Abstract
HDL-like particles in human cerebrospinal fluid (CSF) promote the efflux of cholesterol from astrocytes toward the neurons that rely on this supply for their functions. We evaluated whether cell cholesterol efflux capacity of CSF (CSF-CEC) is impaired in Alzheimer’s disease (AD) by analyzing AD (n = 37) patients, non-AD dementia (non-AD DEM; n = 16) patients, and control subjects (n = 39). As expected, AD patients showed reduced CSF Aβ 1-42, increased total and phosphorylated tau, and a higher frequency of the apoε4 genotype. ABCA1- and ABCG1-mediated CSF-CEC was markedly reduced in AD (−73% and −33%, respectively) but not in non-AD DEM patients, in which a reduced passive diffusion CEC (−40%) was observed. Non-AD DEM patients displayed lower CSF apoE concentrations (−24%) compared with controls, while apoA-I levels were similar among groups. No differences in CSF-CEC were found by stratifying subjects for apoε4 status. ABCG1 CSF-CEC positively correlated with Aβ 1-42 (r = 0.305, P = 0.025), while ABCA1 CSF-CEC inversely correlated with total and phosphorylated tau (r = −0.348, P = 0.018 and r = −0.294, P = 0.048, respectively). The CSF-CEC impairment and the correlation with the neurobiochemical markers suggest a pathophysiological link between CSF HDL-like particle dysfunction and neurodegeneration in AD.
Keywords: ATP-binding cassette A1, ATP-binding cassette G1, apolipoproteins, apolipoprotein A-I, apolipoprotein E, apolipoprotein E4
Dysregulation of cholesterol homeostasis in the CNS has been associated with various neurodegenerative disorders, including Parkinson’s, Huntington’s, and Alzheimer’s disease (AD) (1). Evidence supporting this relationship derives, for example, from recent genomic-wide association studies that have identified several loci involved in lipid metabolism among the AD-susceptible genes (2, 3). For example, the ε4 allele of the APOE gene encoding apoE is undoubtedly the most strong genetic risk factor, but recently other genes have been identified such as BIN1, CLU, PICALM, ABCA7, ABCA1, ABCG1, and SORL1 (4).
However, the exact mechanisms linking cholesterol homeostasis derangement and AD pathogenesis are far from being understood and conflicting data have been released, describing both increased, decreased, or no change of cholesterol levels in different brain sections and the cerebrospinal fluid (CSF) of AD patients compared with control subjects (5).
Approximately 30% of the total body cholesterol is present in the brain, where it plays a crucial role in the synaptogenesis and maintenance of neuronal plasticity and function (6). The brain relies on endogenous local cholesterol synthesis because it is isolated from other body compartments by the blood-brain barrier (7, 8). While cholesterol synthesis in neurons and glial cells is very high during embryogenesis, adult neurons progressively lose this capacity and most exclusively rely on cholesterol produced from other cells such as astrocytes (9, 10), which show at least 2- to 3-fold higher cholesterol synthesis capacity compared with neurons (1).
Brain cholesterol is transported from astrocytes to neurons by lipoproteins that have been identified in the human CSF, with a size and density similar to that of plasma HDL (11). These particles vary widely in size (13–20 nm), are mainly spherical, and contain apoE, apoA-I, apoJ, apoA-II, apoA-IV, and apoD (8, 11, 12). CSF HDL-like particles guarantee the transport of cholesterol from astrocytes to neurons that is necessary for providing the proper amount of cholesterol and phospholipids for neurite formation and synaptogenesis (11). Cholesterol transport occurs thanks to the activity of specific membrane transporters such as ABCA1 and ABCG1 (13). ABCA1 mediates the secretion of nascent discoidal apoE-rich particles from astrocytes; these nascent lipoproteins undergo further cholesterol and phospholipid enrichment through the activity of ABCG1 expressed both in astrocytes and neurons (14). This lipid enrichment, together with the activity of remodeling enzymes, such as the cholesterol esterifying enzyme lecithin cholesterol acyltransferase, leads to the conversion of nascent into mature particles that eventually deliver cholesterol to neurons by interacting with specific apoE receptors, such as the LDL receptor and its family members (15).
Few published data suggest that CSF lipoprotein biogenesis, maturation, and cholesterol transport capacity might be altered in AD (16, 17). In particular, Yassine et al. (16) recently showed that CSF HDL-like particle capacity to promote cholesterol efflux through ABCA1 is impaired in both AD patients and subjects with mild cognitive impairment, indicating a derangement of brain lipoprotein function in the early phases of the disease-associated neurodegeneration.
In the present work, we confirmed and extended the observation of Yassine et al. by evaluating the capacity of the CSF of AD patients and controls to promote cholesterol efflux; namely, cholesterol efflux capacity (CEC), by all major cholesterol efflux pathways of ABCA1 and ABCG1, two transporters largely expressed in the CNS (18), and the unmediated process of passive diffusion. We also evaluated whether the alteration of CSF-CEC may occur in degenerative dementias (DEMs) other than AD. Finally, we looked at the potential relationship between CSF HDL-like particle CEC and the presence of the apoE4 isoform, as well as the neurochemical biomarkers normally utilized for the AD clinical diagnosis.
MATERIALS AND METHODS
Patients and samples
The study included a group of patients diagnosed with AD (n = 37), a group of patients with non-AD DEM (n = 16), and a control group of patients with more than 20 heterogeneous conditions unrelated to DEM (controls; n = 39). CSF samples were obtained by lumbar puncture for routine clinical diagnosis after an informed consent using a form approved by the local ethics committees. Specimens were immediately stored at −80°C and slowly defrosted in ice only at the moment of utilization. None of the samples presented alterations at the macroscopic examination. The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki.
The control group included individuals with psychiatric disorders, hydrocephalus, tumors, peripheral neuropathy, and those who underwent lumbar puncture for suspected demyelinating disorders discharged with no neurological diseases. The AD diagnosis was performed according to NINCDS-ADRDA (19) and subsequent research criteria (20). In the non-AD DEM group, patients were diagnosed as non-AD DEM according to established clinical criteria (21–25) and included behavioral variants of frontotemporal DEM, amyotrophic lateral sclerosis-frontotemporal spectrum disorder, corticobasal syndrome, nonfluent primary progressive aphasia, semantic variant primary progressive aphasia, progressive supranuclear palsy, and Parkinson’s disease DEM.
