/− mice and wild-type littermates used for pain testing and patch-clamp analyses were F1 homozygotes derived from mating heterozygous mice of 129S4/SvJae and C57BL/6J genetic backgrounds. Genotypes were determined by PCR analysis using the following primers: F1, 5′-CTCCACACTCTGCAA-CCTAC-3′; R1, 5′-AGTTACTTCTGGATTTTCAGCC-3′; 22 PGK, 5′-CTGACTAGGGG-AGGAGTAGAA-G-3′. Animal care and handling were performed according to institutional guidelines (Korea Institute of Science and Technology, Seoul, Korea). The mice were allowed access to food and water ad libitum and were maintained under a 12 h light/dark cycle.
Micro-osmotic pump implantation and visceral pain test.
For drug infusion into the brain, micro-osmotic pumps (Alzet) were placed in the mouse brain with the tips targeting the ventroposteromedial/ventroposterolateral (VPM/VPL) region of the thalamus (anteroposterior, 1.6; lateral, ±1.7; ventral, 3.4 mm) using a stereotaxic device (David Kopf Instruments). Micro-osmotic pumps were implanted as described previously (Kim et al., 2003). Infusion positions were confirmed by postmortem histology. Visceral pain was induced by injection of acetic acid (0.6% in saline) into the peritoneum at 6 ml/kg. Pain tests and data analysis were performed in a double-blinded manner. Information about the genotype and drugs infused was not exposed to either the investigator who performed the pain tests or to the evaluators to prevent any bias. Pain responses were recorded with a CCD camera, and the results were analyzed later by evaluators blinded to the mice genotypes or drugs injected. The video was analyzed by at least two evaluators independently. After the evaluation on pain responses were completely done, the information about the genotypes and drugs were pooled with the results.
Single-unit recording of VPM/VPL neurons.
Wild-type and PLCβ4−/− mice were anesthetized with low-dose urethane (1.5 g/kg) and fixed in a stereotaxic device. The level of anesthesia was monitored with limb-withdrawal reflexes. Low-dose urethane anesthesia has been used to record the primary sensory responses or to induce type 2 theta rhythms similar to awake electroencephalogram patterns in animals (Brecht and Sakmann, 2002; Buzsáki, 2002; Miyata et al., 2003; Aguilar and Castro-Alamancos, 2005). To insert an electrode, the scalp was incised, and a hole was drilled into the skull above the region where the VPM/VPL thalamic nuclei are located (anteroposterior, 1.6; lateral, 1.7 mm). Parylene-coated tungsten microelectrodes (2 ΜΩ) were positioned into the VPM/VPL thalamic regions (depth, 3.4–3.7 mm) until unit spikes were observed. The units responsive to intraperitoneal acetic acid injection were further analyzed. Signals were amplified ∼5000-fold and bandpass filtered at 300–3000 Hz with a Cyberamp402 amplifier (Molecular Devices), digitized at a 10 kHz sampling rate with Digidata (Molecular Devices), and stored in a computer for analysis. The recording positions were confirmed by postmortem histology. The number of spikes was quantified using MiniAnalysis software (Synaptosoft).
Preparation of brain slices and acutely dissociated neuronal cells.
Firing patterns of TC neurons were recorded in acutely isolated brain slices from ∼4-week-old mice. The brain was blocked and sectioned in the coronal plane in ice-cold slicing solution (in mm: 124 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 5 MgCl2, 1 CaCl2, 3 KCl, and 10 glucose) and incubated for at least 1 h in slicing solution at room temperature before use. All experiments in which Ca2+ currents were recorded were performed using acutely dissociated cells from 14- to 17-d-old mice. Brain slices obtained using the method described above and incubated for at least 1 h were then treated at 37°C in an oxygenated HEPES-buffered solution [in mm: 150 NaCl, 3 KCl, 2 CaCl2, 10 HEPES, 2 MgCl2, and 10 glucose, pH ∼7.4 (320–330 mOsm)] containing 2 mg/ml protease XIV (Sigma) for 10 min, followed by incubation at room temperature for 5 min. After enzyme treatment, slices containing the VPM/VPL region were dissected by scalpel cuts, and cells were triturated with fire-polished Pasteur pipettes and plated onto glass coverslips.
Whole-cell and perforated patch clamping.
Firing patterns of thalamic relay neurons in brain slices were recorded under current-clamp mode in artificial CSF solution (in mm: 124 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 1.3 MgSO4, 2.4 CaCl2, 3 KCl, and 10 mm glucose) bubbled with 95% O2/5% CO2. Patch electrodes (4–6 MΩ) fabricated from standard-wall borosilicate glass (GC150F-10; Warner Instruments) were filled with an intrapipette solution containing the following (in mm):140 K-gluconate, 10 KCl, 1 MgCl2, 10 HEPES, 0.02 EGTA, 4 Mg-ATP, and 0.3 Na2-GTP, with pH adjusted to 7.35. Signals were amplified with a Multiclamp 700-A amplifier (Molecular Devices) and analyzed using pClamp 9.2 and MiniAnalysis software (Synaptosoft).
Ca2+ currents were recorded with the whole-cell configuration from acutely dissociated neurons. Healthy-appearing neurons with processed dendrites were chosen for recording Ca2+ currents. Cells with membrane capacitance values between 30 and 50 pF were included in the analysis. Recordings were performed in an extracellular solution consisting of the following (in mm): 100 NaCl, 25 tetraethylammonium (TEA)-Cl, 5 CaCl2, 20 HEPES, 2 MgCl2, 5 4- aminopyridine (4-AP), 10 glucose, and 0.001 tetrodotoxin (TTX), pH 7.4 (320–330 mOsm). Patch pipettes were filled with a solution containing 130 mm CsCl, 10 mm HEPES, 5 mm TEA-Cl, 10 mm EGTA, 4 mm MgCl2, 4 mm Mg-ATP, and 0.3 Na2-GTP, pH 7.3. The series resistance compensation function (>60%) was used routinely, with final access resistance ∼10 MΩ. The currents were corrected for capacitive currents and for leak currents using a P/4 leak subtraction protocol. Signals were digitized using an Axopatch 200-B amplifier (Molecular Devices) and analyzed using pClamp 9.2 or 10 software.
For perforated patch recordings, the pipette solution contained 50 μg/ml gramicidin-D, 75 mm Cs2SO4, 10 mm KCl, and 10 mm HEPES, pH 7.3, 310 mOsm. Extracellular solution consists of the following (in mm): 100 NaCl, 25 TEA-Cl, 5 BaCl2, 20 HEPES, 2 MgCl2, 5 4-AP, 10 glucose, and 0.001 TTX, pH 7.4 (320–330 mOsm). It took ∼20–30 min to achieve acceptable perforation, with final series resistances ranging from 15 to 30 MΩ. The membrane holding potential was −60 mV if there was no other specification.
Data analysis.
A two-tailed t test was used for statistical analysis unless otherwise specified. Firing patterns of TC neurons and Ca2+ current data were analyzed using MiniAnalysis software. All values are expressed as means ± SE.
