Abstract
Dopamine D1 receptors in the prefrontal cortex (PFC) are important for prefrontal functions, and it is suggested that stimulation of prefrontal D1 receptors induces an inverted U-shaped response, such that too little or too much D1 receptor stimulation impairs prefrontal functions. Less is known of the role of D2 receptors in cognition, but previous studies showed that D2 receptors in the hippocampus (HPC) might play some roles via HPC–PFC interactions. We measured both D1 and D2 receptors in PFC and HPC using positron emission tomography in healthy subjects, with the aim of elucidating how regional D1 and D2 receptors are differentially involved in frontal lobe functions and memory. We found an inverted U-shaped relation between prefrontal D1 receptor binding and Wisconsin Card Sorting Test performance. However, prefrontal D2 binding has no relation with any neuropsychological measures. Hippocampal D2 receptor binding showed positive linear correlations not only with memory function but also with frontal lobe functions, but hippocampal D1 receptor binding had no association with any memory and prefrontal functions. Hippocampal D2 receptors seem to contribute to local hippocampal functions (long-term memory) and to modulation of brain functions outside HPC (“frontal lobe functions”), which are mainly subserved by PFC, via the HPC–PFC pathway. Our findings suggest that orchestration of prefrontal D1 receptors and hippocampal D2 receptors might be necessary for human executive function including working memory.
Keywords: dopamine, D1 receptors, D2 receptors, prefrontal cortex, hippocampus, positron emission tomography
Introduction
Because dopamine D1 receptors in the prefrontal cortex (PFC) are several times more abundant than D2 receptors (Hall et al., 1994), the relationship between D1 receptors and PFC functions have been widely investigated. Sawaguchi and Goldman-Rakic (1994) demonstrated that local administration of D1 receptor antagonists into PFC induced impairment in working memory task in nonhuman primate. In human, Müller et al. (1998) reported that systemic administration of a mixed D1/D2 agonist facilitated working memory, whereas the selective D2 agonist had no effect, indicating that the dopaminergic modulation of working memory processes is mediated primarily via D1 receptors. The use of positron emission tomography (PET) allows us to quantify dopamine receptors in vivo, and previous studies reported that altered prefrontal D1 receptors in schizophrenia were associated with working memory deficits (Okubo et al., 1997; Abi-Dargham et al., 2002).
In contrast to D1 receptors, relatively less attention has been paid to the role of prefrontal D2 receptors in cognitive functions. It was reported that blockade of D2 receptors in PFC did not impair working memory in nonhuman primate (Sawaguchi and Goldman-Rakic, 1994), but some human studies reported that systemic administration of D2 agonist or antagonist modulated cognitive functions that are subserved by the prefrontal cortex (McDowell et al., 1998; Mehta et al., 1999). Because the density of D2 receptors in extrastriatal regions is very low (Suhara et al., 1999), PET studies investigating the involvement of extrastriatal D2 receptors in cognition have been limited. With the introduction of high-affinity PET radioligands such as [11C]FLB457, it has become possible to quantify extrastriatal D2 receptors by PET (Halldin et al., 1995). Using [11C]FLB457, Kemppainen et al. (2003) reported that a reduction of D2 receptors in the hippocampus (HPC) in Alzheimer's disease patients was correlated with memory impairments. Our recent PET study also showed that D2 receptors in HPC were associated not only with memory function but also with frontal lobe functions (Takahashi et al., 2007), suggesting dopaminergic modulation on HPC–PFC interactions during the cognitive process (Laroche et al., 2000; Thierry et al., 2000; Goto and Grace, 2008).
In this study, we measured both D1 and D2 receptors in PFC and HPC using PET in normal healthy subjects, and aimed to elucidate how regional D1 and D2 receptors are differentially involved in neurocognitive performance including memory and frontal lobe functions. A body of animal studies has indicated that stimulation of D1 receptors in PFC produces an inverted U-shaped dose–response curve, such that too little or too much D1 receptor stimulation impairs PFC functions (Goldman-Rakic et al., 2000; Williams and Castner, 2006; Vijayraghavan et al., 2007). We hypothesized that prefrontal D1 receptors would be more related to frontal lobe functions than prefrontal D2 receptors, and that, specifically, an inverted U-shaped relation between prefrontal D1 receptor binding and prefrontal functions would be observed in the normal physiological condition in healthy volunteers. In addition, we predicted that D2 receptors in HPC would be more related to memory than D1 receptors in HPC.
Materials and Methods
Subjects.
