Abstract
This study investigated the role of sensory experience in the refinement of whisker sensory relay synapses in the ventral posterior medial nucleus (VPm) of the murine thalamus. Sensory deprivation was done by whisker plucking, and synaptic connectivity was determined by whole-cell patch-clamp recording in acute slices. Sensory deprivation started at P12–P13, but not at P16, disrupted the elimination of VPm relay synapses. The majority of deprived neurons received multiple relay inputs, whereas the majority of nondeprived neurons received a single relay input. Sensory deprivation started a few days earlier at P10, however, had no effect on synapse elimination. The disruption of synapse elimination was associated with a delay in synapse maturation. The AMPA/NMDA ratio of EPSC was significantly smaller in deprived neurons. On the other hand, deprivation had no effect on the peak amplitude or decay time constant of the NMDA component, or the I–V relationship of the AMPA component, nor does it affect the paired-pulse ratio of EPSCs. The reduction in the AMPA/NMDA ratio was already evident within 24 h of whisker plucking, and the effect is associated with a reduction in the amplitude of quantal AMPA events. Thus, P12–P13 is a critical period for experience-dependent refinement at the whisker sensory relay synapse in the VPm.
Keywords: somatosensory, thalamus, synapse, refinement, vibrissa, mouse
Introduction
The somatosensory system in rodents has been widely used as a model for studying the formation and plasticity of specific neuronal connections in the brain. Tactile information from large whiskers on the rodent snout is relayed to the neocortex primarily through two distinct pathways (Williams et al., 1994). The first, termed the lemniscal pathway, involves the principal V nucleus (Pr5) in the brainstem and the ventral posterior medial nucleus (VPm) in the thalamus. The second, called the paralemniscal pathway, involves the spinal trigeminal subnucleus that projects to the posterior nucleus of the thalamus (Veinante et al., 2000). The lemniscal pathway is topographically organized, with whisker-specific maps present in the Pr5, VPm, and layer 4 of the barrel cortex.
Whisker-specific maps are established during the first few days after birth (Killackey et al., 1995). As in the visual cortex, early sensory experience plays an important role in the development of connectivity in the barrel cortex. Whisker trimming or plucking started before P5 leads to enlarged receptive fields and reduced angular tuning of layer 4 neurons (Simons and Land, 1987; Fox, 1992). However, the whisker-specific map is maintained in deprived animals, suggesting that sensory experience has a limited role in the formation of the map, but is critical for refinement of thalamocortical synapses. Experience-dependent plasticity is also prominent in layer 2/3 of the barrel cortex. Whisker deprivation started before P14 disrupts receptive field structure of layer 2/3 neurons (Stern et al., 2001), presumably through changes in the projection from layer 4 to layer 2/3 (Allen et al., 2003; Shepherd et al., 2003).
Little is known about the role of experience in the development of whisker sensory pathways in subcortical regions. Neonatal blockade of sensory nerve activity does not disrupt the formation of whisker-specific maps in subcortical regions (Henderson et al., 1992). Whisker trimming started at birth has no effect on response properties of deprived VPm neurons in young adult rats (Simons and Land, 1994). On the other hand, the whisker sensory relay synapse in the VPm undergoes extensive remodeling during the second week after birth (Arsenault and Zhang, 2006). At postnatal day 7–9 (P7–P9), well after whisker-specific innervation pattern is formed, each VPm neuron is innervated by an average of eight lemniscal axons. The number of lemniscal axons per neuron decreases rapidly, and the adult pattern is achieved by P16–P17 where the majority of VPm neurons receive one or two lemniscal afferents. This remodeling process is likely to play a key role in establishing functional specifications of thalamocortical neurons within each whisker-related module. In the present studies, we determined the role of sensory experience in the refinement of the VPm relay connection in the mouse. We show that whisker plucking started at P12–P13 leads to disruptions of synapse elimination and modification of synaptic properties at the VPm relay synapse.
Materials and Methods
Whisker deprivation.
C57BL/6J (B6) mice aged between P10 (10 d postnatal with the day of birth as P0) and P16 were anesthetized by isoflurane. Large whiskers on the snout were pulled out gently with a pair of forceps. This method does not damage follicles. Mice were checked every other day until the day of recording, and whisker plucking was repeated when necessary. All procedures are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and have been approved by The Jackson Laboratory Animal Care and Use Committee.
Slice preparation.
Sagittal sections 300 μm thick were prepared using methods described previously (Arsenault and Zhang, 2006). Slices were kept in artificial cerebral spinal fluid (ACSF) containing (in mm): 124 NaCl, 3.0 KCl, 1.5 CaCl2, 1.3 MgCl2, 1.0 NaH2PO4, 26 NaHCO3, and 20 glucose, saturated with 95% O2 and 5% CO2 at room temperature (21–23°C).
Patch-clamp recording.
Recordings were made at 32–34°C. The pipette solution contained (in mm): 110 Cs methylsulfate, 20 TEA-Cl, 15 CsCl, 4 ATP-Mg, 0.3 GTP, 0.5 EGTA, 10 HEPES, 4.0 QX-314, and 1.0 spermine (pH 7.2, 270–280 mOsm with sucrose). Electrodes had resistances between 2 and 4 MΩ. Whole-cell recordings were made at the soma of VPm neurons with a Multiclamp 700B amplifier (Molecular Devices). The series resistance (Rs), usually between 8 and 18 MΩ, was not compensated. Data was discarded when Rs was >20 MΩ. A concentric bipolar electrode (FHC) was placed in the medial lemniscus, and stimuli (60 or 110 μs, 0.01–1.0 mA, with the center pole being negative) were applied at 0.1 Hz. GABAergic transmission was blocked by 100 μm picrotoxin in the bath. Experiments were conducted using AxoGraph X (AxoGraph Scientific). Data were filtered at 4 kHz and digitized at 20 kHz.
To determine the number of inputs for each VPm neuron, we recorded evoked EPSCs from the same cell over a wide range of stimulus intensity. First we screened the number of steps using increments of 50 or 100 μA. Then we used small increments of 5 or 10 μA near each transition point to ensure that it is indeed a single step. Finally, we applied much stronger stimulus (at least twice the intensity required to evoke the last step). Two or more trials were obtained at each stimulus intensity. Experiments were not performed blind.
