Abstract
In the awake big brown bat, 30 min auditory fear conditioning elicits conditioned heart rate decrease and long-term best frequency (BF) shifts of cortical auditory neurons toward the frequency of the conditioned tone; 15 min conditioning elicits subthreshold cortical BF shifts that can be augmented by acetylcholine. The fear conditioning causes stress and an increase in the cortical serotonin (5-HT) level. Serotonergic neurons in the raphe nuclei associated with stress and fear project to the cerebral cortex and cholinergic basal forebrain. Recently, it has been shown that 5-HT2A receptors are mostly expressed on pyramidal neurons and their activation improves learning and memory. We applied 5-HT, an agonist (α-methyl-5-HT), or an antagonist (ritanserin) of 5-HT2A receptors to the primary auditory cortex and discovered the following drug effects: (1) 5-HT had no effect on the conditioned heart rate change, although it reduced the auditory responses; (2) 4 mm 5-HT augmented the subthreshold BF shifts, whereas 20 mm 5-HT did not; (3) 20 mm 5-HT reduced the long-term BF shifts and changed them into short-term; (4) α-methyl-5-HT increased the auditory responses and augmented the subthreshold BF shifts as well as the long-term BF shifts; (5) in contrast, ritanserin reduced the auditory responses and reversed the direction of the BF shifts. Our data indicate that the BF shift can be modulated by serotonergic neurons that augment or reduce the BF shift or even reverse the direction of the BF shift. Therefore, not only the cholinergic system, but also the serotonergic system, plays an important role in cortical plasticity according to behavioral demands.
Keywords: bat, best frequency, neuromodulatory systems, reorganization, stress, tonotopic map
Introduction
The frequency (cochleotopic) map in the primary auditory cortex (AI) can be modified by auditory experiences including learning and injury (Irvine and Rajan, 1996; Ohl and Scheich, 2005). In the AI of the big brown bat, a long-term “centripetal” best frequency (BF) shift [i.e., the shift toward the frequency of a conditioned tonal stimulus (CS)] is elicited by 30 min auditory fear conditioning (Gao and Suga, 2000). The CS activates the neural circuits of the AI and the corticofugal system to elicit a small short-term BF shift. In the meantime, the paired CS and unconditioned stimulus (US) indirectly increase cortical acetylcholine (ACh) release from the cholinergic basal forebrain and make the BF shift large and long term. This process is modulated by several neuromodulators (Suga and Ma, 2003). The importance of ACh in the BF shift has been well demonstrated (Ji et al., 2001; Ji and Suga, 2003). However, serotonergic modulation of BF shifts has not yet been studied.
The mammalian neocortex has an extensive serotonergic input from the raphe nuclei (Jacobs and Azmitia, 1992; Barnes and Sharp, 1999). Exogenously applied 5-hydroxytryptamine (serotonin or 5-HT) excites GABAergic neurons or suppresses the responses to sensory stimuli of the inferior colliculus (Hurley et al., 2002), lateral amygdala (Stutzmann and LeDoux, 1999), and somatosensory cortex (Waterhouse et al., 1986). Of the 14 subtypes (seven families) of 5-HT receptors (Barnes and Sharp, 1999), cortical 5-HT2A receptors are mostly located on the pyramidal neurons (Liang et al., 1998; Jakab and Goldman-Rakic, 2000) and mediate the depolarization of layer V pyramidal neurons (Araneda and Andrade, 1991; Zhou and Hablitz, 1999; Lambe et al., 2000; Marek et al., 2001), whereas cortical 5-HT3 receptors are exclusively located on the GABAergic neurons (Barnes et al., 1989; DeFelipe et al., 1991; Roerig and Katz, 1997; Puig et al., 2004) and mainly mediate 5-HT-induced inhibition. Importantly, 5-HT2A receptors increase the release of cortical ACh (Quirion et al., 1985; Nair and Gudelsky, 2004) and glutamate (Aghajanian and Marek, 2000; Hasuo et al., 2002; Chen et al., 2003), whereas 5-HT3 receptors decrease ACh release in the cortex (Maura et al., 1992; Crespi et al., 1997; Giovannini et al., 1998).
Serotonergic neurons play a role in reconfiguring neural circuitry in the sensory and motor systems and are associated with anxiety and defensive behaviors (Eison and Eison, 1994; Meneses, 1999). Recently, it has been shown that 5-HT2A receptors alone mediate fear and anxiety states (Castilho and Brandao, 2001; Weisstaub et al., 2006) and that activation of 5-HT2A receptors improves learning and working memory (Williams et al., 2002; Harvey, 2003; Harvey et al., 2004; Romano et al., 2006). Also, 5-HT is involved in the control of both cardiovascular and acute psychological stress reactivity (Davies et al., 2006). We hypothesized that the conditioning-elicited cortical plasticity could be related to stress and be modulated by 5-HT. Thus, we investigated the effects of low and high doses of 5-HT as well as an agonist and antagonist of 5-HT2A receptors on the cortical BF shifts elicited by auditory fear conditioning. We found that cortical BF shifts were augmented, reduced, or even reversed in the shifting direction by activation of 5-HT receptors.
Materials and Methods
Experimental subject.
Forty-eight adult big brown bats (Eptesicus fuscus) were used for the experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis and were the same as those previously described (Gao and Suga, 2000; Ji et al., 2001).
Surgical and recording procedures.
Under neuroleptanalgesia (Innovar, 4.08 mg/kg of body weight; 0.4 ml fentanyl citrate in 20 mg/ml droperidol), a 1.5-cm-long metal post was glued to the dorsal surface of the bat's skull. Experiments began 3–4 d after the surgery. An awake bat was placed in a polyethylene-foam body mold suspended at the center of a soundproof room maintained at 31°C. The bat's head was immobilized by fixing the metal post glued on the skull onto a metal rod with set screws to ensure a uniform and stable position of the head, which directly faced a loudspeaker located 74 cm away. For single-unit recording, a tungsten-wire microelectrode with a tip diameter of ∼7 μm was inserted orthogonally into the AI at a depth between 200 and 700 μm. Local anesthetic (5% Lidocaine; E. Fougera, Melville, NY) and antibiotic (0.2% Nitrofurazone; RXV Products, Westlake, TX) ointments were applied to the surgical wound. The recording session lasted 5–7 h. Water was provided with a dropper, and local anesthesia with lidocaine was refreshed every 2 h. The bat was neither anesthetized nor tranquilized during the experiments. If the bat continued to show signs of discomfort, recordings were terminated and the bat was returned to its cage.
Acoustic stimulation.
