Abstract
Phox2b protein is a specific marker for neurons in the parafacial region of the ventral medulla, which are proposed to play a role in central chemoreception and postnatal survival. Mutations of PHOX2B cause congenital central hypoventilation syndrome. However, there have been no reports concerning electrophysiological characteristics of these Phox2b-expressing neurons in the parafacial region of the neonate immediately after birth. This region overlaps with the parafacial respiratory group (pFRG) composed predominantly of preinspiratory (Pre-I) neurons that are involved in respiratory rhythm generation. We studied (1) whether pFRG neurons are Phox2b immunoreactive or not and (2) whether they show intrinsic CO2 chemosensitivity. We found that most pFRG/Pre-I neurons were Phox2b immunoreactive and depolarized upon increase in CO2 concentration under condition of action potential-dependent synaptic transmission blockade by tetrodotoxin. We also confirmed that these pFRG neurons expressed neurokinin-1 receptor. They were tyrosine hydroxylase negative and presumed to be glutamatergic. Our findings suggest that Phox2b-expressing parafacial neurons play a role in respiratory rhythm generation as well as central chemoreception and thus are essential for postnatal survival.
Keywords: Phox2b, central chemoreceptors, parafacial neurons, respiratory rhythm, congenital central hypoventilation syndrome, medulla oblongata
Introduction
The paired-like homeobox gene PHOX2B encodes a transcription factor which is essential for the development of the central and peripheral autonomic nervous systems (Pattyn et al., 1999). Phox2b-expressing cells are distributed in several regions of the hindbrain involved in the autonomic nervous system in embryonic, neonatal, and adult rodents (Dauger et al., 2003; Kang et al., 2007). Heterozygous mutations of PHOX2B in the form of polyalanine expansions or frameshifts cause congenital central hypoventilation syndrome (CCHS) and late-onset CHS (LO-CHS), symptoms of which manifest in adulthood (Amiel et al., 2003; Blanchi and Sieweke, 2005; Weese-Mayer et al., 2005). Mice bearing mutations with polyalanine expansion (Phox2b27Ala/+) lack Phox2b-expressing glutamatergic neurons in the parafacial region of the ventral medulla (Dubreuil et al., 2008). They die soon after birth from central apnea, indicating that the Phox2b27Ala/+ mice reproduce the phenotype of severe cases of CCHS. Phox2b-expressing neurons in the parafacial region that overlap the retrotrapezoid nucleus (RTN) play an important role in chemosensory integration (Takakura et al., 2008), including central CO2 chemoreception in respiratory control (Nattie et al., 1991; Nattie and Li, 2002; Mulkey et al., 2004; Stornetta et al., 2006). These findings suggest that Phox2b-expressing neurons in the parafacial region are essential in sensing CO2 and producing regular breathing at birth. However, electrophysiological characteristics of these Phox2b-expressing neurons (i.e., burst pattern and membrane potential) have not been examined in neonatal rodent immediately after birth. We have previously defined a parafacial respiratory group (pFRG) in the neonatal rat (which may correspond to the RTN) which consists of neurons with preinspiratory (Pre-I) discharges that may be involved in the primary respiratory rhythm generation (Onimaru and Homma, 2003). Thus, it is an important and interesting theme to clarify relations between the pFRG and Phox2b-positive cell cluster in the rostral ventrolateral medulla.
Here we show that Pre-I neurons in the parafacial region (pFRG/Pre-I) are indeed Phox2b-expressing neurons and intrinsically CO2 sensitive in neonatal rat. We found that most Phox2b-positive pFRG/Pre-I neurons depolarized with increases of CO2 concentration in the presence of tetrodotoxin (TTX). These results emphasize possible central roles of Pre-I neurons for responding to increased CO2 and generating respiratory rhythm during the postnatal period, and dysfunction of Pre-I neurons that may result in CCHS.
Materials and Methods
(For full description, see supplemental Methods, available at www.jneurosci.org as supplemental material.)
Preparations.
