Abstract
The adaptive mechanisms that protect brain metabolism during and after hypoxia, for instance, during hypoxic preconditioning, are coordinated in part by nitric oxide (NO). We tested the hypothesis that acute transient hypoxia stimulates NO synthase (NOS)-activated mechanisms of mitochondrial biogenesis in the hypoxia-sensitive subcortex of wild-type (Wt) and neuronal NOS (nNOS) and endothelial NOS (eNOS)-deficient mice. Mice were exposed to hypobaric hypoxia for 6 h, and changes in immediate hypoxic transcriptional regulation of mitochondrial biogenesis was assessed in relation to mitochondrial DNA (mtDNA) content and mitochondrial density. There were no differences in cerebral blood flow or hippocampal PO2 responses to acute hypoxia among these strains of mice. In Wt mice, hypoxia increased mRNA levels for peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1 α), nuclear respiratory factor-1, and mitochondrial transcription factor A. After 24 h, new mitochondria, localized in reporter mice expressing mitochondrial green fluorescence protein, were seen primarily in hippocampal neurons. eNOS−/− mice displayed lower basal levels but maintained hypoxic induction of these transcripts. In contrast, nuclear transcriptional regulation of mitochondrial biogenesis in nNOS−/− mice was normal at baseline but did not respond to hypoxia. After hypoxia, subcortical mtDNA content increased in Wt and eNOS−/− mice but not in nNOS−/− mice. Hypoxia stimulated PGC-1α protein expression and phosphorylation of protein kinase A and cAMP response element binding (CREB) protein in Wt mice, but CREB only was activated in eNOS−/− mice and not in nNOS−/− mice. These findings demonstrate that hypoxic preconditioning elicits subcortical mitochondrial biogenesis by a novel mechanism that requires nNOS regulation of PGC-1α and CREB.
Keywords: mitochondria, nitric oxide, mitochondrial biogenesis, hypoxia, brain metabolism, energy metabolism
Introduction
The mammalian brain is exquisitely sensitive to neuronal damage by hypoxia because of its high metabolic rate for oxygen (CMRO2), relatively low capillary density, and scant reserves of substrate and high energy phosphate. If the hypoxia is not too severe, the brain recruits protective mechanisms that help meet its continuous demand for energy production by mitochondria (Semenza and Wang, 1992; Levy et al., 1995; Bunn and Poyton, 1996). This is exemplified by hypoxic preconditioning (Sharp et al., 2004), in which previous exposure to hypoxia leads to neuroprotection from subsequent hypoxic exposure. Other highly aerobic tissues, such as the heart, adapt to hypoxia by mechanisms that include increased mitochondrial mass and therefore respiratory capacity (Ou and Tenney, 1970; Meerson et al., 1972, 1973; Eells et al., 2000). Such data suggest that augmentation of bioenergetic capacity through mitochondrial biogenesis, the generation of new mitochondria, could improve the ability of certain cells to survive a hypoxic stress.
Mitochondrial biogenesis requires the expression of several hundred gene products for proteins that make up the functional and structural organelle, and, although the mitochondrial genome encodes for only 13 of these, oxidative phosphorylation depends on them. Two classes of transcriptional regulators also enable communication between the nuclear and mitochondrial genomes: nuclear DNA-binding transcription factors such as nuclear respiratory factors 1 and 2 proteins (NRF-1 and NRF-2), which mediate expression of multiple nuclear genes encoding for mitochondrial proteins, including subunits of the respiratory chain complexes (Scarpulla, 1997), the rate-limiting heme synthesis enzyme (Braidotti et al., 1993), and factors needed for mitochondrial DNA (mtDNA) replication and transcription including mitochondrial transcription factor A (Tfam) (Virbasius and Scarpulla, 1994). Also, nuclear coactivators, typified by peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), impact on nuclear–mitochondrial communication by interacting with DNA-binding transcription factors and integrating nuclear gene expression into a program of mitochondrial biogenesis (Puigserver et al., 1998; Wu et al., 1999; Lehman et al., 2000).
Hypoxia triggers gene transcription for proteins that sustain the O2 supply to tissues and enhance cell survival during O2 deprivation, for instance via hypoxia-inducible factor-1 (Semenza and Wang, 1992; Levy et al., 1995; Bunn and Poyton, 1996). In the brain, some of the adaptive hypoxic responses are regulated by nitric oxide (NO) (Mishra et al., 2006), and the molecule has a key role in mitochondrial biogenesis in adipocyte and fibroblast lines via soluble guanylate cyclase-sensitive (sGC) cGMP-dependent induction of PGC-1α (Nisoli et al., 2003, 2004). The hippocampus is particularly vulnerable to global hypoxia, and we have demonstrated that mitochondrial biogenesis occurs in this brain region in response to the oxidative stress of extreme hyperoxia (Gutsaeva et al., 2006). We therefore considered whether acute transient cerebral hypoxia stimulates mitochondrial biogenesis in the subcortex, specifically the hippocampus, through an NO-dependent mechanism that converges on activation of key nuclear transcription factors and coactivators. To test this hypothesis, we investigated the effect of hypoxia on mitochondrial biogenesis in the mouse brain and determined the requirement for NO as well the NO synthase (NOS) isoform involved in regulating mitochondrial biogenesis in hypoxic subcortex.
Materials and Methods
Animal procedures and hypoxic exposures.
The animal protocol was approved by the Duke University Institutional Animal Care and Use Committee. C57BL/6J wild-type (Wt), endothelial NOS (eNOS)-deficient (eNOS or NOS-3−/[minus]) mice (strain B6.129P2-Nos3tm1Unc/J, C57BL/6J background) and neuronal NOS (nNOS)-deficient (nNOS or NOS-1−/−) mice (strain B6.129S-Nos1tm1Plh, C57BL/6J background) were obtained from The Jackson Laboratory (Bar Harbor, ME), crossbred, and raised in the animal facilities of Duke University. Additional NOS-1-deficient mice were generously supplied by Dr. Paul Huang at Harvard Medical School (Boston, MA). Transgenic mice that express green fluorescent protein (GFP) exclusively in mitochondria (mtGFP-tg mice) were obtained from Tokyo, Japan and bred in our facility (Shitara et al., 2001). The experimental Wt and mutant mice were exposed to hypobaric hypoxia (HH) for 6 h in an altitude chamber at 24,000 feet above sea level (equivalent to 8% O2 normobaric hypoxia). The control mice breathed sea level air in the same chamber. Another group of Wt mice (n = 8) received the nonselective NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) (Sigma, St. Louis, MO), administered (30 mg/kg, i.p.) 30 min before the 6 h period of hypoxia. All animals were killed with an overdose of halothane either immediately at the end of 6 h exposure or after 24 or 48 h. The brains were quickly perfused via the aorta with ice-cold 0.9% NaCl, removed en bloc, and rapidly dissected to isolate the subcortex, which was snap frozen in liquid N2 and stored at −80°C.
PO2 was measured in the hippocampus in the Wt and knock-out strains using a platinum needle microelectrode (10 μm tip). Mice were anesthetized, intubated, ventilated with 30% O2, and placed in a stereotaxic frame. Platinum wire electrodes insulated with epoxy, except the conical tip of 0.5 mm length, were inserted into the dentate gyrus of the hippocampus. O2 electrodes were calibrated after each experiment in three artificial CSF buffers at 37°C, equilibrated with 100% N2 (0% O2), 8% O2 (balance N2), and air (21% O2). Brain PO2 measurements were conducted in Wt and NOS-deficient strains ventilated with room air, 8% O2 (balance N2), and room air after hypoxia. The electrodes were used also for regional cerebral blood flow (rCBF) measurements using hydrogen clearance (Demchenko et al., 2005).
