Abstract
Nonalcoholic fatty liver disease (NAFLD) is one of the most common forms of liver disease worldwide and has emerged as a significant public health concern in China. A better understanding of the etiology of NAFLD can inform effective management strategies for this disease. We examined factors associated with NAFLD in two districts of Hangzhou, China, focusing on the relationship of regional body fat distribution, muscle mass, and NAFLD. We used baseline data to carry out a cross‐sectional analysis among 3,589 participants from the Wellness Living Laboratory (WELL) China study, a longitudinal population‐based study that aims to investigate and promote well‐being among the Chinese population. NAFLD was defined using the widely validated fatty liver index (FLI). Multivariate logistic regressions were performed to assess independent associations between NAFLD and metabolic risk factors (e.g., insulin resistance) and dual x‐ray absorptiometry (DXA)‐derived measures (e.g., android fat ratio [AFR] and skeletal muscle index [SMI]). Of the 3,589 participants, 476 (13.3%) were classified as having FLI‐defined NAFLD (FLI ≥60). Among those, 58.0% were men. According to our analysis, AFR (odds ratio [OR], 10.0; 95% confidence interval [CI], 5.8‐18.5), insulin resistance (OR, 4.0; 95% CI, 3.0‐5.3), high alanine aminotransferase levels (OR, 7.6; 95% CI, 5.8‐10.0), smoking (OR, 2.0; 95% CI, 1.4‐3.0), and male sex (OR, 2.9; 95% CI, 2.0‐4.2) were positively associated with NAFLD risk, while SMI (OR, 0.1; 95% CI, 0.07‐0.13) was inversely associated with NAFLD risk. Conclusion: In addition to known metabolic risk factors, DXA‐derived AFR and SMI may provide additional insights to the understanding of NAFLD. Interventions that aim to decrease AFR and increase SMI may be important to reduce the burden of NAFLD in this population.
Abbreviations
- ABV
alcohol by volume
- AFR
android fat ratio
- ALM
appendicular lean mass
- ALT
alanine aminotransferase
- BMI
body mass index
- CI
confidence interval
- DXA
dual x‐ray absorptiometry
- FFA
free fatty acid
- FLI
fatty liver index
- FPG
fasting plasma glucose
- GGT
gamma‐glutamyltransferase
- HbA1c
hemoglobin A1c
- HBV
hepatitis B virus
- HC
hip circumference
- HOMA‐IR
homeostasis model for insulin resistance
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- SES
sociodemographic
- SMI
skeletal muscle index
- TG
triglycerides
- WC
waist circumference
- WELL
Wellness Living Laboratory
- WHR
waist‐to‐hip ratio
Nonalcoholic fatty liver disease (NAFLD) affects about 15%‐30% of the world population and encompasses a spectrum of histologic liver changes, ranging from simple steatosis to nonalcoholic steatohepatitis, fibrosis, cirrhosis, and liver cancer.1, 2 In addition to being one of the most common forms of liver disease worldwide, NAFLD is also a risk factor for several other chronic diseases, including chronic kidney disease, cardiovascular disease, and osteoporosis.3 Premature mortality associated with NAFLD is due to both liver and cardiovascular deaths.3 In recent years, the prevalence of NAFLD has also increased in Asia,1 including China, where prevalence reaches 43.3% according to one population‐based study conducted in Shanghai and seven other provinces in East China.4 Therefore, a better understanding of the etiology of NAFLD is urgently needed to inform effective prevention and control strategies for NAFLD.
It has been reported that several cardiometabolic and age‐related diseases, such as abdominal obesity,5 type 2 diabetes,5 insulin resistance,6 and sarcopenia,7 are closely associated with NAFLD. Different studies have reported separate associations among fat and muscle mass with NAFLD risk,5, 7 but it is still unclear whether fat mass and muscle mass are independently associated with NAFLD. In addition, most studies that examined the relationship between body fat and NAFLD have used overall body fat, but based on a study that found strong associations of gynoid and android fat patterns with cardiometabolic risk factors, it is possible that differences in regional adiposity may be separately linked to NAFLD.8 In this study, we assessed the independent associations between android fat mass composition, muscle mass, and NAFLD risk, using the fatty liver index (FLI), a widely validated index that has been used to assess NAFLD status.
Participants and Methods
Study Population
In 2016, Zhejiang University and the Stanford Prevention Research Center, part of the Stanford University School of Medicine, collaborated to launch the Wellness Living Laboratory (WELL) China initiative. This longitudinal cohort study set in Hangzhou, China, aims to investigate and promote well‐being among the Chinese population. The city of Hangzhou was selected as the site for the WELL China cohort because it has a mixed urban and rural population of over 7 million people, with stable infrastructure and engaged communities leaders who are committed to carrying out the study successfully.9 We chose to sample from two of the 10 districts in Hangzhou: the Xihu (West Lake) and Shangcheng districts. Together, these two districts have over 800,000 residents. There are two administration levels, subdistrict and community, within each district. To ensure representativeness of the study subjects and variation across age groups, permanent residents within all subdistricts and communities under each subdistrict were sampled. We also applied quota sampling (i.e., age and sex) within each residential area to reflect the population distribution. Eligible permanent residents aged 18‐80 years were identified from residential listings (sampling frame). Community social workers from each district then visited each household to screen for eligible participants for the study. People with decisional or mental impairments were excluded. Pregnant women during the data collection phase were also excluded due to the required dual x‐ray absorptiometry (DXA) screening. All eligible participants provided informed consent prior to enrollment and were invited to Zhejiang University for extensive data collection, clinical examinations, and biospecimen collection; 96.4% of those approached consented to the study. A total of 6,134 residents consented to the study, and all participated.
