During development, oligodendrocyte precursor cells (OPCs) are guided by several cues as they follow distinct migratory routes to target axons of the CNS, where they differentiate into myelinating oligodendrocytes. Recruitment of migrating OPCs also facilitates the repair of demyelinated areas in Multiple Sclerosis (MS) and spinal cord injury. The developmental signals that influence these cells include attractive and repulsive cues such as platelet derived growth factor AA (PDGF) and netrin-1 [for review, see de Castro and Bribián (2005)], but the precise mechanisms by which these cues act are poorly understood. By identifying key components of the intracellular signaling cascade triggered by PDGF, Miyamoto and colleagues (2008) have provided further insight into the processes governing OPC migration.
Rearrangement of the actin cytoskeleton leads to motility in a number of cell types, including OPCs and oligodendrocytes. Modulators of oligodendrocyte morphology that affect actin polymerization include the Src family nonreceptor tyrosine kinases Src, Fyn, Yes, and Lyn (Colognato et al., 2004); the Rho family of small GTPases; and the Wiskott-Aldrich syndrome protein (WASP) family members, N-WASP and WAVE-1 [for review, see Sloane and Vartanian (2007)]. The serine/threonine kinase cyclin-dependent kinase 5 (Cdk5) also plays a role in the differentiation of OPCs into oligodendrocytes (Miyamoto et al., 2007). Miyamoto and colleagues (2008) have proposed that binding of PDGF to its receptor, PDGF-Rα, initiates a signaling cascade involving sequential activation of Fyn, Cdk5, and WAVE-2, which culminates in increased migration.
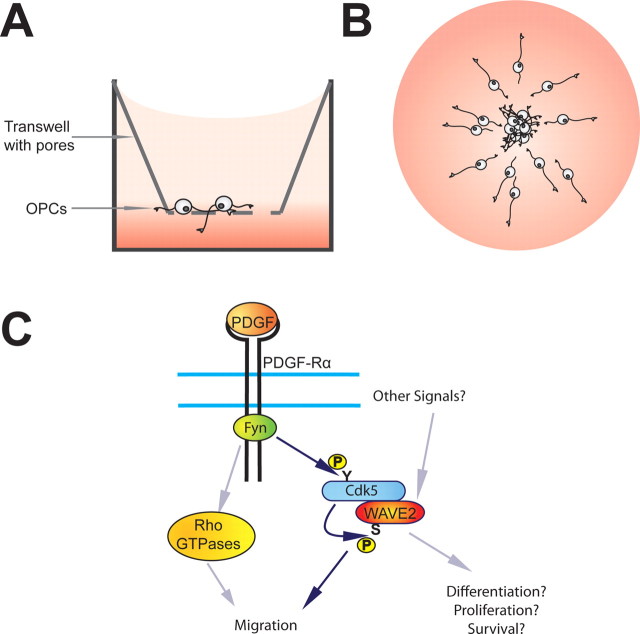
OPC migration in vitro has classically been studied with microchemotaxis assays (Fig. 1A). Miyamoto and colleagues (2008) complemented this method with a modified aggregation assay [Miyamoto et al. (2008), their Fig. 1A (http://www.jneurosci.org/cgi/content/full/28/33/8326/F1)] (Fig. 1B), originally used to study Schwann cell migration in vitro. This method of studying migration in vitro permits simple and effective quantification of OPC migration. Future work can combine live-imaging with this assay to illuminate the real-time response of OPCs to modulators of migration, providing more information about how these cells orient their motile processes as they migrate.
Figure 1.
Investigating the mechanism of OPC migration. A, In a microchemotaxis assay, cells are seeded on a transwell filter containing pores and exposed to a gradient of a chemotactic cue. The numbers of cells that migrate to the bottom of the filter under different conditions are compared. B, In a reaggregation assay, aggregates of OPCs are plated on low attachment plates, and exposed to chemotactic cues. The average distance migrated by the cells is compared under different conditions. C, The signaling cascade induced by PDGF. Blue arrows indicate the interpretations proposed by the authors, gray arrows represent untested alternatives.
