Abstract
Human grasping relies on feedforward control that is monitored and corrected on-line by means of sensory feedback. While much of the sensory mechanisms underpinning hand-object interaction are known, information has been lacking about muscle receptor responses during the phases before and after actual object contact. We therefore let subjects use their thumb and fingers to grasp blocks presented to them while we recorded muscle afferents from the thumb and finger extensor muscles along with wrist and digit kinematics, and electromyographic activity. The kinematics of the task was indistinguishable from “normal” grasping. None of the afferents encoded either object contact or finger apposition. Both primary and secondary afferents were more phase advanced on the parent muscle lengths than expected from previous studies as well as from their responses to imposed length changes of their parent muscles. Thus, the discharges of both primary and secondary afferents were well correlated to the tendon velocity of their parent muscles and that of primary afferents also to acceleration whereas neither appeared to encode muscle length as such. Decoding the velocity of muscle length changes were significantly improved if the discharge of Golgi tendon organ afferents were taken into account along with that of the muscle spindle afferents. We propose that these findings may be explained by the biomechanical properties of contracting muscles. Moreover, we conclude that it seems unlikely that the muscle spindle afferents recorded in this task have any role in providing “proprioceptive” information pertaining to the size of an object grasped.
Keywords: muscle spindles, human, manipulation, hand, sensorimotor mechanisms, motor control
Introduction
Successful object grasping depends on a multitude of neural mechanisms (for review, see Olivier et al., 2007; Castiello and Begliomini, 2008): The location and size of the object must be determined, an appropriate trajectory for reaching the object must be defined as well as a suitable grasp given subsequent actions, the hand must be transported toward the object while being preshaped to allow the selected grasp, and finally, once that hand is in physical contact with the object, a stable grasp must be established. While these motor behaviors characteristically rely on feedfoward control, they are monitored and corrected on-line by means of sensory feedback.
Somatosensory signals of potential relevance for grasping behaviors originate in skin and muscle receptors. Tactile sensors in the glabrous skin are known to play a crucial role for grasp stability (Johansson, 1998). Moreover, they are responsible for initiating adaptive behaviors when mechanical perturbances jeopardize grasp stability in a restrain task (Macefield et al., 1996) whereas muscle receptors, despite significant joint movements, play no role in this respect (Macefield and Johansson, 1996). Less is known about sensory signaling in phases before and after hand-object interaction. We know, however, that stretch-sensitive skin mechanoreceptors in the hairy skin provide good representations of joint configurations and movements as shown specifically for the hand (Edin and Abbs, 1991; Grill and Hallett, 1995), but also the knee (Edin, 2001), and the ankle (Aimonetti et al., 2007). In contrast, there are no recordings of muscle spindle activity in hand or finger muscles during grasping behaviors.
Human muscle spindles in relaxed muscles behave as stretch receptors. Accordingly, their responses to movements imposed on their passive parent muscles can easily be predicted from anatomy (Roll et al., 2000; Ribot-Ciscar et al., 2002, 2003). Natural hand and finger movements are, however, characteristically associated with extensive cocontractions (Johansson and Westling, 1988; Maier and Hepp-Reymond, 1995), that is, during grasping behaviors muscle spindles are subjected not only to length changes but also affected by the fusimotor system and extrafusal muscle contractions. In fact, during active movements both primary (type Ia) and secondary muscle spindle afferents (type II) typically fail to represent joint positions (Vallbo et al., 1981; Hulliger et al., 1982; Jahnke and Struppler, 1990; Jones et al., 2001). In short, extrapolating muscle spindle responses from available data on human muscle spindle afferents would be precarious at best.
We have therefore developed a paradigm that allows microneurography recordings of muscle receptor afferents during unconstrained wrist and digit movements. Subjects grasped blocks of different sizes while neural signals from muscle receptor afferents in the finger extensor and thumb abductor muscles were recorded, along with wrist and digit kinematics, and electromyographic activity. The analyses focused on defining the impacts of the length, velocity, acceleration and electromyographic activity of the parent muscles on the discharge rates of single afferents and populations of afferents as well as the possibility to decode kinematic variables from afferent discharges.
Materials and Methods
Subjects.
Fifteen right-handed subjects (age 20–35, seven females) participated in the experiments. All were healthy and gave their informed consent before experimentation according to the Declaration of Helsinki.
Behavioral task.
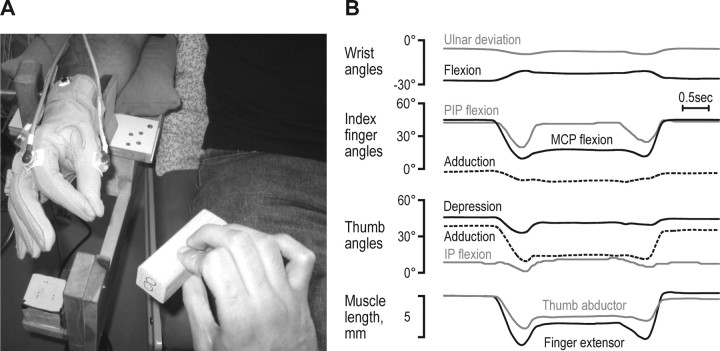
The participants were seated in a dentist's chair with their right arm resting on a mobile ramp and supported by a vacuum pillow around their forearm and a cushioned clamp just proximal to the wrist. In this position, the subjects could move their wrist and digits freely. Seven wooden blocks (i.e., a “set of blocks”), were presented in sequence and they differed only in their widths in increments of 10 mm (30–90 mm). The subjects were instructed to use the thumb and one finger (index or middle) to grasp and hold a block that was first presented ∼30 cm from the subject's hand and then delivered to the hand by the experimenter (Fig. 1A). The subjects thus performed the task under full visual control. The experimenter released the block when the subjects made contact with the object. After holding the block for several seconds, the subjects had received previous instructions to release the block when the experimenter again grasped the block. The digit and wrist angular changes as well as the resulting muscle length changes occurring when a single block was grasped, held, and released are shown in Figure 1B. Each trial started and ended with the finger and thumb in apposition.
Figure 1.
Method. A, Subject wearing the CyberGlove and with Polhemus FasTrak 3D sensors mounted on the wrist, the thumb and the index finger and with the hand in the starting position, prepared to receive and grasp a wooden block. The experimenter is holding the block prepared to drive it into the subject's grasp. B, Recorded angles and at the bottom, the calculated muscle length changes of the finger extensor and the thumb abductor muscles when grasping, holding and then releasing a 60 mm block.
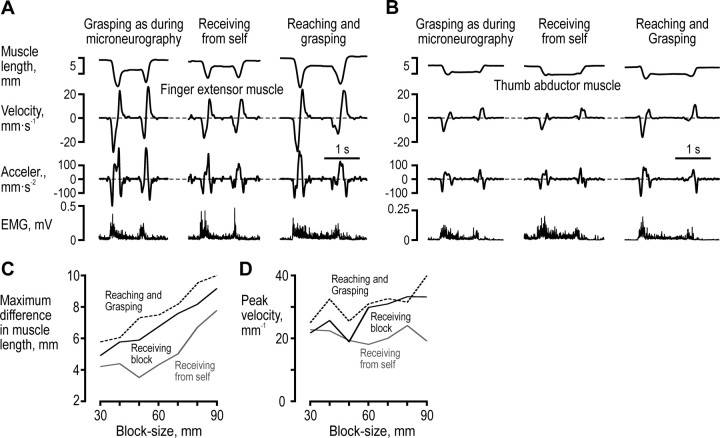
We confirmed that the subjects' grasping behaviors were very similar when the subjects received blocks as during the microneurography recordings, when they themselves transferred the object from the left-hand to the right-hand, and when they performed normal reaching-and-grasping movements (Fig. 2). The participants were familiarized with the task before the microneurography recordings by repeating at least one complete set of blocks. The task was conducted at speeds that the individual participant felt represented normal speeds during the practice trials, i.e., speeds they were comfortable with.