The inclusion criteria work flow of the NAFLD analysis of this cross‐sectional study is shown in Fig. 1. After excluding subjects with missing data on sociodemographic factors (n = 275; 4.5%), biomarkers (n = 26; 0.4%), DXA information (n = 113; 1.8%), and alcohol (n = 154; 2.5%) from our analysis as well as moderate (n = 131) and heavy (n = 863) drinkers, a total of 4,572 subjects of the initial 6, 134 remained for analysis. Among these, we stratified subjects into three groups based on their FLI index: “Yes NAFLD” (FLI >60), “Maybe NAFLD” (FLI 30‐60), and “No NAFLD” (FLI <30). We chose not to include Maybe NAFLD in our final analysis as the 983 subjects in the Maybe NAFLD group did not have a significant effect on our outcome when we conducted a sensitivity analysis that included or excluded the Maybe NAFLD participants. A total of 3,589 subjects were included in our final analysis.
Figure 1.
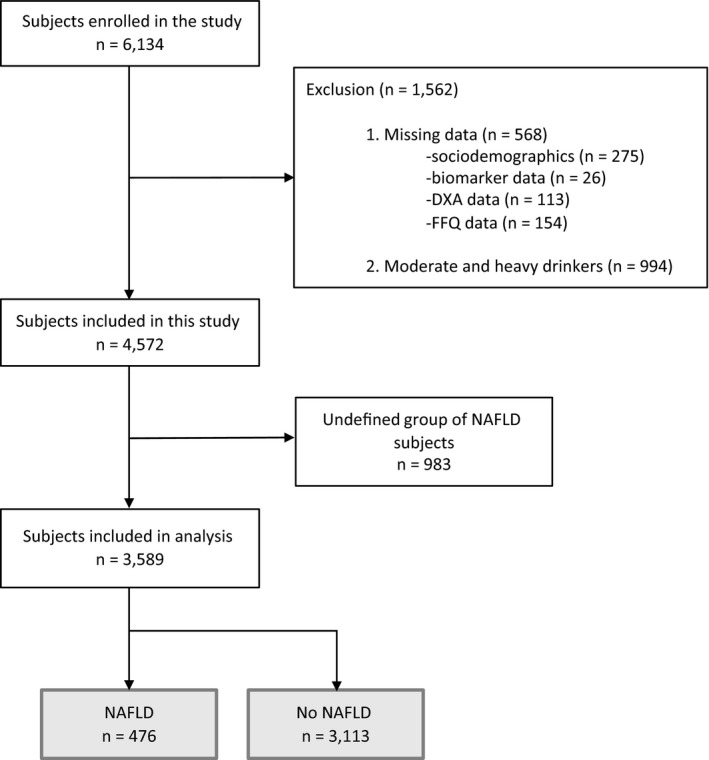
Flow chart for the selection of the study population. Moderate and heavy drinkers: ≥14 g/day for men and ≥7 g/day for women. Abbreviation: FFQ, food frequency questionnaire.
The study was approved by the institutional review boards at both Zhejiang University School of Public Health and Stanford University.
Data Collection and Clinical Examinations
Survey Data Collection
In‐person surveys collected extensive self‐reported data, including demographic characteristics, such as age, sex, income, and educational attainment; lifestyle factors, such as smoking, drinking, and sleep behaviors; well‐being‐related data, such as social connectedness, stress and resilience, and emotional well‐being; and health status, such as diagnoses of chronic conditions, including diabetes, hypertension, and cardiovascular disease.
Anthropometry and Cardiometabolic Measurements
Baseline anthropometric measurements, including height, weight, waist circumference (WC), and hip circumference (HC), were measured 3 times for each subject. For these variables, we used the average of the three measurements for analysis. Overall obesity was defined when body mass index (BMI) was ≥25 kg/m2, according to the World Health Organization (WHO) Asian standard.10 Abdominal obesity was assessed by waist‐to‐hip ratio (WHR), which was calculated by dividing WC (cm) by HC (cm). WHR for each subject was categorized into three groups based on the WHO standard.11 Men with a WHR <0.95 and women with a WHR <0.80 were categorized into low WHR. Men with a WHR between 0.96 and 1.0 and women with a WHR between 0.81 and 0.85 were categorized into moderate WHR. Men with a WHR >1.0 and women with a WHR >0.86 were categorized into high WHR. Metabolic syndrome (MetS) was defined according to the criteria of the International Diabetes Federation12 for the Chinese population, i.e., central obesity (WC ≥90 cm for men and ≥80 cm for women) and at least two of the following factors: (1) serum triglycerides (TG) ≥1.7 mmol/L or ≥150.5 mg/dL; (2) serum high‐density lipoprotein cholesterol <1.0 mmol/L or <38.6 mg/dL in men and <1.3 mmol/L or <50.2 mg/dL in women; (3) systolic blood pressure >130 mm Hg or diastolic blood pressure >85 mm Hg; (4) fasting plasma glucose (FPG) ≥5.6 mmol/L or ≥100.9 mg/dL.
DXA Measurements
We used DXA to assess android fat mass and muscle mass. Whole‐body DXA measurements were made with a GE Lunar Prodigy Scanner (General Electric Medical Systems Lunar, Madison, WI). A whole‐body DXA examination included total body and regional measurements of the head, arms, legs, and trunk (includes ribs, pelvis, and spine) to analyze lean‐ and fat‐mass tissue. The soft tissue analysis was performed using software version 11.40.004 supplied by the manufacturer. DXA scanning was applied in a supine position without any movement.
