Abstract
It is unclear whether eradication of hepatitis C virus (HCV) leads to a reduction in the risk of hematologic malignancies. We aimed to determine the impact of sustained virologic response (SVR) induced by either direct‐acting antivirals (DAAs) or interferon (IFN) on the risk of hematologic malignancies. We identified 69,581 patients who initiated antiviral treatment in the Veterans Affairs national health care system from January 1, 1999, to December 31, 2015, including 40,410 (58%) IFN‐only regimens, 4,546 (6.5%) DAA + IFN regimens, and 24,625 (35%) DAA‐only regimens. We retrospectively followed patients to identify incident cases of hematologic malignancies or monoclonal gammopathy of unknown significance (MGUS), a premalignant precursor of multiple myeloma. Among patients treated with IFN, SVR was significantly associated with a reduction in the risk of lymphoma (adjusted hazard ratio [AHR], 0.70; 95% confidence interval [CI], 0.51‐0.97), multiple myeloma (AHR, 0.40; 95% CI, 0.20‐0.77), MGUS (AHR, 0.65; 95% CI, 0.42‐0.99), or all hematologic malignancies and MGUS combined (AHR, 0.67; 95% CI, 0.53‐0.84) over a mean follow‐up of 10.6 years. In contrast, among patients treated with DAA, SVR was not associated with the risk of lymphoma, multiple myeloma, MGUS, or all hematologic malignancies and MGUS combined (AHR, 1.08; 95% CI, 0.66‐1.78) during a mean follow‐up of 2.9 years. Neither IFN‐induced SVR nor DAA‐induced SVR was associated with risk of colon cancer or prostate cancer, which were chosen a priori as comparison/control malignancies. Conclusion: We describe novel strong associations between IFN‐induced SVR and lymphoma, multiple myeloma, MGUS, and all hematologic malignancies combined. Surprisingly, these associations were not observed with DAA‐induced SVR.
Abbreviations
- AHR
adjusted hazard ratio
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- DAA
direct‐acting antiviral
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICD
International Classification of Diseases
- IFN
interferon
- INR
international normalized ratio
- MGUS
monoclonal gammopathy of unknown significance
- NS
nonstructural protein
- SVR
sustained virologic response
- VA
Veterans Affairs
Multiple observational studies have consistently reported an increased risk of certain B‐cell lymphomas with hepatitis C virus (HCV) infection.1, 2, 3, 4, 5, 6, 7 Chronic antigen stimulation of lymphocytes is a hypothesized mechanism in HCV‐associated lymphoma,8 followed by a second oncogenic or proliferative “hit” that may be antigen independent.9, 10 Although not as strong and consistent as the associations with lymphoma, HCV has also been associated with many other hematologic malignancies, including myeloma, chronic lymphocytic leukemia, and myeloid leukemias,11, 12, 13 as well as with monoclonal gammopathy of unknown significance (MGUS),14, 15 a premalignant precursor of multiple myeloma.
Small‐case series have shown patients in whom eradication of HCV either with interferon (IFN) or direct‐acting antivirals (DAAs) led to regression or even cure of low‐grade non‐Hodgkin lymphoma.16, 17, 18, 19, 20, 21, 22 In addition, eradication of HCV may prevent relapse after successful treatment of high‐grade, diffuse, large B‐cell lymphoma.23
Whether HCV eradication reduces the risk of developing hematologic malignancies has not been firmly established either for IFN‐based treatments or for DAAs. Hematologic malignancies are relatively rare, and a large number of treated patients would need to be followed for a long time for a sufficient number of events to accrue to enable a comparison between patients who eradicate the virus (sustained virologic response [SVR]) and those who do not. Furthermore, studies must be conducted separately for patients treated with IFN versus DAA treatments because IFN has putative antineoplastic and immunomodulatory effects and because IFN and DAAs have very different SVR rates and encompass different nonoverlapping eras. A recent study failed to show a statistically significant association between patients treated with IFN who achieved SVR and those who did not with respect to the risk of non‐Hodgkin lymphoma, although it approached statistical significance (adjusted hazard ratio [AHR], 0.71; P = 0.1).24 However, treatment and follow‐up data in that study only extended to 2009, and hematologic malignancies other than non‐Hodgkin lymphoma were not investigated. To our knowledge, the association between DAA‐induced SVR and hematologic malignancies has not been investigated. DAAs are well tolerated, lead to SVR in the majority of patients, and have led to a dramatic increase in the number of patients infected with HCV undergoing treatment. However, DAAs are extremely expensive, and concerns have been raised that the long‐term clinical benefits of DAA‐based antiviral treatment and SVR have not been adequately ascertained.25
We aimed to compare patients who achieved SVR to those who failed treatment with respect to the risk of developing hematologic malignancies, independently for patients treated with IFN and those treated with DAA. We used data from the Veterans Affairs (VA) national health care system, which provides the greatest number of antiviral treatment of any health care system in the United States.
Patients and Methods
Data Source
The VA health care system is the largest integrated health care system in the United States, currently serving more than 8.9 million veterans at 168 VA medical centers and 1,053 outpatient clinics throughout the country.26 The Veterans Health Administration uses a single, nationwide, comprehensive electronic health care information network (known as the Veterans Information Systems and Technology Architecture [VistA]) that consists of nearly 180 applications of clinical, financial, administrative, and infrastructure needs integrated into a single common database of all veterans’ health information. We obtained electronic data on all patients who initiated antiviral treatment in the VA system, using the VA Corporate Data Warehouse (CDW), a national continually updated repository of data from VistA developed specifically to facilitate research.27 Data extracted included all patient pharmacy prescriptions, demographics, inpatient and outpatient visits, problem lists, procedures, vital signs, diagnostic tests, and laboratory tests. The study was approved by the Institutional Review Board of the VA Puget Sound Health Care System.
Study Population
We identified all HCV antiviral regimens (n = 105,366 regimens in 78,944 patients) initiated in the VA during 17 calendar years from January 1, 1999, to December 31, 2015, with patients followed until June 30, 2018. We excluded 2,762 patients who had a diagnosis hematologic malignancy or any of the control/comparison malignancies shown in Table 1 ever recorded prior to their first HCV antiviral treatment, 114 who died within 180 days from the start date of antiviral treatment or had fewer than 180 days of available follow‐up, and 254 patients who were diagnosed with any one of the malignancies of interest within 180 days from the start date of their antiviral treatment because these cases were very unlikely to be incident (new) cases. We excluded 6,233 patients with missing SVR data, leaving 69,581 patients in the current analysis.