Figure 2.
Comparing grasping behaviors. A, B, The kinematic and EMG signals related to the finger extensor (A) and thumb abductor (B) when subject 67 received and grasped a 60 mm block in three different ways: receiving the block by the experimenter in her stationary hand as during microneurography; when the subject used her left-hand to transfer the block to her stationary hand; and when the subject reached for and grasped a stationary block placed ∼10 cm from her hand. C, D, For the finger extensor muscle, maximum muscle length difference (C) and the absolute peak muscle lengthening velocity during the grasping phase (D) during a complete block series under the different grasping conditions.
Neurophysiological technique.
Single afferent recordings were obtained using microneurography (Vallbo and Hagbarth, 1968). Briefly, a thin electrode (200 μm diameter) was inserted percutaneously into the right radial nerve ∼10 cm proximal to the elbow and small adjustments were made to its location until the activity of a single afferent could be isolated in the neurogram. We included only afferents originating in the finger extensor muscles (FE: m extensor digitorum communis, m indicis proprius) and the thumb abductor muscles (TA: m abductor pollicis longus). The origins of the afferents were determined by palpation of the forearm and by various passive and active finger movements. Muscle spindle afferents were classified as primary (“type Ia”) or secondary (“type II”) and were differentiated from Golgi tendon organ afferents by their different responses to passive “ramp-and-hold” stretches and near-isometric voluntary contractions and relaxations (Edin and Vallbo, 1990). All single-unit recordings were terminated because of accidental electrode dislocation.
All afferents were recorded during at least one set of blocks except for one unit that was lost before the subject grasped the last block (90 mm). The median time spent recording a full block-set (all seven blocks) was 39 s (maximum 59 s). Block sets were repeated for as long as the single afferent recording could be maintained.
Electromyographic signals (EMG) were recorded from the major forearm muscles that are involved in digit movement and also accessible by surface electrodes: FE, TA and the finger flexor muscles (FF: m flexor digitorum). The optimal recording site for each muscle was identified with help of a hand-held EMG recording probe during isometric contractions. Custom-build surface electrodes (Ø 2 mm; 12 mm apart) were coated with electrode jelly and attached to the skin at the optimal sites using double-sided adhesive tape.
Kinematics and tendon excursion estimates.
Digit and wrist kinematics were recorded using the Polhemus FasTrak motion tracking system (Skill Technologies) in 11 of 15 subjects and with the CyberGlove (Immersion Corporation) in all subjects (Fig. 1A,B). Polhemus FasTrak sensors could be fixated anywhere on the hand or digits and provided azimuth, elevation and roll angles with an accuracy of 0.15 degrees. The reference position (i.e., zero angles in all planes) was defined as the posture when the subjects kept the wrist and digits extended and the thumb fully abducted. The CyberGlove allowed recordings of wrist, metacarpophalangeal, and interphalangeal joint angles (flexion, extension, abduction and adduction) with a nominal resolution of ∼0.5°. Each of the 18 sensors of the CyberGlove was calibrated for each subject using custom-build wooden blocks.
The total range of ulnar-radial deviation during a complete block-set was 4.2° (averaged medians across subjects), for wrist flexion-extension 12.9°. The corresponding angular velocities were 10 and 28° · s−1, respectively. The movement range at the finger metacarpophalangeal joint was 32° and for the finger proximal interphalangeal joint 44°. The corresponding maximum angular velocities were 49, and 118° · s−1, respectively. Finger adduction-abduction range was 9.4° and angular velocity was 8.6° · s−1. The averaged range of angular deviation in thumb adduction-abduction was 46 and 26° in elevation-depression, and 27° at the thumb around the thumb interphalangeal joint. The corresponding angular velocities were 48, 81 and 99° · s−1, respectively.
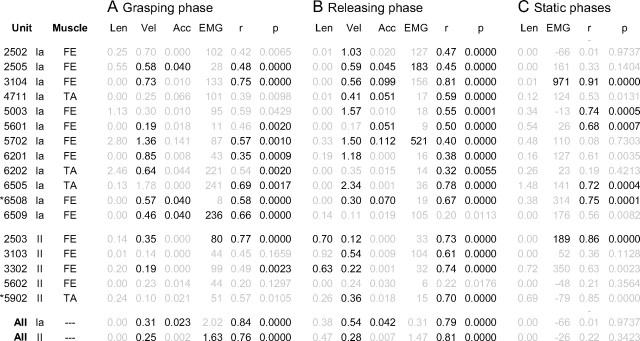
From the angular measurements we calculated the corresponding muscle length changes with zero muscle length corresponding to the muscle length at the reference position. This process involved simple transformations of angular data into tendon excursion estimates (for the FE: Elliot and McGrouther, 1986; for the TA: Smutz et al., 1998). Whereas the angle at the interphalangeal joint of the thumb was taken into account, the effect of finger adduction, wrist radial-ulnar deviation and movements at the distal interphalangeal joints of the fingers were all too small to affect significantly the estimated tendon excursions and were therefore ignored. Since we were unable to accurately measure the joint radii in individual subjects we used reported averaged values (Elliot and McGrouther, 1986). Any resulting errors would affect the magnitude of the reported regression constants in Table 1 for individual afferents but hardly at all affect the estimated averaged kinematics across all subjects (see Fig. 6) and would in neither case have any bearing on our conclusions.
Table 1.
Determinants of kinematic and electromyographic variables on the discharge of muscle spindle afferents
Regression coefficients, correlation coefficients (r) and the probability (p) that r was zero for the grasping phases (A), releasing phases (B), and static phases (C) are reported separately for all muscle spindle afferents but one. The coefficients ″Len,″ ″Vel,″ ″Acc,″ and ″EMG″ correspond to the increase in discharge (imp · s−1) per unit change in length (mm), velocity (mm · s−1), acceleration (mm · s−2), and raw EMG signals (mV), respectively. If the confidence intervals of ±0.9995 did not include zero, the coefficients were considered significant (i.e., p < 0.01 with Bonferroni correction). Text in grayed font represents nonsignificant data. FE, Finger extensor muscle; TA, thumb abductor muscle.
