Abstract
To enable patient- and disease-specific diagnostic and treatment at the intracellular level in real time, it is imperative to engineer a perfect way to locally stimulate selected individual neurons, navigate and dispense a cargo of biomolecules into damaged cells or image sites with relatively high efficacy and with adequate spatial and temporal resolutions. Significant progress has been made using biotechnology; especially with the development of bioinformatics, there are endless molecular databases to identify biomolecules to target almost any disease-specific biomarker. Conversely, the technobiology approach that exploits advanced engineering to control underlying molecular mechanisms to recover biosystem’s energy states at the molecular level as well as at the level of the entire network of cells (i.e., the internet of the human body) is still in its early research stage. The recently developed magnetoelectric nanoparticles (MENPs) provide a tool to enable the unique capabilities of technobiology. Using exemplary studies that could potentially lead to future pinpoint treatment and prevention of cancer, neurodegenerative diseases, and HIV, this article discusses how MENPs could become a vital enabling tool of technobiology.
The rapidly growing interdisciplinary field of nanomedicine promises unprecedented patient- and disease-specific high-precision medical diagnostic and treatment. To underscore the significance of its potential impact, with the rapid growth of genetic engineering, nanomedicine can become the ultimate enabling tool to unlock all the great potential of highly personalized precision medicine. Significant progress has already been achieved using biotechnology. Especially with the development of bioinformatics, today there are endless computational resources and molecular databases to help identify biomolecules that could target almost any specific biomarker. Conversely, the recently emerged technobiology approach that exploits advanced engineering combined with physics, chemistry, and computer science to monitor and control the intracellular and intercellular biology in real time is still in its very early stage of research. The technobiology approach could potentially enable a high-efficacy external control of intrinsic molecular processes that underlie a disease pathology and gene variability at the single-molecule level as well as at the level of entire massive network of trillions of interconnected cells of the human body also known as the internet of the human body. Communication between all the cells in this internet follows a set of laws that eventually define the state of human health. This set of laws remains to be understood; technobiology is the pathway to gaining and then applying the required knowledge. In other words, technobiology aims to exploit the physics of the molecular-level interactions as well as to decipher the science of the complex internet of the human body with the goal to engineer better medicines and make personalized medicine a reality.
The biological processes that underlie medical diagnostic, treatment, and disease pathogenesis at the molecular level are inherently driven by intrinsic electric fields. For example, the brain is one biological system the understanding of which could be substantially improved if the electric fields deep in the brain could be mapped in real time. The neural activity in the central and peripheral neural systems is determined by complex electric circuits and subcircuits that have not been decoded yet. Despite the significant progress in neuroscience over the last few decades, we still know very little about the brain. Most of the modern therapies related to neurodegenerative diseases or brain tumors are not developed at this fundamental level and thus are substantially limited in their capabilities. In addition, the holy-grail task of reverse engineering the brain would also significantly benefit from the ability to map the intrinsic electric fields in real time. Another familiar example relates to the field of cellular oncology. Normal and cancer cells of one type under equivalent conditions have distinctly different electric properties, for example, reflected in different values of the membrane potential, the dielectric breakdown voltage, the electroporation threshold energy, the surface charge, or another parameter that could be used for unique cellular identification and control and eventually high-specificity therapy. One more application of a translational impact could result from using local electric fields to control lineage reprogramming of somatic cells into specific cell types such as vital dopaminergic neurons and others. Last, but not least, in general, the promising and rapidly growing discipline of nanoparticle-based targeted drug delivery indispensably relies on a chemical bond between the nanoparticle carrier and the drug(s); in turn, this bond, regardless of its origin, is always electric-field driven and therefore could be externally controlled provided there is the right technobiology to accomplish this feat. In this regard, for example, using nanoparticles for externally controlled gene transfection could unlock many new life-saving applications.
With this said, the quest for an approach to exploit these molecular- and network-level electric field–driven interactions to enable externally controlled therapy must not be surprising. Unfortunately, achieving a local control of intrinsic electric fields remotely has long been a formidable task. The reason is the fact that remote electric fields cannot be used for a direct cellular level control of local electric properties without causing and/or experiencing a major interference with/from the rest of the electric micro- and macroenvironments of the human body. For example, that is the reason why the current version of deep-brain stimulation (DBS) requires establishment of direct physical contacts/wires to neurons. That is the reason why DBS can be used only with an inadequately small number of electrodes and thus cannot provide a single-neuron control. Obviously, a goal of attaching over 80 billion wires to the brain would be out of the question. In contrast, magnetic fields do not face this challenge. We do have the ubiquitous diagnostic tool of magnetic resonance imaging (MRI) (Fox and Raichle 2007). Even relatively high magnetic fields (on the order of 3 Tesla) only insignificantly interfere with the patient’s physiological system and thus have been designated as safe by the Food and Drug Administration (FDA). However, despite the excellent structural imaging capabilities of MRI, under normal circumstances, magnetic fields can barely couple to local electric fields and thus cannot provide sufficiently rich information associated with intrinsic biological processes, in addition to the temporal resolution of MRI on the order of 1 msec, is not sufficient for imaging in real time. In summary, discovering a novel approach for providing high-efficacy external control of local electric fields in intrinsic physiological microenvironments is vital for enabling technobiology.
One solution to this problem would be to use the recently introduced (in medicine) class of multiferroic nanostructures known as magnetoelectric nanoparticles (MENPs). Unlike any other materials and nanostructures, MENPs have unique properties that allow them to combine the wireless control capability of magnetic fields and the intricate access to intrinsic molecular-level information by local electric fields.
MAGNETOELECTRIC NANOPARTICLES AND THEIR MIGHTY CAPABILITIES
In general, the use of nanoparticles is considered an essential enabling tool of personalized nanomedicine because of their unique and diverse size- and shape-dependent properties. There are many different types of nanoparticle systems. To name a few, they rely on using thermally responsive polymers, electromagnetically or acoustically activated materials, liposomes, electrochemical processes, or other mechanisms to deliver and release the drug or enhance an image contrast (Derfus et al. 2007). However, it is only the MENPs that can unlock all the aforementioned technobiology capabilities. They can do this because of the presence of the magnetoelectric (ME) effect.