Biochemical analyses
The CSF neurobiomarker profile [amyloid β (Aβ) 1-42, total tau, and phosphorylated tau levels] was evaluated by ELISA (Fujirebio, Ghent, Belgium). Total apoE and apoE4 levels were measured in CSF by ELISA (MBL, Nagoya, Japan). The kit measures the amount of human total apoE and apoE4 specifically with high sensitivity using affinity-purified polyclonal antibody against total apoE and monoclonal antibody against apoE4. The minimum detectable concentration is 4 ng/ml and 8 ng/ml for total apoE and apoE4, respectively. Because apoE4 production is discrete and not continuous according to the null, heterozygous, or homozygous genotype, the apoE4/total apoE ratio could be used to identify the apoε4 genotype (26, 27). Based on this concept we stratified subjects in apoε4 carriers when the apoE4/apoE ratio was >0 (26, 28). CSF apoA-I was measured by ELISA (Abcam, Cambridge, UK). The minimum detectable apoA-I concentration of the kit is 59 pg/ml.
CEC
We evaluated CSF-CEC through the cholesterol transporters ABCA1 and ABCG1 that are expressed in CNS and involved in the cholesterol cross-talk between astrocytes and neurons (11). In addition, we also measured CEC through the passive diffusion process, a spontaneous desorption phenomenon that does not require the expression of membrane transporters (29).
CSF was evaluated for its capacity to promote cholesterol efflux by slightly modifying a standard radioisotopic technique commonly used for the evaluation of serum HDL CEC and characterized by the utilization of specific cell models overexpressing the single cholesterol transporters (30–32). In particular, we used J774 mouse macrophages in basal conditions to measure CEC by passive diffusion; J774 cells treated with 0.3 mM cpt-cAMP (Sigma-Aldrich, St. Louis, MO), which upregulates the ABCA1 transporter (33), were used for total CEC; the specific ABCA1 CEC was then calculated as the difference in CEC between ABCA1-expressing J774 and J774 cells in basal conditions. ABCG1-mediated CEC was evaluated in hABCG1-expressing Chinese hamster ovary (CHO)-K1 cells and calculated as the difference in CEC between hABCG1-expressing and parent CHO-K1 control cells (34).
J774 macrophages were grown in 10% FCS containing DMEM (Lonza, Verviers, Belgium) in the presence of 1% penicillin-streptomycin (Thermo Fisher Scientific, Carlsbad, CA). CHO cells were cultured in 10% FCS containing Ham’s F-12 (Lonza) in the presence of antibiotics.
In all assays, cells were plated (density of 120,000 cells/well for J774 and 10,000 cells/well for CHO) and following 24 h were labeled with [1,2-3H]cholesterol (PerkinElmer, Milano, Italy) for 24 h in the presence of an inhibitor of the cholesterol esterifying enzyme acyl-CoA:cholesterol acyltransferase (Sandoz 58035; Sigma-Aldrich) to ensure that all cellular cholesterol would be in the free form. After labeling, cells underwent an equilibration time in medium containing 0.2% free fatty acid BSA (Sigma-Aldrich). During this time, J774 underwent ABCA1 upregulation with a cAMP analogue. Cells were then washed to remove any cell death and subsequently exposed to CSF from controls, AD, and non-AD DEM subjects for 4–24 h depending on the pathway evaluated. Prior to the utilization, we did not perform any sample fractionation to separate the HDL-like particles from the lipid-containing nanoparticles that have also been identified in the CSF (35) because of their negligible contribution to cholesterol efflux (16). The concentration of CSF used as cholesterol acceptor was 30% (v/v) for both cell models. In the case of ABCA1 CEC, this was based on previous reports (16). In the case of ABCG1 CEC, we assessed the optimal concentration of CSF to be used though preliminary dose-response experiments with a pool of control CSF. Like what was seen for ABCA1 CEC (16), incubation with increasing concentrations of CSF resulted in a significant, dose-dependent increase of ABCG1-mediated CEC (r2 = 0.962, P = 0.019; supplemental Fig. S1). CSF-CEC was expressed as a percentage of the radioactivity released into the medium over the total radioactivity incorporated by cells. A parallel set of cells was incubated with medium alone to provide a background efflux that was subtracted from CEC values of CSF samples. To verify the cAMP-mediated induction of ABCA1 expression, reference normal lipid-free human apoA-I (Sigma-Aldrich) was used in each experiment. Similarly, as a control for ABCG1 CEC cell responsiveness, in each experiment we evaluated cholesterol efflux to native plasma HDL isolated from healthy donors by ultracentrifugation (36). In addition, a pool of normal human sera, as a reference standard, was tested in each assay and its CEC value was used to normalize the patients’ CSF-CEC values obtained in different experiments to correct for the interassay variability.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software Inc., La Jolla, CA). Every CSF sample was run in triplicate and average values and SDs were calculated for each percentage of efflux obtained. The D’Agostino and Pearson omnibus normality test was used to verify whether parameters were normally distributed. Normally distributed parameters were presented as means ± SDs, and skewed continuous parameters were expressed as medians (interquartile ranges). The comparison between the three groups of subjects was performed by one-way ANOVA or the Kruskal-Wallis test for data normally and not normally distributed, respectively. In both cases, we corrected the results for multiple comparison by Dunn’s post hoc test. Categorical variables were compared with the Chi-square test. The relationship between parameters was assessed by linear correlation analysis and the correlation coefficients (Spearman r was reported). Statistical significance was defined as P < 0.05.
RESULTS
Demographic and clinical parameters
The demographic data and clinical parameters available for the cohort of the analyzed subjects are shown in Table 1. AD patients were significantly older compared with controls (+15%), while the sex distribution was similar among groups. The AD group presented the typical neurobiomarker CSF profile, characterized by a marked reduction of Aβ 1-42 levels combined with a significant increase of total and phosphorylated tau. Conversely, both the controls and the non-AD DEM groups displayed a nonpathological neurobiochemical profile. As expected, the frequency of the apoε4 genotype was markedly higher in AD patients.
