Abstract
In grassland studies, an intermediate level of grazing often results in the highest species diversity. Although a few hypotheses have been proposed to explain this unimodal response of species diversity to grazing intensity, no convincing explanation has been provided. Here, we build a lattice model of a grassland community comprising multiple species with various levels of grazing. We analyze the relationship between grazing and plant diversity in grasslands under variable intensities of grazing pressure. The highest species diversity is observed at an intermediate grazing intensity. Grazers suppress domination by the most superior species in birth rate, resulting in the coexistence of inferior species. This unimodal grazing effect disappears with the introduction of a small amount of nongrazing natural mortality. Unimodal patterns of species diversity may be limited to the case where grazers are the principal source of natural mortality.
Subject terms: Biodiversity, Community ecology, Grassland ecology, Theoretical ecology, Ecological modelling
Introduction
Grasslands are a vital part of the world’s ecosystems and occupy more than one-quarter of Earth’s land area1. Some grasslands contain more species than tropical forests at a small scale, e.g., 15.5–19.7 species in a 0.01 m2 area of alpine meadow on the Qinghai-Tibet plateau2, 43 species in a 0.1 m2 area of Romanian grassland3, and a new world record with 17 species in 0.0044 m2 of Krkonoše Mountains4. Maintaining the species diversity of grasslands is crucial for avoiding productivity loss, which in turn affects ecosystem processes5,6.
Many previous studies sought to explain the factors influencing the diversity of grasslands. Resource partitioning plays a significant role in promoting diversity7,8. Seed dispersal is also an important factor affecting the coexistence of multiple plant species9–11. Another factor that positively affects diversity is environmental heterogeneity, which exists in all natural systems, including grasslands12–14. However, all these studies concluded a role of niche differentiation or niche partitioning in determining species diversity without explicitly considering the role of grazing.
Many studies of natural, seminatural and managed grasslands have shown that an intermediate level of grazing results in the highest species diversity. Excessive grazing reduces species diversity because it converts grassland to bare land. However, why the species diversity of grasslands increases with the introduction of/an increase in grazing to some point is unclear. Based on previous empirical studies, the introduction of light grazing or an increase in grazing intensity increases the species diversity in some grasslands. For example, in five types of grassland communities in Andorran mountains, the highest plant diversity and resulting functional richness were observed at the intermediate grazing intensity15. In rangelands located in Tibetan alpine meadows, light and moderate grazing promote biodiversity and increase nectar volume at both the individual level and the community level16. In Himalayan rangelands, moderate grazing increases diversity17. Heavy grazing indirectly influences species richness in Inner Mongolian grassland sites18. Thus, explanations of why and how an increase in grazing intensity promotes the coexistence of many more species are needed.
Previous studies have modeled the coexistence of vegetation. The lottery model revealed the importance of environmental variability in promoting coexistence19–21. However, lottery models eventually lead to competitive exclusion over a very long time scale22. Therefore, a lottery model is not appropriate for testing the effects of grazing because of the eventual extinction of all but one species. We need a spatial component in the model to achieve the coexistence of plant species as in lattice models22.
Using a lattice model, Kakishima et al. examined the contribution of animal seed dispersers to the coexistence of tree species23. Tubay et al. applied this lattice model to build a general model of plant communities and reported the importance of spatial heterogeneity in determining the coexistence of many species22. These two models are able to avoid competitive exclusion as a stable state. Therefore, we can test the effects of grazing by using these lattice models. Here, we introduce grazing effects into a lattice model of a grassland community to examine how species diversity is affected by grazing intensity. We modify the lattice community model of Tubay et al. to develop a two-layer lattice model of a grassland community under grazing (with an animal layer)22. Using our model, we examine the effects of animal grazing on grasslands and investigate the underlying mechanism of these effects. The highest species diversity in the grasslands is observed under an intermediate grazing intensity. In contrast, species diversity decreases with an increase in grazer density. Furthermore, this unimodal grazing effect disappears with the introduction of a small amount of nongrazing natural mortality. We discuss when, why and how the highest diversity is achieved at an intermediate grazing intensity. We also discuss the implications of our results for the management of grassland communities.
