Abstract
Acute intermittent hypoxia elicits a form of spinal, brain-derived neurotrophic factor (BDNF)-dependent respiratory plasticity known as phrenic long-term facilitation. Ligands that activate Gs-protein-coupled receptors, such as the adenosine 2a receptor, mimic the effects of neurotrophins in vitro by transactivating their high-affinity receptor tyrosine kinases, the Trk receptors. Thus, we hypothesized that A2a receptor agonists would elicit phrenic long-term facilitation by mimicking the effects of BDNF on TrkB receptors. Here we demonstrate that spinal A2a receptor agonists transactivate TrkB receptors in the rat cervical spinal cord near phrenic motoneurons, thus inducing long-lasting (hours) phrenic motor facilitation. A2a receptor activation increased phosphorylation and new synthesis of an immature TrkB protein, induced TrkB signaling through Akt, and strengthened synaptic pathways to phrenic motoneurons. RNA interference targeting TrkB mRNA demonstrated that new TrkB protein synthesis is necessary for A2a-induced phrenic motor facilitation. A2a receptor activation also increased breathing in unanesthetized rats, and improved breathing in rats with cervical spinal injuries. Thus, small, highly permeable drugs (such as adenosine receptor agonists) that transactivate TrkB receptors may provide an effective therapeutic strategy in the treatment of patients with ventilatory control disorders, such as obstructive sleep apnea, or respiratory insufficiency after spinal injury or during neurodegenerative diseases.
Keywords: plasticity, breathing, neurotrophin, adenosine, transactivation, phrenic
Introduction
The respiratory neural network must accommodate the ever-changing demands on breathing experienced during an individual's lifetime, including adjustments for physical activity, aging, pregnancy, and the onset of disease. Transient adjustments to respiratory motor output are achieved through a variety of feedforward and sensory feedback mechanisms. However, with repeated or chronic perturbations, the respiratory control system exhibits functional plasticity, a change in system performance based on previous experience (Mitchell and Johnson, 2003). Understanding endogenous mechanisms of respiratory plasticity may enable the development of novel therapeutic strategies aimed at improving respiratory function in ventilatory control disorders, such as respiratory insufficiency during spinal cord injury.
Long-term changes in respiratory motoneuron function can occur via plasticity of their synaptic inputs (Mitchell and Johnson, 2003; Golder and Mitchell, 2005). Several models of respiratory synaptic plasticity are induced by neuromodulators such as serotonin and/or norepinephrine versus synaptic activity per se (Feldman et al., 2003; Bocchiaro and Feldman, 2004; Neverova et al., 2007). Downstream signaling mechanisms often involve the neurotrophin brain-derived neurotrophic factor (BDNF) and its high-affinity receptor tyrosine kinase, TrkB (Carter et al., 2002; Baker-Herman et al., 2004; Bramham and Messaoudi, 2005). For example, acute intermittent hypoxia elicits a long-lasting enhancement of phrenic motor activity known as phrenic long-term facilitation (pLTF) (Mitchell et al., 2001). pLTF requires new protein synthesis, most notably new BDNF synthesis (Baker-Herman et al., 2004). BDNF may strengthen excitatory glutamatergic synapses from brainstem respiratory neurons onto phrenic motoneurons, thus enhancing inspiratory motoneuron activity and increasing diaphragm contractions (Feldman et al., 2003). Although intermittent hypoxia has the potential to match motor function with repetitive changes in demand, it is a nonspecific stimulus that may affect many physiological functions and induce pathology (Bass et al., 2004). Thus, models of phrenic motor plasticity that do not require hypoxia are more likely candidates for the development of new therapeutic options to treat respiratory insufficiencies.
BDNF applied to the spinal cord is sufficient to induce phrenic motor facilitation (Baker-Herman et al., 2004). However, CNS administration of proteins is riddled with problems associated with delivery across the blood–brain barrier, penetration to the relevant neural site, and the unintended activation of immune responses against a foreign protein. An alternative approach would be the use of small, highly permeable molecules with properties that mimic the effects of BDNF on respiratory motor output. Adenosine 2a receptor (A2a) activation mimics neurotrophins by transactivating Trk receptors without their neurotrophin ligands in PC12 cells (Lee and Chao, 2001; Lee et al., 2002; Rajagopal et al., 2004). Thus, we hypothesized that spinal A2a receptor activation would transactivate TrkB receptors and mimic the effects of BDNF on phrenic motor output. Here we report that spinal A2a receptor activation elicits phrenic motor facilitation in rats by increasing phosphorylation and intracellular signaling of an immature TrkB receptor isoform near phrenic motoneurons. Furthermore, we report that the capacity to induce phrenic motor facilitation can be harnessed by systemic administration of the A2a receptor agonist, thereby improving breathing in rats with respiratory insufficiency resulting from cervical spinal injury.
Materials and Methods
Animals and experimental groups.
All experiments were conducted on adult (3–6 months of age) male Sprague Dawley rats (308–364 g; Harlan colony 217; Harlan, Indianapolis, IN). The initial experiment was designed to investigate phrenic motor facilitation after spinal A2a receptor activation in anesthetized rats. The second series of experiments explored the relationship between the magnitude of A2a-induced phrenic motor facilitation and strengthening of synaptic pathways onto phrenic motoneurons. In a third series, C4 ventral horn TrkB isoforms were examined with Western analyses for A2a-induced TrkB levels and phosphorylation state, as well as phosphorylation state in its downstream signaling molecules (ERKs 1/2 and protein kinase B). Immunohistochemistry was performed to confirm and localize changes in TrkB protein and phosphorylation within cells of the C4 ventral gray matter. Fourth, RNA interference (RNAi) targeting TrkB and BDNF mRNA was used to inhibit new TrkB and BDNF protein synthesis, thus enabling a test of the hypothesis that new TrkB, but not BDNF, protein synthesis is necessary for A2a-induced phrenic motor facilitation. Finally, the effect of A2a receptor activation on ventilatory function was examined in unanesthetized, freely behaving uninjured rats and in C2 spinally injured rats with decreased tidal volume. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the School of Veterinary Medicine at the University of Wisconsin, Madison.
Measurement of respiratory motor output.
To determine the effects of spinal A2a receptor activation on phrenic motor activity, integrated phrenic amplitude was recorded in anesthetized rats before and after intrathecal drug administration. Anesthesia was induced with isoflurane. Fluid (4 ml · kg−1 · h−1, i.v.; 50:50 mixture of lactated Ringer's and 6% hetastarch) was administered throughout the experiment. The trachea was cannulated, and rats were mechanically ventilated (rodent respirator model 683; Harvard Apparatus, South Natick, MA). Both cervical vagi were transected to prevent entrainment of phrenic activity with the ventilator. A femoral arterial catheter enabled arterial blood pressure monitoring (model P122; Grass Telefactor, West Warrick, RI) and sampling of arterial blood for pH and blood gas analysis (ABL-500; Radiometer, Copenhagen, Denmark). End-tidal pCO2 was measured continuously (Capnoguard; Novametrix Medical Systems, Wallingford, CT). The C2 spinal segment was exposed for intrathecal drug administration and positioning a stimulating electrode in the lateromedial funiculus. To enable intrathecal drug injections, a cannula attached to a Hamilton syringe and filled with the specified drug or vehicle solution was advanced through a C2 durotomy until the tip of cannula rested over the C4 spinal segment.
After surgical preparation, rats were converted to urethane anesthesia (1.8 g · kg−1, i.v.) while isoflurane was discontinued, and then neuromuscularly paralyzed (pancuronium bromide, 1 mg · kg−1, i.v.). Phrenic nerves were isolated, and motor activity was recorded continuously with bipolar silver electrodes, amplified, rectified, moving averaged, digitized, recorded, and analyzed as previously described (Golder and Mitchell, 2005). At least 1 h elapsed after conversion to urethane anesthesia before protocols began.
