Abstract
Among the neuroendocrine neoplasia, the pancreatic somatostatin-producing tumors are very rare. Usually functional, these tumors produce the somatostatinoma syndrome, which encompasses diabetes mellitus, diarrhea/steatorrhoea, and cholelithiasis. Other symptoms may include dyspepsia, weight loss, anemia, and hypochlorhydria. All theses symptoms are explained by the inhibitory actions of the somatostatin released by tumoral cells originated from pancreatic delta cells or endocrine cells of the digestive tract. The diagnosis is easy to overlook since these symptoms are commonly observed in other more common syndromes. Besides the clinical features, diagnosis is based on serum determination of somatostatin, and imaging exams, such as ultrasound, computer tomography and positron emission tomography. Pathologic examination is characterized by the positivity of immunohistochemical reaction for synaptophysin, chromogranin, and somatostatin. These tumors can be classified according to tumor size, mitotic index, neural or vascular invasion, and distant metastases. The authors describe the case of a 61-year-old female patient who sought medical care because of a 6-month history of watery diarrhea, weight loss, and depression. She was diagnosed with diabetes mellitus 3 years ago. Imaging examination revealed a tumoral mass of 4 cm in its longest axis in the topography of the head of the pancreas and calculous cholecistopathy. The patient’s clinical status was unfavorable for a surgical approach. She died after 20 days of hospitalization. The definitive diagnosis was achieved with the autopsy findings, which disclosed a pancreatic somatostatinoma.
Keywords: Somatostatinoma, Weight Loss, Diarrhea, Depression, Diabetes Mellitus, Gallstones
CASE REPORT
A 61-year-old female patient was brought to the hospital with a history of intermittent watery diarrhea for the past 6 months, which became constant during the last month. The diarrhea had no mucus, blood, or steatorrhea, nor was it accompanied by abdominal pain. She referred being previously obese and used to present constipation. During the last 4 years she had been showing discouragement and a progressive loss of appetite, starting progressive anhedonia, failing to seek medical aid, and avoiding socializing. Concomitantly she presented a weight loss of 50 kg, which was more pronounced over the last 6 months. More recently, she could barely sustain her own weight in the upright position due to muscle atrophy. Her past medical history included the diagnoses of diabetes mellitus and hypertension, and was taking captopril and glibenclamide irregularly, without accurate monitoring. She denied smoking and consumption of alcoholic beverages. She was receiving assistance from neighbors who brought her to the hospital.
The initial physical examination showed an ill-looking patient, pale, cachectic (weight = 40.1 kg), height = 1.56 m, and body mass index (BMI) = 16.4. Blood pressure = 160/90 mm Hg, pulse rate was regular = 84 beats per minute, respiratory rate = 16 respiratory movements per minute, axillary temperature 36 °C, room air oximetry = 97%, and capillary glucose = 458 mg/dL (reference value (RV); 80-99 mg/dL). Physical examination of the lungs and heart was normal, and the abdomen was flaccid with tenderness on palpation of the hypogastrium (clinically interpreted as a full urinary bladder); intestinal sounds were normal and no other organ enlargement was noted. Initial laboratory work up is shown in Table 1.
Table 1. – Admission laboratory workup.
| RV | RV | ||||
|---|---|---|---|---|---|
| Hemoglobin | 13.6 | 12.3-15.3 g/dL | Potassium | 2.6 | 3.5-5.0 mEq/L |
| Hematocrit | 41 | 36.0-45.0% | Sodium | 140 | 3.5-5.0 mEq/L |
| Leucocytes | 7200 | 4.4-11.3 103/mm3 | ALT | 15 | 136-146 mEq/L |
| Bands | 0 | 1-5% | AST | 24 | 9-36 U/L |
| Segmented | 74 | 45-70% | γGT | 23 | 1-24 U/L |
| Eosinophils | 0 | 1-4% | AF | 78 | 10-100 U/L |
| Basophils | 0 | 0-2.5% | Amylase | 55 | 20-104 U/L |
| Lymphocytes | 18 | 18-40% | Lipase | 18 | 30-118 U/L |
| Monocytes | 8 | 2-9% | Glucose | 421 | 80-99 mg/dL |
| Platelets | 226.103 | 150-400 × 103/mm3 | HbA1c | 16% | <5.4% |
| Urea | 15 | 10-50 mg/dL | PT (INR) | 1.18 | 1 |
| Creatinine | 0.5 | 0.4-1.3 mg/dL | Cai+ | 1.19 | 1.11-1.40 mmol/L |
AF = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; Cai+ = ionized calcium; γGT = gamma-glutamyl transferase; HbA1c = glycated hemoglobin; INR = international normalized ratio; PT = prothrombin time; RV = reference value.