Data analysis.
AxoGraph X was used for analysis. For evoked mEPSCs recorded in Sr2+, data were filtered at 1 kHz, and events were detected using variable amplitude template functions with the rise time set at 1 ms and decay time set at 5 ms. Statistics was performed using IgorPro (WaveMetrics) and InStat (GraphPad). Throughout, means are given ± SEM. Means were compared using Kruskal–Wallis test (for three groups, with Dunn's multiple-comparisons test) or Mann–Whitney test (for two groups). Distributions of cells with different number of inputs were compared using χ2 test.
Results
Whisker deprivation during a critical period disrupts elimination of relay synapses
The lemniscal whisker pathway from the Pr5 to VPm in the mouse undergoes extensive refinement during the second week after birth, when the animal starts to explore the environment. Whisking, a typical exploratory behavior in mice, begins around P11–P12 in B6 mice (our unpublished observation). The whisker sensory relay synapse in the VPm is likely to be highly active during these early explorations. We investigated the role of sensory experience by examining effects of whisker deprivation on synaptic connectivity. The VPm on one side of the brain receives inputs only from the contralateral whiskers, allowing us to examine effects of whisker deprivation in the same animal. Whisker deprivation was performed at P12 or P13 by removing all whiskers on one side of the snout. The connectivity of VPm relay synapse was examined in contralateral (deprived) and ipsilateral (spared) VPm at P16–P18, when the adult pattern of connectivity is achieved in the mouse. In total, we recorded from 39 cells from deprived VPm, and 34 cells from spared VPm. These data were obtained from 14 mice, with 2–5 cells recorded from either side of the brain. Neurons were voltage-clamped at −70 and +40 mV, and synaptic responses were recorded over a wide range of stimulus intensity. We also recorded 26 VPm neurons from eight untreated normal mice aged P16–P18 to examine the possibility of compensatory changes in spared VPm neurons.
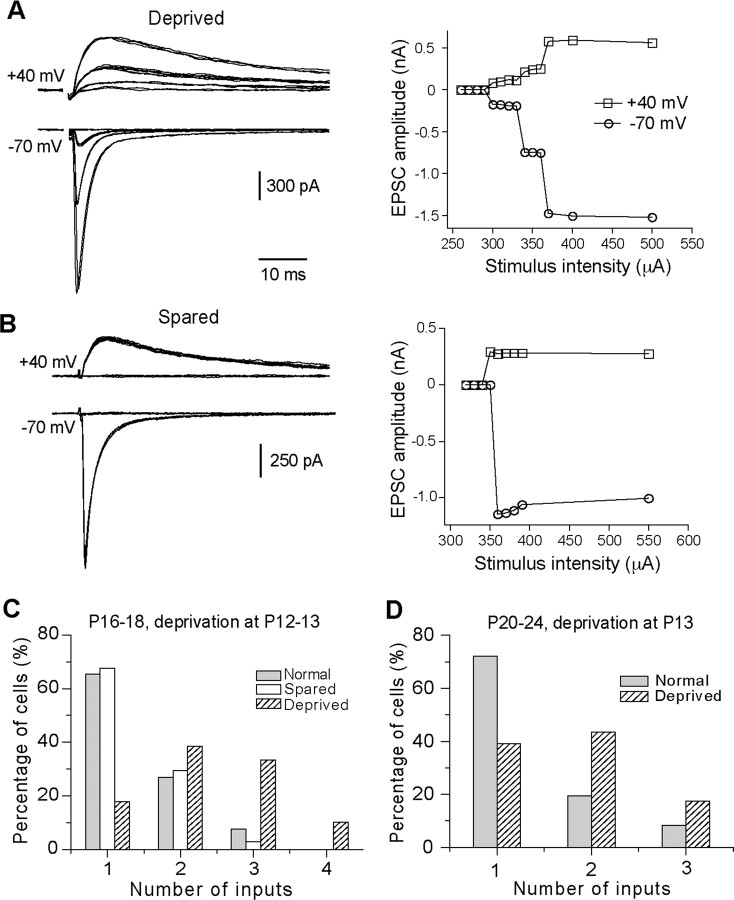
Whisker deprivation started at P12 or P13 disrupted synapse elimination in the VPm. The majority of neurons in the contralateral VPm (deprived) showed 2 or more steps in synaptic responses (Fig. 1A), whereas the majority of neurons in the ipsilateral VPm (spared) showed an all-or-none response (Fig. 1B). We estimated the number of relay inputs for each VPm neuron by counting the number of discrete steps in synaptic responses. These analyses were performed blind, and the results are summarized in Figure 1C. At P16–P18, 68% of neurons in spared VPm received a single lemniscal input (Fig. 1C, empty column), a pattern similar to that of normal mice at the same age (Fig. 1C, in gray) (p > 0.5, χ2 test). In contrast, only 18% of neurons in deprived VPm received a single input (Fig. 1C, hatched), with a distribution significantly different from that of spared VPm or that of normal mice (p < 0.00001, χ2 test). The average number of inputs per neuron was 2.4 ± 0.1 (n = 39) for deprived, 1.4 ± 0.1 (n = 34) for spared, and 1.4 ± 0.1 (n = 26) for normal mice (p < 0.0001, deprived vs spared or normal, Kruskal–Wallis test; p > 0.5 between spared and normal group).
Figure 1.
Whisker deprivation started at P12–P13 disrupts synapse elimination at the lemniscal relay connection in the VPm. A, B, Left panels, Synaptic currents in response to stimuli with a range of intensity in VPm neurons at P17 following one-side whisker deprivation started at P13. A is from a neuron in deprived VPm, and B is from a neuron in spared VPm. The right panels are the plots of peak amplitudes at +40 (empty square) or −70 mV (empty circles) versus stimulus intensity. C, The distributions of neurons at P16–P18 with different number of lemniscal inputs for deprived VPm (hatched; n = 39), spared VPm (empty; n = 34), and VPm in normal mice (gray; n = 26). One-side whisker deprivation was performed at P12–P13. D, The distribution of neurons in deprived VPm (hatched; n = 23) at P20–P24 following whisker deprivation started at P13 and the distribution obtained from normal mice at P20–P24 (gray; n = 36).