Distinction between Magnetoelectric and Traditional Magnetic Nanoparticles
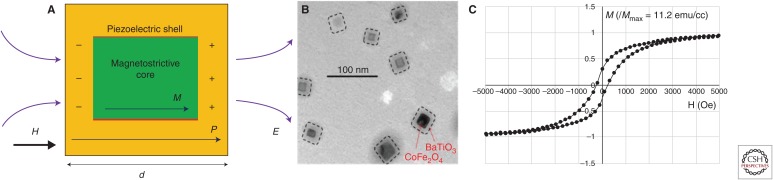
MENPs must not be confused with traditional magnetic nanoparticles (MNPs), which, like MENPs, do have a field-dependent magnetic moment but, unlike MENPs, do not display any ME effect. Although the ME effect has been known for many decades, only recently have we learned to synthesize materials with a nonzero ME effect at room temperature and above. There are many types of MENPs. They differ mostly depending on the material composition. The coreshell ME nanoparticles represent the most widely used type of MENPs. These coreshell nanostructures belong to type I multiferroics and are made of two ferroic components, the ferromagnetic (or ferrimagnetic or antiferromagnetic) core and the ferroelectric shell, respectively, as shown in Figure 1A. The ME effect is the result of a strain-induced lattice match between the two components. Owing to this lattice match, the magnetostrictive effect of the magnetic core is intrinsically coupled (through strain) to the piezoelectric effect of the ferroelectric shell. A transmission electron microscopy (TEM) image of 30-nm CoFe2O4–BaTiO3, made of the magnetostrictive ferrimagnetic spinel core CoFe2O4 and the piezoelectric perovskite shell BaTiO3, is shown in Figure 1B. The size can be controlled in a range from 10 to over 100 nm through temperature conditions, while the magnetic component is fabricated through a hydrothermal method (Guduru and Khizroev 2014; Stewart et al. 2018). This procedure results in MENPs that can be resuspended in aqueous solutions because of the presence of carboxyl groups. The carboxyl functional groups are used for drug/dye conjugation. These structures have been shown not to cause any toxicity when used in adequate doses and particularly when coated with some lipid molecules such as glycerol mono-oleate (GMO) (Nair et al. 2013; Kaushik et al. 2016; Rodzinski et al. 2016). It is noteworthy that the M-H hysteresis loop of these nanostructures does not follow a hysteresis-free dependence typical of superparamagnetic MNPs, as shown in Figure 1C (Islam et al. 2008; Nair et al. 2013). This fact could be explained by an effectively increased anisotropy of the ferrimagnetic core caused by the additional strain-induced interaction with the piezoelectric component.
Figure 1.
Magnetoelectric nanoparticles and their magnetic properties. (A) Illustration of the basic coreshell configuration of multiferroic nanostructures. M and P are the magnetic and electric polarizations, and H and E are the respective magnetic and electric fields; (B) A transmission electron microscopy (TEM) image of CoFe2O4–BaTiO3 MEnTs; and (C) M-H loop of 30-nm MEnTs measured with a vibrating sample magnetometer (VSM).
Like the traditional MNPs, MENPs can be used for image-guided navigation through organs via application of external magnetic field gradients and be image guided with MRI or the recently emerged magnetic particle imaging (MPI) (Senyei et al. 1978; McBain et al. 2008). Using MNPs as contrast-enhancing agents in MRI has been well established for decades. Superparamagnetic iron oxide nanoparticles (SPIONs) and gadolinium nanoparticles are some of the most common MRI contrast-enhancement agents today. As for the application of MNPs for targeted drug delivery, it has also been known for decades. Indeed, any MNPs can be wirelessly guided by magnetic field gradients; the rate of delivery is defined by the external magnetic field source and not limited by the circulatory system and other internal processes. For comparison, many conventional biology-driven approaches (e.g., based on the exocytosis of the drug with an intracellular vesicle) are strongly dependent on the internal microenvironment and thus often fail to adequately regulate cellular phenomena (Batrakova et al. 2011). For instance, as it is related to drug delivery across the blood–brain barrier (BBB), MNP-based delivery provides a unique way to transfer drugs sufficiently fast for the drug-loaded nanoparticles to avoid being engulfed by the reticuloendothelial system (RES) (Saiyed et al. 2010). Unfortunately, like most conventional approaches, this delivery also suffers from uncertainty of the drug release when the nanoparticles reach the target site(s). Another problem with MNPs is the difficulty to efficiently couple their magnetic properties to intrinsic electric-field-driven processes to enable superior diagnostic and treatment. Again, revealing and controlling the intrinsic interaction with the micro- and macroenvironments is vital for enabling personalized diagnostic and recovery or regeneration of all the normal functions. Hence, this review focuses on understanding how the new type of nanoparticles (i.e., MENPs) could be exploited to overcome the stumbling roadblock of external control in nanomedicine.
Unlike MNPs, owing to the presence of the ME effect, MENPs display an entirely new set of unique and important functions. These functions arise from the fact that the ME effect allows to strongly couple local intrinsic electric fields at the intracellular level with magnetic fields to enable a wireless control of cellular processes in any organ (e.g., neural activity deep in the brain, intracellular penetration specifically into cancer cells, and release of RNAs or other biomolecules into specific cells of any specific organ of the body). As described below in more detail, these functions allow for simultaneous imaging, local stimulation, targeted drug delivery, and field-controlled release on demand with a nanoscale 3D precision in real time.
The ME effect, for example, present in some type I multiferroics because of a relatively strong strain-related coupling between the ferroelectric and ferromagnetic components, can be explained thermodynamically according to the Landau theory of multiferroics for the second-order free energy expansion, G (Landau 1937):
| (1) |
where Ei and Hj stand for the ith and jth components of the electric and magnetic fields, respectively, and αij represents the ME coefficient tensor. For simplicity, this expression includes only the cross-term, which depends on both fields.
As a result, in this order of approximation, the induced electric polarization (dipole moment per unit volume), Pi, depends on the applied magnetic field according to this linear expression:
| (2) |
For example, given α of 100 mV cm−1 Oe−1 (Corral-Flores et al. 2010), a relatively small magnetic field on the order of 100 Oe (0.01 T) would generate a local electric field on the order of 10 V/cm, which could be used to stimulate the neural network locally.