PDGF stimulated the migration of OPCs in both the microchemotaxis assay and the aggregation assay [Miyamoto et al. (2008), their Fig. 1A (http://www.jneurosci.org/cgi/content/full/28/33/8326/F1)]. Through pharmacological inhibition and shRNA knockdown, the authors demonstrated that the PDGF-dependent increase in OPC migration required Cdk5 activation [Miyamoto et al. (2008), their Fig. 2 (http://www.jneurosci.org/cgi/content/full/28/33/8326/F2)]. Surprisingly, however, knockdown of p35 and p39, the canonical activators of Cdk5, only partially disrupted PDGF-mediated OPC migration [Miyamoto et al. (2008), their Fig. 2K,M (http://www.jneurosci.org/cgi/content/full/28/33/8326/F2)]. The authors interpret this data to indicate the presence of another coactivator. Inhibition of one potential candidate, Fyn, disrupted both PDGF-dependent activation of Cdk5 and the resulting increase in OPC migration. Biochemical studies indicated that both Cdk5 and Fyn were phosphorylated and activated over a similar time course [Miyamoto et al. (2008), their Fig. 3 (http://www.jneurosci.org/cgi/content/full/28/33/8326/F3)], supporting a role for a functional interaction between the two proteins.
A similar strategy was used to investigate the potential for the WASP family member WAVE2 to act downstream of Cdk5 and mediate its migration-specific activity. Immunoprecipitation performed from 293T cells expressing recombinant Cdk5 and WAVE2 demonstrated that Cdk5 bound to the SH domain of WAVE2, resulting in the phosphorylation of a serine residue (S137) [Miyamoto et al. (2008), their Fig. 6 (http://www.jneurosci.org/cgi/content/full/28/33/8326/F6)]. However, the evidence for such an interaction in OPCs was not as persuasive because the immunoprecipitation of WAVE2 was performed using an antibody that recognizes a consensus sequence phosphorylated by the MAPK and Cdk family members, and not Cdk5 specifically [Miyamoto et al. (2008), their Fig. 7 (http://www.jneurosci.org/cgi/content/full/28/33/8326/F7)].
The authors also demonstrated a functional role for WAVE2 in the promotion of OPC migration. Loss-of-function experiments, such as the knockdown of WAVE2 [Miyamoto et al. (2008), their Fig. 5 (http://www.jneurosci.org/cgi/content/full/28/33/8326/F5)] or the infection of OPCs with a phosphorylation-incompetent WAVE2 (S137A), resulted in disruption of PDGF-induced migration [Miyamoto et al. (2008), their Fig. 7E (http://www.jneurosci.org/cgi/content/full/28/33/8326/F7)]. Infection of OPCs with a phosphomimetic mutant WAVE2 (S137E) promoted migration in the absence of PDGF, strengthening the link between WAVE2 phosphorylation and increased OPC migration. But does WAVE2 act specifically downstream of PDGF? Infection of OPCs with wild-type WAVE2 also increased baseline OPC migration, and interestingly, these cells were unresponsive to PDGF stimulation. This ability of wild-type WAVE2 to increase migration in the absence of PDGF stimulation indicates that the authors may have identified an important signaling modulator that, when present at sufficiently high levels, independently promotes OPC motility. Inhibition or activation of WAVE2 in the presence of other attractive or repulsive guidance cues would enhance our understanding of the specificity of this mechanism.
Miyamoto and colleagues (2008) conclude that PDGF promotes migration by initiating a signaling cascade involving Fyn, Cdk5, and WAVE2. In their discussion, however, they also acknowledge that Fyn could independently stimulate the Rho GTPases to modulate the cytoskeletal changes leading to migration. It therefore unclear whether Fyn, Cdk5 and WAVE2 act sequentially to stimulate migration or whether these components are part of parallel migration-related signaling cascades (Fig. 1C). This issue could be resolved by using roscovitine and PP1 to inhibit both Cdk5 and Fyn in the presence of PDGF. Another informative experiment would be to test the effect of this inhibition on the observed increase in baseline motility of OPCs expressing wild-type or constitutively active WAVE2. In both cases, synergistic effects of the inhibitors on migration would point to the existence of parallel pathways, while redundancy would support the interpretations made in the study.