Reverse-transcription-PCR analysis.
Total RNA was isolated from both whole brain and the VPM/VPL region of F1 wild-type mice by using RNA isolation reagent (Tri reagent; MRC). To produce cDNA, 1 g of total RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) using oligo-dT priming (Invitrogen). PCR primers used to confirm expression were as follows: actin: F1 primer, 5′-AGATCTGGCACCACACCTTC-3′ and R1 primer, 5′-TCTCCAGGGAGGAAGAGGAT-3′ were used to produce a 462 bp cDNA; SK1 channel: F2 primer, 5′-GAAAAGCGTAAACGGCTCAG-3′ and R2 primer, 5′-ACCAACTCCAGCGAGATCAG-3′ were used to produce a 281 bp cDNA; SK2 channel: F3 primer, 5′-CTGTTTGAGAAGCGCAAGC-3′ and R3 primer, 5′-ACGCTCATAAGTCATGGC-3′ were used to produce a 261 bp cDNA; SK3 channel: F4 primer, 5′-CACCAGACTCTGCTCCATCA-3′ and R4 primer, 5′-CGATGATCAAACCAAGCAGG-3′ were used to produce a 319 bp cDNA; BK channel: F5 primer, 5′-GAGGTGCAGTGGCTTCTCTT-3′ and R5 primer, 5′-CCTGCCCACCAGAGAATTAG-3′ were used to produce 311 bp a cDNA; α1C L-type Ca2+ channel: F6 primer, 5′-GCAGTATGGGAAACCCAAGA-3′ and R6 primer, 5′-GCTCCTTTCCCTCCTAGAGC-3′ were used to produce a 435 bp cDNA; and αα1D L-type Ca2+ channel: F7 primer, 5′-AATTCGGGGTGTCATAACCA-3′ and R7 primer, 5′-CACAGCACTCCTCGCTACTG-3′ were used to produce a 355 bp cDNA. PCR conditions consisted of 5 min at 94°C for one cycle and then 40 cycles lasting 40 s each at 94°C, 40 s at 56°C, and 40 s at 72°C, followed by 5 min at 72°C.
Results
Firing pattern of TC neurons in vivo correlates with behavioral evidence of pain
To determine the role of the thalamic mGluR1–PLCβ4 cascade in pain signal processing, we compared visceral pain responses induced by acetic acid injection between PLCβ4−/− mice and their wild-type littermates (Fig. 1A,B) as described previously (Kim et al., 2003; Choi et al., 2007). Pain responses were recorded with a CCD camera, and the results were analyzed later by investigators blinded to the mice genotypes or drugs injected. The number of writhes, including abdominal stretching and constriction, was counted to measure visceral pain responses. Wild-type mice showed typical visceral pain responses characterized by writhing reflexes. In contrast, PLCβ4−/− mice displayed fewer writhing responses (p < 0.001) (Fig. 1A), suggesting that the activity level of the endogenous PLCβ4 pathway modulates pain signal processing. These results were consistent with the previous observation that PLCβ4−/− mice showed a reduced Formalin-induced inflammatory pain response (Miyata et al., 2003).
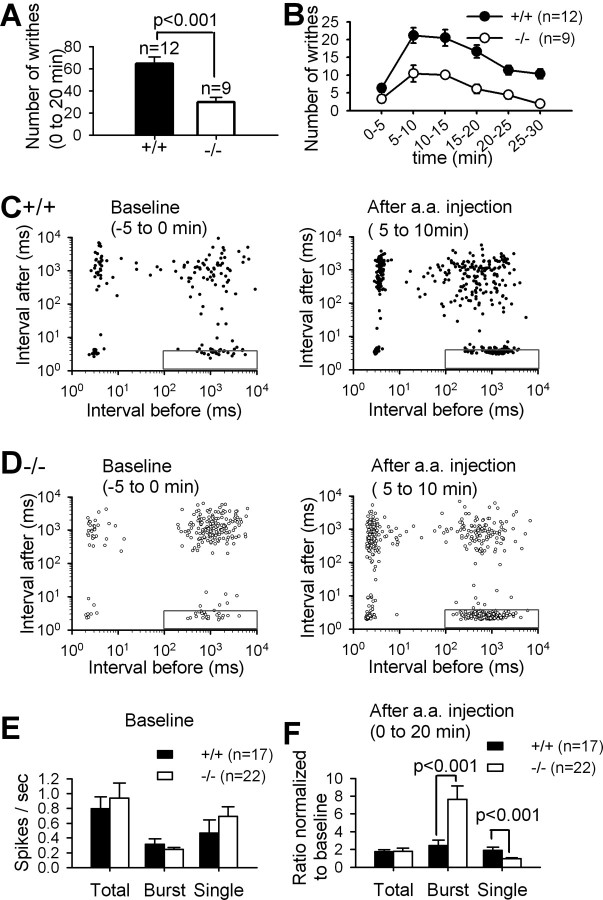
Figure 1.
Visceral pain responses and in vivo firing patterns in VPL/VPM neurons in wild-type and PLCβ4−/− mice. The number of writhes was measured for 20 min (A) and quantified at each 5 min interval (B) after intraperitoneal injection of acetic acid. The number of writhing responses was significantly lower in PLCβ4−/− mice than in wild-type mice (p < 0.001). Error bar represents SEM if there is no specification. Each spike of single-unit activities of VPL/VPM neurons recorded in vivo from wild-type (C) and PLCβ4−/− (D) mice before and after intraperitoneal acetic acid (a.a.) injection was plotted as a point at the interspike interval before the spike on the x-axis and the interspike interval after the spike on the y-axis on a log-log scale. n refers to the number of mice used for in vivo recordings. E, The number of spikes per second of total, burst, and single-spike during the 10 min baseline recordings before acetic acid injection showed no differences between two genotypes. F, The spike numbers per second in each category were normalized to the spike numbers in the baseline recording. The normalized spike numbers referenced to the baseline spike numbers of each neuron revealed significantly higher burst spikes and lower single spikes in PLCβ4−/− mice (open bars) than wild-type mice (filled bars) after acetic acid injection.