The patient was initially treated with volume replacement of saline, the potassium imbalance was corrected, and captopril and insulin were prescribed. The diagnostic investigation comprised an abdominal ultrasonography (USG) examination that revealed the presence of a heterogeneous nodular formation, measuring 3.7 × 2.5 × 4.0 cm, which was evidenced on the topography of the head of the pancreas. The gallbladder presented a thin wall but was distended with multiple images compatible with calculi in its interior. The computed tomography (CT) examination of the abdomen confirmed the USG findings and was complemented with a slight ectasia of the common bile duct (which measured 8 mm). This stressed that the pancreatic lesion had poorly defined limits, showing heterogeneous enhancement after the contrast medium injection, and the duct of Wirsung was somewhat dilated (Figures 1 and 2).
Figure 1. – Axial computed tomography of the abdomen in the arterial phase. A - Heterogeneous mass in the topography of the head of the pancreas (white arrows) and dilation of the common bile duct (black arrow); B - Dilation of the pancreatic duct (white arrow) and the common bile duct (black arrow).
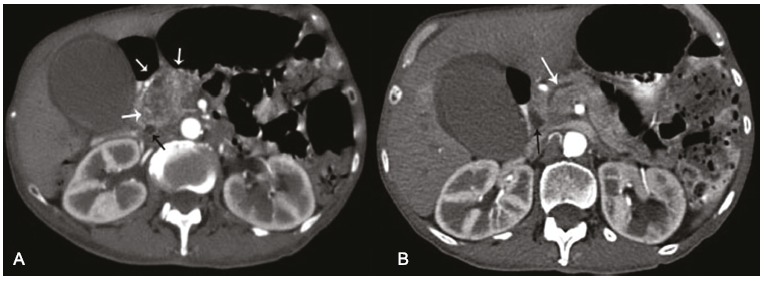
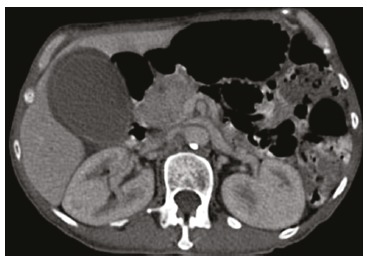
Figure 2. – Axial computed tomography of the abdomen showing the heterogeneous mass in the topography of the head of the pancreas, a hydropic gallbladder surrounded by a thin liquid layer.
CT of the chest and brain, upper gastrointestinal endoscopy, and colonoscopy were normal. Others exams included: determination of serum B12 vitamin, folic acid, and TSH and free T4, which were normal. HIV, hepatitis B and C serologies were all negative. Determination of carcinoembrionic antigen (CEA) = 18 µg/L (reference value (RV) < 3 µg/L), carbohydrate antigen 125 (CA 125) = 7.6 U/mL (RV < 35 U/mL) and CA 19-9 = 14 U/mL (RV < 37 U/mL). Thus the patient was diagnosed with chronic diarrhea, cachexia, uncontrolled diabetes mellitus, depression, calculous cholecystitis, and a tumor mass in the pancreatic head topography. She progressed presenting continuous epigastric pain and fever. A urinary tract infection was diagnosed and treated with ceftriaxone. On the eighth day of hospitalization, laboratory tests changed with elevation of AST, ALT, AP and γGT serum determinations. Total blood count changed to leukocytosis with neutrophilia accompanied by a shift to the left. Surgical treatment was contraindicated considering the patient’s clinical status to a high-grade severity surgery: gastroduodenopancreatectomy. The patient presented a progressive decrease in the level of consciousness and died on the twentieth day of hospitalization.
Despite the long hospitalization stay, definitive diagnosis could not be done, which is why an autopsy was performed.