Overall, the biochemical experiments provide strong evidence for the involvement and interaction of Fyn, Cdk5 and WAVE2 in a PDGF-induced signaling cascade. However, this cascade may have multiple functional consequences because PDGF also increases OPC proliferation in addition to migration (Noble et al., 1988). Miyamoto and colleagues (2008) used the DNA synthesis inhibitor Cytosine arabinoside (AraC), which inhibited cell proliferation in the functional assays but would not have prevented the biochemical activation of a signaling pathway triggered by PDGF. Furthermore, this study did not investigate the effect of their experimental manipulations on cell survival or differentiation. Trypan blue exclusion was used to account for cell viability but this method does not detect the upregulation of early apoptotic markers. Fyn plays an important role in the differentiation of oligodendrocytes (Colognato et al., 2004) and the authors recently demonstrated that Cdk5 is required for oligodendrocyte maturation (Miyamoto et al., 2007). This raises the interesting possibility that manipulation of these proteins could alter migration by promoting the differentiation of OPCs into less motile oligodendrocytes.
The relevance of Fyn, Cdk5, and WAVE2 to OPC migration in vivo is unclear, since OPC migration defects in the respective knock-outs have not been reported. There is no obvious migration defect in the thinly myelinated Fyn null mice (Umemori et al., 1994), although several explanations may account for the lack of a phenotype. One possibility is that while myelination occurs, it is poor because OPCs are frequently misguided. Another possibility is that a requirement for Fyn in vivo is masked by the upregulation of other Src family kinases. Mice lacking Cdk5 show defects in the neuronal progenitor migration, but OPC migration defects have not been reported. WAVE1, WAVE2, and WAVE3 are expressed by cells of the oligodendrocyte lineage, and the authors show that WAVE1 may also play a role in PDGF-mediated migration [Miyamoto et al. (2008), their Fig. 5D (http://www.jneurosci.org/cgi/content/full/28/33/8326/F5)]. However, WAVE1 deficient mice do not show aberrant OPC migration, and the embryonic lethality of WAVE2 mice means that oligodendrocyte-specific conditional knock-outs would be required to address the relevance of WAVE family members to migration.
These caveats should not detract from the significance of the findings, which open a number of new avenues of research. It will be interesting to study whether there is a directional component to the signaling cascade identified by the authors. Does activation of Fyn, Cdk5 or WAVE2 influence the ability of an OPC to orient itself to a gradient of PDGF, thereby facilitating increased migration? This issue could be addressed by adding PDGF to the top compartment of the microchemotaxis well, or by spatially restricting the source of the cue in the aggregation assay. Additionally, these signals could induce changes in cell adhesion to stimulate migration. Cdk5 associates with the focal adhesion marker paxillin in OPCs (Miyamoto et al., 2007), while WASP family members have been observed at the leading edge of an OPC growth cone (Kim et al., 2006; Sloane and Vartanian, 2007). Future work with the aggregation assays could include immunostaining for components of focal complexes at these leading edges, furthering our knowledge of the adhesive mechanisms that result in OPC process extension and migration.
These results have therapeutic implications for demyelinating disorders. Poor recruitment of OPCs to demyelinated lesions in spinal cord injuries and MS is thought to contribute to inefficient remyelination. The cues that contribute to the inhibition of OPC migration may act to modulate the signaling cascade identified by Miyamoto and colleagues (2008). The important role played by this cascade in both neuronal and oligodendrocyte development, combined with the potential use of pharmacological agents that target these molecules, raises the question of how the different CNS cell types would respond to therapies directed at OPC recruitment. Therapies promoting migration may alter neuronal migration, axon extension and axonal sprouting. One way to circumvent such challenges may lie in targeting the cell-type specific molecules that play a supporting role in activating these pathways, such as the Cdk5 coactivators p35 and p39. By identifying novel interactions in a signaling cascade downstream of PDGF, Miyamoto and colleagues (2008) have thus provided several focal points for the future study of OPC migration.
Footnotes
S.R. is supported by a studentship from the Multiple Sclerosis Society of Canada. I would like to thank Dr. Timothy Kennedy, Dr. K. Adam Baker, Katherine E. Horn, Jenea Bin, and Sarah-Jane Bull for their comments.
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
References
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro F, Bribián A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Brain Res Rev. 2005;49:227–241. doi: 10.1016/j.brainresrev.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Chan JR, Okada A, Tomooka Y, Hisanaga S, Tanoue A. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci. 2007;120:4355–4366. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A. Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. J Neurosci. 2008;28:8326–8337. doi: 10.1523/JNEUROSCI.1482-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Vartanian TK. WAVE1 and regulation of actin nucleation in myelination. Neuroscientist. 2007;13:486–491. doi: 10.1177/1073858407299423. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]