Baseline nerve activity was established at ∼2–3 mmHg above the apneic threshold (Golder and Mitchell, 2005). Peak integrated phrenic nerve burst amplitude was recorded for 30 min at baseline conditions and for 120 min after intrathecal drug administration. Arterial blood samples were collected during baseline conditions and 30, 60, 90, and 120 min after intrathecal drug administration. Peak moving-averaged phrenic neurogram burst amplitude and burst frequency (“fictive” respiratory depth and rate) were measured off-line over a 20 s period immediately before blood samples. Arterial pCO2 (PaCO2) was controlled by adjusting the level of inspired CO2 or by making ventilator adjustments; blood pressure was maintained by varying the fluid infusion rate. Animals were included in the final analysis only if PaO2 during the entire experiment remained >150 mmHg, and PaCO2 was within 1 mmHg of the baseline value during postinjection measurement periods.
Drug administration.
2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride (CGS 21680; Sigma-Aldrich, St. Louis, MO) is a relatively specific A2a receptor agonist. CGS 21680 (50 μm) was dissolved in DMSO and administered intrathecally over the C4 spinal segment (2 μg · kg−1) or intraperitoneally (100 μg · kg−1) to anesthetized (series 1–4) and (series 5) unanesthetized rats, respectively. The intrathecal dose of CGS 21680 was chosen based on preliminary dose–response studies, using the minimal dose required to achieve consistent phrenic motor facilitation. The intraperitoneal dose was chosen as the minimal dose required for phrenic motor facilitation in anesthetized rats without significant effects on arterial blood pressure. In some rats, MSX-3 (130 ng · kg−1; 10 μm dissolved in DMSO; Sigma-Aldrich), a specific A2a receptor antagonist, was administered intrathecally 30 min before CGS 21680 injection to confirm that the effects of CGS 21680 were caused by C4 spinal A2a a receptor activation. The dose of A2a antagonist was chosen based on preliminary dose–response studies. K252a, a tyrosine kinase inhibitor, prevents TrkB receptor phosphorylation in response to neurotrophin binding. K252a (1.75 μg · kg−1; 150 μm dissolved in DMSO; Sigma-Aldrich) was administered intrathecally to some rats 30 min before intrathecal CGS 21680 injection to determine whether A2a-induced changes in phrenic motor output required C4 spinal TrkB phosphorylation. The dose of K252a required to block TrkB phosphorylation was determined from preliminary studies in which the minimal dose of K252a needed to block the effects of BDNF on phrenic motor output (Baker-Herman et al., 2004) was identified. Emetine is a translation inhibitor that blocks new protein synthesis. In some rats, emetine was administered intrathecally 30 min before CGS 21680 to investigate the role of new protein synthesis in the effects of CGS 21680 on phrenic motor output. The dose of emetine (1 μg · kg−1; 75 μm dissolved in DMSO; Sigma-Aldrich) was chosen from a previous study (Baker-Herman et al., 2004).
Phrenic evoked compound action potentials.
To determine the effects of spinal A2a receptor activation on the strength of short-latency synaptic pathways to phrenic motoneurons (presumptive monosynaptic premotor inputs), we recorded phrenic evoked potentials before and after intrathecal drug administration. Details of the phrenic evoked potential recording technique have been published previously (Fuller et al., 2003, 2006). Rats were hyperventilated (end-tidal PCO2, 27–33 mmHg) to prevent spontaneous phrenic motor activity, thereby isolating phrenic synaptic inputs from central respiratory drive. A monopolar tungsten electrode was positioned in the ventrolateral C2 spinal funiculus (2.0–2.2 mm below the dorsal root entry zone). Electrode position was selected by maximizing the amplitude of a short-latency (< 0.8 ms) evoked potential in the phrenic nerve ipsilateral to the stimulating electrode. Preliminary experiments of stimulus–response relationships indicated a sigmoid response curve to increasing current pulses (20–1000 μA, 0.2 ms duration) using a stimulator (model S88; Grass Instruments, Quincy, MA) and stimulus isolation unit (model PSIU6E; Grass Instruments). Based on these experiments, four stimulating currents (threshold current and threshold + 100 μA, 500 μA, and 1000 μA) were delivered to anesthetized rats before and after intrathecal drug administration. Phrenic potentials were digitized and analyzed with P-CLAMP software (Molecular Devices, Sunnyvale, CA).
In vivo RNAi. Rats that received small interfering RNAs (siRNAs) were surgically prepared as described above. A pool of siRNAs targeting TrkB mRNA was used to determine the role of new synthesis of TrkB protein in A2a-induced phrenic motor facilitation. TrkB siRNAs were obtained as a pool of four 21 nt duplexes (siGENOME, Dharmacon, Lafayette, CO; gene, NTRK2; GenBank accession number, NM 012731). We also used two separate siRNA sequences as internal negative controls: (1) an siRNA with at least four mismatches with all known human, mouse, and rat genes (siCONTROL NonTargeting siRNA #2; Dharmacon) and (2) a pool of functional control siRNAs targeting BDNF. The sequences for the BDNF siRNA prevent new synthesis of C4 spinal BDNF and have been published in detail previously (Baker-Herman et al., 2004). siRNAs were reconstituted with siRNA Universal Buffer (Dharmacon; final concentration, 50 μm) and stored at −20°C. The minimal concentration of TrkB siRNA required to abolish A2a-induced phrenic motor facilitation (50 nm total RNA) was determined from preliminary dose–response studies. siRNAs (5 μl of 500 nm solution) were combined with Oligofectamine (8 μl; Invitrogen, Carlsbad, CA), a transfection reagent, and RNase-free water (37 μl) and incubated at room temperature for 20 min. The siRNAs were injected over the C4 spinal segment via an intrathecal catheter (two 10 μl injections separated by 5 min). Two hours were allowed before initiating the protocol with intrathecal CGS 21680.
Western analysis.
The C4 spinal segment, which contains the majority of phrenic motoneurons, was removed at the end of electrophysiological experiments, snap-frozen on dry ice, and stored at −80°C before homogenization. The spinal segment was mounted on a freezing microtome and the dorsal spinal cord shaved off until the area around the central canal was visible. Only data from ventral spinal cords are reported here. Tissue samples were homogenized in RIPA buffer (200–400 μl) and centrifuged (7000 × g at 4°C for 10 min). The supernatant was collected and separated into aliquots. Total protein was quantified using the bicinchoninic acid method from a commercially available kit (Pierce Biotechnology, Rockford IL). Samples were combined with 6× SDS-PAGE sample buffer (100 mm Tris, 7.5 mm EDTA, 100 mm DTT, 6% SDS, 30% glycerol, and 1% bromophenol blue) and boiled for 4–5 min, and 50 μg of protein was loaded into each well. Proteins were separated by 8% SDS-PAGE gels and then transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA). For pERK1/2 (1:2500; Cell Signaling Technology, Beverly, MA), ERK1/2 (1:2500; Cell Signaling Technology), pAkt (1:1000; Cell Signaling Technology), and Akt (1:1000; Cell Signaling Technology) immunoblots, membranes were blocked in 6% nonfat milk/TBST (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.05% Tween 20) at 37°C for 30 min, and then probed for 1 h at 37°C and overnight at 4°C with primary antibodies. For pTrkB (1:1000; a gift from R. Rajagopal and M. V. Chao, New York University, New York, NY) and TrkB (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) immunoblots, membranes were incubated in 0.25% gelatin/TBST (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 0.05% Tween 20) at 37°C for 30 min, and then probed for 1 h at 37°C and overnight at 4°C with primary antibodies. Some membranes were incubated with two additional pTrk antibodies [pTrk against Y490 (Genetex, San Antonio, TX) and pTrk against Y674/675 (gifted from R. Segal, Harvard University, Cambridge, MA)] to confirm the presence of pTrk bands and determine whether phosphorylation occurred at multiple sites on the Trk protein. Membranes were then washed three times for 5 min each in TBST and subsequently probed for 1 h with secondary antibodies (goat anti-rabbit, 1:4000; Santa Cruz Biotechnology). The immunoreactive bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) and the Autochemi detection system (UVP, Upland, CA). To ensure equal protein loading, blots were reprobed with anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1000; Santa Cruz Biotechnology).