Autopsy Findings
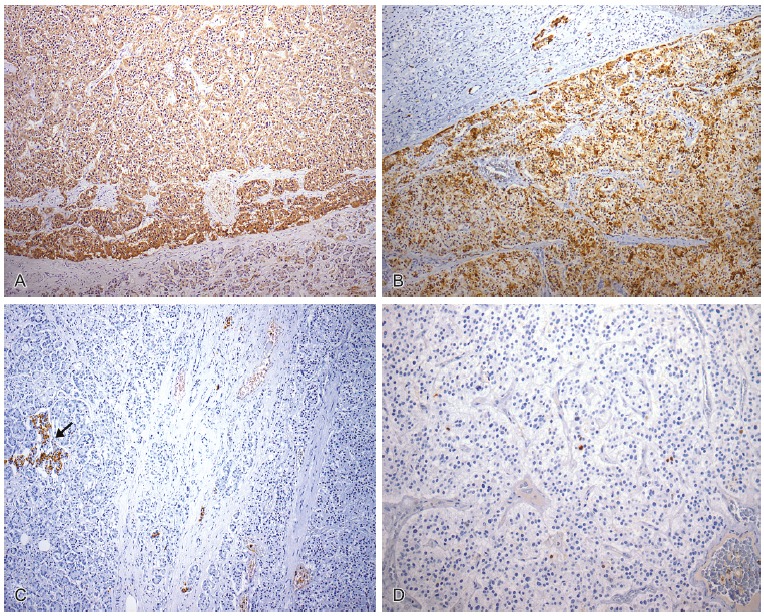
The abdominal cavity showed adhesion between the stomach, gallbladder and duodenum, with serositis. The pancreas weighed 131.0 g (RV range = 60-135 g), had a tumor located in the head, which was nodular and circumscribed, measuring 4.0 cm in its longest axis. The mass was homogeneous showing well-defined contours without areas of necrosis and hemorrhage. Microscopically, the tumor cells were relatively uniform and round, with finely granular eosinophilic cytoplasm and a centrally located round nucleus, arranged in a trabecular or ribbon pattern, separated by delicate fibrovascular stroma. The histological findings were consistent with pancreatic endocrine tumor. The immunohistochemical study was performed and is summarized in Table 2. This research showed tumor cells diffusely positive for somatostatin and chromogranin A, with a low proliferative rate (Ki-67 < 2%), which allowed the diagnosis of a well-differentiated pancreatic somatostatinoma (Figures 3 and 4).
Table 2. – Immunohistochemical panel used for diagnosis.
| Antigen | Result | Antigen | Result |
|---|---|---|---|
| Chromogranin A | Positive | Calcitonin | Negative |
| Somatostatin | Positive | Gastrin | Negative |
| ACTH | Negative | Glucagon | Negative |
| PP | Negative | Serotonin | Negative |
| Insulin | Negative | Ki-67 | Positive (< 2%) |
ACTH = Adrenocorticotropic hormone, PP = pancreatic polypeptide.
Figure 3. – A - Gross macroscopic view of the somatostatinoma located in the head of the pancreas - histological appearance of the pancreatic somatostatinoma; B - Panoramic photomicrography of the tumor showing the well circumscribed border (arrow) and the normal pancreatic parenchyma (HE – 25×); C - Photomicrography of the tumor cells arranged in a trabecular or ribbon pattern, separated by delicate fibrovascular stroma (HE – 100×); D - Photomicrography of tumor cells with a relatively uniform and round shape, with fine granular eosinophilic cytoplasm and a central located round nucleus (HE – 400×).
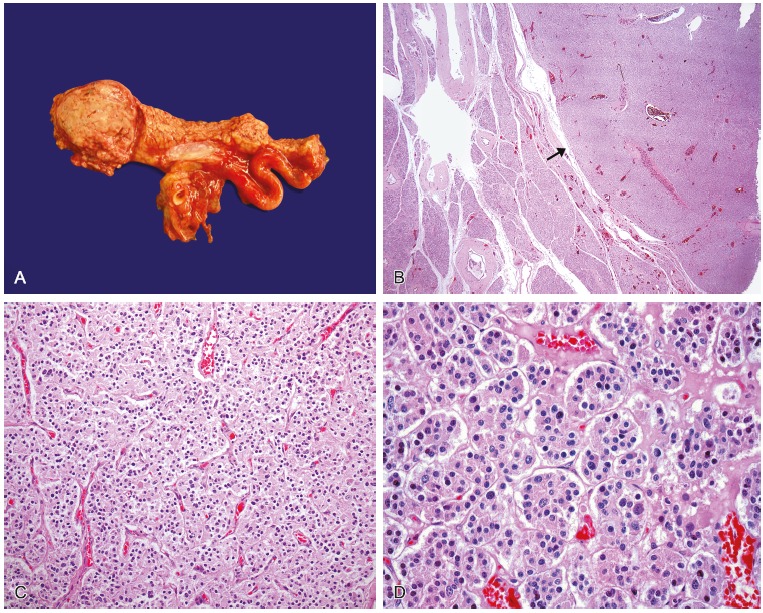
Figure 4. – Immunohistochemical photomicrography of the tumor. A - Somatostatin positive (100×); B - Chromogranin A positive (100×); C - Insulin negative in the tumor cells but focal positivity in a Langerhans islet (arrow) in the non-neoplastic parenchyma (100×); D - Ki-67 positive (<2%) (200×).