Experiments were performed with brainstem–spinal cord preparations from 0- to 2-d-old Wistar rats. The experimental protocols were approved by the Animal Research Committee of Showa University, which operates in accordance with Law No. 105 for the care and use of laboratory animals of the Japanese Government. Newborn rats were deeply anesthetized with ether in a 120 ml glass bottle until nociceptive reflexes induced by tail pinch were abolished. The brainstem and spinal cord were isolated according to methods described previously (Suzue, 1984; Onimaru and Homma, 1992). The preparation was superfused at a rate of 3.0 ml/min with the following artificial CSF (ACSF) (Suzue, 1984) (in mm): 124 NaCl, 5.0 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3, and 30 glucose, equilibrated with 95% O2 and 5% CO2, pH 7.4, at 26–27°C. Inspiratory activity corresponding to phrenic nerve activity was monitored from the fourth cervical ventral root (C4).
Whole-cell patch-clamp recordings.
Membrane potentials and input resistances of neurons in the parafacial region of the rostral medulla were recorded by a blind whole-cell patch-clamp method (Onimaru and Homma, 1992). In brief, we used three types of preparations for the whole-cell recordings for approaches from three directions: (1) an approach from the ventral surface for recording cells at various depths around the level of caudal end of the facial nucleus (n = 21); (2) an approach from the rostral cut surface for recording ventral superficial cells at the level of the rostral half of the facial nucleus (n = 10); and (3) an approach from the caudal cut surface for recording ventral superficial cells at the level of the caudal half of the facial nucleus (n = 22).
Experimental protocol.
After establishment of the whole-cell recordings, the standard solution (5% CO2, pH 7.4) was replaced by an ACSF containing 0.5 μm TTX (Sigma) equilibrated with 2% CO2 (pH 7.8). After a 15 min incubation with the 2% CO2 solution, the superfusate was replaced by a hypercapnic acidic ACSF equilibrated with 8% CO2 (pH 7.2) (Kawai et al., 2006). After a 5–6 min test of membrane potential responses in the 8% CO2 solution, the superfusate was returned to 2% CO2 solution.
Immunofluorescence.
For histologic analysis of the recorded cells, the electrode tips were filled with 0.5% Lucifer yellow (lithium salt; Sigma-Aldrich). After experiments, preparations were fixed for 2–3 h at 4°C in 4% paraformaldehyde in 0.1 m PBS, immersed in 18% sucrose-PBS overnight, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek), then frozen on dry ice, and cut into 30- or 50-μm-thick transverse sections, followed by immunofluorescence. The following primary antibodies were used for immunofluorescence: rabbit anti-Lucifer yellow (1:400 dilution, Molecular Probes/Invitrogen), guinea pig anti-Phox2b (1:1000 dilution) (see supplemental Methods, available at www.jneurosci.org as supplemental material), rabbit anti-tyrosine hydroxylase (TH) (1:500 dilution, Abcam), and rabbit anti-neurokinin-1 receptor (NK1R) (1:2000 dilution, BIOMOL International). The secondary antibodies for fluorescence staining (1:1000 dilution) were Alexa Fluor 488 anti-rabbit IgG or Alexa Fluor 546 anti-rabbit IgG (Molecular Probes/Invitrogen), and Alexa Fluor 633 anti-guinea pig IgG or Alexa Fluor 546 anti-guinea pig IgG. To identify motor neuron nuclei in the medulla, the sections were routinely stained with NeuroTrace (435/455 blue fluorescence, Invitrogen) for Nissl stain. Images of immunofluorescent samples were obtained with 20× or 40× objectives on an Olympus FV1000 confocal microscope (Olympus Optical) or conventional fluorescence microscope (BX60, Olympus Optical).
In situ hybridization.
Digoxigenin (DIG)-labeled riboprobe for in situ detection of vesicular glutamate transporter 2 (VGlut2) mRNA was prepared as reported (Yokota et al., 2007) using the plasmid containing VGlut2 cDNA as a template [kindly provided by Dr. Stornetta (University of Virginia Health System, Charlottesville, VA) (Stornetta et al., 2002)]. In situ hybridization was performed essentially as described previously on 30-μm-thick cryosections (Yokota et al., 2007). Signals were detected with an anti-Dig antibody conjugated to alkaline phosphatase (Roche) and NBT/BCIP (Roche) for chromogen, followed by immunofluorescence using anti-Phox2b and anti-TH antibodies.