Twenty-three healthy male volunteers [mean age 25.7 ± (SD) 4.3 years] were studied. Seven of the 23 subjects had participated in our earlier study (Takahashi et al., 2007). They did not meet the criteria for any psychiatric disorder based on unstructured psychiatric screening interviews. None of the controls were using alcohol at the time, nor did they have a history of psychiatric disorder, significant physical illness, head injury, neurological disorder, or alcohol or drug dependence. All subjects were right-handed according to the Edinburgh Handedness Inventory. All subjects underwent magnetic resonance imaging (MRI) to rule out cerebral anatomic abnormalities. After complete explanation of the study, written informed consent was obtained from all subjects, and the study was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences, Chiba Japan.
PET scanning.
PET studies were performed on ECAT EXACT HR+ (CTI; Siemens). The system provides 63 planes and a 15.5 cm field of view. To minimize head movement, a head fixation device (Fixster) was used. A transmission scan for attenuation correction was performed using a germanium 68–gallium 68 source. Acquisitions were done in three-dimensional mode with the interplane septa retracted. For evaluation of D1 receptors, a bolus of 213.9 ± 20.5 MBq of [11C]SCH23390 with specific radioactivities (52.1 ± 28.9 GBq/μmol) was injected intravenously from the antecubital vein with a 20 ml saline flush. For evaluation of extrastriatal D2 receptors, a bolus of 215.4 ± 24.5 MBq of [11C]FLB457 with high specific radioactivities (171.0 ± 58.0 GBq/μmol) was injected in the same way. The mean injected amounts of [11C]SCH23390 and [11C]FLB457 were 1.18 ± 0.20 μg and 0.47 ± 0.17 μg, respectively. Dynamic scans were performed for 60 min for [11C]SCH23390 and 90 min for [11C]FLB 457 immediately after the injection. All emission scans were reconstructed with a Hanning filter cutoff frequency of 0.4 (full width at half maximum, 7.5 mm). MRI was performed on Gyroscan NT (Philips Medical Systems) (1.5 T). T1-weighted images of the brain were obtained for all subjects. The scan parameters were 1-mm-thick, three-dimensional T1 images with a transverse plane (repetition time/echo time, 19/10 milliseconds; flip angle, 30°; scan matrix, 256 × 256 pixels; field of view, 256 × 256 mm; number of excitations, 1).
Quantification of D1 and D2 receptors in PFC and HPC.
The tissue concentrations of the radioactivities of [11C]SCH23390 and [11C]FLB457 were obtained from regions of interest (ROIs) defined on the PET images of summated activity for 60 and 90 min, respectively, with reference to the individual MRIs that were coregistered on summated PET images and the brain atlas. The regions were PFC, HPC and cerebellar cortex. Each ROI consisted of three axial slices. ROI of PFC occupies the middle third of the middle frontal gyrus and the rostral portion of the inferior frontal gyrus (approximately corresponding to the dorsolateral prefrontal cortex or Brodmann area 46). ROI of HPC was set at the level of the midbrain. The anterior boundary was identified at the level of the inferior horn of the lateral ventricle. The posterior boundary was identified at the level of the collateral sulcus. Although [11C]FLB457 accumulates to a high degree in the striatum, striatal data were not evaluated because the duration of the [11C]FLB457 PET study was not sufficient to obtain equilibrium in the striatum (Olsson et al., 1999; Suhara et al., 1999). Quantitative analysis was performed using the three-parameter simplified reference tissue model (Lammertsma and Hume, 1996). The cerebellum was used as reference region because it has been shown to be almost devoid of D1 and D2 receptors (Farde et al., 1987; Olsson et al., 1999; Suhara et al., 1999). The model provides an estimation of the binding potential (BPND (nondisplaceable)) (Innis et al., 2007), which is defined by the following equation: BPND = k3/k4 = f2 Bmax/{Kd [1 + Σi Fi/Kdi]}, where k3 and k4 describe the bidirectional exchange of tracer between the free compartment and the compartment representing specific binding, f2 is the “free fraction” of nonspecifically bound radioligand in brain, Bmax is the receptor density, Kd is the equilibrium dissociation constant for the radioligand, and Fi and Kdi are the free concentration and the dissociation constant of competing ligands, respectively (Lammertsma and Hume, 1996).
Neuropsychological tests.
