Abstract
Background:
Colorectal Cancer (CRC) is the third most common cancer diagnosed and the second leading cause of cancer-related deaths in the United States. Cancer Stem Cells (CSCs) are believed to be the primary reason for the recurrence of CRC. Specific stem cell marker, doublecortin-like kinase 1 (DCLK1) plays critical roles in the tumorigenesis and progression of CRC. Up-regulation of DCLK1 is correlated with poor prognosis. Whether DCLK1 is correlated with enhanced chemoresistance of CRC cells is unclear. We aim to reveal the association of DCLK1 with chemoresistance of CRC cells and the underlying molecular mechanisms.
Methods:
Stable DCLK1 over-expression cells (DCLK1+) were established using the HCT116 cells (WT). DCLK1+ and WT cells were treated with 5-Fluorouracil (5-Fu) at different doses for 24 or 48 hours. MTT assay was used to evaluate cell viability and IC50 of 5-Fu was determined. Quantitative real-time PCR was applied to determine the gene expression of caspase-3 (casp-3), casp-4, and casp-10. Cleaved casp-3 expression was investigated using Western blot and immunofluorescence.
Results:
Our results demonstrated that IC50 of 5-Fu for the DCLK1+ cells was significantly higher than that of the WT cells for both 24 and 48-hour treatment (p=0.002 and 0.048 respectively), indicating increased chemoresistance of the DCLK1+ cells. Gene expression of casp-3, casp-4, and casp-10 were significantly inhibited in the DCLK1+ cells after 5-Fu treatment compared to the WT cells (p=7.616e-08, 1.575e-05 and 5.307e-08, respectively). Cleaved casp-3 amount and casp-3 positive cells were significantly decreased in the DCLK1+ cells after 5-Fu treatment compared to the WT cells (p=0.015).
Conclusions:
In conclusion, our results demonstrated that DCLK1 overexpression enhanced the chemoresistance of CRC cells to 5-Fu treatment by suppressing gene expression of key caspases in the apoptosis pathway and activation of the apoptosis pathway. DCLK1 can be an intriguing therapeutic target for the effective treatment of CRC patients.
Keywords: Doublecortin-like kinase 1, Colorectal cancer, Chemoresistance, Anti-apoptosis pathway
Introduction
Colorectal Cancer (CRC) is the third most common cancer diagnosed in both men and women and the second leading cause of cancer-related deaths in the United States (http://www.cdc.gov/cancer/colorectal/statistics/). In the last 20 years, progress in the treatment of CRC has improved quality of patients’ life, but up to 50% of patients relapsed after surgical resection and ultimately died of metastatic disease [1]. Adjuvant systemic chemotherapy with cytotoxic drugs is recommended as standard clinical practice for patients with stage III CRC after surgical resection of the local CRC [2] since the survival outcomes of CRC patients with adjuvant systemic chemotherapy combined with surgical resection was significantly higher than those with surgical resection only [3]. The promising progress of systemic chemotherapy for CRC began with the discovery of 5-fluorouracil (5-Fu) in 1957 [4]. Currently, the conventional first-line treatments for CRC patients are the combination of 5-Fu, leucovorin, and oxaliplatin (FOLFOX) or the combination of 5-Fu, leucovorin, and irinotecan(FOLFIRI) [5]. Recently, Curcumin was proven to be effective in the inhibition of cell proliferation and migration of the chemoresistant CRC cells [6]. However, not all of the CRC patients respond to the systemic therapies, and even though for the responsive patients, almost all of them developed resistance [7]. According to the Cancer Stem Cell (CSC) hypothesis, the presence of chemoresistant CSCs (also known as Tumor Stem Cells (TSCs)) is the primary cause [8,9]. CSCs accounts for 0.05% to 1% of the tumor mass, but they can give rise to all of the cell types in the tumor and possess unlimited self-renewal capability [10]. Several specific putative markers have been identified for the stem cell populations in the gastrointestinal tract, including doublecortin-like kinase 1 (DCLK1, also known as KIAA0369 or DCAMKL1 [11,12].