We used an android fat ratio (AFR) to define the distribution of android fat on the body. The android fat region, according to General Electric Medical Systems, included the lower boundary at the pelvis cut, the upper boundary above the pelvis cut by 20% of the distance between the pelvis and neck cuts, and the lateral boundaries at the arm cuts.13 The AFR was calculated by dividing total android fat mass (g) by total fat mass (g). Muscle mass was defined by the skeletal muscle mass index (SMI), which was calculated by dividing the appendicular lean mass (ALM) (g) by total body weight (g) and multiplying by 100.7, 14 ALM was defined as the summation of both arm and leg lean mass. We decided to use an SMI definition that adjusted for total body weight instead of height (m2)15 because the latter definition produced unstable estimates as there were too few subjects (n = 15) within the low SMI and Yes NAFLD cell. Similar to a previous study, we defined low muscle mass as 1 SD below the mean SMI value of a young reference group created from this study (353 men, 648 women, 18‐40 years old).16 The low muscle‐mass cut‐off points were 29.2% for men and 25.1% for women.
Biochemical Measurements and Metabolic Factors
Fasting venous blood samples were obtained for baseline biochemical analysis. Blood samples were processed following a standardized protocol within 24 hours of collection. Biomarkers, including serum TG, FPG, gamma‐glutamyltransferase (GGT), alanine aminotransferase (ALT), and glycated hemoglobin (HbA1c) were measured. High ALT was defined by >33 IU/L in men and >25 IU/L in women.17 Insulin resistance was defined as 2.0 U/L, using the homeostasis model for insulin resistance (HOMA‐IR).18, 19 Diabetes was defined as HbA1c ≥6.5%, and prediabetes was defined as HbA1c between 5.7% and 6.5%.20
FLI Calculation
In this analysis, we used the FLI to estimate NAFLD status.21 The FLI equation is as follows: FLI = exp(0.953 ln[TG] + 0.139 × BMI + 0.718 ln[GGT] + 0.053 × WC – 15.745)/(1 + exp[0.953 ln{TG} + 0.139 × BMI + 0.718 ln{GGT} + 0.053 × WC – 15.745]) × 100. The FLI classifies subjects with a score of >60 as more likely to have NAFLD (Yes NAFLD) and subjects with <30 as less likely to have NAFLD (No NAFLD). Subjects with a score ≥30 and ≤60 were classified as Maybe NAFLD.21
We also excluded moderate to heavy drinkers from the analysis. Moderate drinkers were defined as consuming 14‐20 g/day (men) or 7‐10 g/day (women) of ethanol. Heavy drinkers were defined as consuming >20 g/day (men) or >10 g/day (women) of ethanol. Ethanol content was derived by the following equation: alcohol quantity (g) × alcohol frequency (times/day) × % alcohol by volume (ABV) × density of alcohol (0.8 g/mL). We used a validated food frequency questionnaire to obtain detailed quantity and frequency consumption of the following four common alcoholic beverages: beer, yellow liquor, white liquor, and red wine. Yellow liquor and white liquor are common alcoholic beverages consumed in China and thus are often easily identifiable by study participants. We then individually calculated the ethanol content of each of the four beverages and summed them to obtain the total alcohol consumption for each participant. We used an average percentage of ABV for each alcoholic beverage as follows: 5% ABV for beer, 14% ABV for yellow liquor, 46% ABV for white liquor, and 12.5% for red wine.
Statistical Analysis
For descriptive analysis, means and SDs were calculated for continuous variables and counts and percentages were calculated for categorical variables. Differences between the groups according to nonnumerical values were tested by the chi‐square test and Fisher exact tests. Normal and non‐normal distributions were distinguished visually by histogram plots. Normally distributed values were analyzed by the Student t test, and non‐normally distributed values were analyzed by the Mann‐Whitney U Test. Bivariate unadjusted regression analyses were conducted to assess the associations of each covariate with NAFLD. Multivariate logistic regression analyses were used to determine independent associations between android fat, muscle mass, and NAFLD. Results of three multivariate models built based on a conceptual model of NAFLD are shown in Fig. 2. Independent variables for each model were selected on the basis of clinically related risk factors. For instance, model 1 shows the odds ratios (ORs) of NAFLD risk after adjusting for age, sex, and income. Model 2 additionally adjusted for other known NAFLD‐related risk factors, such as smoking, ALT, and insulin resistance. Model 3 further adjusted for DXA‐related fat‐ and lean‐mass measures to consider the impact of android fat and skeletal muscle mass on NAFLD. Other variables, including MetS, BMI, and WC, were excluded from our models to minimize collinearity with other independent variables and endogeneity with the FLI outcome variable. In both bivariate and multivariate analyses, the outcome measured was Yes NAFLD (vs. No NAFLD; reference group). However, we also conducted sensitivity analyses to evaluate the robustness of the association findings by including Maybe NAFLD individuals in the models (Supporting Table S1). For all models, ORs and 95% confidence intervals (CIs) were calculated to examine predictive power and significance of each independent variable within the regression model. All statistical analyses were performed with RStudio 1.0.153 (Boston, MA). P < 0.05 was considered statistically significant (two‐sided).
Figure 2.