A battery of cognitive tests was given by an experienced clinical neuropsychologist. The neuropsychological tests used were Rey's Auditory Verbal Learning Test (RAVLT), Rey-Osterrieth's Complex Figure Test (ROCFT), Keio version of the Wisconsin Card Sorting Test (WCST) (Igarashi et al., 2002), Verbal Fluency Test, and Raven's Colored Progressive Matrices (RCPM). RAVLT is used to evaluate the performance of verbal memory, and ROCFT is used as a measure of nonverbal visual memory. RAVLT and ROCFT were performed in the standard manner (Lezak, 1995). In RAVLT, 15 words were presented auditorily in the same sequence in five trials, ending with a free recall of the words (immediate recall). After the five trials, an interference list was presented and recalled, and then the subjects were instructed to recall the first list of words (delayed recall). In ROCFT, after the copy trial, subjects were asked to reproduce a figure from memory (immediate recall). After a 15 min pause, the subjects were asked to reproduce the figure from memory again (delayed recall). WCST is a test for executive function or cognitive flexibility involving working memory (Berman et al., 1995). It has been shown to be sensitive to dysfunction of PFC (Nelson, 1976). In WCST, categories achieved (CA), total errors (TE) and perseverative errors of Nelson (PE) were evaluated (Lezak, 1995). In the phonemic verbal fluency test, the subject was requested to retrieve in 1 min as many words as possible beginning with the Japanese syllabic characters (hiragana) “shi,” “i” and “re,” respectively. In the semantic verbal fluency test, the subject was requested to recall in 1 min as many words as possible belonging to a given semantic category (e.g., animals, fruit) (Lezak, 1995). RCPM was used as a general visuospatial intelligence test.
Statistical analyses.
Although the selection of subjects was confined to young males in their 20's and 30's, the possible age effect on the BPND values of [11C]SCH23390 and [11C]FLB457, and neuropsychological performance were examined using Pearson correlation analysis. To explore the relation between D1 and D2 receptors and cognitive functions, linear regression between the BPND values of each ROI and each neuropsychological performance was analyzed, and the threshold for significance was set at p = 0.05/2 = 0.025 to correct for two regions (PFC and HPC). Although a single dominant factor underlying the scores on all tests, i.e., general cognitive ability, might contribute to intercorrelations across the tests, what we measure with neuropsychological tests is, by nature, a dimensionality of cognitive ability. Therefore, correction of p values for multiple comparisons was done only for regions, not for multiple neuropsychological tests. To examine putative nonlinear (inverted U-shaped) relations between prefrontal dopamine receptors and frontal lobe functions, quadratic regression between the BPND values of [11C]SCH23390 and [11C]FLB457 in PFC and neuropsychological performance was analyzed by SPSS package (SPSS).
To confirm the findings of the ROI analysis, parametric images of BPND (Gunn et al., 1997) were analyzed using statistical parametric mapping software (SPM2) (Wellcome Department of Imaging, Institute of Neurology, University College of London, London, UK). Normalized BPND images were smoothed with a Gaussian filter to 16 mm full-width half-maximum. Using each individual cognitive performance as covariate, regression analyses with the BPND images and the covariates were performed.
Results
The mean [11C]SCH23390 BPND values of PFC and HPC were 0.41 ± 0.06 (range: 0.29–0.59) and 0.33 ± 0.09 (range: 0.20–0.53), respectively. The mean [11C]FLB457 BPND values of PFC and HPC were 1.16 ± 0.21 (range: 0.82–1.58) and 1.57 ± 0.28 (range: 0.98–1.92), respectively. The mean scores of the neuropsychological data are shown in Table 1. There was no age effect on the BPND values of [11C]SCH23390 and [11C]FLB457 in the two ROIs, nor on any neuropsychological performance (p > 0.01).
Table 1.