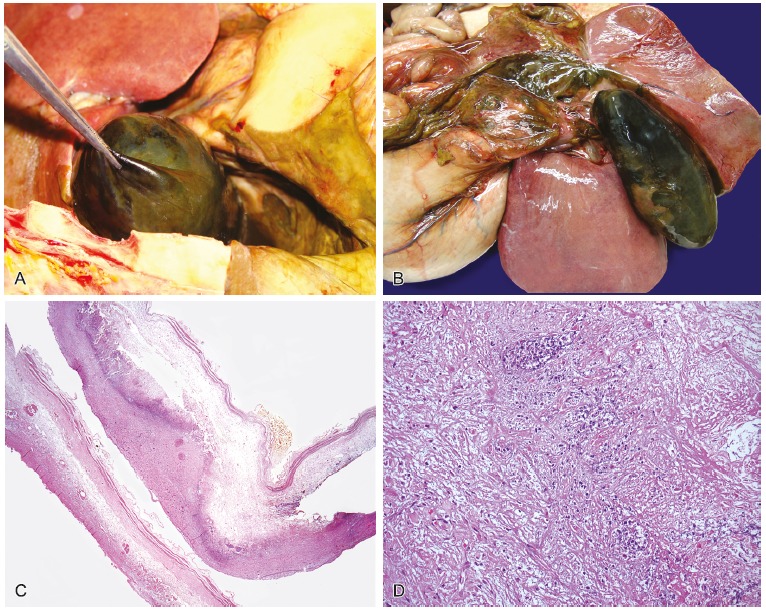
A distended gallbladder with dark greenish external surface was observed with deposition of friable material surrounded by a thick collection of peritoneal fluid. Upon opening, the gallbladder wall was thick, and contained a thick biliary secretion and micro calculi. The microscopic examination evidenced autolysis, acute inflammatory infiltrate in the wall, and serositis. This histological picture was compatible with acute cholecystitis with cholelithiasis (Figure 5).
Figure 5. – A and B - Gross macroscopic view of distended gallbladder with dark greenish and external surfaces, with deposition of friable material, surrounded by a thick collection; C - Photomicrography of the gallbladder showing evident autolysis and acute inflammatory infiltrate (HE – 25×); D - Photomicrography of the gallbladder wall showing, in detail, the acute inflammatory infiltrate (HE – 400×).
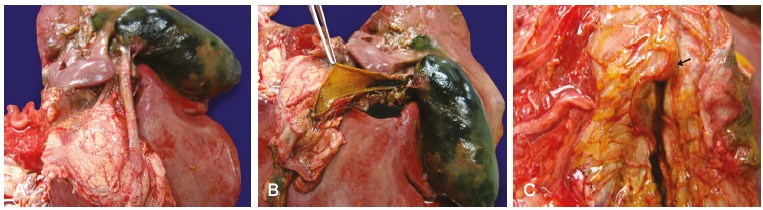
The common bile duct was dilated with a 1.0 cm diameter. At opening of the duodenum, the duodenal papilla was preserved, without calculous impaction, and with normal bile output with the gallbladder expression (Figure 6).
Figure 6. – A and B - Gross macroscopic examination of distended gallbladder and dilated common bile duct; C - Preserved duodenal papilla (arrow) with output of bile with the gallbladder expression.
The history of diarrhea and diabetes mellitus associated with the autopsy findings of cholelithiasis with acute cholecystitis allows the diagnosis of somatostatinoma syndrome.
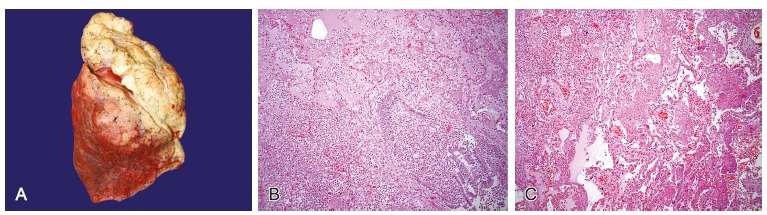
The thoracic cavity showed congestion in both lungs. The right lung weighed 417.0 g (RV range = 360‑570 g) and left lung 452.0 g (RV range = 325-480 g), with friable areas on digital pressure. Microscopic examination diagnosed bronchopneumonia, edema, and areas of diffuse alveolar damage (Figure 7).