Reciprocally, the induced magnetization change of the nanoparticle depends on the local electric field according to the linear expression derived from (1):
| (3) |
In this case, the ME coefficient is known as the converse ME effect coefficient. Although, according to the convention, the designation letter, α, remains the same, it is reasonable to use different units (i.e., G cm V−1 instead of mV cm−1 Oe−1) to make the connection between fields obvious. For example, given the value for α of 0.1 G cm V−1 (Jia et al. 2006; Cho and Priya 2011; Zhang et al. 2014), a local electric field caused by an action potential at the neuronal membrane of 1 V/cm would induce the nanoparticles’ magnetization change on the order of 1 emu/cc if the nanoparticle is in the vicinity of the membrane. Assuming the MENP’s saturation magnetization is on the order of 10 emu/cc (Betal et al. 2015), the relative change of the magnetization on the order of 10% would be quite significant for providing a high-contrast image of an electric-field profile. Therefore, if MENPs are used instead of traditional MNPs (e.g., SPIONs or gadolinium nanoparticles) (Nielson and Thomsen 2012) to enhance the image contrast of MRI or, better yet, MPI (Panagiotopoulos et al. 2015), they could provide an image not only containing structural information but also reflecting local intrinsic electric fields. In turn, because the local electric fields are intrinsically linked to neural activity, MENPs-based magnetic imaging can shed light on understanding the brain.
This paper gives an overview of recent studies on using MENPs as a technobiology enabler to provide another dimension to the fields of targeted drug delivery, DBS, neuroimaging, and functionalized diagnostics that could trigger leapfrog advances in the state of treatment of neurological diseases, cancer, HIV, and other devastating diseases in the near future.
Wireless Stimulation of Central and Peripheral Nervous Systems at a Single-Neuron Level
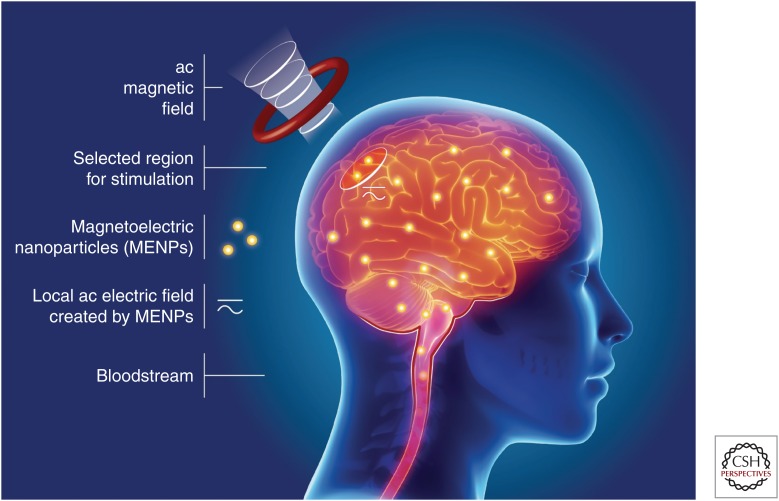
The significance of the capability of MENPs to provide wireless stimulation of selective regions deep in the brain locally at the subneuronal level or vagus nerve stimulation is hard to overestimate (Carreno and Frazer 2017). Such a capability can open a pathway to ultimate treatment of disabilities related to motor and sensory impairments and curing patients suffering from Parkinson’s disease (PD) and any other devastating neurodegenerative disease. Both the central and peripheral nervous systems (CNS and PNS) are driven by electric signals and thus can be represented as electric circuits. A neurodegenerative disease is a result of one or several of electric subcircuits having a defect or being completely broken. Such damaged subcircuits could be repaired by local stimulation through MENPs. It is noteworthy that electric-field-triggered stimulation is the basis for most current stimulation approaches such as various forms of invasive direct-contact DBS and low-efficacy transcranial magnetic stimulation (TMS) techniques (Fregni and Pascual-Leone 2007; Kringelbach et al. 2007). However, all these approaches are severely limited in their capabilities. DBS needs to establish direct physical contacts to the neural network and thus is limited by a finite number of implants. TMS only indirectly interacts with the electric circuitry and thus has very low efficacy and poor spatial resolution. In contrast, wirelessly controlled MENP-based stimulation can be done locally and therefore could be made entirely noninvasive (or only minutely invasive) while achieving unprecedented high efficacy. The idea of using MENPs for wireless stimulation to recover the communication between neurons in patients with PD was discussed in a paper by Yue et al. (2012). Supporting experimental results of an in vivo study on mice were presented in a paper by Guduru et al. (2015). These studies for the first time demonstrated that wireless stimulation with MENPs was indeed feasible. In their in vivo experiments, they administrated a relatively small dose of the nanoparticles into the bloodstream intravenously, through an injection of approximately 100 µg of MENPs in the tail of a mouse. Then they pulled the nanoparticles into the brain across the BBB via application of a magnetic field gradient of approximately 3000 Oe/cm. They confirmed the resulting significantly increased concentration of the nanoparticles in the brain through atomic and magnetic force microscopy (AFM and MFM) as well as through scanning electron microscopy (SEM) imaging of brain slices post euthanasia. Wirelessly controlled stimulation was demonstrated by measuring electroencephalography (EEG) signals from EEG implants in correlation with an applied ac magnetic field of 100 Oe strength at a frequency in a range up to 100 Hz. The concept of MENPs-based wirelessly controlled stimulation is shown in Figure 2.
Figure 2.
Illustration of a wireless electric stimulation with magnetoelectric nanoparticles (MENPs) via application of ac magnetic fields.