To investigate the firing patterns of thalamic neurons during visceral pain induction, we performed in vivo extracellular single-unit recordings in the VPM/VPL regions in lightly anesthetized mice with the low-dose urethane. Previous studies have shown that, under the low-dose urethane anesthesia, the sensory-evoked responses can be measured in thalamocortical neurons and cortical neurons in primary somatosensory cortex (Brecht and Sakmann, 2002; Miyata et al., 2003; Aguilar and Castro-Alamancos, 2005). Neurons in VPM/VPL regions whose firing patterns were changed responding to acetic acid injection into the abdomen of mice were analyzed in this study (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Low-threshold burst spikes were defined by the preceding silent period longer than 100 ms, high-frequency spikes of 200–400 Hz with more than two spikes, a shortening of the first interspike interval (1–4 ms), and a progressive prolongation of successive interspike intervals as described previously in the literatures (Guido et al., 1992; Kim et al., 2003). Each spike was categorized to burst or single spike according to this definition (supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
Two-dimensional interspike interval plots were obtained from 5 min recordings before and after acetic acid injection (Fig. 1C,D). The points within the rectangles at the bottom right clusters indicate the initial spike in each burst event according to our criteria, and the bottom left clusters mostly represent the second to penultimate spikes in bursts. The top left clusters mostly represent the last spikes in bursts. The top right clusters mostly represent single spikes in tonic mode. Some points in these clusters may include single low-threshold spikes, but they were counted as single spikes because they were not distinguishable according to the criteria used in this study. PLCβ4−/− thalamic neurons mostly showed more spikes in the clusters representing the initial spike in bursts than wild-type thalamic neurons after acetic acid injection, which suggested the increased occurrence of burst events (Fig. 1C,D). In fact, the normalized number of burst events in PLCβ4−/− mice was significantly higher than that of wild-type mice only after acetic acid injection, whereas there was no difference in the number of burst events before acetic acid injection (supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
The spike numbers in total, burst-spike, and tonic-spike categories were summed and divided by the recording time to obtain the spikes per second of total, burst spike, and tonic spike, respectively. There were no significant differences in the baseline spikes per second of total (p = 0.762), single spike (p = 0.301), and burst spike (p = 0.336) between PLCβ4−/− and wild-type mice (Fig. 1E). The spikes per second of total, burst, and single spikes of each neuron after the acetic acid injection were normalized to their own baseline spikes per second to obtain the normalized ratios. The total spike numbers were nearly doubled relative to the baseline in both wild-type and PLCβ4−/− mice. Neurons in PLCβ4−/− mice showed significantly higher burst spike numbers (p < 0.001) and lower single spike numbers (p < 0.001) than those in wild-type mice only after intraperitoneal acetic acid injection (Fig. 1F). The number of spikes per burst in PLCβ4−/− mice increased significantly (p < 0.001) and was higher than that in wild-type mice (p < 0.05) after acetic acid injection (supplemental Fig. S1 available at www.jneurosci.org as supplemental material). Single and burst spikes of thalamic neurons have been implicated in the transmission and inhibition of sensory signals to the cortex, respectively. Therefore, our data suggest that the increased burst spikes and decreased single spikes observed in PLCβ4−/− thalamic neurons may correlate with the reduced visceral pain responses of PLCβ4−/− mice.
Thalamocortical neurons in PLCβ4−/− brain slices show increased burst firing and decreased tonic firing
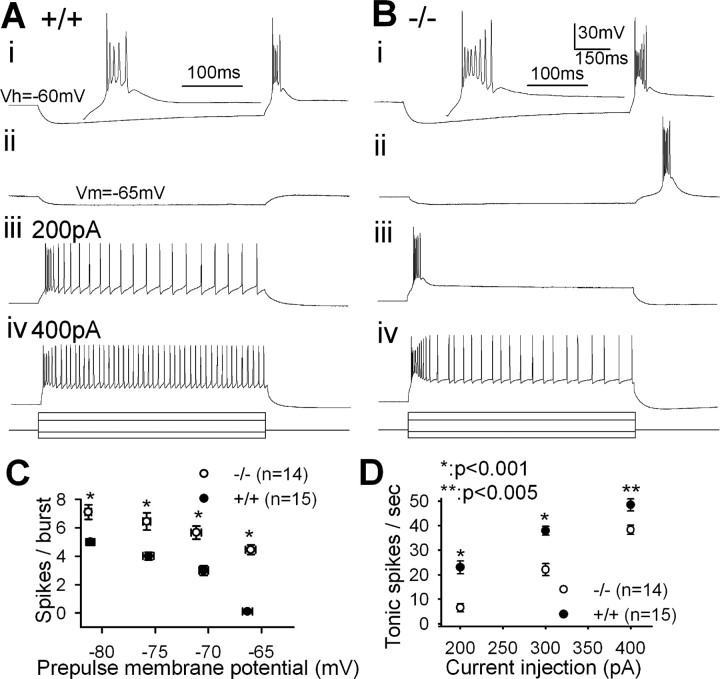
To determine whether the altered firing patterns seen in vivo reflect changes in the intrinsic excitability of TC neurons, we performed whole-cell patch clamping of TC neurons in the VPM/VPL regions. Low-threshold burst firing was elicited from TC neurons when the membrane potential was rebound to the holding potential (−60 mV) after the hyperpolarizing prepulses for 1 s (Fig. 2Ai,Bi). The number of spikes in a burst was significantly increased in PLCβ4−/− TC neurons (p < 0.001) at all of the tested prepulse membrane potentials (Fig. 2C). The most conspicuous finding was that a slight hyperpolarization of the membrane potential, to approximately −65 mV, elicited a low-threshold burst firing in PLCβ4−/− (Fig. 2Bii) but not in wild-type (Fig. 2Aii) TC neurons. Tonic spike numbers were significantly lower in PLCβ4−/− TC neurons with a wide range of depolarizing current injections (Fig. 2D).
Figure 2.
Altered firing properties of PLCβ4−/− TC neurons in the VPM/VPL regions. Representative traces showing the firing pattern of wild-type (A) and PLCβ4−/− (B) TC neurons from whole-cell patch recordings. Low-threshold burst firing was elicited by injecting prepulses hyperpolarizing the membrane potentials to approximately (i) −80 mV and (ii) −65 mV. Traces were then enlarged to show a closer look of spikes in a burst. Tonic firing was induced with depolarizing currents, (iii) 200 pA and (iv) 400 pA. The bottom panel displays the applied current steps. C, The number of spikes in a burst induced after various hyperpolarizing prepulse membrane potentials in wild-type (filled circles) and mutant (open circles). D, Tonic spikes per second induced by various depolarizing currents.
There was no notable difference in the resting membrane potential (−58.21 ± 4.34 mV, n = 15 vs −55.47 ± 6.23 mV, n = 14; p = 0.71) or in input resistance (241.02 ± 17.82 MΩ, n = 13 vs 274.77 ± 24.93 MΩ, n = 10; p = 0.29) between wild-type and PLCβ4−/− TC neurons. The amplitudes and half-widths of action potentials in burst or tonic firing modes did not differ between the two genotypes.
The propensity for increased burst firing and less tonic firing of PLCβ4−/− TC neurons suggested that the increased burst spikes and decreased single spikes in in vivo single-unit recordings of PLCβ4−/− thalamic neurons were primarily attributable to altered firing properties of individual PLCβ4−/− TC neurons. These observations led us to pursue two additional lines of investigation. First, the increase in burst firing led to an investigation of T-type Ca2+ currents. Second, the decrease in tonic firing led to an investigation of afterhyperpolarization (AHP), an important factor that determines the firing rate of neuronal cells (Stocker, 2004).