Lucifer yellow-labeled neurons were reconstructed with the aid of a camera lucida attached to a fluorescence microscope (BX60; Olympus Optical). Neuronal burst rates and nerve activity (bursts/min) were calculated from the mean burst activity for 3–5 min. Values are shown as mean ± SD. Statistical significance of differences (p < 0.05) were determined by Student's t test.
Results
Distribution of Phox2b-expressing cells in the parafacial region of neonatal rat
We confirmed that distribution pattern of Phox2b-immunoreactive (-ir) cells in the rostral medulla to caudal pons of the newborn rat was comparable with those reported in fetus and newborn mouse (Brunet and Pattyn, 2002; Dubreuil et al., 2008); Phox2b expression in the area postrema, nucleus of the solitary tract, facial nucleus, nucleus ambiguous, dorsal motor nucleus of vagus, etc. (supplemental Fig. S1A, available at www.jneurosci.org as supplemental material). The general pattern was basically identical to that of adult rat, whereas expression in the facial nucleus was absent in adult rat (Stornetta et al., 2006; Kang et al., 2007). Phox2b-expressing cells in the parafacial region of the rostral medulla are implicated in sensing CO2 to affect respiratory rhythm generation in adult rat (Stornetta et al., 2006). To explore the relationship between Phox2b-expressing cells and Pre-I neurons that are identified in the rat neonate, it is indispensable to examine the distribution of Phox2b-expressing cells in the neonatal rat. We analyzed the detailed distribution of Phox2b-expressing cells in the parafacial region of the neonatal rat (5 preparations). They were localized most densely in the ventrolateral medulla around the caudal end of the facial nucleus (Fig. 1Ac,Ad). In the more rostral medulla, Phox2b-expressing cells are found in the superficial area just ventrally to the facial nucleus (Fig. 1Af–Ah). In the level of the most rostral medulla, close to the rostral end of the facial nucleus, they formed one of highest density clusters in the region ventrolateral to the facial nucleus (Fig. 1Ai,Aj). We also confirmed that Phox2b-expressing cells (at least partly) in the parafacial region were also VGlut2 mRNA positive (5 preparations) (supplemental Fig. S1B, available at www.jneurosci.org as supplemental material), as reported in neonatal mouse (Dubreuil et al., 2008) and adult rat (Stornetta et al., 2006). Moreover, we examined NK1R expression in cells of the parafacial region, because these cells are responsible for CO2 sensitivity (Nattie and Li, 2002; Takakura et al., 2008) and Phox2b-expressing cells also express NK1R in the neonatal mouse (Dubreuil et al., 2008). We confirmed that Phox2b-ir cells in the parafacial region strongly expressed NK1R (5 preparations) (supplemental Fig. S1B). The distribution of Phox2b-expressing cells in the parafacial region of the neonatal rat is basically consistent with that in the adult rat (Stornetta et al., 2006; Kang et al., 2007).
Figure 1.