DCLK1 is a microtubule-associated serine-threonine protein kinase and functions in facilitating polymerization of tubulin dimers to assemble microtubules [13]. It is predominantly expressed in the nervous system and is correlated with normal nervous system development and general cognition and verbal memory function [14–16]. In the late 2000s, DCLK1 was identified as a stem cell marker for the intestinal stem cells and correlated with stemness of CRC cells [12,17]. It is co-localized with other well-characterized gastrointestinal stem cell markers, such as Lgr5 in the “+4 position” of the crypt of the small intestine where the intestinal stem cells are located [18,19]. Up-regulated expression of DCLK1 was found broadly in solid tumors almost all over the body, including esophageal cancer, pancreatic cancer, liver cancer, CRC, etc. and is correlated with poor prognosis [20–26]. The most recent clinical findings identified that elevated DCLK1+ cells in the blood can be used as a novel non-invasive marker for the diagnosis of incidence, relapse, and metastasis for CRC, liver cancer, pancreatic cancer, and Barrett’s esophagus and esophageal adenocarcinoma [27–30]. DCLK1 played important roles in the initiation, progression, and metastasis of CRC [31–33]. It can promote cell survival via the prevention of cancer cell apoptosis in neuroblastoma and anoikis in mouse colonic epithelial cells [34,35].
Though DCLK1 is such multiple functional proteins in the CRC tumorigenesis, neither association of DCLK1 with chemoresistance in human CRC nor the underlying cellular and molecular mechanism is clear. In this paper, we identified that DCLK1 can significantly increase chemoresistance of CRC cells to 5-Fu treatment, and it functions through inhibition of gene expression of key caspases and activation of the apoptosis pathway. Our results demonstrated that DCLK1 can be used as an intriguing therapeutic target for CRC treatment.
Material and Methods
Cell line and cell culture
Human colorectal carcinoma cell line HCT116 cells were purchased from ATCC (ATCC® CCL-247™) and were maintained in McCoy’s 5A medium (ATCC® 30–2007™) supplemented with 10% FBS in 37°C incubator with 5% CO2. Isogenic DCLK1 over-expressed cells (DCLK1+) were established by transfecting human DCLK1 variant 1 cDNA, which is fused with a turboGFP gene at C-terminal (OriGene, Cat #RG217050) into HCT116 cells. In order to avoid the clonal variance, different DCLK1 over-expressed clones were selected. Control HCT116 cells (WT) were established by transfecting pCMV6-AC-GFP Tagged Cloning Vector (Origene, Cat #PS100010) into HCT116 cells. Both DCLK1 over-expressed cells and control HCT116 cells were selected (400 µg/ml) and maintained (250 µg/ml) using Geneticin (G418).
5-Fu cytotoxicity assay
WT and DCLK1+ cells were plated at 1 × 10^4 cells/well/100 µL in the 96-well plate for 24 hours. Then cells were treated with 5-Fu (Sigma; F6627–1G) at different concentrations with 8 wells per dose concentration for 24 or 48 hours. Cell viability was determined by MTT assay according to Li’s approach with modifications [36]. Briefly, MTT reagent (5 mg/ml) was added into cells at a 1:10 ratio of the culture medium and incubated for 3 hours at 37°C. After incubation, the culture medium with MTT was replaced by dimethyl sulfoxide (DMSO). The plate was sent to the BioTek Synergy 2 multi-mode reader and absorbance was measured at 570 nm and 630 nm. OD value used for cell viability calculation was calculated by subtracting OD630 (background) from OD570. Cell viability was determined by comparing the averaged calculated OD of 5-Fu treated cells to the DMSO-treated control cells. IC50 of 5-Fu was calculated from equation generated using Excel by the cell viability and dose-killing data. Briefly, select the data, insert charts and select “scatter plot”. Then set the Y-axis to “logarithmic”, select “add trend line” and for the trendline options, select “exponential” and “display equation on chart”. Using the equation, you can calculate the IC50.