Modulation of Ca2+ currents in TC neurons of PLCβ4−/− mice
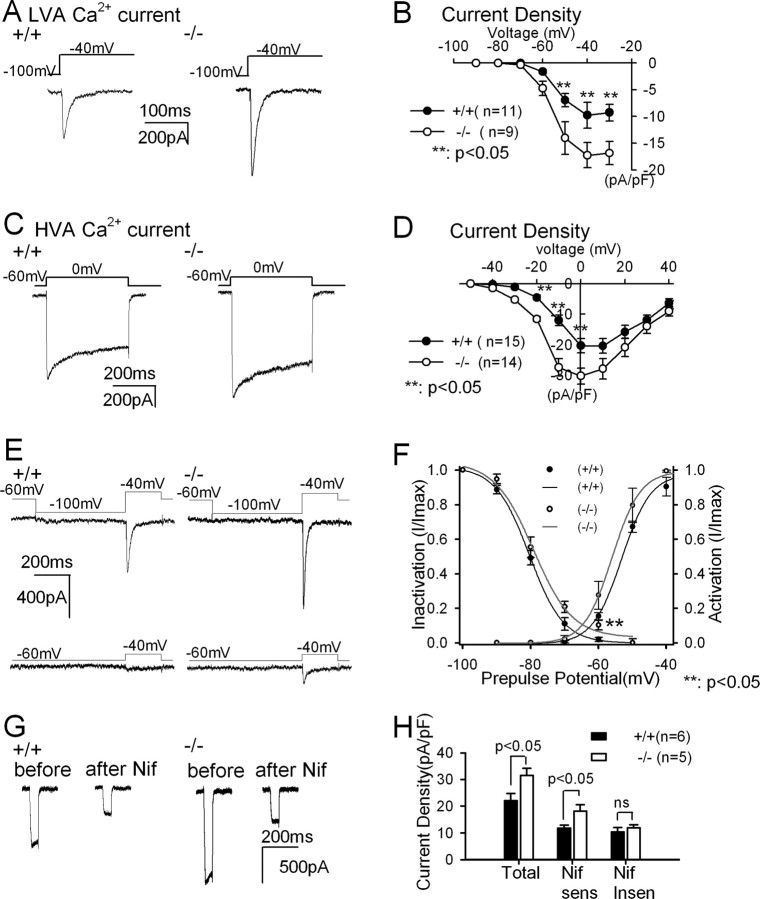
As an initial step in understanding the cellular mechanism(s) underlying changes in the firing patterns of PLCβ4−/− TC neurons, low-voltage-activated (LVA) and high-voltage-activated (HVA) Ca2+ currents were measured sequentially from individual TC neurons (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). The amplitudes of both LVA and HVA Ca2+ currents were significantly higher in mutant than in wild-type TC neurons (p < 0.05) (Fig. 3A–D), whereas the normalized I–V curves were unchanged (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
Figure 3.
Enhanced LVA and HVA Ca2+ currents in PLCβ4−/− TC neurons. LVA (A) and HVA (C) Ca2+ currents were measured from wild-type and PLCβ4−/− TC neurons. I–V relationships of the LVA Ca2+ current density (B) and of the HVA Ca2+ current density (D) in wild-type (filled circles) and PLCβ4−/− (open circles) TC neurons revealed the increased LVA and HVA Ca2+ currents in PLCβ4−/− TC neurons. E, LVA Ca2+ currents from wild-type (left) and PLCβ4−/− (right) TC neurons activated by voltage steps from −100 to −40 mV (top) and from −60 to −40 mV (bottom). F, Steady-state inactivation curves and activation curves for LVA Ca2+ currents in wild-type (filled circles and black lines) and PLCβ4−/− TC neurons (open circles and gray lines) showed that less steady-state inactivation of LVA Ca2+ currents increased the window currents in PLCβ4−/− TC neurons. Ca2+ currents were measured with activation steps to −40 mV from the given prepulse potentials ranged from −100 to −50 mV. Degree of inactivation was measured as the ratio of current density at given prepulse potential divided by maximum current density measured at −100 mV prepulse. Activation curves were obtained from Ca2+ currents measured when the membrane potential was hyperpolarized to −100 mV followed by the activation steps ranged from −90 to −40 mV. Smooth curves represent fits to the data with a Boltzmann equation. G, HVA Ca2+ current was measured in voltage steps from −60 to 0 mV before and after 10 μm nifedipine (Nif) treatment, in wild-type (left) and PLCβ4−/− (right) TC neurons. H, Current density of total, nifedipine-sensitive and nifedipine-insensitive HVA Ca2+ currents.
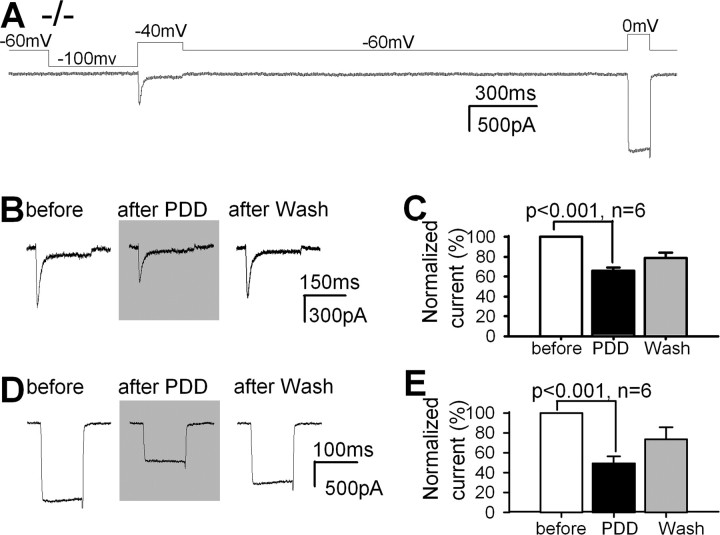
To confirm the involvement of the PLCβ4 signaling system in the modulation of Ca2+ currents, we examined the role of protein kinase C (PKC), a kinase downstream of PLCβ4, in the modulation of Ca2+ currents in TC neurons in vitro and in the pain response in vivo (Fig. 4). Amplitudes of both LVA and HVA Ca2+ currents of PLCβ4−/− TC neurons were decreased to 65.7 ± 3.4 and 49.1 ± 7.3%, respectively, after treating cells with 10 nm phorbol 12,13-didecanoate (PDD), a PKC activator (Fig. 4B–D), whereas 4α-PDD, an inactive structural analog of PDD, at the same concentration did not affect Ca2+ currents (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). LVA and HVA Ca2+ currents were significantly decreased with PDD (p < 0.001) at various concentrations (1–200 nm), and this effect was reversible with PDD at concentrations lower than 50 nm, although the full recovery could not be seen within the 1 h recording time, probably because of the long recovery time from the PKC activation and the slow current rundown from long recording time. Although we could not show the full recovery of currents, it would be important to point out that our data showed the clear tendency to reverse the downregulation of Ca2+ currents by PDD with washout step. These results suggest that the attenuated PKC pathway is responsible for the increased LVA and HVA Ca2+ currents observed in PLCβ4−/− TC neurons.
Figure 4.