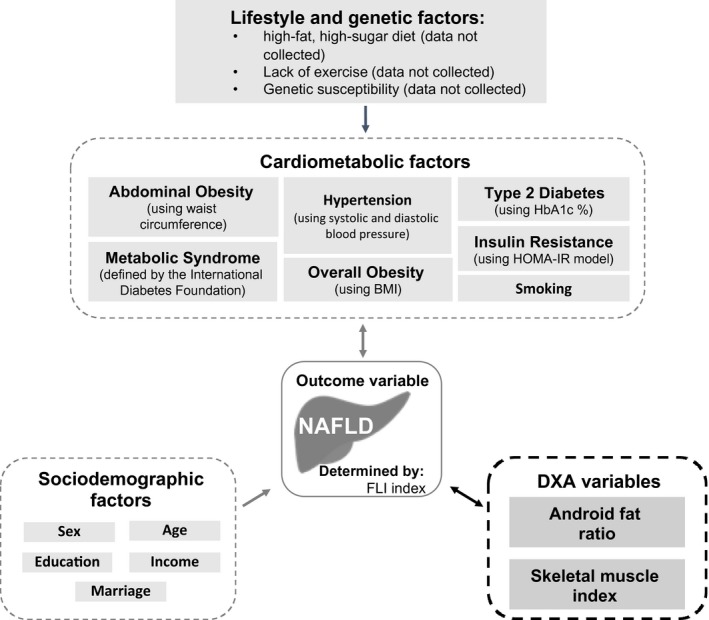
NAFLD conceptual model. Theoretical framework of the relationship of novel DXA‐related cardiometabolic factors and other known cardiometabolic, lifestyle, genetic, and sociodemographic factors that are associated with NAFLD.
Results
Selected characteristics of the 3,589 subjects by NAFLD status defined by the FLI are shown in Table 1. Of these, 3,113 (68.1%) subjects had an FLI score <30 (No NAFLD), 983 (21.5%) had an FLI score between 30 and 60 (Maybe NAFLD), and 476 (10.4%) had an FLI score >60 (Yes NAFLD). The mean FLI was 75.1 (SD, 10.2) for those classified as Yes NAFLD and 11.8 (SD, 3.8) for those classified as No NAFLD. As shown, there were more male subjects in the Yes NAFLD group compared to those in the No NAFLD group (58.4% vs. 24.9%).
Table 1.
Selected characteristics of 3,589 study participants by nafld status
| Total, N | Yes NAFLD | No NAFLD | P value* | |||
|---|---|---|---|---|---|---|
| 3,589 | n | % | n | % | ||
| Variables | 476 | 13.3 | 3,113 | 86.7 | ||
| Sex | ||||||
| Male | 278 | 58.4 | 774 | 24.9 | <0.001 | |
| Female | 198 | 41.6 | 2,339 | 75.1 | ||
| Age (years) | ||||||
| <50 | 167 | 35.1 | 1,314 | 42.2 | <0.05 | |
| 50‐65 | 206 | 43.3 | 1,156 | 37.1 | ||
| >65 | 103 | 21.6 | 643 | 20.7 | ||
| Marital status | ||||||
| Single, divorced,† or widowed | 50 | 10.5 | 389 | 12.5 | 0.26 | |
| Married or remarried | 426 | 89.5 | 2,724 | 87.5 | ||
| Education | ||||||
| Middle school and less | 239 | 50.2 | 1,421 | 45.6 | 0.12 | |
| High school | 106 | 22.3 | 714 | 22.9 | ||
| College and above | 131 | 27.5 | 978 | 31.4 | ||
| Smoking (cigarettes) | ||||||
| Never smokers | 295 | 62.0 | 2,657 | 85.4 | <0.001 | |
| Former smokers | 54 | 11.3 | 166 | 5.3 | ||
| Current smokers | 127 | 26.7 | 290 | 9.3 | ||
| Annual income (US $)‡ | ||||||
| <3,030 | 75 | 15.8 | 493 | 15.8 | <0.05 | |
| 3,030‐12,121 | 313 | 65.8 | 2,193 | 70.4 | ||
| >12,121 | 88 | 18.5 | 427 | 13.7 | ||
| Anthropometry measured at baseline | Mean | SD | Mean | SD | ||
| Height (cm) | 164.8 | 8.9 | 160.2 | 7.4 | <0.001 | |
| Weight (kg) | 77.6 | 10.8 | 56.6 | 7.8 | <0.001 | |
| BMI (kg/m2) | 28.5 | 3.2 | 22.0 | 2.4 | <0.001 | |
| WC (cm) | 98.3 | 7.6 | 78.0 | 7.4 | <0.001 | |
| HC (cm) | 100.9 | 6.9 | 90.2 | 5.1 | <0.001 | |
| WHR | 1.0 | 0.1 | 0.9 | 0.1 | <0.001 | |
| AFR | 0.1 | 0.01 | 0.1 | 0.0 | <0.001 | |
| ALM (kg) | 21.3 | 4.3 | 16.4 | 3.3 | <0.001 | |
| SMI (%) | 27.4 | 3.7 | 28.9 | 3.8 | <0.001 | |
| FLI | ||||||
| FLI >60 | 75.1 | 10.2 | 11.8 | 8.0 | <0.001 | |
| BMI | ||||||
| Underweight (<18.5) | 0 | 0.0 | 210 | 6.7 | <0.001 | |
| Normal (18.5‐22.9) | 9 | 1.9 | 1,873 | 60.2 | ||
| Overweight (23‐24.9) | 38 | 8.0 | 701 | 22.5 | ||
| Pre‐obese (25‐29.9) | 303 | 63.7 | 325 | 10.4 | ||
| Obese (≥30) | 126 | 26.5 | 4 | 0.1 | ||
| Overall obesity | ||||||
| BMI ≥25 | 429 | 90.1 | 329 | 10.6 | <0.001 | |
| WHR | ||||||
| Low ≤0.95 (men), ≤0.80 (women) | 39 | 8.2 | 1,070 | 34.4 | <0.001 | |
| Moderate 0.96‐0.99 (men), 0.91‐0.85 (women) | 124 | 26.1 | 1,003 | 32.2 | ||
| High ≥1.0 (men), ≥0.86 (women) | 313 | 65.8 | 1,040 | 33.4 | ||
| Abdominal obesity | ||||||
| WHR >0.9 (men), >0.85 (women) | 463 | 97.3 | 1,576 | 50.6 | <0.001 | |
| Hypertension12 | ||||||
| SBP >130 mm Hg or DBP >85 mm Hg | 74 | 15.5 | 157 | 5.0 | <0.001 | |
| HbA1c | ||||||
| Normal (<5.7%) | 257 | 54.0 | 2,459 | 79.0 | <0.001 | |
| Prediabetes (5.7%‐6.4%) | 123 | 25.8 | 495 | 15.9 | ||
| Diabetes (≥6.5%) | 96 | 20.2 | 159 | 5.1 | ||
| MetS12 | ||||||
| MetS§ | 266 | 55.9 | 86 | 2.8 | <0.001 | |
| WC ≥90 cm (men), ≥80 cm (women) | 451 | 94.7 | 909 | 29.2 | ||
| TG >1.7 mmol/L | 366 | 76.9 | 378 | 12.1 | ||
| HDL cholesterol <1.0 mmol/L (men), <1.3 mmol/L (women) | 179 | 37.6 | 371 | 11.9 | ||
| SBP >130 mm Hg and/or DBP >85 mm Hg | 74 | 15.5 | 157 | 5.0 | ||
| FPG >5.6 mmol/L | 223 | 46.8 | 505 | 16.2 | ||
| AFR | ||||||