Results
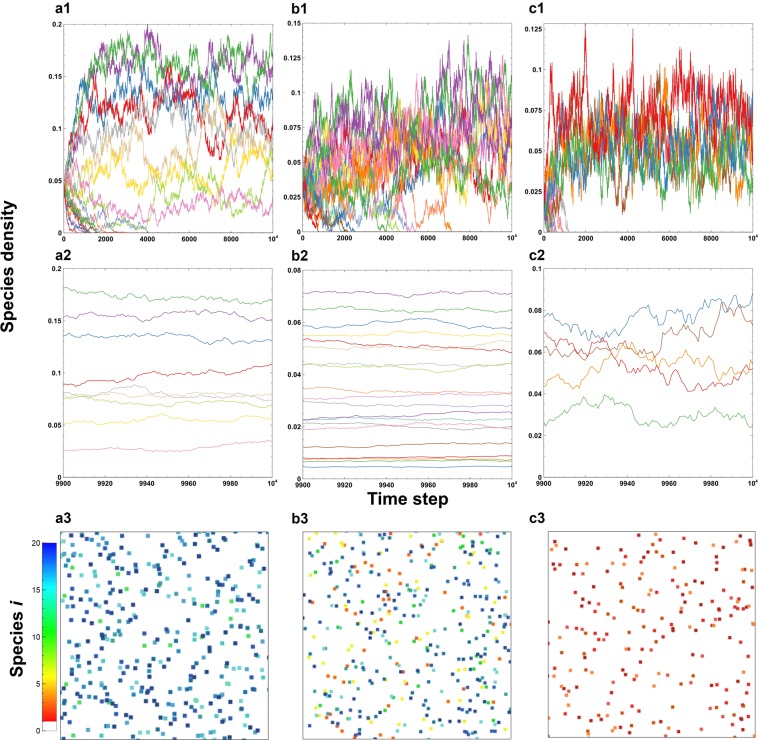
First, we simulate a lattice grassland model with 20 plant species under various levels of grazing intensity (G) without nongrazing natural mortality, i.e., di = 0 (Fig. 1, Supplementary Figure 1). The temporal dynamics exhibit large fluctuations in the number of individuals of each species over a long period of time (Fig. 1a1,b1,c1). In contrast, this number is fairly stable over a short timescale (Fig. 1a2,b2,c2). Based on the short-term temporal dynamics (Fig. 1a2,b2,c2) and snapshots of the plant distributions (Fig. 1a3,b3,c3), the number of surviving plant species is highest at an intermediate grazing intensity (G).
Figure 1.
The temporal dynamics of species density and a snapshot of the final density composition. a1-c3 The grazing intensity of a species decreases as the initial birth rate decreases, i.e., and Bi = B−0.002(i-1) for i = 1, 2, …, s), indicating a trade-off between the birth rate and grazing intensity of a species. a2, b2, c2 Temporal dynamics of the last one hundred steps. a3, b3, c3 A snapshot of the final surviving species composition. The value of the species-dependent factor is G′ = 0.0005. The basal grazing intensities are (a1, b1, c1) G = 0.05, (a2, b2, c2) G = 0.15 and (a3, b3, c3) G = 0.4. The lattice size is 100 × 100. The dispersal distance is P = 40. The initial species density is Ii = 0.03 (same for all species). The density of grazer cells is Iy = 0.4. Error bars indicate the standard deviations.