Table 1.
Diagnostic Criteria Based on ICD‐9 and ICD‐10 Codes for Different Hematologic Malignancies, MGUS, and Two Solid Malignancies (Colon Cancer and Prostate Cancer) that Served as Negative Controls
| Disease | ICD‐9 | ICD‐10 |
|---|---|---|
| Lymphoma | 200.x, 201.x, 202.0x, 202.1x, 202.2x, 202.7x, 202.8x, 273.3 | C81.x, C82.x, C83.x, C84.x, C85.x, C88.0, C88.4 |
| Multiple myeloma and plasma cell diseases | 203.x, 238.6 | C90.x |
| Other hematologic malignancies | ||
| Histiocyte/mast cell diseases | 202.3x, 202.5x, 202.6x | C96.0x, C96.1x, C96.2x, C96.3x |
| Chronic lymphocytic leukemia | 204.1x | C91.1x |
| Acute lymphocytic leukemia | 204.0x | C91.0x |
| Acute nonlymphocytic leukemia | 205.0x, 206.0x | C92.0x, C92.4x, C92.5x, C93.0x |
| Other leukemias | 202.4x, 204.2x, 204.8x, 204.9x, 205.1x, 205.2x, 205.8x, 205.9x, 206.1x, 206.2x, 206.8x, 206.9x, 207.8x, 208.0x, 208.1x, 208.2x, 208.8x, 208.9x | C91.4x, C91.Zx, C91.9x, C92.1x, C92.3x, C92.Zx, C92.9x, C93.1x, C93.9x, C93.Zx, C94.3x, C94.8x, C95.x |
| Unclassified hematologic malignancy | 202.9x | C96.7x, C96.9x |
| MGUS | 273.1 | D47.2 |
| Selected solid organ malignancies | ||
| Colon cancer | 153.x | C18.x |
| Prostate cancer | 185.x | C61.x |
Antiviral Treatment Regimens
The regimens were divided into the following: IFN only, which included pegylated (PEG)‐IFN and regular IFN with or without ribavirin but without DAAs; DAA only, which included only IFN‐free DAA regimens (with or without ribavirin).
An additional category of DAA + IFN regimens used for a brief period from 2011 to 2013 was identified that included PEG in combination with a DAA (nonstructural protein [NS]3/4, NS5A, or NS5B inhibitors). This category was analyzed separately because we did not want to contaminate either the IFN‐only or the DAA‐only categories. The DAA + IFN category included only a small number of regimens and outcomes (e.g., a total of 17 incident lymphomas), precluding robust conclusions; hence, the results are reported in Supporting Table S1. All VA pharmacy data are included in the CDW; dispensed drugs (rather than just prescribed drugs) were used to define antiviral treatment regimens, as we previously reported.28, 29, 30, 31, 32, 33
Baseline Patient Characteristics
For each HCV treatment regimen, we collected baseline data, including age, sex, body mass index (BMI), HCV genotype, HCV viral load, and receipt of prior antiviral treatment. We extracted relevant laboratory tests prior to treatment and recorded the value of each test closest to the treatment starting date within the preceding 6 months. We defined hepatitis B virus (HBV) coinfection by a positive HBV surface antigen test or viral load. We also determined the presence of cirrhosis, manifestations of decompensated cirrhosis (ascites, encephalopathy, gastroesophageal varices, and hepatorenal syndrome), type 2 diabetes mellitus, alcohol use disorders, substance use disorders, human immunodeficiency virus (HIV) infection, and liver transplantation based on appropriate International Classification of Diseases (ICD)‐9 or ICD‐10 codes recorded at least twice on two separate dates before treatment initiation in any inpatient or outpatient encounter (these codes are shown in Supporting Table S2). The ICD‐based definitions of these comorbidities have been widely used and validated in studies using VA medical records.34, 35, 36, 37, 38, 39, 40
SVR
We defined SVR as a serum HCV RNA viral load test below the lower limit of detection performed at least 12 weeks after the end of HCV treatment.41
Incident Hematologic Malignancies, MGUS, Colon Cancer, and Prostate Cancer
We identified incident hematologic malignancies (including lymphoma, multiple myeloma, and other hematologic malignancies) and MGUS diagnosed for the first time at least 180 days after the initiation of antiviral treatment based on appropriate ICD‐9 and ICD‐10 codes (see Table 1) and documented at least twice on two separate dates, some of which were validated in the VA data.4, 24 Malignancies diagnosed within 180 days from antiviral treatment initiation were excluded because eradication of HCV would be unlikely to prevent the development of a malignancy so quickly, while conversely, the presence of occult malignancy during the antiviral treatment might reduce the chances of SVR, thus creating a spurious association between SVR and lower malignancy risk.
We further extracted two additional malignancies, colon cancer and prostate cancer, as negative controls (Table 1). These are malignancies that are relatively common and have no plausible association with HCV or with IFN or DAAs. Therefore, any association between SVR and reduced risk in these malignancies would likely be spurious and would alert us to residual bias in our analyses.
Statistical Analysis
We compared patients who achieved SVR to those who did not with respect to the risk of developing the malignancies of interest, using Cox proportional hazards regression with or without adjusting for potential confounders. We calculated time starting from 180 days after initiation of antiviral treatment because cancers diagnosed within 180 days of the treatment start date could not possibly have been prevented by antiviral treatment and might have been present but undiagnosed at the time of antiviral treatment initiation (i.e., not truly incident cancers). In sensitivity analyses, we extended this period to 2 years after initiation of antiviral treatment. Follow‐up for incident malignancies extended until June 30, 2018, so that even the patients treated in late 2015 (i.e., the most recent in our cohort) would have 2.5 years of follow‐up. Patients without incident events were censored at the time of death or last follow‐up in the VA.
We considered using the date treatment ended or the date at which SVR was ascertained as the starting times for the time‐to‐event analysis; however, we decided against that because of the long and variable duration of the treatment and the interval from treatment end date to ascertainment of SVR, both of which are strongly related to SVR and would therefore introduce significant bias.