RNA isolation and mRNA expression.
RNA was extracted from brain subcortex using Trizol Reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed with 1 μg of total RNA from each sample, random primers, and avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) in a final volume of 25 μl. PCR amplification of mitochondrial and nuclear-encoded cDNA fragments were accomplished using gene-specific primers (Table 1). The number of PCR cycles for each set of primers was optimized during the exponential phase of PCR by titration on GelStar-stained 2% agarose gels. The 18S rRNA was used to control for variation in efficiency of RNA extraction, reverse transcription, and PCR for nuclear and mitochondrial RNA gene expression. Quantification of amplified product was performed by gel densitometry, and band intensities were expressed relative to 18S rRNA (Suliman et al., 2003; Gutsaeva et al., 2006).
Table 1.
Primer sequences used to amplify nuclear mRNA
| Gene | Sense (5′-3′) | Antisense |
|---|---|---|
| PGC-1 | CACGCAGCCCTATTCATTGTTCG | GCTTCTCGTGCTCTTTGCGGTAT |
| NRF-1 | CCACATTACAGGGCGGTGAA | AGTGGCTCCCTGTTGCATCT |
| NRF-2 | GCACAGAAGAAAGCATTG | AGTGTGGTGAGGTCTATATC |
| Tfam | AGTTCATACCTTCGATTTTC | TGACTTGGAGTTAGCTGC |
DNA extraction and mtDNA content.
Genomic DNA was extracted from brain subcortex of normoxic and hypoxic Wt, NOS-1−/−, and NOS-3−/− mice using GenElute Genomic DNA kit (Sigma) and stored at −20°C. The mtDNA content was quantified by amplification of a region of the mouse cytochrome b gene. Portions of the nuclear-encoded 18S rRNA gene were used to normalize for DNA loading. Quantification of amplified product was performed by gel densitometry and band intensities expressed relative to 18S rRNA.
Nuclear protein preparation.
For nuclear protein extracts, brain subcortex of control (0 h) and hypoxic (6 h) Wt, NOS-1−/−, and NOS-3−/− mice were homogenized and lysed in 0.6% NP-40, 10 mm HEPES, pH 7.6, 1.5 mm MgCl2, 10 mm KCl, 0.5 mm PMSF, 1 mm Na3VO4, and phosphatase inhibitor cocktails (Sigma). After 5 min on ice, samples were centrifuged for 5 min at 5000 × g. Pellets were resuspended in 80 μl of buffer containing 10% glycerol, 20 mm HEPES, pH 7.6, 420 mm NaCl, 1.2 mm MgCl2, 0.2 mm EDTA, 0.5 mm PMSF, 0.5 mm DTT, 1 mm Na3VO4, and phosphatase inhibitor cocktail (Sigma). The samples were mixed vigorously and incubated on ice for 30 min and then centrifuged for 5 min at 14,000 × g. The nuclear extracts were stored at −80°C.
Western blot analysis.
Protein concentration was determined by the BCA method (Pierce, Rockford, IL) using BSA as a standard. Nuclear proteins (15 μg) were separated by SDS-PAGE and electroblotted onto Immobilin-P membranes (Millipore, Bedford, MA). Nonspecific binding was blocked with Tris-buffered saline (TBS)/Tween 20 (TBST) containing 5% nonfat dry milk for 12 h at 4°C. Membranes were incubated with antibody as follows: rabbit polyclonal anti-PGC-1 (1:500; Bethyl Laboratories, Montgomery, TX), rabbit polyclonal anti-phospho-cAMP response element binding (pCREB) protein (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-phospho-cAMP-dependent protein kinase (PKA) α/β/γ catalytic (1:500; Santa Cruz Biotechnology), rabbit polyclonal PKA catalytic (1:800; Santa Cruz Biotechnology), and mouse monoclonal anti-α-tubulin (1:5000; Sigma). After five washes in TBST, membranes were incubated with anti-rabbit (Santa Cruz Biotechnology) or anti-mouse (Jackson ImmunoResearch, West Grove, PA) horseradish peroxidase-conjugated secondary antibodies. Specific complexes were detected by enhanced chemiluminescence using the ECL system (GE Healthcare, Little Chalfont, UK). Protein expression was analyzed by densitometry (Bio-Rad, Hercules, CA) and normalized to tubulin in the same sample for at least four samples per group.
Immunohistochemistry.
For immunohistochemistry and fluorescence microscopy, the brains of control and post-hypoxia mice were perfused transcardially with cold PBS followed by 4% paraformaldehyde in 0.1 m phosphate, pH 7.4. The brains were left in situ for 10 min, removed, postfixed, embedded in paraffin, and sectioned. Brain sections were deparaffinized, hydrated, and intrinsic peroxidase blocked with 3% H2O2 in methanol. Incubation with 10% normal goat serum in TBS, pH 7.6, for 30 min was used to block nonspecific binding. Sections were incubated with 1:200 anti-pCREB (Santa Cruz Biotechnology) in TBS containing 10% normal goat serum and then with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at 1:200 for 1 h and avidin-biotinylated horseradish peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories) in TBS for 1 h. The slides were developed with DAB and counterstained with hematoxylin. Immunoreactive specificity was confirmed by omitting the primary antibody.
Fluorescence imaging.
Fluorescence microscopy was performed on Nikon (Tokyo, Japan) Optiphot-2B microscope. Brain sections from control mtGFP-tg mice and at 24 or 48 h after a 6 h period of hypoxia were deparaffinized, rinsed in PBS, pH 7.4, and coverslipped. Fluorescence images were collected using appropriate bandpass filters at excitation wavelengths of 488 nm.
Data analysis.
Data are expressed as mean ± SEM. Statistical analysis was performed using Student's t test or ANOVA with Fisher's post hoc comparison. An α of p < 0.05 was considered significant.