Figure 7. – A - Gross macroscopic view of right lung showing congested lower lobe; B - Photomicrography of the pulmonary parenchyma showing edema and acute inflammatory infiltrate in the alveolar spaces and bronchus (HE – 100×); C - Area of diffuse alveolar damage with fibrin deposition in the alveolar lumen (HE – 100×).
Acute cholecystitis and bronchopneumonia with diffuse alveolar damage led to septic shock. Microscopically, the kidneys showed acute tubular necrosis, which may have contributed to the final events.
The kidneys also showed benign nephrosclerosis. The heart weighed 334.0 g (RV range = 179-385 g) and showed the left ventricle wall with a mild concentric hypertrophy. The abdominal aorta showed calcified, not ulcerated, atherosclerosis, which was consistent with the previous systemic arterial hypertension diagnosis.
The remaining organs and tissues showed no significant alterations on gross and microscopic examination.
DISCUSSION
Somatostatin-producing neuroendocrine tumor is a rare neoplasm usually arising in the pancreas and duodenum. Since its first description in 1977,1,2 less than 200 cases have been reported in the literature up until 2008. The annual estimated incidence is 1 in 40 million, with the median age at onset of 54 years (range, 24-84 years).2-4
Somatostatinoma derive from the somatostatin-producing delta cells of the pancreas or the endocrine cells of the digestive tract5,6 and may be sporadic (93.1%) or familial (6.9%) in association with neurofibromatosis type 1 (NF1), multiple endocrine neoplasia type 1 (MEN1), and Von Hippel–Lindau syndromes.4,7-10 Most somatostatinomas (56%) originate in the pancreas and are usually functional. Extra-pancreatic somatostatinomas (44%) are located in the duodenum and occasionally in the biliary tract or small bowel.4,11-15
In most patients, these tumors are symptomatic, with variable nonspecific clinical presentation. Only a few patients may present with the typical clinical somatostatinoma syndrome, which consists of diabetes mellitus, diarrhea/steatorrhoea, and cholelithiasis. Somatostatinoma syndrome was described 2 years after the first somatostatinoma was reported by Krejs in 1979, being more common in pancreatic somatostatinomas than in the duodenal counterpart.16-18 Additional symptoms have been identified and include dyspepsia, weight loss, anemia, and hypochlorhydria.16 All these symptoms can be explained by the inhibitory actions of somatostatin in the release or action of insulin, glucagon, gastrin, secretin, somatotrophin, thyrotropin, gastric inhibitory peptide, vasoactive intestinal peptide (VIP), pancreatic polypeptide (PP), and cholecystokinin.16,17
The patient of this report presented chronic diarrhea, cachexia, diabetes mellitus, depression, and calculous cholecystitis, which could be considered as symptoms of typical somatostatinoma syndrome. However, these symptoms seemingly were not observed together during the diagnostic process, and the diagnosis of pancreatic somatostatinoma was not taken into consideration.
We suspected that the mood disorder (depression), which was probably a result of the pancreatic tumor, explained the delay in seeking medical attention. The unmotivated patient only sought treatment because she was being taken care of by neighbors, reaching the medical facility presenting a debilitating nutritional status. During the diagnostic workup, the image of the tumor in the head of the pancreas added to the intense weight loss, probably led the surgeons to think of pancreatic adenocarcinoma. For a curative treatment, this would require a gastroduodenopancreatectomy, which represents a high-grade surgical procedure. As the patient was not clinically prepared for such a procedure, surgical treatment was disregarded.
USG is the most sensitive method to demonstrate somatostatinomas of pancreas and duodenum. The sensitivity of trans abdominal USG for detection varies from less than 20% to as high as 80%.19-22 They appear as well circumscribed round or oval hypoechoic masses with smooth margins.
The CT and positron emission tomography (PET) images show circumscribed solid masses that tend to displace surrounding structures. Smaller lesions tend to be more homogeneous, and larger lesions are more likely to demonstrate heterogeneous enhancement, a finding due to areas of cystic degeneration, necrosis, fibrosis, and calcification.20 When contrast material is administered, they demonstrate a hyper-vascular pattern.23.
The diagnosis is confirmed by documentation of elevated plasma concentrations of somatostatin and the presence of the somatostatinoma syndrome. If the plasmatic basal levels of somatostatin do not indicate values at least three times the normal concentration, diagnostic tests, both stimulatory (with tolbutamide, calcium/pentagastrin, or secretin) and inhibitory (with diazoxide), can be performed.4,24,25 However, serum determination of somatostatin is often overlooked when patients present nonspecific symptoms, as it may happen with the somatostatinoma syndrome.
