Abstract
Two families of cell-adhesion molecules, predominantly presynaptic neurexins and postsynaptic neuroligins, are important for the formation and functioning of synapses in the brain, and mutations in several genes encoding these transmembrane proteins have been found in autism patients. However, very little is known about how neurexins are targeted to synapses and which mechanisms regulate this process. Using various epitope-tagged neurexins in primary hippocampal neurons of wild-type and knock-out mice in vitro and in transgenic animals in vivo, we show that neurexins are trafficked throughout neurons via transport vesicles and the plasma membrane insertion of neurexins occurs preferentially in the axonal/synaptic compartment. We also observed that exit of neurexins from the ER/Golgi and correct targeting require their PDZ-binding motif at the C terminus, whereas two presumptive ER retention signals are inactive. The ubiquitous presence of neurexin-positive transport vesicles and absence of bassoon colabeling demonstrate that these carriers are not active zone precursor vesicles, but colocalization with CASK, RIM1α, and calcium channels suggests that they may carry additional components of the exocytotic machinery. Our data indicate that neurexins are delivered to synapses by a polarized and regulated targeting process that involves PDZ-domain mediated interactions, suggesting a novel pathway for the distribution of neurexins and other synaptic proteins.
Keywords: secretory pathway, PDZ domain, neuroligin, autism, synaptic plasticity, neurotransmission
Introduction
Alignment and organization of presynaptic and postsynaptic compartments are essential for the formation of synapses. Due to the asymmetry of synapses, heterophilic complexes formed by cell-adhesion molecules are candidates to mediate contact formation and differentiation (Scheiffele, 2003; Yamagata et al., 2003; Piechotta et al., 2006) but the processes that target polarized proteins are not fully understood (Horton and Ehlers, 2003). Evidence is accumulating, however, that targeting to either side of the synapse involves different pathways (Zhai et al., 2001; Sampo et al., 2003), sequence motifs (Standley et al., 2000; Cheng et al., 2002), and protein–protein interactions (Wenthold et al., 2003; Kim and Sheng, 2004).
One synaptic adhesion complex consists of neurexin (Nrxn) and neuroligins (Fairless et al., 2006; Craig and Kang, 2007). This heterophilic complex is important because Nrxns and neuroligins are required for synaptic transmission (Missler et al., 2003; Varoqueaux et al., 2006), and are involved in synapse formation (Scheiffele et al., 2000; Dean et al., 2003; Graf et al., 2004; Dudanova et al., 2007). The interdependence of their roles in synaptic function (neurotransmission/release) and in synaptic structure (contact formation/stabilization) is not clarified but these effects appear evolutionarily conserved (Li et al., 2007; Zeng et al., 2007), and both molecules represent candidate genes for autism (Jamain et al., 2003; Szatmari et al., 2007). Therefore, understanding their targeting to presynaptic and postsynaptic compartments appears mandatory. Nrxns exist and function predominantly at the presynaptic terminal (Ushkaryov et al., 1992; Dean et al., 2003; Missler et al., 2003; Graf et al., 2004; Dudanova et al., 2006; Berninghausen et al., 2007; Li et al., 2007; Taniguchi et al., 2007), although a small but relevant population at the postsynapse is possible (Kattenstroth et al., 2004; Taniguchi et al., 2007). Neuroligins are present almost exclusively at the postsynaptic compartment (Ichtchenko et al., 1995; Song et al., 1999; Scheiffele et al., 2000; Varoqueaux et al., 2006; Berninghausen et al., 2007). However, while insight into somatodendritic targeting of neuroligin is available (Dresbach et al., 2004; Rosales et al., 2005), little is known about the trafficking of Nrxns.
Three Nrxn genes give rise to two major isoforms called α- and β-Nrxns that differ in length and composition of their extracellular domains but possess identical intracellular sequences, including a C-terminal PDZ-recognition motif (Missler and Südhof, 1998). Through this motif, Nrxns can interact with PDZ proteins, such as CASK (Hata et al., 1996), Mint1 (Biederer and Südhof, 2000), syntenin (Grootjans et al., 2000), and CIPP (Kurschner et al., 1998).
We examined the intracellular trafficking of tagged Nrxns in culture and in transgenic mice. Our results show that Nrxns are distributed via transport vesicles throughout the cell, but insertion into the plasma membrane occurs primarily in the axonal/synaptic compartment. In addition, we demonstrate that interaction through their C-terminal PDZ-recognition motif is an essential step for targeting of Nrxns to synapses.
Materials and Methods
Antibodies.
Primary antibodies for immunocytochemistry were as follows: rabbit anti-GAD65 (1:500, Millipore Bioscience Research Reagents), rabbit anti-vGlut1 and anti-vGat (1:1000; Synaptic Systems), mouse anti-bassoon (1:400; Stressgen), mouse anti-MAP2 (1:400; Sternberger), mouse anti-GFP (1:500; Covance), rabbit anti-pan-synapsin E028 (1:500; courtesy of T. C. Südhof, Center for Basic Neuroscience, University of Texas Southwestern Medical Center, Dallas, TX), mouse anti-myc 9E10 (1:500; Sigma-Aldrich), mouse anti-RIM (1:500; BD Transduction Laboratories), mouse anti-HA (1:500; Roche), and rabbit anti-Mint1 P932 (1:500; courtesy of T. C. Südhof). Secondary antibodies used were Cy3-conjugated goat anti-mouse and goat anti-rabbit antibodies (1:500; Invitrogen and Sigma), goat anti-mouse Alexa568 (1:500; Invitrogen), and goat anti-rabbit Oyster647 (1:500; Luminartis). Secondary antibodies used for Western blots were HRP-conjugated goat anti-mouse or goat anti-rabbit antibodies (1:5000; Bio-Rad).
Expression constructs.
EGFP-tagged Nrxns were generated by amplifying the EGFP coding sequence from pEGFP-C1 (Clontech) using primers which inserted an NheI overhang at both ends. The product was inserted into NheI restriction sites engineered into four different positions of pCMV-L2 or pCMV-L13 (Ushkaryov et al., 1992) as described in the Results section, using QuikChange XL Site-Directed Mutagenesis Kit (Stratagene). All Nrxn deletion constructs were derived from the N-terminally tagged pCMV-EGFP-Nrxn1α construct (Ν-EGFP-Nrxn1α, carrying the tag after the signal sequence), by subcloning into a modified pBluescript SK for manipulation. Deletion of extracellular domains was performed by digesting with MluI and SalI, removing amino acids 49–1309, followed by blunt ending and religation, and then returned to pCMV (ΔEC). C-terminal deletion constructs of Nrxn were generated by subcloning a small fragment of DNA containing the C terminus. Expand Long Template PCR (Roche Applied Science) was then used to amplify this region, using appropriately designed reverse primers to introduce mutations, consisting of either an introduced stop codon (for Δ3 and Δ10), or replacement of several nucleotides (for Δ55 + 10). This resulted in the generation of the following constructs that are all based on Ν-EGFP-Nrxn1α: Δ3, removing amino acids 1477–1479; Δ10, removing amino acids 1470–1479; and Δ55 + 10, removing amino acids 1427–1469. Site-directed mutagenesis of these constructs was then used to create more mutants for the PDZ recognition and putative ER retention motifs as described in the text.
Cell culture.
Primary hippocampal neurons were prepared and cultured as described previously (Dresbach et al., 2003). Briefly, neurons were dissociated from the hippocampi of newborn control (wild-type or single Nrxn 2α KO) and triple α-Nrxn knock-out mice, and plated at a density of 10,000 cells per 10 mm2 coverslip. Neurons were maintained at 37°C with 5% CO2 in MEM (Invitrogen) supplemented with glucose (5 mg/ml), NaHCO3 (0.2 mg/ml), transferrin (0.1 mg/ml), FBS (5%), l-glutamine (0.5 mm), and B27 (2%). To study the targeting of Nrxns, transfection was performed on day 3 after plating using a calcium phosphate method adapted for neurons (Dresbach et al., 2003). Cells were then maintained for a further 3 d before fixation.
tsA201 cells (a derivative of the HEK293 line) were grown at 37°C with 5% CO2 in DMEM supplemented with 10% fetal bovine serum and penicillin-streptomycin. One day before transfection, tsA201 cells were plated onto 16 mm coverslips and then transfected with 3 μg of the appropriate constructs using a standard calcium phosphate protocol. 12 h after transfection, cells were provided with fresh medium and maintained at 37°C for a further 24 h.
Labeling of transfected cells and light microscopy.
Cultured neurons and heterologous cells were fixed using 4% paraformaldehyde in phosphate buffer for 10 min. Permeabilization was performed using 0.3% Triton X-100 for 15 min. Primary and secondary antibodies were applied for 1–24 h at appropriate dilutions. Cell surface staining was performed by incubating nonpermeabilized cells with primary antibodies for 1 h at 37°C before fixation. Between each step, coverslips were washed several times in PBS, and mounted using Vectashield mounting medium (Vector Laboratories) or fluorescent mounting medium (Dako).