| Tertile 1 (0, 0.093) | 16 | 3.4 | 1,031 | 33.1 | <0.001 | |
| Tertile 2 (0.093, 0.1) | 82 | 17.2 | 1,020 | 32.8 | ||
| Tertile 3 (0.1, 1.0) | 378 | 79.4 | 1,062 | 34.1 | ||
| SMI | ||||||
| Low (men ≤29; women ≤25) | 231 | 48.5 | 449 | 14.4 | <0.001 | |
| High (men >29; women >25) | 245 | 51.5 | 2,664 | 85.6 | ||
P values by Student t test (for continuous variables) or chi‐square test (for categorical variables); comparing cases and controls.
ndivorced = 25 subjects.
Chinese renminbi (RMB) was converted into US $ based on the average exchange rate in December 2017 (US $1 = RMB 6.6).
MetS defined by having WC ≥90 cm in men and ≥80 cm in women and at least two of the following four factors listed under MetS.
Abbreviations: DBP, diastolic blood pressure; HDL, high‐density lipoprotein; SBP, systolic blood pressure.
Associations of various risk factors with the possibility of NAFLD in both bivariate and multivariate logistic regression models are shown in Table 2. In unadjusted bivariate analysis, male participants had a 4.2‐fold risk of having NAFLD (95% CI, 3.5‐5.2) compared to their female counterparts. NAFLD risk was also higher among individuals who currently smoked (OR, 3.9; 95% CI, 3.1‐5.0) or quit smoking (OR, 2.9; 95% CI, 2.1‐4.0) relative to individuals who had never smoked. Individuals with high serum ALT levels (men >33; women >25) had a 9.0‐fold risk of NAFLD compared to those with lower ALT levels, and the risk of NAFLD was also higher among those with insulin resistance (HOMA‐IR >2.0 U/L) compared to those without insulin resistance (HOMA‐IR ≤2.0 U/L). High AFR (>0.1) was significantly and positively associated with NAFLD (OR, 22.9; 95% CI, 14.3‐29.7), while high SMI (>29.1% in men; >25.1% in women) was significantly and inversely associated with NAFLD (OR, 0.2; 95% CI, 0.1‐0.2).
Table 2.
Unadjusted and adjusted ORs for NAFLD in relation to AFR and SMI
| NAFLD Status | Bivariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||
| Yes | No | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| n | 476 | 3,113 | ||||||||
| Sex | ||||||||||
| Female | 198 | 2,339 | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| Male | 278 | 774 | 4.2* | 3.5‐5.2 | 4.2* | 3.5‐5.2 | 3.3* | 2.4‐4.4 | 2.9* | 2.0‐4.2 |
| Age (years) | ||||||||||
| <50 | 167 | 1,314 | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| 50‐65 | 206 | 1,156 | 1.4* | 1.1‐1.7 | 1.4* | 1.1‐1.7 | 1.7* | 1.3‐2.2 | 1.2 | 0.9‐1.6 |
| >65 | 103 | 643 | 1.3 | 0.9‐1.6 | 1.1 | 0.8‐1.4 | 1.9 | 1.4‐2.6 | 1.2 | 0.83‐1.7 |
| Income (US $)† | ||||||||||
| <3,030 | 75 | 493 | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| 3,030‐12,121 | 313 | 2,193 | 0.9 | 0.7‐1.2 | 0.8 | 0.6‐1.0 | 0.9 | 0.7‐1.3 | 0.9 | 0.7‐1.4 |
| >12,121 | 88 | 427 | 1.4 | 0.9‐1.9 | 0.9 | 0.7‐1.4 | 0.9 | 0.6‐1.5 | 1.0 | 0.6‐1.6 |
| Smoking (cigarettes) | ||||||||||
| Never smokers | 295 | 2,657 | 1.0 | – | 1.0 | – | 1.0 | – | ||
| Quit smoking | 54 | 166 | 2.9* | 2.1‐4.0 | 1.4 | 0.9‐2.0 | 1.3 | 0.8‐2.1 | ||
| Current smokers | 127 | 290 | 3.9* | 3.1‐5.0 | 1.9* | 1.4‐2.7 | 2.0* | 1.4‐3.0 | ||
| ALT (IU/L) | ||||||||||
| Low (men ≤33; women ≤25) | 239 | 2,805 | 1.0 | – | 1.0 | – | 1.0 | – | ||
| High (men >33; women >25) | 237 | 308 | 9.0* | 7.3‐11.2 | 8.7* | 6.9‐11.1 | 7.6* | 5.8‐10.0 | ||
| Insulin resistance | ||||||||||
| Low (≤2.0) | 279 | 2,718 | 1.0 | – | 1.0 | – | 1.0 | – | ||
| High (>2.0) | 197 | 395 | 4.9* | 3.9‐6.0 | 4.9* | 3.8‐6.3 | 4.0* | 3.0‐5.3 | ||
| AFR‡ | ||||||||||
| Tertile 1 (0, 0.093) | 16 | 1,031 | 1.0 | – | 1.0 | – | ||||
| Tertile 2 (0.093, 0.1) | 82 | 1,020 | 5.2* | 3.1‐9.2 | 3.2* | 1.8‐5.3 | ||||
| Tertile 3 (0.1, 1.0) | 378 | 1,062 | 22.9* | 14.3‐39.7 | 10.0* | 5.8‐18.5 | ||||
| SMI§ | ||||||||||
| Low (men <29.1%; women <25.1%) | 231 | 449 | 1.0 | – | 1.0 | – | ||||
| High (men ≥29.1%; women ≥25.1%) | 245 | 2,664 | 0.2* | 0.1‐0.2 | 0.1* | 0.07‐0.13 | ||||
Denotes significant associations where P < 0.05.