To determine whether the effect of sensory deprivation is long lasting, we performed whisker deprivation at P13, repeated the procedure every 2 d, and made recordings at P20–P24. In total, we recorded 23 cells from 7 deprived mice, and 36 cells from 13 untreated normal mice at P20–P24. The majority of neurons in deprived VPm received 2 or 3 lemniscal inputs, with a distribution significantly different from that of normal mice at the same ages (Fig. 1D) (p < 0.001, χ2 test). The average number of inputs per neuron was 1.8 ± 0.2 (n = 23) for deprived, and 1.4 ± 0.1 (n = 36) for normal mice (p < 0.04, Mann–Whitney test).
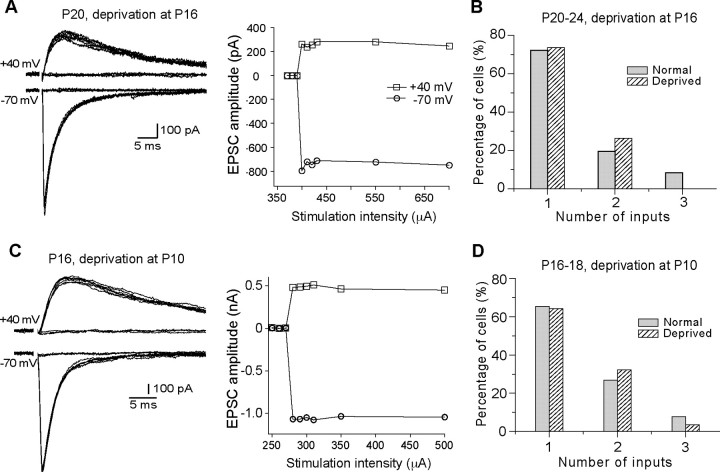
To determine whether there is a critical period for the effect of sensory deprivation, we performed whisker deprivation at P16 and recorded VPm neurons at P20–P24. As illustrated in Figure 2, A and B, the majority of neurons in deprived VPm received a single lemniscal input, with a distribution similar to that of normal mice at the same ages (p > 0.1, χ2 test). The average number of inputs per neuron was 1.3 ± 0.1 (n = 19) for deprived VPm, not significantly different from that of normal mice at the same age (p > 0.8, Mann–Whitney test). This finding indicates that the critical period ends by P16 in the VPm.
Figure 2.
A critical window for the effect of whisker deprivation on synapse elimination. A, Left panel, Synaptic currents at different stimulus intensity recorded from a neuron at P20 following whisker deprivation at P16; right panel, plot of peak amplitude versus stimulus intensity. B, The distribution of neurons (n = 19) in deprived VPm at P20–P24 following whisker deprivation started at P16 and that from normal mice at P20–P24 (in gray; the same data as in Fig. 1D). C, Synaptic responses and the plot of current versus intensity obtained from a neuron at P16 following whisker deprivation at P10. D, The distribution of neurons in deprived VPm (hatched; n = 28) at P16–P18 following whisker deprivation started at P10 and that from normal mice at P16–P18 (gray; the same data as in Fig. 1C).
Surprisingly, whisker deprivation started at P10 had no effect on synapse elimination. We recorded 28 cells from seven mice at P16–P18 with whisker deprivation started at P10. The majority of neurons in the deprived VPm received a single lemniscal input, and the distribution is similar to that of normal P16–P18 mice (Fig. 2C,D) (p > 0.1, χ2 test). The average number of inputs per neurons was 1.4 ± 0.1 (n = 28; p > 0.8 vs normal, Mann–Whitney test). This result is consistent with our previous findings that whisker deprivation beginning at P5 had no effect on synapse elimination in the VPm (Arsenault and Zhang, 2006).
Whisker deprivation disrupts maturation of relay synapses
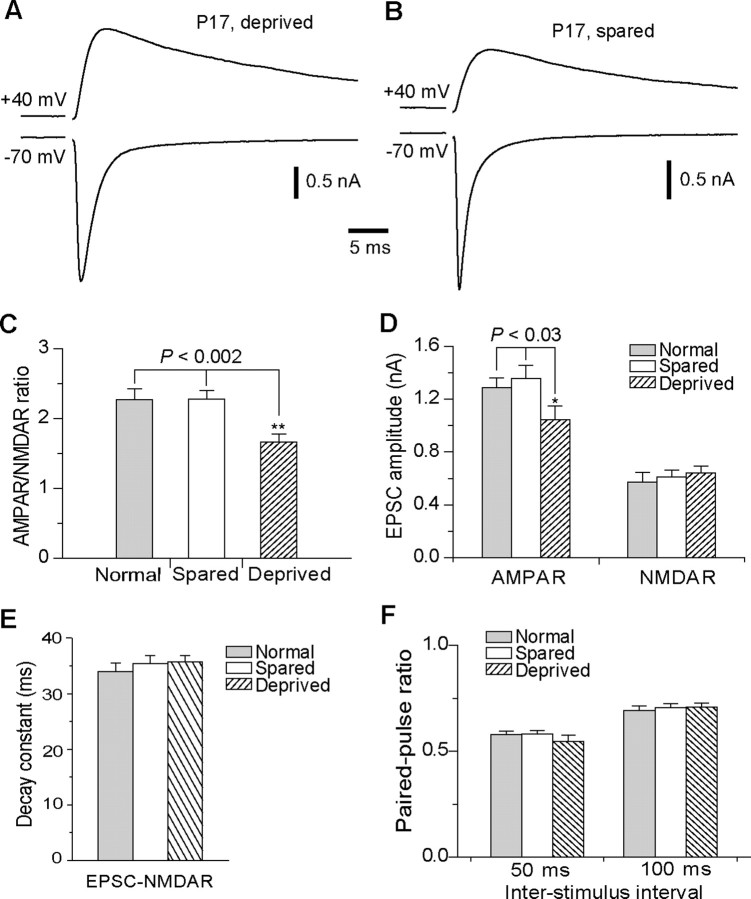
Previous studies have shown that concurrent with synapse elimination, whisker relay synapses in the VPm undergo extensive modification during the second week after birth. Specifically, the ratio of functional AMPA to NMDA receptors (AMPAR/NMDAR ratio) at the synapse increases by approximately threefold between P7 and P16. To determine whether whisker deprivation disrupts synapse maturation, we examined functional properties of VPm relay synapses at P16–P18 in mice that had undergone one-side whisker deprivation at P12 or P13. The AMPAR-mediated component of EPSC (EPSC-AMPAR) was estimated by measuring the peak amplitude of EPSC at −70 mV. At +40 mV, EPSC-AMPAR was very small due to a strong inward rectification, and it decays rapidly (Arsenault and Zhang, 2006). Thus, the peak current of EPSC at +40 mV, typically achieved at 5 ms after the beginning of EPSC, is almost entirely mediated by NMDARs. We estimated the NMDAR-mediated component (EPSC-NMDAR) at +40 mV by measuring the amplitude of EPSC at 8 ms after the beginning of EPSC. The AMPAR/NMDAR ratio was calculated with EPSC-AMPAR and EPSC-NMDAR obtained in the same cell at the same stimulus intensity. As illustrated in Figure 3, A–C, the AMPAR/NMDAR ratio in deprived VPm was significantly smaller than that in spared VPm or that of normal mice (1.67 ± 0.11, n = 24, for deprived; 2.28 ± 0.12, n = 27, for spared; 2.27 ± 0.15, n = 23, for normal mice; p < 0.002, Kruskal-Wallis test), whereas the ratio for spared VPm was the same as that for normal mice (p > 0.5).