Mean scores of neuropsychological tests and linear relations between and neuropsychological measures and BPND values of [11C]SCH23390 and [11C]FLB457 in the prefrontal cortex and hippocampus
| Neuropsychological tests | Mean scores | Prefrontal cortex r (p) |
Hippocampus r (p) |
||
|---|---|---|---|---|---|
| [11C]SCH23390 | [11C]FLB457 | [11C]SCH23390 | [11C]FLB457 | ||
| RALVT immediate | 57.3 ± 6.2 | 0.07 (0.74) | 0.16 (0.47) | 0.10 (0.66) | 0.37 (0.09) |
| RALVT delayed | 13.0 ± 1.5 | 0.14 (0.53) | 0.02 (0.94) | 0.08 (0.72) | 0.28 (0.20) |
| ROCFT immediate | 27.7 ± 3.9 | 0.11 (0.63) | 0.31 (0.15) | 0.21 (0.34) | 0.73 (p < 0.001)** |
| ROCFT delayed | 27.3 ± 4.8 | 0.12 (0.58) | 0.38 (0.07) | 0.11 (0.60) | 0.67 (p < 0.001)** |
| WCST CA | 5.4 ± 1.2 | 0.42 (0.049)* | 0.03 (0.89) | 0.21 (0.33) | 0.30 (0.17) |
| WCST TE | 11.3 ± 3.7 | −0.41 (0.049)* | −0.15 (0.51) | −0.30 (0.16) | −0.51 (0.01)** |
| WCST PE | 0.8 ± 1.4 | −0.27 (0.21) | −0.18 (0.42) | −0.31 (0.15) | −0.59 (0.003)** |
| Phonemic verbal fluency | 30.9 ± 9.3 | 0.21 (0.35) | 0.21 (0.34) | 0.20 (0.36) | 0.47 (0.02)** |
| Semantic verbal fluency | 46.1 ± 7.9 | −0.07 (0.76) | 0.09 (0.69) | 0.06 (0.77) | 0.17 (0.45) |
| RCPM (sec) | 188.5 ± 36.0 | 0.10 (0.65) | −0.04 (0.87) | 0.11 (0.64) | 0.08 (0.70) |
*p < 0.05.
**Significant after correction for multiple statistical tests (new significance threshold: p < 0.025[0.05/2]).
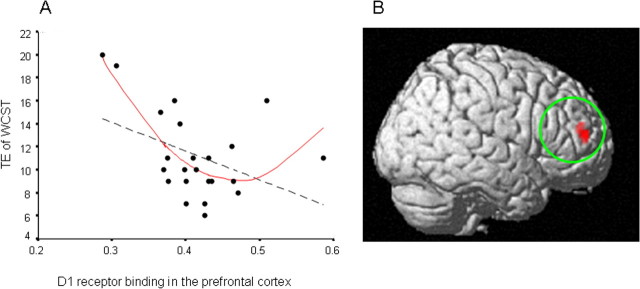
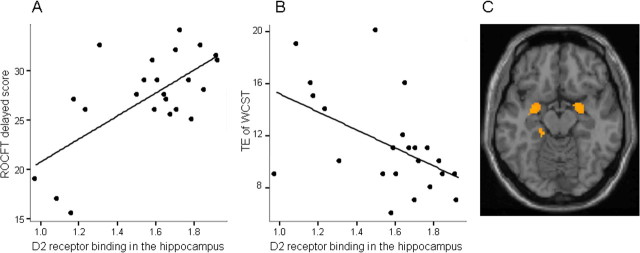
Quadratic regression analysis revealed a significant “U-shaped” relation between the BPND value of [11C]SCH23390 in PFC and TE of WCST (p < 0.001, r = 0.72). (Because TE of WCST is a negative measure of frontal lobe function, the relation is not “inverted”) (Fig. 1). The BPND value of [11C]SCH23390 in PFC and CA of WCST also showed significant quadratic (inverted U-shaped) relation (p < 0.001, r = 0.78). However, no quadratic relation was found between the BPND value of [11C]FLB 457 in PFC and any neuropsychological measures. The linear relations between neuropsychological measures and the BPND value of each ROI are shown in Table 1. As for D1 receptors, the BPND value of [11C]SCH23390 in PFC was positively correlated with CA of WCST (p = 0.049, r = 0.42), and negatively correlated with TE of WCST (p = 0.049, r = −0.41) although these relations did not survive a threshold corrected for multiple comparisons. The BPND value of [11C]SCH23390 in HPC was not correlated with any neuropsychological measures. With regard to D2 receptors, the BPND value of [11C]FLB457 in HPC was positively correlated with immediate and delayed recall scores of ROCFT and phonemic verbal fluency, and negatively correlated with CA and TE of WCST. The BPND value of [11C]FLB457 in PFC was not correlated with any neuropsychological measures. Figure 2 shows these relationships.
Figure 1.
Quadratic (inverted U-shaped) relation between D1 receptor binding in PFC and performance of WCST. A, ROI analysis revealed a significant quadratic regression between the BPND value of [11C]SCH23390 in PFC (BP D1 PFC) and TE of WCST. Red solid line, quadratic regression; black broken line, linear regression. Based on ROI analysis, the relation between BP D1 PFC and TE can be expressed as follows: TE = 326.92(BP D1 PFC −0.47) 2+9.10. B, Using this equation, SPM analysis also revealed a significant quadratic regression between prefrontal D1 receptor binding and TE of WCST (p < 0.001, uncorrected, extent threshold >30 voxels).