Light Microscopic analysis was performed on an Axioscope 2 epifluorescent microscope (Zeiss), using 63× and 100× Plan Apo NA 1.4 oil-immersion objectives. Images were captured with an AxioCam digital camera and processed using AxioVision software (Zeiss). Confocal images were captured as single stack-pictures on a Leica SP2 laser-scanning microscope (Leica), using a 63× Plan Apo CS 1.4 oil-immersion objective. Surface expression of extracellularly EGFP-tagged constructs was quantified using ImageJ analysis software (NIH), following surface labeling with an anti-GFP antibody. Briefly, five random areas per cell of a set unit size were measured for fluorescence intensity and an average background reading was subtracted. Experiments were repeated at least in triplicate. For comparison of different cells and constructs, similarly exposed images were used.
Nrxn expression in transgenic mice.
Several transgenic mouse lines expressing either Nrxn1α or Nrxn1β with an internal horseradish peroxidase (HRP) epitope tag were characterized previously (Zhang et al., 2005). Deeply anesthetized animals were perfusion-fixed with freshly prepared 4% paraformaldehyde in 0.1 m phosphate buffer, postfixed for 2 h in the same solution, and cryoprotected in 25% sucrose. For light microscopy of adult mice, 30 μm free-floating cryosections were labeled overnight with anti-HRPaff and other primary antibodies in blocking buffer (0.1% Triton X-100, 50% normal goat serum, PBS), followed by appropriate fluorescent secondary antibodies. For electron microscopic immunohistochemistry of HRP-Nrxns, 100 μm vibratome sections were labeled with HRP antibodies overnight with anti-HRPaff antibodies in blocking buffer (0.1% Triton X-100, 50% normal goat serum, PBS). Secondary goat anti-rabbit antibody and PAP antibodies (rabbit PAP) were incubated with sections in blocking solution (both antibodies from Sternberger), and visualized with diaminobenzidine (DAB, 0.05% w/v) plus H2O2 (0.005% v/v) and heavy metal enhancement (NiCl2, 0.15% w/v). After DAB staining, the samples were postfixed overnight with 4% paraformaldehyde/1% glutaraldehyde in 0.1 m phosphate buffer. Osmicated sections containing the hippocampal areas were flat-embedded in epoxy resin following standard techniques, and ultramicrotome sections with uranyl acetate/lead citrate contrast were analyzed on a transmission electron microscope EM10 (Zeiss).
Results
Polarized targeting of neurexins to the synapse
The discovery of neurexin (Nrxn) as the α-latrotoxin receptor (Ushkaryov et al., 1992) and impairment of neurotransmitter release in α-Nrxn null-mutant mice (Missler et al., 2003) suggested a predominantly presynaptic localization for these molecules. However, our existing antisera against endogenous neurexins were not suitable to study their subcellular distribution in detail because (1) they could not discriminate between α- and β-Nrxn, and (2) they failed to work in immunoelectron microscopy. More recently, a chicken antiserum produced by Peter Scheiffele's group convincingly demonstrated a presynaptic localization by immunogold EM techniques at least in young animals (P7–P8) but still did not allow them to distinguish between Nrxn isoforms (Dean et al., 2003; Taniguchi et al., 2007). Such a differentiation is necessary, since a small but functional postsynaptic (sub)population of Nrxn has been proposed (Kattenstroth et al., 2004; Taniguchi et al., 2007), and α- and β-Nrxns are not functionally redundant (Zhang et al., 2005). Thus, no antibodies against specific endogenous Nrxn variants are currently available to comprehensively analyze their trafficking to and role at presynaptic and/or postsynaptic compartments.
To detect and monitor the expression and targeting of a defined isoform/splice variant, we first generated a full-length recombinant Nrxn1α with an N-terminal EGFP tag positioned just after the signal peptide. Young neuronal cultures were transfected at 3–5 DIV and analyzed at 6–12 DIV because we were interested in the early events of Nrxn trafficking to the synapse before extensive formation of contacts had occurred. Upon expression of the tagged Nrxn construct in hippocampal neurons, numerous intracellular pools of Nrxn1α became apparent in punctate-like clusters by light microscopy (Fig. 1A–F). Surprisingly, with respect to its presumed presynaptic localization (Ushkaryov et al., 1992; Taniguchi et al., 2007) and function (Missler et al., 2003), N-EGFP-Nrxn1α was seen along the lengths of both axons (Fig. 1B) and dendrites (Fig. 1C). In addition to the processes, Nrxn was present within large somatic clusters (Fig. 1A, open arrowhead), presumably corresponding to the endoplasmic reticulum (ER)/Golgi complex. This ubiquitous occurrence of Nrxn-positive puncta was subsequently confirmed by quantifying their density in the somata, proximal and distal dendrites, and distal axons (Fig. 1J). To brace against the possibility that such a distribution pattern was an artifact of overexpression, we next repeated the experiment in neurons lacking all endogenous α-Nrxns: a quantitative comparison of transfected neurons taken from either control or triple α-neurexin knock-out (TKO) mice (Missler et al., 2003), did not show any variation in terms of the distribution and the number of Nrxn-positive puncta (Fig. 1J). Due to the difference in the endogenous Nrxn levels between control and TKO cultures, this suggests that the distribution pattern of tagged Nrxn1α was not significantly influenced by the level of Nrxn expressed. Also, overexpression of other synaptic markers, such as GFP-tagged neuroligin, synaptotagmin, or bassoon, did not result in such ubiquitously distributed intracellular structures (data not shown).
Figure 1.
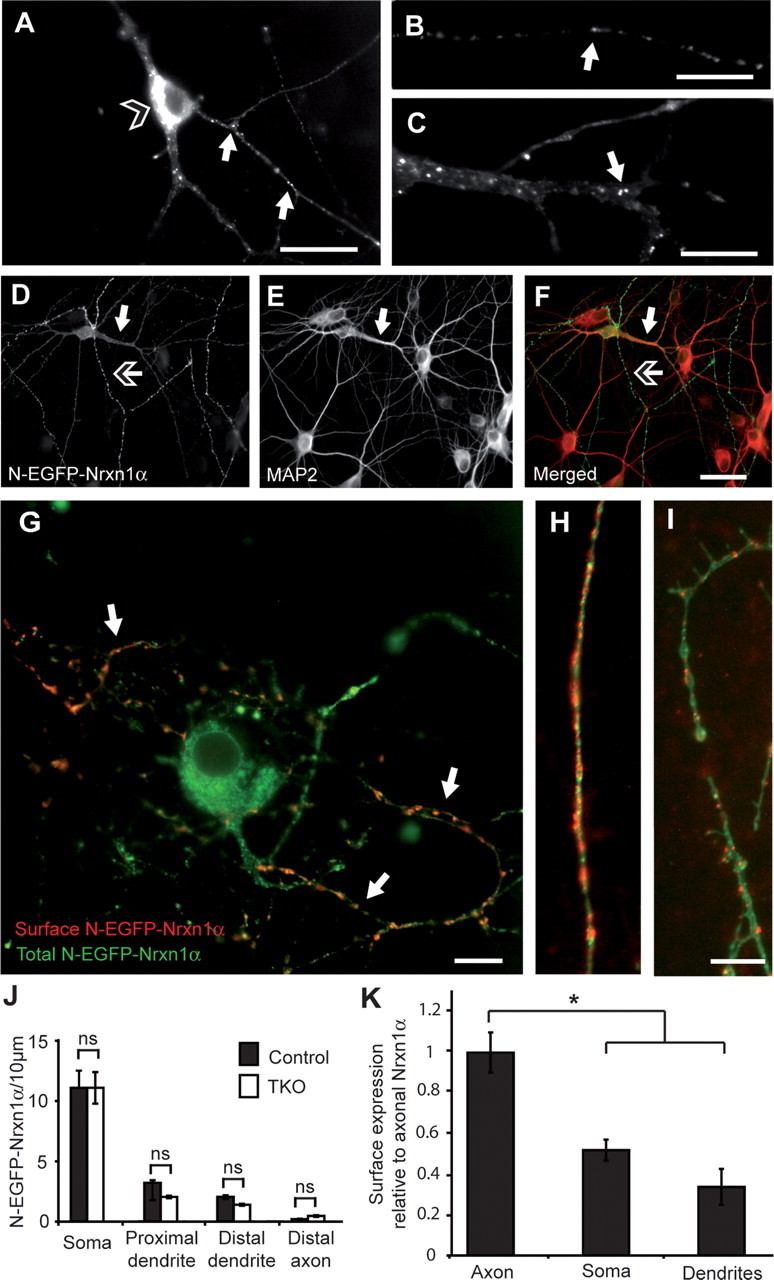
Epitope-tagged Nrxn1α occurs intracellularly and at the axonal surface of primary hippocampal neurons. A–C, N-EGFP-Nrxn1α is visualized by its autofluorescence, and is present in intracellular pools within the soma (open-headed arrow, A) and in puncta (white arrows) along axons (B) and dendrites (C). D–F, Colabeling of MAP2 (E, and red in F) to distinguish tagged Nrxn (D, and green in F) in dendrites (white arrow) and in axons (open-headed arrow). G–I, Colabeling of the surface population of EGFP-Nrxn1α (red). Surface Nrxn is mostly present at the plasma membrane of axons (H, G; white arrows) and less at soma (G) or dendrites (I). J, K, Quantification of total expression (J) and surface labeling (K) of Nrxn1α in different compartments (n = 4 transfected cultures); *p < 0.05. Scale bars: A, F, 40 μm; B, C, G, I, 20 μm.