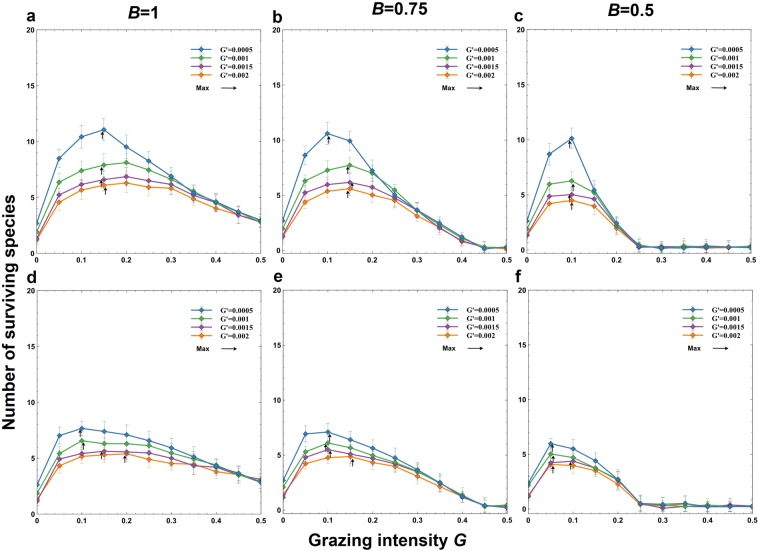
We vary the basal grazing intensity G to examine the effects of grazing (Fig. 2, Supplementary Figures 3, 4, Supplementary Table 1). The number of species is highest at an intermediate grazing intensity (G) for a given fecundity B and interspecific grazing difference G′ (Fig. 2; Supplementary Figure 2b,c,e,f). The interspecific grazing difference G′ is the difference in animal preference or selectiveness in grazing, e.g., palatable herbs or unpalatable grass. To examine the effects of a trade-off between birth rate and grazing intensity, we consider the following opposite relations between the species-specific expected birth rate Bi and grazing rate gi: (1) the trade-off case: species with a high Bi have a high gi (grazing is subject to a trade-off) and (2) the opposite case: species with a high Bi have a low gi. In both cases, the number of surviving species exhibits unimodal behavior in response to the grazing intensity G. The number of coexisting species at peak diversity is larger under the first case (with the trade-off) than under the second case (the opposite) (Fig. 2).
Figure 2.
The number of surviving species as a function of the basal grazing intensity G for various values of interspecific grazing differences (G′). Three different values of the basal birth rate are shown: (a,d) B = 1; (b,e) B = 0.75; and (c,f) B = 0.5. (a–c) The grazing intensity of a species decreases as the initial birth rate decreases, i.e., and Bi = B−0.002(i-1) for i = 1, 2, …, s), indicating a trade-off between the birth rate and grazing intensity of a species. (d–f) The grazing intensity increases as the initial birth rate decreases, i.e., and Bi = B−0.002(i-1) for i = 1, 2, …, s), indicating that inferior species are eaten more often by grazers. The lattice size is 100 × 100. The dispersal distance is P = 40. The initial species density is Ii = 0.03 (same for all species). The density of grazer cells is Iy = 0.4. Error bars indicate the standard deviations.
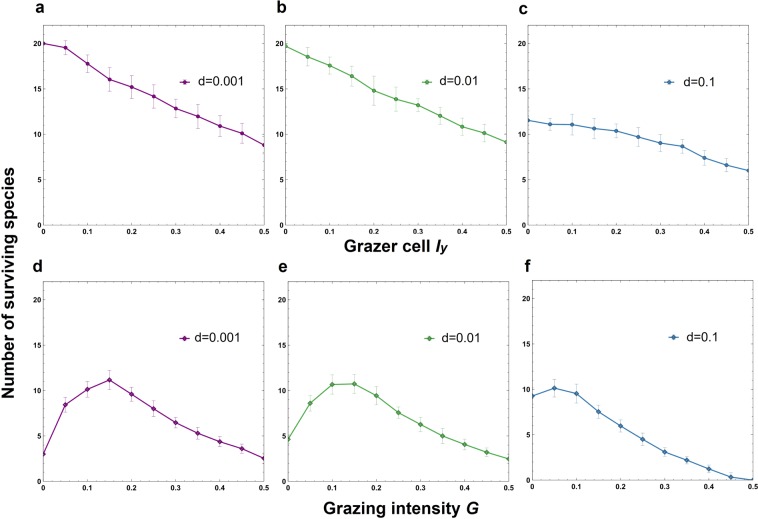
If there is no interspecific grazing difference (G′ = 0), then the number of surviving species decreases monotonically with an increase in the grazing intensity G (Supplementary Figures 2a,d, 3a, 4a,c). However, if a slight difference (e.g., G′ = 0.0005) is introduced, then the number of species responds unimodally (Fig. 2, Supplementary Figures 2b,c,e,f, 3b, 4b,d). Interestingly, the peak height (the number of surviving species) decreases as the interspecific grazing difference G′ is further increased (differences in colored lines in Fig. 2, Supplementary Figures 3b,c). The number of coexisting species decreases as the density of grazer cells Iy increases (Fig. 3a–c; Supplementary Figure 4a). However, the unimodal behavior is preserved as long as the density of grazer cells is kept constant (Fig. 3d–f; Supplementary Figure 4b,d). Natural mortality di (i.e., di = 0.1) also suppresses the unimodal behavior (Fig. 3f).