In the case presented here, if the somatostatinoma syndrome had been taken into account, the somatostatin could have been determined. In turn, and the correct diagnosis would have been made, what which possibly could have changed the adopted approach.
Pathological examination of the surgical specimen provides the definitive diagnosis. Grossly, somatostatinomas usually occur as solitary (up to 90%), are generally large by the time they are detected (average diameter, 5-6 cm in the pancreas and 2-5 cm in the duodenum), are well-circumscribed, but not encapsulated, are soft, and nodules are gray-white to yellow-tan in color.1,4,26 Microscopically, the growth pattern can be solid, nested, trabecular, ribbon like, tubuloacinar, or glandular, and mixed patterns are common in the same tumor.27,28 Psammoma bodies are commonly observed in duodenum somatostatinoma, but rarely in the pancreas.29,30 Immunohistochemical stains for synaptophysin and chromogranin A typically show diffuse positivity. In most cases, the histologic pattern does not determine the functional status of the tumors.31 The accurate diagnosis of somatostatinoma depends on the intense positive immunohistochemical reaction for somatostatin32.
In the recent World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Endocrine Organs, Heitz et al.27 proposed criteria for the clinicopathological classification of pancreatic endocrine tumors. Well-differentiated tumors are divided into those with ‘‘benign’’ behavior and those with “uncertain” behavior. The tumors considered as benign include those confined to the pancreas that are non-angioinvasive, without perineural invasion, and less than 2 cm in diameter, with fewer than 2 mitotic figures per 10 high-power fields and less than 2% Ki-67-positive cells. The tumors of uncertain behavior include those confined to the pancreas with any of the following features: greater than or equal to 2 cm in diameter, 2-10 mitoses per 10 high-power fields, greater than 2% Ki-67-positive cells, angioinvasion, or perineural invasion. Well-differentiated endocrine carcinomas are diagnosed when there is local invasion or metastasis. Poorly differentiated endocrine carcinoma is diagnosed when there are more than 10 mitotic figures per 10 high-power fields.27
The patient of this report had a tumor classified as a well-differentiated tumor with uncertain behavior as it had 4 cm on its longest axis and no metastases. Most somatostatinomas are malignant (71.2%), presenting metastases commonly in the liver, peripancreatic lymph nodes, and bone.3,33
Tumor size is a pertinent prognostic factor.4 The diameter of 2 cm is considered the cut-off value, above which the risk of metastasis significantly increases.26,34 Indeed, by the time they are detected, nearly 30% of duodenal and 70% of pancreatic somatostatinomas have already metastasized to the regional lymph nodes or the liver.30
Treatment largely depends on the site and size of the tumor and the extent of the disease at the time of diagnosis.4 Surgical resection is the treatment of choice.35,36 In cases of locally advanced disease or widespread metastases, tumor debulking, chemo-embolization of the primary tumor and of hepatic metastases, chemotherapy, somatostatin receptor subtype-selective (SSTR-analogs, octreotide), and interferon–alpha (IFN-α) can be used to control the clinical symptoms.37
CONCLUSION
Early detection of pancreatic somatostatinoma could be difficult, in part, by the rarity of the disease and the presence of variable symptoms. This case report not only presents the autopsy findings of a rare entity, but also alerts physicians to the importance of recognizing all symptoms as being part of a syndrome that is usually overlooked. In this case, if the correct diagnosis had been made in her lifetime, perhaps the patient would have benefited from surgical treatment.