Western blotting
WT and DCLK1+ cells were plated at 2 × 10^6 cells per T-25 flask and cultured for 24 hours. Then cells were treated with/without 5-Fu and cultured for 48 more hours. Whole cell lysates were harvested using ice-cold RIPA buffer with 1X protease inhibitor (Sigma, P8340) and 1X phosphate inhibitor (Sigma, P5726). Protein concentration was determined using Pierce™ BCA Protein Assay Kit according to the manufacture’s manual (ThermoFisher Scientific, Catalog number: 23227). 40 µg of protein from each sample was loaded onto the 4% to 15% Mini-PROTEAN® TGX™ Gel (BioRad, Catalog number: 4561083) for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 100V for 60 minutes. Proteins were transferred to nitrocellulose membrane using the Trans-Blot Turbo System (Bio-Rad). Then the membrane was blocked in 3% non-fat milk in 1X PBS with 0.1% tween 20 (1X PBST) at room temperature with constant shaking for one hour and was probed with primary antibodies (caspase-3 (Life Biosciences, LS-C331947) and cleaved caspase-3 (Life Biosciences, LS-C380472)) in 1X PBST with 3% non-fat milk at 4°C overnight. After the primary antibody incubation, the membrane was washed with 1X PBST for 5 minutes, 3 times total. Then the membrane was probed with a secondary antibody with 1% non-fat milk at room temperature for an hour. The membrane was washed with 1X PBST for 5 minutes, 4 times total and sent for imaging using the LI-COR Odyssey Imaging System.
Immunofluorescence
WT and DCLK1+ cells were plated at 3 × 10^5 cells/well into 8-well Millicell EZ chamber slides (Millipore, Catalogue number: PEZGS0816) overnight. Cells were treated with/without 5-Fu for 24 hours and cellular cleaved caspase-3 protein was labeled immunofluorescently. Briefly, the culture medium was removed and cells were washed 3X with 1X PBS. Then cells were fixed with 4% paraformaldehyde for 10 minutes on ice. Wash cells 3X with 1X PBS, and block with 1% BSA by incubating cells for 5 min at 40°C. Wash cells 3X with 1X PBS and incubate cells with the primary antibody in 1X PBS with 1% BSA overnight at 4°C. Wash cells 5X with 1X PBS and probed with secondary antibody by incubating for 1 hour at room temperature in dark. Wash cells 5X with 1X PBS and did nuclei counterstain using 1X DAPI (Sigma Catalogue number: D9542) by incubating for 15 min at room temp. Wash cells with ddH2O, remove the gasket, mounting with Prolong Gold Anti-fade reagent (#9071) and cover with a coverslip. Images were taken using a Life Technologies EVOS FL fluorescent microscope.
Quantitative real time polymerase chain reaction [(q)RT-PCR]
Total cellular RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions from the WT and DCLK1+ cells. First strand cDNA was generated using the Reverse Transcription System (Promega, Madison, WI) according to the manufacturer’s instruction. 5 µL cDNA from reverse transcription PCR was added to a 25 µL reaction containing sybr green. Primers for the human GAPDH, caspase 3, 4 and 10 are listed in (Table 1) (q)RT-PCR was carried out on a Stratagene Mx3005 quantitative real-time PCR thermocycler according to the manufacturer’s instruction. Expression of the gene of interests was normalized to GAPDH first, and then a comparison of fold change between DCLK1+ and WT cells was carried out using the 2-ΔΔCt approach [37].
Table 1:
Primers used for the quantitative real-time PCR.
| GAPDH_F | 5’-CGACCACTTTGTCAAGCTCA-3’ |
| GAPDH_R | 5’-AGGGGTCTACATGGCAACTG-3’ |
| Casp_3_F | 5’-GGCTGAGCTGCCTGTAACTT-3’ |
| Casp_3_R | 5’-GGCAGCATCATCCACACATA-3’ |
| Casp_4_F | 5’-CACAACGTGTCCTGGAGAGA-3’ |
| Casp_4_R | 5’-ACTTCCTCTAGGTGGCAGCA-3’ |
| Casp_10_F | 5’-CCGTATCCATCGAAGCAGAT-3’ |
| Casp_10_R | 5’-GTGGCCAGACCAAGTAGGAA-3’ |
| F: forward, R: reverse | |
Statistical analysis
All data was expressed as Non-paired one-side t-test, two-side t-test or paired two-side t-test was applied. p<0.05 was considered as statistical significant.