Figure 2.
Correlations between D2 receptor binding in the hippocampus and memory. A, B, Significant positive linear correlations between the BPND value of [11C]FLB457 in the hippocampus and the delayed recall score of ROCFT and (B) TE of WCST revealed by ROI analysis. C, The SPM result of a positive linear correlation between hippocampal D2 receptor binding and the delayed recall score of ROCFT is shown (p < 0.005, uncorrected, extent threshold >30 voxels).
D1 binding in PFC showed significant correlation with D1 binding in HPC (r = 0.74, p < 0.001) and trend level correlation with D2 binding in PFC (r = 0.41, p = 0.05), but no correlation with D2 binding in HPC (r = 0.27, p = 0.22). D2 binding in HPC showed significant correlation with D2 binding in PFC (r = 0.50, p = 0.02) and trend level correlation with D1 binding in HPC (r = 0.36, p = 0.09). D2 binding in PFC showed no correlation with D1 binding in HPC.
Using SPM2, we conduced standard voxel-based morphometry without modulation (Ashburner and Friston, 2000) to test whether the BPND values of [11C]SCH23390 and [11C]FLB457 in PFC and HPC were related to the prefrontal and hippocampal gray matter concentration in the normalized images, respectively. The age and total gray matter (GM) volume were treated as confounding covariates in an analysis of covariance. The total GM volume was given by the total number of voxels within the GM compartment of each subject. The analysis revealed that there were no significant correlations between the BP values of [11C]SCH23390 and [11C]FLB457 in PFC and HPC and the concentration of gray matter in the prefrontal and hippocampal regions, respectively, at a threshold of p = 0.01, uncorrected.
Discussion
Although D1 receptor binding in PFC showed trend-level positive linear correlations with WCST performance, quadratic regression analysis revealed significant inverted U-shaped relations between D1 receptors in PFC and WCST performance. That is, a too high or too low level of D1 receptor expression in PFC leads to high errors and a low number of categories achieved. However, D2 receptor binding in PFC did not show significant relation with any neuropsychological measures. With regard to dopamine receptors in HPC, D2 receptor binding in HPC showed positive liner correlations not only with memory function but also with frontal lobe functions, whereas D1 receptor binding in HPC did not show significant relation with any neuropsychological measures. WCST involves a set-shifting component as well as a working memory component, although the two abilities are not mutually exclusive (Konishi et al., 1999). Working memory requires the active maintenance and manipulation of trial-unique information in a short-term memory buffer (Goldman-Rakic, 1995; Fuster, 2000). Thus, set-shifting could be regarded as updating of working memory content, and it has been demonstrated that updating of working memory content and shifting of cognitive set have a similar cognitive aspect in common (Konishi et al., 1998). Thus, in normal human subjects, the individual difference of working memory capacity could contribute to the difference in the performance of tests for cognitive flexibility.
Previous animal studies demonstrated that local injection of D1 receptor antagonists into PFC induced impairment in working memory task in nonhuman primate (Sawaguchi and Goldman-Rakic, 1994). In a human study, systemic administration of a mixed D1/D2 agonist, pergolide, facilitated working memory, but the selective D2 agonist bromocriptine had no effect, indicating that the dopaminergic modulation of working memory is mediated primarily via stimulation of D1 receptors (Müller et al., 1998). Subsequent animal studies indicated that stimulation of D1 receptors in PFC produces an inverted U-shaped response in working memory, with the response being optimized within a narrow range of D1 receptor stimulation (Goldman-Rakic et al., 2000; Lidow et al., 2003; Castner and Goldman-Rakic, 2004; Seamans and Yang, 2004; Vijayraghavan et al., 2007). Recent human studies have investigated the effect of a functional polymorphism in the catechol O-methyltransferase gene, which has been shown to modulate the prefrontal dopamine level, on prefrontal function. The results also suggested that dopamine transmission in PFC produces an inverted U-shaped response, meaning that too little or too much dopamine signaling would impair prefrontal functions, although these studies could not identify the receptor subtype that plays a central role in this effect (Mattay et al., 2003; Williams-Gray et al., 2007).