To determine whether and at which sites N-EGFP-Nrxn1α was transported to the plasma membrane, we applied an antibody against the extracellular EGFP tag to living, nonpermeabilized cells that selectively labeled the cell surface population of this Nrxn (Fig. 1G–I). Interestingly, even though intracellular Nrxn-positive puncta could be seen along both axons and dendrites, its membrane insertion occurred primarily in the axon (Fig. 1G,H), whereas much less insertion was found in the dendrite (Fig. 1G,I). Quantification of area densities demonstrated that axonal insertion was at least twofold to threefold higher than insertion in the somatic and dendritic membranes (Fig. 1K). Thus, despite the presence of a large, ubiquitously distributed intracellular pool of Nrxn1α, it was preferentially presented only at the axonal surface, constituting a case of polarized targeting.
Since our tagged Nrxn1α was successfully transported to the axonal plasma membrane, we next investigated whether it also localized to synapses in vitro. Counterstaining transfected neurons with antibodies against bassoon (Fig. 2A–C) or synapsin (Fig. 2D–I) did not colabel the majority of Nrxn-positive puncta, resulting in a low degree of colocalization (Fig. 2V). These data suggest that most Nrxn-positive puncta were nonsynaptic, and probably represented transport vesicles, consistent with their presumed intracellular distribution (Fig. 1A–F). Furthermore, the bassoon-negative nature of these vesicles indicates that Nrxn is probably transported via a different mechanism to that characterized for the bassoon-piccolo complex (Zhai et al., 2001), instead involving an independent pathway. In addition to these potentially novel transport vesicles, some Nrxn-positive puncta did cluster at synaptic sites, particularly when Nrxn-positive axons were presented with sufficiently large postsynaptic contact sites. An example is shown in Figure 2J–L, where a Nrxn-positive axon lies across a neighboring soma, resulting in a strong colocalization with synapsin. Therefore, the majority of Nrxn-positive clusters are nonsynaptic, though N-EGFP-Nrxn1α does have the ability to be recruited to appropriate synaptic contact sites. To enhance the synaptic locations to which Nrxn1α might be recruited, overexpression of neuroligin 1, shown before to be a potent inducer of presynaptic clustering (Scheiffele et al., 2000; Dean et al., 2003), was performed in conjunction with Nrxn1α transfection. By identifying Nrxn1α-expressing axons in proximity to neuroligin 1-expressing dendrites (visualized by antibodies against a C-terminal myc tag), enlarged aggregates of Nrxn1α were observed (Fig. 2M–O). Thus, in line with previous studies our N-EGFP-Nrxn1α could be recruited to these neuroligin 1-positive contacts. The synaptic nature of these clusters was confirmed by counterstaining with synapsin (Fig. 2P–U), and colocalization increased about twofold (Fig. 2V).
Figure 2.
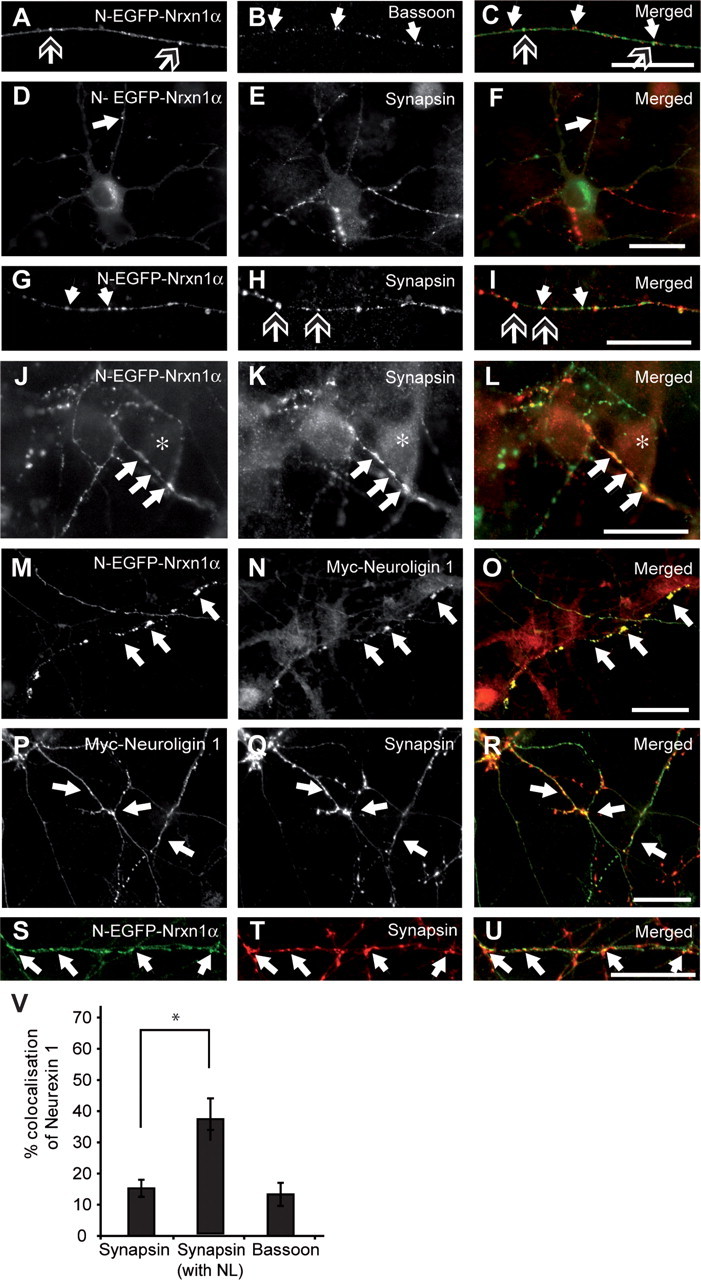
EGFP-tagged Nrxn1α is recruited to synapses. Nrxn1α-transfected neurons (A, C, D, F, G, I, J, L, M, O, S, U; green in merged pictures) were colabeled with antibodies against bassoon (B, C) or synapsin (E, F, H, I, K, L; red in merged pictures). Alternatively, neurons were cotransfected with both N-EGFP-Nrxn1α and myc-tagged neuroligin 1, and labeled for neuroligin (N, O) or synapsin (Q, R, T, U). A–C, Tagged Nrxn1α (open-headed arrows) does not colocalize with bassoon (white arrows) along the length of axons. D–I, Tagged Nrxn1α (white arrows) does not colocalize with synapsin (open-headed arrows) in intracellular puncta along the axon (D–F), but colocalization is observed at presumed synaptic contact sites (G–I). J–L, Asterisks denote the soma of a neighboring neuron, over which an axon with Nrxn1α-positive puncta is positioned, resulting in colocalization between synapsin (K) with Nrxn1α (J, L; white arrows). M–U, Inducing presynaptic differentiation by cotransfecting neuroligin 1 results in enlarged Nrxn-positive clusters (M, O; white arrows) colocalizing with neuroligin 1 (N, O), and synapsin (S–U, white arrows). V, Quantification of colocalization between Nrxn1α and synapsin or bassoon. *p < 0.05; scale bars, 40 μm.
To investigate whether Nrxn1α undergoes similar trafficking in excitatory and inhibitory neurons, we compared N-EGFP-Nrxn1α expression patterns in different neurons in culture (Fig. 3). We found that the pattern of Nrxn expression described above occurred similarly in both cells that were GAD65 positive, representing the inhibitory population of neurons (Fig. 3A–C), and in GAD65-negative neurons, representing the excitatory fraction (Fig. 3D–F). Furthermore, as illustrated by labeling of extracellular GFP, Nrxn1α was successfully inserted into the plasma membrane of both excitatory and inhibitory neurons, where subpopulations colocalized either with the vesicular glutamate (vGlut) (Fig. 3G–J) or GABA transporters (vGat) (Fig. 3K–N) at synaptic contacts. The majority of Nrxn1α puncta along processes, however, did not colocalize with the vGlut/vGat puncta, suggesting that Nrxn1α is not cotransported with either of the vesicular transporters. These results illustrate that Nrxn1α can be inserted into both excitatory and inhibitory synapses and that the trafficking mechanism is not exclusive for one type of neuron, consistent with the ubiquitous phenotype in null-mutant mice (Missler et al., 2003; Zhang et al., 2005; Dudanova et al., 2006, 2007).
Figure 3.