Results
DCLK1 increases chemoresistance of colorectal cancer cells
In order to investigate whether DCLK1 modifies the sensitivity of CRC cells to chemotherapy, the WT, and DCLK1+ cells were treated with different doses of 5-Fu for 24 and 48 hours. Cell viability was measured using MTT assay after the designed time period of drug treatment. Our results demonstrated that as 5-Fu concentration increased, cell viability of the WT and DCLK1+ cells decreased for both 24 and 48-hour treatment. Cell viability of DCLK1+ was higher than the WT cells at every dose for both 24 and 48-hour treatment (Figure 1A). IC50 of 5-Fu for the DCLK1+ cells was significantly higher than that of the WT cells for both 24 and 48-hour treatment (p=0.002 and 0.048 respectively, Figure 1B), indicating increased chemoresistance of the DCLK1+ cells.
Figure 1.
Chemosensitivity of HCT116 wild type cells (WT) and DCLK1 over-expressed cells (DCLK1+) to 5-Fu treatment.
DCLK1 suppresses gene expression of multiple critical caspases transcriptionally
DCLK1 might be correlated with apoptosis pathway since knockdown of DCLK1 gene expression induced apoptosis of neuroblastoma cells and anoikis-resistant mouse colonic epithelial cells expressed an increased amount of DCLK1 [34,35]. Our RNA-Seq data indicated that DCLK1 over-expression modulates gene expression of multiple key caspases, specifically casp-4 and 10 (Figure 2A), which might due to antibiotics G418 selection. In order to find out whether DCLK1 can affect gene expression of casp-4 and 10 without G418 selection but with 5-Fu treatment, and whether casp-3 is also controlled by DCLK1 transcriptionally, (q)RT-PCR was carried out. Our results demonstrated that with G418 selection but without 5-Fu treatment, gene expression of casp-3 and 4 was very similar in the WT and the DCLK1+ cells. Casp-10 was significantly lower in the DCLK1+ cells, which is consistent with the RNA-Seq data. However, in the cells without G418 selection but with 5-Fu treatment, gene expression of all three genes was significantly inhibited in the DCLK1+ cells compared to the WT cells (p=7.616e-08, 1.575e-05 and 5.307e-08, respectively, Figure 2B) and casp-10 was further inhibited compared to G418 selection, indicating DCLK1 can significantly inhibit gene expression of casp-3, 4 and 10 transcriptionally, which may be responsible for the higher cell survival rate after 5-Fu treatment and contribute to the increased chemoresistance of the DCLK1+ cells.
Figure 2.
Gene expression of multiple caspases in HCT116 wild type (WT) and DCLK1 over-expressed (DCLK1+) cells.
DCLK1 suppresses activation of the apoptosis pathway
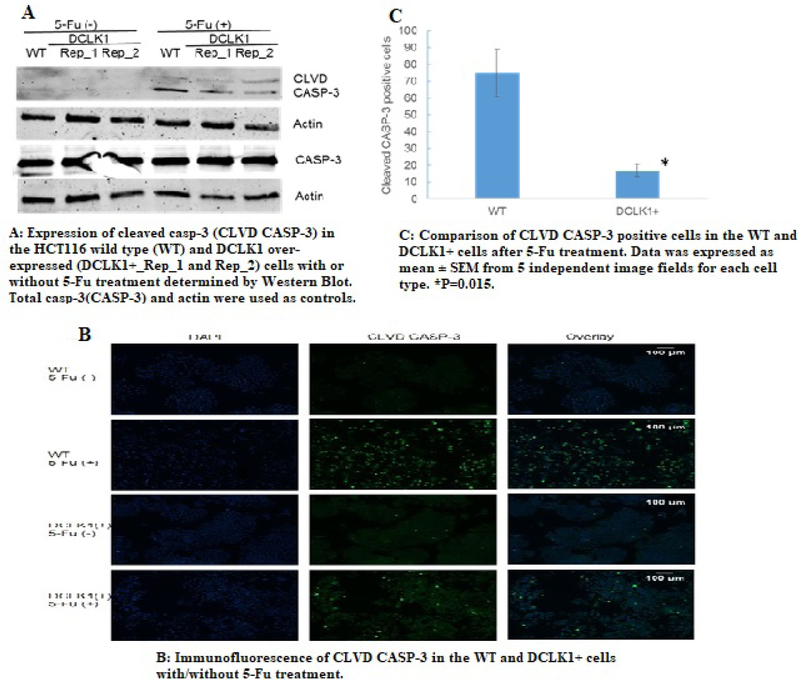
In order to find out whether DCLK1 also modulate the activation of the apoptosis pathway, we evaluated the cleaved casp-3 protein expression level in the WT and the DCLK1+ cells before and after 5-Fu treatment for 24 hours. Our results demonstrated that before 5-Fu treatment, both WT and the DCLK1+ cells demonstrated undetectable cleaved casp-3 expression, indicating no or low apoptosis events. However, with 5-Fu treatment, the WT cells showed a much higher cleaved casp-3 expression level compared to the DCLK1+ cells (Figure 3A), and results of semi-quantification using Image J software demonstrated that amount of cleaved casp-3 is 3 ± 0.8 fold in the WT cells compared to the DCLK1+ cells, indicating much higher apoptosis rate in the WT cells after 5-Fu treatment. When compared the total casp-3 expression level in the WT and DCLK1+ cells, there is no significant difference either with or without 5-Fu treatment.