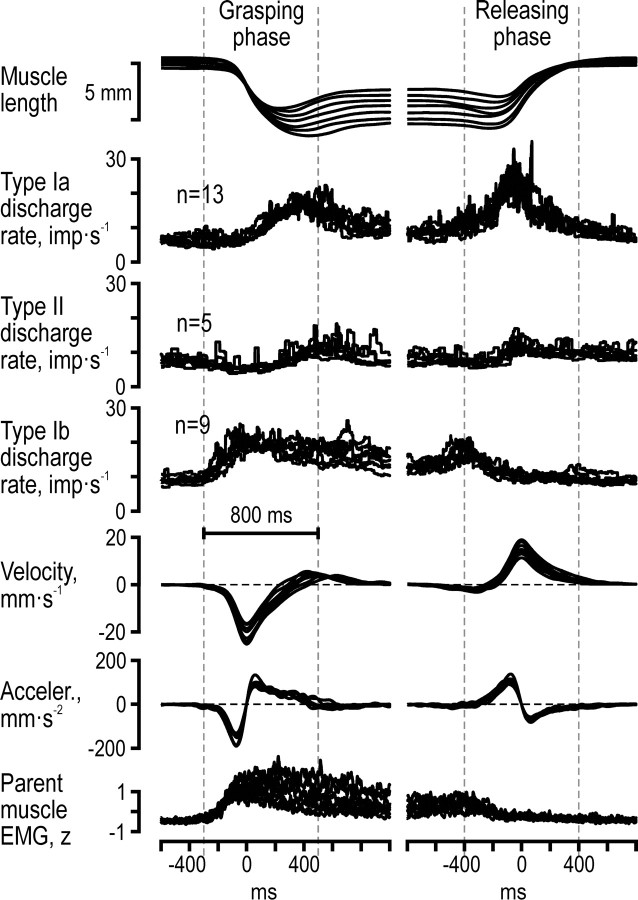
Figure 6.
Afferent population responses during the blockgrasping task. Superimposed averaged ensemble responses across all type Ia, II and Ib afferents during the grasping (left column) and releasing phases (right column) across all block-sizes. The displayed kinematic and EMG signals were averaged across all subjects who contributed with an afferent recording. The periods between the vertical dashed lines mark the 800 ms periods used in the regression analyses. Note the apparent lack of any significant length dependence of the afferent discharges.
Regression analyses.
We performed two kinds of regression analyses: one to determine the impact of the kinematic variables and EMG on the afferents' discharge rates (Table 1), and one to predict kinematic variables from the afferent discharges (Fig. 7). The former analyses were performed on both the discharge of individual muscle spindle afferents and on ensemble discharges (described below). All regression analyses were conducted on data down-sampled to 20 Hz corresponding to the “effective” sampling rate to take into account auto-correlations among the variables and thereby to avoid inflated p values (Dawdy and Matalas, 1964).
Figure 7.
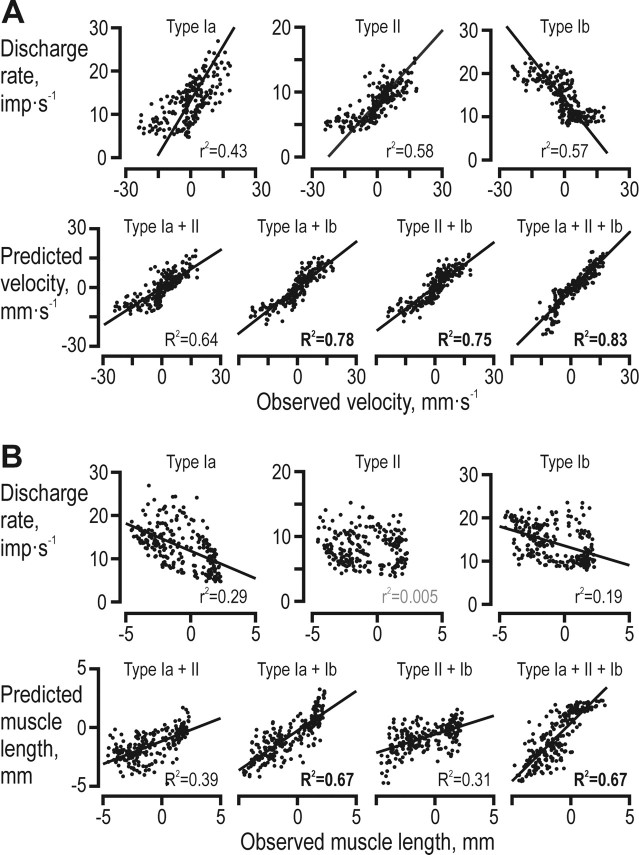
Population encoding of muscle length and velocity. A, Muscle velocity versus instantaneous discharge rates of individual afferent populations (top row) and observed versus the velocity predicted from linear regression with various combinations of populations as the independent variable (bottom row). Data from both the grasping and releasing phases were included. Solid lines indicate significant regression lines (p < 10−6). The R2 values for combinations which included Ib afferents were significantly higher than those of any other combination including, in particular, the combination of only type Ia and II. B, As in A but for muscle length instead of muscle velocity. The R2 value of the combination between Ia and Ib afferents was significantly higher than that for both the combination of Ia and II and the combination of II and Ib (p < 10−4).
The impacts of kinematic variables and EMG on the discharge rates were determined by means of nonlinear regression analyses under the assumption that if all other factors were constant, neither length, nor velocity or acceleration could have had a negative impact on the discharge of spindle afferents. The effect of EMG however was not constrained and could thus have either a negative or a positive impact. Since the grasping phase was characterized by stronger parent muscle activation (Fig. 3C,G), and presumably stronger fusimotor effects on spindle afferents, than the release phase, we performed separate regression analyses for the two phases. The grasping phase included fingertip spacing and grasping a block and the release phase included the release of the block and the return of the digits into apposition. Both periods were 800 ms long and were fixed in time with respect to the peak negative and positive muscle velocity, respectively, with the grasping phase starting 400 ms before and the release phase 300 ms before this event (Fig. 5). For afferents recorded during more than one complete block set, averages were calculated for these phases across repetitions. The nonlinear regression model for the impact analyses is represented by the following equation: Discharge rate = k1 + | k2 | · Len + | k3 | · Vel + | k4 | · Acc + k5 · EMG, where Len, Vel, and Acc represent the instantaneous muscle length, velocity and acceleration, respectively, and k1–k5 represent the constants determined by the regression (the absolute values of k2–k4 are reported). The number of data points available for each regression was 7 block sizes × 16 (= 800/50 ms) = 112 samples.
Figure 3.