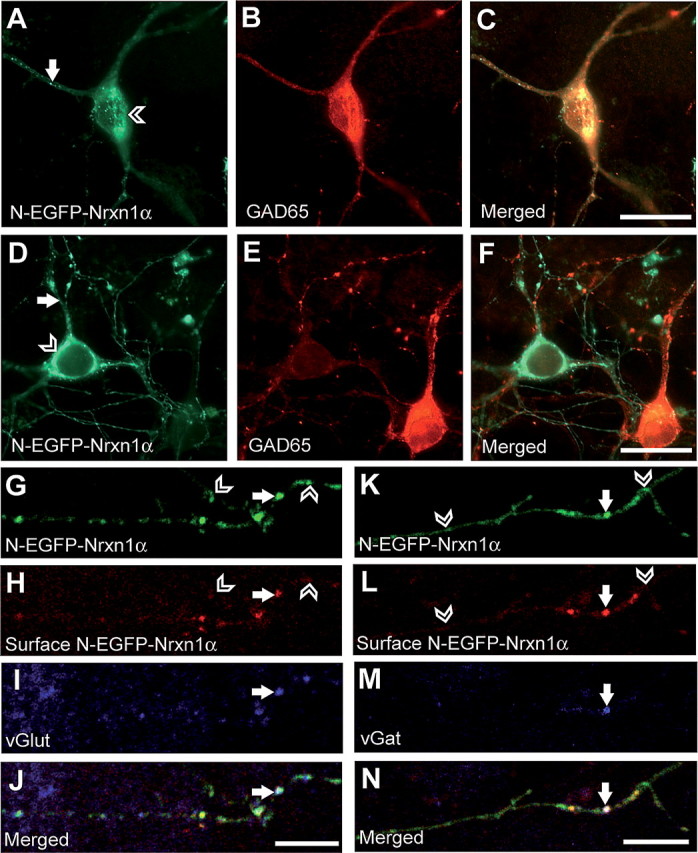
Excitatory and inhibitory neurons display the same pattern of Nrxn1α expression. A–F, Hippocampal neurons were transfected with N-EGFP-Nrxn1α on DIV7 and labeled with an antibody against GAD65 on DIV10 to distinguish between excitatory and inhibitory neurons. Both GAD65-positive (A–C) and GAD65-negative (D–F) cells show Nrxn1α expression in intracellular pools (open arrowheads) and as puncta along their processes (white arrows). G–N, Colabeling of surface Nrxn and the synaptic markers vGlut and vGat in N-EGFP-Nrxn1α-expressing neurons. Nrxn1α is transported along axons (G, K) and is inserted into the membrane (white arrows) as illustrated by extracellular GFP staining (H, L). Open arrowheads point to sites where Nrxn1α has not been inserted into the membrane, and is therefore present in transport vesicles. Membrane insertion occurs at excitatory and inhibitory synapses, as demonstrated by colocalization with vGlut (I) and vGat (M). Scale bars: A–F, 20 μm; G–N, 10 μm.
To clarify the nature of the labeling over cell bodies and to investigate the punctate labeling seen with tagged Nrxn1α, we performed preembedding immunoelectron microscopy of horseradish peroxidase (HRP)-tagged Nrxn transgenic mice, using nontransgenic littermates as controls (Fig. 4). Similar to the EGFP epitope, the HRP tag was necessary to distinguish between Nrxn variants. Transgenic mice expressing HRP-tagged Nrxn1α have previously been shown to rescue the phenotype of the triple Nrxn knock-out (Zhang et al., 2005), demonstrating that these tagged Nrxns are functionally active. Consequently, since this tag is a different epitope to the EGFP used in our culture studies, and that it is placed in a different position, being just extracellular to the transmembrane region of Nrxn rather than at the N terminus, it also served as an additional control for the tagging strategy used in vitro.
Figure 4.
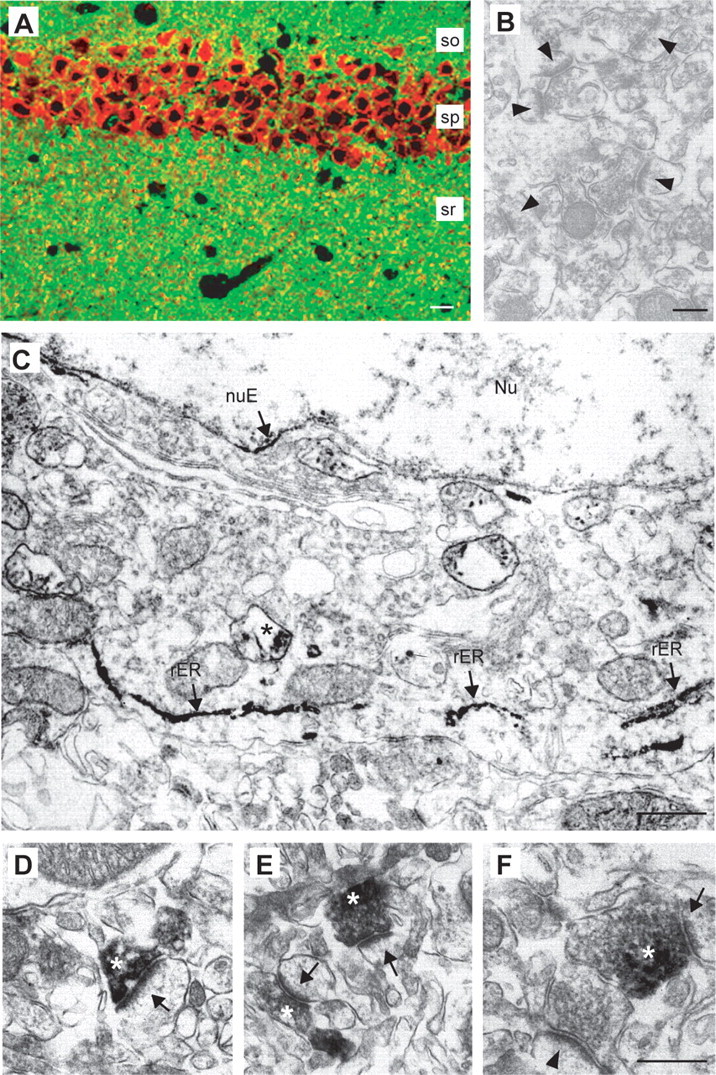
HRP-tagged Nrxn1α expressed in transgenic mice reveals polarized targeting mostly to presynaptic terminals. A, Double labeling of a hippocampal section with antibodies against the HRP-tag (red) and synaptophysin (green) shows strong expression of the transgene in pyramidal neurons of the CA1 region and partial colabeling of punctate clusters in the stratum radiatum (sr). so, Stratum oriens; sp, stratum pyramidale. B, C, Preembedding immuno-EM from nontransgenic (B, control, arrowheads point to unlabeled synapses) and Nrxn1α-HRP transgenic mice (C). C, Staining in somata is present in the rough endoplasmic reticulum (rER, arrows) and in vesicular structures (asterisk). nuE, Nuclear envelope; Nu, nucleus. D–F, Electron micrographs from the neuropil (sr of CA1) of transgenic mice show Nrxn-HRP at synapses (arrows), extending over the plasma membrane and part of the presynaptic terminals (asterisks). Scale bars: A, 40 μm; B, 500 nm; C, 800 nm; D, F, 250 nm (for D–F).
Immunoelectron microscopy of adult transgenic mice revealed that the staining for transgenic Nrxns observed over neuronal cell bodies (Fig. 4A; and in culture, Figs. 1–3) was mainly due to intense labeling of the rough endoplasmic reticulum (rER) (Fig. 4C). Of the neuronal cell bodies documented from the hippocampal pyramidal layer of transgenic mice (n = 34 cells/4 mice), >90% contained detectable transgenic Nrxn1α in the ER. In cases where the nucleus was also included in the section, ∼45% showed immunoreactivity for transgenic Nrxn in the nuclear membrane (nuE) (Fig. 4C). We conclude from these findings that a large amount of expressed Nrxn is present at early stages in the secretory pathway, possibly due to tight regulation of the amount of Nrxn at the cell surface.
We next examined by immunoelectron microscopy the nature of the punctate labeling observed by light microscopy (Fig. 4A over stratum radiatum; and in culture, Figs. 1–3). We found that outside of the ER, predominant labeling was present in vesicle-like structures in the soma and processes (asterisk in Fig. 4C, and data not shown), and in presynaptic terminals of Nrxn1α transgenic neurons (Fig. 4D–F). Although the percentage of synapses that were labeled in the hippocampus was low (∼5% of all synapses in the stratum radiatum), in all labeled synapses (n = 57/4 mice) the staining was exclusively presynaptic. In the labeled synapses, we examined ∼300 μm of synaptic plasma membranes, of which ∼90 μm were identifiable as postsynaptic and ∼210 μm as presynaptic. Of these membranes, no postsynaptic element contained adjacent immunoreactivity, whereas ∼30–40% of the presynaptic membrane was in direct contact with the immunoreactive product. Labeling of presynaptic terminals included the plasma membrane but was also present over vesicular structures inside the terminal. Despite greater diffusion of reaction product in the preembedding technique, labeled vesicular structures occurred mostly at the periphery of the presynaptic terminal (Fig. 4E,F, asterisks), excluding many clear synaptic vesicles, which may reflect the presence of Nrxns on transport/carrier vesicles and/or endosomes. Unfortunately, the affinity-purified HRP antisera were unsuitable for immunogold labeling (data not shown), and could not be used to determine the precise distribution of HRP-tagged transgenic Nrxns within the presynaptic terminals and putative transport vesicles at the highest resolution. Although similar results were also obtained for neurons from transgenic mice expressing HRP-Nrxn1β lacking an insert in splice site #4 (data not shown) (Zhang et al., 2005), the possibility of a small population of postsynaptic Nrxns, e.g., with another Nrxn variant, in earlier developmental ages or in different brain regions, cannot be excluded by our experiments. However, our results indicate that transgenic HRP-tagged Nrxns are present intracellularly within the ER, but also that a fraction is specifically transported from the ER to presynaptic nerve terminals via transport vesicles, both in agreement with our in vitro studies in transfected hippocampal neurons (Figs. 1–3).