In our primary analyses, we analyzed each patient’s first antiviral treatment regimen (i.e., an intention‐to‐treat analysis). A significant proportion (42.7%) of patients who did not achieve SVR after their first treatment received more than one antiviral treatment during the study period. These patients were censored at the time of initiation of a subsequent antiviral regimen that resulted in SVR, if such a regimen existed. Additionally, we performed a secondary analysis in which we analyzed all treatments that each patient received clustered by patient. The intragroup correlation induced by clustering was accounted for by using robust variance estimation. Follow‐up time was censored at the start of a regimen of a different type.
To determine if SVR was independently associated with the malignancies of interest, our multivariable proportional hazards models were adjusted for the following characteristics that may be associated with both SVR and malignancy risk, ascertained at the time of treatment initiation: cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, BMI, HCV viral load, HIV coinfection, type 2 diabetes mellitus, alcohol use disorders, substance use disorders, liver transplantation, platelet count, serum bilirubin, serum creatinine, serum albumin, serum aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, blood international normalized ratio (INR), and blood hemoglobin levels. Continuous variables were categorized and modeled as dummy categorical variables. In addition, we considered adjusting for the duration of IFN treatment to account for any direct antineoplastic effects of IFN itself when comparing patients who achieved SVR versus those who did not.
Results
Characteristics of the Study Population
Of 69,581 patients, 40,410 received the IFN‐only regimen (of whom 36% achieved SVR), 4,546 received DAA + IFN (of whom 61% achieved SVR), and 24,625 received the DAA‐only regimen (of whom 90% achieved SVR). The distribution of individual regimens is shown in Supporting Table S3. The most common DAA‐only regimen was sofosbuvir/ledipasvir, which accounted for 58% of all DAA‐only regimens.
Patients were mostly male individuals (96.5%), with a majority being white (56.4%) but a significant representation of other racial/ethnic groups. Mean age was 55.6 years, and 17.1% had cirrhosis, 5% decompensated cirrhosis, and 1.6% were liver transplant recipients. Genotype 1 HCV infection predominated (72%), followed by genotype 2 (12%) and 3 (8%).
Among all three regimen types, patients who achieved SVR were less likely to have diabetes, cirrhosis, or decompensated cirrhosis than patients who did not achieve SVR (Table 2). Among DAA‐only regimens, patients who achieved SVR were more likely to have genotype 1 infection. Conversely, among IFN‐only regimens, patients who achieved SVR were more likely to have genotypes 2 and 3 infection.
Table 2.
Baseline Characteristics of Patients Infected with HCV who Received Their First Antiviral Treatment from 1999 to 2015 According to Whether they Achieved SVR
| Baseline characteristics | All Patients (N = 69,581) | IFN Only | DAA + IFN | DAA Only | |||
|---|---|---|---|---|---|---|---|
| No SVR (n = 26,078) | SVR (n = 14,332) | No SVR (n = 1,785) | SVR (n = 2,761) | No SVR (n = 2,524) | SVR (n = 22,101) | ||
| Age, years (mean [SD]) | 55.6 [7.7] | 52.3 [6.2] | 52.1 [6.8] | 57. [5.9] | 57.2 [6.7] | 60.3 [6.9] | 60.9 [6.7] |
| BMI, kg/m2 (mean [SD]) | 28.2 [5.3] | 28.4 [5.2] | 28.2 [5.2] | 28.6 [5.3] | 28.3 [5.0] | 28.5 [5.8] | 27.9 [5.4] |
| Male (%) | 96.5 | 96.9 | 95.6 | 95.5 | 96.3 | 98.1 | 96.5 |
| Race/ethnicity (%) | |||||||
| White, non‐Hispanic | 56.4 | 52.7 | 67.4 | 50.6 | 61 | 53.7 | 53.9 |
| Black, non‐Hispanic | 25.1 | 25.5 | 12.3 | 35.7 | 24.6 | 29.8 | 31.5 |
| Hispanic, Asian, Pacific Island, AIAN, other | 7.7 | 8.6 | 7.8 | 7.7 | 5.9 | 9 | 6.7 |
| Declined to answer/missing | 10.8 | 13.2 | 12.5 | 5.9 | 8.5 | 7.4 | 7.9 |
| Genotype (%) | |||||||
| Genotype 1 | 71.9 | 73 | 42.8 | 98.7 | 95 | 71.7 | 84.4 |
| Genotype 2 | 12.2 | 9.4 | 25.9 | 0.1 | 0.8 | 12.9 | 9 |
| Genotype 3 | 7.8 | 7.8 | 13.8 | 0.7 | 2.3 | 12.4 | 4.7 |
| Genotype ≥4 or missing | 8.1 | 9.8 | 17.5 | 0.6 | 2 | 3 | 1.9 |
| HCV RNA viral load >6 million IU/mL (%) | 16.5 | 14.8 | 14.7 | 24.1 | 21.1 | 19.9 | 17.9 |
| HIV coinfection | 3.2 | 3.3 | 1.7 | 2.1 | 1.5 | 3.9 | 4.2 |
| HBV coinfection | 1 | 0.6 | 1 | 1.8 | 1.7 | 0.9 | 1.3 |
| Cirrhosis (%) | 17.1 | 13.5 | 7.6 | 29 | 20.8 | 36.6 | 23.9 |
| Decompensated cirrhosis (%) | 5 | 4.2 | 2.3 | 6.9 | 4 | 13.4 | 6.7 |
| Liver transplantation (%) | 1.6 | 1.5 | 1.3 | 0.3 | 0.5 | 1.1 | 2.2 |
| Diabetes (%) | 21.6 | 19.5 | 13.6 | 25.4 | 20.4 | 32.1 | 27.7 |
| Alcohol use disorder (%) | 38.7 | 34.9 | 33.8 | 42.4 | 40.7 | 50.1 | 44.4 |
| Substance use disorder (%) | 31.1 | 27 | 26.1 | 34.6 | 32.6 | 40.7 | 37.7 |
| Laboratory results(mean [SD]) | |||||||
| Hemoglobin, g/dL | 14.9 [1.5] | 15.0 [1.5] | 15.1 [1.4] | 14.9 [1.4] | 15.0 [1.4] | 14.3 [1.7] | 14.5 [1.6] |
| Platelet count, k/µL | 191.7 [71.9] | 196.5 [72.7] | 210.1 [69.2] | 173.1 [64.0] | 187.8 [63.1] | 159.2 [74.5] | 180.2 [70.4] |
| Creatinine, mg/dL | 1.0 [0.6] | 1.0 [0.7] | 1.0 [0.4] | 1.0 [0.7] | 0.9 [0.3] | 1.0 [0.5] | 1.0 [0.5] |
| Bilirubin, g/dL | 0.7 [0.5] | 0.7 [0.5] | 0.6 [0.5] | 0.7 [0.5] | 0.6 [0.4] | 0.8 [0.7] | 0.7 [0.5] |
| Albumin g/dL | 4.0 [0.5] | 4.0 [0.5] | 4.1 [0.4] | 3.9 [0.5] | 4.0 [0.4] | 3.7 [0.6] | 3.9 [0.5] |
| INR | 1.1 [1.0] | 1.1 [0.9] | 1.1 [1.0] | 1.2 [1.3] | 1.2 [1.1] | 1.2 [1.0] | 1.2 [0.9] |
| AST/ALT | 0.9 [0.4] | 0.9 [0.4] | 0.8 [0.3] | 1.0 [0.4] | 0.8 [0.3] | 1.0 [0.4] | 1.0 [0.4] |
Abbreviation: AIAN, American Indian and Alaskan Native.