Figure 3.
Whisker deprivation started at P13 disrupts maturation of the VPm relay synapse. A, B, Synaptic currents recorded from two neurons in the deprived (A) and spared (B) VPm at P17 following whisker deprivation started at P13. For each cell, synaptic currents at +40 and −70 mV were obtained at the same stimulation intensity. C, The AMPAR/NMDAR ratio obtained from neurons at P16–P18 following whisker deprivation at P13. The ratio obtained from deprived VPm neurons (n = 24; hatched) was significantly smaller than that from spared neurons (n = 27; empty) or neurons in normal mice at the same age (n = 23; gray) (**p < 0.002, Kruskal–Wallis test). D, The peak amplitude of the maximal EPSC-AMPAR and EPSC-NMDAR for deprived (hatched), spared (empty), and normal (gray) VPm neurons at P16–P18 (*p < 0.03, Kruskal–Wallis test). E, Decay constant of EPSC-NMDAR for deprived (hatched), spared (empty), and normal (gray) VPm neurons at P16–P18. F, Paired-pulse ratios of EPSC-AMPAR at interstimulus intervals of 50 and 100 ms.
The difference in the AMPAR/NMDAR ratio may be caused by changes in EPSC-AMPAR or EPSC-NMDAR or both. We examined separately EPSC-AMPAR and EPSC-NMDAR by measuring the peak amplitude of the maximal responses (when all inputs for a given cell were activated by stimulus). The mean maximal response at −70 mV in deprived neurons was significantly smaller than that of spared or that of normal neurons (Fig. 3D) (1044 ± 105 pA, n = 26 cells for deprived; 1357 ± 99 pA, n = 27 for spared; 1285 ± 77 pA, n = 26 for normal group; p < 0.03, Kruskal–Wallis test). In contrast, no difference was detected for the maximal EPSC-NMDAR among the three groups (643 ± 52 pA for deprived; 613 ± 48 pA for spared; 574 ± 71 pA for normal; p > 0.4, Kruskal–Wallis test).
We measured the decay constant of EPSC-NMDAR, and no difference was detected (Fig. 3E). The decay constant was 35.7 ± 1.2 ms (n = 29) for deprived, 35.4 ± 1.4 ms (n = 30) for spared, and 34.0 ± 1.5 ms (n = 24) for normal mice (p > 0.5, Kruskal–Wallis test).
We also measured the rise time and decay time constant of EPSC-AMPAR recorded in the presence of d-APV (100 μm). Again no difference was found among the three groups. The rise time (from 20 to 80% of the peak) was 0.21 ± 0.01 ms (n = 7) for deprived, 0.19 ± 0.01 ms (n = 9) for spared, and 0.21 ± 0.01 ms (n = 7) for normal mice (p > 0.18, Kruskal–Wallis test). The decay constant was 2.0 ± 0.2 ms (n = 7) for deprived, 2.2 ± 0.1 (n = 9) for spared, and 2.2 ± 0.2 (n = 7) for normal mice (p > 0.6, Kruskal–Wallis test).
Recent studies in the barrel cortex have shown that whisker deprivation alters the paired-pulse ratio (PPR) of EPSCs presumably through modifications of presynaptic release probability (Bender et al., 2006). We examined this possibility using paired pulses at interstimulus intervals of 50 and 100 ms. Whisker deprivation had no effect on PPR of EPSC at −70 mV (Fig. 3F). At 50 ms interval, PPR was 0.55 ± 0.03 (n = 18) for deprived neurons, 0.58 ± 0.02 (n = 19) for spared neurons, and 0.58 ± 0.02 (n = 14) for normal mice (p > 0.6, Kruskal–Wallis test). At 100 ms interval, PPR was 0.70 ± 0.02 (n = 29) for deprived neurons, 0.71 ± 0.02 (n = 30) for spared neurons, and 0.69 ± 0.02 (n = 31) for normal mice (p > 0.7, Kruskal–Wallis test).