PKC modulates LVA and HVA Ca2+ currents in TC neurons. A, LVA and HVA Ca2+ currents were measured by gramicidin D perforated patch recording from PLCβ4−/− TC neurons. B, LVA Ca2+ currents measured before and after adding 10 nm PDD, a PKC agonist, and after washing. C, Effect of PDD on LVA current amplitudes. D, HVA Ca2+ currents measured before and after the addition of PDD and after washing. E, Effect of PDD on HVA Ca2+ current amplitude.
We next tested whether modulation of PKC activity in thalamic neurons alters the visceral pain response in vivo. The visceral pain response was greater in PLCβ4−/− mice infused with PDD (10 nm) in the thalamic relay region than in PLCβ4−/− mice infused with vehicle (49.23 ± 7.47, n = 11 vs 31.57 ± 5.68, n = 9; p < 0.05). Furthermore, the visceral pain response was reduced in wild-type mice receiving thalamic infusion of chelerythrine (1 μm), a PKC antagonist (38.13 ± 5.67, n = 9 vs 61.25 ± 6.24, n = 12; p < 0.05). Therefore, the lower pain sensitivity phenotype of PLCβ4−/− mice could be rescued by thalamic infusion of a PKC agonist and could be induced by infusion of the same region of wild-type mice with a PKC antagonist. Collectively, these results suggest that the reduced visceral pain response in PLCβ4−/− mice is primarily attributable to the downregulation of PKC activity in TC neurons.
To explore the possibility that altered calcium current kinetic properties might also affect the burst firing tendency of TC neurons, we examined the kinetics of T-type Ca2+ currents of TC neurons. PLCβ4−/− TC neurons showed almost 10% of the maximum current with a prepulse potential of −60 mV, indicating that T-type Ca2+ channels were not fully inactivated (Fig. 3E, bottom trace of right panel). In contrast, wild-type TC neurons showed little T-type Ca2+ current (Fig. 3E, bottom trace of left panel). The window currents represented by the overlapping area under the inactivation and activation curves in PLCβ4−/− (gray lines) were larger than those in wild-type TC neurons (Fig. 3F, black lines). This reduced steady-state inactivation of the T-type Ca2+ current is probably responsible for the vigorous burst firing even after a slight hyperpolarization in PLCβ4−/− TC neurons (Fig. 2). The fast inactivation recovery properties of T-type Ca2+ channels, critical for their participation in rebound burst firing (Perez-Reyes, 2003), were unchanged in PLCβ4−/− TC neurons compared with wild-type TC neurons (τ = 97 and 92 ms, respectively). These results suggested that reduced steady-state inactivation of T-type Ca2+ currents in PLCβ4−/− TC neurons increases the propensity of TC neurons for burst firing.
Pharmacological dissection of HVA Ca2+ currents demonstrated that a large portion of the HVA Ca2+ currents in the cell body and proximal dendrites are mediated by L-type Ca2+ channels (Fig. 3G,H). Both total and nifedipine-sensitive HVA Ca2+ currents were greater in PLCβ4−/− TC neurons (p < 0.05), whereas there was no difference in nifedipine-insensitive HVA Ca2+ currents (Fig. 3H). This observation revealed that most of the increased HVA Ca2+ currents observed in PLCβ4−/− TC neurons were nifedipine-sensitive L-type Ca2+ currents.
Enhanced AHP in PLCβ4−/− TC neurons
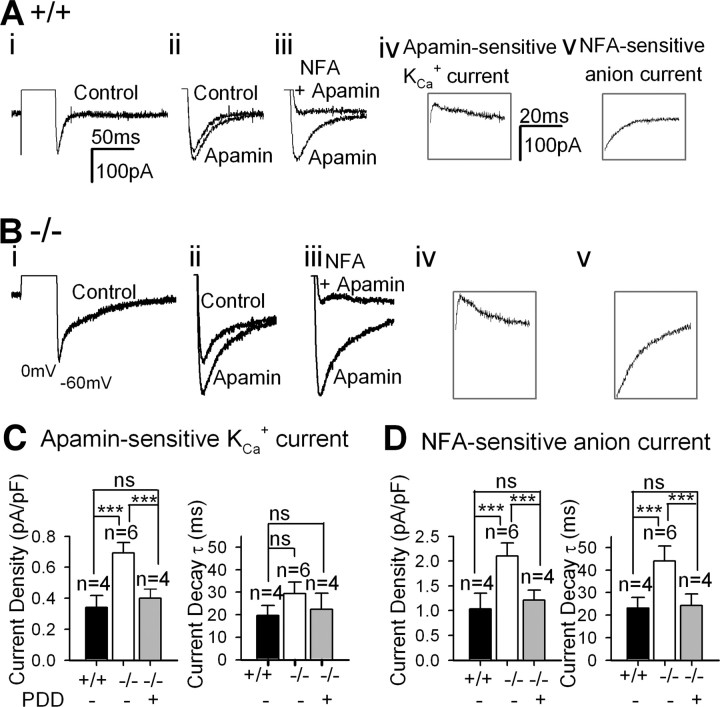
To examine whether the increased L-type Ca2+ currents have enhanced AHP activated by Ca2+ (Stocker, 2004), we measured AHP of TC neurons in brain slices. We observed that AHP after the tonic firing induced by depolarizing currents was greater in PLCβ4−/− than in wild-type TC neurons (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Then the components of AHP were quantified in a voltage-clamping mode. The AHP in TC neurons was observed as a net inward tail current, dissected pharmacologically (Fig. 5A,B). We measured the currents mediated by apamin-sensitive Ca2+-activated K+ channels (KCa) by trace subtraction (Fig. 5Aiv,Biv). The measured reversal potential of the inward tail current appeared to be approximately −50 mV and turned out to be −59 mV with junction potential correction (n = 5) in the presence of 100 nm apamin, close to the calculated ECl (−60 mV). This inward current was mostly suppressed by 100 μm niflumic acid (NFA), a Ca2+-activated Cl− current blocker (Fig. 5Aiii,Biii). The amplitude of NFA-sensitive current was obtained by subtraction (Fig. 5Av,Bv). The third component of AHP was insensitive to either apamin or NFA, and its amplitude was relatively small. The AHP current was greatly reduced in Ca2+-free extracellular buffer (data not shown), suggesting that Ca2+ influx is required for the activation of AHP current.
Figure 5.
Enhanced Ca2+-activated AHP in PLCβ4−/− TC neurons. Representative traces of AHP of wild-type (A) and PLCβ4−/− (B) TC neurons. Panels from i to v show traces recorded sequentially from a single TC neuron. i, AHP; a tail current was generated after a short depolarization in the presence of TTX. ii, Extended view of the tail current shown (i) before (control) and after addition of 100 nm apamin, an SK channel blocker. iii, AHP in the presence of 100 μm niflumic acid (NFA), a Ca2+-activated Cl− channel blocker, and apamin. iv, Apamin-sensitive K+ current, obtained by trace subtraction. v, NFA-sensitive current, obtained by trace subtraction. C, Amplitudes (left bar graph) and decay time constants τ (right bar graph) of apamin-sensitive KCa current of wild-type (filled bars), PLCβ4−/− TC neurons with (gray bars) or without (open bars) PDD. ***p < 0.001 by t test. D, Amplitudes (left bar graph) and decay time constants τ (right bar graph) of NFA-sensitive anion currents current of wild-type (filled bars), PLCβ4−/− TC neurons with (gray bars) or without (open bars) PDD.