Compared to patients treated in the IFN‐only category, those treated only with DAA had a higher mean age (by 8 years) and were more likely to have cirrhosis, alcohol use disorders, and substance use disorders.
IFN‐Induced SVR was Associated with Reduction in Hematologic Malignancies
Of the 40,410 patients treated only with IFN with mean follow‐up of 10.6 years from treatment initiation, 348 developed lymphoma, 121 multiple myeloma, 159 other hematologic malignancies, 225 MGUS, 260 colon cancer, and 1,361 prostate cancer. Among patients treated with IFN, SVR was significantly associated with a reduction in the risk of lymphoma (AHR, 0.70; 95% confidence interval [CI], 0.51‐0.97), multiple myeloma (AHR, 0.40; 95% CI, 0.20‐0.77), MGUS (AHR, 0.65; 95% CI, 0.42‐0.99), or all hematologic malignancies and MGUS combined (AHR, 0.67; 95% CI, 0.53‐0.84) in both crude and adjusted analyses (Table 3; Fig. 1). Among patients treated with IFN, SVR was not associated with colon cancer (AHR, 1.13; 95% CI, 0.81‐1.58) or prostate cancer (AHR, 1.14; 95% CI, 0.98‐1.33), the two negative control malignancies that we studied.
Table 3.
Association Between Eradication of HCV (SVR) and Risk of Lymphoma, Multiple Myeloma, Other Hematologic Malignancies, MGUS, Colon Cancer, and Prostate Cancer, Presented Separately According to Treatment with IFN‐only Versus DAA‐only Regimens
| Number of Patients | Patient Years | Number Who Developed Malignancy (%) | Incidence per 100 Patient Years | Crude HR (95% CI) | AHR* (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Lymphoma | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 263,705 | 256 (1.0) | 0.1 | 1 | 1 |
| SVR | 14,332 (35.5) | 144,887 | 92 (0.6) | 0.06 | 0.66 (0.52‐0.83) | 0.70 (0.51‐0.97) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,140 | 12 (0.5) | 0.2 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,706 | 82 (0.4) | 0.15 | 0.78 (0.43‐1.43) | 0.91 (0.47‐1.76) | |
| Multiple myeloma | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 264,195 | 100 (0.4) | 0.04 | 1 | 1 |
| SVR | 14,332 (35.5) | 145,178 | 21 (0.1) | 0.01 | 0.38 (0.24‐0.61) | 0.40 (0.20‐0.77) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,155 | 1 (0.0) | 0.02 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,772 | 19 (0.1) | 0.04 | 2.17 (0.29‐16.18) | 1.85 (0.23‐14.71) | |
| Other hematologic malignancies (except lymphoma and myeloma) | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 264,104 | 123 (0.5) | 0.05 | 1 | 1 |
| SVR | 14,332 (35.5) | 145,111 | 36 (0.3) | 0.02 | 0.53 (0.37‐0.77) | 0.65 (0.38‐1.12) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,153 | 3 (0.1) | 0.05 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,786 | 21 (0.1) | 0.04 | 0.80 (0.24‐2.68) | 0.90 (0.25‐3.16) | |
| MGUS | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 263,891 | 174 (0.7) | 0.07 | 1 | 1 |
| SVR | 14,332 (35.5) | 145,020 | 51 (0.4) | 0.04 | 0.54 (0.39‐0.74) | 0.65 (0.42‐0.99) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,151 | 5 (0.2) | 0.08 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,689 | 71 (0.3) | 0.13 | 1.60 (0.64‐3.96) | 1.54 (0.56‐4.27) | |
| Hematologic malignancies or MGUS | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 262,325 | 574 (2.2) | 0.22 | 1 | 1 |
| SVR | 14,332 (35.5) | 144,534 | 183 (1.3) | 0.13 | 0.58 (0.49‐0.69) | 0.67 (0.53‐0.84) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,133 | 20 (0.8) | 0.33 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,561 | 179 (0.8) | 0.33 | 1.02 (0.64‐1.62) | 1.08 (0.66‐1.78) | |
| Colon cancer | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 263,834 | 165 (0.6) | 0.06 | 1 | 1 |
| SVR | 14,332 (35.5) | 144,846 | 95 (0.7) | 0.07 | 1.05 (0.81‐1.35) | 1.13 (0.81‐1.58) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,153 | 2 (0.1) | 0.03 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,757 | 41 (0.2) | 0.08 | 2.31 (0.56‐9.53) | 4.60 (0.62‐33.93) | |
| Prostate cancer | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 259,757 | 902 (3.5) | 0.35 | 1 | 1 |
| SVR | 14,332 (35.5) | 142,813 | 459 (3.2) | 0.32 | 0.93 (0.83‐1.04) | 1.14 (0.98‐1.33) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,121 | 28 (1.1) | 0.46 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,428 | 288 (1.3) | 0.54 | 1.18 (0.80‐1.74) | 1.03 (0.68‐1.56) | |
| Colon cancer or prostate cancer | |||||||
| IFN‐only regimens | No SVR | 26,078 (64.5) | 259,017 | 1,059 (4.1) | 0.41 | 1 | 1 |
| SVR | 14,332 (35.5) | 142,440 | 548 (3.8) | 0.38 | 0.94 (0.85‐1.05) | 1.14 (0.99‐1.31) | |
| DAA‐only regimens | No SVR | 2,524 (10.2) | 6,119 | 30 (1.2) | 0.49 | 1 | 1 |
| SVR | 22,101 (89.8) | 53,381 | 329 (1.5) | 0.62 | 1.25 (0.86‐1.82) | 1.18 (0.79‐1.77) | |
Adjusted for cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, BMI, HCV genotype, length of treatment, HCV viral load, HIV coinfection, type 2 diabetes mellitus, alcohol use disorders, substance use disorder, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/ALT ratio, blood INR, and blood hemoglobin levels. Laboratory tests were categorized into quartiles and modeled as dummy categorical variables.