Footnotes
Vianna PM, Ferreira CR, Campos FPF. Somatostatinoma syndrome: a challenging differential diagnosis among pancreatic tumors. Autopsy Case Rep [Internet]. 2013;3(1): 29-37. http://dx.doi.org/10.4322/acr.2013.005
REFERENCES
- 1.Larsson LI, Hirsch MA, Holst JJ, et al. . Pancreatic somatostatinoma: clinical features and physiological implications. Lancet. 1977;309:666-8. 10.1016/S0140-6736(77)92113-4 [DOI] [PubMed] [Google Scholar]
- 2.Soga J, Yakuwa Y.. Somatostatinoma/inhibitory syndrome: a statistical evaluation of 173 reported cases as compared to other pancreatic endocrinomas. J Exp Clin Cancer Res. 1999;18:13-22. . [PubMed] [Google Scholar]
- 3.Marakis G, Ballas K, Rafailidis S, Alatsakis M, Pat-siaoura K, Sakadamis A. Somatostatin-producing pancreatic endocrine carcinoma presented as relapsing cholangitis: a case report. Pancreatology. 2005;5:295-9. 10.1159/000085286 [DOI] [PubMed] [Google Scholar]
- 4.Nesi G, Marcucci T, Rubio CA, Brandi ML, Tonelli F. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. J Gastroenterol Hepatol. 2008;23:521-6. 10.1111/j.1440-1746.2007.05053.x [DOI] [PubMed] [Google Scholar]
- 5.Ganda OP, Weir GC, Soeldner JS, et al. . Somatostatinoma: a somatostatin-containing tumor of the endocrine pancreas. N Engl J Med. 1977;296: 963-7. 10.1056/NEJM197704282961703 [DOI] [PubMed] [Google Scholar]
- 6.Kaneko H, Yanaihara N, Ito S, et al. . Somatostatinoma of the duodenum. Cancer. 1979;44:2273-9. [DOI] [PubMed] [Google Scholar]
- 7.Kainuma O, Ito Y, Taniguchi T, et al. . Ampullary somatostatinoma in a patient with von Recklinghausen’s disease. J Gastroenterol. 1996;31:460-4. 10.1007/BF02355041 [DOI] [PubMed] [Google Scholar]
- 8.Karasawa Y, Sakaguchi M, Minami S, et al. . Duodenal somatostatinoma and erythrocytosis in a patient with von Hippel-Lindau disease type 2. Intern Med. 2001;40:38-43. 10.2169/internalmedicine.40.38 [DOI] [PubMed] [Google Scholar]
- 9.Tomassetti P, Migliori M, Lalli S, Campana D, Tomassetti V, Corinaldesi R. Epidemiology, clinical features and diagnosis of gastroenteropancreatic endocrine tumours. Ann Oncol. 2001;12(Suppl. 2):S95-9. 10.1093/annonc/12.suppl_2.S95 [DOI] [PubMed] [Google Scholar]
- 10.Usui M, Matsuda S, Suzuki H, Hirata K, Ogura Y, Shiraishi T. Somatostatinoma of the papilla of Vater with multiple gastrointestinal stromal tumors in a patient with von Recklinghausen’s disease. J. Gastroenterol. 2002;37:947-53. 10.1007/s005350200159 [DOI] [PubMed] [Google Scholar]
- 11.Takashi M, Matsuyama M, Furuhashi K, et al. . Composite tumor of mucinous cystadenoma and somatostatinoma of the kidney. Int J Urol. 2003;10:603-6. 10.1046/j.1442-2042.2003.00698.x [DOI] [PubMed] [Google Scholar]
- 12.Walsh IK, Kernohan RM, Johnston CF, Keane PF. Somatostatinoma in a horseshoe kidney. Br J Urol. 1996;78:958-9. 10.1046/j.1464-410X.1996.131935.x [DOI] [PubMed] [Google Scholar]
- 13.Grundmann R, Thul P, Krestin GP, Krueger GR. Somatostatinoma of the liver. Leber Magen Darm. 1985;15:81-4. . [PubMed] [Google Scholar]
- 14.Ohwada S, Joshita T, Ishihara T, et al. . Primary liver somatostatinoma. J Gastroenterol Hepatol. 2003;18:1218-9. 10.1046/j.1440-1746.2003.03151.x [DOI] [PubMed] [Google Scholar]
- 15.Harris GJ, Tio F, Cruz Junior AB. Somatostatinoma: a case report and review of the literature. J Surg Oncol. 1987;36:8-16. 10.1002/jso.2930360104 [DOI] [PubMed] [Google Scholar]
- 16.Krejs GJ, Orci L, Conlon JM. Somatostatinoma syndrome: biochemical, morphologic, and clinic features. N Engl J Med. 1979;301:285-92. 10.1056/NEJM197908093010601 [DOI] [PubMed] [Google Scholar]
- 17.Green BT, Rockey DC. Duodenal somatostatinoma presenting with complete somatostatinoma syndrome. J Clin Gastroenterol. 2001;33:415-7. 10.1097/00004836-200111000-00015 [DOI] [PubMed] [Google Scholar]
- 18.Hamy A, Heymann MF, Bodic J, et al. . Duodenal somatostatinoma. Anatomic/clinical study of 12 operated cases. Ann Chir. 2001;126:221-6. 10.1016/S0003-3944(01)00493-X [DOI] [PubMed] [Google Scholar]