TABLE 1.
Demographic data and diagnostic parameters of studied subjects
| Controls (n = 39) | AD (n = 37) | non-AD DEM (n = 16) | P | |
| Age (years) | 60 ± 16 | 69 ± 9a | 63 ± 8 | aP < 0.01 vs. controls |
| Males [n (%)] | 20 (51) | 19 (49) | 8 (50) | 0.960 |
| Aβ 1-42 (ng/l) | 966 (782–1,390) | 472 (381–562)a | 742 (555–1,414) | aP < 0.0001 vs. controls and non-AD DEM |
| Total tau (ng/l) | 120 (98–181) | 607 (297–978)a,b | 216 (103–549) | aP < 0.0001 vs. controls |
| bP = 0.004 vs. non-AD DEM | ||||
| Phosphorylated tau (ng/l) | 30 (26–38) | 84 (62–104)a | 34 (16–50) | aP < 0.0001 vs. controls and non-AD DEM |
| apoE4 carriers [n (%)] | 7 (18) | 26 (70)a | 5 (31) | aP < 0.0001 vs. controls and non-AD DEM |
Normally distributed parameters are presented as means ± SDs, and skewed continuous parameters are expressed as medians (interquartile ranges). Statistically different values are reported in bold. CSF neurobiomarker (Aβ 1-42, total tau, and phosphorylated tau) values were available for 12/39 subjects in the control group and 13/16 subjects in the non-AD DEM group.
CSF-CEC
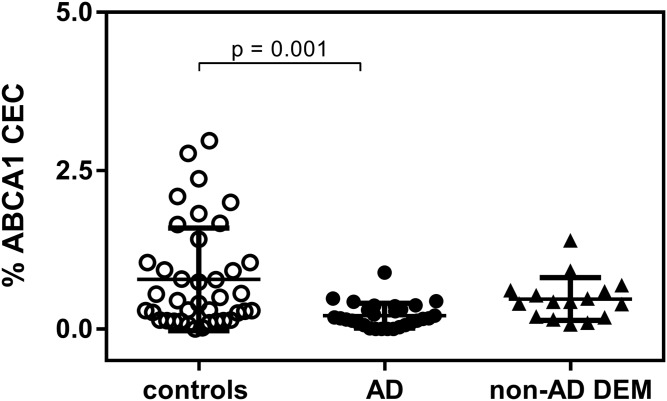
We evaluated the capacity of CSF to promote cell cholesterol efflux though the membrane transporter ABCA1. The actual ABCA1 expression in cells was demonstrated by the internal quality control obtained by cholesterol efflux induction with apoA-I and normal human serum (mean ± SD effluxes are shown in supplemental Table S1). CSF from AD patients displayed a lower ABCA1-mediated CEC compared with controls (−73%; P = 0.001; Fig. 1). Conversely, non-AD DEM ABCA1-mediated CEC did not significantly differ from that of controls (P > 0.999). To rule out the possibility that the difference between the control and AD groups was driven by the highest nine CEC values in the control group, we excluded them from the analysis and confirmed the significant, although attenuated, difference between the two groups (mean ± SD 0.39 ± 0.05% in controls compared with 0.21 ± 0.036% in AD; P = 0.048).
Fig. 1.
CSF ABCA1-mediated CEC in control subjects, AD patients, and non-AD DEM patients. Each point of the scatter plot represents the mean of a triplicate analysis of each CSF sample. The plot reports the mean ± SD within each group. ABCA1 CEC was performed on 29/39 CSF samples from AD patients.
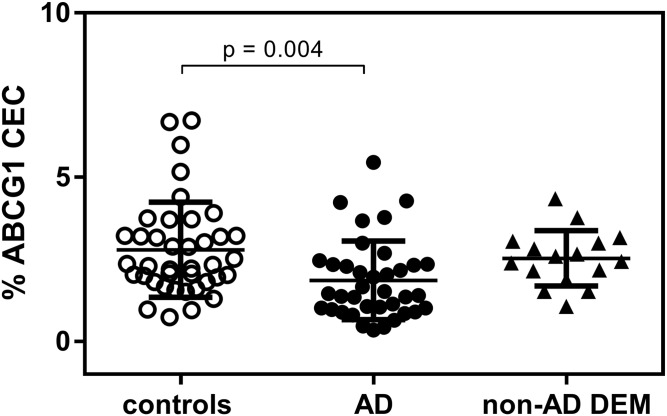
We also measured the capacity of CSF to promote cell cholesterol efflux through the membrane transporter ABCG1. The actual ABCG1 expression in cells was demonstrated by the internal quality control obtained by cholesterol efflux induction with isolated normal human HDL and normal human serum (supplemental Table S1). The measurement of CSF ABCG1-mediated CEC showed that, like what was observed for ABCA1 CEC, values relative to AD patients were significantly lower than those of controls (−33%; P = 0.004; Fig. 2). The reduction appeared to be specific for AD, as CSF from patients with non-AD DEM displayed CEC values comparable to those of the control group (P > 0.999).
Fig. 2.
CSF ABCG1 CEC in control subjects, AD patients, and non-AD DEM patients. Each point of the scatter plot represents the mean of a triplicate analysis of each CSF sample. The plot reports the mean ± SD within each group.
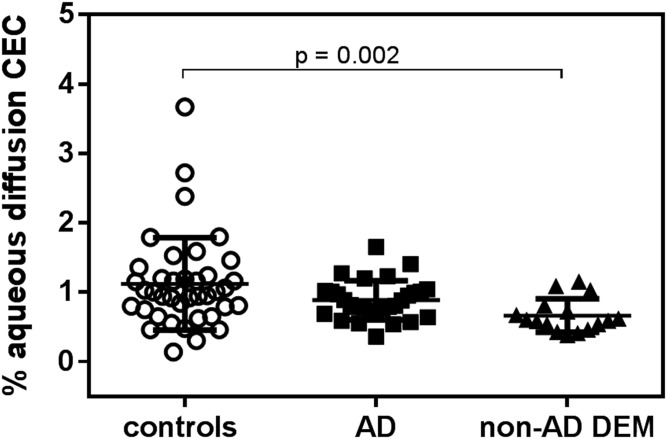
Finally, by evaluating CFS-CEC mediated by the passive diffusion process, we found no differences between AD and control groups, while non-AD DEM patients displayed significantly lower values compared with control subjects (−40%; P = 0.002; Fig. 3).