Figure 1.
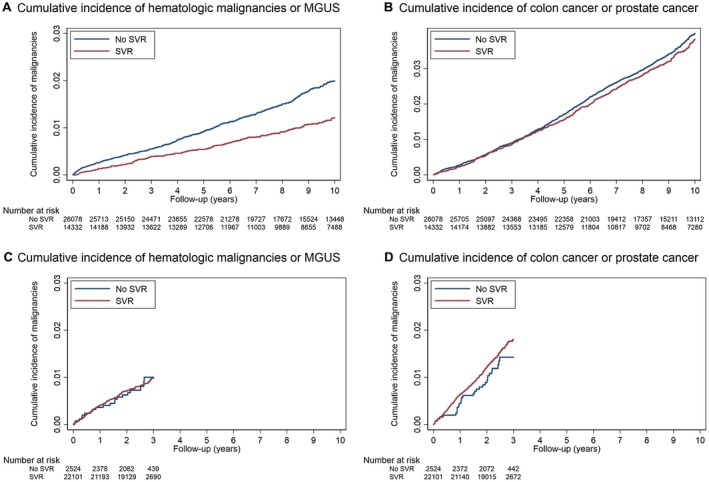
Cumulative incidence curves comparing patients who achieved SVR versus those who did not after treatment with IFN (panels A and B) or DAAs (panels C and D). Patients with IFN‐induced SVR have lower cumulative incidence of hematologic malignancies or MGUS compared to patients who did not achieve SVR (A). Furthermore, the difference between the “SVR” and “No SVR” cumulative incidence curves in panel A continues to expand as more time accrues after treatment up to 10 years. In contrast, patients with DAA‐induced SVR have almost identical cumulative incidence of hematologic malignancies as the patients who did not achieve SVR (C). Although follow‐up only extends for 3 years from the time point 6 months after DAA treatment initiation, the equivalent curves appear to separate by 3 years after IFN treatment in panel A. As expected, there is no difference between patients with and without SVR following either IFN or DAA in the cumulative incidence of the “negative control” malignancies of colon and prostate cancer – which have no putative relationship with HCV or IFN (B and D). [Corrections added 13 June 2019. In the original publication the captions for Figure 1 panels A through D were omitted.]
DAA‐Induced SVR was not Associated with Reduction in Hematologic Malignancies
Of the 24,625 patients treated only with DAA with mean follow‐up of 2.9 years from treatment initiation, 94 developed lymphoma, 20 multiple myeloma, 24 other hematologic malignancies, 76 MGUS, 43 colon cancer, and 316 prostate cancer. Among patients treated with DAA, SVR was not associated in unadjusted or adjusted models with a reduction in any of these malignancies individually or when all hematologic malignancies or all solid malignancies were combined (Table 3; Fig. 1).
Additional Analyses and Considerations Attempting to Explain the Discrepancy Between IFN‐Induced SVR And DAA‐Induced SVR
A test of statistical interaction for risk of hematologic malignancy or MGUS versus HCV treatment and SVR, after adjusting for other predictors, was performed (P = 0.05) and supported the role of effect modification by regimen type rather than differences in sample size. To evaluate the possibility of effect modification by age in which SVR could exert a protective effect against hematologic malignancies only among young patients (and thus result in a greater impact in the younger IFN‐treated cohort), stratification by age groups was performed (Supporting Table S4). Even when limiting our analyses to patients <65 or <60 years old, there was no association between DAA‐induced SVR and hematologic malignancies, while the association between IFN‐induced SVR and reduced risk of hematologic malignancies persisted unchanged.
We selected 20 confounding variables a priori as those potentially associated with both SVR and hematological malignancy. To ensure that simultaneously adjusting for a large number of variables did not result in overfitting, we conducted secondary analyses that modeled different levels of adjustment. These included unadjusted; adjusted for the most important predictors of SVR, including cirrhosis, decompensated cirrhosis, HCV genotype, and diabetes; adjusted for the most important predictors of SVR and critical demographics comprising cirrhosis, decompensated cirrhosis, HCV genotype, diabetes, age, sex, race/ethnicity, and BMI; and adjusted for all 20 potential confounders selected a priori. AHRs were consistent across the different levels of adjustment (Supporting Table S5).
We performed additional analyses in which we excluded the first 2 years of follow‐up after treatment instead of only 180 days. IFN regimens were associated with multiple side effects, including cytopenias, which frequently led to early discontinuation and failure to achieve SVR.35 It is possible that hematologic malignancies might have been present but occult during the antiviral treatment or present in premalignant forms, exacerbating treatment‐related cytopenias and leading to early discontinuation and/or treatment failure. We reasoned that such hematologic malignancies that were present but occult at the time of treatment would be diagnosed within 2 years. However, the association between IFN‐induced SVR and reduced risk of lymphoma, multiple myeloma, or MGUS was even stronger after excluding the first 2 years, thus ruling out this potential source of bias (Supporting Table S6). Furthermore, the cumulative incidence curves of the patients treated with IFN who achieved SVR and those who did not continued to separate from each other even up to 10 years of follow‐up (Fig. 1). It is extremely unlikely that this would be caused by occult malignant or premalignant conditions present so many years before the antiviral treatment.
IFN has been used as treatment of some hematologic malignancies (e.g., multiple myeloma, lymphoma, chronic myeloid leukemia). It is therefore conceivable that the IFN that was given to treat HCV might have also treated an occult or developing hematologic malignancy. However, both patients with and without SVR received IFN, and any such effect would have canceled out. We additionally adjusted for duration of IFN treatment in the event that patients who achieved SVR received longer IFN treatment and hence more antineoplastic benefits, but the associations with IFN‐related SVR persisted.
Among patients treated with IFN, those who achieved SVR had identical mean follow‐up duration (10.6 years) as those who did not (10.6 years) and were treated during the same calendar years. Therefore, unequal follow‐up time or changes over time in the ascertainment of malignancies cannot explain the associations between IFN‐induced SVR and lower risk of hematologic malignancies.