Sensory deprivation may alter EPSC-AMPAR through changes in the subunit composition of AMPA receptors at the synapse. Specifically, sensory deprivation may regulate the expression and insertion of GluR2 subunit at the synapse. To examine this possibility, we determined the current–voltage (I–V) relationship for EPSC-AMPAR in the presence of d-APV (50 μm). As illustrated in Figure 4A, the AMPA component showed strong inward rectification. The I–V curves, established using normalized responses, were very similar among the three groups (Fig. 4B), indicated that sensory deprivation does not affect the I–V relationship. To quantify the degree of rectification, we calculated for each cell, the ratio of the peak amplitude of EPSCs recorded at +34 mV to that recorded at −36 mV. The average ratio was 0.18 ± 0.03 (n = 7) for deprived, 0.21 ± 0.2 (n = 7) for spared, and 0.15 ± 0.02 (n = 7) for normal group (p > 0.13, Kruskal–Wallis test). These results suggest that few AMPA receptors at the VPm relay synapse contain GluR2, and that sensory deprivation does not alter the number of GluR2-containing receptors at the synapse.
Figure 4.
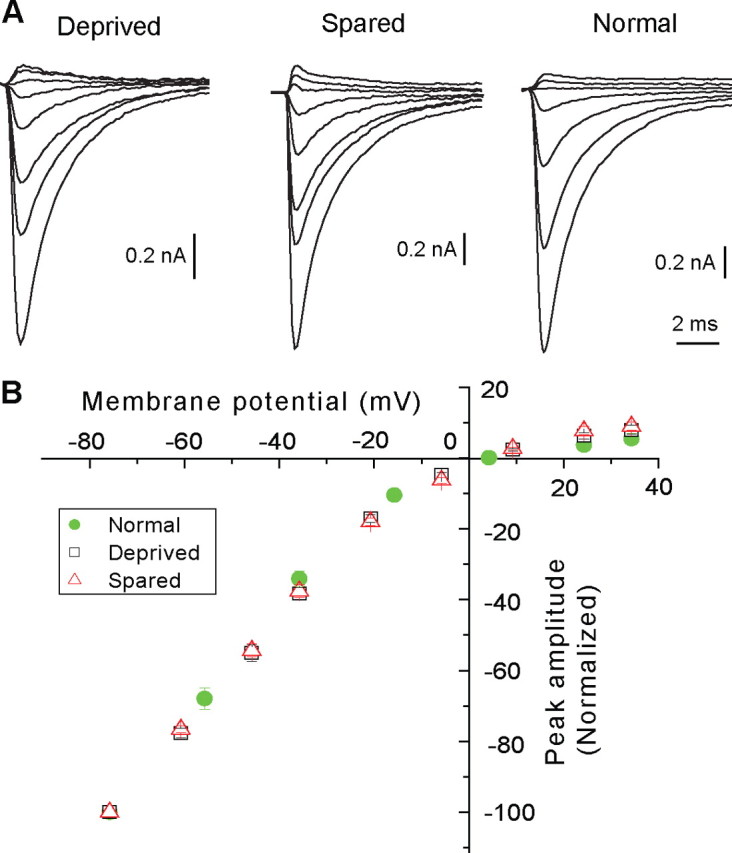
Whisker deprivation does not alter the I–V relationship of EPSC-AMPAR. A, EPSC-AMPAR recorded at various holding potentials from deprived (left), spared (middle), and normal (right) neuron recorded in the presence of d-APV (50 μm). For A (left and middle), the holding potentials, from the top to bottom, were +34, +24, +9, −6, −21, −36, −46, and −76 mV; for A (right), the holding potentials were +34, +24, +4, −16, −36, −56, and −76 mV. B, I–V plots for deprived (black square, n = 7 cells), spared (red triangle, n = 7), and normal (green filled circle, n = 6) groups. For each cell, the peak amplitude was normalized to that recorded at −76 mV. The liquid junction potential, estimated to be +6 mV, was corrected for all cells.
Rapid modifications of synaptic properties by whisker deprivation
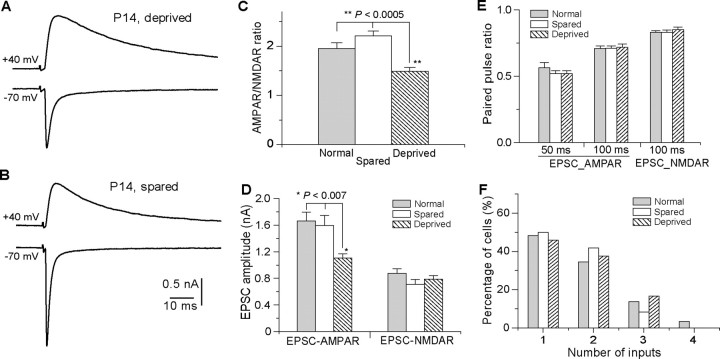
Previous studies at the retinocollicular synapse have shown that EPSC-AMPAR increases rapidly following eye opening (Lu and Constantine-Paton, 2004). To determine whether sensory deprivation induces rapid modification at the VPm relay synapse, one-side whisker deprivation was performed at P13 and recordings were performed 18–22 h later (P14) in both deprived and spared VPm neurons. For comparison, we also recorded VPm neurons from normal mice at P14. We examined EPSC-AMPAR and EPSC-NMDAR at −70 and +40 mV, respectively (Fig. 5A,B). The AMPAR/NMDAR ratio in deprived VPm was significantly smaller than that in spared VPm or that in normal mice (Fig. 5C) (1.49 ± 0.08, n = 19 cells from six mice, for the deprived; 2.19 ± 0.09, n = 17 from six mice, for spared VPm; 1.95 ± 0.12, n = 20 from five normal mice; p < 0.0005, Kruskal–Wallis test). This reduction in the AMPAR/NMDAR ratio was associated with a decrease in the amplitude of EPSC-AMPAR (Fig. 5D). The maximal EPSC-AMPAR was 1102 ± 68 pA for deprived (n = 24); 1594 ± 150 pA for spared VPm (n = 21), and 1663 ± 134 pA for normal mice (n = 20; p < 0.007, Kruskal–Wallis test). On the other hand, no difference was observed for the maximal EPSC-NMDA (Fig. 3D) (785 ± 57 pA, n = 19 for deprived, 711 ± 71 pA, n = 17, for spared VPm; 873 ± 70 pA, n = 20, for normal; p > 0.25, Kruskal–Wallis test), or the decay constant of EPSC-NMDAR (32. 0 ± 1.7 ms for deprived; 27.9 ± 1.9 ms for spared; 33.8 ± 1.3 ms for normal; p > 0.2, Kruskal–Wallis test).