Fig. 3.
CSF passive diffusion CEC in control subjects, AD patients, and non-AD DEM patients. Each point of the scatter plot represents the mean of a triplicate analysis of each CSF sample. The plot reports the mean ± SD within each group. Passive diffusion CEC was performed on 29/39 CSF samples from AD patients.
Measurement of apoE and apoA-I CSF levels
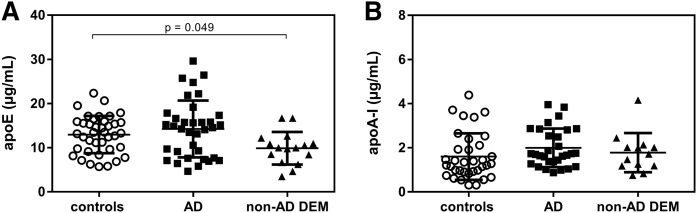
The measurement of apoE levels in the CSF of the three analyzed groups showed no difference comparing AD to controls (Fig. 4A). However, non-AD DEM values were slightly but significantly lower than those relative to the control group (P = 0.049). The quantification of CSF apoA-I did not reveal significant differences between groups (Fig. 4B).
Fig. 4.
CSF levels (µg/ml) of (A) apoE and (B) apoA-I in control subjects, AD patients, and non-AD DEM patients. Each point of the scatter plot represents the mean of a duplicate analysis of each CSF sample. The plot reports the mean ± SD within each group. ApoE levels were evaluated in 35/39 CSF samples from AD patients. apoA-I levels were evaluated in 30/39 CSF samples from control subjects, 35/37 samples from AD patients, and 13/16 samples from non-AD DEM patients.
CSF-CEC by apoE4 status
Stratifying all subjects of the study based on the absence or presence of the apoE4 isoform, we did not observe significant differences in any of the CSF-CEC pathways (Table 2).
TABLE 2.
CSF cholesterol efflux values stratified between carriers and noncarriers of the apoE4 isoform
| apoE4 carriers | apoE4 noncarriers | ||
| % | P | ||
| ABCG1 CEC | 2.27 ± 1.24 | 2.39 ± 1.41 | 0.969 |
| ABCA1 CEC | 0.60 ± 0.70 | 0.43 ± 0.52 | 0.162 |
| Passive diffusion CEC | 0.82 ± 0.36 | 1.03 ± 0.60 | 0.090 |
Data are presented as means ± SDs.
Correlations
Finally, we evaluated whether some correlations exist between the CSF-CEC mediated by each pathway analyzed and age, apolipoproteins levels, and neurobiomarkers of AD diagnosis. Results of linear-regression analyses considering all subjects together are reported in Table 3. None of the efflux pathways correlated with age. ABCG1 CEC was positively associated with CSF apoE levels. However, by analyzing the three study groups separately, a significant positive correlation was conserved only in the control group (r = 0.358, P = 0.027). A similar behavior was observed for the correlation between apoA-I and passive diffusion, significant in the whole series of subjects but driven only by the control group (r = 0.413, P = 0.021).
TABLE 3.
Correlations
| ABCG1 CEC | ABCA1 CEC | Passive diffusion CEC | |
| Age | r = −0.078 | r = −0.102 | r = −0.123 |
| P = 0.463 | P = 0.358 | P = 0.268 | |
| apoE | r = 0.370 | r = 0.011 | r = 0.170 |
| P < 0.001 | P = 0.920 | P = 0.132 | |
| apoE4 | r = 0.046 | r = −0.153 | r = −0.174 |
| P = 0.675 | P = 0.176 | P = 0.121 | |
| apoA-I | r = 0.048 | r = −0.061 | r = 0.354 |
| P = 0.692 | P = 0.626 | P = 0.003 | |
| Aβ 1-42 | r = 0.305 | r = 0.203 | r = 0.068 |
| P = 0.025 | P = 0.172 | P = 0.647 | |
| Total tau | r = −0.212 | r = −0.348 | r = 0.092 |
| P = 0.127 | P = 0.018 | P = 0.542 | |
| Phosphorylated tau | r = −0.225 | r = −0.294 | r = 0.269 |
| P = 0.106 | P = 0.048 | P = 0.070 |
The relationship between parameters was established by correlation analysis, and the Spearman coefficients are reported. Significant associations are shown in bold.
None of the CSF-CEC pathways correlated with apoE4 CSF levels. ABCG1 CSF-CEC correlated positively with Aβ 1-42, while ABCA1 CSF-CEC inversely correlated with both total and phosphorylated tau levels. All of these correlations were absent analyzing the three groups separately (data not shown). The neurobiomarkers Aβ 1-42, total tau, and phosphorylated tau did not correlate with age, neither considering all subjects together nor the three groups separately (data not shown).
DISCUSSION
Disturbances in brain cholesterol homeostasis, including total content, trafficking between cells, and intracellular metabolism, are involved in AD pathogenesis (1). Considering brain cholesterol trafficking, the integrity of cholesterol fluxes between brain cells relies both on a normal membrane expression of cholesterol transporters/receptors on the cell side and on a proper functionality of HDL-like particles on the CSF side. In AD, alterations of genes coding for the membrane cholesterol transporters ABCA1 or ABCG1 and loss-of-function ABCA1 variants are risk factors for disease onset (4, 37, 38). We evaluated the brain cholesterol trafficking studying the function of CSF HDL-like particles in AD and non-AD DEM by testing CSF-CEC on standardized cellular models expressing ABCA1 or ABCG1 transporters and analyzing its relationship with other AD-related laboratory parameters.
Our results not only confirm the previously reported reduction of CSF ABCA1-mediated CEC (16) but also describe an impairment of ABCG1-mediated CEC specifically occurring in AD.