C-terminal sequences are essential for targeting of neurexins
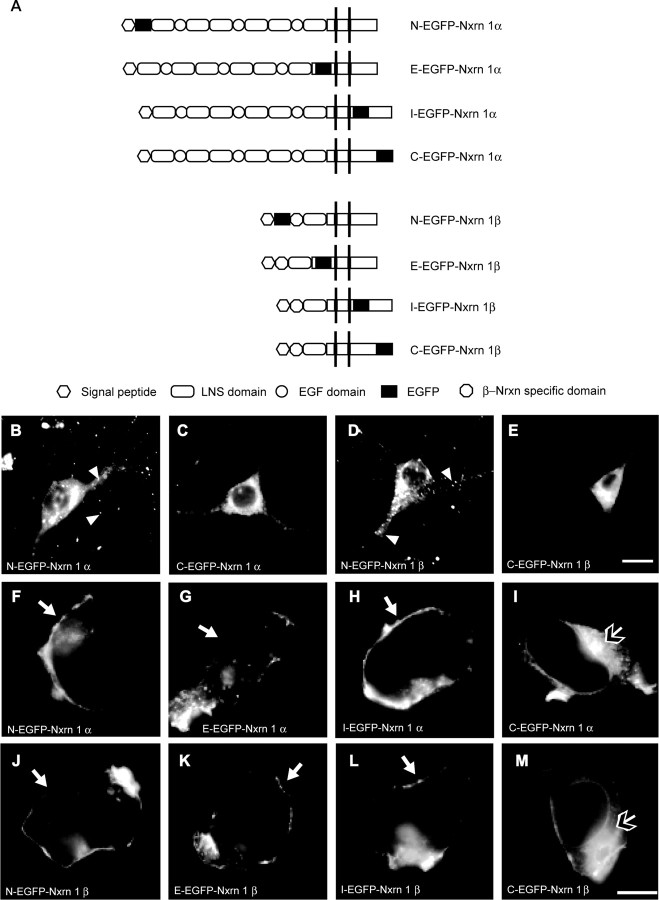
Our transgenic rescue of the null-mutant phenotype had revealed that α- and β-Nrxns are not functionally redundant, and suggested that the extracellular domains in Nrxn1α are most likely responsible for their strong effect on synaptic transmission (Missler et al., 2003; Zhang et al., 2005). In contrast, the identical C-terminal domains of α- and β-Nrxns and very similar localization in Nrxn1α-HRP and 1β-HRP transgenic mice raise the possibility that the intracellular sequences are involved in targeting of Nrxns. To directly test this hypothesis, we generated a series of Nrxn1α and 1β expression constructs with EGFP placed at different positions (Fig. 5A), including the N terminus after the signal peptide (N-EGFP-Nrxn, as used for Nrxn1α in Figs. 1–3), just extracellular to the transmembrane region [E-EGFP-Nrxn, similar to the HRP-tagged Nrxns in transgenic mice (Fig. 4)], just intracellular to the transmembrane region (I-EGFP-Nrxn), and at the very C terminus after the –YYV PDZ-recognition motif (C-EGFP-Nrxn). Upon transfecting these tagged Nrxns into hippocampal neurons, EGFP-tagged Nrxn1β produced an identical pattern to Nrxn1α (Fig. 5B,D). In fact, all EGFP-Nrxn1α and 1β constructs resulted in the distribution pattern of intracellular transport vesicle-like puncta (arrowheads in Fig. 5B,D; and data not shown), except for the C-terminally tagged Nrxns: C-EGFP-Nrxn1α (Fig. 5C) and C-EGFP-Nrxn1β (Fig. 5E) both failed to localize into a punctate pattern, and instead became rather homogenously distributed in the soma and proximal dendrites. Thus, expression of these constructs in hippocampal neurons pointed to a role of C-terminal sequences in targeting Nrxns because blocking access, for example to the PDZ-recognition motif at the C terminus, may prevent export of Nrxns into presumptive transport vesicles. To determine whether this mistargeting of C-terminally tagged Nrxns was due to their expression in neurons (e.g., because of the required presence of a specific chaperone) or whether it revealed a more general property of their sequence motifs, we next transfected heterologous cells with the eight EGFP-Nrxn constructs: Both Nrxn1α and 1β were efficiently exported to the plasma membrane of transfected tsA201 cells (a HEK293 derivative) when the EGFP tag was placed at any position except for the C terminus (Fig. 5F–M). The majority of C-EGFP-Nrxn1α (Fig. 5I) and C-EGFP-Nrxn1β (Fig. 5M) was retained intracellularly around the nucleus, presumably in the endoplasmic reticulum, though in contrast to neurons, a small cell surface population could be observed. These data suggest that the C terminus of Nrxns is required for correct targeting, and that motifs in the intracellular sequences of Nrxns presumably are involved in regulating delivery to the plasma membrane.
Figure 5.
C-terminally placed EGFP tags cause mistargeting of α- and β-Nrxns. A, Schematic representation of EGFP tagged Nrxn1α and Nrxn1β. B–E, Constructs were expressed in hippocampal neurons and their localization visualized by autofluorescence. N-terminally tagged Nrxns are present as numerous puncta along the axons and dendrites (B, D; white arrowheads), presumably reflecting a large number of transport/cargo vesicles and some synaptic contact sites. The punctate pattern is lost upon tagging Nrxn1α and 1β at the C terminus (C, E), resulting in a diffuse distribution of C-EGFP-Nrxn around the nucleus and in proximal dendrites. F–M, All constructs (A) were also expressed in tsA201 cells (a HEK293 derivative) where a distinct plasma membrane ring (white arrow) is visible for all constructs, except for those tagged at the C terminus. I, M, C-EGFP-Nrxn1α and C-EGFP-Nrxn1β are mostly retained intracellularly around the nucleus (open-headed arrow, also note the delineated nuclear envelope), with only a small proportion reaching the plasma membrane. Scale bars, 20 μm.
To brace against the possibility that insertion of the EGFP at the C terminus itself caused the mistargeting and to determine which parts of the protein are essential for targeting, we then generated deletion constructs based on the N-terminally tagged Nrxn1α (Fig. 6A) that also served as the standard for these experiments (N-EGFP-Nrxn1α) (Fig. 6B). The deletion constructs were lacking (1) all the extracellular sequences (ΔEC), (2) all the C-terminal sequences apart from the last 10 aa containing the PDZ-binding motif (Δ55 + 10), or (3) the PDZ-binding motif itself by removing the last 3 aa (Δ3) (Fig. 6A). The N-terminally truncated Nrxn (ΔEC) did not affect targeting compared with full-length Nrxn1α (Fig. 6C). ΔEC was present within an intracellular pool, consisting of large somatic clusters and numerous small puncta in soma and processes. In addition, ΔEC could be detected at the cell surface of axons by anti-GFP labeling of unpermeabilized neurons at similar levels as full-length Nrxn1α (Fig. 6F,H,Q), suggesting that the remaining C terminus was sufficient to direct targeting of Nrxns.
Figure 6.