Figure 3.
The effects of the animal density Iy on the number of surviving species under various basal grazing intensities (G) and natural mortalities (di). (a–c) The grazing intensity of a species decreases as the fecundity decreases, i.e., and Bi = B−0.002(i-1) for i = 1, 2, …, s), indicating a trade-off between the birth rate and grazing intensity of a species. The lattice size is 100 × 100. (a–c) The grazing intensity is 0.15. The dispersal distance is P = 40. The initial species density is Ii = 0.03 (same for all species). (d–f) The density of grazer cells is Iy = 0.4. Error bars indicate the standard deviations.
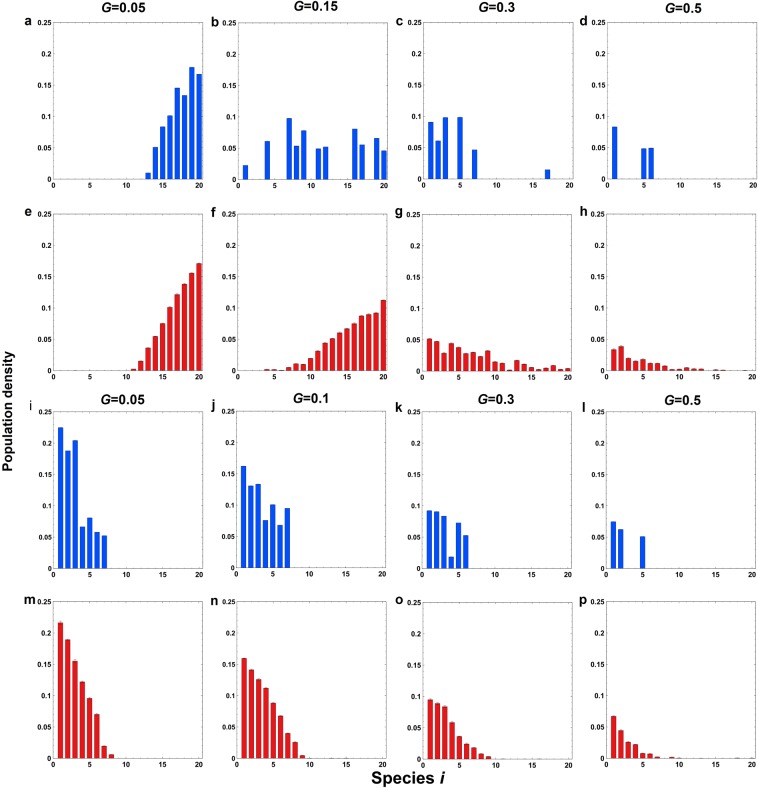
To understand the effect of grazing on population density, we examine the composition of surviving species (Fig. 4). In the first (trade-off) case, the species with the lowest grazing rate dominates at a low grazing intensity (Fig. 1a3), and that with the highest birth rate dominates at a high grazing intensity (Fig. 1c3), while various species survive at low densities at an intermediate grazing intensity (Fig. 1b3). In contrast to the second case (opposite to the trade-off case) (Fig. 4i–p), however, the results of the first case (with the trade-off) reveal a large fluctuation when the grazing intensity G is high (Fig. 4a–h). Here, which species survive depends on the simulation run (Fig. 4c,d,g,h). In any case, the results converge, and monotonic behavior is recovered as G′ decreases to zero (Supplementary Figure 2a,d). Accordingly, the results indicate that the species dependence of grazing G′ is important for unimodality but the trade-off between the birth rate and grazing intensity is not essential.