Our PET finding is the first direct evidence in human that demonstrated an inverted U-shaped relation between D1 receptors in PFC and executive function including working memory in normal healthy subjects. Our previous PET study revealed that, compared with normal controls, D1 receptors in PFC were decreased in schizophrenia, which was associated with poor performance on WCST (Okubo et al., 1997). However, another PET study reported that an increase in D1 receptors in PFC was associated with working memory deficits in schizophrenia (Abi-Dargham et al., 2002). It has been discussed that these inconsistent results might stem from several factors including differences in radioligands and patient demographics. Although the reasons for these inconsistent results need to be clarified in the future, an inverted U-shaped response can account for working memory deficits in schizophrenia whether D1 receptors in PFC are increased or decreased in patients, because the D1 receptor inverted U-shaped response is observed within a narrow range of the normal physiological condition (Williams and Castner, 2006; Vijayraghavan et al., 2007). An inverted U-shaped response has been suggested based on cognitive and behavioral studies, but the exact physiological mechanism of this effect has not yet been fully understood. A recent monkey electrophysiology study has demonstrated a neuron-level mechanism that constitutes the inverted U-shaped response whereby too much or too little stimulation of prefrontal D1 receptors leads to working memory deficits. D1 receptor stimulation had a suppressive effect on the PFC neural activities involved in a spatial working memory task. Moderate D1 receptor stimulation spatially tunes PFC neurons that process target signals by preferentially suppressing nontarget (noisy) neural activities, whereas excessive D1 receptor stimulation induces nonselective suppression of PFC neural activities regardless of whether the neural activities are task-related or not (Vijayraghavan et al., 2007).
Animal studies have suggested that the inverted U-shaped principle of D1 receptor stimulation mediating working memory does not necessarily apply to other prefrontal functions (Floresco and Magyar, 2006). Therefore, it is noteworthy that prefrontal D1 receptors were not associated with other prefrontal measures besides WCST, because fluency task by phonetic or semantic cues and problem-solving test with visuospatial analysis are less dependent on the working memory process.
Considering that D1 binding in PFC was not correlated significantly with D2 binding either in PFC or HPC, D1- and D2-mediated working memory processes are considered to contribute differently to the completion of WCST. Although previous animal studies showed that working memory or executive function mainly depends on D1 receptors, not on D2 receptors in PFC (Sawaguchi and Goldman-Rakic, 1994; Seamans et al., 1998), a recent rat study demonstrated that D2 receptors in PFC were necessary for set-shifting ability (Floresco et al., 2006). It has been suggested that when the dopamine level is high under a novel circumstance, the prefrontal network is mainly modulated by D2 receptors. In such state, the network is likely to process multiple information (Seamans and Yang, 2004; Floresco et al., 2006). During the set-shifting stage of WCST, one needs to disengage from the previous strategy and compare alternative options under a new condition. After shifting attentional sets, one needs to learn and maintain a new strategy of WCST. In such condition, the dopamine level is considered to be moderate and D1 receptors play a central role in stabilizing the network (Seamans and Yang, 2004; Floresco et al., 2006). We did not find any correlation between D2 binding in PFC and WCST performances, possibly attributable to the fact that the working memory component and the set-shifting component are not entirely dissociable in WCST (Konishi et al., 1999). Instead, D2 binding in HPC was related to WCST performances. Although the role of hippocampal D2 receptors in set-shifting is not known, a possible interpretation is that in the initial set-shifting stage of WCST, D2 receptors in HPC might play a role in quick learning and comparison to guide future behaviors, and once a new strategy is learned, D1 receptors in PFC might contribute to the stability and maintenance of the novel strategy.
The association between hippocampal D2 receptors and memory is consistent with the findings of previous PET studies (Kemppainen et al., 2003; Takahashi et al., 2007). The finding that hippocampal D2 binding was more related to visuospatial memory than to verbal memory might stem from the fact that verbal learning is dependent on regions other than HPC, such as anterior, lateral and superior temporal lobes, which are involved in human language, although HPC plays a central role in both types of memory (Hodges and Graham, 2001). Umegaki et al. (2001) reported that injection of a D2 receptor antagonist into HPC impaired memory performance and that the memory impairment was ameliorated by coinjection of a D2 receptor agonist. They also found that local infusion of D2 agonist into HPC stimulated acetylcholine release in HPC and ameliorated scopolamine-induced memory impairment (Fujishiro et al., 2005). In addition, hippocampal D2 receptors appear to be involved in synaptic plasticity. It has been reported that D2 antagonist inhibited long-term potentiation in HPC (Frey et al., 1990; Manahan-Vaughan and Kulla, 2003), the key mechanism underlying memory consolidation (Jay, 2003; Lynch, 2004). There is some evidence from animal studies that hippocampal D1 receptors are also involved in memory (Hersi et al., 1995a,b; Bach et al., 1999), but supporting our PET data, Wilkerson and Levin (1999) reported that hippocampal D1 receptors were not as responsible as D2 receptors for memory functions.