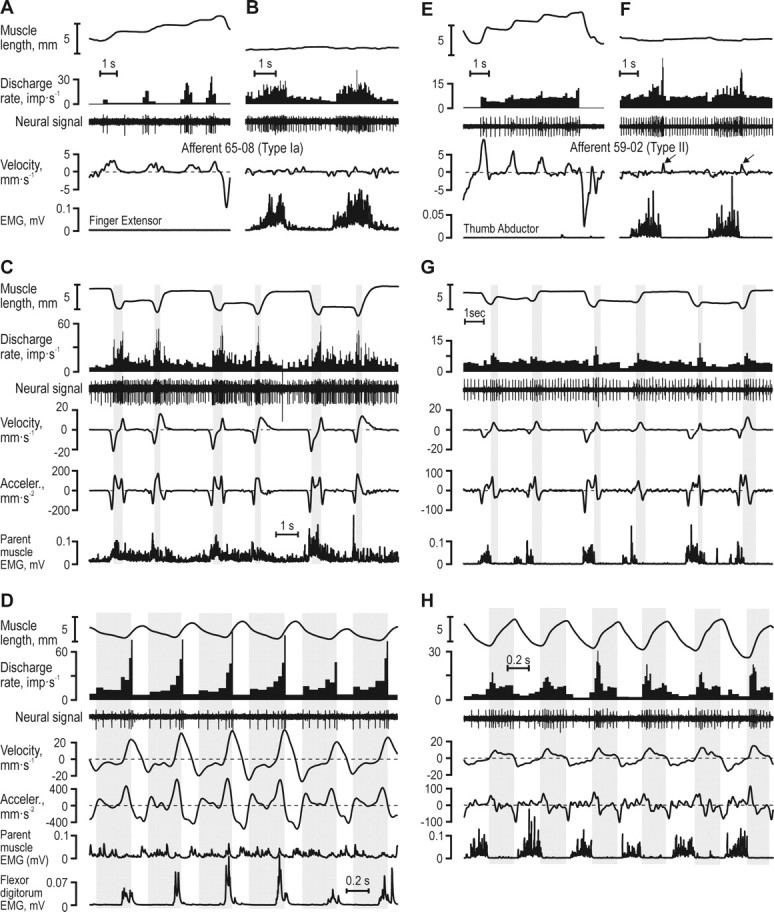
Responses of typical muscle spindle afferents. A–D, Ia afferent from the finger extensor and E–H, type II afferent from the thumb abductor. A, E, Responses to passive muscle length changes. B, F, Responses during near-isometric contractions. C, G, Record of the subjects grasping, holding and releasing blocks of increasing widths (40, 50 and 60 mm). Whereas the type Ia afferent discharged preferentially when the second time derivative of muscle length (acceleration) was positive (vertical gray bars in C) and in particular when both acceleration and velocity were positive, the type II afferent discharged preferentially during phases of muscle stretch (vertical gray bars in G). The discharge of neither afferent displayed an obvious relationship to muscle length. D, H, Both afferents showed the same dependence on kinematics during active sinusoidal joint movement and block grasping.
Figure 5.
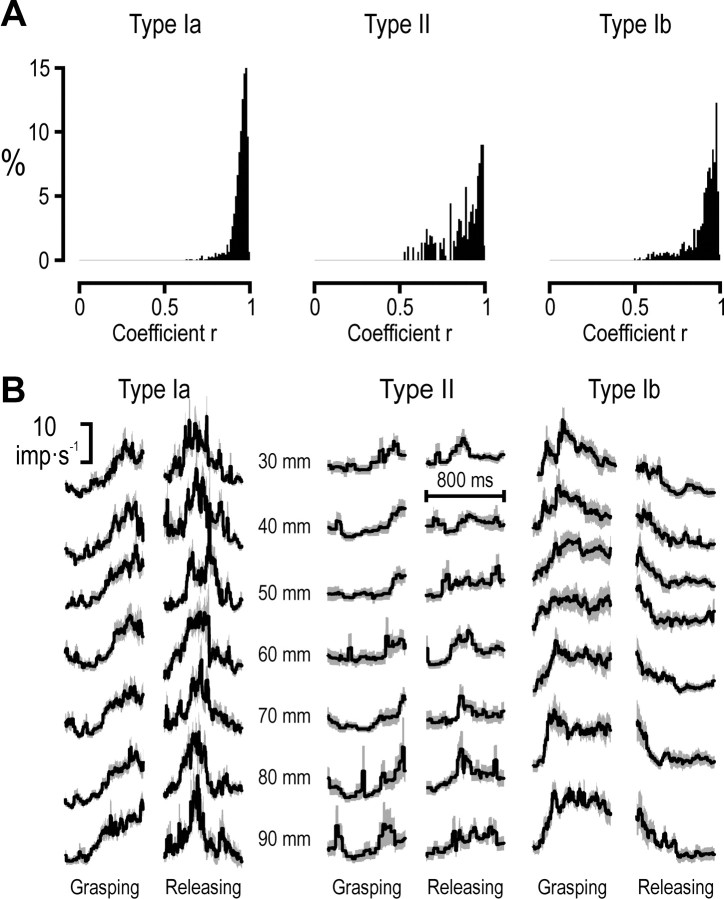
Cross-validation of generated afferent populations. A, The distribution of r values of correlations between the responses averaged across all recorded afferents and the responses averaged over randomly drawn subsets of afferents (∼60% of the available afferents). B, The averaged population discharge rate ±SD (solid line and grayed area) for block sizes 30–90 mm during the grasping and releasing phases.
Kinematic variables were predicted from the ensemble discharges of the afferents using the following equation: Kinematic variable = k1 + k2 · Ia + k3 · II + k4 · Ib, where “Kinematic variable” corresponds to the instantaneous muscle length, velocity or acceleration; “Ia,” “II” and “Ib” correspond to the instantaneous ensemble discharge of type Ia, type II and type Ib afferents, respectively; and k1–k4 represent the constants determined by the regression. In these regressions both the grasping and releasing phases were included and the data points available for each regression was thus 7 block sizes × 32 (= 2 · 800/50 ms) = 224 samples.
Ensemble responses.
Averaged “population responses,” kinematics and EMG variables were generated across all block sizes and afferent types. The averaged population responses were cross-validated by using a bootstrapping methodology: a data matrix containing one data series for each recorded afferent, variable, block size and phase was first compiled. From this matrix 100 averaged responses of populations were created, each comprising a random sample of ∼60% of the available afferents (8 of 13 type Ia; 3 of 5 type II afferents; 5 of 9 type Ib; a total of 3 afferent types × 2 phases × 7 block sizes × 100 replicates = 4200 generated population responses). The median r obtained when correlating all averaged population responses from a subset of type Ia afferents with the corresponding average calculated from all type Ia afferents was 0.96 (range between the upper and lower quartiles was 0.04); for type II 0.91 (range 0.17); and for type Ib 0.93 (range 0.09). Averaged kinematic and parent EMG signals were generated in a similar way but based on subjects not on single afferents. All median r values obtained when correlating each randomly generated kinematic or EMG average against the relevant grand mean were >0.98 and all ranges between upper and lower quartiles were <0.09. We therefore felt justified to claim that the population responses and averaged kinematics and EMG signals we used were fair estimates of the true averages.
Data sampling, processing and analyses.
Data were digitally sampled using SC/ZOOMΤΜ (Physiology Section, Department of Integrative Medical Biology, Umeå University, Umeå, Sweden). The neural signals were stored at 12.8 kHz after amplification close to the recording site (×10,000, band-width 0.47–5.0 kHz). Identification of single action potentials was made semiautomatically under visual control (Edin et al., 1988). Each EMG channel was root-mean-square processed with a rise-time constant of 1 ms and a decay-time constant of 3 ms and digitally sampled at 1 kHz. The 18 channels from the CyberGlove were digitally sampled at 86 Hz. Wrist pitch, yaw and rotation angles generated from the Polhemus FasTrak were sampled at 30 Hz. First and second time derivatives were calculated with moving windows that corresponded to low-pass filtering DC-17 Hz, i.e., including all significant frequency components of the subjects' movements.
For each recorded afferent unit and for each phase and block-size, the extracted variables used for statistical analyses were (1) the discharge rate of the afferent (2) the parent muscle length (i.e., tendon excursion) and its first and second time derivatives (“velocity” and “acceleration”), and (3) the EMG signals recorded from FE, TA and FF.
Statistical analyses were performed with STATISTICA (version 7.1; StatSoft) and Matlab (version 2007b; The MathWorks). The significance level was set to p < 0.01. Only adjusted R2 values are reported. Since muscle length, velocity and acceleration could not have had a negative impact on any of the afferents' discharges, the relevant nonlinear regressions involved forcing the corresponding coefficients to be ≥0.