Acoustic stimuli were 20 ms tone bursts with a 0.5 ms rise-decay time delivered to the bat at a rate of four per second from a leaf tweeter with Real-Time processors version 2.1 (Tucker-Davis Technologies, Alachua, FL). Their frequencies and amplitudes were manually varied or computer controlled to measure the BF and the minimum threshold (MT) of a given neuron. The computer-controlled frequency scan consisted of 21 250 ms time blocks. The frequency of tone bursts was randomly varied by the stimulus control and recording software (Brainware version 8.0) and was delivered every 250 ms in 0.5 or 1.0 kHz steps. The amplitude of the tone bursts delivered from a leaf tweeter was calibrated with a microphone (Brüel & Kjær Instruments, Naerum, Denmark). It was flat within ±5 dB from 10.0 to 70.0 kHz. The amplitude of the tone burst was set at 10 dB above the MT of the neuron to obtain its frequency-response curve and to easily detect a BF shift. The sound pressure level was expressed in decibels sound pressure level (dB SPL) referred to 20 μPa. An identical frequency scan was repeated 50 times.
Auditory fear conditioning.
To evoke the BF shift of a cortical neuron, the bat was exposed to the auditory fear conditioning over 15 or 30 min. The CS was a train of tone bursts which were 50 dB SPL, 10 ms long, 33 per second over 1.0 s, and 5.0 kHz lower than the BF of a given neuron. The US was a 50 ms long, 0.10–0.40 mA monophasic electric pulse applied to the bat's leg with a 1.0 s delay from the CS. The CS–US pair was delivered every 30 s, so that the CS–US pair was delivered 30 or 60 times in the 15 or 30 min conditioning, respectively. The intensity of electric foot shock was just above the threshold for eliciting a just noticeable leg flexion monitored with a strain gauge. In the big brown bat, the 30 min conditioning elicits a large centripetal BF shift, but the 15 min conditioning elicits a “subthreshold” BF shift that is not large enough to be reliably detected but can be augmented to a large long-term BF shift by ACh applied to the AI (Ji et al., 2001). The CS evokes the largest centripetal BF shift when it is ∼5.0 kHz lower than the BF of a given neuron. Therefore, the centripetal BF shift has a negative value (Gao and Suga, 2000). In each 1 d experiment, tone bursts alone were delivered at different frequencies and at a rate of four per second over 180 min to record single-unit activity. Only one neuron was studied in a 1 d experiment, and the same animal was used with a 3 d interval to minimize the cumulative effect of the conditioning. This period presumably caused the extinction of BF shifts if there were any remaining after a previous conditioning experiment.
Heart rate recording and analysis.
An electrocardiogram was recorded during and after a conditioning session. Tab electrodes (Physician Sale & Services, Jacksonville, FL) were attached to the chest and wing of a bat. Electrocardiograms were amplified by a RP2.1 enhanced real-time processor (Tucker-Davis Technologies) and stored by Brainware version 8.0 software. The heart rate change evoked by the conditioning was plotted as a function of time.
Drug applications.
In the big brown bat, auditory responses can be recorded within a 2.4 mm diameter of the cortical area, which is mostly the AI (Dear et al., 1993). The approximate center of the AI is dorsoventrally crossed by the 30 kHz iso-BF line. The approximate midpoint of this iso-BF line was first electrophysiologically located by recording auditory responses at five to six cortical loci. Then a ∼1.0 mm hole was made there for single-unit recording and drug applications. The drug applied was 0.2 ml of 4 mm 5-HT, 20 mm 5-HT, 10 mm α-methyl-5-HT, or 10 mm ritanserin (Sigma, St. Louis, MO) dissolved in a 0.9% saline solution. Different from other drugs, ritanserin hardly dissolved in the saline solution. Therefore, the ritanserin solution was sonicated immediately before its use (Williams et al., 2002). Each of these drugs was applied to the surface of the recording site in the AI before or after the conditioning with a 1.0 μl Hamilton syringe. [Williams et al. (2002) used ritanserin and MDL100,907 as the antagonists of 5-HT2A receptors and reported that ritanserin had the same effect as MDL100,907 on associate learning. Therefore, we used ritanserin.]
Data acquisition.
Action potentials of a single cortical neuron tuned to a specific frequency were amplified (Medusa base station; Tucker-Davis Technologies) and selected with a time-amplitude window discriminator (Brainware version 8.0). During and after the conditioning and/or drug application, the action potentials discharged by the neuron were continuously compared with the template stored and displayed on the monitor screen at the beginning of the study of the neuron. An array of poststimulus-time histograms displays the auditory responses to the 21 tone bursts of the frequency scan repeated 50 times. It was acquired every 15 min up to 240 min after the onset of the conditioning as long as action potentials visually matched the template.
Off-line data processing.
The magnitude of auditory responses of a neuron was expressed by the number of spikes per 50 identical stimuli and was plotted as a function of frequency to show the frequency-response curve of the neuron. The BF of the neuron was defined as the frequency at which the frequency-response curve peaked. To study the development of a BF shift, the BFs determined from the frequency-response curves obtained every 15 min were plotted as a function of time. The time courses of BF shifts obtained from many single neurons were averaged. Therefore, each data point in an averaged time course curve represents the mean and SE (mean ± SE) based on 50 responses multiplied by the number of single neurons used for averaging. To determine whether there were differences in response magnitude between a BF and its adjacent frequencies, or between the BFs obtained before and after the electric stimulation and/or drug application, a two-tailed unpaired t test was used for a significant difference of p < 0.01 or p < 0.05.
According to the definition by Gao and Suga (2000), the BF shift that disappeared (i.e., recovered) within 210 min after the onset of a conditioning session is designated as short term, whereas the BF shift showing no sign of recovery at 210 min is considered long term. The conditioning usually causes an ∼10% decrease in overall responses right after the conditioning. The recovery of an auditory response was defined as the changed response at the BF recovered to that of the control response within ±10%.
Results
No effect of 5-HT applied to the AI on the conditioned heart rate change
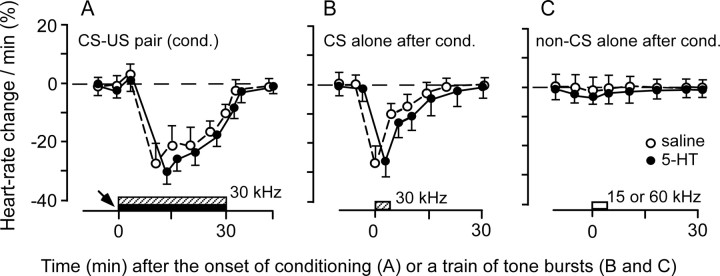
Without a drug application, 30 min auditory fear conditioning elicited a tone-specific heart rate decrease in all four bats tested. When 30.0 kHz tone bursts (CS) paired with electric leg stimulation (US) were delivered to the bats, their heart rate decreased 28.0 ± 7.5% (n = 4) (Fig. 1A, open circles). The bats showed a heart rate decrease to the 30.0 kHz tone bursts alone ∼2 min after this conditioning (Fig. 1B, open circles), but not to the non-CS tone bursts, such as 15.0 and 60.0 kHz tone bursts (Fig. 1C, open circles). Therefore, the heart rate decrease was a conditioned autonomic response specific to the CS. 5-HT (4 mm or 20 mm) applied to the AI before the conditioning had no effect on this conditioned heart rate decrease (30.0 ± 5.8%; n = 4; two-tailed t test; p > 0.05) (Fig. 1, filled circles).