Figure 3.
Modulation of activation of apoptosis pathway by DCLK1 in the colorectal cancer cells.
We further confirmed the Western blot findings with immunofluorescence label of cleaved casp-3 protein inside the WT and DCLK1+ cells after 5-Fu treatment. We found significantly more cleaved casp-3 positive cells in the WT cells than the DCLK1+ cells p=0.015, (Figure 3B and 3C). In summary, our data indicated that DCLK1 significantly inhibits activation of the apoptosis pathway post-translationally as well.
Discussion
Using the DCLK1 over-expressed cells, we identified that DCLK1 can enhance the chemoresistance of CRC cells to the 5-Fu treatment significantly through the anti-apoptosis pathway by inhibiting gene expression of critical caspases transcriptionally and post-translationally. This is the first time that the association of DCLK1 with chemoresistance in the CRC cells and the underlying molecular mechanism were clarified.
Chemoresistance is a big challenge for the effective treatment of colorectal cancer. CRC cells have evolved multiple mechanisms to resist and escape from anticancer drugs treatment. Hypoxia tumor microenvironment and enhancement of autophagy procedure both contribute to the chemoresistance of CRC cells [38,39]. CRCs can also manipulate the expression of multiple microRNAs to facilitate their chemoresistance, including suppressing the expression of anti-tumor microRNAs, such as miR-181a/135a/302c, miR-874–3p, miR-200c; and increasing tumorigenic miRNAs, such as miR-196b-5p, miR-315b, miR-10b [40–45]. Several cellular pathways were identified to abnormally modulated to facilitate CRC cells chemoresistant procedure, including activation of Wnt/β-catenin pathway, Akt pathway, and Notch signaling pathway and inactivation of Hippo signaling pathway and caspase-dependent apoptosis pathway [41,46–49]. Recently, it was identified that DCLK1 might be associated with chemoresistance of cancer cells. miR-539, a tumor suppressor microRNA, directly inhibits DCLK1 expression to enhance chemosensitivity of non-small cell lung cancer to cisplatin [50]. Over-expression of DCLK1 significantly increased chemoresistance of kidney cancer cells to the receptor tyrosine kinase inhibitors and mTOR inhibitors [51]. miR-15a might overcome chemoresistance of CRC stem cells to 5-FU through suppression of DCLK1 [52]. However, how DCLK1 enhances chemoresistance of CRC cells is unclear.
In this paper, we identified that DCLK1 can increase the chemoresistance of CRC cells through modulating apoptosis pathway. Firstly, it can significantly suppress the gene expression of several critical caspases, including casp-3, 4 and 10, after 5-Fu treatment. Secondly, it can significantly inhibit activation of the apoptosis pathway, demonstrated by the decreased expression of cleaved casp-3 after 5-Fu treatment in the DCLK1+ cells. We are not the first one to find that DCLK1 was involved in the apoptosis pathway. It was identified that DCLK1 is essential for the survival of neuroblastoma cells since knockdown of DCLK1 gene expression induced apoptosis of the cells [34] and in the anoikis-resistant mouse colonic epithelial cells, DCLK1 was up-regulated [35]. However, it is the first time that DCLK1 was demonstrated to increase the chemoresistance of CRC cells via anti-apoptosis pathway.