Peptide blocking experiments were performed to determine whether the bands identified by the TrkB antibody represented nonspecific binding. TrkB antibody (SC-119) and its blocking peptide were obtained from Santa Cruz Biotechnology. The primary antibody and blocking peptide (1:40 ratio of antibody/peptide) were incubated together in blocking solution overnight at 4°C before being used for Western analysis.
Deglycosylation of TrkB.
Trk proteins have multiple glycosylation sites, which increase their molecular weight from a core protein (∼50–60 kDa) to a mature isoform expressed on extracellular membranes (∼140–150 kDa) (Watson et al., 1999). PNGase digestion of C4 ventral spinal cords was performed to determine whether the bands identified as TrkB proteins on Western analysis were glycosylated. The digestion protocol has been published in full previously (Watson et al., 1999). Briefly, five C4 ventral spinal cords were homogenized in a 1.5 ml of reaction buffer [10× G7 buffer, 300 μl; 10× denaturing buffer, 30 μl; 10% NP-40, double-distilled H2O (ddH2O), 2.1 ml; New England Biolabs, Beverly, MA] and centrifuged (7000 × g at 4°C for 10 min). The supernatant was boiled for 2 min at 100°C and then separated into two 600 μl aliquots. After cooling, 5000 U of PNGase (New England Biolabs) was added to one aliquot (digestion sample), and 10 μl of ddH2O was added to the other aliquot (control sample). The aliquots were incubated at 37°C and sampled at 15 min. Samples were immediately boiled to denature the enzyme and stored at −80°C until prepared for TrkB Western analysis as described above.
BDNF ELISA.
C4 ventral spinal BDNF protein concentration was measured using a commercially available ELISA kit (Quantikine BDNF ELISA; R & D Systems, Minneapolis, MN) in nine rats that had received intrathecal CGS 21680 and six rats that had received intrathecal DMSO. All tissues were harvested at 120 min after CGS 21680 and prepared in RIPA buffer as described for Western analysis. All samples were diluted fourfold using calibrator diluent RD5K (R & D Systems). A 100 μl of assay diluent RD1S and 50 μl of sample was added to each well. The plate was incubated for 2 h at room temperature. One hundred microliters of conjugate were then added to each well and incubated for 1 h at room temperature. The plate was then washed three times with prepared wash buffer (R & D Systems). Two hundred microliters of substrate solution were added, and the plate was incubated for 30 min away from light. Fifty microliters of stop solution (R & D Systems) were added to each well, and the plate was read in a plate reader using a 540/570 nm wavelength.
Immunohistochemistry.
The cellular localization of TrkB and phospho-TrkB protein was evaluated in C4 spinal segments collected from rats (n = 4) at 120 min after intrathecal delivery of vehicle (DMSO) or CGS 21680 (n = 2 for each treatment). Rats were perfused transcardially with 0.01 m PBS, pH 7.4, followed by 4% buffered paraformaldehyde, pH 7.4. The cervical spinal cords were removed and cryoprotected with 30% sucrose in 0.1 m PBS, and 25 μm transverse sections were cut using a freezing microtome. Four representative sections were selected for each animal. The sections were rinsed in 0.1 m Tris-buffered saline with 0.1% Triton X-100 (TBS-T), incubated in 1% H2O2 for 30 min, and rewashed in TBS-T. The sections were then incubated in 5% normal goat serum for 1 h and overnight in rabbit polyclonal TrkB (1:1000; Santa Cruz Biotechnology) or rabbit polyclonal phospho-TrkB (1:500; courtesy of M. V. Chao). The sections were rewashed in TBS-T and incubated in biotinylated goat anti-rabbit antibody (1:1000; Vector Laboratories, Burlingame, CA) for 1 h. Conjugation with avidin–biotin complex (1:100; Vectastain Elite ABC kit; Vector Laboratories) was followed by visualization with the mixture 3,3′-diaminobenzidine-hydrogen peroxidase with nickel chloride solution (DAB Substrate Kit; Vector Laboratories). To identify cell morphology, all sections were counterstained with 0.1% cresyl-violet, dehydrated, and coverslipped. Sections incubated without primary or secondary antibodies served as negative control.
Double immunofluorescence was used to examine adenosine 2a receptor expression near phrenic motoneurons. Parallel sets of sections from each C4 spinal segment were blocked with 5% donkey serum in 0.1 m TBS-T for 1 h and incubated overnight in a combination of adenosine 2a receptor antibody (1:500; mouse monoclonal; Millipore) with antibody against the mature neuronal marker ChAT (sheep polyclonal; 1:500; Millipore). Sections were washed with TBS-T and then incubated with the secondary antibodies conjugated to donkey anti-mouse (red fluorescent, Alexa 495; 1:200; Invitrogen) and donkey anti-sheep (green fluorescent, Alexa 488; 1:200, Invitrogen) for 2 h at room temperature. After extensive washes, sections were mounted with antifade medium (Invitrogen) and examined under a fluorescent microscope (E600; Nikon, Osaka, Japan). All images were captured and analyzed with a digital camera (SPOT II; Diagnostic Instruments, Sterling Heights, MI). Photoshop software (version 7.0; Adobe Systems, San Jose, CA) was used to create the photomicrographs. Equivalent adjustments to tone scale, gamma, and sharpness were made across all images.
Spinal cord hemisection.
Details of the surgical technique have been published previously (Golder et al., 2003; Golder and Mitchell, 2005). Rats were anesthetized, intubated, and mechanically ventilated. Surgical exposure of the C2 spinal segment permitted aspiration of spinal tissue leaving a 1-mm-long left-sided hemisection. All animals received postsurgical pain control as previously described. Rats recovered for 8 weeks after surgery before use in the whole-body plethysmography experiments.
Whole-body plethysmography.
To investigate whether A2a receptor activation improves ventilatory function in spinally injured rats, ventilation was measured using flow-through, barometric plethysmography before and after CGS 21680 administration. Uninjured and spinally injured rats were placed in a whole-body plethysmograph (model PLY3213; Buxco Electronics, Wilmington, NC). Pressurized air flowed through the chamber providing a bias airflow of 2 L/min, allowing control of inspired gas composition and preventing CO2 accumulation. A pressure calibration signal (obtained before placing a rat into the chamber), plethysmograph temperature, rat body temperature, ambient and chamber pressures, and rat body mass were used in the Drorbaugh and Fenn equation (Drorbaugh and Fenn, 1955) to calculate breath-by-breath minute ventilation (ml · min−1 · 100 g−1), frequency (breaths/min), and tidal volume (ml/100 g) (Biosystems XA software; Buxco Electronics, Wilmington, NC). Baseline recordings were obtained for at least 2 h, during which rats alternated between awake and subjective sleep. Rats were then removed from the chamber and received three injections of CGS 21680 (50 μm, i.p.; each separated by 5 min). Preliminary experiments in anesthetized rats revealed a dose of CGS 21680 that had minimal effects on arterial blood pressure while eliciting A2a-induced phrenic motor facilitation. After the third intraperitoneal injection, rats were returned to the chamber and ventilatory recordings continued for an additional 150 min. Plethysmography data were analyzed in 10 min bins during subjective sleep during baseline conditions and at 60 and 120 min after CGS 21680 administration. Comparisons between baseline and postdrug ventilatory measurements were matched for body position during sleep (curled lateral or head-tucked sternal) because preliminary date demonstrated an effect of body position on the pattern of breathing.
Statistical analysis.