Figure 1.
A conditioned heart rate change was not affected by 5-HT applied to the AI. A, A heart rate decrease elicited by the 30 min conditioning (cond.): a CS (30 kHz; hatched rectangle) paired with a US (filled rectangle). B, A heart rate decrease for the CS alone ∼2 min after the conditioning. C, No heart rate decrease for non-CS (open rectangle) tone bursts at 15 or 60 kHz. A 5-HT (filled circles) or saline (open circles) solution applied to the AI (arrow) before the conditioning had no effect on the conditioned autonomic response (filled circles compared with open circles; p > 0.05). Each symbol and bar represents a mean and SE in the percentage of heart rate change. The data were obtained from four bats.
Serotonergic effects on cortical auditory responses
Without conditioning, 97 cortical neurons with BFs ranging between 19.0 and 44.0 kHz (mean ± SE, 28.0 ± 2.5 kHz) were examined for the effects of 5-HT (48 neurons), α-methyl-5-HT (20 neurons), ritanserin (19 neurons), or saline (10 neurons) applied to the AI (Table 1).
Table 1.
Effects of 5-HT, α-methyl-5-HT, and ritanserin on cortical neurons
| Drugs applied to the AI | % Change in resp. at BF | BF shift (kHz) elicited by: |
|
|---|---|---|---|
| 15 min conditioning | 30 min conditioning | ||
| Saline | −2.0 ± 1.0 (10/10) | 0.0 ± 0.1 (12/12) | −1.8 ± 0.1 (10/10) |
| 4 mm 5-HT | −28.6 ± 4.8 (11/14)** | −1.5 ± 0.2 (16/20)** | Not studied |
| 20 mm 5-HT | −58.8 ± 4.3 (25/34)** | 0.0 ± 0.1 (12/12) | −0.7 ± 0.1 (17/22)* |
| 10 mm α-methyl-5-HT | 26.9 ± 5.6 (15/20)** | −1.8 ± 0.2 (18/27)** | −1.8 ± 0.2 (14/18) |
| 10 mm ritanserin | −20.5 ± 4.3 (16/19)* | +1.0 ± 0.2 (10/12)* | +1.0 ± 0.1 (18/23)** |
Changes in response magnitude and conditioning-elicited cortical BF shift evoked by 5-HT, α-methyl-5-HT, and ritanserin. Response (resp.) magnitude (number of spikes per 50 tone bursts) is expressed in percentage of change compared with the response in the control condition. A BF shift (mean ± SE) was either centripetal (negative) or centrifugal (positive). The numbers in parentheses are the number of neurons affected by a drug out of the total number of neurons studied. The drug effect is compared with the effect of saline (i.e., the control) for *p < 0.05 or **p < 0.01.
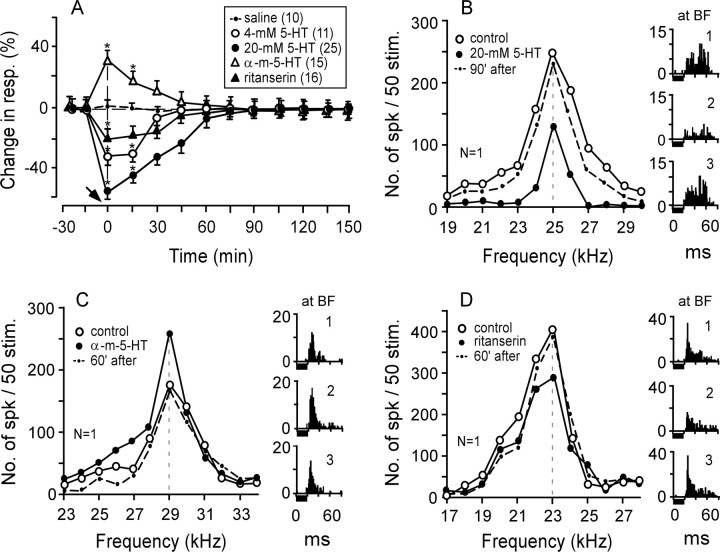
Four millimolar 5-HT reduced the auditory responses at the BFs of 11 of 14 neurons studied by 29%. The responses recovered to the control ∼45 min after the application (Fig. 2A, open circles). Twenty millimolar 5-HT reduced the auditory responses at the BFs of 25 of 34 neurons studied by 59%. The recovery time was ∼90 min (Fig. 2A, filled circles). Therefore, the amount and duration of the effect of 5-HT were dose dependent. An agonist of 5-HT2A receptors, α-methyl-5-HT, increased the auditory responses of 15 of the 20 neurons studied by 27%. These responses recovered ∼60 min later (Fig. 2A, open triangles). Conversely, an antagonist of 5-HT2A receptors, ritanserin, decreased the auditory responses of 16 of the 19 neurons studied by 21%. These responses recovered ∼60 min later (Fig. 2A, filled triangles). All of these changes in the auditory responses were significant (t test; p < 0.05). The effect of each drug on a single neuron is shown in Figure 2B–D.
Figure 2.
Changes in response magnitude evoked by 5-HT, α-methyl-5-HT, or ritanserin. A, The change (mean ± SE) in response (number of spikes per 50 tone bursts) is expressed in percentage of spikes per 50 tone bursts compared with the response in the control condition (*p < 0.01). The arrow along the time axis indicates when a drug was applied to the AI. The inset shows the symbols indicating the drugs applied to the AI and the number of single neurons affected by a drug. α-m-5-HT, α-Methyl-5-HT. B–D, The frequency-response curves and poststimulus-time histograms (1–3) displaying the responses at the BFs of three single neurons were recorded before (control), 5 min after, and 60 or 90 min after a drug application. Each horizontal bar under the histogram represents a 20 ms tone burst fixed at 10 dB above the MT of a given neuron. N, Number of neurons studied; resp., response; stim., stimulation; No., number; spk, spike.
Figure 2B shows the frequency-response curves of a cortical neuron tuned to 25.0 kHz (open circles and histogram 1). The auditory responses of all frequencies were reduced by 20 mm 5-HT (filled circles and histogram 2) and then recovered ∼90 min after the 5-HT application (dashed curve and histogram 3). The responses at the BF were decreased by ∼46%. Figure 2C shows that α-methyl-5-HT increased the response at the BF of a neuron tuned to 29.0 kHz (open and filled circles; histograms 1 and 2). The response recovered ∼60 min after the application (dashed curve and histogram 3). In Figure 2D, ritanserin reduced the response at the BF of a neuron tuned to 23.0 kHz by 24% (open vs filled circles; histogram 1 vs 2). The response recovered ∼60 min after the drug application (dashed curve and histogram 3).
Without acoustic stimulation, none of the drugs applied to the AI evoked a BF shift as did ACh applied to the AI (Ji et al., 2001). To evoke the BF shift (Zhang and Suga, 1997) and the shift of delay-tuning curves (Yan and Suga, 1999), the selective activation of a small group of cortical neurons by a tonal stimulus was necessary.