Results
We recorded from 27 muscle receptor afferents originating in the FE and TA muscles during block grasping: 13 primary muscle spindle afferents (type Ia, 3 TA units), 5 secondary muscle spindle afferents (type II, one TA unit), and 9 Golgi tendon organ afferents (type Ib, 3 TA units). Type Ia afferents were recorded from 9 subjects, type II from 5 subjects and type Ib from 7 subjects (Table 1). Across all afferents we obtained data during a total of 335 block-grasping movements. After describing characteristic discharge patterns of several single afferents we report the impact of kinematic variables and EMG on the discharge of all single afferents and on population responses, and then demonstrate the possibility of decoding kinematic variables from various combinations of population responses.
Single afferent responses
Figure 3 shows responses from a type Ia and a type II afferent. The type Ia afferent (65–08) from the FE muscle behaved as a typical type Ia when the parent muscle was relaxed: it responded to imposed muscle stretch, was silent during muscle shortening, and showed a poor static response (Fig. 3A), while it increased its discharge during isometric voluntary contractions (Fig. 3B). During the block grasping task, however, high discharge rates were not seen during periods of muscle elongation per se, but rather when the second derivative of muscle length (i.e., acceleration) was positive (Fig. 3C, shaded gray areas). The highest discharge rates were evident when both velocity and acceleration were positive. The afferent showed a similar dependence on acceleration and velocity during fast voluntary sinusoidal movements (Fig. 3D).
During repeated stretch-and-hold of its relaxed parent muscle, the type II afferent 59–02 from the TA displayed a degree of sensitivity to static length changes as well as to velocity (Fig. 3E). This afferent also increased its discharge during near-isometric voluntary muscle activations (Fig. 3F). When the subject sequentially grasped the same blocks as above (40–60 mm), this afferent showed its highest discharge rates during phases when its parent muscle was lengthening (Fig. 3G, shaded gray areas), and responded similarly during voluntary sinusoidal movements (Fig. 3H).
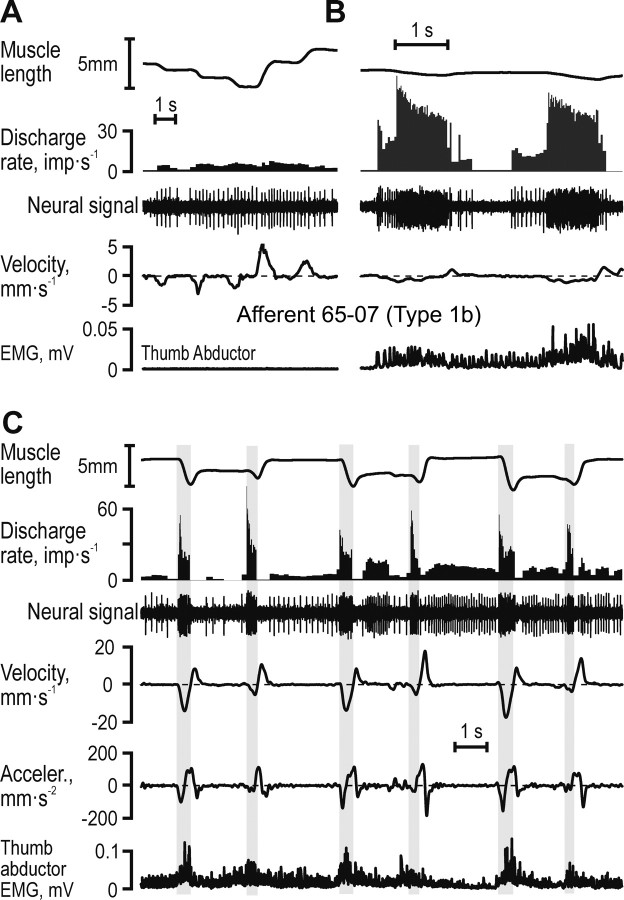
Figure 4 displays responses in a type Ib afferent (65–07) from TA tendon that was “spontaneously” active. As is characteristic for Golgi tendon organs, the Ib afferent responded poorly to imposed elongation and shortening of the relaxed muscle (Fig. 4A), but discharged prominently during voluntary contractions (Fig. 4B). During the grasp and release of three consecutive blocks (40–60 mm), this afferent discharged most when the muscle was undergoing concentric contractions (i.e., “active” muscle shortening, shaded gray areas in Fig. 4C). The highest discharge rates were observed during the initial phases of these concentric contractions.
Figure 4.
Discharges of a Golgi tendon organ afferent from the thumb abductor. A, It was spontaneously active and showed inconsistent responses to static length and muscle stretch; B, it discharged vigorously when the muscle was actively contracting; and C, when the subject grasped, held and subsequently released wooden blocks of increasing widths (40, 50, and 60 mm) the afferent discharged preferentially during active muscle contraction, especially during periods of active muscle shortening (vertical gray bars).
In summary, Figures 3–4 present three afferents that given their types responded as expected during both imposed stretch of their passive parent muscles and during voluntary, “isometric” contractions. In contrast, during both the block-grasping task and during sinusoidal joint movements, the type Ia afferent appeared to respond to both velocity and acceleration and the type II afferent seemed to be particularly responsive to velocity. Only the type Ib afferent responded as expected during the block-grasping task.
Impact of kinematic variables and EMG on muscle spindle afferent discharges
Regression analyses were performed during the grasping and releasing phases for all spindle afferents (but one for which EMG was lacking), and the results are shown in Table 1A, B. To summarize, parent muscle velocity displayed a significant impact on the discharge rates of the majority of both type Ia and II afferents during at least one phase of the block grasping task. Moreover, and unexpectedly, acceleration had a highly significant impact on the discharge of 7/12 type Ia afferents during at least one phase while length lacked a significant impact on all type Ia afferents and had a weak impact on only two type II afferents. Finally, EMG had a significant impact on the discharge rates of only 4 of 17 muscle spindle afferents and then only during either the grasping or the releasing phases.
Specifically, during the grasping and releasing phases of the block grasping series during which type Ia 65–08 (Fig. 3C) was recorded, the range of lengths, velocities and accelerations was 6.3 mm, 31 mm · s−1 and 645 mm · s−2, respectively. For this afferent only velocity and acceleration had significant impacts on the discharge rates (Table 1). Given the range of velocities observed during the grasping phase, velocity was responsible for modulating the afferent's discharge during the studied behavior with 23 imp · s−1 and during the release phase with 15 imp · s−1. The corresponding modulation accounted for by acceleration was 17 imp · s−1 during the grasping phase and 45 imp · s−1 during releasing phase. For both phases of the block grasping task, the impact of velocity and acceleration on this afferent's discharge was substantial compared with the total range of discharge rates observed: 54 and 72 imp · s−1 during grasping and releasing, respectively. The same type of analysis of type II afferent 59–02 (Fig. 3G) showed that only velocity had a significant impact and this only during the releasing phase.
Velocity had a significant impact on a majority of type Ia afferents: 8 of 12 during the grasping phase and 9 of 12 during the releasing phase (Table 1). The median modulation accounted for by velocity across both phases was 26 imp · s−1. Acceleration showed a significant impact on 3 of 12 and 6 of 12 type Ia afferents during the same phases and the median modulation accounted for by acceleration was 20 imp · s−1. Velocity had a significant impact on 2 of 5 type II afferents during the grasping phase and 4 of 5 during the releasing phase. The median modulation of the type II afferents accounted for by velocity was 14 imp · s−1 across both phases. Just 2 of 5 type II afferents showed a statistically significant positive impact of length, but this only during the releasing phases (i.e., muscle lengthening), but the impact was modest in terms of total discharge modulation given the range of muscle lengths (6 and 9 imp · s−1, respectively).