- 19.Pitre J, Soubrane O, Palazzo L, Chapuis Y. Endoscopic ultrasonography for the preoperative localization of insulinomas. Pancreas. 1996;13:55-60. 10.1097/00006676-199607000-00007 [DOI] [PubMed] [Google Scholar]
- 20.Buetow PC, Miller DL, Parrino TV, Buck JL. Islet cell tumors of the pancreas: clinical, radiologic, and pathologic correlation in diagnosis and localization. Radiographics. 1997;17:453 72. . [DOI] [PubMed] [Google Scholar]
- 21.Fidler JL, Fletcher JG, Reading CC, et al. . Preoperative detection of pancreatic insulinomas on multi- phasic helical CT. AJR Am J Roentgenol. 2003;181:775-80. 10.2214/ajr.181.3.1810775 [DOI] [PubMed] [Google Scholar]
- 22.Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res. 2004;120:139-61. 10.1016/j.jss.2003.12.007 [DOI] [PubMed] [Google Scholar]
- 23.Malagò R, D’Onofrio M, Zamboni GA, et al. . Contrast-enhanced sonography of nonfunctioning pancreatic neuroendocrine tumors. AJR Am J Roentgenol. 2009;192:424 30. 10.2214/AJR.07.4043 [DOI] [PubMed] [Google Scholar]
- 24.Farr CM, Price HM, Bezmalinovic Z. Duodenal somatostatinoma with congenital pseudoarthrosis. J Clin Gastroenterol. 1991;13:195-7. . [PubMed] [Google Scholar]
- 25.Roy J, Pompilio M, Samama G.. Pancreatic somatostatinoma and MEN 1. Apropos of a case. Review of the literature. Ann Endocrinol (Paris). 1996;57:71-6. French [PubMed] [Google Scholar]
- 26.Tanaka S, Yamasaki S, Matsushita H, et al. . Duodenal somatostatinoma: a case report and review of 31 cases with special reference to the relationship between tumor size and metastasis. Pathol Int. 2000;50:146-52. 10.1046/j.1440-1827.2000.01016.x [DOI] [PubMed] [Google Scholar]
- 27.Heitz PU, Komminoth P, Perren A, et al. . Tumors of the endocrine pancreas. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press; 2004. p. 175-208. (World Health Organization Classification of Tumours). [Google Scholar]
- 28.Kloppel G, Heitz PU. Tumors of the endocrine pancreas. In: Fletcher CD, editor. Diagnostic histopathology of tumors. 2nd ed. London: Churchill Livingstone; 2000. p. 1083-98. [Google Scholar]
- 29.House MG, Yeo CJ, Schulick RD. Periampullary pancreatic somatostatinoma. Ann Surg Oncol. 2002;9:869 74. 10.1007/BF02557523 [DOI] [PubMed] [Google Scholar]
- 30.Mao C, Shah A, Hanson DJ, Howard JM. Von Recklinghausen’s disease associated with duodenal somatostatinoma: contrast of duodenal versus pancreatic somatostatinomas. J Surg Oncol. 1995;59:67-73. 10.1002/jso.2930590116 [DOI] [PubMed] [Google Scholar]
- 31.Frankel WL. Update on pancreatic endocrine tumors. Arch Pathol Lab Med. 2006;130:963-6. . [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Xie QP, Gao SL, et al. . Pancreatic somatostatinoma with obscure inhibitory syndrome and mixed pathological pattern. J Zhejiang Univ Sci B. 2010;11:22-6. 10.1631/jzus.B0900166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomono H, Kitamura H, Iwase M, Oyama T, Inui Y, Aoki J.. A small, incidentally detected pancreatic somatostatinoma: report of a case. Surg Today. 2003;33:62-5. 10.1007/s005950300012 [DOI] [PubMed] [Google Scholar]
- 34.Madeira I, Terris B, Voss M, et al. . Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422-7. 10.1136/gut.43.3.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azimuddin K, Chamberlain RS. The surgical management of pancreatic neuroendocrine tumors. Surg Clin North Am. 2001;81:511-25. 10.1016/S0039-6109(05)70140-7 [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Konishi K, Kimura H, et al. . Strategy for pancreatic endocrine tumors. Hepatogastroenterology. 2000;47:537-9. . [PubMed] [Google Scholar]
- 37.Angeletti S, Corleto VD, Schillaci O, et al. . Use of the somatostatin analogue octreotide to localise and manage somatostatin-producing tumours. Gut. 1998;42:792-4. 10.1136/gut.42.6.792 [DOI] [PMC free article] [PubMed] [Google Scholar]