Figure 5.
Rapid modifications of synaptic properties by whisker deprivation. A and B are evoked synaptic currents from neurons in the deprived (A) and spared (B) VPm of a P14 mouse following one-side whisker deprivation at P13. C, The AMPAR-NMDAR ratio for spared (empty; n = 17), deprived (hatched; n = 19), and normal (gray; n = 20) VPm neurons following acute deprivation (**p < 0.0005, Kruskal–Wallis test). D, Peak amplitudes of EPSC-AMPAR and EPSC-NMDAR in deprived (hatched; n = 24), spared (empty; n = 21), and normal (gray; n = 20) VPm neurons at P14 (*p < 0.007, Kruskal–Wallis test). E, Paired-pulse ratios at 100 ms interval for EPSC-AMPAR and EPSC-NMDAR in deprived (hatched; n = 19), spared (empty; n = 17), and normal (gray; n = 20) VPm neurons at P14. F, Distributions of neurons at P14 with different number of inputs for deprived, spared, and normal groups.
We examined the effect of whisker deprivation on PPR for both EPSC-AMPAR and EPSC-NMDAR. As illustrated in Figure 5E, whisker deprivation for 18–22 h had no effect on PPR at the interstimulus interval of 100 ms. PPR of EPSC-AMPAR was 0.72 ± 0.02 for deprived (n = 19), 0.71 ± 0.02 for spared VPm (n = 17), and 0.71 ± 0.02 for normal mice (n = 20; p > 0.5, Kruskal–Wallis test). PPR of EPSC-NMDAR was 0.85 ± 0.02 (n = 15) for deprived, 0.83 ± 0.02 (n = 17) for spared VPm, and 0.83 ± 0.01 (n = 19) for normal mice (p > 0.5, Kruskal–Wallis test). We also measured PPR of EPSC-AMPAR at the interstimulus interval of 50 ms, and again no difference was detected between among the three groups. PPR of EPSC-AMPAR was 0.52 ± 0.02 (n = 10) for deprived, 0.52 ± 0.02 (n = 11) for spared VPm, and 0.56 ± 0.04 for normal mice (p > 0.5, Kruskal–Wallis test). Because of the slow decay of EPSC at +40 mV, we did not examine PPR of EPSC-NMDAR at 50 ms interval. Together, these findings suggest that whisker deprivation has no effect on presynaptic functions at VPm relay synapses.
To determine whether whisker deprivation for 18–22 h is sufficient to disrupt synapse elimination, we estimated the number of inputs at P14 using the step counting method described above. As illustrated in Figure 5F, the distribution of input number per neuron was not different among deprived, spared, and normal group (p > 0.1, χ2 test). The average number of inputs per neuron was 1.7 ± 0.2 (n = 24) for deprived, 1.6 ± 0.1 (n = 24) for spared, and 1.7 ± 0.2 (n = 20) for normal mice (p > 0.8, Kruskal–Wallis test). These results suggest that whisker deprivation for 24 h was not sufficient to alter the number of inputs for the neuron.
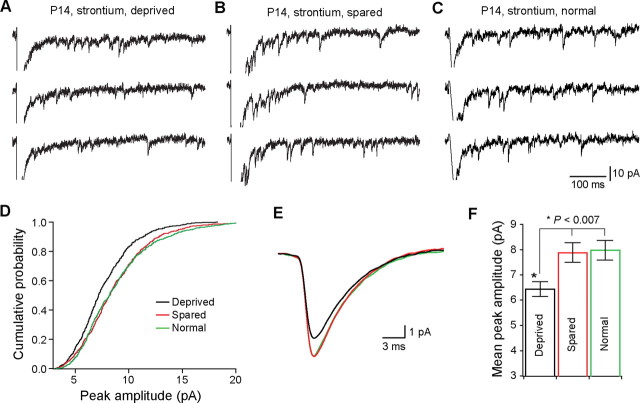
The difference in EPSC-AMPAR between deprived and spared VPm neurons may be the result of altered quantal events at the relay synapse. To examine this possibility, we recorded evoked EPSC at the synapse in ACSF that contains 3 mm Sr2+ and 0 mm Ca2+. Replacing Ca2+ with Sr2+ reduced peak amplitude of EPSC at −70 mV, and caused a large number of asynchronous miniature EPSC (Sr-EPSC) (Fig. 6 A–C). We analyzed neurons with comparable series resistance (14.7 ± 0.7 MΩ, n = 15 for deprived; 14.6 ± 0.8 MΩ, n = 15 for spared; 14.1 ± 1.0 MΩ, n = 11 for normal). Sr-EPSCs were detected within 700 ms after the stimulation. Sr-EPSCs in deprived VPm neurons were significantly smaller than those in spared VPm neurons (Fig. 6D,E). The distribution of the peak amplitude for the deprived shifted to the left compared with those of spared or normal group. The mean peak amplitude was 6.4 ± 0.3 pA (n = 15 cells from seven mice) for deprived, 7.9 ± 0.4 pA (n = 15 cells from seven mice) for spared VPm neurons, and 8.0 ± 0.4 pA (n = 11 cells from four mice) for normal mice (p < 0.007, Kruskal–Wallis test). On the other hand, decay time constant of Sr-EPSC was not different between the three groups (Fig. 6E) (4.1 ± 0.1 ms for deprived; 4.0 ± 0.1 ms for spared VPm; 4.1 ± 0.1 ms for normal mice; p > 0.6, Kruskal–Wallis test). These findings suggest that whisker deprivation reduces the amplitude of AMPAR-mediated quantal events at the synapse.
Figure 6.