Figure 4.
Population densities of species with respect to the basal grazing intensity G. (a–h) The grazing intensity of a species increases as the fecundity increases, i.e., and Bi = B−0.002(i-1) for i = 1, 2, …, s), indicating a trade-off between the birth rate and grazing intensity of a species. (i–p) The grazing intensity decreases as the fecundity increases, i.e., and Bi = B−0.002(i-1) for i = 1, 2, …, s), indicating that inferior species are eaten more often by grazers. (a–d,i–l) The result of a single run. (e–h,m–p) The average of 30 runs. The basal grazing rates are (a,e,i,m) G = 0.05, (b,f,j,n) G = 0.1, (c,g,k,o) G = 0.3, and (d,h,l,p) G = 0.5. The parameters are as follows: interspecific grazing difference, G′ = 0.0005; basal fecundity, B = 1; initial species density, Ii = 0.03 (for all species); density of grazer cells, Iy = 0.4; and dispersal distance, P = 40. The lattice size is 100 × 100.
We also examine the effects of dispersal distance and lattice size on the number of surviving species (Supplementary Figure 5). We find no effects of dispersal distance on surviving species (Supplementary Figure 5a). Furthermore, the number of surviving species increases with the lattice size (Supplementary Figure 5b).
Discussion
The current report demonstrates that the empirically observed promotion of diversity by grazing is possible. This paper presents the first possible mechanism to explain the enhancement of species diversity by grazing. Our result confirms that the coexistence of plant species is possible under grazing in grasslands (Fig. 1). The results reveal unimodality in which the peak species diversity occurs at an intermediate grazing intensity (Fig. 2). When there is a trade-off between the birth rate and grazing rate, the number of coexisting species is large (more than 10 species) (Fig. 2a–c). Therefore, this trade-off promotes coexistence by balancing competitiveness among all plant species. We also test the case in which the grazing rate is negatively correlated with the birth rate. Surprisingly, unimodality in response to grazing intensity is still observed (Fig. 2d–f). In this case, the intermediate grazing rate allows less superior species to persist by occupying the new vacant sites produced by grazers (Fig. 4j,n), while it is not strong enough to exclude them (as in Fig. 4l,p). Thus, the coexistence of species is mechanically promoted because (weak) grazing produces vacant sites available for other species. The number and composition of coexisting species vary depending on simulation runs (Fig. 4a–d,i–l), while on average they are determined by the birth rate and grazing mortality rate (Fig. 4e–h,m–p). The trade-off between the birth rate and grazing rate is not necessary for the unimodality in response to grazing intensity.
The promotion of coexistence by grazing disappears when nongrazing mortality is introduced (Fig. 3d–f, Supplementary Figures 2a, 2d, 3). Therefore, an increase in diversity due to grazing does not occur when grazing is not the principal source of mortality in grasslands, i.e., when the nongrazing mortality is not negligible. This prediction should be tested empirically in future field studies. The interspecific grazing difference G′ also plays an important role in the promotion of coexistence. If the difference is absent or very minute, then the promotion of coexistence by grazing disappears (Supplementary Figure 3a vs Supplementary Figure 3b,c).
Which species survive under grazing depends on the interspecific relationship between the birth rate and grazing mortality rate (Fig. 4). In the trade-off case, inferior species (with low birth rates) exclude superior species (with high birth rates) (Fig. 2a–c). In the opposite case, superior species dominate the grassland (Fig. 2d–f). The composition of surviving species is highly variable in the trade-off case but stable in the opposite case (Fig. 4). These results should be verified empirically in managed grasslands. Furthermore, the current simplified assumptions about grazing rate gi may be modified explicitly to include the behavior of grazing animals, e.g., the frequency dependence in food-plant selection.