Assumptions of normality and equal variance were tested before parametric analyses were performed. When these assumptions could not be confirmed, nonparametric analysis was performed with Kruskal–Wallis ANOVAs followed by Mann–Whitney U tests. Otherwise, statistical inferences were based on a two-way ANOVA with a repeated-measures design [factors: time (repeated measures) and treatment], with individual comparisons made using Student–Newman–Keuls post hoc test when indicated. Western analysis data were compared between treatment groups and vehicles using one-sample t tests. Differences were considered significant if p < 0.05. All values are expressed as mean ± SEM.
Results
Cervical A2a receptor activation elicits persistent phrenic motor facilitation
Phrenic inspiratory burst amplitude increased above baseline values for >120 min after cervical (C4) intrathecal administration of the A2a receptor agonist CGS 21680 (2 μg · kg−1; 50 μm) in anesthetized rats (Fig. 1a,b). Phrenic burst frequency (fictive respiratory rate) also increased to a small extent (baseline, 41 ± 1; 120 min, 44 ± 1; p < 0.05), but this effect was independent of drug treatment. To confirm the role of cervical spinal A2a receptors near phrenic motoneurons (Fig. 1c) in CGS 21680-induced phrenic motor facilitation, rats were pretreated with cervical intrathecal MSX-3, a specific A2a receptor antagonist. MSX-3 (10 μm) abolished A2a-induced phrenic motor facilitation (Fig. 1b), but only when administered before CGS 21680. When MSX-3 was administered 120 min after CGS 21680, A2a-induced phrenic motor facilitation was unaffected (n = 3; CGS 21680 + MSX-3, 87 ± 16% of baseline), suggesting that cervical spinal A2a receptor activation initiates, but does not maintain, phrenic motor facilitation in anesthetized rats. Alone, MSX-3 (n = 4) did not alter phrenic motor output compared with time controls (MSX-3, 9 ± 7%; time controls, 6 ± 5%, of baseline).
Figure 1.

C4 spinal A2a receptor activation facilitates phrenic motor output in anesthetized rats. a, Representative traces of integrated phrenic discharge before and 120 min after intrathecal injection of the A2a receptor agonist, CGS 21680 (2 μg · kg−1). b, Average data depicting the percentage change in phrenic burst amplitude after intrathecal injections of CGS 21680 (filled circles; n = 12), vehicle (DMSO; squares; n = 6), and the A2a receptor antagonist, MSX-3 (130 ng · kg−1; open circles; n = 5) followed by CGS 21680 (CGS). Intrathecal CGS 21680 significantly increased phrenic burst amplitude. MSX-3 blocks CGS 21680 effects on phrenic motor output, confirming that the effects result from A2a receptor activation. c, Immunofluorescence and representative photomicrographs of back-labeled C4 phrenic motoneurons and A2a receptor protein. A2a receptor protein was localized on and near phrenic motoneurons. *Significantly different from other groups (p < 0.05). Data are mean ± SEM.
A2a receptors strengthens spinal synaptic pathways to phrenic motoneurons
Multiple factors influence phrenic motoneuron activity (Feldman and McCrimmon, 2002), including peripheral and central chemoreflexes. Thus, it is essential to maintain precise blood gas regulation during studies of respiratory plasticity (Mitchell and Johnson, 2003). Measurements of arterial pCO2 and pO2 were similar across treatment groups and did not change over time (data not shown). Mean arterial blood pressure decreased with time, but the change was similar across all experimental groups (data not shown). To assure that A2a-induced phrenic motor facilitation was not the result of drug reaching the brainstem, we recorded respiratory activity in the hypoglossal nerve after cervical intrathecal CGS 21680. No hypoglossal facilitation (−4 ± 9% of baseline; n = 6) was observed at the standard dose of CGS 21680. On the other hand, A2a-induced hypoglossal motor facilitation could be elicited at higher doses of CGS 21680 (5 mm; 155 ± 22 of baseline; n = 2), suggesting that hypoglossal motoneurons express A2a-induced motor facilitation and are an appropriate control for supraspinal drug spread.
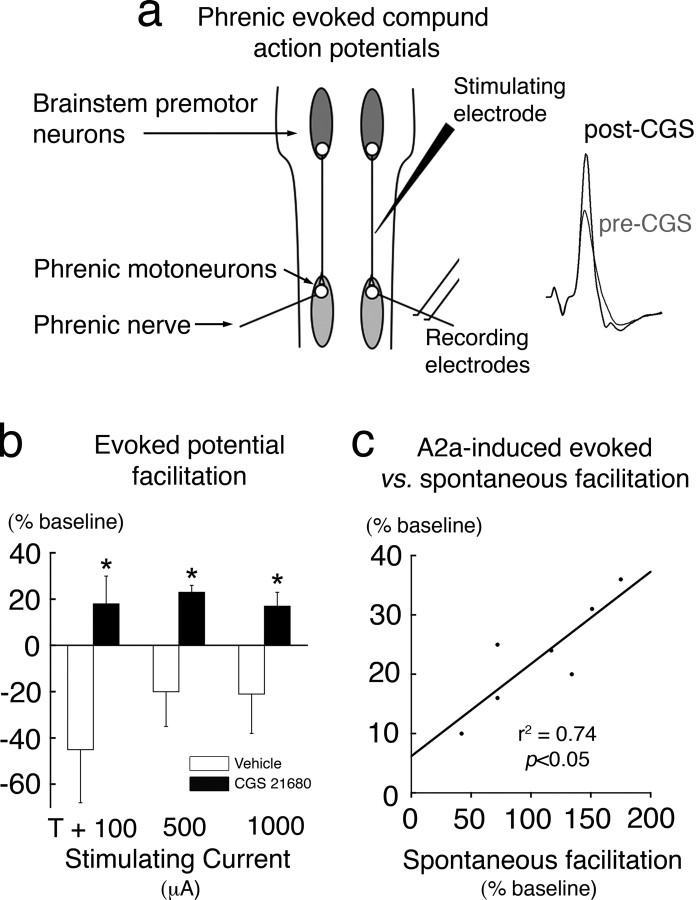
Synaptic plasticity can strengthen spinal pathways to phrenic motoneurons after intermittent hypoxia, thereby contributing to pLTF (Fuller et al., 2003; Baker-Herman et al., 2004; Golder and Mitchell, 2005). To demonstrate similar synaptic plasticity during A2a-induced phrenic motor facilitation, short-latency phrenic potentials evoked by electrical stimulation of the ventrolateral funiculus were recorded during hypocapnia-induced apnea from anesthetized rats before and after cervical spinal A2a receptor activation (Fig. 2a). Facilitation of phrenic evoked potentials was observed after A2a receptor activation (Fig. 2b). A strong, positive correlation existed between A2a-induced evoked potential facilitation and phrenic motor facilitation (Fig. 2c), suggesting that strengthened spinal synaptic pathways (vs increased descending respiratory drive) underlie A2a-induced facilitation of spontaneous phrenic motor activity.
Figure 2.
C4 spinal A2a receptor activation strengthens synaptic pathways to phrenic motoneurons. a, Schematic representation of stimulating and recording electrode positions and representative phrenic evoked potentials. A stimulating electrode was positioned in the C2 ventrolateral funiculus. Stimulus current-evoked short-latency potentials were recorded from the phrenic nerve ipsilateral to the stimulating electrode. b, Average data depicting the percentage change in phrenic evoked potential amplitude at multiple stimulating currents after intrathecal CGS 21680 (n = 7) or vehicle (DMSO; n = 5) injection in anesthetized rats. Intrathecal CGS 21680 elicited significant facilitation of phrenic evoked potential amplitude, suggesting that A2a receptor activation strengthens short-latency (putative monosynaptic) connections to phrenic motoneurons. T, Threshold current. c, Regression analysis of A2a-induced facilitation of the evoked response versus spontaneous phrenic burst facilitation from the same rats. The magnitude of evoked facilitation positively correlated with the magnitude of spontaneous phrenic facilitation. *Significantly different from other groups (p < 0.05). Data are mean ± SEM.