Patients treated only with DAA had much shorter mean follow‐up duration (2.9 years) than patients treated with IFN. It is possible that follow‐up in the patients treated with DAA was not long enough for the effects of DAA‐induced SVR on hematologic malignancy to manifest. While this may be true, it appears that among the patients treated with IFN, the cumulative incidence curves of the patients who achieved SVR and those who did not separate from each other even within the first 3 years (Fig. 1), whereas the curves for the patients treated with DAA do not. We performed a secondary analysis in which follow‐up of patients treated with IFN and those treated with DAA was truncated at 3 years (Supporting Table S7). An association of reduced risk of hematologic malignancies or MGUS in IFN‐induced SVR was observed in the unadjusted model; in the adjusted model, the association did not meet statistical significance, likely due to fewer incident hematologic malignancies in this period of time.
Our primary analysis included only the first antiviral treatment that patients received. However, many patients received multiple treatment courses, especially many patients treated with DAA who had previously failed treatments with IFN. For this reason, we performed additional analyses that included all treatment courses received (n = 85,200) among the 69,581 patients in our study, using cluster analysis (Supporting Table S8). The associations with IFN‐induced SVR persisted and if anything were slightly stronger, whereas again no associations were identified with DAA‐induced SVR.
Discussion
We report for the first time that IFN‐induced SVR is associated with a significant reduction in the risk of hematologic malignancies (including lymphoma and multiple myeloma) and MGUS during a mean follow‐up of 10.6 years from treatment. In contrast, DAA‐induced SVR was not associated with a reduction in the risk of hematologic malignancies, albeit during a shorter mean follow‐up of 2.9 years from treatment. This suggests that the eradication of HCV may have different long‐term benefits depending on whether it is achieved by IFN or DAAs.
Multiple observational studies have consistently reported an increased risk of certain B‐cell lymphomas with HCV infection.1, 2, 3, 4, 5 HCV has also been associated with the development of MGUS14, 15 and many other hematologic malignancies, including myeloma, T‐cell lymphomas, chronic lymphocytic leukemia, and myeloid leukemias, but these associations have not been confirmed across studies.11, 12, 13 Neither the pathogenic mechanism of HCV contributing risk to hematologic malignancies nor the effect on this risk of SVR is well characterized.42 Chronic antigen stimulation of lymphocytes is a hypothesized mechanism in HCV‐associated lymphoma.8 Alternatively or in combination, a direct oncogenic effect by HCV may occur through infection of B cells by virus or engagement by HCV‐E2 protein of B‐cell and T‐cell surface receptor clusters of differentiation (CD)81.43 Furthermore, eradication of HCV either with IFN or DAAs has led to regression or prevention of relapse in certain cases of lymphoma.16, 17, 18, 19, 20, 21, 22, 23
Even assuming that HCV is causatively associated with hematologic malignancies, it does not necessarily follow that eradication of HCV should reduce the risk. Most patients have been infected for decades before undergoing treatment and eradication. It is plausible that groundwork for tumorigenesis was already laid during the long period of infection and the occurrence of future mutagenic hits that complete a cell’s neoplastic conversion are unrelated to HCV and may occur even after HCV eradication. A Japanese study extending from 1969 to 2006 reported that none of the 1,048 patients who achieved SVR with IFN developed lymphoma compared to 12 cases of lymphoma among 1,660 patients treated with IFN who failed to achieve SVR.44 These results suggest that SVR induced by IFN may be protective for the development of HCV‐related lymphoma; however, no multivariable adjustments for baseline characteristics that could be confounders were performed, and the likelihood of diagnosis of lymphoma might have changed during the almost 40‐year study period. Recently, Mahale et al.24 reported that patients with IFN‐induced SVR had significantly lower risk of non‐Hodgkin lymphoma compared with patients who were untreated (AHR, 0.64; 95% CI, 0.43‐0.95) but not compared with patients who received IFN and did not achieve SVR (AHR, 0.71; 95% CI, 0.45‐1.11). Like ours, this study was also based on national VA data but only extended from 1999 to 2009; our study extended from 1999 to 2018, which may explain why that study did not reach statistical significance even though the AHR for lymphoma was remarkably similar to the one we reported (AHR, 0.70; 95% CI, 0.51‐0.97). In addition to showing a significant association with lymphoma, our results demonstrated a significant association between IFN‐induced SVR and a reduction in multiple myeloma (AHR, 0.40; 95% CI, 0.20‐0.77) and MGUS (AHR, 0.65; 95% CI, 0.42‐0.99) as well as all hematologic malignancies and MGUS combined (AHR, 0.67; 95% CI, 0.53‐0.84). In fact, the magnitude of the association between IFN‐induced SVR and reduced multiple myeloma or MGUS was greater than that for lymphoma. We are not aware of prior studies that investigated the effect of HCV antiviral treatments on hematologic malignancies other than lymphoma.
In contrast, we found no association between DAA‐induced SVR and lymphoma, myeloma, MGUS, or all hematologic malignancies and MGUS combined. Mean follow‐up (2.9 years from treatment) was unavoidably shorter in the patients treated with DAA than in the patients treated with IFN, but it was as long as could possibly be at the time the analysis was done because we extended follow‐up to June 30, 2018, and began with the earliest DAA‐only regimens available. We are not aware that this association has been tested in other large community‐based databases of DAA treatments, and it merits confirmation. Also, it would be important to repeat this analysis in 2‐3 years when more follow‐up time has accrued. This finding is surprising because we are not aware of other long‐term outcomes where IFN‐induced SVR has been convincingly shown to have different implications than DAA‐induced SVR. For example, we have previously shown that DAA‐induced SVR and IFN‐induced SVR have similar effects on reducing the risk of hepatocellular carcinoma.45
Critical to understanding the relationship between IFN‐induced SVR and reduced incidence of hematologic malignancies is the direct role of IFN in either treating early stage or subclinical malignancy or enhancing immune surveillance as a prophylactic intervention. IFN is well established as a promoter of immune response to malignancy as evidenced, for example, by the increased rate of cancer in mice lacking the IFN signaling gene interferon alpha and beta receptor subunit 1 (Ifnar1).46 IFN has a long history of use treating various hematologic malignancies, albeit with limited success, including chronic myeloid leukemia, myeloma, and lymphoma.47, 48, 49, 50 Therefore, it is plausible that IFN‐induced SVR has a particularly strong effect on hematologic malignancies through the positive interaction of both the pharmacologic effects of IFN itself and the effects of viral eradication. Such an interaction needs to be invoked because patients with SVR and those without SVR were exposed to IFN, and hence the pharmacologic effects of IFN alone cannot explain our finding. Although we went to great lengths to exclude a spurious or biased finding, as explained in the Results section (e.g., including negative control malignancies, excluding 6 months and 2 years of follow‐up, adjusting for duration of IFN treatment as well as 20 other potential confounders, ensuring a sufficiently long follow‐up time, performing cluster analyses as well as first‐treatment analyses), we cannot completely exclude the possibility that patients who failed IFN treatments also happened to have characteristics that we did not adjust for that predisposed them to hematologic malignancies many years later.