Effects of different doses of 5-HT on BF shifts
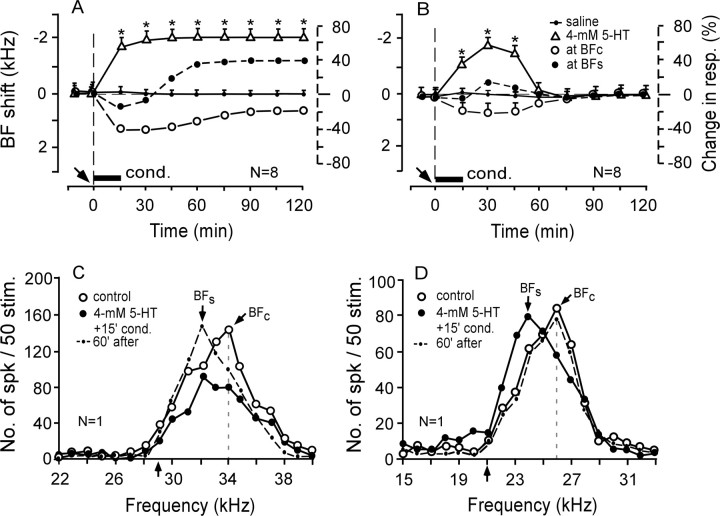
As described in the Materials and Methods, 15 min conditioning elicited subthreshold cortical BF shifts. A saline solution applied to the AI had no effect on these subthreshold BF shifts (n = 12; p > 0.05) (Table 1). To examine the effect of a low dose of 5-HT on the subthreshold BF shift, 4 mm 5-HT was applied to the AI before the 15 min conditioning. It was found that subthreshold BF shifts were augmented in 16 of the 20 neurons studied, and the remaining four neurons showed no changes in the BF and response magnitude. Eight of the 16 neurons showed long-term BF shifts (Fig. 3A, open triangles). Their response at the control BF (BFc) decreased by 22 to 40% after the conditioning (Fig. 3A, open circles), whereas that at the shifted BF (BFs) initially decreased by ∼18% and then increased up to 38% (Fig. 3A, filled circles). Another eight neurons showed short-term BF shifts (Fig. 3B, open triangles). The changes in the responses at the BFc and BFs were small and short (Fig. 3B, open and filled circles).
Figure 3.
Effects of 4 mm 5-HT on the frequency-response curves and subthreshold BF shifts elicited by the 15 min conditioning. A–D, Four millimolar 5-HT evoked either a long-term centripetal BF shift (A, C) or short-term centripetal BF shift (B, D). A, B, BF shifts refer to the left vertical axes, percentage of changes in response at the BFc (open circles) and BFs (filled circles) refer to the right vertical axes. N, Number of neurons studied. The arrow and horizontal bar along the time axis indicates the time of a drug application to the AI and the conditioning (cond.), respectively. C, D, Changes in the frequency-response curves of two single neurons tuned to 34.0 or 26.0 kHz, respectively. The upward arrows along the frequency axis indicate the frequencies of CS tone bursts. See Figure 2 legend for the symbols and abbreviations.
Figure 3, C and D, respectively, show the two types of changes in the frequency-response curves of single neurons evoked by 4 mm 5-HT applied to the AI before the 15 min conditioning. In Figure 3C, the auditory response of the neuron tuned to 34.0 kHz (open circles) was initially reduced between 30.0 and 36.0 kHz (filled circles). This reduction was caused by the applied drug. However, ∼60 min after the onset of the conditioning, the response at 32.0 kHz increased by 48%. Thus, the BF of the neuron shifted from 34.0 to 32.0 kHz (dashed curve) (i.e., toward the 29.0 kHz CS). This BF shift was long term and caused by the conditioning. Of the 16 neurons studied in the same way, eight showed identical changes as those shown in Figure 3C. In Figure 3D, the neuron was tuned to 26.0 kHz (open circles). Its BF first shifted to 24.0 kHz (filled circles) (i.e., toward the 21.0 kHz CS) and then recovered ∼60 min after the onset of the conditioning (dashed curve). The remaining eight of the 16 neurons showed the same changes as those shown in Figure 3D.
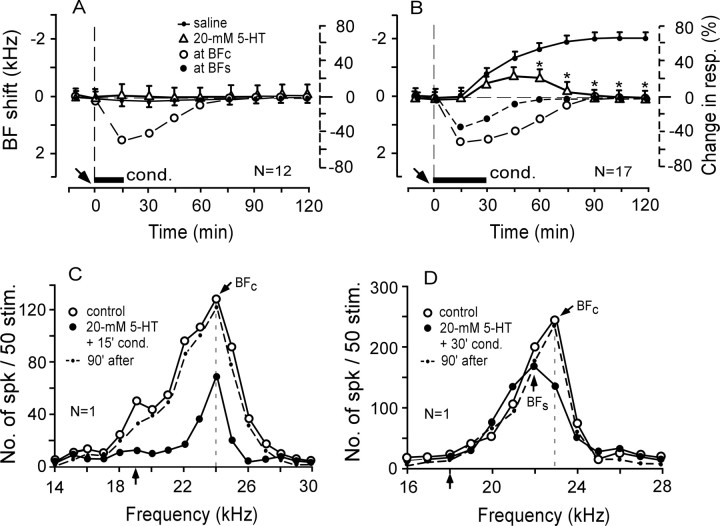
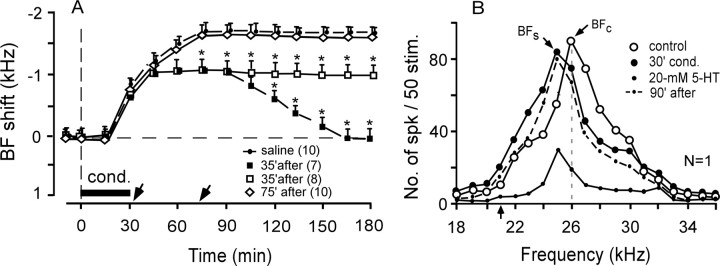
With the expectation that a high dose of 5-HT might augment the subthreshold BF shift more than the 4 mm 5-HT did, 20 mm 5-HT was applied to the AI before the 15 min conditioning. It was then found that the subthreshold BF shifts of all 12 cortical neurons studied were not augmented. That is, no BF shifts were elicited (Table 1; Fig. 4A, open triangles). In Figure 4C, 20 mm 5-HT applied to the AI before the 15 min conditioning significantly reduced the overall response of a single neuron tuned to 24.0 kHz, but it did not evoke any BF shift (open vs filled circles).
Figure 4.
Effects of 20 mm 5-HT on the subthreshold BF shifts elicited by the 15 min conditioning and the large long-term BF shifts elicited by the 30 min conditioning. A, C, Twenty millimolar 5-HT did not augment the subthreshold BF shift and reduced the auditory response. B, D, Twenty millimolar 5-HT changed the long-term BF shift into a short-term BF shift. C, D, Changes in the frequency-response curves of two single neurons tuned to 24.0 or 23.0 kHz, respectively. See Figures 2 and 3 legends for the symbols and abbreviations.