Results
Hypoxia activates transcriptional regulation of mitochondrial biogenesis
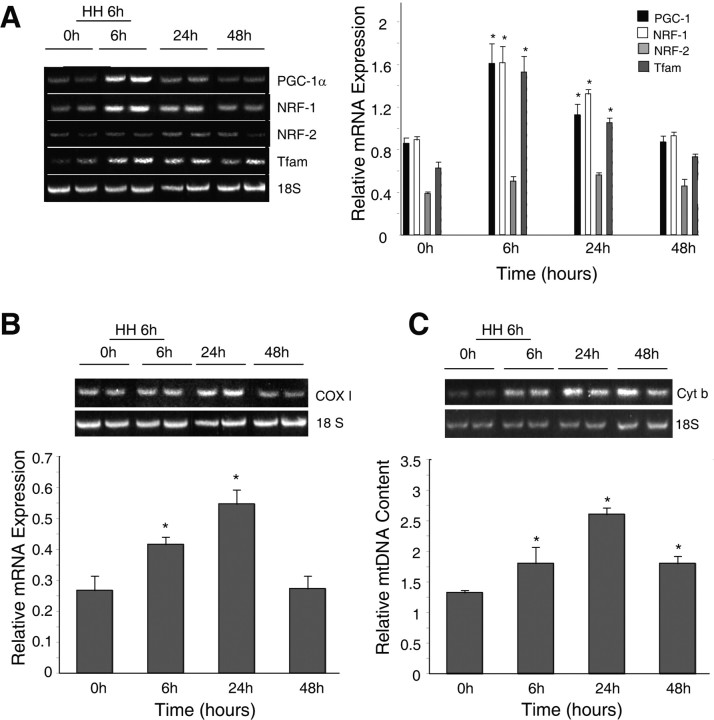
To test the hypothesis that hypoxia activates mitochondrial biogenesis in the brain subcortex, we evaluated the expression of NRF-1 and NRF-2, key nuclear transcription factors that regulate critical proteins involved in mitochondrial biogenesis. The mRNA expression levels of the coactivator PGC-1α and the mitochondrial transcription factor Tfam, a nuclear gene under transcriptional control by these factors, was also measured in the subcortex of Wt mice before and immediately after 6 h of hypoxia and at 24 and 48 h after exposure onset. These data are shown in Figure 1A. PGC-1α mRNA expression increased twofold after 6 h of hypoxia, remained significantly elevated at 24 h, and returned to control levels by 48 h. A twofold increase in NRF-1 mRNA expression was also detected after 6 h hypoxia, which persisted for 24 h but returned to control levels by 48 h. NRF-2 mRNA levels did not change significantly after hypoxia. Similar to PGC-1α and NRF-1, Tfam mRNA expression increased threefold at the end of hypoxia compared with nonhypoxic controls, remained elevated at 24 h, and returned to baseline by 48 h.
Figure 1.
Activation of mitochondrial biogenesis in the brain subcortex of Wt mice exposed to HH. A, mRNA expression for transcription factors and the PGC-1α cofactor. Left, Representative GelStar-stained 2% gels showing PGC-1α, NRF-1, NRF-2, and Tfam transcripts by reverse transcription (RT)-PCR before and at different times after hypoxia. Right, Densitometry of PGC-1α, NRF-1, NRF-2, and Tfam mRNA expression normalized to the 18S rRNA. Values are expressed as means ± SEM of four animals at each time (*p < 0.05 compared with control). B, Mitochondria encoded mRNA expression. Top, Representative gel of RT-PCR for mitochondria-encoded subunit I of cytochrome c oxidase. RNA was obtained from subcortex of control mice (0 h) and mice at 6, 24, and 48 h after HH. Nuclear 18S rRNA was used to control for RNA loading and RT-PCR efficiency. Bottom, Histogram of COX I mRNA expression by densitometry. Values are mean ± SEM for four animals at each time. White bars, Control; gray bars, HH-exposed mice (*p < 0.05 compared with control). C, MtDNA content. Top, Representative gel of PCR product for mitochondrial cytochrome b before and after hypoxia. Bottom, Densitometry of cytochrome b normalized to 18S rRNA. Values are mean ± SEM for four animals at each time (*p < 0.05 compared with control).
Tfam expression should enhance mitochondrial gene transcription and mitochondrial mRNA levels; therefore, steady-state mRNA levels for mito-chondria-encoded subunit I of cytochrome c oxidase (COX I) were measured in Wt mice after hypoxia (Fig. 1B). COX I expression increased significantly in the subcortex after hypoxia and was twice the control at 24 h before returning to baseline at 48 h. Because mtDNA replication should increase mtDNA content, the ratio of cytochrome b DNA to nuclear 18S rRNA was measured as a quantitative index of mtDNA content (Suliman et al., 2005, 2006). Figure 1C shows that, after 6 h hypoxia, mtDNA content increased and reached a maximum by 24 h (approximately twofold) and remained elevated at 48 h. These data indicate that hypoxic signaling through NRF-1 and PGC-1α activates mitochondrial biogenesis in the subcortex.
Hypoxia increases PGC-1α and CREB protein expression
The expression of PGC-1α protein was evaluated by Western analysis of nuclear extracts from brain subcortex in control conditions and immediately after 6 h of hypoxia. Western blotting was performed with antibody to PGC-1α, and the band densities were normalized to tubulin to verify equal protein loading. PGC-1α protein was expressed at low levels under normoxic conditions, and exposure to hypoxia enhanced PGC-1α protein expression in brain subcortex by threefold (Fig. 2A,B).
Figure 2.

Western blot analysis for proteins involved in mitochondrial biogenesis in brain subcortex of Wt mice exposed to HH. A, B, Representative Western blots of PGC-1α expression (A) and CREB phosphorylation (B), in brain nuclear extracts of controls and mice after hypoxia (6 h). Histograms show densitometry values for PGC-1α and CREB relative to tubulin, expressed as means ± SEM of four animals at each group (*p < 0.05 compared with control). C, Immunohistochemical localization of pCREB. Paraformaldehyde-fixed sagittal sections prepared from normoxic mouse brain and at 6 h after hypoxia were incubated with anti-pCREB. Controls (a) showed minimal pCREB staining in the dentate gyrus. After 6 h exposure to HH (b), numerous cells in the dentate gyrus showed robust staining for pCREB. Original magnification, 400×.
CREB is an important transcription factor that regulates PGC-1α gene expression (Herzig et al., 2001) and is a prime candidate for activating mitochondrial biogenesis in response to hypoxia. The levels of activated (phosphorylated) CREB protein were quantified by Western in brain subcortex in Wt mice exposed to hypoxia. Nuclear extracts from the subcortex of normoxic and 6 h hypoxic mice were probed with CREB phospho-specific antibodies, and band densities were normalized to tubulin. Hypoxia increased CREB phosphorylation in the subcortex of Wt mice by 2.2-fold compared with normoxic controls (Fig. 2A,B).
To test the hypothesis that hippocampus would be particularly receptive to hypoxic activation, immunohistochemistry was used to localize pCREB within the subcortex in Wt animals before and after 6 h hypoxia (Fig. 2C). Minimal staining for pCREB was found in control sections, but hypoxia led to a significant increase in neuronal immunoreactivity in the hippocampus. In high-magnification images of the dentate gyrus region (Fig. 2C), staining for pCREB in control sections was low (a), but dense nuclear staining was seen in many neurons in the hypoxic animals (b), indicating nuclear activation of CREB in the hypoxic hippocampus. Specific immunoreactivity was confirmed by the absence of immunostaining when primary antibody was omitted and by demonstrated loss of immunostaining with progressive dilutions of pCREB antisera (data not shown).
Hypoxia stimulates mitochondrial biogenesis in mtGFP-tg mice
Our data indicated that CREB activation in the hypoxic brain subcortex is associated with activation of NRF-1 and PGC-1α and mtDNA replication. To localize mitochondrial biogenesis in the brain definitively, we performed fluorescence microscopy in mtGFP-tg mice before and after exposure to acute hypoxia. These animals express fluorescence exclusively in mitochondria and are ideal for localizing and quantifying the organelles (Shitara et al., 2001). At 24 and 48 h after the onset of 6 h of hypoxia, we found that the main site of increased subcortical mitochondrial fluorescence was the hippocampus. Figure 3A shows representative fluorescence photomicrographs of the hippocampus in control mtGFP-tg mice and at 24 and 48 h after hypoxia. In nonhypoxia exposed controls, mitochondrial localized GFP was present throughout the neuronal cytoplasm in the hippocampus. The nucleus is visible as a central area devoid of staining (Fig. 3Aa,Ad). By 24 h, mitochondrial fluorescence was enhanced in a perinuclear distribution in focal areas of the hippocampus (Fig. 3Ab,Ae). Notably, hippocampal neurons did not respond uniformly to hypoxia, and some showed little fluorescence. At 48 h, hippocampal mitochondrial fluorescence still remained above control (Fig. 3Ac,Af). By comparison, neuronal fluorescence was scattered and an occasional bright cell was detected in the brain cortex (Fig. 3B). Typically, no significant increase in cortical fluorescence was detected 24 h after 6 h of acute hypoxia in photomicrographs comparing cortex (Fig. 3Ba,Bb) and hippocampus (Bc,Bd) in the same mice.