A2a receptor activation transactivates TrkB protein in the cervical ventral horn
A2a receptor activation has been reported to phosphorylate the tyrosine kinase domain of Trk receptors in PC12 cells in the absence of neurotrophins and induces neurotrophin-like effects on cellular function (Lee and Chao, 2001; Lee et al., 2002; Rajagopal et al., 2004). To determine whether A2a-induced phrenic motor facilitation requires tyrosine kinase activation, the tyrosine kinase inhibitor K252a was delivered intrathecally before CGS 21680. Because TrkB receptors are the primary neurotrophin receptor expressed in spinal motoneurons (Yan et al., 1993), the dose of K252a was selected based on its ability to inhibit BDNF effects on phrenic motor output (Baker-Herman et al., 2004). K252a (150 μm) inhibited A2a-induced phrenic motor facilitation at 60 min after CGS 21680 administration and beyond, but not at earlier time points (Fig. 3a). Alone, K252a (n = 3) did not alter phrenic motor output compared with time controls (K252a, −5 ± 8%; time controls, 6 ± 5%, of baseline). Thus, A2a-induced phrenic motor facilitation has an early and late phase, and only the late phase appears to require receptor tyrosine kinase activation.
Figure 3.

A2a-induced facilitation of phrenic motor output requires tyrosine kinase activation, and C4 A2a receptor activation phosphorylates immature TrkB80 protein near phrenic motoneurons. a, Average data demonstrating that intrathecal K252a (1.75 μg · kg−1; squares; n = 5), a tyrosine kinase inhibitor, blocked A2a-induced phrenic motor facilitation (circles; n = 12), but only 60 min after intrathecal CGS 21680 injection. b, Representative immunoblot for TrkB and phospho-TrkB (pTrkB) protein, and Akt and phospho-Akt protein from C4 ventral spinal cords collected from two rats at 120 min after intrathecal vehicle (DMSO) or CGS 21680 injections. c, Average data depicting percentage change from vehicle of C4 ventral spinal TrkB (normalized to GAPDH) and pTrkB (normalized to total TrkB) protein isoforms after intrathecal vehicle (n = 6) or CGS 21680 (CGS; n = 9) injection. Intrathecal CGS 21680 increased mature (150 kDa) and immature (∼80 kDa) isoforms of TrkB protein. Intrathecal CGS 21680 also selectively phosphorylated the low-molecular-weight TrkB isoform. d, Average data depicting percentage change from vehicle (n = 6) of C4 ventral spinal Akt, ERK1, and ERK2 after intrathecal vehicle or CGS 21680 (n = 9) injection. Intrathecal CGS 21680 activated Akt but not ERK1/2 or PLC-γ. e, Immunoblot from the peptide blocking experiment. TrkB antibody was preincubated with TrkB-blocking protein to decrease TrkB antibody binding to TrkB receptor protein on the C4 ventral spinal immunoblots. f, Immunoblot from the deglycosylation experiment. PNGase was added to C4 ventral spinal protein. The intensity of the ∼80 kDa TrkB isoform decreased, and the ∼50 kDa core protein intensity increased, after the digestion. *Significantly different from other groups (p < 0.05). Data are mean ± SEM.
TrkB and phosphorylated TrkB protein were identified by immunoblots of C4 ventral spinal tissue, a region that contains the greatest density of phrenic motoneurons. Two TrkB splice variants have been reported in rat motoneurons (Armanini et al., 1995). Three TrkB protein isoforms were observed in the C4 ventral cord, including TrkB150, TrkB100, and TrkB80 (Fig. 3b). These same bands were confirmed with a second TrkB antibody (from a different commercial source and directed to a different epitope on TrkB), and were absent after peptide blocking experiments (Fig. 3e). Trk protein is glycosylated after its initial synthesis, before insertion into the neuronal membrane (Watson et al., 1999). Thus, we performed PNGase deglycosylation experiments. PNGase digestions shifted TrkB bands toward a core protein of ∼52 kDa (Fig. 3f).
TrkB150 was phosphorylated after intrathecal BDNF administration (n = 2; phospho-TrkB-to-TrkB ratio increased 43 ± 10%, compared with vehicle) and is likely the full-length catalytic form of TrkB protein expressed on extracellular membranes. TrkB100 was not detected by the phospho-TrkB antibodies and is likely the truncated isoform lacking the tyrosine kinase domain (Armanini et al., 1995). Lower-molecular-weight Trk isoforms have been described as hypoglycosylated immature protein, intracellularly localized, and inaccessible for neurotrophin binding (Watson et al., 1999; Mutoh et al., 2000; Rajagopal et al., 2004). Similarly, TrkB80 may represent an intracellular, hypoglycosylated TrkB isoform that is unavailable to BDNF. Indeed, the TrkB80 phosphorylation state was unaffected by intrathecal BDNF (n = 2; pTrkB-to-TrkB ratio, −13 ± 5%, compared with vehicle).
Cervical A2a receptor activation increased TrkB80, but not TrkB150, phosphorylation in the C4 ventral cord (Fig. 3c); TrkB phosphorylation state was detected with a phospho-TrkB antibody for the Y-816 phosphorylation site (gift from R. Rajagopal and M. V. Chao). Selective phosphorylation of TrkB80 was confirmed with two other phospho-TrkB antibodies directed against the Y-490 and Y-674/675 phosphorylation sites (data not shown). Thus, A2a receptor activation selectively phosphorylates the TrkB80 isoform, and does so at multiple tyrosine residues. In this study, we normalized phospho-TrkB expression to total TrkB protein (Figs. 3, 5). However, phosphorylated proteins can also be normalized to loading controls, such as GAPDH. Regardless of the method used, A2a receptor activation increased phospho-TrkB80 expression in C4 ventral spinal segments (phospho-TrkB80/total TrkB80, 24 ± 5%; phospho-TrkB80/GAPDH, 69 ± 16%; p < 0.05). To confirm and localize TrkB activation within the C4 ventral cord, spinal sections were exposed to the same pTrkB antibody. pTrkB protein was observed throughout the ventral gray matter in small fibers and, to a lesser degree, within presumptive motoneuron somata (Fig. 4a). Spinal A2a receptor activation increased pTrkB immunoreactivity within motoneuron somata and in adjacent fibers.
Figure 5.
New TrkB protein synthesis near phrenic motoneurons is necessary for A2a-induced facilitation of phrenic motor output. a, Average data depicting percentage change in phrenic burst amplitude after intrathecal CGS 21680 only (squares), and in rats that received intrathecal siRNA targeting either BDNF mRNA (open circles; n = 5) or TrkB mRNA (filled circles; n = 5) or emetine (open circles; n = 3) 2 h before intrathecal CGS 21680 injection. RNAi targeting TrkB mRNA blocked late A2a-induced phrenic motor facilitation. RNAi targeting BDNF mRNA did not alter A2a-induced facilitation. b, Average data for percentage change in TrkB protein (normalized to GAPDH) for the study described in a. RNAi to BDNF mRNA did not block A2a-induced TrkB synthesis. However, TrkB synthesis was blocked by pretreatment with siRNAs targeting TrkB mRNA. c, Average data for percentage change in pTrkB protein (normalized to TrkB) for the study described in a. RNAi to BDNF mRNA did not block A2a-induced selective phosphorylation of immature TrkB isoforms. Low-molecular-weight TrkB protein was not phosphorylated when an siRNA targeting TrkB mRNA was administered before intrathecal CGS 21680. *Significantly different from other groups (p < 0.05). Data are mean ± SEM.
Figure 4.

Representative photomicrographs of ventral C4 spinal cords collected from rats perfused at 120 min after intrathecal vehicle (DMSO) or CGS 21680. Top, Immunohistochemical photomicrographs of TrkB protein (gray-black) with a Nissl counterstain (blue) (400×). TrkB protein was observed near large neurons (presumptive phrenic motoneurons) in the ventral gray matter. Intrathecal CGS 21680 increased TrkB protein immunoreactivity around these motoneurons, but most notably within neuron somata. Bottom, Immunohistochemical examination for pTrkB protein (400×). pTrkB protein was observed near, and sometimes within, presumptive motoneurons. Intrathecal CGS 21680 increased TrkB immunoreactivity around and, most notably, within neuron somata. Arrows indicate presumptive motoneurons. Insets, enlarged area near arrows (600×).