The use of VA data enabled this large study; however, the cohort is heavily skewed to men. It would be important for other large community‐based cohorts of patients treated for HCV to confirm our findings. The accuracy of the data herein is contingent on coding of malignancies by health care providers in the VA electronic health records system. Efforts to optimize this included the stringent definition of a diagnosis of malignancy or MGUS by recording ICD‐9 or ICD‐10 codes 2 or more times on two different dates. Validated, previously published, diagnostic coding libraries were used when available, including specifically from VA‐based studies. Certain ICD codes, such as ICD‐9 202.8x, designate B‐cell non‐Hodgkin lymphoma but can be used for multiple histologic subtypes. This can result in the dilution of rare lymphoma subtypes more commonly associated with HCV. Although some diagnostic inaccuracies and imprecision are inevitable, there is no reason to suspect that they would be in a particular direction and more likely to occur in the SVR versus the no SVR group. Similarly, while the IFN‐treated cohort and the DAA‐treated cohort differed by era (and, potentially, changes in cancer screening practices) and factors associated with cancer incidence, including age and prevalence of cirrhosis, the comparison of SVR and no SVR groups by treatment modality are contemporaneous and not subject to these differences. Strengths of the study include large sample size, long duration of follow‐up, adequate number of incidence malignancies, appropriate comparison/control groups, and adequate adjustment for almost all factors known to be associated with SVR. Although there may be additional factors associated with hematologic malignancies and HCV, a characteristic can be a confounder only if it is associated with both SVR and the future development of hematologic malignancies. For example, mixed and polyclonal cryoglobulinemia are important extrahepatic complications of HCV and may represent a manifestation of certain hematologic malignancies. Cryoglobulinemia is not routinely documented by laboratory and diagnostic evaluations, however, and it was omitted in the analyses as there is no reason to suspect an association with SVR.
We demonstrate that IFN‐induced SVR is associated with dramatic reductions in the risk of hematologic malignancies, including lymphoma and multiple myeloma, and the premalignant condition of MGUS. In contrast, DAA‐induced SVR was not associated with such risk reductions, at least within the study’s mean follow‐up of 2.9 years. We hope that studies from other large population‐based cohorts will replicate our findings and that our study will be repeated with the accrual of longer follow‐up since the introduction of DAAs. Our study cautions that we should not automatically assume that any benefits of viral eradication derived from IFN‐based treatments apply to DAA‐based treatments. It is critical to continue to evaluate the long‐term clinical benefits that patients derive from DAA treatments.
Supporting information
Supported by the National Institutes of Health/National Cancer Institute (grant R01CA196692 to G.N.I.) and Veterans Affairs Clinical Science Research and Development (grant I01CX001156 to G.N.I.).
The funding source played no role in study design, collection, analysis or interpretation of data. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Potential conflict of interest: Nothing to report.
References
- 1. de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM, et al. Hepatitis C and non‐Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 2008;6:451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iqbal T, Mahale P, Turturro F, Kyvernitakis A, Torres HA. Prevalence and association of hepatitis C virus infection with different types of lymphoma. Int J Cancer 2016;138:1035‐1037. [DOI] [PubMed] [Google Scholar]
- 3. Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longombardo G, et al. Hepatitis C virus infection in patients with non‐Hodgkin's lymphoma. Br J Haematol 1994;88:392‐394. [DOI] [PubMed] [Google Scholar]
- 4. Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El‐Serag H, et al. Risk of non‐Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 2007;297:2010‐2017. [DOI] [PubMed] [Google Scholar]
- 5. Gisbert JP, García‐Buey L, Pajares JM, Moreno‐Otero R. Prevalence of hepatitis C virus infection in B‐cell non‐Hodgkin's lymphoma: systematic review and meta‐analysis. Gastroenterology 2003;125:1723‐1732. [DOI] [PubMed] [Google Scholar]
- 6. Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, et al. Low‐grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood 1994;84:3047‐3053. [PubMed] [Google Scholar]
- 7. Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta‐analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2006;15:2078‐2085. [DOI] [PubMed] [Google Scholar]
- 8. Zucca E, Bertoni F, Vannata B, Cavalli F. Emerging role of infectious etiologies in the pathogenesis of marginal zone B‐cell lymphomas. Clin Cancer Res 2014;20:5207‐5216. [DOI] [PubMed] [Google Scholar]
- 9. Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol 2010;8:1017‐1029. [DOI] [PubMed] [Google Scholar]
- 10. Charles ED, Brunetti C, Marukian S, Ritola KD, Talal AH, Marks K, et al. Clonal B cells in patients with hepatitis C virus‐associated mixed cryoglobulinemia contain an expanded anergic CD21low B‐cell subset. Blood 2011;117:5425‐5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bianco E, Marcucci F, Mele A, Musto P, Cotichini R, Sanpaolo MG, et al. Italian Multi‐Center case‐control study. Prevalence of hepatitis C virus infection in lymphoproliferative diseases other than B‐cell non‐Hodgkin's lymphoma, and in myeloproliferative diseases: an Italian multi‐center case‐control study. Haematologica 2004;89:70‐76. [PubMed] [Google Scholar]
- 12. Fiorino S, Bacchi‐Reggiani L, de Biase D, Fornelli A, Masetti M, Tura A, et al. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: a systematic review. World J Gastroenterol 2015;21:12896‐12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Li Y, Zhang L, Li W. Hepatitis C virus infection and risk of multiple myeloma: evidence from a meta‐analysis based on 17 case‐control studies. J Viral Hepat 2017;24:1151‐1159. [DOI] [PubMed] [Google Scholar]
- 14. Andreone P, Zignego AL, Cursaro C, Gramenzi A, Gherlinzoni F, Fiorino S, et al. Prevalence of monoclonal gammopathies in patients with hepatitis C virus infection. Ann Intern Med 1998;129:294‐298. [DOI] [PubMed] [Google Scholar]