Figure 3.

Fluorescence microscopy for mitochondrial localization in targeted GFP transgenic mice. Aa, Ad, Mitochondrial distribution in the CA1 and CA2 regions of hippocampus in unexposed mice. Ab, Ae, CA1 and CA2 regions of hippocampus with increased mitochondrial fluorescence 24 h after hypoxia. Ac, Af, The CA1 and CA2 regions of hippocampus showing increased mitochondrial fluorescence 48 h after hypoxia. Ba and Bb show scattered fluorescence in the cortex that is similar before (a) and 24 h after (b) hypoxia, whereas the hippocampus in the same mice (c) shows a generalized increase after hypoxia (d). Original magnification, 400×.
Role of NOS in activation of mitochondrial biogenesis by hypoxia
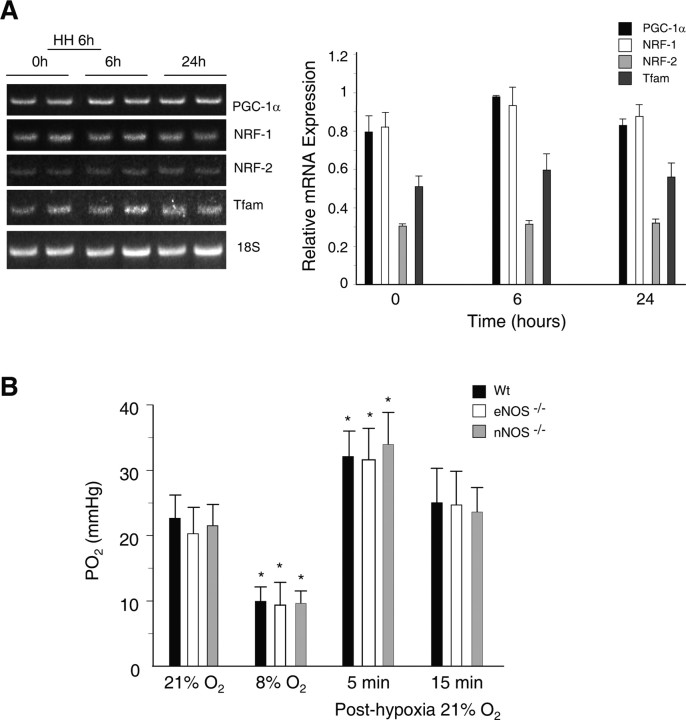
To determine whether NO regulates hypoxic activation of mitochondrial biogenesis in the brain, we blocked NOS activity by injection of Wt mice with the nonspecific NOS inhibitor l-NAME before hypoxia. In l-NAME-treated mice, hypoxia did not increase mRNA expression for PGC-1α, NRF-1, NRF-2, or Tfam (Fig. 4A).
Figure 4.
l-NAME blocks mitochondrial biogenesis in brain subcortex in Wt mice exposed to HH, and PO2 values are similar in Wt and NOS-deficient strains. A, Left, GelStar-stained 2% agarose gel of RT-PCR products for PGC-1α, NRF-1, NRF-2, and Tfam RNA from subcortex of control and l-NAME-treated mice after HH. Right, Gene expression after normalization to 18S rRNA. Densitometry values are means ± SEM of four animals at each time (no significant differences compared with control). B, Hippocampal PO2 in Wt and eNOS−/− and nNOS−/− mice measured after 15 min breathing 8% O2 and after return to 21% O2. PO2 decreased equally after hypoxia, increased transiently after reexposure to 21% O2, and then returned to preexposure levels (*p ≤ 0.01).
Because constitutive NOS inhibition with l-NAME prevented hypoxic induction of the mRNA for the major regulatory factors of mitochondrial biogenesis, a role for one or both constitutive isoforms was indicated. We used two NOS-deficient mouse strains, nNOS−/− (NOS-1) and eNOS−/− (NOS-3) to determine whether one isoform regulates mitochondrial biogenesis in acute hypoxia. We measured resting rCBF and PO2 in the hippocampus to determine whether the values were comparable in the three strains of mice (Fig. 4B). The resting blood flow in hippocampus was 0.76–0.81 ml · g−1 · min−1 and did not differ significantly among the Wt, nNOS−/−, or eNOS−/− animals. The rCBF responses to hypoxia/reoxygenation did not differ significantly in Wt and mutant mice. In all three strains, hippocampal PO2 values were closely similar in mice breathing air (21% O2). Within 15 min of ventilation with 8% O2, hippocampal PO2 decreased to 6–9 mmHg in all strains, where it remained for at least 15 min. After reoxygenation at 30 min, PO2 returned to normal with a small and similar hyperemic overshoot in all three strains.