Chinese RMB was converted into US $ based on the average exchange rate in December 2017 (US $1 = RMB 6.6).
AFR is android fat mass/total fat mass.
SMI is ALM/weight × 100, where ALM is arms lean mass + legs lean mass.
In the multivariate analysis, we ran three separate multivariate logistic regression models: model 1 adjusted for various sociodemographic (SES) factors; model 2 adjusted for SES factors and NAFLD‐related risk factors, including smoking, ALT liver enzyme levels, and insulin resistance; and model 3 further adjusted for DXA‐derived NAFLD risk factors, such as AFR and SMI. In all models, men had an increased risk of NAFLD relative to women. When adjusted for SES factors, men had a 4.2‐fold risk of NAFLD and individuals between 50 and 65 years of age had a 1.4‐fold risk relative to individuals younger than 50 years. These associations stayed consistent even after further adjusting for smoking, ALT levels, and insulin resistance in model 2. In this model, current smokers had the highest risk of NAFLD compared to those who never smoked (OR, 1.9; 95% CI, 1.4‐2.7). Subjects who quit smoking had a 1.4‐fold risk of NAFLD relative to those who never smoked (95% CI, 0.9‐2.0). Moreover, high ALT levels had a positive association with NAFLD risk, independent of insulin resistance (Table 2). In model 3, we found individuals with a high AFR had a 10.0‐fold risk of NAFLD (95% CI, 5.8‐15.5) compared to those with a low AFR. In contrast, individuals with a high SMI had a reduced risk of NAFLD relative to those with a low SMI (OR, 0.1; 95% CI, 0.07‐0.13).
Risk patterns for the Yes NAFLD group as reported in model 3 persisted even when we included the 983 Maybe NAFLD individuals in the multinomial regression sensitivity analysis (Supporting Table S1). The magnitude for the NAFLD risk estimates among the Yes NAFLD group was greater than that for the Maybe NAFLD group.
Discussion
Using data from the WELL China study, we uniquely show that there is an independent positive association of AFR and an inverse association of SMI with NAFLD. It has been debated whether regional adiposity sites are better predictors of cardiometabolic diseases than measures such as BMI and WC.8 Previous studies have demonstrated that greater WC increases metabolic or cardiovascular risks.22 However, generalized anthropometric fat measurements, such as WC, cannot distinguish between fat and muscle mass, both of which have structural and functional differences that contribute to disease risk.23 In the present study, we used DXA scanning as a way to accurately and precisely measure regional android fat mass and skeletal muscle mass to better understand their associations with NAFLD risk.
Recent studies have shown that high android fat was a major determinant of the development of metabolic and cardiovascular disease risk.24 NAFLD is thought to be a hepatic manifestation of MetS25; therefore, it is not surprising that we also found a significant positive association between the AFR and NAFLD. Although the pathogenesis of NAFLD is unclear, it is likely that the AFR plays a key role. A possible mechanism may be because android fat stores readily undergo lipolysis and release free fatty acids (FFAs) into the blood to be circulated throughout the body.26 As a result, the increased concentration of FFAs in the arterial circulation system may increase the risk of cardiovascular disease. Similarly, FFAs exposed to the liver through the hepatic portal system may increase the risk of developing NAFLD. Therefore, certain exercise or dietary interventions targeting decreased android fat may be important in the prevention and management of NAFLD.
Insulin resistance has been reported to be an important factor in mediating the progression of NAFLD, primarily due to its strong association with intra‐abdominal fat.6 Studies have found that the accumulation of intra‐abdominal fat was positively correlated with liver fat27 and hepatic insulin resistance in both men and women.28 In the present study, we found a positive association between insulin resistance and NAFLD independent of abdominal android fat, which is consistent with other studies. In addition to abdominal fat, skeletal muscle is also a target organ of insulin through glucose metabolism.26 Under normal conditions, muscles respond to changes in insulin levels that lead it to either breakdown or store glucose. However, when cells in the muscle fail to respond normally to insulin, partly due to increased levels of FFAs in the blood, peripheral insulin resistance in muscles results, leading to the reduction of protein synthesis and muscle mass.26 The independent and inverse association between SMI and NAFLD in this study suggests that having higher skeletal muscle mass might be important when investigating NAFLD. This finding is of great interest and relevance to the Chinese population. Sarcopenia is an age‐related disorder that is characterized by the loss of skeletal muscle mass and strength and is a common public health problem in the Chinese population.29 Some studies have argued that sarcopenia shares several pathophysiologic processes with NAFLD, including that of insulin resistance and chronic inflammation.7 Similar to the results of our study, Kim et al.7 also found a significant inverse association between skeletal muscle mass and NAFLD. To better understand the relationship between SMI and NAFLD, further exploration of the mechanism of how an increase in muscle mass and strength may reverse or prevent the progression of NAFLD in individuals with hepatic steatosis is warranted.