It is important to note that to improve the control and maximize the efficacy of the MENPs-based wireless stimulation, it makes sense to have nanoparticles be positioned on the neuronal membrane surface, right where action potentials start. As a reminder, a typical value of the membrane potential at rest is approximately −70 mV. Increasing the membrane potential by approximately +15 mV triggers firing of an action potential. The exact value of the electric field required to achieve this threshold depends on the neuron type and on the specific location on the membrane surface. MENPs on the membrane should be able to locally generate an electric field strong enough to overcome the potential threshold to fire action potentials. Using a back-of-the-envelope-type estimation, assuming an applied magnetic field of 1000 Oe, an MENP with an α of 100 mV cm−1 Oe−1 would generate an electric field of 100 V/cm (104 V/m). Generating such a field across the membrane would be sufficient to trigger firing of an action potential by a single nanoparticle (Ye and Steiger 2015). Furthermore, when acted collectively and under application of periodic signals corresponding to periodic rhythms of brain waves, MENPs could easily provide high-efficacy stimulation.
Learning from the recent development of DBS and TMS, with MENPs, application of a magnetic field as a near-dc (10 to 100 Hz) train of relatively sharp 100 Oe pulses with a pulse width ranging from 10 to 100 μsec would provide an ideal local stimulation. Although providing a relatively low-frequency periodic train of narrow pulses could very efficiently stimulate the neural network locally, such a relatively sustained stimulation process involving a collection of coherent action potentials would be limited only to several applications (e.g., to treat depression, PD, and a few other neurodegenerative diseases). Ideally, to perfectly simulate/recover any operation of the neural network (e.g., for recovering movement of limbs and repairing senses, etc.), it is necessary to trigger individual action potentials through any channel in any different region in the neural system at any time instance on demand. With MENPs, such high-precision control could be accomplished through implementation of advanced electromagnetic theory and signal processing.
Hence, further increasing the ME coefficient is critical. The ME coefficient can be significantly increased through improving materials properties. There are claims of the coefficient value above 1 V cm−1 Oe−1 (Palneedi et al. 2016). Further, the ME coefficient can be increased through a dc field biasing (Islam et al. 2008). In addition, it is noteworthy that the ME effect strongly depends on the frequency (Popov et al. 2008; Cho and Priya 2011; Liu et al. 2012). In fact, it could be substantially increased (by orders of magnitude) at a frequency corresponding to a natural resonance, whether the resonance is the result of mechanical vibrations, ferromagnetic precession, or a combination of the two or not (Yu et al. 2008). Ideally, the highest resonance would take place when both phases, magnetic and electric components, respectively, resonate at the same frequency (Popov et al. 2014). However, most of these resonances in such small nanostructures typically occur in a GHz range (e.g., from below 5 to over 10 GHz). At the same time, it is known that electromagnetic waves in this frequency range strongly attenuate in this frequency range because of the absorption by water (Chandra et al. 2014; Ziskin et al. 2018). Hopefully, integration of extremely sensitive nanotechnologies with advanced signal processing and antenna technologies might be able to address the challenge.
Last but not least, it is noteworthy that posttreatment MENPs can be removed from the brain the same way they were brought in (i.e., through application of magnetic field gradients). During the removal process, the gradient directions should be reversed so that the nanoparticles are pulled back to the bloodstream. Independently, nanoparticles can be cleared from the brain naturally. Indeed, it has been shown on animal models that the nanoparticles are excreted within a 2-month period depending on their size (Hadjikhani et al. 2017). Finally, it is likely that some type of biodegradable MENPs, possibly based on carbon nanostructures, will be developed in the future (Pridgen et al. 2007).
Externally Controlled Targeted Drug Delivery and Release across the Blood–Brain Barrier
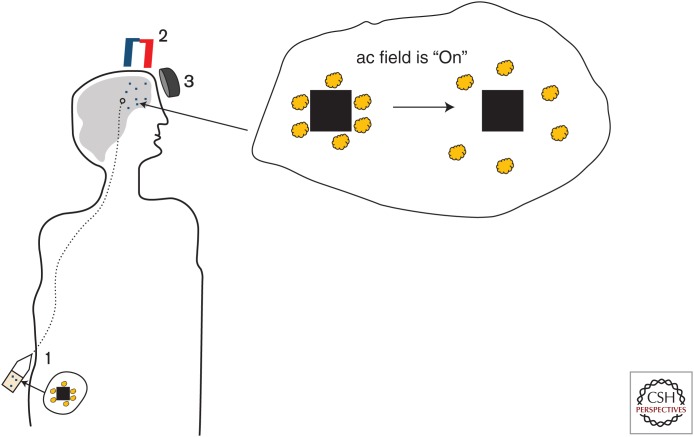
Probably, one of the most important properties of MENPs is not only their ability to deliver a therapeutic load across the BBB with a very high efficacy but also their ability to release the load in any place at any time on demand. As discussed above, like any other nanoparticles, MENPs can be used to deliver drugs across the BBB (Lockman et al. 2002; Guduru et al. 2015). However, for the delivered drug to be bioactive, it is important to release it off the carrier nanoparticles when they reach the target site (Veronese and Pasut 2005). Although there are approaches in which they formulate or functionalize nanoparticles to allow for triggering drug release by thermal activation, application of magnetic fields, electromagnetic, or acoustic waves through a change in intracellular pH or intracellular enzymes, in most cases, the release mechanism cannot be controlled with adequately high efficacy (Torchilin 2005; Arruebo et al. 2007). To ensure the drug gets released at the target site, the conjugation strength between the drug and the nanoparticles is often kept relatively weak. As a result, most of the drug is prematurely released off the nanoparticles in the plasma or interstitial space, not at the intended target site (Zhang et al. 2010). In contrast, with MENPs, the conjugation strength, defined by electric fields between the drug and the nanoparticles, can be wirelessly controlled via application of dc and ac magnetic fields. In the paper by Nair et al. (2013), they showed that owing to the ME effect, application of an ac magnetic field is equivalent to shaking the drug off the nanoparticles. Therefore, the conjugation strength between the drug and the nanoparticles can be made adequately strong to ensure no drug is released before the nanoparticles reach the target site. Only after the nanoparticles with the drug are pulled across the BBB and reach the target site in the brain, an ac magnetic field can be applied to trigger the desired high-efficacy release. The concept of the ac magnetic field–controlled drug release is shown in Figure 3. In their study, they used an in vitro BBB model to show that this MENPs concept could be used to deliver and release the well-known antiretroviral therapy AZTTP to eliminate HIV-1 virus hidden deep in the brain. They used a dc magnetic field gradient of approximately 3000 Oe/cm to pull the drug-loaded nanoparticles across the BBB. Then, when the loaded nanoparticles reached the brain, they applied a 100 Oe ac magnetic field at a frequency of 100 Hz to trigger the drug release.