The effects of herbivores on plant diversity in grasslands have been analyzed by using empirical data24–26. The proposed grazing model integrated with a microhabitat locality model22 should be sufficient as a canonical model with which to investigate the functional mechanisms of herbivore grazing effects mathematically. In terms of maintaining and increasing diversity, our results are in line with those of some empirical studies2,15–18,27,28. For example, Komac et al. stated that maintaining an adequate grazing intensity (avoiding both abandonment and overgrazing) is necessary to preserve diversity in grasslands15. Mu et al. also found that light and moderate grazing promote plant biodiversity16. In addition, Chen et al. showed that heavy grazing has an indirect influence on biodiversity18. Furthermore, Chen et al. based on empirical observation inferred that grazing type and vegetation structure that affect spatial variation are the reasons for the high species richness in the Qinghai alpine meadow2. The two reasons are in line with our models and results. Moreover, by modifying the lattice setting, the current model can be applied to other terrestrial communities while considering predators in the system. However, this mechanism of grazer or predator effects should be directly verified by using empirical data in the future.
We should also note that this grazing model presents similar results with the intermediate disturbance models13,19,20,29,30. In these models, there may be several specific underlying mechanisms promoting coexistence. Qualitatively the current grazing model appears to conform to these models. However, whether the current model is considered as one such specific mechanism or not is a future issue.
Methods
Lattice Model
Following the lattice models of Tubay et al.22, we analyzed a modified lattice Lotka-Volterra (LV) competition model of a grassland community with grazing:
| 1 |
| 2 |
| 3 |
The parameters bi, di and gi denote the birth rate, nongrazing death rate and grazing mortality rate of plant species i, respectively. The model of Tubay et al. represents only the situation where plant species compete for space (i.e., direct sunlight and soil)22. In the modified model, we add another two-dimensional lattice (100×100) of grazers that randomly feed on plants. The grazer site Y, i.e., the site occupied by a population of grazers, moves randomly to feed on plants. Death by grazing (Eq. (3)) occurs only in site Y. The density Iy of cells occupied by grazers (Y) and the initial density Ii of plant cells (Xi) are kept constant. The stronger the grazing intensity is, the higher the gi is. One site is either vacant or occupied by a single plant species. In contrast, one grazer site may represent multiple grazing animals, depending on the grazing intensity. Nongrazing mortality is assumed negligible, i.e., di = 0, unless otherwise stated (Fig. 3).
Following Tubay et al.22, we assume that the birth rate is given by bi = Bi ɛi[m,n], where ɛi[m,n] is a random number between 0 and 1 that specifically depends both on species i and site [m,n], and the range of seed dispersal (dispersal distance: P) is kept constant, i.e., P = 40, unless specified. Depending on species i, the expected birth rate Bi is given by Bi = B−0.002(i-1) (i = 1, 2, …, 20), i.e., species 1 (20) is the strongest (weakest) among 20 species.
We consider the following two cases of species dependence of the grazing mortality gi:
Trade-off case: .
Opposite case: .
The parameters G and G′ are the (basal, species-independent) grazing intensity and the species-dependent factor, respectively. In the first (second) case, the grazing intensity is strongest for the weakest (strongest) of 20 species.
We analyze the effect of grazing mortality (gi) on the number of surviving plant species according to the following simulation procedure:
- Initial distributions
- Individuals of plant species i are randomly distributed over the square-lattice cells with the initial density Ii ( = 0.03).
- Grazer cells are also randomly distributed over the square-lattice cells with a given density Iy.
- Reaction dynamics
- Birth process (Eq. (1)): Two cells are chosen randomly. If the cells are Xi and O, then cell O is changed to Xi with probability bi. Otherwise, the cells remain unchanged.
- Death process (Eq. (2)): One cell is chosen randomly. If the cell is Xi, then it is changed to O with probability di. Note that this process is omitted when di = 0.
- Grazing process (Eq. (3)): One cell is chosen randomly. If the cell is occupied by a grazer (Y) and species i (Xi), then Xi is changed to O with probability gi. Otherwise, the cell remains unchanged.
- Repetition process
- Steps 2a-2b and 2c are repeated L×L and k×L×L times, respectively, where L×L is the total number of square-lattice sites and the number of grazing episodes is fixed at k = 3. As a default, we set L = 100. This whole process is called a Monte Carlo step.