We used the widely validated FLI to assess NAFLD status in a large sample in this community‐based study. The FLI is a reliable algorithm used in Western populations to predict NAFLD based on WC, BMI, TG, and GGT.21 It was first proposed by Bedogni et al.21 and has since been validated by liver biopsy or ultrasonography in various studies of different races, age groups, and ethnicities.30, 31 Although liver biopsy is currently the gold standard for diagnosing NAFLD, it is invasive and costly, making it less suitable for screening NAFLD in large population studies. Thus, noninvasive scoring indices, such as the FLI, have been developed based on important and relevant risk factors for NAFLD. The FLI was shown to be an accurate and precise predictor for NAFLD in a population of middle‐aged and elderly Chinese people from Shanghai, China, which is geographically close to Hangzhou.30 This suggests that the FLI may be a valuable tool in helping to screen for NAFLD risk in large population studies. For reasons similar to the FLI, we used the widely reported HOMA‐IR model as a reliable clinical and epidemiologic tool to detect insulin resistance in our population.
This study has several strengths. First, we collected data from a large number of subjects in two districts in Hangzhou across a wide range of age and education levels with a high response rate. Second, we used DXA to objectively assess fat and muscle mass directly. DXA is an accurate and precise tool for measuring body fat mass. Third, with extensive biomarker and body measurement data, we were able to adjust for several potential confounding factors that were objectively measured, including ALT, diabetes, insulin resistance, and MetS.
Limitations of the study should be mentioned. First, because this was a cross‐sectional study, we could not establish temporal relationships and thus causal relationships. Second, subjects with missing data were excluded from our analysis, which might have caused bias if data were not missing at random. Such bias, if any, should be minimal as the extent for each variable is less than 5%. Third, the use of the FLI in this study has several limitations. Although it is widely validated, the FLI does not conclusively define NAFLD, and in this analysis we did not use other techniques, such as abdominal ultrasound30 or magnetic resonance spectroscopy,32 to validate each subject’s NAFLD status in our cohort. This potentially could have led to diagnostic misclassification. Validation studies of the FLI in other Chinese populations have reported areas under the receiver operating curve (AUROC) ranging from 0.76 to 0.88,30, 33, 34, 35 but given that we used a higher FLI cut‐off value than that recommended for the Chinese population,33 we expect to have smaller misclassification bias. Furthermore, because our study population is relatively ethnically homogeneous (Han Chinese), we anticipate a higher AUROC of the FLI in this analysis. The use of the FLI in this study to stratify patients is another potential limitation as several of the components that make up the FLI are associated with our predictor variables (e.g., WC, which is part of the FLI, is likely to correlate with the AFR because both are related to abdominal obesity). Future studies may consider including other relevant outcomes, such as the fibrosis‐4 index or the NAFLD fibrosis score, to minimize potential correlations between outcomes and predictors. Finally, the lack of viral hepatitis data is a limitation worth nothing. In China, the prevalence of hepatitis C virus is <1%36 while the prevalence of hepatitis B virus (HBV) is 7%‐10%,37, 38 so it would have been ideal to at least have data on the HBV carrier status in our population study. Furthermore, because those with chronic HBV infection have a higher risk of steatosis, it is possible some subjects in our study had a high FLI score that was related to HBV infection instead of NAFLD.38 Although the effect of hepatic steatosis on the disease course of HBV and the pathophysiology between HBV and NAFLD are unclear, future studies should measure viral hepatitis and exclude those with viral hepatitis infections to confirm our findings.
In conclusion, android fat mass was independently and positively associated with NAFLD, while muscle mass was independently and inversely associated with NAFLD after accounting for a comprehensive list of covariates. These findings suggest that interventions focusing on decreasing android fat and increasing muscle mass may be important for the prevention and reversal of NAFLD.
Supporting information
Acknowledgment
We acknowledge all participants involved in the WELL China project and the Community Health Service Centers, China Center for Disease Control and Prevention (China CDC), and the Health Bureaus of Xihu and Shangcheng Districts, Hangzhou, China. We also thank Dr. Davis Maahs for his knowledge and expertise with respect to the manuscript.
Initial funding for the Stanford Well Living Laboratory (WELL) was provided by Amway via a gift through the Nutrilite Health Institute Wellness Fund (GHEEV 1174806‐104 to A.W.H., S.J.W., Y.M., X.Z., D.G., and S.Z.). This study was also supported by funding from the Cyrus Tang Foundation (100000‐11028 [2018]; 100000‐11028 [2019] to S.Z.) and the Zhejiang University Education Foundation (419600‐1102 to S.Z.).
Potential conflict of interest: Dr. Nguyen advises and received grants from Bristol‐Myers Squibb, Janssen, Gilead, Laboratory for Advance Medicine, and Exact Sciences Laboratories; she advises Intercept, Roche, Dynavax, Alnylam, Novartis, Eisai, Bayer, and Spring Bank and received grants from the National Cancer Institute and the B.K. Kee Foundation. The other authors have nothing to report.