Figure 3.
Illustration of steps using magnetoelectric nanoparticles (MENPs) to deliver drugs across the blood–brain barrier (BBB): (1) Intravenous (IV) injection of drug-loaded nanoparticles, (2) the drug-loaded MENPs are pulled across the BBB via application of a dc magnetic field gradient (on the order of 3000 Oe/cm), (3) when, optionally through image guiding, MENPs can be localized at the intended site, a relatively weak ac magnetic field, with a strength of 100 Oe at a frequency of 100 Hz, is applied to release the drug.
High-Specificity Intracellular Targeted Drug Delivery
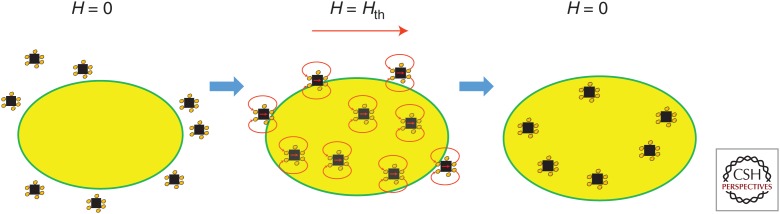
Again, one of the most important properties of MENPs is the capability they provide to control local electric fields wirelessly via application of magnetic fields. It is well known that different cells, particularly their membranes, could be distinguished through their electric properties such as membrane potential, dielectric permittivity, conductivity, and others. For example, the membrane potentials of cancer cells can be quite different from those of their normal counterparts (Yang and Brackenbury 2013). The membrane potential defines the energy required to break through the membrane for entering the cell. That is the reason there is a well-established high-specificity process, called electroporation, used to deliver biomolecules specifically into cancer cells, without affecting the surrounding normal cells of the same type (Prausnitz et al. 1993). One challenge with this approach is the need to apply relatively high electric fields, on the order of 1000 V/cm, to electroporate cancer cells. When applied to a relatively large region in the body, with a characteristic size on the order of a few millimeters, such high fields can be damaging also to surrounding normal tissues. In contrast, if MENPs are used to induce electroporation, the high fields are applied only in a local nanoscale region around the nanoparticles. Thus, the MENP-triggered electroporation, known as nanoelectroporation, does not produce any field-sensitive side effects. The MENPs-triggered nanoelectroporation was for the first time used to deliver the well-known mitotic inhibitor paclitaxel into ovarian cancer cells while sparing the surrounding normal ovarian cells. Such experiments were conducted both in vitro and in vivo (Guduru et al. 2013; Rodzinski et al. 2016). The high specificity of this effect was explained by the substantial difference in the membrane potential between the two cell types. The membrane potentials of ovarian cancer and normal cells are on the order of −5 and −50 mV, respectively. In other words, the field required to “break into” the normal cells needs to be higher by a factor of ten compared to that for the cancer cells. The underlying physics of the nanoelectroporation was discussed in the paper by Stimphil et al. (2017). To directly measure the tissue specificity of this approach and detect the presence of MENPs with a nanoscale precision, the mode of SEM known as the energy-dispersive spectroscopy (EDS) was used (Rodzinski et al. 2016). The EDS-SEM imaging combines the advantages of the elemental compositional analysis on par with that by mass spectroscopy and the high spatial resolution by SEM (Hadjikhani et al. 2017). The concept of MENP-triggered nanoelectroporation used to deliver drugs specifically into cancer cells via application of a 100 Oe dc magnetic field is shown in Figure 4. To ensure the required specificity, it is important to keep the dc field between the nanoelectroporation thresholds for the cancer and normal cells, respectively. After the loaded nanoparticles were delivered into the cancer cells, a relatively weak ac magnetic field, with a strength of 30 Oe at a frequency of 100 Hz, was applied to release the drug on demand.
Figure 4.
Illustration of steps of the concept of the field-controlled MENP-based nanoelectroporation to deliver drugs specifically into cancer cells. (1) Drug-loaded MENPs are administrated into the cellular microenvironment of interest; (2) a dc magnetic field above the threshold value for the particular cancer cell line is applied to induce the process of nanoelectroporation; and (3) the magnetic field is turned off to trap the drug-loaded nanoparticles inside the cancer cells.
It is noteworthy that because of the fundamental nature of the approach, MENPs could be used for intracellular delivery of any biomolecules, including nucleic acids for enabling genetic engineering or certain antitumor peptides for treatment of glioblastomas, as described in the paper by Stewart et al. (2018).
HIGH-CONTRAST FUNCTIONALIZED IMAGING AND HIGH-SPECIFICITY BIOMOLECULAR DIAGNOSTICS
According to the principle of reciprocity, if MENPs can be used to “write” information (or stimulate locally), then the same nanoparticles should be able to “read back” (or record) the information because of local cellular or neural activity (Khizroev et al. 1997). Just applied to the brain alone, the importance of the capability to record the neural activity with the subneuronal spatial resolution at an adequately high temporal resolution with the goal to monitor the brain in real time is hard to overestimate (Fox and Raichle 2007; Marblestone et al. 2013). Such a capability would not only revolutionize the large area of diagnostics of neurodegenerative diseases but would also pave the way to fundamental understanding and reverse engineering the brain (Koch and Reid 2012).
There are already a number of technologies available for functionalized brain imaging, including (1) EEG (Coenen 1995), (2) functionalized MRI (fMRI), and diffusion MRI (dMRI) also known as diffusion tensor imaging (DTI), or a combination of these two (DfMRI) (Yassa et al. 2010), (3) positron emission tomography (Grafton et al. 1992), (4) magnetoencephalography (MEG) (de Pasquale et al. 2010), (5) neuronal optogenetics (Toettcher et al. 2010), and (6) molecular recording (Zamft et al. 2012). However, all these approaches are strongly limited in their capabilities because of inadequate spatial and temporal resolutions. In other words, to date, there is no viable way to map local electric fields caused by neural activity deep in the brain.