Overall, the results of our study support the existence of a pathophysiological link between the impairment of brain cholesterol fluxes mediated by CSF HDL-like particles and AD. The dysfunction of CSF HDL-like particles in promoting ABCA1- and ABCG1-mediated cholesterol efflux may have deleterious consequences on neurons. In fact, these cells, unable to synthetize all cholesterol they need (1), rely on cholesterol uptake from CSF HDL particles, which, in turn, enrich in cholesterol through the interaction with the astrocyte membrane cholesterol transporters ABCA1 and ABCG1 (13). Thus, a defect in CEC of CSF HDL-like particles may end up in lower supply of cholesterol to neurons, leading to their apoptosis and eventually to neurodegeneration (39). As ABCA1 and ABCG1 are also expressed by neurons, one could argue that reduced CSF-CEC might also end up in less cholesterol removal from these cells. However, as the requirement of neurons for cholesterol is very high and satisfied only by a continuous supply from astrocytes, it is likely that the net effect of CSF-CEC impairment is that of neuron cholesterol depletion. This effect is even more important considering the increase in PCSK9 CSF levels in AD, which has been recently reported by us and by others (26, 40). PCSK9, in fact, reduces neuronal expression of receptors for cholesterol uptake (41, 42). Thus, increased PCSK9 CSF levels and reduced CSF-CEC may concur in neuronal cholesterol depletion and neurodegeneration.
Our finding of a multiple pathway impairment of CSF-CEC only in AD patients points to a role of CSF HDL-like particles dysfunction specifically in AD neuronal damage. The theoretical role of CSF lipid nanoparticles and albumin in cell cholesterol efflux promotion is in fact extremely unlikely, as indicated by previous studies on their concentration in the brain and cholesterol efflux ability (16, 43). In AD, HDL-like particle dysfunction might be due to the interference on lipoprotein-mediated brain cholesterol trafficking by Aβ metabolism and the formation of neurofibrillary tangles, as discussed below.
CSF-CEC likely depends not only on CSF HDL-like particle concentration but also on their size and composition, as widely demonstrated for serum HDL (44). CSF content in apoE, produced in CNS (45) and in apoA-I, which derives from the peripheral circulation through a cholesterol-dependent process (46, 47), reflects HDL-like particle concentration and was comparable among groups. Moreover, we did not find any correlation between CEC and CSF apolipoprotein levels in AD, while ABCG1 CEC and passive diffusion correlated with apoE and apoA-I only in the control group, respectively.
The similarity of apoE and apoA-I levels in controls and AD that we found is in line with the results of some previous studies (48–50) but not with others (16, 48, 50), making necessary further investigation to clarify this aspect.
From our data we may speculate that modifications of CSF-CEC in AD appear to be related to changes in HDL-like particle quality and function rather than concentration. Concerning the difference between the control and AD groups, impaired phospholipid or triglyceride enrichment of HDL-like particles might derive from the reduced CSF lecithin cholesterol acyltransferase activity reported in AD patients (17) and be associated with a specific impairment of the subclass of larger particles, representing the major lipoprotein fraction in the cerebrospinal fluid (12, 51), with consequent altered affinity for the ABCG1 transporter (52, 53). Our data showing in the control group low values of ABCA1 CEC compared with those of ABCG1 CEC are consistent with the notion that in human CSF mainly large and spherical particles are present (11, 12). An alternative explanation for ABCG1 CEC impairment in AD is the altered Aβ protein cargo previously described in AD CSF particles (54) and specifically on the large HDL1 subfraction (54, 55). This hypothesis is somehow consistent with the relationship that we previously observed between an increased HDL content of serum amyloid A, another misfolded protein involved in the acute-phase response, and a reduced capacity of serum HDL to promote ABCG1-mediated cholesterol efflux (31).
The mentioned specificity of CSF-CEC alterations in AD and the differences with other types of DEM are further underlined by our finding that only non-AD subjects presented a reduction in CSF-CEC mediated by passive diffusion. This finding is likely explained by the lower CSF apoE levels of these patients, as cell passive diffusion is a process known to be driven by relative gradients of cholesterol and extracellular acceptors (56). The reduced concentration of apoE observed in non-AD DEM are consistent with previous findings (50, 57).
Our data suggest also that the impairment of CSF-CEC in AD is independent of the presence of the apoE4 isoform, one of the major genetic risk factors for AD (4). Our results are in line with Yassine et al. (16), who also did not find significant correlations between CSF ABCA1-mediated CEC and the apoε genotype. In addition, our data are also consistent with previous work performed with isolated apoE4 or apoE4-containing synthetic HDL on ABCA1- and ABCG1-mediated efflux (58, 59). However, also taking into account other previous data on the reduced capacity of the isolated human apoE4 isoform to promote neuron cholesterol efflux (60, 61), a further confirmation of CSF-CEC independence to the apoε4 genotype in a larger population is needed.
The correlations that we found between CSF-CEC and the neurochemical biomarkers used for AD diagnosis are suggestive of a mechanistic link between HDL-like particle quality and biochemical abnormalities involved in Aβ peptide deposition and protein tau phosphorylation. This association seems to be independent of age because age did not correlate with either CSF-CEC or with the AD biomarkers. In detail, the ABCG1 CSF-CEC was directly associated with Aβ levels, which decrease with Aβ deposition in the insoluble form, and ABCA1 CEC was inversely correlated to phosphorylated tau levels. Given that no cause-and-effect relationship can be inferred, it is intriguing considering, for example, that neuronal cholesterol content and distribution have been involved in Aβ metabolism, with cholesterol depletion reducing Aβ solubility (62, 63).
On the other hand, tau is present in lipid rafts, which are specialized cholesterol- and receptor-rich membrane domains (64). Membrane raft disruption in neurons caused by cholesterol depletion has been shown to cause perturbations of membrane raft-associated cytoskeleton proteins, including tau (65). In addition, reduced raft cholesterol has been found to promote tau hyperphosphorylation in Niemann-Pick C1-deficient cells (66). Thus, impaired CSF HDL-like particle-mediated brain cholesterol trafficking might be somehow involved in the complex and mostly yet ununderstood processes leading to the formation of amyloid plaques and neurofibrillary tangles in AD.
The main limitations are relative to the descriptive nature of the work and to the fact that our cell models are different from the cerebral cells with which the CSF is in contact, except for the expression of the cholesterol transporter. For these reasons, our findings need to be integrated with future studies to obtain mechanistic insights.