Similar to in vitro studies using PC-12 cells (Lee et al., 2002; Rajagopal et al., 2004), Akt phosphorylation (but not ERK-1, ERK-2, or PLC-γ) was increased after cervical A2a receptor activation (Fig. 3d). The A2a-induced phosphorylation pattern differed from intrathecal BDNF, where Akt, ERK-1, and ERK-2 were all phosphorylated (pAkt-to-Akt ratio, 125 ± 2%; pERK-1-to-ERK-1 ratio, 122 ± 30%; ERK-2-to-ERK-2 ratio, 118 ± 7%, compared with vehicle; n = 3).
TrkB activation was not attributable to new BDNF synthesis, because BDNF concentration in the ventral C4 cord was unaffected by A2a receptor activation (control, 412 ± 28; n = 9; CGS 21680, 398 ± 12 ng/g; n = 6); this result was confirmed with BDNF immunoblots (CGS 21680, 149 ± 75%, compared with vehicles; n = 6). Furthermore, siRNAs directed at BDNF mRNA to prevent translation of new BDNF protein were ineffective at blocking A2A receptor-induced phrenic motor facilitation (see below). Collectively, these data demonstrate cervical A2a receptor-induced transactivation of TrkB protein in the C4 spinal cord does not require new BDNF synthesis (unlike phrenic long-term facilitation after intermittent hypoxia).
New TrkB synthesis is necessary for A2a-induced phrenic motor facilitation
New protein synthesis contributes to multiple forms of synaptic plasticity (Sajikumar et al., 2005; White-Grindley and Si, 2006), including pLTF after intermittent hypoxia (Baker-Herman et al., 2004). New protein synthesis is also necessary for A2a receptor-induced phrenic motor facilitation in anesthetized rats, because it was abolished by intrathecal administration of the translation inhibitor emetine (Fig. 5a).
Immunoblots revealed increased TrkB protein levels after A2a receptor activation, suggesting new TrkB synthesis (Fig. 3b,c) similar to reports in PC12 cells after A2a receptor activation. C4 spinal sections were examined for TrkB immunoreactivity in the ventral gray matter. TrkB protein was observed primarily in small fibers and, occasionally, in presumptive motoneuron somata (Fig. 4b). A2a receptor activation increased TrkB immunoreactivity in the ventral gray matter, including within presumptive motoneuron somata.
To determine whether new TrkB synthesis was necessary for A2a-induced phrenic motor facilitation, we used translational inhibition via RNAi directed against TrkB mRNA. RNAi blocked phrenic motor facilitation, but only 60 min after CGS 21680 administration and beyond (Fig. 5a). These results again suggest mechanistically distinct phases of A2a-induced phrenic motor facilitation: (1) a late phase requiring tyrosine kinase activation and new TrkB protein synthesis; and (2) an early phase requiring new protein synthesis, but not tyrosine kinase activation or new TrkB synthesis. As a control, two siRNA sequences (50 nm total with Oligofectamine) directed against BDNF mRNA [and known to block intermittent hypoxia-induced BDNF synthesis in C4 spinal segments (Baker-Herman et al., 2004)], were administered intrathecally before A2a receptor activation, and phrenic motor facilitation was unaffected (Fig. 5a). In addition, we used an siRNA with at least four mismatches with all known human, mouse, and rat genes (siCONTROL NonTargeting siRNA #2; Dharmacon) as a control sequence before injecting CGS 21680 intrathecally. The siCONTROL siRNA also did not alter A2a-induced phrenic motor facilitation (facilitation, 120 ± 13% above baseline amplitude) compared with CGS 21680 alone. Thus, siRNAs targeting TrkB mRNA are selective in their effects on A2a-induced phrenic motor facilitation. Furthermore, the mechanisms of A2a-induced phrenic motor facilitation and intermittent hypoxia phrenic long-term facilitation converge only downstream from TrkB signaling.
Western analyses of ventral C4 spinal segments exposed to siRNAs targeting TrkB or BDNF mRNA identified differences in protein expression after A2a receptor activation. TrkB80 synthesis and phosphorylation by A2a receptor activation were both blocked by TrkB siRNAs, but not BDNF siRNAs (Fig. 5b,c). Thus, new TrkB protein synthesis is necessary for transactivation of TrkB80 and A2a-induced phrenic motor facilitation.
Systemic A2a receptor activation increases tidal volume in intact and spinally injured rats
Inspiratory phrenic activity is strongly correlated with inspiratory pressure and tidal volume in spontaneously breathing mammals (Eldridge, 1975). C2 hemisection impairs breathing (Golder et al., 2001; Fuller et al., 2006) by disrupting descending synaptic pathways from the brainstem to spinal cord, thereby compromising the ability to generate normal tidal volumes (VTs). In compensation, rats typically increase their respiratory frequency (f) to preserve pulmonary ventilation. Consistent with earlier reports, spinally injured rats in the current study maintained minute ventilation by using a rapid shallow breathing pattern (uninjured, f = 71 ± 3 min−1; VT = 6.8 ± 0.3 ml · kg−1; pulmonary ventilation = 459 ± 18 ml · kg−1 · min−1; n = 6; C2 spinally hemisected, f = 86 ± 2 min−1; VT = 4.9 ± 0.3 ml · kg−1; minute ventilation = 411 ± 15 ml · kg−1 · min−1; n = 3). A2a receptor activation increases VT in uninjured rats and restores normal tidal volumes in spinally hemisected rats 60 and 120 min after intraperitoneal CGS 21680 (100 μg · kg−1; 50 μm administered in three doses separated by 5 min). CGS 21680 increased pulmonary ventilation above baseline values in spinally injured rats at 60 and 120 min after administration, and above DMSO vehicle control rats at all time points (Fig. 6a,b). The relative magnitude and time course of A2a-induced ventilatory facilitation was similar in control and hemisected rats. Increased VT and f (uninjured, 60 min postdrug f = 16 ± 10%; C2 spinally hemisected, 60 min postdrug f = 19 ± 6%; of predrug value) both contributed to ventilatory facilitation at 60 min after intraperitoneal CGS 21680, whereas only VT remained elevated above baseline values at 120 min (frequency uninjured, 120 min postdrug f = 0 ± 1%; C2 spinally hemisected, 120 min postdrug f = 1 ± 1%; of predrug value) (Fig. 6a–c). Thus, A2a receptor activation increases inspiratory volume in unanesthetized intact and spinally hemisected rats, restoring inspiratory volumes toward normal in injured rats (uninjured, VT = 6.8 ± 0.3 ml · kg−1; C2 spinally hemisected, 5.8 ± 0.2 ml · kg−1). The percentage change in tidal volume after systemic CGS 21680 was similar in uninjured and C2 hemisected rats (uninjured, 21 ± 3%; injured, 18 ± 2%; of baseline values). An important caveat is that spontaneously breathing rats decrease arterial pCO2 levels during ventilatory facilitation, thereby minimizing the apparent A2a-induced ventilatory facilitation caused by inhibitory chemoreceptor feedback (Olson et al., 2001). If arterial pCO2 levels were controlled, predicted changes in VT are larger.
Figure 6.
A2a receptor activation with systemic CGS 21680 increases tidal volume in uninjured and spinally injured unanesthetized rats. a, Representative airflow traces measured in a whole-body plethysmograph from an uninjured (top trace) and C2 spinally hemisected rat (bottom trace) at 8 weeks after injury, during baseline breathing, and 120 min after intraperitoneal CGS 21680 (100 μg · kg−1). I, Inspiration; E, expiration. b, c, Average data for percentage change in minute pulmonary ventilation (frequency × tidal volume) and tidal volume from baseline values after intraperitoneal CGS 21680 (uninjured, filled circles; n = 4; injured, open circles; n = 4) or vehicle (DMSO; squares; n = 4). Systemic CGS 21680 elicited ventilatory facilitation with a time course similar to phrenic motor facilitation that was primarily a result of increased tidal volume. *Significantly different from other groups (p < 0.05). Data are mean ± SEM.