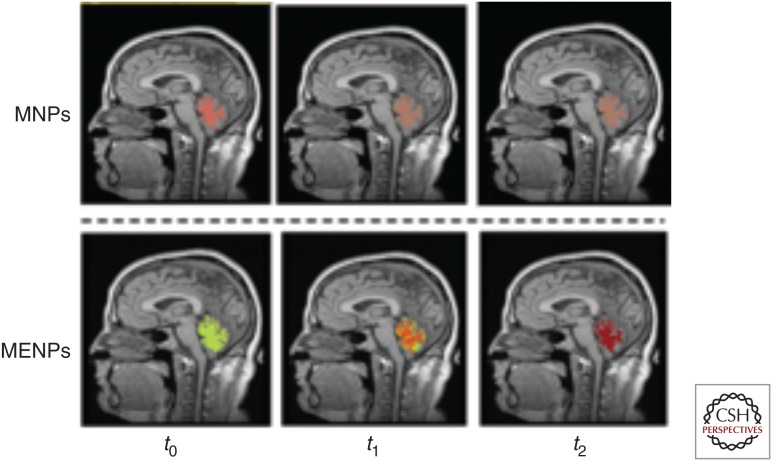
Again, owing to their ME effect, MENPs allow to fill in this gap. Particularly, MENPs allow to take advantage of both the magnetic field’s capability to penetrate through the brain without a significant interference with the complex electric circuitry of the brain and the electric field’s capability to couple to neural activity locally with the subneuronal resolution. Therefore, if MENPs are integrated with a magnetic imaging approach, they could be used to map electric fields in the brain. For example, if MENPs are used instead of the traditional MNPs (e.g., SPIONS, as image contrast enhancement agents), they will not only enhance the contrast caused by the structural image but also will provide additional information caused by the local electric activity. However, to be able to record neural activity in real time, it is important to have a sufficiently high temporal resolution, arguably, in the microsecond range. Unfortunately, MRI cannot achieve such resolution. Its resolution is limited by the nuclear spin relaxation time to the hundreds of milliseconds range. Therefore, in the study by Guduru et al. (2018), they propose to couple the advantageous properties of MENPs with the recently developed approach of MPI (Gleich and Weizenecker 2005; Goodwill et al. 2009; Weizenecker et al. 2009). The MPI’s temporal resolution is limited by the ferromagnetic resonance of the nanoparticles, which in turn is defined by the magnetic anisotropy and can be in the nanosecond range. As an illustration, numerically simulated MPI images taken with traditional MNPs and equivalent MENPs at three consecutive time instances from the same brain region under equivalent conditions are shown in Figure 5. As a result of the ME effect, MENPs can detect some time-dependent process, which cannot be detected by MNPs. For example, this process could result from some kind of neuroinflammation dynamic, which would be characteristic of a disease progression.
Figure 5.
Simulated magnetic particle imaging (MPI) images taken with traditional magnetic nanoparticles (MNPs) (top row) and equivalent magnetoelectric nanoparticles (MENPs) at three consecutive time instances, t0, t1, and t2, respectively. The computation was performed by Dr. Rakesh Guduru of Florida International University (FIU).
Using the same underlying physics, MENPs could be used for early-stage diagnostic and/or rapid screening of diseases. For example, the paper by Nagesetti et al. (2017) describes an in vitro study in which they used MENPs together with the nuclear magnetic resonance (NMR) spectroscopy to diagnose cancer. In their experiment, they mixed MENPs into different cell media for further NMR measurements. The study showed not only that cancer cells have distinctly different NMR spectra compared to their normal counterparts but also that different cancer cell lines can be distinguished from each other through their signature NMR spectra. They compared several breast cancer, ovarian cancer, and brain tumor (glioblastoma) cell lines.
CONCLUSION
In summary, MENPs have proven as a formidable enabling tool, implementation of which can pave the way for many unprecedented technobiology capabilities in medicine. Technobiology’s capabilities are complementary to those of the traditional approach of biotechnology. The discussed research on MENPs during the last several years has been instrumental to shed light on these capabilities in the fields of targeted drug delivery, cancer, HIV/AIDS, neurodegenerative diseases, neuroimaging, diagnostic, and other fields. In a layman’s perspective, these novel capabilities can be summarized as a technology platform allowing for ultimate pinpoint treatment and prevention of any disease. With MENPs, the human body can be wirelessly connected to a computer at the subcellular level so that its fundamental electric circuitry can be continuously monitored and repaired in real time. In other words, MENPs allow us to directly see and control the complex dynamics of electric fields that govern all the biological processes underlying any medical disease. Further, such a capability opens a pathway to understanding the basic principles of the intra- and intercellular communication within the human body, which in turn define the operation of the internet of the human body. In the future, the resulting potential applications of technobiology would not only revolutionize medicine but also lead to leapfrog advances in science and technology. Reciprocally, it is likely that what we will learn from the human body at this technobiology level will also improve our understanding of the nature and change the way we build our technologies.
ACKNOWLEDGMENTS
I dedicate this work to the memory of my friend and colleague Professor Robert Haddon of the University of California–Riverside. His unyielding dedication to science and constantly pushing the boundaries had always inspired me and was instrumental in defining my vision to conduct research at the intersection of medicine and nanotechnology. Special thanks go to all the members of my team at Florida International University (FIU) with whom I have been privileged to work during the last several years (in alphabetical order): Dr. Rakesh Guduru, Dr. Ali Hadjikhani, Dr. Kevin Luongo, Dr. Abhignyan Nagesetti, Brayan Navarette, Krystine Pimentel, Dr. Alexandra Rodzinski, Dr. Tiffanie Stewart, Dr. Emmanuel Stimphil, Dr. Mark Stone, and Ping Wang for conducting most of the experiments described in the paper. I am particularly indebted to Dr. Rakesh Guduru, my first PhD Graduate Student in the field of technobiology. Without his multifaceted talent, dedication, and contagious passion for engineering innovations, this work would not have been possible. I acknowledge vital collaborations with a multidisciplinary team of scientists at FIU and Neuroscience Centers of Florida Foundation (NSCFF). I am particularly grateful to my friend and colleague Professor Ping Liang of the University of California–Riverside. His extraordinary vision for research excellence, talent and passion for research have been instrumental in spearheading this effort. I am grateful to Professor Vish Prasad of the University of North Texas for his inspiration and help to organize the first journal dedicated to this new field called Technobiology. Last but not least, I thank Professor James Tien of The University of Miami for coining the new term of technobiology and his continuous support of our research in this emerging field. I acknowledge partial financial support from National Science Foundation (NSF) awards #ECCS-1408063, ECCS-0939514, and IIP-1237818, National Institutes of Health (NIH) R21-MH101025-01 and R01-DA034547-01, and numerous other awards from Department of Defense (DoD) agencies and the NSCFF.