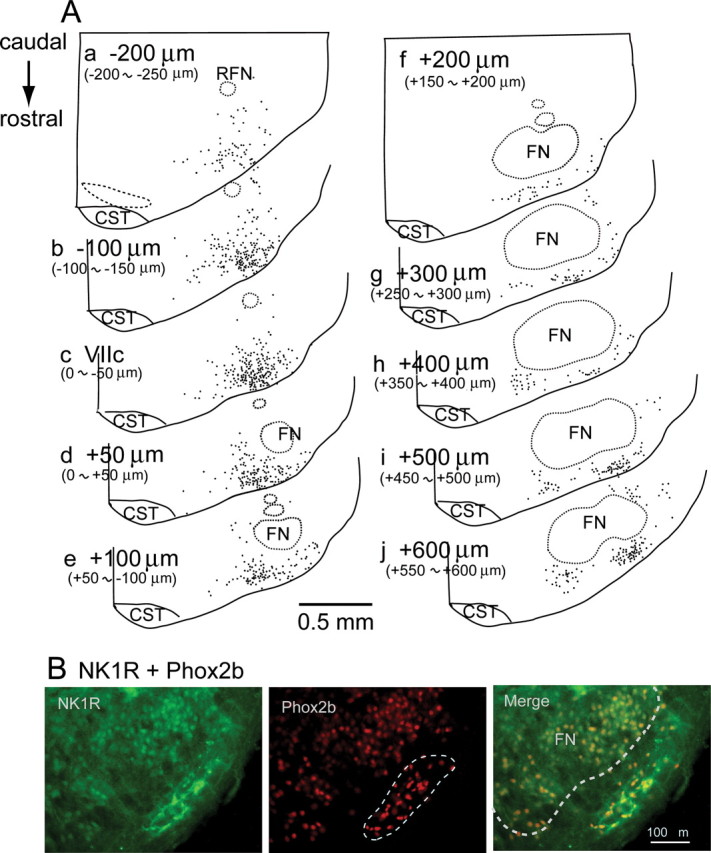
Phox2b-immunoreactive (-ir) cells and NK1R expression in the parafacial region of neonatal rat medulla. A, Distribution Phox2b-ir cells. Each dot represents a single Phox2b-ir nucleus plotted on the 50-μm-thick coronal section. Phox2b-ir cells in the facial and retrofacial nuclei were not plotted. Panels were arranged in a direction corresponding to the most caudal (upper left) to most rostral (lower right) sections. Values denote distance from the level of the caudal end of facial nucleus (VIIc) (Ruangkittisakul et al., 2006). B, Phox2b immunoreactivity and NK1R expression in the most rostral medulla, corresponding to i or j in A. NK1R expression (left), Phox2b immunoreactivity (middle) and merge of both (right). Dotted line in middle panel denotes a rostral cluster of Phox2b-immunoreactive cells which also express NK1R (right column). FN, Facial nucleus; RFN, retrofacial nucleus; CST, corticospinal tract.
Phox2b immunoreactivity and CO2 sensitivity of preinspiratory neurons
To investigate whether Pre-I neurons express Phox2b, we performed whole-cell patch clamp recordings from 34 Pre-I neurons. The resting potential was −47.5 ± 3.6 mV and input resistance was 649 ± 241 MΩ in the standard solution (5% CO2). They were marked by Lucifer yellow in the rostral ventrolateral medulla followed by Phox2b immunostaining and were confirmed to not be facial motor neurons by Nissl stain (see Materials and Methods). We found that 82% of Pre-I neurons were Phox2b-ir. These Phox2b-ir Pre-I neurons (n = 28) were localized in the area of the Phox2b-expressing cell cluster in the parafacial region (Fig. 2; supplemental Fig. S2, available at www.jneurosci.org as supplemental material). These neurons were depolarized in response to hypercapnia (2%→8%) in the presence of TTX (Fig. 2B). The average membrane potential change was +7.8 ± 6.1 mV (p < 0.001) compared with resting membrane potentials (−47.3 ± 3.9 mV) in 2% CO2, accompanied with an increase in input resistance (580 ± 309 MΩ in 2% CO2 vs 728 ± 389 MΩ in 8% CO2, p < 0.001). Three Phox2b-ir Pre-I neurons were further tested for NK1R expression, and they were NK1R positive (supplemental Fig. S2B, available at www.jneurosci.org as supplemental material). In contrast, Phox2b-negative Pre-I neurons (n = 6) were localized caudally to the caudal end of the facial nucleus level and dorsally to Phox2b-positive cell cluster (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Their averaged resting membrane potentials and input resistances were −45.3 ± 3.7 mV and 460 ± 312 MΩ in 2% CO2. They did not show any significant membrane potential change in response to hypercapnia in the presence of TTX (supplemental Fig. S3B, available at www.jneurosci.org as supplemental material). The input resistance in standard solution (452 ± 292 MΩ) was significantly less than that of Phox2b-ir Pre-I neurons (691 ± 211 MΩ, p < 0.05). C1 adrenergic neurons reside around the caudal end of the facial nucleus and some of them are Phox2b positive (Kang et al., 2007; Takakura et al., 2008). Therefore, some Pre-I neurons (4 Phox2b positive and 2 Phox2b negative) were further examined for TH immunoreactivity, and they were TH negative, i.e., not C1 adrenergic neurons (supplemental Fig. S4, available at www.jneurosci.org as supplemental material).