Another limitation concerns the small sample size, especially for the non-AD DEM group. For example, the small numbers may not be sufficiently statistically powered to detect differences in the stratification of CSF-CEC based on the genotype or in correlation studies within groups. In particular, the small sample size has conditioned the impossibility to make stratified analyses on control patients, presenting a wide distribution of CEC values. The particular nature of CSF makes obviously difficult obtaining samples from healthy subjects but renders also difficult assembling a homogeneous control group. However, we were able to confirm the presence of a reduced but still statistically significant difference in CEC values between AD and controls after excluding from the analyses those control samples giving the highest ABCA1-CEC values. On the other hand, no particular diagnosis was associated with the highest ABCA1-CEC values and, most importantly, the high heterogeneity of diagnoses in our control group reduced the possibility of specific bias. A further limitation of our study derives from the limited CSF sample volume, which did not allow us to study physicochemical characteristics of CSF HDL-like particles.
CONCLUSION
We demonstrated that CSF capacity to promote cell cholesterol efflux via the active transporters ABCA1 and ABCG1 is specifically impaired in AD, possibly due to qualitative modifications in CSF HDL-like particles. Such impairment correlates with the main neurobiochemical markers of AD, Aβ, and p-tau, suggesting the existence of pathophysiological links between reduced neuronal cholesterol supply and neurodegeneration in AD. Our data, together with further information worth obtaining on the precise mechanisms of CSF HDL-like particle dysfunction, may open the way to developing new lipoprotein-based pharmacological approaches, such as modulators of HDL-like particle maturation, to ameliorate brain cholesterol trafficking in AD.
Supplementary Material
Footnotes
Abbreviations:
- AD
- Alzheimer’s disease
- Aβ
- amyloid β
- CEC
- cholesterol efflux capacity
- CHO
- Chinese hamster ovary
- CSF
- cerebrospinal fluid
- DEM
- dementia
This work was partially supported by funding for basic activities related to research from the Italian National Agency for the Evaluation of Universities and Research Institutes (F.Z.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Arenas F., Garcia-Ruiz C., and Fernandez-Checa J. C.. 2017. Intracellular cholesterol trafficking and impact in neurodegeneration. Front. Mol. Neurosci. 10: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong H. K., Gim J. A., Yeo S. H., and Kim H. S.. 2017. Integrated late onset Alzheimer’s disease (LOAD) susceptibility genes: cholesterol metabolism and trafficking perspectives. Gene. 597: 10–16. [DOI] [PubMed] [Google Scholar]
- 3.Lambert J. C., Ibrahim-Verbaas C. A., Harold D., Naj A. C., Sims R., Bellenguez C., DeStafano A. L., Bis J. C., Beecham G. W., Grenier-Boley B., et al. 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45: 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picard C., Julien C., Frappier J., Miron J., Theroux L., Dea D., United Kingdom Brain Expression Consortium and for the Alzheimer’s Disease Neuroimaging Initiative, Breitner J. C. S., and Poirier J.. 2018. Alterations in cholesterol metabolism-related genes in sporadic Alzheimer’s disease. Neurobiol. Aging. 66: 180.e181–180.e189. [DOI] [PubMed] [Google Scholar]
- 5.Wood W. G., Li L., Muller W. E., and Eckert G. P.. 2014. Cholesterol as a causative factor in Alzheimer’s disease: a debatable hypothesis. J. Neurochem. 129: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietschy J. M., and Turley S. D.. 2004. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397. [DOI] [PubMed] [Google Scholar]
- 7.Dietschy J. M. 2009. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 390: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang T. Y., Yamauchi Y., Hasan M. T., and Chang C.. 2017. Cellular cholesterol homeostasis and Alzheimer’s disease. J. Lipid Res. 58: 2239–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan G., Xie C., Dietschy J. M., and Turley S. D.. 2003. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res. Dev. Brain Res. 146: 87–98. [DOI] [PubMed] [Google Scholar]
- 10.Ikonen E. 2008. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9: 125–138. [DOI] [PubMed] [Google Scholar]
- 11.Vitali C., Wellington C. L., and Calabresi L.. 2014. HDL and cholesterol handling in the brain. Cardiovasc. Res. 103: 405–413. [DOI] [PubMed] [Google Scholar]
- 12.Koch S., Donarski N., Goetze K., Kreckel M., Stuerenburg H. J., Buhmann C., and Beisiegel U.. 2001. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 42: 1143–1151. [PubMed] [Google Scholar]
- 13.Chen J., Zhang X., Kusumo H., Costa L. G., and Guizzetti M.. 2013. Cholesterol efflux is differentially regulated in neurons and astrocytes: implications for brain cholesterol homeostasis. Biochim. Biophys. Acta. 1831: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarr P. T., and Edwards P. A.. 2008. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J. Lipid Res. 49: 169–182. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., and Eckel R. H.. 2014. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 25: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yassine H. N., Feng Q., Chiang J., Petrosspour L. M., Fonteh A. N., Chui H. C., and Harrington M. G.. 2016. ABCA1-mediated cholesterol efflux capacity to cerebrospinal fluid is reduced in patients with mild cognitive impairment and Alzheimer’s disease. J. Am. Heart Assoc. 5: e002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demeester N., Castro G., Desrumaux C., De Geitere C., Fruchart J. C., Santens P., Mulleners E., Engelborghs S., De Deyn P. P., Vandekerckhove J., et al. 2000. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J. Lipid Res. 41: 963–974. [PubMed] [Google Scholar]
- 18.Vance J. E., and Hayashi H.. 2010. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 1801: 806–818. [DOI] [PubMed] [Google Scholar]