For the 30 min conditioning, the BF shift slowly developed to a plateau of 1.8 ± 0.1 kHz ∼75 min after the onset of the conditioning and was sustained for >3 h (Ji et al., 2001). Such a BF shift was not affected by a saline solution applied to the AI (n = 10) (Table 1). Because 20 mm 5-HT did not augment the subthreshold BF shift elicited by the 15 min conditioning, it was applied to the AI before the 30 min conditioning. Twenty millimolar 5-HT completely blocked the development of long-term BF shifts in five of the 22 cortical neurons studied (data not shown in Fig. 4) and reduced and shortened the BF shifts in the remaining 17 neurons (Table 1; Fig. 4B, open triangles). For these 17 cortical neurons, the BF shifts peaked at −0.7 ± 0.1 kHz and lasted 90 ± 5.0 min. In Figure 4D, 20 mm 5-HT applied to the AI before the 30 min conditioning significantly reduced the auditory response of a neuron tuned to 23.0 kHz (open circles) and temporarily shifted its BF toward the 18.0 kHz CS (filled circles and dashed curve).
Effect of 20 mm 5-HT applied after the 30 min conditioning on long-term BF shifts
Because 20 mm 5-HT drastically reduced the development of the long-term BF shift as described above, its effect was further studied on the BF shift that had developed to the midpoint of the plateau or to the plateau after the 30 min conditioning. Without a drug application, the BF shift elicited by the 30 min conditioning develops up to ∼50% of the plateau value ∼35 min after the onset of the conditioning and reaches the plateau at ∼75 min after the onset of the conditioning (Fig. 5A, dashed curve with dots) (Ji and Suga, 2003).
Figure 5.
Effects on the BF shifts elicited by the 30 min conditioning of 20 mm 5-HT applied to the AI after the 30 min conditioning. A, 5-HT applied to the AI 35 min after the onset of the conditioning decreased the magnitude of the long-term BF shift in eight neurons (open squares) and changed the long-term BF shift into a short-term BF shift in seven neurons (filled squares). 5-HT applied 75 min after the onset of the conditioning had no effect on the long-term BF shift (open diamonds). B, A cortical neuron tuned to 26.0 kHz (open circles) shifted its BF to 25.0 kHz (filled circles) at the end of the conditioning. 5-HT applied to the AI 35 min after the onset of the conditioning transiently reduced its auditory responses, but its BF stayed at 25.0 kHz (small dots). See Figures 2 and 3 legends for the symbols and abbreviations.
Twenty millimolar 5-HT applied to the AI at 35 min after the onset of the conditioning evoked a 1.0 ± 0.2 kHz BF shift instead of a 1.8 ± 0.1 kHz BF shift in 15 of the 19 cortical neurons studied (p < 0.05). Seven of these 15 neurons maintained this 1.0 kHz BF shift for 45 min at the plateau. Then, their BFs fully recovered ∼165 min after the onset of the conditioning (Fig. 5A, filled squares). However, the remaining eight neurons maintained the 1.0 kHz BF shift for ≥135 min (Fig. 5A, open squares). In Figure 5B, a cortical neuron tuned to 26.0 kHz showed a BF shift from 26.0 to 25.0 kHz [i.e., toward the 21.0 kHz CS, at the end of the 30 min conditioning (open vs filled circles)]. When 20 mm 5-HT was applied to the AI at this time, the auditory response of the neuron drastically decreased at the frequencies between 19.0 and 34.0 kHz, but the BFs stayed at 25.0 kHz (small dots). The response recovered ∼90 min after the onset of the conditioning (dashed curve). However, 20 mm 5-HT applied at ∼75 min after the onset of the 30 min conditioning had no effect on the long-term BF shifts of all 10 neurons studied (p > 0.01) (Fig. 5A, open diamonds), although it drastically reduced their auditory responses.
Effect of 10 mm α-methyl-5-HT applied before the 15 or 30 min conditioning on BF shifts
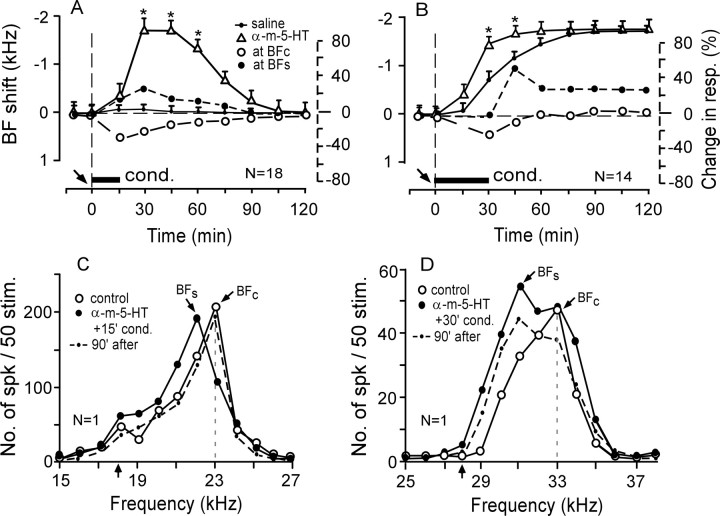
An agonist of 5-HT2A receptors, α-methyl-5-HT, applied to the AI before the 15 min conditioning augmented the subthreshold BF shift and evoked a short-term centripetal BF shift in 18 of the 27 cortical neurons studied (Table 1). The BF shift was 1.8 ± 0.2 kHz at the peak and lasted 105 ± 3.5 min (n = 18; p < 0.01) (Fig. 6A, open triangles). There was no effect on the auditory responses and BFs of the remaining nine neurons (data not shown in Fig. 6). In Figure 6C, α-methyl-5-HT reduced the response at the BFc (23.0 kHz) of a cortical neuron and augmented the response at 22.0 kHz (filled circles). These frequency-dependent changes in the responses caused the BF to shift from 23.0 to 22.0 kHz (i.e., toward the 18.0 kHz CS). They disappeared (i.e., the responses recovered) ∼90 min after the onset of the conditioning (dashed curve).
Figure 6.
Effects of α-methyl-5-HT (an agonist of 5-HT2A receptors) on the subthreshold BF shifts elicited by the 15 min conditioning (A, C) or the long-term BF shifts elicited by the 30 min conditioning (B, D). α-Methyl-5-HT augmented the subthreshold-BF shifts elicited by the 15 min conditioning (A, open triangles) and the BF shift elicited by the 30 min conditioning (B, open triangles). C, D, The effect of 10 mm α-methyl-5-HT applied before 15 or 30 min conditioning on the frequency-response curves of two single neurons tuned to 23.0 or 33.0 kHz. See Figures 2 and 3 legends for the symbols and abbreviations.
α-methyl-5-HT applied to the AI before the 30 min conditioning augmented the centripetal BF shift in 14 of the 18 cortical neurons studied (Table 1). The augmented BF shift developed faster than the BFc shift (Fig. 6B, open triangles vs small dots) (p < 0.05), but the amount of the augmented BF shift at the plateau was the same as that of the BFc shift (p > 0.05). In Figure 6D, a cortical neuron tuned to 33.0 kHz (open circles) increased its response at 31.0 kHz by ∼68%, which led to a 2.0 kHz long-term centripetal BF shift (filled circles). The overall response was reduced slightly ∼210 min after the onset of the conditioning (dashed curve).