PLCβ4−/− TC neurons showed larger amplitudes of both apamin-sensitive KCa currents (p < 0.001) and NFA-sensitive anion currents (p < 0.001) than wild-type neurons (Fig. 5C,D). The decay time constant τ for the NFA-sensitive current was larger in PLCβ4−/− than wild-type TC neurons (p < 0.001) (Fig. 5D), whereas there was no significant difference in τ for apamin-sensitive KCa currents (p = 0.329) (Fig. 5C) between PLCβ4−/− and wild-type TC neurons. PDD reversed these enlarged Ca2+-activated AHP currents in PLCβ4−/− TC neurons (Fig. 5C,D), which suggested that the enhanced Ca2+-activated AHP currents were probably attributable to the downregulation of PKC activity in TC neurons.
Blocking thalamic L-type Ca2+ channels or SK channels increases the tonic firing rate of TC neurons in brain slices and increases the visceral pain response in vivo
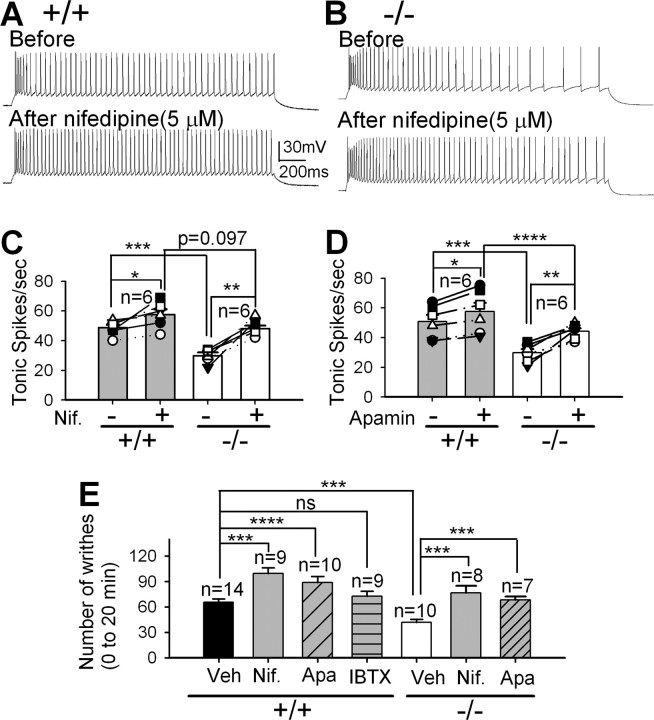
We next examined whether the modulation of either T-type or L-type Ca2+ current in TC neurons alters the pain response as expected from our initial observations in PLCβ4−/− mice. Our previous study demonstrated that a lack of Cav3.1 T-type Ca2+ channels in thalamocortical neurons increases the visceral pain response (Kim et al., 2003). Here, we examined the influence of L-type Ca2+ channels on the firing rate of TC neurons and on the visceral pain response by blocking the L-type channels with nifedipine (1–10 μm). The tonic firing rate of TC neurons in brain slices increased by ∼19% in wild-type (48.7 ± 2.0 to 57.5 ± 3.6, p < 0.005 by paired t test) (Fig. 6A,C) and by ∼65% in PLCβ4−/− (29.3 ± 2.1 to 48.0 ± 1.9, p < 0.001 by paired t test) (Fig. 6B,C) TC neurons in the presence of nifedipine (5 μm) without affecting the burst firing rate (supplemental Fig. S3, available at www.jneurosci.org as supplemental material), which indicates that the effect of nifedipine at this concentration was selective for L-type Ca2+ channels. It is noteworthy that the difference in the tonic firing rate between wild-type and PLCβ4−/− TC neurons was abolished by nifedipine treatment (5 μm), suggesting that the reduced tonic firing rate in PLCβ4−/− TC neurons was mainly attributable to increased L-type Ca2+ currents. The tonic firing rate was also increased in wild-type (50.7 ± 4.6 to 57.8 ± 5.9, p < 0.005 by paired t test) and in PLCβ4−/− (30.5 ± 2.6 to 44.2 ± 2.1, p < 0.001 by paired t test) TC neurons in the presence of apamin (100 nm) (Fig. 6D) without affecting the burst firing rate (supplemental Fig. S3, available at www.jneurosci.org as supplemental material).
Figure 6.
Blocking either thalamic L-type Ca2+ channels or SK channels increases the firing rate of TC neurons in vitro and the visceral pain response in vivo. A, Tonic firing of a wild-type TC neuron before and after adding nifedipine (5 μm). B, Tonic firing of a PLCβ4−/− TC neuron before and after adding nifedipine (5 μm). C, There was a significant increase in the tonic firing rate in both wild-type TC neurons (*p < 0.005 by paired t test on 6 pairs) and PLCβ4−/− TC neurons (**p < 0.001 by paired t test on 6 pairs) after treating with nifedipine (Nif; 5 μm). There was no difference in tonic firing rate (p = 0.097 by t test) between wild-type and PLCβ4−/− TC neurons after nifedipine treatment, whereas the tonic firing rate of PLCβ4−/− TC neurons was lower than wild-type TC neurons (***p < 0.005 by t test) before treatment with nifedipine (5 μm). D, There was a significant increase in the tonic firing rate in both wild-type TC neurons (*p < 0.005 by paired t test on 6 pairs) and PLCβ4−/− TC neurons (**p < 0.001 by paired t test on 6 pairs) before and after treating apamin (100 nm). There was a difference in tonic firing rate between wild-type and PLCβ4−/− TC neurons before (***p < 0.005 by t test) and after (****p < 0.01 by t test) apamin treatment. E, Visceral pain responses of wild-type and PLCβ4−/− mice with thalamic infusion of 5 μm nifedipine (Nif), 100 nm apamin (Apa), 50 nm iberiotoxin (IBTX), or vehicle (Veh) (***p < 0.005 and ****p < 0.01 by t test), respectively.
To determine whether the increased tonic firing rate of TC neurons caused by blockade of L-type Ca2+ channels or Ca2+-dependent small-conductance SK-type K+ channels alters pain responses in vivo, we examined the visceral pain response of mice after infusing the pharmacologic antagonist into the thalamic relay regions. The number of writhing response was significantly enhanced in wild-type mice infused with 5 μm nifedipine (99.6 ± 6.7, p < 0.005) and apamin (88.6 ± 7.5, p < 0.01) compared with those infused with vehicle (65.3 ± 4.2), whereas blockage of large conductance Ca2+-activated K+ BK channels with iberiotoxin (50 nm) did not affect visceral pain responses (72.5 ± 6.0) (Fig. 6E). It was also increased in PLCβ4−/− mice infused with 5 μm nifedipine (76.4 ± 8.6, p < 0.005) and with 100 nm apamin (68.2 ± 4.0, p < 0.01) compared with those with vehicle (41.9 ± 3.5).