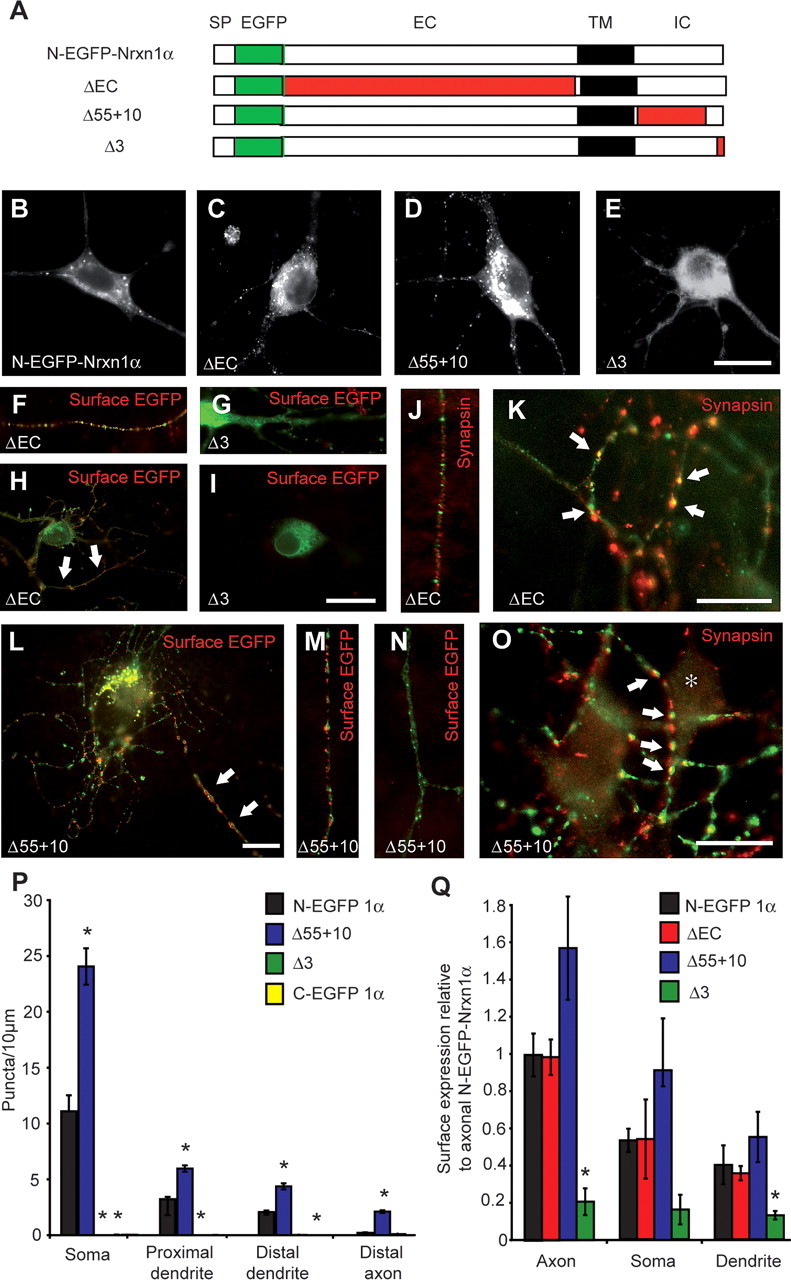
C-terminal sequences are involved in targeting of Nrxns. A, Schematic representation of full-length N-terminally EGFP-tagged Nrxn1α and of N-EGFP-Nrxn1α carrying various deletions (in red). SP, Signal peptide; EGFP, enhanced GFP tag; EC, extracellular region; TM, transmembrane region; IC, intracellular region. B–O, Hippocampal neurons were transfected with full-length or deletion constructs, and visualized by autofluorescence (B–E) or counterstained (in red) for surface EGFP (F–I, L–N) and synapsin (J, K, O). F, H, L, M, Nrxn constructs are inserted into the plasma membrane mainly in the axon (white arrows) except when the PDZ-binding motif was deleted (G, I). ΔEC and Δ55 + 10 constructs do not colocalize with synapsin in intracellular transport vesicles along the axon (J) but do so where appropriate synaptic contact sites are formed (K, O; white arrows). P, Quantification of Nrxn1α-positive puncta. Transfection of control (SKO) and triple (TKO) knock-out neurons with N-EGFP-Nrxn1α yielded similar levels and distribution. In contrast, the construct Δ55 + 10 was present at levels approximately twofold to threefold those of N-EGFP-Nrxn1α, and addition of a C-terminal tag (C-EGFP-Nrxn1α) or deleting the PDZ motif (Δ3) significantly reduced the number of puncta present at all subcellular locations (n ≥9 cultures). Q, Quantification of the surface expression of extracellularly tagged Nrxn1α constructs (relative to axonal N-EGFP-Nrxn1α) demonstrated that deleting the extracellular portion of Nrxn1α (ΔEC) had no effect on its surface presentation, whereas the construct Δ55 + 10 had increased surface labeling, and Δ3 was significantly decreased (n ≥ 3 cultures). *Significantly different to N-EGFP-Nrxn1α (p < 0.05). Scale bars, 20 μm.
In contrast, deletion of the PDZ-binding motif (Δ3) resulted in a largely diffuse distribution around the nucleus (Fig. 6E), and completely abolished its presence within discrete puncta (Fig. 6P). In agreement with the idea that Nrxns are targeted to the plasma membrane via transport/cargo vesicles, Δ3 was not able to reach the plasma membrane because a cell surface population could not be observed (Fig. 6G,I,Q). In addition, deleting all of the C-terminal tail apart from the last 10 aa (Δ55 + 10), produced a construct that not only was successfully packaged into intracellular puncta (Fig. 6D), but resulted in a significant increase in the number of these vesicles: quantification demonstrated that the number of Nrxn-positive puncta was approximately twofold to threefold higher than that of full-length Nrxn 1α at all locations analyzed (Fig. 6P). However, the general distribution pattern was not disturbed since the percentage of puncta found in each compartment was unchanged (Fig. 6P), and membrane insertion of Δ55 + 10 still occurred mainly along the axon (Fig. 6L,M,Q). The synaptic localization of constructs targeted to the axonal membrane was then investigated by counter-staining transfected hippocampal neurons with an antibody against synapsin. Similar to full-length Nrxn1α (Fig. 2D–I), ΔEC and Δ55 + 10 were present predominantly in nonsynaptic puncta (Fig. 6J,N), though they could be detected when synaptic contacts were made (Fig. 6K,O). These results demonstrate that first, the PDZ-binding motif was necessary to direct targeting of Nrxn1α via transport vesicles to the axonal/synaptic membrane, and second, additional intracellular sequences may be involved in the regulation of the amount of Nrxn1α export from the ER/Golgi complex. In contrast, although extracellular domains appear important for α-Nrxn function in neurotransmission (Zhang et al., 2005), they are not essential for targeting to synapses. Similar results were obtained upon expressing the Nrxn deletion constructs in heterologous tsA201 cells, where all constructs efficiently reached the plasma membrane, apart from when the PDZ-binding motif was deleted (Δ3) (see supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
Our finding that the PDZ-binding motif is necessary for directing Nrxn export to the axonal membrane prompted the mechanistic question whether this is actively achieved through a protein–protein interaction at the motif itself or by exposure of previously hidden ER retention signals as has been shown for NMDA receptor trafficking (Standley et al., 2000). Therefore, we generated additional expression constructs where potential ER retention signals alone and in combination with the PDZ-binding motif were mutated (Fig. 7A). Two such potential sequences included the double-lysine motif KKxx or KxKx sequence, positioned at the C terminus (Teasdale and Jackson, 1996), and the sequence RxR which can be more distant from the C terminus (Zerangue et al., 1999). The latter sequence was shown in NMDA receptor to interplay with the PDZ-binding motif in regulating NMDAR export from the ER, and thus, removal of the PDZ-binding domain in Nrxn1α may have unmasked the RNR sequence positioned near the transmembrane region. In addition, removal of the PDZ-binding motif inadvertently placed the motif KDKE at the active C-terminal position (in Δ3) (Fig. 7A), where it could now act as an artificially created ER retention motif. However, disruption of RNR and C-terminal KKNKDKE by replacement with ANA and DDRDDKE, respectively, had no effect on the export of full-length Nrxn1α, which was transported to the plasma membrane similar to wild-type N-EGFP-Nrxn1α (Fig. 7B–E), and at comparable levels (Fig. 7M). Likewise, mutating the potential ER retention motifs in deletion mutants Δ3 and Δ10 had no effect since they were still retained within the ER (Fig. 7G–M). However, replacing the PDZ-binding motif YYV of full-length Nrxn1α with the nonbinding sequence AAD resulted in blocking the transport of Nrxn from the ER (Fig. 7F,M), providing further evidence that the PDZ-binding motif is necessary for targeting. In addition, similar results were again obtained when the mutated constructs were expressed in heterologous tsA201 cells, where Nrxn1α AAD was not inserted into the plasma membrane, and mutating any potential ER retention motifs did not allow membrane insertion to occur in constructs lacking the PDZ-binding motif (see supplemental Fig. S2, available at www.jneurosci.org as supplemental material). Therefore, it can be concluded that disruption of Nrxn1α export from the ER toward the axonal membrane occurred due to the lack of binding to a PDZ-domain containing interaction partner, and not through the unmasking of a preexisting, or generation of an artificial ER retention signal. Thus, the integrity of the PDZ-binding motif itself is essential for transport along the secretory pathway to the target axonal membrane, and subsequent synaptic recruitment of Nrxns.
Figure 7.