In line with our previous study (Takahashi et al., 2007), we also found hippocampal D2 receptors to be involved in the performance of WCST and phonemic verbal fluency, which is more dependent on PFC than semantic verbal fluency. Patients with lesions in HPC sometimes show deficits in WCST (Corkin, 2001; Igarashi et al., 2002). These observations suggest that hippocampal D2 receptors could modulate PFC activity by the HPC–PFC pathway, which plays a significant role in the cognitive process (Laroche et al., 2000; Thierry et al., 2000). Accumulating evidence has suggested the modulatory effects of dopamine on HPC–PFC interactions (Seamans et al., 1998; Aalto et al., 2005; Tseng et al., 2007; Goto and Grace, 2008). Conceivably, dopamine influences PFC neurons directly by prefrontal D1 receptors and indirectly by hippocampal D2 receptors via the HPC–PFC pathway.
Müller et al. (1998) reported that the systemic administration of the mixed D1/D2 agonist pergolide facilitated working memory, whereas selective D2 agonist had no effect. However, there is converging evidence from human and animal studies to suggest the involvement of D2 receptors in cognitive functions. It was reported that the systemic administration of D2 agonist in human improved cognitive functions including working memory and executive functions (McDowell et al., 1998), and the administration of D2 antagonist impaired those functions (Mehta et al., 1999). In an animal study, it was reported that mice lacking D2 receptors showed a working memory deficit (Glickstein et al., 2002). These studies, however, did not reveal the regions most responsible for these effects. Moreover, although the involvement of D1 receptors in working memory is widely recognized, it was not clear whether D1 receptor stimulation alone or the combination of D1 and D2 receptor stimulation is most effective. Our finding suggested that orchestration of prefrontal D1 receptors and hippocampal D2 receptors might be necessary for executive functions including working memory.
The current study has several limitations. First, although BPND is the complex value of receptor density and affinity (the inverse of Kd), previous studies indicated that the affinity does not differ according to region (Suhara et al., 1999) and that extrastriatal binding of current PET ligands is not sensitive to endogenous dopamine (Abi-Dargham et al., 1999; Okauchi et al., 2001). Still, we should keep in mind that the BPND values of [11C]SCH23390 and [11C]FLB457 might not necessarily be equivalents for D1 and D2 receptor functions, respectively. This emphasizes the need for PET investigations of the relation of BPND and presynaptic function or second messenger beyond dopamine receptors. Alternatively, multimodal imaging study combining the current method with other modalities such as functional MRI might also be advantageous in investigating the direct relation between dopamine receptor function and PFC functions. Second, we measured the level of dopamine receptor binding during a resting state rather than during cognitive tasks. It is difficult to measure endogenous dopamine release in extrastriatal regions with the current PET ligands (Abi-Dargham et al., 1999; Okauchi et al., 2001). Future study with radioligands more sensitive to endogenous dopamine release will enable us to examine its degree of receptor occupancy. Finally, attributable to limitations of the [11C] radioligand, the data of [11C] FLB457 binding in the striatum was not available. The striatum plays an important role in the prefrontal-hippocampus pathway. PET data in the striatum would lead to a better understanding of the interaction of these three regions. Future study with triple radioligands such as [11C]SCH23390, [11C] FLB457 and [11C] raclopride will enable us to examine striatal and extrastriatal D1 and D2 receptors in the same subject.
In summary, we found that an inverted U-shaped relation existed between D1 receptor binding in PFC and WCST performance, indicating an inverted U-shaped relation between prefrontal D1 receptors and working memory, and that prefrontal D2 receptor binding was not related to any frontal lobe functions. Hippocampal D2 receptors seem to contribute to local hippocampal functions (long-term memory) and to modulation of brain functions outside HPC (frontal lobe functions), which are mainly subserved by PFC, via the HPC–PFC pathway. Our findings suggest that prefrontal D1 receptors and hippocampal D2 receptors might be targets for pharmacological therapeutics for cognitive and memory impairments observed in neuropsychiatric disorders such as Alzheimer's disease, Parkinson's disease and schizophrenia.