Effect of 10 mm ritanserin applied before the conditioning on BF shifts
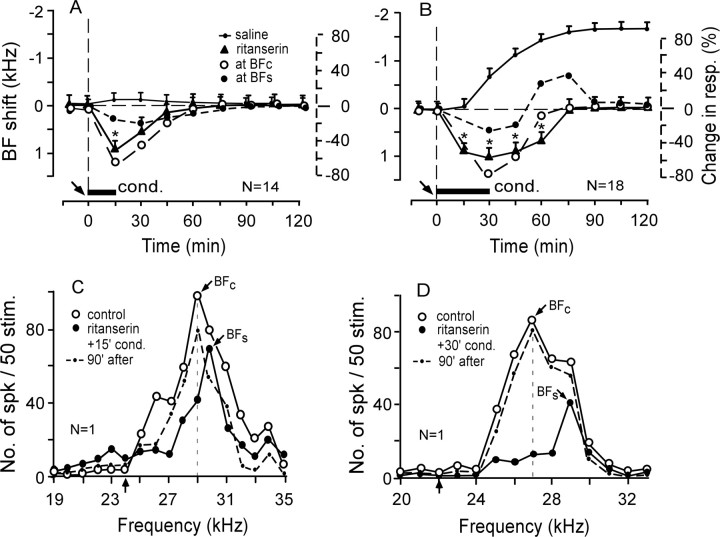
An antagonist of 5-HT2A receptors, ritanserin, applied to the AI before the 15 min conditioning evoked a small short-term “centrifugal” BF shift in 10 of the 12 neurons studied. The BF shift was 1.0 ± 0.2 kHz and the recovery time was 45 ± 8.5 min (Fig. 7A, filled triangles). Ritanserin did not change the responses and BFs of the remaining two neurons. Figure 7C shows the centrifugal BF shift of a single neuron tuned to 29.0 kHz. The BF at 29.0 kHz (open circles) shifted to 30.0 kHz [i.e., away from the 24.0 kHz CS (filled circles)].
Figure 7.
Effects of ritanserin (an antagonist of 5-HT2A receptors) on the subthreshold BF shifts elicited by the 15 min conditioning (A, C) or the long-term BF shifts elicited by the 30 min conditioning (B, D). A, B, The time courses of the BF shifts (solid lines) and changes in response magnitude (dashed lines). Ritanserin changed the subthreshold BF shift to the small centrifugal BF shift (A, filled triangles) and reversed the direction of the BF shift evoked by the 30 min conditioning (B, filled triangles). C, D, The effect of 10 mm ritanserin applied before the 15 or 30 min conditioning on the frequency-response curves of two single neurons tuned to 29.0 or 27.0 kHz. See Figures 2 and 3 legends for the symbols and abbreviations.
Ritanserin applied to the AI before the 30 min conditioning also evoked the centrifugal BF shift in 18 of the 23 neurons studied. This centrifugal BF shift was 1.0 ± 0.1 kHz and disappeared (i.e., recovered) in ∼75 min after the onset of the conditioning (Table 1; Fig. 7B, filled triangles). The centrifugal BF shift at the peak was the same as that evoked by ritanserin paired with the 15 min conditioning but lasted ∼30 min longer than the latter. In Figure 7D, a cortical neuron tuned to 27.0 kHz (open circles) decreased its responses to tone bursts between 25.0 and 29.0 kHz after a ritanserin application to the AI before the 30 min conditioning. The decrease in response was largest at 27.0 kHz and smallest at 29.0 kHz (filled circles). Accordingly, the BF of the neuron shifted from 27.0 to 29.0 kHz (i.e., away from the 22.0 kHz CS). The recovery time of the BF shift was ∼75 min.
Discussion
Effect of 5-HT applied to the AI on the conditioned heart rate change
Both the AI and auditory thalamus project to the amygdala, which in turn projects to many places in the brain to evoke conditioned autonomic and behavioral responses (LeDoux et al., 1984; Inoue et al., 1993; Stutzmann et al., 1999). The thalamo-amygdala projection plays an essential role in a conditioned heart rate change, but the cortico-amygdala projection does not, because a lesion of the neocortex does not interfere with the acquisition and maintenance of conditioned autonomic responses (DiCara et al., 1970; Berntson et al., 1983). Therefore, our data, which showed no effect of 5-HT applied to the AI on the conditioned heart rate change, is compatible with the above finding. Although auditory fear conditioning elicits an increase in the 5-HT level of the AI (Stark and Scheich, 1997), this increase is apparently not directly related to the conditioned heart rate change, but to the modulation of cortical plasticity as described below.
Neural mechanism of serotonergic modulation of the BF shift elicited by auditory fear conditioning
To evoke the cortical BF shift, the selective activation of a small group of cortical neurons by a tonal or electrical stimulation is necessary. The amount and duration of the BF shift are modulated by the serotonergic and cholinergic systems. Without the tonal or cortical electric stimulation, neither ACh nor 5-HT applied to the AI evokes the BF shift.
The effect of any of the drugs used on the responses to tone bursts was largest immediately after their application to the AI and disappeared within 60 min thereafter. It was frequency-independent. However, the cortical BF shift elicited by the 30 min conditioning gradually increased over 60 min after the onset of conditioning and reached a plateau which lasted more than several hours. It was frequency-dependent because it depended on the decrease in the response at the BFc and the increase in the response at the BFs. Therefore, the BF shift occurred regardless of a frequency-independent decrease or increase in the response evoked by 4 mm 5-HT or α-methyl-5-HT. Because the development of the cortical BF shift greatly depends on the increase in cortical ACh that occurs within 60 min after the onset of the conditioning (Ji et al., 2001; Ji and Suga, 2004), the drugs used in our current research affected the development of the BF shift in the initial 40 min or so and had a long-lasting effect on the BF shift. Therefore, the time course of the drug effect on the response magnitude was quite different from that on the BF shift.
Our current data are not sufficient enough to propose a model for serotonergic modulation of the cortical BF shift. However, we may speculate the mechanism for the serotonergic modulation. The direction of the BF shift depends on the balance between excitation and inhibition occurring in the AI, because muscimol (an agonist of GABAA receptors) changes the centripetal BF shift into a centrifugal BF shift (Ma and Suga, 2004), and bicucullin (an antagonist of GABAA receptors) changes the centrifugal BF shift into a centripetal BF shift (Xiao and Suga, 2002; Ma and Suga, 2004). Ritanserin (a 5-HT2A receptor antagonist) suppressed the cortical auditory response and changed the centripetal BF shift into a centrifugal BF shift. It presumably reduced the activity of 5-HT2A receptors and led to a relative increase in the cortical inhibition by the GABAergic neurons. α-methyl-5-HT (a 5-HT2A receptor agonist), which augmented the cortical auditory response and centripetal BF shift, presumably increased the excitation of the cortical pyramidal neurons through 5-HT2A receptors. α-methyl-5-HT has a moderate affinity for 5-HT2C receptors but a high affinity for 5-HT2A receptors (Garnovskaya et al., 1995). Therefore, we simply interpreted that the effect of α-methyl-5-HT was because of the activation of 5-HT2A receptors.