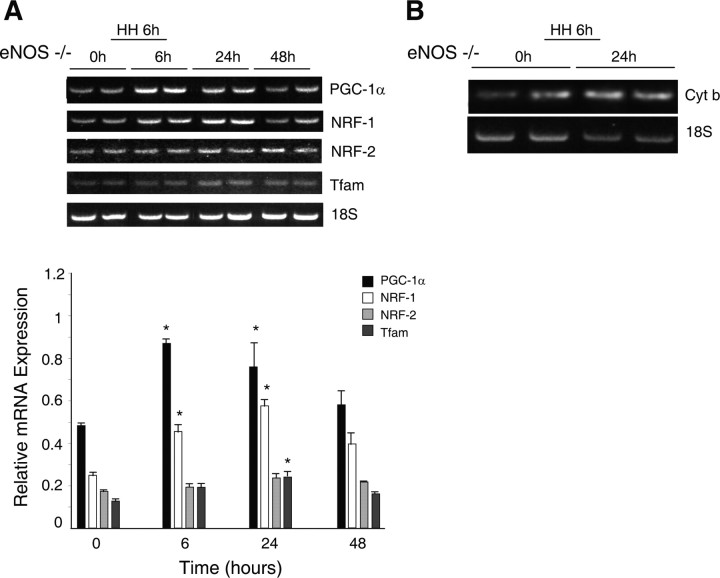
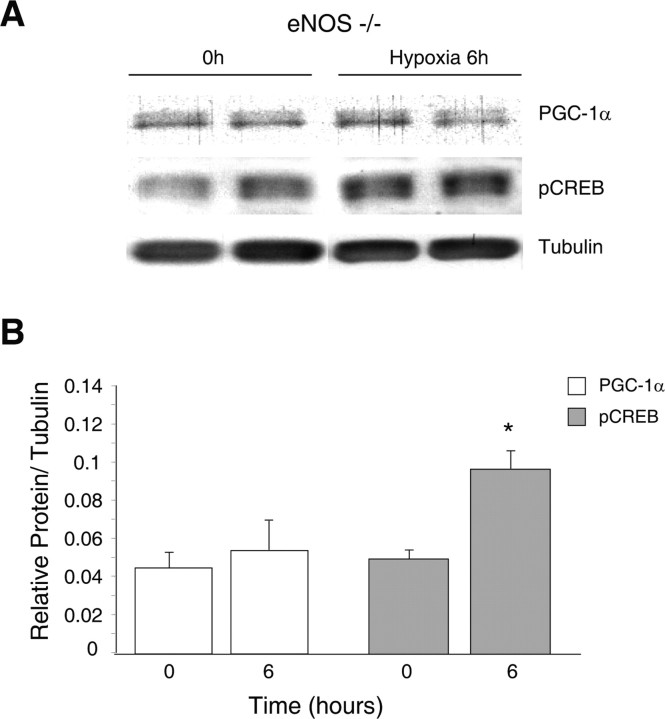
PGC-1α, NRF-1, NRF-2, and Tfam mRNA expression was measured in the brains of the NOS-deficient mice after 6 h hypoxia and then 24 and 48 h after the onset of exposure. Figure 5A displays mRNA expression levels of transcriptional activators of mitochondrial biogenesis in eNOS−/− mouse brain before and after hypoxia. Basal levels of PGC-1α, NRF-1, NRF-2, and Tfam mRNA expression, relative to 18S RNA, were less in eNOS-deficient mice compared with Wt mice (compare Fig. 1A), confirming a role for eNOS in basal maintenance of mitochondrial biogenesis in the subcortex. PGC-1α and NRF-1 mRNA expression increased by twofold in the eNOS−/− mice after 6 h hypoxia, remained elevated after 24 h, and returned to preexposure levels by 48 h (Fig. 5A). As in the Wt, NRF-2 mRNA expression did not respond significantly to hypoxia. Tfam mRNA levels did not increase significantly in eNOS−/− mice (Fig. 5A). Increased NRF-1 and PGC-1α correlated with increased mtDNA content assessed at 24 h after the onset of hypoxia when the increase in subcortical mtDNA content in the Wt strain was the greatest. In eNOS−/− mice, the basal mitochondrial cytochrome b to 18S ratio was lower than in Wt mice, but these animals displayed an increase in mtDNA content at 24 h after hypoxia (Fig. 5B). Densitometry of cytochrome b normalized to 18S rRNA showed a 1.7-fold increase (p < 0.01) in mtDNA content. Also after 6 h of hypoxia, PGC-1α protein in eNOS−/− mice did not increase significantly relative to tubulin levels and compared with controls (Fig. 6A,B).
Figure 5.
Mitochondrial biogenesis in the brains of eNOS−/− mice exposed to HH. A, mRNA levels. Top, GelStar-stained 2% agarose gels of RT-PCR products of RNA prepared from controls and after HH (6, 24, and 48 h). Nuclear gene expression for PGC-1α, NRF-1, NRF-2, and Tfam was measured by RT-PCR. The mRNA for 18S rRNA was used to control for RNA loading and RT-PCR efficiency. Bottom, mRNA levels after normalization to 18S rRNA. Graph represents four samples in each group expressed as means ± SEM (*p < 0.05 compared with control). B, mtDNA content. Representative gel of PCR product for mitochondrial cytochrome b. DNA was obtained from subcortex of eNOS−/− control mice and at 6 and 24 h after HH.
Figure 6.
Western blot analysis for proteins involved in mitochondrial biogenesis in subcortex of eNOS−/− mice exposed to HH. A, Representative Western blots of PGC-1α expression and CREB phosphorylation in nuclear extracts of brains of control eNOS−/− mice and eNOS−/− mice after hypoxia (6 h). B, Histogram shows densitometry values for PGC-1α and CREB relative to tubulin, expressed as means ± SEM of four animals at each group (*p < 0.05 compared with control).
Whether or not CREB activation during hypoxia is regulated by NOS was assessed by examining the role of eNOS in CREB activation. Significantly less pCREB protein was present in subcortex of normoxic eNOS−/− mice compared with the Wt (∼50%). However, eNOS−/− mice displayed a twofold increase in CREB phosphorylation after 6 h of hypoxia (Fig. 6A,B), similar to the Wt. These data indicate a constitutive role for eNOS in CREB activation in the subcortex but that it is not essential for the CREB activation in hypoxia. It is notable that, although the eNOS−/− mice did show augmentation of the mitochondrial biogenic program, the overall response was blunted compared with that of Wt mice, indicating that the basal capacity for biogenesis restricts the ability to develop a full response to a hypoxic preconditioning regimen.
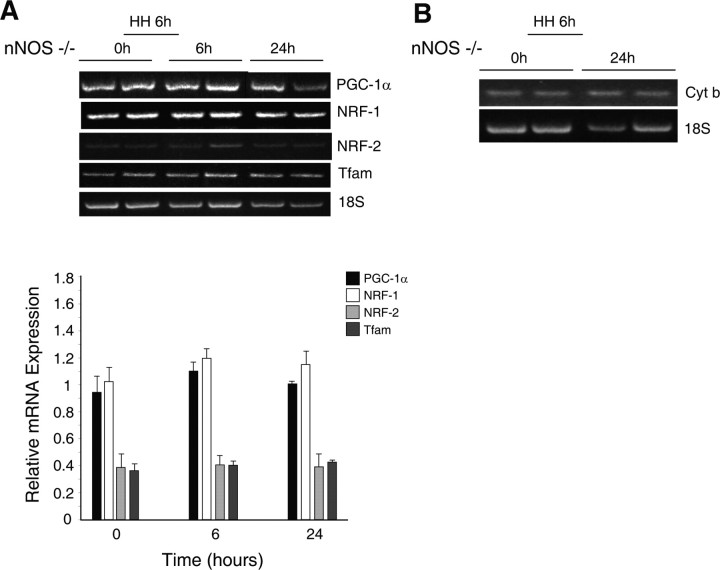
Because eNOS did not explain the activation of mitochondrial biogenesis after hypoxia, we examined the nNOS-deficient mouse strain and observed a different mRNA expression pattern for the transcriptional program of mitochondrial biogenesis in the subcortex compared with eNOS−/− or Wt mice. Basal PGC-1α, NRF-1, and NRF-2, and Tfam mRNA expression was similar to Wt mice and higher than in eNOS−/− mice. In contrast, however, these mice displayed minimal changes in PGC-1α, NRF-1, NRF-2, or Tfam mRNA levels after 6 h hypoxia or at 24 h after the onset of the hypoxic exposure (Fig. 7A). This translated to no significant change in mtDNA content in the nNOS−/− strain before and 24 h after hypoxia (Fig. 7B).
Figure 7.
Mitochondrial biogenesis in the brains of nNOS−/− mice exposed to HH. A, mRNA levels for mitochondrial biogenesis markers. GelStar-stained 2% agarose gels demonstrating RT-PCR products from subcortex in nNOS−/− mice are shown on top. RNA was prepared from control brain (0 h) and after hypoxia (6 and 24 h). Gene expression for PGC-1α, NRF-1, NRF-2, and Tfam was measured with RT-PCR using 18S rRNA to control for RNA loading and amplification (bottom). Values are mean ± SEM for four animals at each time (no significant differences compared with control). B, mtDNA content. Representative gel showing PCR products for mitochondrial cytochrome b. DNA was obtained from subcortex of nNOS−/− control mice and at 6 and 24 h after HH.