Discussion
Collectively, these results demonstrate that Gs-protein-coupled A2a receptors induce new TrkB protein synthesis and transactivation of an immature TrkB isoform in cervical spinal regions associated with respiratory motor control (Fig. 7). The functional consequence of TrkB transactivation in or near phrenic motoneurons is a robust phrenic motor facilitation of similar duration and magnitude to BDNF-dependent phrenic long-term facilitation after acute intermittent hypoxia. The ability to simulate BDNF with a small, more easily delivered molecule suggests that A2a receptor agonists have considerable potential in the treatment of respiratory motor deficits caused by, for example, cervical spinal injury.
Figure 7.
A schematic figure comparing and contrasting the proposed mechanisms contributing to intermittent hypoxia-induced phrenic LTF and adenosine A2a receptor-induced LTF. Intermittent hypoxia stimulates serotonin (5-HT) release from raphe-spinal terminals near phrenic motoneurons. Serotonin receptor and Gq-protein activation increases PKC activity, which in turn increases BDNF synthesis within phrenic motoneurons. BDNF is secreted extracellularly and binds to mature, fully glycosylated (CHO) TrkB receptors expressed on the outer cell membrane. BDNF phosphorylates (P) mature TrkB receptors, which in turn strengthen excitatory glutamatergic synapses onto phrenic motoneurons via activated MAP kinases (pERK1/2). A2a receptor agonists activate Gs-protein and increase synthesis and phosphorylation of intracellular, hypoglycosylated, immature TrkB protein without the need for BDNF (transactivation). Phosphorylated intracellular TrkB strengthens excitatory glutamatergic synapses onto phrenic motoneurons via activated PI3K and protein kinase B (pAkt).
Interactions between Gs-protein-coupled receptors and Trk receptors have been described in vitro (Lee and Chao, 2001; Lee et al., 2002; Rajagopal et al., 2004). For example, A2a receptor agonists increase cell survival in cell cultures by activating immature Trk receptors (Rajagopal et al., 2004), which are restricted in their distribution to intracellular membranes (Watson et al., 1999; Mutoh et al., 2000). Intracellular Trk protein is hypoglycosylated and has a lower molecular weight than mature Trk protein at the cell surface. Low-molecular-weight (∼79 kDa) TrkB protein has been previously described in the rat spinal cord (Skup et al., 2002), consistent with our conclusion that spinal A2a receptor activation phosphorylated a low-molecular-weight (∼80 kDa) TrkB isoform. Our conclusion that the 80 kDa band represents immature TrkB protein is supported by multiple observations, including the following: (1) multiple TrkB and phospho-TrkB antibodies targeting different epitopes of TrkB protein identify the same band, (2) the band is absent when a blocking peptide is applied to the TrkB antibody, (3) the ∼80 kDa band (or bands) represent a glycosylated protein that converges toward a low-molecular-weight band after deglycosylation, and (4) a similar band is reported by others in rat spinal cord and in PC-12 cells that overexpress TrkB. Collectively, our data strongly suggest that A2a receptor activation induces new TrkB synthesis and selectively phosphorylates an immature, likely intracellular TrkB isoform within cervical spinal regions associated with the phrenic motor nucleus.
A2a-induced Trk signaling requires new protein synthesis and tyrosine kinase activation in PC12 cells (Lee et al., 2002). In agreement, new protein synthesis is necessary for A2a receptor-induced phrenic motor facilitation, because the translation inhibitor emetine blocked the response. On the other hand, because tyrosine kinase inhibition with k252a and RNAi targeting TrkB mRNA block only a late phase of the facilitation, at least two distinct mechanisms must give rise to phrenic motor facilitation after A2a receptor activation. An important caveat limiting interpretation of this study is that K252a inhibits multiple kinases; consequently, some K252a effects may reflect inhibition of receptor tyrosine kinases other than TrkB, or possibly other kinases. Nevertheless, the critical role of Trk receptor signaling is supported by evidence from the RNAi experiments, because siRNAs targeting TrkB mRNA (but not BDNF mRNA) reduced new TrkB80 synthesis, the increase in TrkB80 phosphorylation, the increase in Akt phosphorylation, and phrenic motor facilitation. Increasing intracellular TrkB protein can induce constitutive Trk signaling (Watson et al., 1999) in the absence of ligand, possibly because increased Trk monomer concentrations enable autodimerization and subsequent phosphorylation.
A2a-induced Trk signaling occurs in the absence of neurotrophin–Trk interactions, has a slow onset (>60 min), and selectively activates PI3-kinase/Akt signaling in PC12 cells (Huang and Reichardt, 2003). Similarly, A2a-induced phrenic motor facilitation occurs without new BDNF synthesis; although we cannot rule out an involvement of BDNF–TrkB interactions, several lines of evidence argue against this possibility: (1) BDNF protein concentration did not change after A2a receptor activation; (2) RNAi to BDNF mRNA did not alter A2a-induced phrenic motor facilitation; (3) BDNF selectively activates the mature, fully glycosylated TrkB150 isoform, whereas A2a receptor activation selectively phosphorylates the TrkB80 isoform; and (4) BDNF-induced TrkB activation increases both Akt and ERK1/2 phosphorylation, whereas A2a receptor-induced TrkB activation increases Akt phosphorylation alone.
Similar mechanisms of A2a receptor-induced TrkB transactivation may be operative in motoneurons subserving other motor functions, such as locomotion and upper airway patency. For example, facilitation of respiratory motor output was observed in hypoglossal motoneurons after A2a receptor activation. Respiratory motor output to the tongue and other upper airway muscles plays a critical role in maintaining upper airway patency during breathing. When upper airway patency is compromised during sleep, varied forms of sleep disordered breathing result. Thus, A2a receptor activation may represent a novel therapy for snoring and/or sleep apnea, disorders that afflict a large proportion of the human population and for which there are currently no effective pharmacological therapeutic approaches.
Our data suggest that spinal mechanisms contribute to A2a-induced phrenic motor facilitation, at least in part, because spinal administration of CGS 21680 strengthened short-latency synaptic inputs onto phrenic motoneurons. Most respiratory bulbospinal neurons convey excitatory drive to phrenic motoneurons via monosynaptic connections in the rat. Thus, we propose that spinal A2a receptor activation increases phrenic motor output by strengthening respiratory premotor inputs. On the other hand, the effects of spinal A2a receptor activation on spinal interneurons or other polysynaptic circuits were not investigated. Thus, it is possible that at least some of the facilitation observed in these experiments could result from modulation of other afferent inputs [i.e., intercostal-to-phrenic afferents (Eldridge et al., 1981, 1987; Bolser et al., 1988)].
We believe supraspinal mechanisms are not involved in A2a-induced phrenic motor facilitation because spinal administration of CGS 21680 did not change phrenic burst frequency (fictive respiratory rate), nor elicit hypoglossal motor facilitation (a respiratory motoneuron population located within the brainstem). On the other hand, in awake, freely behaving rats, intraperitoneal CGS 21680 did increase respiratory frequency in the short-term after drug administration. The mechanism(s) responsible for this frequency effect is (are) unknown.