Footnotes
Editors: Valentin A. Pavlov and Kevin J. Tracey
Additional Perspectives on Bioelectronic Medicine available at www.perspectivesinmedicine.org
REFERENCES
- Arruebo M, Fernandez-Pacheco R, Ibarra MR. 2007. Magnetic nanoparticles for drug delivery. Nano Today 2: 22–32. [Google Scholar]
- Batrakova EV, Gendelman HE, Kabanov AV. 2011. Cell-mediated drugs delivery. Expert Opin Deliv 8: 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betal S, Dutta M, Cotica LF, Bhalla A, Guo R. 2015. BaTiO3 coated CoFe2O4-core-shell magnetoelectric nanoparticles (CSMEN) characterization. Integr Ferroelectr 166: 225–231. [Google Scholar]
- Carreno FR, Frazer A. 2017. Vagus nerve stimulation for treatment-resistant depression. Neurotherapeutics 14: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra CR, Bharani PSN, Kumar YR. 2014. Frequently dependent attenuation of EM radiation on biological tissues. Int J Adv Res EEIE 3: 12094–12099. [Google Scholar]
- Cho KH, Priya S. 2011. Direct and converse effect in magnetoelectric laminate composites. Appl Phys Lett 98: 232904. [Google Scholar]
- Coenen AM. 1995. Neuronal activities underlying the electroencephalogram and evoked potentials of sleeping and waking: Implications for information processing. Neurosci Biobehav Rev 19: 447–463. [DOI] [PubMed] [Google Scholar]
- Corral-Flores V, Bueno-Baques D, Ziolo R. 2010. Synthesis and characterization of novel CoFe2O4–BaTiO3 multiferroic core–shell-type nanostructures. Acta Materialia 58: 764–769. [Google Scholar]
- de Pasquale F, Penna SD, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, et al. 2010. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci 107: 6040–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derfus AM, von Maltzahn G, Harris TJ, Duza T, Vecchio KS, Ruoslahti E, Bhatia SN. 2007. Remotely triggered release from magnetic nanoparticles. Adv Mater 19: 3932–3936. [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. 2007. Technology insight: Noninvasive brain stimulation in neurology—Perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 3: 383–393. [DOI] [PubMed] [Google Scholar]
- Gleich B, Weizenecker J. 2005. Tomographic imaging using the nonlinear response of magnetic particles. Nature 435: 1214–1217. [DOI] [PubMed] [Google Scholar]
- Goodwill PW, Scott GC, Stang PP, Conolly SM. 2009. Narrowband magnetic particle imaging. IEEE Trans Med Imag 28: 1231–1237. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME. 1992. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci 12: 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guduru R, Khizroev S. 2014. Magnetic field-controlled release of paclitaxel drug from functionalized magneto-electric nanoparticles. Particle 31: 605–611. [Google Scholar]
- Guduru R, Liang P, Runowicz C, Nair M, Atluri V, Khizroev S. 2013. Magneto-electric nanoparticles to enable field-controlled high-specificity drug delivery to eradicate ovarian cancer cells. Sci Rep 3: 2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guduru R, Liang P, Hong J, Rodzinski A, Hadjikhani A, Horstmyer J, Levister E, Khizroev S. 2015. Magnetoelectric “spin” on stimulating the brain. Nanomed (Lond) 10: 2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guduru R, Liang P, Yousef M, Horstmyer J, Khizroev S. 2018. Mapping the brain’s electric fields with magnetoelectric nanoparticles. Bioelectron Med 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani A, Rodzinski A, Wang P, Nagesetti A, Guduru R, Liang P, Runowicz C, Shahbazmohamadi S, Khizroev S. 2017. Mapping the brain’s electric fields with magnetoelectric nanoparticles. Nanomedicine (Lond) 12: 1801–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam RA, Bedekar V, Poudyal N, Liu JP, Priya S. 2008. Magnetoelectric properties of core-shell particulate nanocomposites. J Appl Phys 104: 104111. [Google Scholar]
- Jia J, Or SW, Chan HLW, Zhao X, Luo H. 2006. Converse magnetoelectric effect in laminated composites of PMN-PT single crystal and terfenol-D alloy. Appl Phys Lett 88: 9202–9204. [Google Scholar]
- Kaushik A, Jayant RD, Nikkhah-Moshaie R, Bhardwaj V, Roy U, Huang Z, Ruiz A, Yndart A, Atluri V, El-Hage N, et al. 2016. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci Rep 6: 25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khizroev S, Bain J, Kryder M. 1997. Considerations in the design of probe heads for 100 Gbit/in2 recording density. IEEE Trans Magn 33: 2893–2895. [Google Scholar]
- Koch C, Reid RC. 2012. Neuroscience: Observatories of the mind. Nature 483: 397–398. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. 2007. Translational principles of deep brain stimulation. Nat Rev Neurosci 8: 623–635. [DOI] [PubMed] [Google Scholar]
- Landau LD. 1937. Collected papers of L.D. Landau. Zh Exper Theor Fiz 7: 19. [Google Scholar]
- Li S, Wang C, Chu XM, Miao GX, Xue Q, Zou W, Liu M, Xu J, Li Q, Dai Y, et al. 2016. Engineering optical mode ferromagnetic resonance in FeCoB films with ultrathin Ru insertion. Sci Rep 6: 33349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Miroshnichenko AE, Neshev DN, Kivshar YS. 2012. Broadband unidirectional scattering by magneto-electric core-shell nanoparticles. ACS Nano 6: 5489–5497. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Mumper RJ, Khan MA, Allen DD. 2002. Nanoparticle technology for drug delivery across the blood–brain barrier. Drug Dev Ind Pharm 28: 1–13. [DOI] [PubMed] [Google Scholar]