- 19.Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., et al. 2007. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6: 734–746. [DOI] [PubMed] [Google Scholar]
- 20.Dubois B., Feldman H. H., Jacova C., Hampel H., Molinuevo J. L., Blennow K., DeKosky S. T., Gauthier S., Selkoe D., Bateman R., et al. 2014. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13: 614–629. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong M. J., Litvan I., Lang A. E., Bak T. H., Bhatia K. P., Borroni B., Boxer A. L., Dickson D. W., Grossman M., Hallett M., et al. 2013. Criteria for the diagnosis of corticobasal degeneration. Neurology. 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., Ogar J. M., Rohrer J. D., Black S., Boeve B. F., et al. 2011. Classification of primary progressive aphasia and its variants. Neurology. 76: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litvan I., Agid Y., Jankovic J., Goetz C., Brandel J. P., Lai E. C., Wenning G., D’Olhaberriague L., Verny M., Chaudhuri K. R., et al. 1996. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology. 46: 922–930. [DOI] [PubMed] [Google Scholar]
- 24.Rascovsky K., Hodges J. R., Knopman D., Mendez M. F., Kramer J. H., Neuhaus J., van Swieten J. C., Seelaar H., Dopper E. G., Onyike C. U., et al. 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134: 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strong M. J., Abrahams S., Goldstein L. H., Woolley S., McLaughlin P., Snowden J., Mioshi E., Roberts-South A., Benatar M., Hortobágyi T., et al. 2017. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph. Lateral Scler. Frontotemporal Degener. 18: 153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimetti F., Caffarra P., Ronda N., Favari E., Adorni M. P., Zanotti I., Bernini F., Barocco F., Spallazzi M., Galimberti D., et al. 2017. Increased PCSK9 cerebrospinal fluid concentrations in Alzheimer’s disease. J. Alzheimers Dis. 55: 315–320. [DOI] [PubMed] [Google Scholar]
- 27.Calero O., Garcia-Albert L., Rodriguez-Martin A., Veiga S., and Calero M.. 2018. A fast and cost-effective method for apolipoprotein E isotyping as an alternative to APOE genotyping for patient screening and stratification. Sci. Rep. 8: 5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukumoto H., Ingelsson M., Garevik N., Wahlund L. O., Nukina N., Yaguchi Y., Shibata M., Hyman B. T., Rebeck G. W., and Irizarry M. C.. 2003. APOE epsilon 3/epsilon 4 heterozygotes have an elevated proportion of apolipoprotein E4 in cerebrospinal fluid relative to plasma, independent of Alzheimer’s disease diagnosis. Exp. Neurol. 183: 249–253. [DOI] [PubMed] [Google Scholar]
- 29.Favari E., Chroni A., Tietge U. J., Zanotti I., Escola-Gil J. C., and Bernini F.. 2015. Cholesterol efflux and reverse cholesterol transport. Handb. Exp. Pharmacol. 224: 181–206. [DOI] [PubMed] [Google Scholar]
- 30.Adorni M. P., Ferri N., Marchiano S., Trimarco V., Rozza F., Izzo R., Bernini F., and Zimetti F.. 2017. Effect of a novel nutraceutical combination on serum lipoprotein functional profile and circulating PCSK9. Ther. Clin. Risk Manag. 13: 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimetti F., De Vuono S., Gomaraschi M., Adorni M. P., Favari E., Ronda N., Ricci M. A., Veglia F., Calabresi L., and Lupattelli G.. 2017. Plasma cholesterol homeostasis, HDL remodeling and function during the acute phase reaction. J. Lipid Res. 58: 2051–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigna G. B., Satta E., Bernini F., Boarini S., Bosi C., Giusto L., Pinotti E., Tarugi P., Vanini A., Volpato S., et al. 2014. Flow-mediated dilation, carotid wall thickness and HDL function in subjects with hyperalphalipoproteinemia. Nutr. Metab. Cardiovasc. Dis. 24: 777–783. [DOI] [PubMed] [Google Scholar]
- 33.Favari E., Zimetti F., Bortnick A. E., Adorni M. P., Zanotti I., Canavesi M., and Bernini F.. 2005. Impaired ATP-binding cassette transporter A1-mediated sterol efflux from oxidized LDL-loaded macrophages. FEBS Lett. 579: 6537–6542. [DOI] [PubMed] [Google Scholar]
- 34.Greco D., Kocyigit D., Adorni M. P., Marchi C., Ronda N., Bernini F., Gurses K. M., Canpinar H., Guc D., Oguz S. H., et al. 2018. Vitamin D replacement ameliorates serum lipoprotein functions, adipokine profile and subclinical atherosclerosis in pre-menopausal women. Nutr. Metab. Cardiovasc. Dis. 28: 822–829. [DOI] [PubMed] [Google Scholar]
- 35.Harrington M. G., Fonteh A. N., Oborina E., Liao P., Cowan R. P., McComb G., Chavez J. N., Rush J., Biringer R. G., and Huhmer A. F.. 2009. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal Fluid Res. 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havel R. J., Eder H. A., and Bragdon J. H.. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beecham G. W., Hamilton K., Naj A. C., Martin E. R., Huentelman M., Myers A. J., Corneveaux J. J., Hardy J., Vonsattel J. P., Younkin S. G., et al. 2014. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 10: e1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordestgaard L. T., Tybjaerg-Hansen A., Nordestgaard B. G., and Frikke-Schmidt R.. 2015. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimers Dement. 11: 1430–1438. [DOI] [PubMed] [Google Scholar]
- 39.Koudinov A. R., and Koudinova N. V.. 2005. Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. J. Neurol. Sci. 229–230: 233–240. [DOI] [PubMed] [Google Scholar]
- 40.Courtemanche H., Bigot E., Pichelin M., Guyomarch B., Boutoleau-Bretonniere C., Le May C., Derkinderen P., and Cariou B.. 2018. PCSK9 concentrations in cerebrospinal fluid are not specifically increased in Alzheimer’s disease. J. Alzheimers Dis. 62: 1519–1525. [DOI] [PubMed] [Google Scholar]
- 41.Kysenius K., Muggalla P., Matlik K., Arumae U., and Huttunen H. J.. 2012. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell. Mol. Life Sci. 69: 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poirier S., Mayer G., Benjannet S., Bergeron E., Marcinkiewicz J., Nassoury N., Mayer H., Nimpf J., Prat A., and Seidah N. G.. 2008. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 283: 2363–2372. [DOI] [PubMed] [Google Scholar]