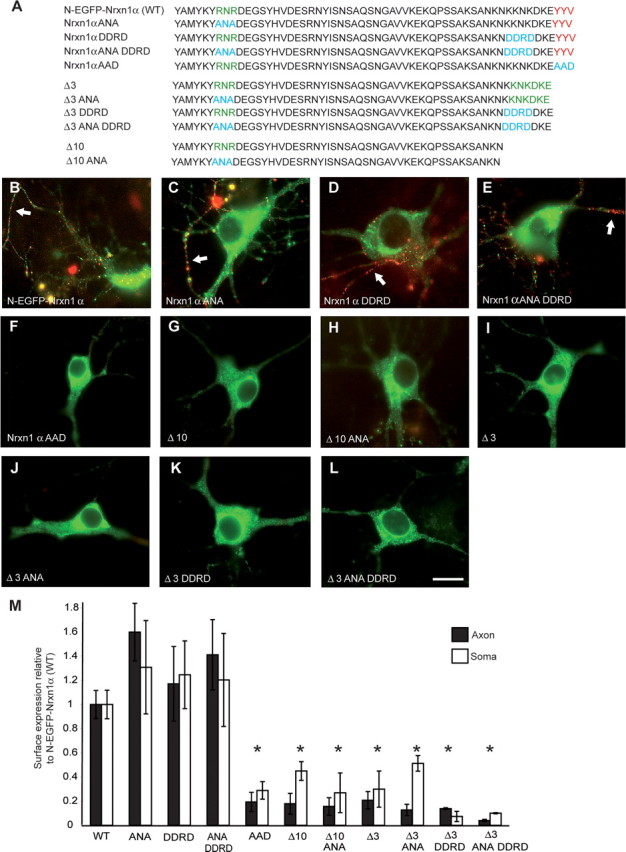
PDZ-binding motif is primarily responsible for targeting of Nrxns. A, Intracellular sequences of wild-type and mutated Nrxn constructs that all contain the identical N-EGFP-Nrxn1α sequences extracellularly (data not shown). Amino acids in red are part of the PDZ-binding motif, green are potential ER retention signals, and blue indicates residues which have been changed. Hippocampal neurons were transfected with either wild-type Nrxn1α (B), Nrxn1α ANA (C), Nrxn1α DDRD (D), Nrxn1α ANA DDRD (E), or Nrxn1α AAD (F). Alternatively, mutants were made from Δ3 already lacking the last three amino acids (I, Δ3; J, Δ3 ANA; K, Δ3 DDRD; or L, Δ3 ANA DDRD), or Δ10 constructs already lacking the last 10 aa (G, Δ10; H, Δ10 ANA). The potential ER retention motif RNR was replaced with ANA, whereas the C-terminal KDKE motif in Δ3 was targeted with the sequence DDRD to disrupt a second potential dilysine motif, KKNK, which partially overlapped with KDKE. All transfected neurons were counterstained with an extracellularly applied antibody to EGFP to detect successful cell-surface insertion (red), and labeling of axonal Nrxn is indicated (white arrows). C–E, Mutating all potential ER retention motifs individually (C, D) or together (E) has no effect on the trafficking of N-EGFP-Nrxn1α. Similarly, mutating these motifs in the Δ3 (J, K, L) or Δ10 deletion constructs (H) does not reverse the mistargeting seen after removing the PDZ-recognition motif (G, I), indicating that the interactions at the motif itself are actively required for correct transport of Nrxns. Consistently, mutating the PDZ-recognition motif YYV to a nonbinding AAD in N-EGFP-Nrxn1α abolished its export in transport vesicles and its insertion into the axonal membrane (F). M, Surface expression of constructs was quantified demonstrating that all mutations affecting the PDZ motif significantly reduced the surface labeling of neuronal axons and soma. *Significantly different to N-EGFP-Nrxn1α at p < 0.05 (n ≥ 3). Scale bar, 20 μm.
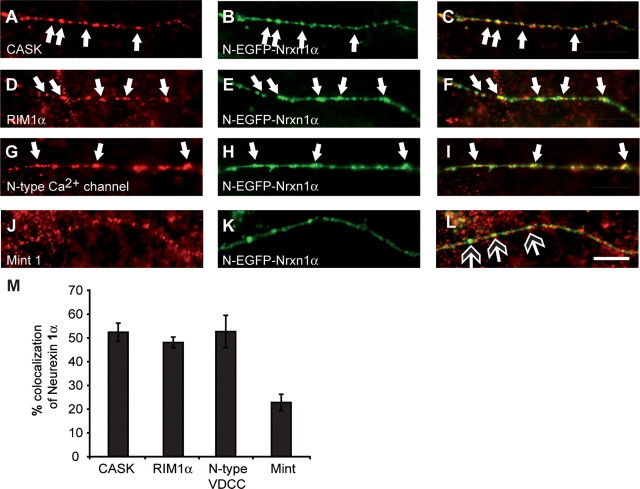
Since we observed that polarized targeting of Nrxns is regulated by their C-terminal PDZ-binding motif, we started to investigate whether Nrxns were cotransported with presynaptic proteins containing PDZ-domains. N-EGFP-Nrxn1α present along axons of hippocampal neurons without obvious synaptic contact sites were analyzed to assess the nonsynaptic pool of Nrxns. A high-degree of colocalization was seen between transfected Nrxn and endogenous CASK (Fig. 8A–C,M) and RIM1α (Fig. 8D–F,M). A similar degree of colocalization was also seen with an HA-tagged N-type Ca2+ channel (Fig. 8G–I,M) [N-HA-Cav2.2; for characterization of this channel, see Dudanova et al. (2006)], which despite not containing a PDZ motif, has been shown to be functionally dependent upon neurexin at the presynaptic terminal (Missler et al., 2003). This indicates that the Nrxn-positive transport vesicles carry additional components of the active zone and/or exocytotic machinery. In contrast, much less colocalization was seen with Mint1, although they have been shown to interact biochemically (Biederer and Südhof, 2000), suggesting the possibility that the C terminus of Nrxns may interact with different binding partners during their passage through the secretory pathway and at the synapse.
Figure 8.
Colocalization of Nrxns with additional presynaptic components on transport vesicles. Hippocampal neurons were transfected with N-EGFP-Nrxn1α alone (A–F; J–L), or cotransfected with N-EGFP-Nrxn1α and HA-tagged N-type calcium channel along with α2δ and β1b subunits) (G–I). Nrxn was visualized by its EGFP tag (autofluorescence; B, E, H, K), and endogenous or transfected proteins were labeled with appropriate antibodies (red), with colocalization appearing yellow in the merged pictures (C, F, I, L). Colocalization is seen between nonsynaptic Nrxn1α and CASK (C), RIM1α (F), and HA-tagged N-type calcium channels (I), but not between Nrxn1α and Mint1 (L, open-headed arrows). M, Quantification of colocalization between Nrxn1α and CASK, RIM1α, N-type calcium channel, or Mint1. Scale bar, 20 μm.
Discussion
Evidence for presynaptic Nrxns first arose through their ability to bind α-latrotoxin which acts presynaptically (Ushkaryov et al., 1992). While immunogold EM labeling has shown a postsynaptic localization for their binding partner neuroligins soon after discovery (Ichtchenko et al., 1995; Song et al., 1999), a presynaptic localization of Nrxns has only recently been demonstrated ultrastructurally (Taniguchi et al., 2007). Consistently, cell culture assays revealed transsynaptic binding of Nrxns to postsynaptic neuroligin, resulting in the recruitment of presynaptic proteins (Scheiffele et al., 2000; Dean et al., 2003). Similarly consistent, studies in null-mutant mice revealed that α-Nrxns play a role in presynaptic Ca2+-dependent neurotransmitter release at excitatory and inhibitory synapses (Missler et al., 2003; Zhang et al., 2005), and in Ca2+-dependent release from NMJ and endocrine melanotrophs where no neuronal postsynaptic terminals exists (Dudanova et al., 2006; Sons et al., 2006).
By tagging Nrxns extracellularly, we could separately visualize intracellular and cell surface Nrxns, demonstrating for the first time that targeting of Nrxn1α is polarized and plasma membrane insertion occurs primarily in the axonal/synaptic compartment (Figs. 1,2) of both excitatory and inhibitory neurons (Fig. 3). Ultrastructural analysis of transgenic mice expressing functional HRP-tagged Nrxns (Zhang et al., 2005) validated their presence in the secretory pathway and at presynaptic terminals (Fig. 4). These results are unlikely to reflect artifacts from overexpression of tagged molecules because they were independent of (1) the nature and extracellular position of the epitope and (2) the amount of exogenous or endogenous Nrxns present, and they agree well with other studies where epitope-tagged Nrxns were recruited to synapses (Dean et al., 2003; Graf et al., 2004; Boucard et al., 2005; Nam and Chen, 2005; Taniguchi et al., 2007). Recent studies have also raised the possibility of an additional postsynaptic role of Nrxns because earlier work showed a dependence of postsynaptic NMDA currents on α-Nrxns (Kattenstroth et al., 2004). In agreement, some postsynaptic Nrxn has been visualized by immuno EM, and reported to interact in cis-configuration with neuroligin (Taniguchi et al., 2007). Here, we also observed small amounts of Nrxns inserted into the plasma membrane of dendrites, although this population appeared significantly less than at axons. Future work will have to clarify whether the small postsynaptic population represents particular variants of Nrxns, and to further investigate its physiological role.
Several mechanisms for asymmetric distribution of synaptic proteins have been elucidated for dendritically targeted (Cheng et al., 2002) and axonally targeted proteins (Sampo et al., 2003). For example, polarized targeting of NgCAM/L1 occurs by preferential delivery to the axonal membrane, whereas VAMP2 is delivered to both dendritic and axonal membranes, only to be subsequently endocytosed from the dendritic membrane (Sampo et al., 2003). Since we show here that Nrxn is transported to both axons and dendrites, but surface presentation occurs predominantly at the axonal/synaptic compartment, it remains unclear at present whether the axonal membrane population results from a selective stabilization or axonal-specific insertion.