We also confirmed the expression of L-type Ca2+ channels [α1C (Cav 1.2) and α1D (Cav 1.3)] and SK channel subunits in thalamic neurons (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). These results support our hypothesis that increased Ca2+ influx via L-type Ca2+ channels in PLCβ4−/− TC neurons augments AHP, which eventually leads to both decreased tonic firing rates in TC neurons and diminished pain responses.
Discussion
Thalamic PLCβ4–PKC pathway tunes the firing modes of TC neurons
Here we have demonstrated that thalamic PLCβ4–PKC pathway regulates the firing properties of individual TC neurons and thereby controls visceral pain responses. This was confirmed by experiments using electrophysiological, pharmacological, and genetic tools, in vitro as well as in vivo.
Previous studies have shown that activation of thalamic mGluR1s produces a long-term enhancement of thalamic excitatory responses (McCormick and von Krosigk, 1992; von Krosigk et al., 1999) and switches the visual response mode of LGN cells from burst to tonic firing (Godwin et al., 1996; Golshani et al., 1998). On the basis of these findings in the thalamus as well as a putative mechanism suggested previously in the hippocampus (Charpak et al., 1990), it has been proposed that activation of mGluR1 enhances the excitability of thalamic neurons by slow evoked EPSPs (McCormick and von Krosigk, 1992) or synaptic facilitation (Pedroarena and Llinás, 2001). It was reported that PLCβ4 is extensively linked to mGluR1 in thalamic relay neurons evidenced by the fact that thalamic infusion of mGluR antagonist and PLC blocker had the same effect on Formalin-induced inflammatory pain responses in mice, whereas mGluR1 and PLCβ4 are predominant subtypes (Miyata et al., 2003). Therefore, our results may present a new mechanism for the corticothalamic control, one mediated via the mGluR1–PLCβ4–PKC pathway. We performed other types of pain responses such as tail-flick test and acute mechanical pain responses, which are known to measure spinal reflexes mostly, and found no significant difference between wild-type and PLCβ4−/− mice (data not shown), which was consistent with the literature (Miyata et al., 2003). Therefore, we believe that there is no significant change in ascending pain input pathway at the spinal level or periphery in PLCβ4−/− mice. Our model suggests that the mGluR1–PLCβ4–PKC signal transduction cascade acts as a switch regulating the firing modes of TC neurons (supplemental Fig. S4, available at www.jneurosci.org as supplemental material).
The role of low-threshold burst firing of TC neurons in signal transmission has been controversial, whereas tonic firing putatively serves as a relay mode. Thalamic burst firing has been associated with drowsy/sleep states or loss of consciousness in many studies (von Krosigk et al., 1993; Kim et al., 2001; Crunelli and Leresche, 2002) and has been considered a “closed gate” state that afferent sensory signals encounter (Kim et al., 2003). However, it has also been suggested that burst firing represents a stronger signal transmission from LGN neurons to cortex, serving as a cortical “wake-up call” (Sherman, 2001; Swadlow and Gusev, 2001). Burst firing in rabbit LGN neurons has also been associated with inattentive states (Bezdudnaya et al., 2006). In our experiments, burst firing was dramatically increased in PLCβ4−/− TC neurons, accompanying the decreased pain response that was observed in the PLCβ4−/− mice. This paradigm thus produced a state that is the opposite of that encountered in our previous study, wherein the genetic deletion of the thalamic burst firing resulted in an enhanced visceral pain response in the mouse (Kim et al., 2003). Together, these findings support the idea that burst firing acts as an inhibitor of visceral pain signal transmission to the cortex.
Concomitant modulation of T- and L-type Ca2+ currents regulates the firing properties of TC neurons
Our results suggest that the activity level of thalamic PLCβ4–PKC pathway controls the firing properties of TC neurons by simultaneously regulating T-type and L-type Ca2+ currents. Our data show that a PKC agonist, but not an inactive structural analog, decreases T-type and L-type Ca2+ currents reversibly and that the pain phenotype of PLCβ4−/− mice could be rescued by thalamic infusion of a PKC agonist. The present experiments also demonstrate that the pain phenotype can be reproduced by infusion of the same region in a wild-type mice with a PKC antagonist. Together, these findings strongly support the notion that the phenotype of mutant mice was primarily caused by disruption of the PLCβ4–PKC pathway and not by developmental or compensatory changes. This model was further supported by our findings that blocking thalamic L-type Ca2+ channels recapitulated the expected alteration in both firing properties of TC neurons and the visceral pain response (Fig. 6).
Previous reports have identified altered Ca2+ currents in several knock-out mice. For example, mice lacking P/Q-type Ca2+ channels show increased T-type Ca2+ currents in TC neurons (Zhang et al., 2002; Song et al., 2004) and enhanced N-type (Cav2.2) Ca2+ currents in cerebellar Purkinje neurons and hippocampal neurons (Jun et al., 1999). Estrogen receptor-deficient mice exhibit increased expression of L-type Ca2+ channels (Johnson et al., 1997). However, in no case has disturbance of one pathway that simultaneously altered both LVA and HVA Ca2+ currents been reported.
Previous studies have yielded conflicting results in regard to PKC modulation of T-type Ca2+ channels; both upregulation of the T-type currents in dorsal root ganglion neurons (Schroeder et al., 1990) and downregulation of currents expressed in Xenopus oocytes (Park et al., 2003) or no effect on T-type Ca2+ channel activity in hippocampal neurons cultured from rats or guinea pigs (O'Dell and Alger, 1991) have been reported. Our results support the idea that PKC downregulates the Cav3.1 T-type Ca2+ current. This discrepancy might come from different subtypes of channels or different cell types. However, the precise molecular mechanism and site of PKC-mediated phosphorylation remain to be clarified (Catterall, 2000; Perez-Reyes, 2003).
The increased amplitude of T-type Ca2+ current in PLCβ4−/− TC neurons correlates well with an increase in the number of spikes per burst. In addition, PLCβ4−/− TC neurons display a greater tendency for burst firing near the resting membrane potential, probably attributable to less steady-state inactivation of the T-type Ca2+ current. The ability of T-type channels in PLCβ4−/− TC neurons to open at potentials where they are normally inactive provides an opportunity to generate an enlarged window current, that is, the overlap region between the activation and steady-state inactivation curves. All three T-type channel subtypes are predicted to generate window currents mediated by less than ∼1% of the channels in physiological conditions (Crunelli et al., 2006). Therefore, enlarged window current in PLCβ4−/− TC neurons could contribute to the dramatic increase in the frequency of burst spikes after inhibitory inputs observed in PLCβ4−/− mice.
Inhibitory role of thalamic L-type Ca2+ channels in pain sensory transmission
Our data support the model of an inhibitory role for L-type Ca2+ channels in the firing of TC neurons and transmission of pain sensation, via interaction with KCa channels. This is demonstrated by our findings that blocking L-type Ca2+ channels increased the tonic firing rate and visceral pain responses both in wild-type and PLCβ4−/− mice. In particular, a stronger effect of nifedipine on both tonic firing rate and pain responses in PLCβ4−/− mice supports our hypothesis that increased L-type Ca2+ currents in PLCβ4−/− TC neurons decrease the tonic firing rate, resulting in a decreased pain response.