A role for nNOS in CREB activation during hypoxia was checked next. Western analysis for PGC-1α protein expression and CREB phosphorylation in nNOS−/− mice showed no significant responses of these proteins after 6 h of hypoxia (Fig. 8A,B), indicating that nNOS was required for CREB activation by hypoxia. A summary of the mRNA, protein, and mtDNA responses in the three strains of mice is provided in Table 2.
Figure 8.
Western blot analysis for proteins involved in mitochondrial biogenesis in subcortex of nNOS−/− mice exposed to HH. A, Representative Western blots of PGC-1α expression and CREB phosphorylation in nuclear extracts of brains of control nNOS−/− mice and nNOS−/− mice after hypoxia (6 h). B, Histogram shows densitometry values for PGC-1α and CREB relative to tubulin, expressed as means ± SEM of four animals at each group.
Table 2.
Changes in mRNA and protein expression for regulation of subcortical mitochondrial biogenesis and mtDNA content in response to acute hypoxia
| Strain | mRNA |
Protein |
|||||
|---|---|---|---|---|---|---|---|
| PGC-1α | NRF-1 | NRF-2 | Tfam | PGC-1α | pCREB | mtDNA Content | |
| Wt | ++ | ++ | − | ++ | ++ | ++ | ++ |
| eNOS−/− | ++ | ++ | − | + | − | ++ | ++ |
| nNOS−/− | − | − | − | − | − | − | − |
Role of protein kinase A in mitochondrial biogenesis
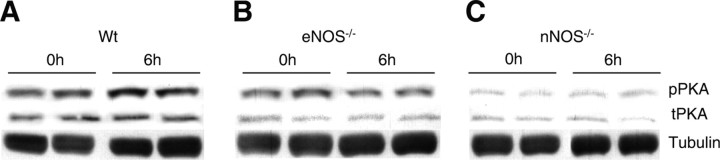
After finding an nNOS requirement for CREB activation and mitochondrial biogenesis in acute transient hypoxia, we investigated the cAMP/PKA signaling pathway as a possible regulator of CREB activation. This kinase is activated by hypoxia (Benzi and Villa, 1976) and plays a pivotal role in CREB phosphorylation (Gonzalez and Montminy, 1989). Because phosphorylation of Thr 197 in the catalytic PKA subunit is necessary for maturation and optimal biological activity of the kinase (Steinberg et al., 1993), we measured the phosphorylated PKA catalytic subunit by Western analysis in subcortical nuclear extracts of Wt and NOS-deficient mice before and after hypoxia. Hypoxia increased phospho-PKA level in the subcortex of Wt mouse brain after 6 h (Fig. 9A). Basal brain PKA phosphorylation was lower in nNOS−/− mouse subcortex compared with Wt and eNOS−/− mice (Fig. 9). However, neither the nNOS- nor eNOS-deficient strain showed PKA activation after hypoxia (Fig. 9B,C). Because these strains show divergent mitochondrial biogenesis responses, PKA did not appear to be a central regulator of the hypoxic response under the acute test conditions.
Figure 9.
PKA phosphorylation in brain subcortex after hypoxia. Western analysis for total and phosphorylated PKA catalytic subunit in brain nuclear extracts of Wt (A), eNOS−/− (B), and nNOS−/− (C) mice. For all three strains, brain nuclear extracts of controls (0 h) and mice after 6 h hypoxia were probed with phospho-PKA antibodies and compared with total PKA and tubulin expression.
Discussion
This study reports three novel findings about the response of brain mitochondria to transient global hypoxia. First, our hypoxic regimen stimulates mitochondrial biogenesis in the brain subcortex by activating the nuclear-encoded regulatory program for mitochondrial biogenesis, including the NRF-1 transcription factor, the PGC-1α coactivator, and the mitochondrial transcription factor Tfam. This program led to a subsequent increase in mtDNA content, especially in the hippocampus, which is especially vulnerable to hypoxic injury. Second, NOS enzyme activity is required for both the maintenance of mtDNA and the induction of mitochondrial biogenesis in brain subcortex by acute hypoxia. Third, the NO bioactivity for these two functions is specifically derived from different NOS isoforms.
The acute and chronic adaptive responses to hypoxia include the initiation of gene transcription for proteins involved in angiogenesis, anaerobic glucose metabolism, and oxygen transport. These responses sustain O2 supply to tissues and enhance cell survival during O2 deprivation (Semenza and Wang, 1992; Levy et al., 1995; Bunn and Poyton, 1996; Wick et al., 2002). We demonstrate for the first time that a sensitive brain region responds to acute hypoxia by activating critical nuclear and mitochondrial pathways for mitochondrial biogenesis. These responses are accompanied by increased mitochondrial DNA transcription and content, followed by structural evidence of neuronal mitochondrial biogenesis, demonstrated in the hippocampus using transgenic mice with GFP targeting to mitochondria (Shitara et al., 2001). This novel demonstration of the capacity to increase neuronal mitochondrial number and/or volume density in response to hypoxia indicates differential sensitivity of hippocampal neurons to acute hypoxia, likely reflecting phenotypic or functional differences among the cells.
Activation of only one of the two main nuclear respiratory transcription factors in the subcortex, NRF-1, is implicated in the hypoxic mitochondrial response. Although NRF-1 and NRF-2 both regulate aspects of mitochondrial biogenesis and binding sites for both are present in the promoter regions of many nuclear-encoded mitochondrial genes, including Tfam and several electron transport chain proteins (Scarpulla, 1997), NRF-2 did not increase in brain subcortex after hypoxia. In contrast, we found previously that extreme hyperoxia activated both transcription factors in this same region in association with significant mitochondrial oxidative stress (Gutsaeva et al., 2006). This indicates that both extremes of oxygen partial pressure activate parts of the transcriptional program for mitochondrial biogenesis but that the mechanisms and implications of hypoxia and hyperoxia are not the same.
The transcriptional activity of both NRF-1 and NRF-2 is enhanced by the PGC-1α coactivator in the process of mitochondrial biogenesis (Puigserver et al., 1998; Wu et al., 1999). The PGC-1α requirement has been demonstrated in cultured myoblasts, cardiac cells, and brown fat tissue during adaptive thermogenesis (Puigserver et al., 1998; Wu et al., 1999; Lehman et al., 2000). PGC-1α is widely expressed in rodent brain (Tritos et al., 2003), and we found that, in the hippocampus, elevated O2 increased its expression only after extreme hyperbaric hyperoxia (Gutsaeva et al., 2006). The present data also suggest that PGC-1α helps coordinate gene activation and facilitate mitochondrial biogenesis in this brain region in hypoxia.
The nuclear transcriptional program activated by hypoxia includes Tfam expression, which heralded an increase in mtDNA content and confirmed effective nuclear–mitochondrial communication. Tfam is necessary for mtDNA replication and transcription (Parisi et al., 1993; Virbasius and Scarpulla, 1994; Larsson et al., 1998), and, in addition to the brain after hyperbaria, it is activated in the heart and liver after lipopolysaccharide-induced mtDNA depletion (Suliman et al., 2003, 2004).