Our data show for the first time that synaptic targeting of Nrxns requires their C-terminal sequences and relies on an intact class II PDZ-binding motif (Figs. 5–7). Disrupting the Nrxn PDZ-binding motif resulted in a diffuse distribution pattern, suggesting that these defective Nrxns have not reached the stage of packaging into trafficking vesicles, and are retained in the ER. This regulation of trafficking contrasts with the postsynaptic binding partner of Nrxns, neuroligin, which is exclusively targeted to dendrites by a short C-terminal motif excluding its class I PDZ-binding domain (Rosales et al., 2005). Although a certain controversy exists whether subsequent targeting of neuroligin to synapses does require PDZ-mediated interactions (Dresbach et al., 2004; Prange et al., 2004), it apparently does not involve regulated export from the ER/Golgi via transport vesicles (Rosales et al., 2005).
Another PDZ motif-containing synaptic protein well studied for trafficking is the NMDA receptor, which interacts with PDZ-containing proteins during successful transport from the ER to the synapse (Setou et al., 2000; Standley et al., 2000). Alternatively, NMDAR interaction with PSD-95 may be essential for concentration at the postsynapse (Washbourne et al., 2002). Studies on NMDAR targeting also identified an ER retention signal (RxR) which could be suppressed by binding of a PDZ protein (Setou et al., 2000; Standley et al., 2000). A similar mechanism was addressed in our study through the creation of the Δ55 + 10 construct, which retained the PDZ-recognition motif, while lacking most of the C terminus (Fig. 6). This construct resulted in increased numbers of Nrxn-positive puncta, raising the possibility that an inhibitory sequence was removed. However, mutating potential ER retention motifs did not mimic the Δ55 + 10 construct, which may have alternative explanations: (1) The actual identity of a second regulatory motif has not been uncovered, or (2) removing the sequences upstream of the PDZ-recognition site reduced its specificity, allowing more PDZ proteins to interact with the C terminus of Nrxns, and, possibly, facilitating trafficking.
In any case, disrupting potential PDZ-binding events caused mis-targeting of α- and β-Nrxn, demonstrating that their trafficking relies on similar C-terminal mechanisms. Consistently, the extracellularly truncated ΔEC construct was efficiently transported to synapses and distribution of Nrxn1α-HRP and Nrxn1β-HRP in transgenic mice was indistinguishable, indicating that the cytoplasmic C terminus, common to both α- and β-Nrxns, was sufficient for targeting. Surprising at first, Nrxns epitope-tagged at the C terminus were used to demonstrate their synaptogenic properties (Graf et al., 2004), challenging our notion that the C terminus is required for targeting. However, in these studies, non-neuronal cells were transfected to generate chimeric synapses, and it was also our observation that heterologous cells are prone to “leaking,” resulting in some protein reaching the plasma membrane (Fig. 5; supplemental Fig. S1, available at www.jneurosci.org as supplemental material). In contrast, Graf et al. (2004) had to move the CFP tag to extracellular sequences to show synaptic localization, and consistently, transfected neurons with a disrupted PDZ-binding motif did not present Nrxn at the surface (Figs. 6, 7).
Since the PDZ-binding motif is essential for targeting, PDZ-containing proteins may regulate this process. Due to the promiscuous nature of PDZ-mediated binding, several candidates exist. For example, biochemical interactions have been demonstrated between neurexins and CASK, CIPP, Mint, and syntenin (Hata et al., 1996; Kurschner et al., 1998; Biederer and Südhof, 2000; Grootjans et al., 2000). Such interactions might occur, not only at the mature synapse, but also earlier during the trafficking process, as suggested here by our colabeling of Nrxn-positive transport vesicles. Indeed, CASK has been shown to be important for the trafficking of several proteins, including calcium and potassium channels (Maximov et al., 1999; Leonoudakis et al., 2004). However, when comparing the phenotypes of α-Nrxn knock-outs (Missler et al., 2003) and null-mutants of CASK, important differences are revealed since the latter displays unchanged evoked neurotransmission (Atasoy et al., 2007). Therefore, although we observed the presence of CASK on Nrxn-positive transport vesicles, consistent with their known interaction (Hata et al., 1996; Mukherjee et al., 2008), CASK is unlikely to be essential for Nrxn trafficking. The identity and roles that PDZ-containing proteins may play in Nrxn trafficking remain unclear at present, and remain to be the focus of further work.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB629-B11 (M.M.). M.A. was a recipient of a Lichtenberg fellowship awarded by the International MSc/PhD Program Neurosciences (Göttingen). We thank Dr. Craig Garner and members of his laboratory for comments on a previous version of this manuscript, and Sandra Gerke, Kai Kerkhoff, and Ilka Wolff for excellent technical help.
References
- Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, Kavalali ET, Südhof TC. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104:2525–2530. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninghausen O, Rahman MA, Silva JP, Davletov B, Hopkins C, Ushkaryov YA. Neurexin Ibeta and neuroligin are localized on opposite membranes in mature central synapses. J Neurochem. 2007;103:1855–1863. doi: 10.1111/j.1471-4159.2007.04918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Südhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275:39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Cheng C, Glover G, Banker G, Amara SG. A novel sorting motif in the glutamate transporter excitatory amino acid transporter 3 directs its targeting in Madin-Darby canine kidney cells and hippocampal neurons. J Neurosci. 2002;22:10643–10652. doi: 10.1523/JNEUROSCI.22-24-10643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresbach T, Hempelmann A, Spilker C, tom Dieck S, Altrock WD, Zuschratter W, Garner CC, Gundelfinger ED. Functional regions of the presynaptic cytomatrix protein bassoon: significance for synaptic targeting and cytomatrix anchoring. Mol Cell Neurosci. 2003;23:279–291. doi: 10.1016/s1044-7431(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Neeb A, Meyer G, Gundelfinger ED, Brose N. Synaptic targeting of neuroligin is independent of neurexin and SAP90/PSD95 binding. Mol Cell Neurosci. 2004;27:227–235. doi: 10.1016/j.mcn.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Dudanova I, Sedej S, Ahmad M, Masius H, Sargsyan V, Zhang W, Riedel D, Angenstein F, Schild D, Rupnik M, Missler M. Important contribution of α-neurexins to Ca2+-triggered exocytosis of secretory granules. J Neurosci. 2006;26:10599–10613. doi: 10.1523/JNEUROSCI.1913-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I, Tabuchi K, Rohlmann A, Südhof TC, Missler M. Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J Comp Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- Fairless R, Reissner C, Missler M. The role of neuroligin binding to neurexins in synaptic organization. In: Dityatev A, El-Husseini A, editors. Molecular mechanisms of synaptogenesis. New York: Springer; 2006. pp. 111–124. [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans JJ, Reekmans G, Ceulemans H, David G. Syntenin-syndecan binding requires syndecan-synteny and the co-operation of both PDZ domains of syntenin. J Biol Chem. 2000;275:19933–19941. doi: 10.1074/jbc.M002459200. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Südhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenstroth G, Tantalaki E, Südhof TC, Gottmann K, Missler M. Postsynaptic N-methyl-d-aspartate receptor function requires alpha-neurexins. Proc Natl Acad Sci U S A. 2004;101:2607–2612. doi: 10.1073/pnas.0308626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kurschner C, Mermelstein PG, Holden WT, Surmeier DJ. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Mol Cell Neurosci. 1998;11:161–172. doi: 10.1006/mcne.1998.0679. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem. 2004;279:19051–19063. doi: 10.1074/jbc.M400284200. [DOI] [PubMed] [Google Scholar]
- Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Südhof TC, Bezprozvanny I. Association of neuronal calcium channels with modular adaptor proteins. J Biol Chem. 1999;274:24453–24456. doi: 10.1074/jbc.274.35.24453. [DOI] [PubMed] [Google Scholar]
- Missler M, Südhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Südhof TC, Wahl MC. CASK Functions as a Mg2+-independent neurexin kinase. Cell. 2008;133:328–339. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechotta K, Dudanova I, Missler M. The resilient synapse: insights from genetic interference of synaptic cell adhesion molecules. Cell Tissue Res. 2006;326:617–642. doi: 10.1007/s00441-006-0267-4. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales CR, Osborne KD, Zuccarino GV, Scheiffele P, Silverman MA. A cytoplasmic motif targets neuroligin-1 exclusively to dendrites of cultured hippocampal neurons. Eur J Neurosci. 2005;22:2381–2386. doi: 10.1111/j.1460-9568.2005.04400.x. [DOI] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sons MS, Busche N, Strenzke N, Moser T, Ernsberger U, Mooren FC, Zhang W, Ahmad M, Steffens H, Schomburg ED, Plomp JJ, Missler M. alpha-Neurexins are required for efficient transmitter release and synaptic homeostasis at the mouse neuromuscular junction. Neuroscience. 2006;138:433–446. doi: 10.1016/j.neuroscience.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28:887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale RD, Jackson MR. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Sans N, Standley S, Prybylowski K, Petralia RS. Early events in the trafficking of N-methyl-d-aspartate (NMDA) receptors. Biochem Soc Trans. 2003;31:885–888. doi: 10.1042/bst0310885. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett. 2007;581:2509–2516. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rohlmann A, Sargsyan V, Aramuni G, Hammer RE, Südhof TC, Missler M. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci. 2005;25:4330–4342. doi: 10.1523/JNEUROSCI.0497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]