Rapid change of quantal events after whisker deprivation. A, B, evoked quantal EPSCs from deprived (A) and spared (B) neuron at P14 following whisker deprivation at P13. C, Evoked quantal EPSCs in a neuron from a normal untreated mouse at P14. Evoked quantal EPSCs (Sr-EPSCs) were recorded at −70 mV in the presence of 3 mm Sr2+ and 0 mm Ca2+. D, Cumulative distributions of the peak amplitude for deprived (black; n = 15 cells), spared (red; n = 15), and normal (green; n = 11) group. For each group, the distribution was established using 50 consecutively detected events from each cell. E, Averaged Sr-EPSCs for deprived (black; n = 15 cells), spared (red; n = 15), and normal (green; n = 11) groups. F, Mean peak amplitudes of Sr-EPSC recorded from deprived, spared, and normal groups (*p < 0.007, Kruskal–Wallis test).
Discussion
The present study revealed that whisker deprivation at the onset of whisking disrupts both elimination and maturation of VPm relay synapses. These findings underscore the role of sensory experience in fine-tuning of whisker sensory pathways. Whisker deprivation rapidly reduced peak amplitudes of EPSC-AMPAR and AMPAR-mediated quantal events, suggesting that synaptic strengthening by experience plays a key role in synaptic refinement during early life.
Experience-dependent synaptic refinement at VPm relay synapse
In the VPm of rodents, whisker-specific pattern of innervation is established by P4–P5 (Belford and Killackey, 1979). However, VPm relay synapses undergo extensive remodeling well after the formation of whisker-specific map. The average number of lemniscal axons innervating each VPm neuron decreases from 8 at P7–P9 to 4 at P11–P13. The adult pattern emerges at P16–P17 where the majority of neurons receive a single lemniscal axon (Arsenault and Zhang, 2006). Previous studies in the rat have shown that neurons in a single whisker-specific unit in the VPm (barreloid) have different angular preferences to whisker motion, although they all receive inputs from the same whisker (Brecht and Sakmann, 2002; Temereanca and Simons, 2003; Timofeeva et al., 2003). This angular preference is likely to arise from selective innervation by directional sensitive neurons in the Pr5 (Minnery et al., 2003). The remodeling process at VPm relay synapses may play a key role in establishing angular selectivity of VPm neurons within the same barreloid.
Our results show that whisker deprivation started at P12–P13 disrupted synapse elimination in the VPm. When examined at P16–P18, 82% of neurons in deprived VPm received 2–4 lemniscal inputs, whereas 68% of neurons in spared VPm received a single lemniscal input. The deficit in synaptic elimination was also observed at P20–P22, suggesting that the effect is long lasting. In contrast, whisker deprivation starting at P16 did not affect synapse elimination, indicating that the critical period ends by P16. Because the adult pattern of innervation is established around P16, our findings also suggest that the maintenance of innervation pattern in the VPm does not require sensory experience. Together with previous studies in the barrel cortex (Simons and Land, 1987; Fox, 1992), our findings underscore the role of early sensory experience in establishing functional specifications among neurons within each whisker-related modules.
There are two main concerns about the step-counting method used here to estimate the number of inputs for each neuron. First, the step-counting method becomes unreliable when there are a large number of steps (e.g., more than eight). In the present studies, all cells showed 1–4 steps with >90% having 1–3 steps. In this range, each step can be reliably identified. Therefore, the results obtained here provide a good estimation of the number of inputs for cells in slices. The second concern is the loss of synapses in slices. Indeed, some synapses must have been lost during slice sectioning. Previous studies have shown that Pr5 axons preferentially target the proximal dendrites and soma of VPm neurons (Williams et al., 1994). Thus, the loss of relay synapses in slices may be relatively minor. Nevertheless our results represent an underestimation. However, the objective of these studies was to determine the differences among deprived, spared, and normal neurons. It is unlikely that the loss of synaptic contacts selectively affects the results obtained from spared and normal VPm. Therefore, we think that the differences among the three groups can be identified using this method.
Pr5 axons in normally reared rats preferentially contact the proximal dendrites and soma of VPm relay neurons (Peschanski et al., 1985; Williams et al., 1994); however, whether this selective synaptic targeting requires normal sensory experience is unknown. It is possible that whisker deprivation causes some of Pr5 terminals to contact distal dendrites instead of the soma and proximal dendrites of VPm neurons. Such a change would result in a reduction of response size recorded at the soma (as in the case of this study), and thereby lead to an underestimation of the number of inputs in deprived neurons.
Whisker deprivation started at P10 had no effect on innervation pattern of the lemniscal pathway in the VPm. This is consistent with our previous findings in mice that had undergone whisker deprivation at P5 (Arsenault and Zhang, 2006). Together, our results at VPm relay synapse are comparable with those obtained from the lateral geniculate nucleus in the mouse (Hooks and Chen, 2006). At the retinogeniculate synapse, chronic dark rearing had no effect on synaptic remodeling, but acute dark rearing at later ages was effective. This similarity suggests that the same form of experience-dependent plasticity may be involved in the two systems, although the onset of sensitivity is much earlier at the VPm synapse (P12) than that at the retinogeniculate synapse (P20). So far it is not clear why sensory deprivation started at early ages failed to influence synaptic remodeling. As suggested by Hooks and Chen (2006), early experience may be required to prime the synapse for experience-dependent plasticity at later ages. Another possibility, however, is that sensory deprivation started at earlier but not later age activates mechanisms that compensate for the loss of sensory experience.
The sensitive period (P12–P13) at the VPm relay synapse coincides with the critical period in layer 2/3 of the barrel cortex for experience-dependent plasticity. Whisker deprivation between P11 and P14 reduces motility of dendritic spines and disrupts receptive field structures of layer 2/3 neurons (Lendvai et al., 2000; Stern et al., 2001). Because whisker deprivation started after P5 does not alter receptive field structures of layer 4 neurons that are the primary targets of VPm neurons (Fox, 1992), it is unlikely that these changes in the VPm contribute directly to experience-dependent plasticity of layer 2/3 neurons. On the other hand, the fact that experience-dependent plasticity occurs in both the VPm and layer 2/3 of the barrel cortex around the onset of whisking suggests that this active exploratory behavior is important for the refinement of whisker sensory pathways during early life.