The most prominent consequence of the increase in Ca2+ influx through L-type Ca2+ channels in PLCβ4−/− TC neurons is the subsequent increase in Ca2+-activated AHP, which decreases the firing rate (Stocker, 2004). The observation that TC neurons express L-type Ca2+ channels and SK channel subunits (supplemental Fig. S4, available at www.jneurosci.org as supplemental material) supports a role for functional coupling between these two channels. Coupling between voltage-gated Ca2+ channels and Ca2+-activated potassium channels has also been reported (Stocker, 2004). In CA1 pyramidal neurons, L-type (but not P/Q- or N-type) Ca2+ channels and SK channels are colocalized in the soma, whereas N-type Ca2+ channels are colocalized with BK channels (Marrion and Tavalin, 1998). Recently, it was reported that Cav1.3 L-type Ca2+ channels are physically linked to SK channels in atrial tissue (Lu et al., 2007). In these reports, most of the AHP current has been attributed to Ca2+-activated K+ channels including BK and SK channels, as well as K+ current components whose molecular identity is currently unknown (Bond et al., 2004; Stocker, 2004). Surprisingly, we found that, in addition to an apamin-sensitive SK component, there was a large NFA-sensitive anion component in Ca2+-activated AHP of TC neurons (Fig. 5). Additional study is needed to determine the molecular identity of the channels mediating this NFA-sensitive Ca2+-activated anion current.
A molecular switch for thalamic pain sensory gating
Recently, it was shown that the state of thalamic neurons plays a critical role in controlling sensory information processing in humans (Schiff et al., 2007). This finding is consistent with the data presented in this paper. Our results provide new insights into how cortical excitatory inputs to the thalamus modulate the flow of sensory information to the cortex. Our study suggests that a single cellular signal transduction pathway, PLCβ4–PKC, is linked to both T- and L-type Ca2+ channels and simultaneously regulates both burst and tonic firing. These findings also highlight the functional significance of the thalamic mGluR1–PLCβ4–PKC pathway as a “molecular switch” to set the state of TC neurons in favor of either tonic or burst firing, which would efficiently sharpen the transition between open and closed gate status in pain sensory processing.
Footnotes
This work was supported by a National Honor Scientist Grant from the Ministry of Science and Technology, Korea, by the Centre of Excellence program, and by the Top-Brand program at Korea Institute of Science and Technology. We especially thank our colleagues J. Lee and C. Kim for their helpful technical support and valuable discussion.
References
- Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci. 2005;25:10990–11002. doi: 10.1523/JNEUROSCI.3229-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol. 2002;543:49–70. doi: 10.1113/jphysiol.2002.018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Charpak S, Gähwiler BH, Do KQ, Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347:765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Attenuated pain responses in mice lacking Ca(V)3.2 T-type channels. Genes Brain Behav. 2007;6:425–431. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–190. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin DW, Vaughan JW, Sherman SM. Metabotropic glutamate receptors switch visual response mode of lateral geniculate nucleus cells from burst to tonic. J Neurophysiol. 1996;76:1800–1816. doi: 10.1152/jn.1996.76.3.1800. [DOI] [PubMed] [Google Scholar]
- Golshani P, Warren RA, Jones EG. Progression of change in NMDA, non-NMDA, and metabotropic glutamate receptor function at the developing corticothalamic synapse. J Neurophysiol. 1998;80:143–154. doi: 10.1152/jn.1998.80.1.143. [DOI] [PubMed] [Google Scholar]
- Guido W, Lu SM, Sherman SM. Relative contributions of burst and tonic responses to the receptive field properties of lateral geniculate neurons in the cat. J Neurophysiol. 1992;68:2199–2211. doi: 10.1152/jn.1992.68.6.2199. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Zheng W, Korach KS, Scheuer T, Catterall WA, Rubanyi GM. Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J Gen Physiol. 1997;110:135–140. doi: 10.1085/jgp.110.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annu Rev Neurosci. 2000;23:1–37. doi: 10.1146/annurev.neuro.23.1.1. [DOI] [PubMed] [Google Scholar]
- Jun K, Piedras-Rentería ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin HS. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci U S A. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin HS. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302:117–119. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- Le Masson G, Renaud-Le Masson S, Debay D, Bal T. Feedback inhibition controls spike transfer in hybrid thalamic circuits. Nature. 2002;417:854–858. doi: 10.1038/nature00825. [DOI] [PubMed] [Google Scholar]
- Llinás R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- McCormick DA, von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci U S A. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M, Kashiwadani H, Fukaya M, Hayashi T, Wu D, Suzuki T, Watanabe M, Kawakami Y. Role of thalamic phospholipase Cβ4 mediated by metabotropic glutamate receptor type 1 in inflammatory pain. J Neurosci. 2003;23:8098–8108. doi: 10.1523/JNEUROSCI.23-22-08098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Alger BE. Single calcium channels in rat and guinea-pig hippocampal neurons. J Physiol. 1991;436:739–767. doi: 10.1113/jphysiol.1991.sp018577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Jeong SW, Perez-Reyes E, Lee JH. Modulation of Ca(v)3.2 T-type Ca2+ channels by protein kinase C. FEBS Lett. 2003;547:37–42. doi: 10.1016/s0014-5793(03)00665-3. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM, Llinás R. Interactions of synaptic and intrinsic electroresponsiveness determine corticothalamic activation dynamics. Thalamus Relat Syst. 2001;1:3–14. [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O'Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Schroeder JE, Fischbach PS, McCleskey EW. T-type calcium channels: heterogeneous expression in rat sensory neurons and selective modulation by phorbol esters. J Neurosci. 1990;10:947–951. doi: 10.1523/JNEUROSCI.10-03-00947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. A wake-up call from the thalamus. Nat Neurosci. 2001;4:344–346. doi: 10.1038/85973. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Song I, Kim D, Choi S, Sun M, Kim Y, Shin HS. Role of the α1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004;24:5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Arousal: revisiting the reticular activating system. Science. 1996;272:225–226. doi: 10.1126/science.272.5259.225. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of “bursting” thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Vidnyanszky Z, Gorcs TJ, Negyessy L, Borostyankio Z, Knopfel T, Hamori J. Immunocytochemical visualization of the mGluR1a metabotropic glutamate receptor at synapses of corticothalamic terminals originating from area 17 of the rat. Eur J Neurosci. 1996;8:1061–1071. doi: 10.1111/j.1460-9568.1996.tb01273.x. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Monckton JE, Reiner PB, McCormick DA. Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus. Neuroscience. 1999;91:7–20. doi: 10.1016/s0306-4522(98)00557-0. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y. Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cbeta in mouse brain. Eur J Neurosci. 1998;10:2016–2025. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mori M, Burgess DL, Noebels JL. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci. 2002;22:6362–6371. doi: 10.1523/JNEUROSCI.22-15-06362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]