There are at least three types of cortical GABAergic neurons: those with 5-HT3 or 5-HT2A receptors and those without 5-HT3/5-HT2A receptors (Jakab and Goldman-Rakic, 2000). 5-HT applied to the AI decreased the auditory response. This decrease in the response may be because of 5-HT2A and/or 5-HT3 receptors located on GABAergic neurons. Layer V pyramidal neurons have abundant 5-HT2A receptors and may be excited by 5-HT (Aghajanian and Marek, 1997, 2000). Four millimolar 5-HT augmented the BF shift, but 20 mm 5-HT reduced the BF shift. To explain these observations, we have to assume that the affinity of 5-HT3 receptors on the GABAergic neurons for 5-HT was lower than that of 5-HT2A receptors (Glennon et al., 2000) and that only higher levels of 5-HT activated GABAergic neurons through 5-HT3 receptors. Four millimolar 5-HT weakly excited the GABAergic neurons and excited the pyramidal neurons via 5-HT2A receptors, which in turn activated cortical cholinergic endings so that the subthreshold BF shift changed into a suprathreshold BF shift. However, 20 mm 5-HT strongly excited the GABAergic neurons via 5-HT3 receptors so that the BF shift was reduced. The serotonergic modulation of the cortical BF shift presumably depends on the balance between the excitation evoked by the pyramidal neurons through the activation of 5-HT2A receptors and the inhibition evoked by the GABAergic neurons through the activation of 5-HT3 receptors.
The effects of 5-HT and stress on cortical plasticity
It has been shown that (1) a 30 min electric footshock induces 5-HT release in the brain, but a 15 min electric footshock does not (Dunn, 1988; Inoue et al., 1993, 1994); (2) a 60 min electric foot-shock induces severe stress (Malyszko et al., 1994) and; (3) the 30 min auditory fear conditioning with tone bursts and an electric footshock evokes a large, long-lasting cortical BF shift (Gao and Suga, 2000). These findings indicate that the cortical 5-HT level is influenced by the level of stress and suggest that the large, long-lasting BF shift occurs in a mildly stressful situation caused by the 30 min conditioning. Our current studies show that 20 mm 5-HT blocked the long-term BF shift, whereas 4 mm 5-HT changed the subthreshold BF shift into a suprathreshold BF shift. These findings may be interpreted as follows: the 30 min conditioning mildly stressed the animal and evoked a small increase in the cortical 5-HT levels, which increased the development of the long-term BF shift augmented by ACh. However, 20 mm 5-HT applied to the AI before the 30 min conditioning caused a large increase in the 5-HT level, as did strong stressors, and suppressed the development of the BF shift. However, the 15 min conditioning weakly stressed the animal and evoked the subthreshold BF shift. Four millimolar 5-HT applied to the AI before the conditioning caused a small increase in the cortical 5-HT level and changed the subthreshold BF shift into the suprathreshold BF shift. We hypothesize that a functional role of cortical 5-HT is to suppress the plasticity of the AI (i.e., auditory learning) in strongly stressful situations but to facilitate it in weakly stressful situations. It has been reported that cortical plasticity is influenced by not only stress but also attention, arousal, and motivation (Polley et al., 2006).
Interaction between ACh and 5-HT for modulation of cortical BF shifts
Each cortical neuron has several thousand synapses on the dendrites for information processing (Kandel, 2000). The BF of a neuron is probably determined by the most dominant excitatory input (synapse) in an array of frequency-labeled inputs. The BF shift occurs presumably because of a shift of the dominant input along the array of the inputs according to a change in synaptic connectivity and/or the formation of new synapses (Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999). Serotonergic and cholinergic synapses are mostly located on the spines and dendritic shafts of pyramidal neurons and modulate activity-dependent synaptic changes (Rasmusson, 2000; Gu, 2002). Thus, these two neuromodulators presumably interact with each other during the development of the BF shift.
The CS (tone bursts) excites a specific cortical input. Then, the cortical neural net and corticofugal feedback produce the cortical BF shift, which is, in turn, augmented by ACh released from the neurons in the nucleus basalis (Gao and Suga, 2000; Suga and Ma, 2003). Muscarinic ACh receptors play an important role in this augmentation because atropine applied to the AI immediately before the 30 min conditioning abolishes the development of the long-term cortical BF shift (Ji et al., 2001). Atropine applied to the AI ∼35 min after the onset of the conditioning reduces the long-term cortical BF shift and changes it to short term; atropine applied to the AI ∼55 min after the onset of the conditioning reduces the amount of the cortical BF shift without changing it into short term; and atropine applied to the AI ∼70 min after the onset of the conditioning transiently reduces the cortical BF shift elicited by the conditioning (Ji and Suga, 2003). In comparison, 20 mm 5-HT applied to the AI ∼35 min after the 30 min conditioning reduced the long-term BF shift and, in addition, made it short term in 33% of the neurons studied; and 5-HT applied to the AI ∼75 min after the conditioning had no effect on it at all. These findings indicate that the cholinergic and serotonergic neuromodulator systems can alter the cortical BF shift, interacting with each other only during the development of the BF shift.
Serotonergic neurons in the raphe nuclei project both to the cortical sensory neurons (Barnes and Sharp, 1999) and the cholinergic neurons in the nucleus basalis (Gasbarri et al., 1999). A lesion of the cholinergic basal nucleus decreases the cortical ACh level and 5-HT2A receptor binding in layer IV of the cortex (Quirion et al., 1985). Furthermore, 5-HT2A and/or 5-HT3 receptors are located on cholinergic terminals in the cortex so that serotonergic neurons as well as cholinergic neurons can regulate the cortical function (Maura et al., 1992; Crespi et al., 1997; Giovannini et al., 1998; Dringenberg and Zalan, 1999). Our current data indicate that the cholinergic augmentation of the BF shift is modulated by serotonergic neurons, which can augment or reduce the BF shift or even reverse the direction of the BF shift, and that both the serotonergic and cholinergic systems play an important role in cortical plasticity according to behavioral demands.
Footnotes
This work was supported by the National Institute on Deafness and Other Communicative Disorders Grant DC-000175. We thank S. E. Miller for editing our current manuscript.