One may argue that the insignificant impact of length on the discharge rates of spindle afferents during the dynamic grasping and releasing phases could be explained by an overwhelming effect of the velocity, the acceleration and either the unloading due to extrafusal contractions or the fusimotor drive. In other words, the spindle afferents could have been substantially sensitive to the static length but that this effect was small compared with other factors during the block grasping task. To address this issue, we analyzed the relationship between the spindle discharges and the static length during finger apposition and while the subjects stably held the object in their grasp (Table 1C). Under these circumstances, only static muscle length and any ongoing EMG activity (coupled to receptor unloading, fusimotor drive, etc) should affect the afferent's responses: both type Ia and II population discharge rates were negatively correlated to muscle length and positively correlated to EMG. Like for the regression of the dynamic phases we assumed, however, that length could not have had a negative impact on the discharge rate of any afferent but that EMG could have had a positive or a negative impact. The results were clear-cut: muscle length lacked a statistically significant impact (p > 0.05) on all single afferents as well as on any of the population responses. Thus, the effect of length, if any, must have been small. Furthermore, this negative result could not be explained by a lack of statistical power because the median modulation of discharge across all afferents using the length impacts and the maximum static length difference observed with the individual afferents (median 5.5 mm) was <0.01 imp · s−1 (the mean was 1.8 imp · s−1).
Averaging across populations of sensory neurons has been proposed as a means by which the CNS counters the effects of nonlinearities and noise that is present at the single sensory afferent level (Scott and Loeb, 1994; Faisal et al., 2008). Although the single afferents in this study were recorded at different times, in different subjects, and in different muscles, averaging across all recorded afferents seemed justified. First, the kinematics and EMG activity during the periods of interest, i.e., the grasping and the releasing phases were similar across subjects and across muscles. Second, as indicated by Table 1, the impacts of kinematic and EMG variables were similar in magnitude across the recorded afferents. All population signals produced by simple averaging were in addition cross-validated through comparisons with population responses generated through bootstrapping methodology (Fig. 5) (see Materials and Methods for details).
The results of the nonlinear regressions using the population responses during the grasping and releasing phases confirmed the results at the single afferent level (Figs. 3–4, Table 1), as well as those inferred from visually inspecting the population responses (Fig. 6): velocity and acceleration had significant impacts on the discharge rate of the type Ia population, whereas EMG lacked a significant effect altogether (Table 1). For the type II population discharges, velocity but not length had a significant impact, and EMG had a significant impact but only during the grasping phase (i.e., during shortening of their parent muscles). Notably, length did not show a significant impact on either type Ia or type II afferents for any of the analyzed dynamic phases of the block grasping task. Finally, the type Ib population was significantly affected only by parent EMG activity. The length of the parent muscles did not have a significant impact on any of the population responses either during the dynamic or the static phases.
Decoding kinematics from afferent responses
In the impact analyses above we took for granted that neither increased length, nor positive velocity or acceleration could cause a decreased discharge in any of the afferent types. If we instead want to analyze to what extent muscle length, velocity, or acceleration can be decoded from the afferent discharges, such restrictions are not necessarily relevant because consistent correlations allow predictions whether the correlations are positive or negative.
We used multiple linear regressions to predict the instantaneous acceleration and velocity during the grasping and releasing phases from the discharge rates of all possible combinations of the three afferent type populations. As could be predicted from the analyses reported in Table 1, the discharges of type Ia afferents were significantly correlated with acceleration (r2 = 0.20, p < 10−6), whereas this was not true for either type II or Ib. The results of the regressions predicting parent muscle velocity were, however, more complex (Fig. 7A). The population discharge rates of all afferent types were significantly correlated to velocity (muscle spindle afferents positively and type Ib negatively; p < 10−6) (Fig. 7A) but the r2 values did not differ between them (p > 0.3). Moreover, when the population discharges of both type Ia and II were used to predict velocity, the resulting R2 was not significantly higher than those obtained when using single populations (Fig. 7A). In contrast, whenever the type Ib population discharge was combined with either type Ia or type II, the resulting R2 values (≥0.75) were significantly higher (p < 0.01) than either single afferent population or the combination of type Ia and II (R2≤0.64).
While the discharge rates of type II afferents were uncorrelated with the length of their parent muscles, both type Ia and Ib population discharge rates were negatively correlated (Fig. 7B). Using the discharge of single afferent populations evidently yielded poor predictions (e.g., r2 = 0.29 obtained for type Ia). A significantly better prediction was obtained, however, when the discharge rates of both type Ia and Ib were combined (R2 = 0.67).
Moment of object contact and fingertip apposition
Finally, we investigated if any of the muscle receptor afferents encoded the mechanical transients associated with object contact during the grasping phase or the moment of finger apposition at the end of the release phase. For each trial the moment of contact at the end of the grasping phase was defined as the moment when the velocity approached zero and there was a zero-crossing in the acceleration signal (Fig. 8); the moment of fingertip appositions was identified in a similar manner. No single afferent showed any sign of change in its discharges close to the moment when the fingertips made contact with the object or at fingertip apposition. Likewise, no sign of systematic changes in the discharge could be discerned in spike histograms anchored in time on the moment of contact, histograms to which all recorded afferents contributed. We therefore conclude that during normal grasping, muscle receptors convey no information about either the time of contact with grasped object or the time of fingertip apposition.
Figure 8.
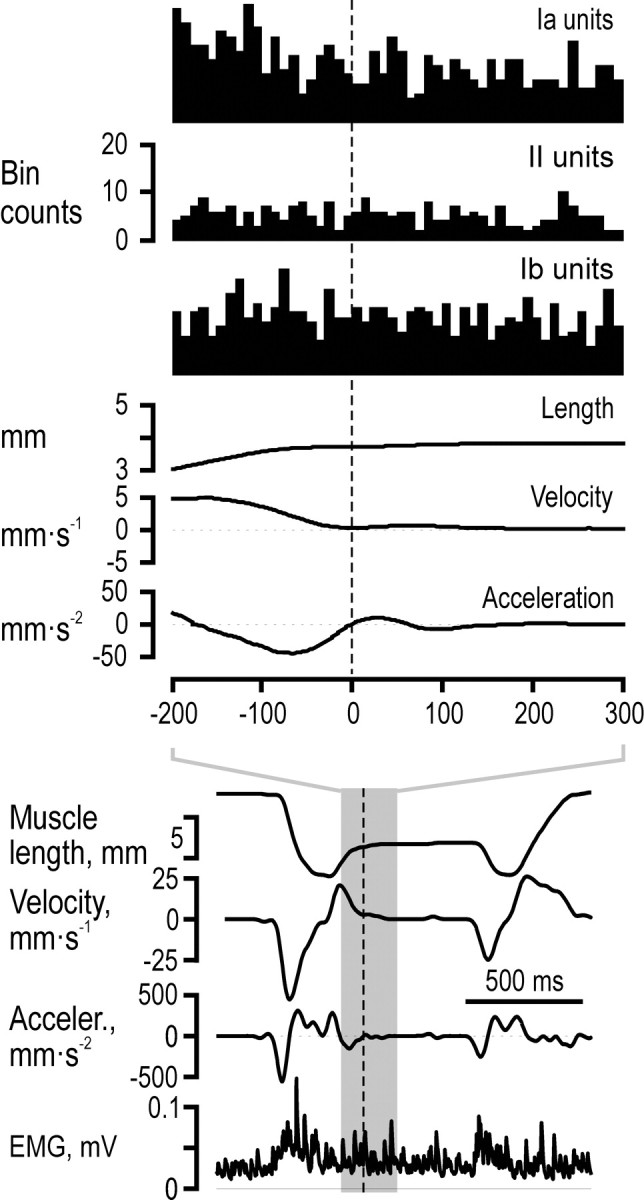
Lack of responses to object contact. All recorded afferents contributed to the histograms synchronized at the estimated time of object contact. There were no sign of either an increased or decreased discharge close to the time of contact neither in any single afferent nor in the population histogram. The results were identical for digit apposition.