Process 3 is repeated for a specific number (e.g., 10,000 times) of Monte Carlo steps.
Supplementary information
Acknowledgements
This work was partly supported by grants-in-aid from the Japan Society for Promotion of Science (nos. 26257405 and 15H04420 to JY; no. 18K03453 to SM; nos. 17J06741 and 17H04731 to HI; nos. 25257406 and 16H04839 to TT), the UP System Enhanced Creative Work and Research Grant (ECWRG 2016-1-008) to JMT, and a Mitsubishi Scholarship (MISTU1722) to MKAG.
Author Contributions
M.A.P., S.S. and J.Y. conceived the study. M.A.P., S.S. and M.K.A.G. built and analyzed the model. M.A.P., S.S. and J.M.T. built a program and ran the numerical simulations. M.A.K.G., J.M.T., G.I. and S.M. verified the mathematical properties of the models. J.Y., A.I., M.S., T.T., T.O. and H.I. developed biological interpretations of the model. M.A.P., S.S., M.K.A.G., J.M.T., T.O., M.N. and J.Y. wrote the manuscript, with M.A.P. and S.S. as the lead authors. All authors reviewed the manuscript and gave final approval for publication.
Data Availability
Simulation program is provided in Supplementary Information. All data are derived from the simulation program.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Almaududi Pulungan, Email: almaududi.plg@gmail.com.
Jin Yoshimura, Email: yoshimura.jin@shizuoka.ac.jp.
Satoru Morita, Email: morita.satoru@shizuoka.ac.jp.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47635-1.
References
- 1.Loveland TR, et al. Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data. Int. J. Remote Sens. 2000;21:1303–1330. doi: 10.1080/014311600210191. [DOI] [Google Scholar]
- 2.Chen J, et al. Small-scale species richness and its spatial variation in an alpine meadow on the Qinghai-Tibet Plateau. Ecol. Res. 2007;23:657–663. doi: 10.1007/s11284-007-0423-7. [DOI] [Google Scholar]
- 3.Wilson JB, Peet RK, Dengler J, Pärtel M. Plant species richness: The world records. J. Veg. Sci. 2012;23:796–802. doi: 10.1111/j.1654-1103.2012.01400.x. [DOI] [Google Scholar]
- 4.Chytrý M, et al. The most species-rich plant communities in the Czech Republic and Slovakia (with new world records) Preslia. 2015;87:217–218. [Google Scholar]
- 5.Hector A, et al. Plant Diversity and Productivity Experiments in European Grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 6.Tilman D, et al. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science. 1997;277:1300–1302. doi: 10.1126/science.277.5330.1300. [DOI] [Google Scholar]
- 7.Sala OE, Golluscio RA, Lauentroth WK, Soriano A. Resource partitioning between shrubs and grasses in the Patagonian steppe. Oecologia. 1989;81:501–505. doi: 10.1007/BF00378959. [DOI] [PubMed] [Google Scholar]
- 8.McKane RB, et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- 9.Collins SL, Uno GE. Seed Predation, Seed Dispersal, and Disturbance in Grasslands: A Comment. Am. Nat. 1985;125:866–872. doi: 10.1086/284384. [DOI] [Google Scholar]
- 10.Vandvik V, Goldberg DE. Sources of diversity in a grassland metacommunity: quantifying the contribution of dispersal to species richness. Am. Nat. 2006;168:157–167. doi: 10.1086/505759. [DOI] [PubMed] [Google Scholar]
- 11.Pinto SM, Pearson DE, Maron JL. Seed dispersal is more limiting to native grassland diversity than competition or seed predation. Journal of Ecology. 2014;102:1258–1265. doi: 10.1111/1365-2745.12282. [DOI] [Google Scholar]
- 12.Tilman, D. Resource Competition and Community Structure (Princeton Univ. Press, Princeton, New Jersey, 1982). [PubMed]
- 13.Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. doi: 10.1146/annurev.ecolsys.31.1.343. [DOI] [Google Scholar]