In patients with high cervical spinal cord injury, enhancing respiratory motor function would markedly improve quality of life and survival (Winslow and Rozovsky, 2003). One viable approach to enhance respiratory motor function in spinal cord injury patients is to harness the inherent plasticity of residual synaptic inputs to respiratory motor neurons below an injury. For example, exogenous neurotrophins enhance synaptic strength in residual synaptic pathways to lumbar motoneurons after experimental spinal injuries (Arvanian et al., 2006). Similarly, BDNF may improve respiratory function below cervical spinal injury by strengthening surviving bulbospinal pathways to phrenic motoneurons. For example, acute intermittent hypoxia strengthens synaptic pathways to phrenic motoneurons and improves spontaneous phrenic activity by increasing endogenous BDNF synthesis and release (Golder and Mitchell, 2005). Thus, intermittent hypoxia may have beneficial effects in restoring respiratory motor function after cervical spinal injury. However, intermittent hypoxia is a nonspecific stimulus and may affect multiple physiological systems and, in excess, may induce severe morbidity such as systemic hypertension, hippocampal apoptosis, and learning deficits (Bass et al., 2004).
Whereas exogenous BDNF delivery to the cervical spinal cord is sufficient to elicit phrenic long-term facilitation (Baker-Herman et al., 2004), and may exert beneficial effects on respiratory function after spinal injury, protein delivery to the CNS is often complicated by poor penetration, immune reactions, and complications attendant to receptor downregulation. Small, highly permeable molecules, such as A2a receptor agonists, that mimic BDNF effects on respiratory motor output could be a useful alternative to intermittent hypoxia and/or exogenous BDNF, because it is easy to deliver at relevant sites and improves respiratory function in animals with cervical spinal injuries.
Adenosinergic drugs are reported to improve respiratory function after cervical spinal injury (Bascom et al., 2005; Nantwi and Goshgarian, 2005). For example, systemic theophylline, a nonselective adenosine receptor antagonist, increases phrenic motor output below a C2 hemisection in rats via supraspinal mechanisms, but only when administered shortly after injury (Nantwi and Goshgarian, 2005). In fact, 8 weeks after C2 hemisection, theophylline effects are reversed, and theophylline now inhibits phrenic motor output (Nantwi and Goshgarian, 2005). Thus, after chronic C2 hemisection, theophylline may be detrimental to respiratory function. Our data suggest that adenosinergic agonists may be a more appropriate strategy in chronically injured patients.
Our observations that systemic A2a receptor activation stimulates ventilation with a magnitude and time course similar to phrenic motor facilitation after spinal A2a receptor activation supports the feasibility of this approach to achieve functional benefits in patients with multiple ventilatory control disorders (Mitchell, 2007). CGS 21680 has a short half-life (T1/2 < 15 min), and continued A2a receptor occupancy is an unlikely contributor to ventilatory facilitation 60 min after injection. Any prolonged ventilatory effects after drug administration most likely arise from enduring effects such as protein synthesis-dependent transactivation of Trk receptors. Although we suggest that A2a-induced ventilatory facilitation after systemic CGS 21680 administration results largely from the same spinal mechanisms of A2a-induced phrenic motor facilitation, we cannot completely rule out actions at other sites, such as the carotid body chemoreceptor (Gozal, 1998; Vandier et al., 1999; Kobayashi et al., 2000; Bae et al., 2005). Although A2a-induced ventilatory facilitation appears modest, even small increments in respiratory function can significantly enhance quality of life and reduce morbidity in ventilator-dependent patients, particularly if the functional gain tips the balance, allowing even partial ventilator independence.
Footnotes
This work was supported by National Institutes of Health Grants HL69064, HL80209, Training Grant HL07654 (M.H.), and the Francis Families Foundation (T.L.B.-H.). We gratefully acknowledge the generous gift of phospho-TrkB antibody from M. V. Chao and R. Rajagopal (School of Medicine, New York University, New York, NY) and pTrk antibody from R. Segal (School of Medicine, Harvard University, Cambridge, MA). We also gratefully acknowledge important contributions to these studies by B. Hodgeman, J. Dahlberg, D. Harrigan, S. Mahamed, and V. Brautigam. Some control rat experiments were completed at the University of Pennsylvania.
References
- Armanini MP, McMahon SB, Sutherland J, Shelton DL, Phillips HS. Truncated and catalytic isoforms of trkB are co-expressed in neurons of rat and mouse CNS. Eur J Neurosci. 1995;7:1403–1409. doi: 10.1111/j.1460-9568.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Bowers WJ, Anderson A, Horner PJ, Federoff HJ, Mendell LM. Combined delivery of neurotrophin-3 and NMDA receptors 2D subunit strengthens synaptic transmission in contused and staggered double hemisected spinal cord of neonatal rat. Exp Neurol. 2006;197:347–352. doi: 10.1016/j.expneurol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bae H, Nantwi KD, Goshgarian HG. Recovery of respiratory function following C2 hemi and carotid body denervation in adult rats: influence of peripheral adenosine receptors. Exp Neurol. 2005;191:94–103. doi: 10.1016/j.expneurol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005;127:658–661. doi: 10.1378/chest.127.2.658. [DOI] [PubMed] [Google Scholar]
- Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, Wilker RE, Stehle S, Kinane TB. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Lindsey BG, Shannon R. Respiratory pattern changes produced by intercostal muscle/rib vibration. J Appl Physiol. 1988;64:2458–2462. doi: 10.1152/jappl.1988.64.6.2458. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Carter AR, Chen C, Schwartz PM, Segal RA. Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J Neurosci. 2002;22:1316–1327. doi: 10.1523/JNEUROSCI.22-04-01316.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Eldridge FL. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975;39:567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE, Waldrop TG. Spinal inhibition of phrenic motoneurones by stimulation of afferents from peripheral muscles. J Physiol (Lond) 1981;311:67–79. doi: 10.1113/jphysiol.1981.sp013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop T. Spinal inhibition of phrenic motoneurones by stimulation of afferents from leg muscle in the cat: blockade by strychnine. J Physiol (Lond) 1987;389:137–146. doi: 10.1113/jphysiol.1987.sp016650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, McCrimmon DR. Neural control of breathing. In: Squire LR, editor. Fundamental neuroscience. Ed 2. Academic; 2002. pp. 967–990. [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D. Potentiation of hypoxic ventilatory response by hyperoxia in the conscious rat: putative role of nitric oxide. J Appl Physiol. 1998;85:129–132. doi: 10.1152/jappl.1998.85.1.129. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Conforti L, Millhorn DE. Gene expression and function of adenosine A(2A) receptor in the rat carotid body. Am J Physiol Lung Cell Mol Physiol. 2000;279:L273–L282. doi: 10.1152/ajplung.2000.279.2.L273. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic basis for respiratory control disorders. New York: Springer; 2007. [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Hamano T, Tokuda A, Kuriyama M. Unglycosylated Trk protein does not co-localize nor associate with ganglioside GM1 in stable clone of PC12 cells overexpressing Trk (PCtrk cells) Glycoconj J. 2000;17:233–237. doi: 10.1023/a:1026597408790. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Adenosinergic mechanisms underlying recovery of diaphragm motor function following upper cervical spinal cord injury: potential therapeutic implications. Neurol Res. 2005;27:195–205. doi: 10.1179/016164105X21977. [DOI] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of α1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27:4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Frey JU. Protein synthesis-dependent long-term functional plasticity: methods and techniques. Curr Opin Neurobiol. 2005;15:607–613. doi: 10.1016/j.conb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Skup M, Dwornik A, Macias M, Sulejczak D, Wiater M, Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Vandier C, Conway AF, Landauer RC, Kumar P. Presynaptic action of adenosine on a 4-aminopyridine-sensitive current in the rat carotid body. J Physiol (Lond) 1999;515:419–429. doi: 10.1111/j.1469-7793.1999.419ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Porcionatto MA, Bhattacharyya A, Stiles CD, Segal RA. TrkA glycosylation regulates receptor localization and activity. J Neurobiol. 1999;39:323–336. doi: 10.1002/(sici)1097-4695(199905)39:2<323::aid-neu15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- White-Grindley E, Si K. RISC-y memories. Cell. 2006;124:23–26. doi: 10.1016/j.cell.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Yan Q, Elliott JL, Matheson C, Sun J, Zhang L, Mu X, Rex KL, Snider WD. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993;24:1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]