- Marblestone AH, Zamft BM, Maguire YG, Shapiro MG, Cybulski TR, Glaser JI, Amodei D, Stranges PB, Kalhor R, Dalrymple DA, et al. 2013. Physical principles for scalable neural recording. Front Comput Neurosci 7: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain SC, Yiu HP, Dobson J. 2008. Magnetic nanoparticles for gene and drug delivery. Int J Nanomed 3: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesetti A, Rodzinski A, Stimphil E, Stewart T, Khanal C, Wang P, Guduru R, Liang P, Agoulnik I, Horstmyer J, et al. 2017. Multiferroic coreshell magnetoelectric nanoparticles as NMR sensitive nanoprobes for cancer cell detection. Sci Rep 7: 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. 2013. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat Commun 4: 1707. [DOI] [PubMed] [Google Scholar]
- Nielson YW, Thomsen HS. 2012. Contrast-enhanced peripheral MRA: Technique and contrast agents. Acta Radiologica 53: 769–777. [DOI] [PubMed] [Google Scholar]
- Palneedi H, Annapureddy V, Priya S, Ryu J. 2016. Status and perspectives of multiferroic magnetoelectric composite materials and applications. Actuators 5: 1–31. [Google Scholar]
- Panagiotopoulos N, Duschka RL, Ahlborg M, Bringout G, Debbeler C, Graeser M, Kaethner C, Ludtke-Buzug K, Medimagh H, Stelzner J, et al. 2015. Magnetic particle imaging: Current developments and future directions. Int J Nanomed 10: 3097–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov C, Chang H, Record PM, Abraham E, Whatmore RW, Huang Z. 2008. Direct and converse magnetoelectric effect at resonant frequency in laminar piezoelectric-magnetostrictive composite. J Electroceramics 20: 53–58. [Google Scholar]
- Popov M, Sreenivasulu G, Petrov VM, Chavez FA, Srinivasan G. 2014. High frequency magneto-dielectric effects in self-assembled ferrite-ferroelectric core-shell nanoparticles. AIP Adv 4: 097117. [Google Scholar]
- Prausnitz MR, Bose VG, Langer R, Weaver JC. 1993. Electroporation of mammalian skin: A mechanism to enhance transdermal drug delivery. Proc Nat Acad Sci 90: 10504–10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgen EM, Langer R, Farokhzad OC. 2007. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine (London) 2: 669–680. [DOI] [PubMed] [Google Scholar]
- Rodzinski A, Guduru R, Liang P, Hadjikhani A, Stewart T, Stimphil E, Runowicz C, Cote R, Altman N, Datar R, et al. 2016. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci Rep 6: 20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyed ZM, Gandhi NH, Nair MP. 2010. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood–brain barrier. Int J Nanomedicine 5: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyei A, Widder K, Czerlinski C. 1978. Magnetic guidance of drug carrying microspheres. J Appl Phys 49: 3578–3583. [Google Scholar]
- Stewart TS, Nagesetti A, Guduru R, Liang P, Stimphil E, Hadjikhani A, Salgueiro L, Horstmyer J, Cai R, Schally A, et al. 2018. Magnetoelectric nanoparticles for delivery of antitumor peptides into glioblastoma cells by magnetic fields. Nanomedicine (Lond) 13: 423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimphil E, Nagasetti A, Guduru R, Stewart T, Rodzinski A, Liang P, Khizroev S. 2017. Physics considerations in targeted anticancer drug delivery by magnetoelectric nanoparticles. Appl Phys Rev 4: 021101. [Google Scholar]
- Toettcher JE, Voigt CA, Weiner OD, Lim WA. 2010. The promise of optogenetics in cell biology: Interrogating molecular circuits in space and time. Nat Methods 8: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. 2005. Recent advances with liposomes as pharmaceutical carriers. Nat Rev 4: 145–160. [DOI] [PubMed] [Google Scholar]
- Veronese FM, Pasut G. 2005. PEGylation, successful approach to drug delivery. Drug Discov Today 10: 1451–1458. [DOI] [PubMed] [Google Scholar]
- Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. 2009. Three-dimensional real-time in vivo magnetic particle imaging. Phys Med Biol 54: L1. [DOI] [PubMed] [Google Scholar]
- Yang M, Brackenbury WJ. 2013. Membrane potential and cancer progression. Front Physiol 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. 2010. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci 107: 12687–12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Steiger A. 2015. Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J Neuroeng Rehabil 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Pechan MJ, Srivastava S, Palmstrom CJ, Biegaslski M, Brooks C, Schlom D. 2008. Ferromagnetic resonance in ferromagnetic/ferroelectric Fe/BaTiO3/SrTiO3(001). J Appl Phys 103: 07B108. [Google Scholar]
- Yue K, Guduru R, Hong J, Liang P, Nair M, Khizroev S. 2012. Magneto-electric nano-particles for non-invasive brain stimulation. PloS ONE 7: e44040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamft BM, Marblestone AH, Kording K, Schmidt D, Martin-Alarcon D, Tyo K, Boyden ES, Church G. 2012. Measuring cation dependent DNA polymerase fidelity landscapes by deep sequencing. PLoS ONE 7: e43876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gilstrap K, Wu L, et al. 2010. Synthesis and characterization of thermally responsive Pluronic F127-chitosan nanocapsules for controlled release and intracellular delivery of small molecules. ACS Nano 4: 6747–6759. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu G, Shi H, Li M, Dong S. 2014. Converse magnetoelectric effect in laminated composite of metglas and Pb(Zr,Ti)O3 with screen-printed interdigitated electrodes. AIP Adv 4: 067106. [Google Scholar]
- Ziskin MC, Alekseev SI, Foster KR, Balzano Q. 2018. Tissue models for RF exposure evaluation at frequencies above 6 GHz. Bioelectromagnetics 39: 173–189. [DOI] [PubMed] [Google Scholar]