- 14.Maestre FT, Reynolds JF. Spatial heterogeneity in soil nutrient supply modulates nutrient and biomass responses to multiple global change drivers in model grassland communities. Glob. Change Biol. 2006;12:2431–2441. doi: 10.1111/j.1365-2486.2006.01262.x. [DOI] [Google Scholar]
- 15.Komac B, Pladevall C, Doménech M, Fanlo R. Functional diversity and grazing intensity in sub-alpine and alpine grasslands in Andorra. Appl. Veg. Sci. 2015;18:75–85. doi: 10.1111/avsc.12119. [DOI] [Google Scholar]
- 16.Mu J, Zeng Y, Wu Q, Niklas KJ, Niu K. Traditional grazing regimes promote biodiversity and increase nectar production in Tibetan alpine meadows. Agr. Ecosyst. Environ. 2016;233:336–342. doi: 10.1016/j.agee.2016.09.030. [DOI] [Google Scholar]
- 17.Niu K, He J, Zhang S, Lechowicz MJ. Tradeoffs between forage quality and soil fertility: Lessons from Himalayan rangelands. Agr. Ecosyst. Environ. 2016;234:31–39. doi: 10.1016/j.agee.2016.04.023. [DOI] [Google Scholar]
- 18.Chen J, Shiyomi M, Wuyunna, Hori Y, Yamamura Y. Vegetation and its spatial pattern analysis on salinized grasslands in the semiarid Inner Mongolia steppe. Grassl. Sci. 2014;61:121–130. doi: 10.1111/grs.12084. [DOI] [Google Scholar]
- 19.Chesson PL, Warner RR. Environmental Variability Promotes Coexistence in Lottery Competitive Systems. Am. Nat. 1981;117:923–943. doi: 10.1086/283778. [DOI] [Google Scholar]
- 20.Snyder RE, Chesson P. Local dispersal can facilitate coexistence in the presence of permanent spatial heterogeneity. Ecol. Lett. 2003;6:301–309. doi: 10.1046/j.1461-0248.2003.00434.x. [DOI] [Google Scholar]
- 21.Takenaka A. Dynamics of seedling populations and tree species coexistence in a forest: a simulation study. Ecol. Res. 2006;21:356–363. doi: 10.1007/s11284-006-0165-y. [DOI] [Google Scholar]
- 22.Tubay JM, et al. Microhabitat locality allows multi-species coexistence in terrestrial plant communities. Sci. Rep. 2015;5:15376. doi: 10.1038/srep15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakishima S, et al. The contribution of seed dispersers to tree species diversity in tropical rainforests. R. Soc. Open Sci. 2015;2:150330. doi: 10.1098/rsos.150330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olff H, Ritchie ME. Effects of herbivores on grassland plant diversity. Tree. 1998;13:7. doi: 10.1016/s0169-5347(98)01364-0. [DOI] [PubMed] [Google Scholar]
- 25.Bakker ES, Ritchie ME, Olff H, Milchunas DG, Knops JMH. Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol. Lett. 2006;9:780–788. doi: 10.1111/j.1461-0248.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, et al. Impacts of grazing by different large herbivores in grassland depend on plant species diversity. J. Appl. Ecol. 2015;52:1053–1062. doi: 10.1111/1365-2664.12456. [DOI] [Google Scholar]
- 27.Collins SL, Knapp AK, Briggs JM, Blair JM, Steinauer EM. Modulation of diversity by grazing and mowing in native tallgrass prairie. Science. 1998;280:5364. doi: 10.1126/science.280.5364.745. [DOI] [PubMed] [Google Scholar]
- 28.Xiong D, Shi P, Zhang X, Zou CB. Effects of grazing exclusion on carbon sequestration and plant diversity in grasslands of China—A meta-analysis. Ecol. Eng. 2016;94:647–655. doi: 10.1016/j.ecoleng.2016.06.124. [DOI] [Google Scholar]
- 29.Chesson P, Huntly N. The Roles of Harsh and Fluctuating Conditions in the Dynamics of Ecological Communities. Am. Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson DM. The Disturbing History of Intermediate Disturbance. Oikos. 1999;84:145–147. doi: 10.2307/3546874. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Simulation program is provided in Supplementary Information. All data are derived from the simulation program.