In addition to changes in the number of inputs, whisker deprivation started at P12–P13 also affected the properties of VPm relay synapses. Specifically, the AMPA/NMDA ratio was significantly reduced in deprived neurons. However, the peak amplitudes of EPSC-AMPAR or EPSC-NMDAR showed little change (Fig. 3D). This discrepancy may be caused by the technical difficulties in determining the full response at the synapse. Anatomical studies have shown that each Pr5 axon makes multiple synaptic contacts with VPm neurons on their proximal dendrites and soma (Peschanski et al., 1985; Williams et al., 1994; Veinante and Deschênes, 1999). Because we measure synaptic responses in acute slices, it is likely that in some cells, part of the synaptic contacts was lost during slice sectioning. Thus the peak amplitude of EPSCs obtained with this method is likely to show large variations (as we saw here). In contrast, the AMPAR/NMDAR ratio is a more reliable index of synaptic properties, because this ratio is derived from AMPA and NMDA responses from the same groups of synaptic contacts for each cell, therefore independent of the number of synaptic contacts.
Mechanisms of experience-dependent synaptic refinement
How does whisker deprivation disrupt synapse elimination? Whisker deprivation may cause a halt, a delay, or even a regression in synaptic refinement. The average number of inputs per neuron (2.4 ± 0.1) for deprived VPm at P16–P18 falls between that of normal P11–P13 (4.2 ± 0.8) (Arsenault and Zhang, 2006) and that of normal P16–P18 mice (1.4 ± 0.1). The AMPA/NMDA ratio of deprived neurons at P16–P18 is also between that of normal P11–P13 and that of normal P16–P18 mice. These findings indicate a delay of development in deprived VPm neurons. However, we cannot exclude the possibility of a regression because it may be particularly difficult to detect newly formed synapses in slices prepared from older mice. Detailed analyses of synaptic structures are required to provide definitive answers to this question.
At VPm relay synapses, whisker deprivation started at P13 reduced within 24 h the AMPA/NMDA ratio, peak amplitudes of both the maximal EPSC-AMPAR and AMPAR-mediated quantal events, without any effect on EPSC-NMDA or PPR. These findings differ significantly from those observed in the barrel cortex. At layer 4 to layer 2/3 synapses, synaptic depression induced by whisker deprivation is associated with an increase in PPR, with no change observed for quantal amplitude or the AMPAR/NMDAR ratio. Thus, a reduction of presynaptic function is responsible for the effects of deprivation at layer 4 to layer 2/3 synapses, whereas postsynaptic modifications are involved at VPm relay synapses. However, presynaptic changes, such as a reduction in the number of synaptic contacts made by a single Pr5 axon on each VPm neuron, may also contribute to the effects of whisker deprivation at VPm relay synapses. Previous studies in rats have shown that each Pr5 axon gives rise to a cluster of large terminals in the VPm, and each of these large terminals often makes more then one synaptic contacts (Peschanski et al., 1985; Veinante and Deschênes, 1999). Whisker deprivation may lead to reductions in the number of terminals and number of synaptic contacts by individual terminals.
The effects of sensory deprivation on synaptic function in the VPm are much quicker than those observed at the retinogeniculate synapse (Hooks and Chen, 2008), but are comparable to those obtained in the rat superior colliculus (SC). Eye opening increases within 24 h amplitudes of EPSC-AMPAR and AMPAR-mediated quantal events in SC neurons (Lu and Constantine-Paton, 2004). Accordingly, activity-dependent receptor trafficking is thought to play a key role in synaptic refinement in the SC. VPm synapses at P12–P13 are still immature with smaller AMPAR components than those at P16–P18. Sensory experience during this critical period can play an instructive role in synaptic refinement by promoting AMPAR trafficking at functionally appropriate connections.
Footnotes
We thank Martin Deschênes and Rob Burgess for comments on a previous version of this manuscript.
References
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Arsenault D, Zhang ZW. Developmental remodelling of the lemniscal synapse in the ventral basal thalamus of the mouse. J Physiol. 2006;573:121–132. doi: 10.1113/jphysiol.2006.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979;188:63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–4165. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Whisker maps of neuronal subclasses of the rat ventral posterior medial thalamus, identified by whole-cell voltage recording and morphological reconstruction. J Physiol. 2002;538:495–515. doi: 10.1113/jphysiol.2001.012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Woolsey TA, Jacquin MF. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Brain Res Dev Brain Res. 1992;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28:4807–4817. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Lu W, Constantine-Paton M. Eye opening rapidly induces synaptic potentiation and refinement. Neuron. 2004;43:237–249. doi: 10.1016/j.neuron.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Bruno RM, Simons DJ. Response transformation and receptive-field synthesis in the lemniscal trigeminothalamic circuit. J Neurophysiol. 2003;90:1556–1570. doi: 10.1152/jn.00111.2003. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Roudier F, Ralston HJ, 3rd, Besson JM. Ultrastructural analysis of the terminals of various somatosensory pathways in the ventrobasal complex of the rat thalamus: an electron-microscopic study using wheatgerm agglutinin conjugated to horseradish peroxidase as an axonal tracer. Somatosens Res. 1985;3:75–87. doi: 10.3109/07367228509144578. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Neonatal whisker trimming produces greater effects in nondeprived than deprived thalamic barreloids. J Neurophysiol. 1994;72:1434–1437. doi: 10.1152/jn.1994.72.3.1434. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Local field potentials and the encoding of whisker deflections by population firing synchrony in thalamic barreloids. J Neurophysiol. 2003;89:2137–2145. doi: 10.1152/jn.00582.2002. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Mérette C, Emond C, Lavallée P, Deschênes M. A map of angular tuning preference in thalamic barreloids. J Neurosci. 2003;23:10717–10723. doi: 10.1523/JNEUROSCI.23-33-10717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Deschênes M. Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. J Neurosci. 1999;19:5085–5095. doi: 10.1523/JNEUROSCI.19-12-05085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Jacquin MF, Deschênes M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J Comp Neurol. 2000;420:233–243. doi: 10.1002/(sici)1096-9861(20000501)420:2<233::aid-cne6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. Eur J Neurosci. 1994;6:429–453. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]