Contributor Information
Shankuan Zhu, Email: zsk@zju.edu.cn.
C. Jason Wang, Email: cjwang1@stanford.edu.
References
Author names in bold designate shared co‐first authorship.
- 1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 2. Paul S, Davis AM. Diagnosis and management of nonalcoholic fatty liver disease. JAMA 2018. 10.1001/jama.2018.17365 [DOI] [PubMed] [Google Scholar]
- 3. VanWagner LB, Rinella ME. Extrahepatic manifestations of nonalcoholic fatty liver disease. Curr Hepatol Rep 2016;15:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhai HL, Wang NJ, Han B, Li Q, Chen Y, Zhu CF, et al. Low vitamin D levels and non‐alcoholic fatty liver disease, evidence for their independent association in men in East China: a cross‐sectional study (Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT‐China)). Br J Nutr 2016;115:1352‐1359. [DOI] [PubMed] [Google Scholar]
- 5. Dietrich P, Hellerbrand C. Non‐alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014;28:637‐653. [DOI] [PubMed] [Google Scholar]
- 6. Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2006;91:4753‐4761. [DOI] [PubMed] [Google Scholar]
- 7. Kim HY, Kim CW, Park CH, Choi JY, Han K, Merchant AT, et al. Low skeletal muscle mass is associated with non‐alcoholic fatty liver disease in Korean adults: the Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis Int 2016;15:39‐47. [DOI] [PubMed] [Google Scholar]
- 8. Okosun IS, Seale JP, Lyn R. Commingling effect of gynoid and android fat patterns on cardiometabolic dysregulation in normal weight American adults. Nutr Diabetes 2015;5:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hangzhou Statistical Yearbook 2017 . Beijing, China: China Statistics Press; 2017. [Google Scholar]
- 10. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157‐163. Erratum. In: Lancet 2004;363:902. [DOI] [PubMed] [Google Scholar]
- 11. WHO . Waist circumference and waist‐hip ratio: report of a WHO Expert Consultation, Geneva, 8–11 December 2008. Geneva, Switzerland: World Health Organization; 2011. https://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 12. International Diabetes Federation . The IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. https://www.pitt.edu/~super1/Metabolic/IDF1.pdf. [Google Scholar]
- 13. GE Healthcare . Lunar enCORE‐based x‐ray bone densitometer User Manual. http://medicaloutfitter.net/wp-content/uploads/2014/09/enCORE_V13.5_EN_English.pdf. Revised January 2010. Accessed on November 28, 2018 [Google Scholar]
- 14. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean longitudinal study on health and aging (KLoSHA). Diabetes Care 2019;33:1652‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al.; Health ABC Study Investigators . Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602‐1609. [DOI] [PubMed] [Google Scholar]
- 16. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772‐1778. [DOI] [PubMed] [Google Scholar]
- 17. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18‐35. [DOI] [PubMed] [Google Scholar]
- 18. Salgado AL, Carvalho Ld, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA‐IR) in the differentiation of patients with non‐alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol 2010;47:165‐169. [DOI] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Published 2011. Accessed on December 11, 2018 [Google Scholar]
- 21. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi‐Sunyer X, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (Silver Spring) 2006;14:727‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11‐18. [DOI] [PubMed] [Google Scholar]
- 24. Samsell L, Regier M, Walton C, Cottrell L. Importance of android/gynoid fat ratio in predicting metabolic and cardiovascular disease risk in normal weight as well as overweight and obese children. J Obes 2014;2014:846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai. China. J Hepatol 2005;43:508‐514. [DOI] [PubMed] [Google Scholar]
- 26. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93(Suppl. 1):S57‐S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen‐Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 2003;284:E1065‐E1071. [DOI] [PubMed] [Google Scholar]
- 28. Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2002;283:E1135‐E1143. [DOI] [PubMed] [Google Scholar]
- 29. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014;15:95‐101. [DOI] [PubMed] [Google Scholar]
- 30. Huang X, Xu M, Chen Y, Peng K, Huang Y, Wang P, et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle‐aged and elderly Chinese. Medicine (Baltimore) 2015;94:e1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015;41:65‐76. [DOI] [PubMed] [Google Scholar]
- 32. Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee‐Moradie F, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H‐magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin‐resistant individuals. Eur J Endocrinol 2014;171:561‐569. [DOI] [PubMed] [Google Scholar]
- 33. Xia MF, Yki‐Järvinen H, Bian H, Lin HD, Yan HM, Chang XX, et al. Influence of ethnicity on the accuracy of non‐invasive scores predicting non‐alcoholic fatty liver disease. PLoS One 2016;11:e0160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang BL, Wu WC, Fang KC, Wang YC, Huo TI, Huang YH, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large‐scale cross‐sectional study in Taiwan. PLoS One 2015;10:e0120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu J, He M, Zhang Y, Li T, Liu Y, Xu Z, et al. Validation of simple indexes for nonalcoholic fatty liver disease in western China: a retrospective cross‐sectional study. Endocr J 2018;65:373‐381. [DOI] [PubMed] [Google Scholar]
- 36. Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161‐176. [DOI] [PubMed] [Google Scholar]
- 37. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Reprint of: epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine 2013;31(Suppl. 9):J21‐J28. [DOI] [PubMed] [Google Scholar]
- 38. Seto WK, Hui R, Mak LY, Fung J, Cheung KS, Liu K, et al. Association between hepatic steatosis, measured by controlled attenuation parameter, and fibrosis burden in chronic hepatitis B. Clin Gastroenterol Hepatol 2018;16:575‐583.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