Figure 2.
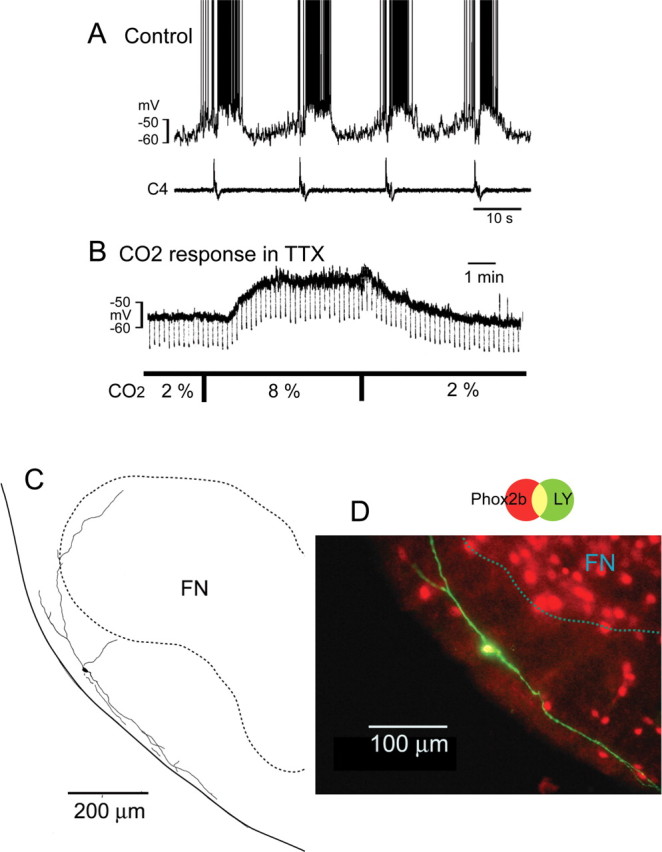
A Phox2b-immunoreactive Pre-I neuron in parafacial region. The neuron was recorded at the level of 400 μm rostral to the caudal end of facial nucleus. A, Membrane potential trajectory and C4 inspiratory activity. B, Membrane potential change in response to hypercapnia in the presence of 0.5 μm TTX. CO2 concentration was changed from 2% to 8%. Square current pulse (500 ms, 0.1 Hz, 20 pA) was applied to monitor change of input resistance. Negative deflections of the baseline membrane potential are proportional to input resistance. Note that application of 8% CO2 induced membrane depolarization and increase of input resistance. C, Camera lucida drawing of the neuron. D, Phox2b immunoreactivity of this neuron. The neuron labeled with Lucifer yellow (green) in the electrode solution showed Phox2b immunoreactivity (red). FN, facial nucleus.
Phox2b immunoreactivity and CO2 sensitivity of tonic firing neurons
In the parafacial region, tonic firing neurons are also frequently encountered. We recorded 18 tonic firing neurons of which membrane potential was −41.5 ± 3.8 mV and input resistance was 698 ± 162 MΩ in the standard solution (5% CO2). Firing of these tonic neurons was modulated with respiratory activity; typically with tendency of higher firing frequency during preinspiratory phase (Fig. 3). Twelve of 18 tonic neurons were Phox2b-ir (Fig. 3D) and, except for two, depolarized with hypercapnia. The average membrane potential change was +8.5 ± 8.3 mV (p < 0.01) compared with resting membrane potentials (−44.6 ± 3.0 mV) in 2% CO2, accompanied with an increase in input resistance (482 ± 114 MΩ in 2% CO2 vs 689 ± 262 MΩ in 8% CO2, p < 0.05). Six tonic neurons were Phox2b negative and did not show significant depolarization to hypercapnia. Their averaged resting membrane potentials and input resistances were −43.5 ± 1.8 mV and 580 ± 344 MΩ in 2% CO2.