A highly novel aspect of this work is linkage of specific NOS isoforms to basal and inducible mitochondrial biogenesis in the brain. Although pronounced increases in NRF-1 and PGC-1α mRNA and mtDNA content were found in eNOS−/− mice after 6 h of hypoxia, Tfam mRNA levels responded, but increases in PCG-1α protein were not detected in this strain. Although eNOS-deficient mice might regulate mitochondrial biogenesis in response to hypoxia through PGC-1α-independent mechanisms, increased protein levels may not have been detected because we focused on early events. However, eNOS−/− mice do show diminished brain mitochondrial biogenesis, and it is not surprising that some aspects of the hypoxia response would be attenuated or absent.
Hypoxia activates nNOS and increases its expression, thereby implicating it as an important trigger for the hypoxic responses in the nervous system (Castro-Blanco et al., 2003; Encinas et al., 2004; Mishra et al., 2006). In other tissues, such as brown fat, NO derived from eNOS is required for basal and cold-induced mitochondrial biogenesis (Nisoli et al., 2003). Here eNOS in the hippocampus was necessary for basal mitochondrial biogenesis, whereas nNOS was required for the hypoxic response. These data raise important questions about differential functions of the NOS isoforms in the brain, related for instance to differences in intracellular enzyme expression in different cell types, leading to spatial constraints on NO signaling pathways. It is also possible that nNOS has a lower Km for oxygen, allowing it to respond more robustly to hypoxia.
Functional cAMP response element (CRE) sites are present in the promoter regions of nuclear-encoded mitochondrial genes such as cytochrome c (Gopalakrishnan and Scarpulla, 1994) and manganese superoxide dismutase (Bedogni et al., 2003), as well as the PGC-1α coactivator gene (Herzig et al., 2001). Thus, the prosurvival transcription factor CREB is a candidate sensor for energy insufficiency and has been implicated in support of mitochondrial biogenesis (Herzig et al., 2000). During hypoxia, CREB activation in the mouse brain correlates with mitochondrial biogenesis and requires nNOS. CREB is also upregulated in hypoxia in other tissues through NO-dependent mechanisms (Beitner-Johnson and Millhorn, 1998; Mishra et al., 2002). In the brain, nNOS overexpression in neuronal cells enhances CREB activity and promotes cell survival (Ciani et al., 2002b), and, in cortical and hippocampal neurons, NO promotes CREB binding to CRE promoter sites through S-nitrosylation of nuclear proteins that associate with CREB target genes (Riccio et al., 2006).
In neurons, CREB activity is regulated by phosphorylation via several kinases, including PKA, Akt/PKB, PKC, and calcium/calmodulin-dependent kinase (for review, see Vo and Goodman, 2001; Lonze and Ginty, 2002). Thus, NO-dependent CREB activation could occur in hypoxia by any of several mechanisms, including S-nitrosylation or activation of cAMP and cGMP pathways. We investigated one possibility, that CREB activation was cAMP/PKA dependent, because PKA is activated by loss of mitochondrial function and energy depletion (Benzi and Villa, 1976). We observed that nNOS is important for basal PKA activation in the subcortex, raising the possibility of crosstalk between cyclic nucleotides pathways, for instance, by a cGMP influence on cAMP hydrolysis through the phosphodiesterases (Steinberg et al., 1993; Sutor et al., 1998; Pelligrino and Wang, 1998). We also found, however, that the PKA response during hypoxia was absent in both NOS knock-out strains. Because Wt and eNOS−/− strains had comparable increases in pCREB in hypoxia, an alternate pathway of CREB activation by hypoxia is implicated in eNOS−/− mice. Moreover, because nNOS- and eNOS-deficient strains display similar depression of PKA activation, alternate pathways of NOS-mediated activation of mitochondrial biogenesis in eNOS−/− mice are likely.
The sGC/cGMP/PKG pathway is a potential candidate for regulating hypoxic activation of mitochondrial biogenesis. In some non-neuronal tissues, PKG mediates NOS-mediated mitochondrial biogenesis (Nisoli et al., 2003). PKG also directly regulates CREB in vitro and indirectly in vivo through NO–cGMP activity (Wu et al., 2002; Yang et al., 2002); this can upregulate prosurvival mitochondrial proteins and is involved in hypoxic preconditioning in some tissues (Chiueh et al., 2005). NO-dependent PKG activation has also been implicated in CREB phosphorylation and survival of cerebellar granule cells (Ciani et al., 2002a). In neurons, sGC inhibition diminishes pCREB levels and the protective effects of nNOS overexpression (Ciani et al., 2002b).
Finally, it should be said that the activation of mitochondrial biogenesis after acute transient hypoxia does not necessarily predict the response to prolonged hypoxia. An increased mitochondrial mass early in hypoxia could indicate that new or remodeled mitochondria are distributed differently either with respect to capillaries or to meet different functions in various cells in hypoxia. For the first possibility, the transcription factor NRF-1 does regulate several of the mitochondrial fission and fusion genes, and it is feasible, but unknown, whether it regulates mitochondrial position in the cell. Although we did not examine whether mitochondrial biogenesis occurs in areas close to blood vessels, we did see that it occurs in specific regions and individual cells, suggesting that the response is attributable to changes in the distribution of energy-requiring functions. For the second possibility, it is reasonable that some older mitochondria are relieved of work, whereas new ones take on extra work somewhere else. This means the CMRO2 could remain stable while the work distribution changes. Once hypoxia is relieved, the brain would gradually de-adapt; however, if hypoxia is prolonged, original “normoxic” organelles may undergo mitophagy, in which case, the chronic picture may look quite different. Moreover, new mitochondria may have unique phenotypes in hypoxia (Fukuda et al., 2007), perhaps younger organelles are more efficient or better at nonenergy-producing functions thus providing an advantage.
In summary, we report that a representative hypoxia preconditioning regimen stimulates mitochondrial biogenesis in the brain subcortex of mice in part by upregulation of PGC-1α, NRF-1, and Tfam. The process is NO dependent and is mediated by the nNOS isoform. Moreover, NO signaling of mitochondrial biogenesis in hypoxia appears to be linked to CREB-stimulated target gene transcription. These cellular responses appear to be a part of a regional adaptive program to optimize oxygen utilization, energy production, and/or mitochondrial phenotype during cerebral O2 limitation.
Footnotes
This work was supported by the Office of Naval Research and National Institutes of Health Grant P01 HL42-444 (C.A.P.).