DCLK1 plays important roles in the initiation, progression, and metastasis of CRC, and targeting DCLK1 is efficient in the inhibition of developed tumor growth in an animal model. Using Apcmin/+ mouse model, Chandrakesan and colleagues demonstrated that DCLK1 was over-expressed in the small intestine of the elderly mice compared to normal control mice, and the over-expression of DCLK1 facilitated Epithelial-Mesenchymal Transition (EMT), which in turn resulted in the colorectal tumorigenesis [31]. Most recently, they identified that DCLK1 over-expression is correlated with enhanced pluripotency and self-renewal capability of intestinal epithelial cells [53]. When DCLK1 was conditionally over-expressed by crossing Dclk1creERT2 Rosa26R mice to the Apcmin/+ mice, the over-expression of DCLK1 significantly increased the incidence of intestinal polyps compared to the normal control mice [32]. DCLK1+ tuft cells are responsible for the tumorigenesis of colon cancer in the DCLK1-CreERT transgenic mice and over-expression of DCLK1 in human pancreatic cancer stem cells facilitated the tumor invasion and metastasis [33,54]. When DCLK1 expression was specifically knockdown in a mouse model, fewer polyps and decreased dysplasia were observed and when DCLK1+ cells were specifically targeted in the developed polyps, the CSCs died and the established polyps were rapidly collapsed [31,32]. All of these findings indicate that DCLK1 can become a promising therapeutic target for CRC treatment.
Conclusion
In summary, DCLK1 was identified to be associated with increased chemoresistance in the CRC cells, and its functions through the anti-apoptosis pathway. It can become a new novel therapeutic target for the efficient treatment of CRC patients. Further in vivo studies need to be carried out to determine the association of DCLK1 with chemoresistance and its underlying molecular mechanisms, which will provide more evidence for its clinical application and finally become beneficial to CRC patients treatment.
Acknowledgment
LL designed and did most of the experiments. KJ was an undergraduate student in Dr. Li’s lab and did the Western Blot experiment. MM did the statistical analysis. LL and MM analyzed the data and drafted the manuscript. All authors discussed and interpreted the results. The final manuscript was read and approved by all authors.
Funding
This work was supported by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103476. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of General Medical Sciences or the National Institutes of Health.
Abbreviations
- DCLK1
Doublecotin-Like Kinase 1
- CRC
Colorectal Cancer
- CSC
Cancer Stem Cell
- DCLK1+
DCLK1 Over-Expression Cells
- WT
Wild Type HCT116 Cells
- 5-Fu
5-Fluorouracil
- Casp-3
Caspase-3
- Casp-4
Caspase-4
- Casp-10
Caspase-10
- (Q)RT-PCR
Quantitative Real-Time Polymerase Chain Reaction
- RNA-Seq
RNA-Sequencing
- EMT
Epithelial-Mesenchymal Transition
References
- 1.De Dosso S, Sessa C, Saletti P (2009) Adjuvant therapy for colon cancer: present and perspectives. Cancer Treat Rev 35: 160–166. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Engstrom PF, Arnoletti JP, Benson AB, Chen YJ, Choti MA, et al. (2009) NCCN clinical practice guidelines in oncology: Rectal cancer. J Nat Compr Cancer Net 7: 838–881. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson NW, Yothers G, Lopa S, Costantino JP, Petrelli NJ, et al. (2010) Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05: A baseline from which to compare modern adjuvant trials. Ann Sur Oncol 17: 959–966. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, et al. (1957) Fluorinated pyrimidines, a new class of tumor-inhibitory compounds. Nature 179: 663–666. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Lenz HJ (2008) First-line combination treatment of colorectal cancer with hepatic metastases: Choosing a targeted agent. Cancer Treat Rev 34: 3–7. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Hosseini SA, Zand H, Cheraghpour M (2019) The influence of curcumin on the downregulation of MYC, Insulin, and IGF-1 Receptors: A possible mechanism underlying the anti-growth and anti-migration in chemoresistant colorectal cancer cells. Medicina (Kaunas) 55: 1–12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, et al. (2009) Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res 69: 1951–1957. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5: 275–284. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Zeuner A, Todaro M, Stassi G, De Maria R (2014) Colorectal cancer stem cells: From the crypt to the clinic. Cell Stem Cell 15: 692–705. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Yamakage K, Omori Y, Piccoli C, Yamasaki H (1998) Growth control of 3T3 fibroblast cell lines established from connexin 43-deficient mice. Mol Carcinog 23: 121–128. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Omori Y, Suzuki M, Ozaki K, Harada Y, Nakamura Y, et al. (1998) Expression and chromosomal localization of KIAA0369, a putative kinase structurally related to Doublecortin. J Hum Genet 43: 169–177. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.May R, Riehl TE, Hunt C, Sureban SM, Anant S, et al. (2008) Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26: 630–637. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA (2000) DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci 20: 9152–9161. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess HA, Martinez S, Reiner O (1999) KIAA0369, doublecortin-like kinase, is expressed during brain development. J Neurosci Res 58: 567–575. [PubMed] [PubMed] [Google Scholar]
- 15.Mizuguchi M, Qin J, Yamada M, Ikeda K, Takashima S (1999) High expression of doublecortin and KIAA0369 protein in fetal brain suggests their specific role in neuronal migration. Am J Pathol 155: 1713–1721. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Hellard S, Havik B, Espeseth T, Breilid H, Lovlie R, et al. (2009) Variants in doublecortin- and calmodulin kinase like 1, a gene up-regulated by BDNF, are associated with memory and general cognitive abilities. PloS One 4: e7534 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Bellows CF (2013) Doublecortin-like kinase 1 exhibits cancer stem cell-like characteristcs in a human colon cancer cell line. Chin J Cancer Res 25: 134–142. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, et al. (2006) Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem 281: 11292–11300. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, et al. (2009) Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cell 27: 2571–2579. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vega KJ, May R, Sureban SM, Lightfoot SA, Qu D, et al. (2012) Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol 27: 773–780. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, et al. (2011) DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res 71: 2328–2338. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, et al. (2014) DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterol 146: 245–256. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali N, Allam H, Bader T, May R, Basalingappa KM, et al. (2013) Fluvastatin interferes with hepatitis C virus replication via microtubule bundling and a doublecortin-like kinase-mediated mechanism. PloS One 8: e80304 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagliardi G, Goswami M, Passera R, Bellows CF (2012) DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol 5: 35–42. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirzaei A, Tavoosidana G, Modarressi MH, Rad AA, Fazeli MS, et al. (2015) Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients. Tumour Biol 36: 4801–4810. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Weygant N, Ge Y, Qu D, Kaddis JS, Berry WL, et al. (2016) Survival of patients with gastrointestinal cancers can be predicted by a surrogate microRNA signature for cancer stem-like cells marked by DCLK1 kinase. Cancer Res 76: 4090–4099. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantara C, O’Connell MR, Luthra G, Gajjar A, Sarkar S, et al. (2015) Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis. Lab Invest 95: 100–112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sureban SM, Madhoun MF, May R, Qu D, Ali N, et al. (2015) Plasma DCLK1 is a marker of hepatocellular carcinoma (HCC): Targeting DCLK1 prevents HCC tumor xenograft growth via a microRNA-dependent mechanism. Oncotarget 6: 37200–37215. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu D, Johnson J, Chandrakesan P, Weygant N, May R, et al. (2015) Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells. PLoS One 10: e0118933 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whorton J, Sureban SM, May R, Qu D, Lightfoot SA, et al. (2014) DCLK1 is detectable in plasma of patients with barrett’s esophagus and esophageal adenocarcinoma. Dig Dis Sci 60: 509–513. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandrakesan P, Weygant N, May R, Qu D, Chinthalapally HR, et al. (2014) DCLK1 facilitates intestinal tumor growth via enhancing pluripotency and epithelial-mesenchymal transition. Oncotarget 5: 9269–9280. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, et al. (2013) Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 45: 98–103. [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, et al. (2014) Long-lived intestinal tuft cells serve as colon-cancer-initiating cells. J Clin Invest 124: 1283–1295. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verissimo CS, Molenaar JJ, Meerman J, Puigvert JC, Lamers F, et al. (2010) Silencing of the microtubule-associated proteins doublecortin-like and doublecortin-like kinase-long induces apoptosis in neuroblastoma cells. Endocr Relat Cancer 17: 399–414. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki H, Yoshida T, Horiguchi K, Ohama T, Sato K (2013) Characterization of anoikis-resistant cells in mouse colonic epithelium. J Vet Med Sci 75: 1173–1180. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Li L, Sevinsky JR, Rowland MD, Bundy JL, Stephenson JL, et al. (2010) Proteomic analysis reveals virus-specific Hsp25 modulation in cardiac myocytes. J Proteome Res 9: 2460–2471. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Tang YA, Chen YF, Bao Y, Mahara S, Yatim S, et al. (2018) Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci USA 115: E5990–E5999. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang P, Lai ZL, Chen HF, Zhang M, Wang A, et al. (2017) Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J Exp Clin Cancer Res 36: 190 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Shi L, Li X, Wu Z, Li X, Nie J, et al. (2018) DNA methylation-mediated repression of miR-181a/135a/302c expression promotes the microsatellite-unstable colorectal cancer development and 5-FU resistance via targeting PLAG1. J Genet Genomics 45: 205–214. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Que K, Tong Y, Que G, Li L, Lin H, et al. (2017) Downregulation of miR-874–3p promotes chemotherapeutic resistance in colorectal cancer via inactivation of the Hippo signaling pathway. Oncol Rep 38: 3376–3386. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heydari K, Saidijam M, Sharifi MR, Dermani FK, Soleimani Asl S, et al. (2018) The effect of miR-200c inhibition on chemosensitivity (5-FluoroUracil) in colorectal cancer. Pathol Oncol Res 24: 145–151. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Ren D, Lin B, Zhang X, Peng Y, Ye Z, et al. (2017) Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget 8: 49807–49823. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Y, Wang J, Wang J, Yung VY, Hsu E, et al. (2015) MicroRNA-135b regulates apoptosis and chemoresistance in colorectal cancer by targeting large tumor suppressor kinase 2. Am J Cancer Res 5: 1382–1395. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishida N, Yamashita S, Mimori K, Sudo T, Tanaka F, et al. (2012) MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann Surg Oncol 19: 3065–3071. [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Zhang K, Li M, Huang H, Li L, Yang J, et al. (2017) Dishevelled1–3 contribute to multidrug resistance in colorectal cancer via activating Wnt/beta-catenin signaling. Oncotarget 8: 115803–115816. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proutski I, Stevenson L, Allen WL, McCulla A, Boyer J, et al. (2009) Prostate-derived factor-a novel inhibitor of drug-induced cell death in colon cancer cells. Mol Cancer Ther 8: 2566–2574. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Wang R, Ye X, Bhattacharya R, Boulbes DR, Fan F, et al. (2016) A disintegrin and metalloproteinase domain 17 regulates colorectal cancer stem cells and chemosensitivity via notch1 signaling. Stem Cells Transl Med 5: 331–338. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu H, Wang M, Guan X, Yuan Z, Liu Z, et al. (2018) Loss of ABCB4 attenuates the caspase-dependent apoptosis regulating resistance to 5-Fu in colorectal cancer. Biosci Rep 38: BSR20171428. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng H, Qianqian G, Ting J, Aimin Y (2018) miR-539 enhances chemosensitivity to cisplatin in non-small cell lung cancer by targeting DCLK1. Biomed Pharmacother 106: 1072–1081. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Ge Y, Weygant N, Qu D, May R, Berry WL, et al. (2018) Alternative splice variants of DCLK1 mark cancer stem cells, promote self-renewal and drug-resistance and can be targeted to inhibit tumorigenesis in kidney cancer. Int J Cancer 143: 1162–1175. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fesler A, Liu H, Ju J (2018) Modified miR-15a has therapeutic potential for improving treatment of advanced stage colorectal cancer through inhibition of BCL2, BMI1, YAP1 and DCLK1. Oncotarget 9: 2367–2383. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandrakesan P, Yao J, Qu D, May R, Weygant N, et al. (2017) Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells. Mol Cancer 16: 30 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, et al. (2016) Dominant expression of DCLK1 in human pancreatic cancer stem cells accelerates tumor invasion and metastasis. PLoSOne 11: e0146564 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]