- 43.Li X. M., Tang W. H., Mosior M. K., Huang Y., Wu Y., Matter W., Gao V., Schmitt D., Didonato J. A., Fisher E. A., et al. 2013. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 33: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothblat G. H., and Phillips M. C.. 2010. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 21: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao F., Yoon H., and Kim J.. 2017. Apolipoprotein E metabolism and functions in brain and its role in Alzheimer’s disease. Curr. Opin. Lipidol. 28: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiesa G., Parolini C., Canavesi M., Colombo N., Sirtori C. R., Fumagalli R., Franceschini G., and Bernini F.. 1998. Human apolipoproteins A-I and A-II in cell cholesterol efflux: studies with transgenic mice. Arterioscler. Thromb. Vasc. Biol. 18: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 47.Zhou A. L., Swaminathan S. K., Curran G. L., Poduslo J. F., Lowe V. J., Li L., and Kandimalla K. K.. 2019. Apolipoprotein A-I crosses the blood-brain barrier through clathrin-independent and cholesterol-mediated endocytosis. J. Pharmacol. Exp. Ther. 369: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talwar P., Sinha J., Grover S., Agarwal R., Kushwaha S., Srivastava M. V., and Kukreti R.. 2016. Meta-analysis of apolipoprotein E levels in the cerebrospinal fluid of patients with Alzheimer’s disease. J. Neurol. Sci. 360: 179–187. [DOI] [PubMed] [Google Scholar]
- 49.Song H., Saito K., Seishima M., Noma A., Urakami K., and Nakashima K.. 1997. Cerebrospinal fluid apo E and apo A-I concentrations in early- and late-onset Alzheimer’s disease. Neurosci. Lett. 231: 175–178. [DOI] [PubMed] [Google Scholar]
- 50.Johansson P., Almqvist E. G., Bjerke M., Wallin A., Johansson J. O., Andreasson U., Blennow K., Zetterberg H., and Svensson J.. 2017. Reduced cerebrospinal fluid concentration of apolipoprotein A-I in patients with Alzheimer’s disease. J. Alzheimers Dis. 59: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 51.Kontush A. 2004. Apolipoprotein Abeta: black sheep in a good family. Brain Pathol. 14: 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gelissen I. C., Harris M., Rye K. A., Quinn C., Brown A. J., Kockx M., Cartland S., Packianathan M., Kritharides L., and Jessup W.. 2006. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 26: 534–540. [DOI] [PubMed] [Google Scholar]
- 53.Sankaranarayanan S., Oram J. F., Asztalos B. F., Vaughan A. M., Lund-Katz S., Adorni M. P., Phillips M. C., and Rothblat G. H.. 2009. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 50: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koudinov A. R., Berezov T. T., and Koudinova N. V.. 2001. The levels of soluble amyloid beta in different high density lipoprotein subfractions distinguish Alzheimer’s and normal aging cerebrospinal fluid: implication for brain cholesterol pathology? Neurosci. Lett. 314: 115–118. [DOI] [PubMed] [Google Scholar]
- 55.Guyton J. R., Miller S. E., Martin M. E., Khan W. A., Roses A. D., and Strittmatter W. J.. 1998. Novel large apolipoprotein E-containing lipoproteins of density 1.006–1.060 g/ml in human cerebrospinal fluid. J. Neurochem. 70: 1235–1240. [DOI] [PubMed] [Google Scholar]
- 56.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., and Rothblat G. H.. 2007. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 57.Landén M., Hesse C., Fredman P., Regland B., Wallin A., and Blennow K.. 1996. Apolipoprotein E in cerebrospinal fluid from patients with Alzheimer’s disease and other forms of dementia is reduced but without any correlation to the apoE4 isoform. Dementia. 7: 273–278. [DOI] [PubMed] [Google Scholar]
- 58.Dafnis I., Raftopoulou C., Mountaki C., Megalou E., Zannis V. I., and Chroni A.. 2018. ApoE isoforms and carboxyl-terminal-truncated apoE4 forms affect neuronal BACE1 levels and Abeta production independently of their cholesterol efflux capacity. Biochem. J. 475: 1839–1859. [DOI] [PubMed] [Google Scholar]
- 59.Kim W. S., Rahmanto A. S., Kamili A., Rye K. A., Guillemin G. J., Gelissen I. C., Jessup W., Hill A. F., and Garner B.. 2007. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J. Biol. Chem. 282: 2851–2861. [DOI] [PubMed] [Google Scholar]
- 60.Minagawa H., Gong J. S., Jung C. G., Watanabe A., Lund-Katz S., Phillips M. C., Saito H., and Michikawa M.. 2009. Mechanism underlying apolipoprotein E (ApoE) isoform-dependent lipid efflux from neural cells in culture. J. Neurosci. Res. 87: 2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okoro E. U., Zhao Y., Guo Z., Zhou L., Lin X., and Yang H.. 2012. Apolipoprotein E4 is deficient in inducing macrophage ABCA1 expression and stimulating the Sp1 signaling pathway. PLoS One. 7: e44430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eckert G. P., Reik C., and Muller W. E.. 2013. Simvastatin alters membrane cholesterol distribution and beta-amyloid levels in brains of female APP751SL mice. Pharmazie. 68: 590–594. [PubMed] [Google Scholar]
- 63.Phan H. T., Vestergaard M. C., Baek K., Shimokawa N., and Takagi M.. 2014. Localization of amyloid beta (Abeta1–42) protofibrils in membrane lateral compartments: effect of cholesterol and 7-ketocholesterol. FEBS Lett. 588: 3483–3490. [DOI] [PubMed] [Google Scholar]
- 64.Kawarabayashi T., Shoji M., Younkin L. H., Wen-Lang L., Dickson D. W., Murakami T., Matsubara E., Abe K., Ashe K. H., and Younkin S. G.. 2004. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 24: 3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitehead S. N., Gangaraju S., Aylsworth A., and Hou S. T.. 2012. Membrane raft disruption results in neuritic retraction prior to neuronal death in cortical neurons. Biosci. Trends. 6: 183–191. [DOI] [PubMed] [Google Scholar]
- 66.Sawamura N., Gong J. S., Chang T. Y., Yanagisawa K., and Michikawa M.. 2003. Promotion of tau phosphorylation by MAP kinase Erk1/2 is accompanied by reduced cholesterol level in detergent-insoluble membrane fraction in Niemann-Pick C1-deficient cells. J. Neurochem. 84: 1086–1096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.