Discussion
We have analyzed the discharge of muscle receptor afferents during object grasping. The discharge of muscle spindle afferents was more phase advanced with respect to the length of their parent muscles than expected. Moreover, when we attempted to predict muscle lengths and velocities from the afferent discharges we found that these predictions improved significantly with the inclusion of Golgi tendon organ afferents.
The discharge rates of cat spindle afferents in the physiological range of frequencies (<10 Hz) and amplitudes show a phase advance on the length of the parent muscles significantly above 0° but below 90° (Hulliger et al., 1977). Accordingly, they can be said to encode both length and velocity. The discharge rates of primary and secondary muscle spindles in our recordings were, however, ∼90° more phase advanced then expected from results in animal experiments. That is, the phase advance of secondary afferents appeared closer to 90° than to 0°, and accordingly, their discharges were better correlated with the velocity than with the length of their parent muscles. Similarly, primary afferents were significantly correlated with both velocity and acceleration, i.e., they showed a phase advance somewhere between 90 and 180°. In animal experiments such large phase advances have been reported for both primary and secondary endings but only during static fusimotor stimulation and during small-amplitude (≤100 μm) sinusoidal muscle stretching at frequencies above 20 Hz (Goodwin et al., 1975), that is, at amplitudes much smaller and at frequencies much beyond those observed in our experiments.
We have no reason to believe that the properties of human muscle spindles are fundamentally different from those in the cat with respect to their encoding properties. In other words, if the experiments that have been performed in the cat were performed in humans, we would expect the same phase advances as those observed in animal studies (Kakuda, 2000). What is different in our experiments from most animal studies and practically all previous human studies is that we have observed spindle discharges under “natural” conditions, i.e., during varying shortening and lengthening velocities and contraction levels of their parent muscles. Aware that a complete account would require detailed quantitative biomechanical analyses and further experimentation, we will try to offer an explanation based on qualitative reasoning and known relationships.
Let us consider a muscle actively contracting against a load. The length changes of the whole muscle, and thereby the position of the load, will comprise length changes in the tendon as well as in the muscle fascicles. If the load is a spring then the generated force will be proportional to the whole muscle length change, will be proportional to the velocity of the whole muscle if the load is viscous, and will be proportional to acceleration if the load is a mass, i.e., be 0, 90 and 180° phase advanced, respectively, on whole muscle length changes. The mechanical properties of both the tendon and the contracting muscle fascicles are dominated by stiffness. The tendon and the muscle fascicle will therefore change their lengths in direct proportion to the generated force, and accordingly, the length of the muscle fascicle will presumably display the same phase relationship to the whole muscle length as will the force. The length of the muscle fascicle, i.e., the compartment “measured” by muscle spindle afferents will thus show a load dependent phase relationship. If this reasoning is correct, the phase advances we observed in the muscle spindle afferents would be explained if their parent muscles were acting on a load dominated by viscosity constituted by the antagonists, skin and connective tissues.
The biomechanical situation in the block grasping task was, of course, much more complicated than what was considered above: the load was most likely changing during the movements, e.g., because of varying degrees of cocontractions (Weiss and Flanders, 2004). Moreover, whenever the parent muscle was deactivated, elastic recoil of its tendon might elongate the muscle fascicle whether the whole muscle was shortening or lengthening (Hoffer et al., 1989; Roberts et al., 1997). Moreover, the muscle itself is part of the “load” and its mechanical properties were certainly changing during the task. These complications do not, however, invalidate the basic conjecture, viz., that the phase relationship between the muscle spindle discharges and the length of their parent muscles might depend on the load experienced by the muscle. In the reflex control of human standing, larger reflex phase advances have been observed than could be accounted for by the known velocity-sensitive properties of muscle spindles (Fitzpatrick et al., 1996). While some of this phase advance may be explained by central nervous processes it is possible that muscle spindle afferents in humans may in fact display more phase advance then previously thought.
There is also the possibility that the fusimotor system is activated in a manner that generates increased spindle discharges during phases of high acceleration. It is of particular interest that fusimotor stimulation applied just before stretch of a passive muscle can substantially enhance such responses (Brown et al., 1969). Perhaps a similar result is obtained when a muscle is contracting to shorten, relaxes, and then is lengthened by its antagonists, as has been suggested for spindle afferents during chewing in alert monkeys (Goodwin and Luschei, 1975). If so, there is a possibility that the apparent responsiveness to acceleration displayed by type Ia afferents in our study is the result of stretching spindles that have just previously been exposed to fusimotor activity concomitant to a shortening contraction of their parent muscle. In fact, the above can explain why more type Ia afferents were sensitive to acceleration during the release phase rather than the block-grasp phase, since the former was characterized by a brief contraction and then stretch of a relatively more relaxed parent muscle (compare Table 1 and Fig. 6).
Both secondary and primary afferents display an “initial burst” at the onset of stretch under some conditions. While this has been interpreted as an acceleration response (Schäfer, 1967) it is most likely a phenomenon unrelated to our findings. It can be explained by stretch and sudden yield of stable cross-bridges that form spontaneously in the intrafusal muscles, but can be invoked both by repeated stretching (Proske, 1975; Edin and Vallbo, 1988), and previous fusimotor stimulation (Brown et al., 1969), whereas ongoing fusimotor stimulation usually eliminates it (Jansen and Matthews, 1962).
The two parent muscles of this study have comparatively long tendons. It is well known that changes in overall muscle-tendon length may differ significantly from those of the muscle fascicles due to series elasticity represented by tendons (Hoffer et al., 1989; Loram et al., 2005). Moreover, the relative contribution of muscle fascicle length and tendon length to imposed whole-muscle length changes depends on the contraction level and this effect is evident already at low contraction levels (Herbert et al., 2002). Hence, even if we ignore dynamic phase changes when acting on loads, estimates of muscle lengths cannot rely solely on receptors encoding the length of muscle fascicles. It is therefore of considerable interest that predictions of velocity significantly improved when we incorporated the responses of Ib afferents in the models (Fig. 7A). Spinal interneurons and spinocerebellar neurons receive both “length-related” and “force-related” sensory inputs and this has been interpreted as a mixed kinematic-kinetic representation (Windhorst, 2007). Perhaps afferent inputs from the Golgi tendon organs are necessary for disambiguating spindle inputs.