- 15. Mangia A, Clemente R, Musto P, Cascavilla I, La Floresta P, Sanpaolo G, et al. Hepatitis C virus infection and monoclonal gammopathies not associated with cryoglobulinemia. Leukemia 1996;10:1209‐1213. [PubMed] [Google Scholar]
- 16. Gisbert JP, Garcia‐Buey L, Pajares JM, Moreno‐Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther 2005;21:653‐662. [DOI] [PubMed] [Google Scholar]
- 17. Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache‐Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med 2002;347:89‐94. [DOI] [PubMed] [Google Scholar]
- 18. Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, et al. Role of anti‐hepatitis C virus (HCV) treatment in HCV‐related, low‐grade, B‐cell, non‐Hodgkin's lymphoma: a multicenter Italian experience. J Clin Oncol 2005;23:468‐473. [DOI] [PubMed] [Google Scholar]
- 19. Arcaini L, Besson C, Frigeni M, Fontaine H, Goldaniga M, Casato M, et al. Interferon‐free antiviral treatment in B‐cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood 2016;128:2527‐2532. [DOI] [PubMed] [Google Scholar]
- 20. Carrier P, Jaccard A, Jacques J, Tabouret T, Debette‐Gratien M, Abraham J, et al. HCV‐associated B‐cell non‐Hodgkin lymphomas and new direct antiviral agents. Liver Int 2015;35:2222‐2227. [DOI] [PubMed] [Google Scholar]
- 21. Hattori N, Ikeda H, Nakano H, Matsumoto N, Watanabe T, Shigefuku R, et al. Curative effects for B‐cell lymphoma accomplished by direct‐acting antiviral agents of hepatitis C. Open Forum. Infect Dis 2017;4:ofx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossotti R, Travi G, Pazzi A, Baiguera C, Morra E, Puoti M. Rapid clearance of HCV‐related splenic marginal zone lymphoma under an interferon‐free, NS3/NS4A inhibitor‐based treatment. A case report. J Hepatol 2015;62:234‐237. [DOI] [PubMed] [Google Scholar]
- 23. La Mura V, De Renzo A, Perna F, D'Agostino D, Masarone M, Romano M, et al. Antiviral therapy after complete response to chemotherapy could be efficacious in HCV‐positive non‐Hodgkin's lymphoma. J Hepatol 2008;49:557‐563. [DOI] [PubMed] [Google Scholar]
- 24. Mahale P, Engels EA, Li R, Torres HA, Hwang LY, Brown EL, et al. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut 2018;67:553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakobsen J, Nielsen E, Feinberg J, Katakam K, Fobian K, Hauser G, et al. Direct‐acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev 2017;6: CD012143. Update. Cochrane Database Syst Rev 2017;9:CD012143.28922704 [Google Scholar]
- 26. U.S. Department of Veterans Affairs . Veterans Health Administration. http://www.va.gov/health/findcare.asp. Updated January 23, 2018. Accessed December 2018.
- 27. U.S. Department of Veterans Affairs . Veterans Affairs Corporate Data Warehouse. http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm. Updated March 28, 2014. Accessed December 2016.
- 28. Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology 2017;65:426‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend 2016;169:101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology 2016;151:457‐471.e5. Erratum. In: Gastroenterology 2016;151:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol 2017;67:32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hum J, Jou JH, Green PK, Berry K, Lundblad J, Hettinger BD, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care 2017;40:1173‐1180. [DOI] [PubMed] [Google Scholar]
- 33. Moon AM, Green PK, Berry K, Ioannou GN. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Aliment Pharmacol Ther 2017;45:1201‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kramer JR, Kanwal F, Richardson P, Giordano TP, Petersen LA, El‐Serag HB. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol 2011;106:483‐491. [DOI] [PubMed] [Google Scholar]
- 35. Beste LA, Ioannou GN, Larson MS, Chapko M, Dominitz JA. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clin Gastroenterol Hepatol 2010;8:972‐978. [DOI] [PubMed] [Google Scholar]
- 36. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology 2007;46:37‐47. [DOI] [PubMed] [Google Scholar]
- 37. Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus‐infected veterans in the United States. Ann Intern Med 2011;154:85‐93. [DOI] [PubMed] [Google Scholar]
- 38. Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC andcirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011;140:1182‐1188.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27(Suppl. 2):B10‐B21. [DOI] [PubMed] [Google Scholar]
- 40. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471‐1482.e5. [DOI] [PubMed] [Google Scholar]
- 41. Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks post‐treatment with sofosbuvir‐containing regimens for hepatitis C virus. Hepatology 2015;61:41‐45. [DOI] [PubMed] [Google Scholar]
- 42. Couronne L, Bachy E, Roulland S, Nadel B, Davi F, Armand M, et al. From hepatitis C virus infection to B‐cell lymphoma. Ann Oncol 2018;29:92‐100. [DOI] [PubMed] [Google Scholar]
- 43. Marcucci F, Mele A. Hepatitis viruses and non‐Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117:1792‐1798. [DOI] [PubMed] [Google Scholar]
- 44. Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, et al. Viral elimination reduces incidence of malignant lymphoma in patients with hepatitis C. Am J Med 2007;120:1034‐1041. [DOI] [PubMed] [Google Scholar]
- 45. Ioannou GN, Green PK. Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2017;pii:S0168‐8278(17)32273‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol 2005;6:722‐729. [DOI] [PubMed] [Google Scholar]
- 47. Mellstedt H, Aahre A, Bjorkholm M, Cantell K, Holm G, Johansson B, et al. Interferon therapy in myelomatosis. Lancet 1979;2:697. [DOI] [PubMed] [Google Scholar]
- 48. Solal‐Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, et al. Recombinant interferon alfa‐2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d'Etude des Lymphomes de l'Adulte. N Engl J Med 1993;329:1608‐1614. [DOI] [PubMed] [Google Scholar]
- 49. Talpaz M, Kantarjian HM, McCredie K, Trujillo JM, Keating MJ, Gutterman JU. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N Engl J Med 1986;314:1065‐1069. [DOI] [PubMed] [Google Scholar]
- 50. Medrano RFV, Hunger A, Mendonca SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget 2017;8:71249‐71284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