Figure 3.
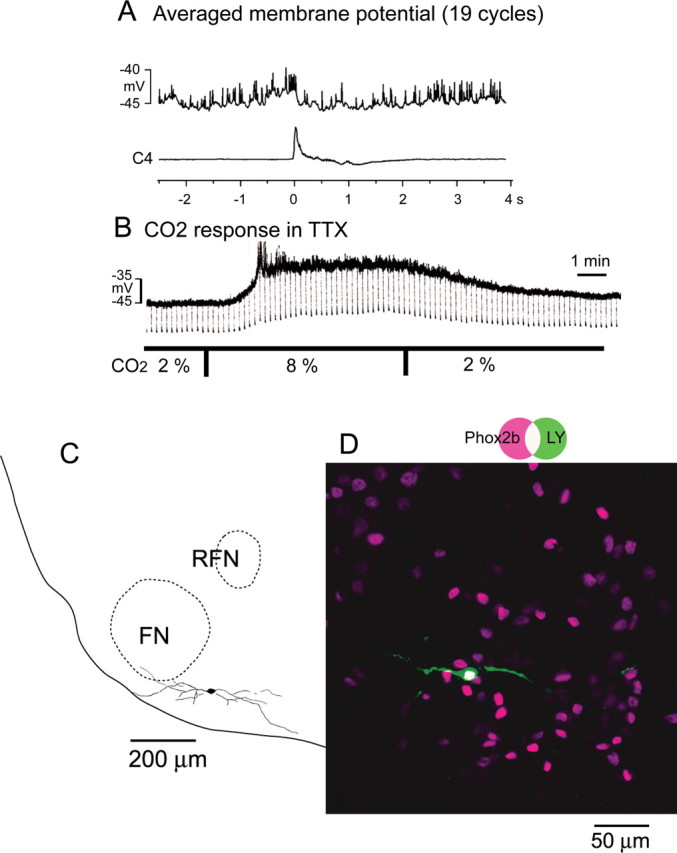
A Phox2b-immunoreactive tonic firing neuron in the parafacial region. The neuron was recorded at the level of 150 μm rostral to the caudal end of facial nucleus. A, Averaged membrane potential (19 cycles) triggered by the C4 activity. Note modulation of the membrane potential showing slight depolarization during the preinspiratory phase. B, Membrane potential change in response to hypercapnia in the presence of 0.5 μm TTX. CO2 concentration was changed from 2% to 8%. Square current pulse (500 ms, 0.1 Hz, 20 pA) was applied to monitor the change of input resistance. Negative deflections of the baseline membrane potential are proportional to input resistance. Note that application of 8% CO2 induced membrane depolarization and increase of input resistance. C, Camera lucida drawing of the neuron. D, Phox2b immunoreactivity of this neuron. The neuron labeled with Lucifer yellow (green) showed Phox2b immunoreactivity (magenta). FN, Facial nucleus; RFN, retrofacial nucleus.
Distribution of recorded neurons
Figure 4 shows plots of recorded neurons (Pre-I and tonic) in the parafacial region. Most Pre-I and many tonic neurons in the ventral medulla superficial region that overlaps the region of the Phox2b-expressing cell cluster (Fig. 1A) were Phox2b positive, whereas some tonic neurons (23%) were Phox2b negative. It is notable that we did not find Phox2b-negative Pre-I neuron in the ventral superficial region in the rostral medulla. In contrast, Phox2b-negative Pre-I neurons were localized more dorsally to Phox2b-ir neurons. Many of Phox2b-expressing superficial cells have dendrites extending predominantly in the mediolateral direction close to the ventral surface (Fig. 2C).