References
- Bedogni B, Pani G, Colavitti R, Riccio A, Borrello S, Murphy M, Smith R, Eboli ML, Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003;278:16510–16519. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Millhorn DE. Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism. J Biol Chem. 1998;273:19834–19839. doi: 10.1074/jbc.273.31.19834. [DOI] [PubMed] [Google Scholar]
- Benzi G, Villa RF. Adenyl cyclase system and cerebral energy state. J Neurol Neurosurg Psychiatry. 1976;39:77–83. doi: 10.1136/jnnp.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidotti G, Borthwick IA, May B. Identification of regulatory sequences in the gene for 5-aminolevulinate synthase from rat. J Biol Chem. 1993;268:1109–1117. [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Castro-Blanco S, Encinas JM, Serrano J, Alonso D, Gomez MB, Sanchez J, Rios-Tejada F, Fernandez-Vizarra P, Fernandez AP, Martinez-Murillo R, Rodrigo J. Expression of nitrergic system and protein nitration in adult rat brains submitted to acute hypobaric hypoxia. Nitric Oxide. 2003;8:182–201. doi: 10.1016/s1089-8603(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Andoh T, Chok PB. Induction of thioredoxin and mitochondrial survival proteins mediates preconditioning-induced cardioprotection and neuroprotection. Ann NY Acad Sci. 2005;1042:403–418. doi: 10.1196/annals.1338.034. [DOI] [PubMed] [Google Scholar]
- Ciani E, Guidid S, Bartesaghi R, Contestabile A. Nitric oxide regulates cGMP-dependent cAMP-responsive element binding protein phosphorylation and Bcl-2 expression in cerebellar neurons: implication for a survival role of nitric oxide. J Neurochem. 2002a;82:1282–1289. doi: 10.1046/j.1471-4159.2002.01080.x. [DOI] [PubMed] [Google Scholar]
- Ciani E, Guidid S, Valle GD, Perini G, Bartesaghi R, Contestabile A. Nitric oxide protects neuroblastoma cells from apoptosis induced by serum deprivation through cAMP-response element-binding protein (CREB) activation. J Biol Chem. 2002b;277:49896–49902. doi: 10.1074/jbc.M206177200. [DOI] [PubMed] [Google Scholar]
- Demchenko IT, Luchakov YI, Moskvin AN, Gutsaeva DR, Allen BW, Thalmann ED, Piantadosi CA. Cerebral blood flow and brain oxygenation in rats breathing oxygen under pressure. J Cereb Blood Flow Metab. 2005;25:1288–1300. doi: 10.1038/sj.jcbfm.9600110. [DOI] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Gross GJ, Baker JE. Increased mitochondrial K(ATP) channel activity during chronic myocardial hypoxia: is cardioprotection mediated by improved biogenesis? Circ Res. 2000;87:915–921. doi: 10.1161/01.res.87.10.915. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Fernandez AP, Salas E, Castro-Blanco S, Munoz P, Rodrigo J, Serrano J. Nitric oxide synthase and NADPH-diaphorase after acute hypobaric hypoxia in the rat caudate putamen. Exp Neurol. 2004;186:33–45. doi: 10.1016/j.expneurol.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan L, Scarpulla RC. Differential regulation of respiratory chain subunits by a CREB-dependent signal transduction pathway. Role of cyclic AMP in cytochrome c and COX IV gene expression. J Biol Chem. 1994;269:105–113. [PubMed] [Google Scholar]
- Gutsaeva DR, Suliman HB, Carraway MS, Demchenko IT, Piantadosi CA. Oxygen-induced mitochondrial biogenesis in the rat hippocampus. Neuroscience. 2006;137:493–504. doi: 10.1016/j.neuroscience.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Herzig RP, Scacco S, Scarpulla RC. Sequential serum-dependent activation of CREB and NRF-1 leads to enhanced mitochondrial respiration through the induction of cytochrome c. J Biol Chem. 2000;28:13134–13141. doi: 10.1074/jbc.275.17.13134. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala UC, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Larsson NL, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Bursh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Meerson FZ, Pomoinitsskii VD, Iampol'skaia BA. Role of mitochondrial biogenesis in adaptation to altitude hypoxia. Dokl Akad Nauk SSSR. 1972;203:973–976. [PubMed] [Google Scholar]
- Meerson FZ, Gomzakov OA, Shimkovich MV. Adaptation to high altitude hypoxia as a factor preventing development of myocardial ischemic necrosis. Am J Cardiol. 1973;31:30–34. doi: 10.1016/0002-9149(73)90806-0. [DOI] [PubMed] [Google Scholar]
- Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. Phosphorylation of cAMP response element binding (CREB) protein during hypoxia in cerebral cortex of newborn piglets and the effect of nitric oxide synthase inhibition. Neuroscience. 2002;115:985–991. doi: 10.1016/s0306-4522(02)00275-0. [DOI] [PubMed] [Google Scholar]
- Mishra OP, Mishra R, Ashraf QM, Delivoria-Papadopoulos M. Nitric oxide-mediated mechanism of neuronal nitric oxide synthase and inducible nitric oxide synthase expression during hypoxia in the cerebral cortex of newborn piglets. Neuroscience. 2006;140:857–863. doi: 10.1016/j.neuroscience.2006.02.060. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Moncada S, Carruba MO. Mitochondrial biogenesis as a cellular signaling framework. Biochem Pharmacol. 2004;67:1–15. doi: 10.1016/j.bcp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Ou LC, Tenney SM. Properties of mitochondria from hearts of cattle acclimatized to high altitude. Respir Physiol. 1970;8:151–159. doi: 10.1016/0034-5687(70)90011-3. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Xu B, Clayton DA. A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol Cell Biol. 1993;13:951–1961. doi: 10.1128/mcb.13.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Riccio A, Alvania RS, Lonz BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Moll Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory chain expression in mammalian cells. J Bioenerg Biomembr. 1997;29:109–119. doi: 10.1023/a:1022681828846. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, Ardizzone T, Bernaudin M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx. 2004;1:26–35. doi: 10.1602/neurorx.1.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara H, Kaneda H, Sato A, Iwasaki K, Hayashi JI, Taya C, Yonekawa H. Non-invasive visualization of sperm mitochondria behavior in transgenic mice with introduced green fluorescent protein (GFP) FEBS Lett. 2001;500:7–11. doi: 10.1016/s0014-5793(01)02574-1. [DOI] [PubMed] [Google Scholar]
- Steinberg RA, Cauthron RD, Symcox MM, Shunton H. Autoactivation of catalytic (C alpha) subunit of cyclic AMP-dependent protein kinase by phosphorylation of threonine 197. Mol Cell Biol. 1993;13:2332–2341. doi: 10.1128/mcb.13.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. LPS stimulates mitochondria biogenesis via activation of nuclear respiratory factor-1. J Biol Chem. 2003;278:41510–41518. doi: 10.1074/jbc.M304719200. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Welty-Wolf KE, Carraway MS, Tatro L, Piantadosi ClA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res. 2004;64:279–288. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Welty-Wolf KE, Carraway MS, Piantadosi CA. TLR4 regulates mitochondrial biogenesis after lipopolysaccharide. FASEB J. 2005;19:1531–1534. doi: 10.1096/fj.04-3500fje. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Tatro L, Piantadosi CA. A novel activating role for carbon monoxide in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- Sutor B, Mantell K, Bacher B. Evidence for the activity of five adenosine-3′, 5′-monophosphate-degrading phosphodiesterase isozymes in the adult rat neocortex. Neurosci Lett. 1998;252:57–60. doi: 10.1016/s0304-3940(98)00551-5. [DOI] [PubMed] [Google Scholar]
- Tritos NA, Mastaitis JW, Kokkotou EG, Puigserver P, Spiegelman BM, Maratos-Flier E. Characterization of the peroxisome proliferator activated receptor coactivator 1 alpha (PGC 1alpha) expression in the murine brain. Brain Res. 2003;961:255–260. doi: 10.1016/s0006-8993(02)03961-6. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Basse-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Anderson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yang SN, Yang CH, Huang LT, Wu YT, Wang CL. Enhancement of CREBSerine-133 phosphorylation through nitric oxide-mediated signaling induced by bacterial lipopolysaccharide in vascular smooth muscle cells from rats. Chin J Physiol. 2002;45:69–74. [PubMed] [Google Scholar]