The significant predictions of muscle length (Fig. 7B) may be spurious for at least two reasons. First, it is paradoxical that primary afferents showed a significant negative relationship whereas this was lacking with secondary afferents when there was no demonstrable positive impact of length on the discharge of primary afferents and only a weak impact of length on secondary afferents (Table 1). Second, the negative relationship observed for primary afferents, i.e., that they increased their discharges with decreasing muscle lengths, implies that they were strongly driven by fusimotor activation (and perhaps affected by extrafusal contractions). If so, the observed relationship does not reflect muscle length as such but rather the concomitant fusimotor drive and muscle activity and would be different if the task conditions had been different (e.g., requiring more cocontractions).
It has been taken for granted that muscle spindle afferents provide information about the hand posture adopted during prehension, i.e., proprioceptive information about the size of the grasped object (Berryman et al., 2006). Our results suggest that such information can be retrieved from muscle spindles only by integrating their responses during the dynamic phases since their length sensitivity was practically zero. Moreover, if the conjectures presented above are true, such integration can only lead to correct length estimates if the continuously varying load somehow is monitored e.g., by means of Golgi afferents. While the possibility of a contribution by muscle afferents remains open, more likely candidates for providing information about hand postures are stretch-sensitive skin mechanoreceptors as shown in neurophysiological (Edin and Abbs, 1991; Edin, 1992, 2001, 2004; Aimonetti et al., 2007) and behavioral studies (Edin and Johansson, 1995; Collins and Prochazka, 1996; Collins et al., 2005).
Footnotes
This work was supported by grants from the Swedish Research Council (projects 08667 and 2005-6994) and the 6th Framework Program of the European Union (SENSOPAC, IST-001917). We thank Anders Bäckström and Göran Westling for technical support, and Profs. G. E. Loeb and P. B. C. Matthews for valuable discussions about our results.
References
- Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol. 2007;580:649–658. doi: 10.1113/jphysiol.2006.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman LJ, Yau JM, Hsiao SS. Representation of object size in the somatosensory system. J Neurophysiol. 2006;96:27–39. doi: 10.1152/jn.01190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Goodwin GM, Matthews PBC. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969;205:677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U, Begliomini C. The cortical control of visually guided grasping. Neuroscientist. 2008;14:157–170. doi: 10.1177/1073858407312080. [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Dawdy DR, Matalas NC. Statistical and probability analysis of hydrologic data, part III: Analysis of variance, covariance and time series. In: Chow VT, editor. Handbook of applied hydrology, a compendium of water-resources technology. New York: McGraw-Hill; 1964. pp. 68–90. [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. J Neurophysiol. 2004;92:3233–3243. doi: 10.1152/jn.00628.2004. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Stretch sensitization in human muscle spindles. J Physiol. 1988;400:101–111. doi: 10.1113/jphysiol.1988.sp017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Dynamic response of human muscle spindle afferent to stretch. J Neurophysiol. 1990;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Edin BB, Bäckström PA, Bäckström LO. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. J Neurosci Methods. 1988;24:137–144. doi: 10.1016/0165-0270(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Elliot D, McGrouther DA. The excursions of the long extensor tendons of the hand. J Hand Surg [Br] 1986;11:77–80. doi: 10.1016/0266-7681(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Luschei ES. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol. 1975;38:560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Hulliger M, Matthews PBC. The effect of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975;253:175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill SE, Hallett M. Velocity sensitivity of human muscle spindle afferents and slowly adapting type II cutaneous mechanoreceptors. J Physiol. 1995;489:593–602. doi: 10.1113/jphysiol.1995.sp021075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert RD, Moseley AM, Butler JE, Gandevia SC. Change in length of relaxed muscle fascicles and tendons with knee and ankle movement in humans. J Physiol. 2002;539:637–645. doi: 10.1113/jphysiol.2001.012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer JA, Caputi AA, Pose IE, Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res. 1989;80:75–85. doi: 10.1016/s0079-6123(08)62201-3. discussion 57–60. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Matthews PB, Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitudes. J Physiol. 1977;267:811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Vallbo AB. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. J Physiol. 1982;322:167–179. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke MT, Struppler A. Responses of human muscle spindle afferents during isotonic position holding and active movements. Brain Res. 1990;515:181–186. doi: 10.1016/0006-8993(90)90594-2. [DOI] [PubMed] [Google Scholar]
- Jansen JKS, Matthews PBC. The central control of the dynamic response of muscle spindel receptors. J Physiol. 1962;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS. Sensory input and control of grip. Novartis Found Symp. 1998;218:45–59. doi: 10.1002/9780470515563.ch4. discussion 59–63. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988;71:59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo AB. Directional tuning of human forearm muscle afferents during voluntary wrist movements. J Physiol. 2001;536:635–647. doi: 10.1111/j.1469-7793.2001.0635c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N. Response of human muscle spindle afferents to sinusoidal stretching with a wide range of amplitudes. J Physiol. 2000;527(Pt 2):397–404. doi: 10.1111/j.1469-7793.2000.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, non-spring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? J Physiol. 2005;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Johansson RS. Control of grip force during restraint of an object held between finger and thumb: responses of muscle and joint afferents from the digits. Exp Brain Res. 1996;108:172–184. doi: 10.1007/BF00242914. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Häger-Ross C, Johansson RS. Control of grip force during restraint of an object held between finger and thumb: responses of cutaneous afferents from the digits. Exp Brain Res. 1996;108:155–171. doi: 10.1007/BF00242913. [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res. 1995;103:108–122. doi: 10.1007/BF00241969. [DOI] [PubMed] [Google Scholar]
- Olivier E, Davare M, Andres M, Fadiga L. Precision grasping in humans: from motor control to cognition. Curr Opin Neurobiol. 2007;17:644–648. doi: 10.1016/j.conb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Proske U. Stretch-evoked potentiation of responses of muscle spindles in the cat. Brain Res. 1975;88:378–383. doi: 10.1016/0006-8993(75)90403-5. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Roll JP. The preferred sensory direction of muscle spindle primary endings influences the velocity coding of two-dimensional limb movements in humans. Exp Brain Res. 2002;145:429–436. doi: 10.1007/s00221-002-1135-4. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll JP. Proprioceptive population coding of limb position in humans. Exp Brain Res. 2003;149:512–519. doi: 10.1007/s00221-003-1384-x. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Roll JP, Bergenheim M, Ribot-Ciscar E. Proprioceptive population coding of two-dimensional limb movements in humans: II. Muscle-spindle feedback during “drawing-like” movements. Exp Brain Res. 2000;134:311–321. doi: 10.1007/s002210000472. [DOI] [PubMed] [Google Scholar]
- Schäfer SS. The acceleration response of a primary muscle-spindle ending to ramp stretch of the extrafusal muscle. Experientia. 1967;23:1026–1027. doi: 10.1007/BF02136428. [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. The computation of position sense from spindles in mono- and multiarticular muscles. J Neurosci. 1994;14:7529–7540. doi: 10.1523/JNEUROSCI.14-12-07529.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutz WP, Kongsayreepong A, Hughes RE, Niebur G, Cooney WP, An KN. Mechanical advantage of the thumb muscles. J Biomech. 1998;31:565–570. doi: 10.1016/s0021-9290(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hulliger M, Nordh E. Do spindle afferents monitor joint position in man? A study with active position holding. Brain Res. 1981;204:209–213. doi: 10.1016/0006-8993(81)90666-1. [DOI] [PubMed] [Google Scholar]
- Weiss EJ, Flanders M. Muscular and postural synergies of the human hand. J Neurophysiol. 2004;92:523–535. doi: 10.1152/jn.01265.2003. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]