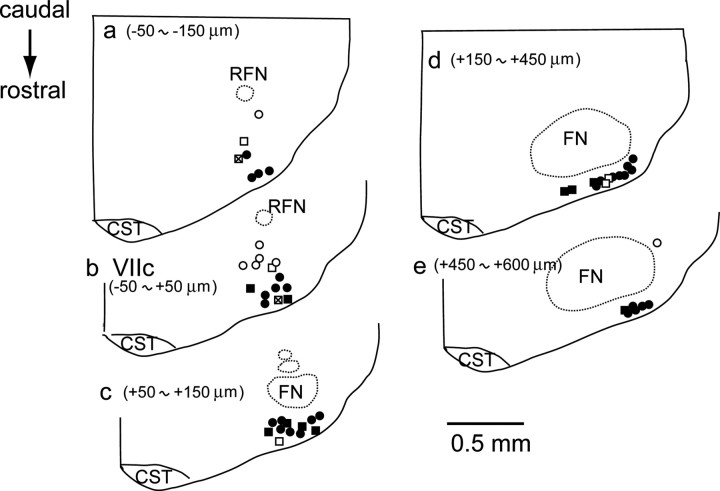
Figure 4.
Distribution of recorded neurons in the parafacial region. Location of neurons are plotted on 50 μm coronal sections. Values denote distance from level of the caudal end of facial nucleus (VIIc). Circles, Preinspiratory neurons; squares, tonic firing neurons; solid, Phox2b immunoreactive and CO2 sensitive; cross, Phox2b immunoreactive and CO2 insensitive; open, Phox2b negative and CO2 insensitive; FN, facial nucleus; RFN, retrofacial nucleus; CST, corticospinal tract.
Discussion
Characteristics of parafacial neurons in the ventral medulla
We found that the Pre-I neurons in the parafacial region of the rostral medulla were Phox2b-ir and postsynaptically sensitive to CO2 concentration changes in an action potential-independent manner. Especially, all of the Pre-I neurons in the ventral superficial region of the rostral medulla were Phox2b-ir neurons. In contrast, Phox2b-negative Pre-I neurons that were CO2 insensitive were localized dorsally to Phox2b-ir cell cluster at the level of the caudal end of the facial nucleus. The existence of such heterogeneous subpopulations with regard to CO2 responsiveness has been reported in a previous study (Kawai et al., 2006) where 36% of the Pre-I neurons that were recorded in the caudal part of the pFRG fully retained the depolarizing response to hypercapnia in the presence of TTX. Consequently, they might be Phox2b-ir cells. A recent study (Fortuna et al., 2008) reported that a population of rostral ventral respiratory neurons (i.e., glycinergic expiratory augmenting neurons) develops a preinspiratory discharge during hypercapnic hypoxia in adult rat in vivo preparations. Despite differences between experimental conditions, these results also support existence of heterogeneous subpopulations of Pre-I neurons in the rostral medulla around the caudal end of the facial nucleus. They may be glycinergic, GABAergic, or adrenergic, and detailed characterization remains to be done. We showed that Pre-I neurons recorded in the caudal part of pFRG were not adrenergic regardless of whether they were Phox2 positive or negative.
In the parafacial region, Phox2b-ir tonic neurons were also found and they were CO2 sensitive. Their firing pattern showed respiratory modulation, typically with higher firing frequency during preinspiratory phase, and was thus reminiscent of the firing pattern of Pre-I neurons. This firing pattern of tonic neurons is similar to that observed in the RTN of the adult rat in vivo (Guyenet et al., 2005; Stornetta et al., 2006). In the latter study, neurons with typical neonatal Pre-I firing patterns were not reported, probably due to differences in the experimental conditions and developmental stage. Indeed, neurons with firing patterns similar to neonatal Pre-I neurons have been reported in the parafacial region of adult cat preparations (Connelly et al., 1990). Recent findings in the adult rat and neonatal mouse suggest that the Phox2b-ir cluster in the rostral ventrolateral medulla is composed of glutamatergic neurons (Weston et al., 2003; Stornetta et al., 2006) and expresses NK1R (Dubreuil et al., 2008; Takakura et al., 2008) which is identical to the RTN (Guyenet et al., 2008). We showed that Phox2b-ir neurons in the parafacial region of the neonatal rat consist mainly of Pre-I neurons and tonic firing neurons, and that they express NK1R and are presumed to be glutamatergic, so that the pFRG closely overlaps the RTN.