Footnotes
This work was supported by a consignment expense for Molecular Imaging Program on “Research Base for PET Diagnosis” from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Japanese Government, and a Grant-in-Aid for Scientific Research from MEXT (18790858). We thank Katsuyuki Tanimoto, Takahiro Shiraishi, and Toshio Miyamoto for their assistance in performing the PET experiments at the National Institute of Radiological Sciences. We also thank Yoshiko Fukushima of the National Institute of Radiological Sciences for her help as clinical research coordinator.
References
- Aalto S, Brück A, Laine M, Någren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van Heertum R, Mann JJ, Foged C, Halldin C, Laruelle M. PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse. 1999;32:93–109. doi: 10.1002/(SICI)1098-2396(199905)32:2<93::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J Neurosci. 2004;24:1446–1450. doi: 10.1523/JNEUROSCI.3987-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. Beware of frontal lobe deficits in hippocampal clothing. Trends Cogn Sci. 2001;5:321–323. doi: 10.1016/s1364-6613(00)01709-5. [DOI] [PubMed] [Google Scholar]
- Farde L, Halldin C, Stone-Elander S, Sedvall G. PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology (Berl) 1987;92:278–284. doi: 10.1007/BF00210831. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Umegaki H, Suzuki Y, Oohara-Kurotani S, Yamaguchi Y, Iguchi A. Dopamine D2 receptor plays a role in memory function: implications of dopamine-acetylcholine interaction in the ventral hippocampus. Psychopharmacology (Berl) 2005;182:253–261. doi: 10.1007/s00213-005-0072-x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- Halldin C, Farde L, Högberg T, Mohell N, Hall H, Suhara T, Karlsson P, Nakashima Y, Swahn CG. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med. 1995;36:1275–1281. [PubMed] [Google Scholar]
- Hersi AI, Rowe W, Gaudreau P, Quirion R. Dopamine D1 receptor ligands modulate cognitive performance and hippocampal acetylcholine release in memory-impaired aged rats. Neuroscience. 1995a;69:1067–1074. doi: 10.1016/0306-4522(95)00319-e. [DOI] [PubMed] [Google Scholar]
- Hersi AI, Richard JW, Gaudreau P, Quirion R. Local modulation of hippocampal acetylcholine release by dopamine D1 receptors: a combined receptor autoradiography and in vivo dialysis study. J Neurosci. 1995b;15:7150–7157. doi: 10.1523/JNEUROSCI.15-11-07150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Graham KS. Episodic memory: insights from semantic dementia. Philos Trans R Soc Lond B Biol Sci. 2001;356:1423–1434. doi: 10.1098/rstb.2001.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Oguni H, Osawa M, Awaya Y, Kato M, Mimura M, Kashima H. Wisconsin card sorting test in children with temporal lobe epilepsy. Brain Dev. 2002;24:174–178. doi: 10.1016/s0387-7604(02)00024-4. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kemppainen N, Laine M, Laakso MP, Kaasinen V, Någren K, Vahlberg T, Kurki T, Rinne JO. Hippocampal dopamine D2 receptors correlate with memory functions in Alzheimer's disease. Eur J Neurosci. 2003;18:149–154. doi: 10.1046/j.1460-9568.2003.02716.x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex. 1999;9:745–753. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Ed 3. New York: Oxford UP; 1995. Neuropsychological assesment. [Google Scholar]
- Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: Immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–135. doi: 10.1093/cercor/13.2.123. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121:1155–1164. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson's disease. Psychopharmacology (Berl) 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Müller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. J Neurosci. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Okauchi T, Suhara T, Maeda J, Kawabe K, Obayashi S, Suzuki K. Effect of endogenous dopamine on extrastriatal [(11)C]FLB 457 binding measured by PET. Synapse. 2001;41:87–95. doi: 10.1002/syn.1063. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, Okubo Y, Nakashima Y, Ito H, Tanada S, Halldin C, Farde L. Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmcol. 1999;2:73–82. doi: 10.1017/S1461145799001431. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Hayashi M, Okubo Y, Takano A, Ito H, Suhara T. Memory and frontal lobe functions; possible relations with dopamine D2 receptors in the hippocampus. Neuroimage. 2007;34:1643–1649. doi: 10.1016/j.neuroimage.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umegaki H, Munoz J, Meyer RC, Spangler EL, Yoshimura J, Ikari H, Iguchi A, Ingram DK. Involvement of dopamine D(2) receptors in complex maze learning and acetylcholine release in ventral hippocampus of rats. Neuroscience. 2001;103:27–33. doi: 10.1016/s0306-4522(00)00542-x. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wilkerson A, Levin ED. Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience. 1999;89:743–749. doi: 10.1016/s0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]