Phox2b-ir neurons in the pFRG/RTN region have been reported to be intrinsically CO2 sensitive (Mulkey et al., 2004; Guyenet et al., 2005; Stornetta et al., 2006; Mulkey et al., 2007). We confirmed these previous findings in the present study. It is notable that our findings that hypercapnia depolarizes these neurons in the presence of TTX which blocks action-potential-dependent synaptic transmission were done using for Phox2b-ir neurons of which burst pattern was identified in the brainstem–spinal cord preparation which preserved intact respiratory networks as opposed to slice preparation. The depolarization was accompanied with an increase in input resistance of parafacial neurons, consistent with an involvement of potassium channels in response to hypercapnia as suggested in previous studies (Kawai et al., 2006; Guyenet et al., 2008). Our findings further support a strong link between CO2 sensitivity and expression of the Phox2b protein.
Functional considerations of parafacial neurons
Dubreuil et al. (2008) developed an animal (mouse) model of CCHS. This mouse has heterozygous mutations of the PHOX2B transcription factor—expansions of polyalanine tracts that correspond to mutations most frequently observed in human CCHS patients. This mouse does not respond to an increase in CO2 concentrations, has an irregular and slowed-down breathing pattern and dies soon after birth from central apnea. Moreover, the mutant mouse lacks in particular a population of glutamatergic (and NK1R positive) Phox2b-expressing neurons in the parafacial region. Therefore, the pFRG/Pre-I neurons as well as tonic neurons are probably missing in this mutant mouse. Thus, our findings suggest that respiratory disorder in this mutant mouse is due to direct effect of the lack of parafacial neurons on respiratory rhythm generation and problems in central chemoreception, although the present results indicate that it is difficult to separate both factors of the rhythm generation and chemoreception.
The pFRG/Pre-I neurons are hypothesized to trigger onset of bursting in the preBötzinger Complex (preBötC) that is an inspiratory center located in more caudal medulla (Smith et al., 1991; Mellen et al., 2003; Onimaru and Homma, 2003; Feldman and Del Negro, 2006). Recent studies also suggest functional coupling between subgroups of Pre-I neurons and expiratory neuronal activity (Janczewski et al., 2002; Onimaru et al., 2006). Our previous study in Atp1a2 knock-out mice shows that decreased functional connection between the pFRG and preBötC induced abnormal respiratory rhythm generation (Ikeda et al., 2004; Onimaru et al., 2007). Findings from the present and above-mentioned previous studies support that coupling between the pFRG and preBötC is important in the generation of regular respiratory rhythm (Mellen et al., 2003) and that the pFRG is necessary for postnatal survival because of its' involvement in primary rhythm generation and central chemoreception. These findings may provide an important key to understanding associated with the neuronal mechanisms of the onset of breathing after birth, as well as of respiratory disorders in CCHS.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology. We thank Drs. Ruth L. Stornetta and Shigefumi Yokota for providing the plasmid.
References
- Amiel J, Laudier B, Attié-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Blanchi B, Sieweke MH. Mutations of brainstem transcription factors and central respiratory disorders. Trends Mol Med. 2005;11:23–30. doi: 10.1016/j.molmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol. 1990;258:L33–L44. doi: 10.1152/ajplung.1990.258.2.L33. [DOI] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Onimaru H, Yamada J, Inoue K, Ueno S, Onaka T, Toyoda H, Arata A, Ishikawa TO, Taketo MM, Fukuda A, Kawakami K. Malfunction of respiratory-related neuronal activity in Na+, K+-ATPase α2 subunit-deficient mice is attributable to abnormal Cl− homeostasis in brainstem neurons. J Neurosci. 2004;24:10693–10701. doi: 10.1523/JNEUROSCI.2909-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li AH, St John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflugers Arch. 1992;420:399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Defective interaction between dual oscillators for respiratory rhythm generation in Na+,K+-ATPase α2 subunit-deficient mice. J Physiol. 2007;584:271–284. doi: 10.1113/jphysiol.2007.136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Marazita ML. In pursuit (and discovery) of a genetic basis for congenital central hypoventilation syndrome. Respir Physiol Neurobiol. 2005;149:73–82. doi: 10.1016/j.resp.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol. 2003;460:525–541. doi: 10.1002/cne.10663. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]