References
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:31302–31312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Naylor RJ, Tyers MB. 5-HT3 receptors mediate inhibition of acetylcholine release in cortical tissue. Nature. 1989;338:762–763. doi: 10.1038/338762a0. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Tuber DS, Ronca AE, Bachman DS. The decerebrate human: associative learning. Exp Neurol. 1983;81:77–88. doi: 10.1016/0014-4886(83)90158-9. [DOI] [PubMed] [Google Scholar]
- Castilho VM, Brandao ML. Conditioned antinociception and freezing using electrical stimulation of the dorsal periaqueductal gray or inferior colliculus as unconditioned stimulus are differentially regulated by 5-HT2A receptors in rats. Psychopharmacology (Berl) 2001;155:154–162. doi: 10.1007/s002130100697. [DOI] [PubMed] [Google Scholar]
- Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience. 2003;119:53–63. doi: 10.1016/s0306-4522(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Crespi D, Gobbi M, Mennini T. 5-HT3 serotonin hetero-receptors inhibit [3H]acethylcholine release in rat cortical synaptosomes. Pharmacol Res. 1997;35:351–354. doi: 10.1006/phrs.1997.0143. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Hood SD, Argyropoulos SV, Morris K, Bell C, Witchel HJ, Jackson PR, Nutt DJ, Potokar JP. Depleting serotonin enhances both cardiovascular and psychological stress reactivity in recovered patients with anxiety disorders. J Clin Psychopharmacol. 2006;26:414–418. doi: 10.1097/01.jcp.0000227704.79740.c0. [DOI] [PubMed] [Google Scholar]
- Dear SP, Fritz J, Haresign T, Ferragamo M, Simmons JA. Tonotopic and functional organization in the auditory cortex of the big brown bat, Eptesicus fuscus. J Neurophysiol. 1993;70:1988–2009. doi: 10.1152/jn.1993.70.5.1988. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Hashikawa T, Jones EG. Synaptic relationships of serotonin-immunoreactive terminal baskets on GABA neurons in the cat auditory cortex. Cereb Cortex. 1991;1:117–133. doi: 10.1093/cercor/1.2.117. [DOI] [PubMed] [Google Scholar]
- DiCara LV, Braun JJ, Pappas BA. Classical conditioning and instrumental learning of cardiac and gastrointestinal responses following removal of neocortex in the rat. J Comp Physiol Psychol. 1970;73:208–216. doi: 10.1037/h0030200. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Zalan RM. Serotonin-dependent maintenance of spatial performance and electroencephalography activation after cholinergic blockade: effects of serotonergic receptor antagonists. Brain Res. 1999;837:242–253. doi: 10.1016/s0006-8993(99)01669-8. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Changes in plasma and brain tryptophan and brain serotonin and 5-hydroxyindoleacetic acid after footshock stress. Life Sci. 1988;42:1847–1853. doi: 10.1016/0024-3205(88)90023-9. [DOI] [PubMed] [Google Scholar]
- Eison AS, Eison MS. Serotonergic mechanisms in anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:47–62. doi: 10.1016/0278-5846(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: Role of the corticofugal system. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnovskaya MN, Nebigil CG, Arthur JM. Spurney RF, Raymond JR. 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharmacol. 1995;48:230–237. [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Pacitti C, McGaugh JL. Serotonergic input to cholinergic neurons in the substantia innominata and nucleus basalis magnocellularis in the rat. Neuroscience. 1999;91:1129–1142. doi: 10.1016/s0306-4522(98)00672-1. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Ceccarelli I, Molinari B, Cecchi M, Goldfarb J, Blandina P. Serotonergic modulation of acetylcholine release from cortex of freely moving rats. J Pharmacol Exp Ther. 1998;285:1219–1225. [PubMed] [Google Scholar]
- Glennon RA, Dukat M, Westkaemper RB. Serotonin receptor subtypes and ligands. Neuropsychopharmacology: The Fourth Generation of Progress. 2000. Retrieved April 6, 2004 from http://www.acnp.org/G4/GN401000039/
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Role of the serotonin 5-HT (2A) receptor in learning. Learn Mem. 2003;10:355–362. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA, Quinn JL, Liu R, Aloyo VJ, Romano AG. Selective remodeling of rabbit frontal cortex: relationship between 5-HT2A receptor density and associative learning. Psychopharmacology (Berl) 2004;172:435–442. doi: 10.1007/s00213-003-1687-4. [DOI] [PubMed] [Google Scholar]
- Hasuo H, Matsuoka T, Akasu T. Activation of presynaptic 5-hydroxytryptamine 2A receptors facilitates excitatory synaptic transmission via protein kinase C in the dorsolateral septal nucleus. J Neurosci. 2002;22:7509–7517. doi: 10.1523/JNEUROSCI.22-17-07509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hear Res. 2002;168:1–11. doi: 10.1016/s0378-5955(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Inoue T, Koyama T, Yamashita I. Effect of conditioned fear stress on serotonin metabolism in the rat brain. Pharmacol Biochem Behav. 1993;44:371–374. doi: 10.1016/0091-3057(93)90476-a. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol Biochem Behav. 1994;49:911–920. doi: 10.1016/0091-3057(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R. Injury- and use-related plasticity in the primary sensory cortex of adult mammals: possible relationship to perceptual learning. Clin Exp Pharmacol Physiol. 1996;23:939–947. doi: 10.1111/j.1440-1681.1996.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J Neurophysiol. 2003;90:1904–1909. doi: 10.1152/jn.00363.2003. [DOI] [PubMed] [Google Scholar]
- Ji W, Gao E, Suga N. Effect of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophy. 2001;86:211–225. doi: 10.1152/jn.2001.86.1.211. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Nerve cell and behavior. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science, 4/e. New York: McGraw-Hill; 2000. p. 25. [Google Scholar]
- Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Arvanov VL, Wang RY. Inhibition of NMDA-receptor mediated response in the rat medial prefrontal cortical pyramidal cells by the 5-HT3 receptor agonist SR 57227A and 5-HT: intracellular studies. Synapse. 1998;29:257–268. doi: 10.1002/(SICI)1098-2396(199807)29:3<257::AID-SYN8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Lateral inhibition for center-surround reorganization of the frequency map of bat auditory cortex. J Neurophysiol. 2004;92:3192–3199. doi: 10.1152/jn.00301.2004. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Malyszko J, Urano T, Yan D, Serizawa K, Kozima Y, Takada Y, Takada A. Foot shock-induced changes in blood and brain serotonin and related substances in rats. Jpn J Physiol. 1994;44:35–47. doi: 10.2170/jjphysiol.44.35. [DOI] [PubMed] [Google Scholar]
- Maura G, Andrioli GC, Cavazzani P, Raiteri M. 5-Hydroxytryptamine3 receptors sited on cholinergic axon terminals of human cerebral cortex mediate inhibition of acetylcholine release. J Neurochem. 1992;58:2334–2337. doi: 10.1111/j.1471-4159.1992.tb10983.x. [DOI] [PubMed] [Google Scholar]
- Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gudelsky GA. Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse. 2004;53:202–207. doi: 10.1002/syn.20054. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Learning-induced plasticity in animal and human auditory cortex. Curr Opin Neurobiol. 2005;15:470–477. doi: 10.1016/j.conb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Santana N, Celada P, Mengod G, Artigas F. In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex. 2004;14:1365–1375. doi: 10.1093/cercor/bhh097. [DOI] [PubMed] [Google Scholar]
- Quirion R, Richard J, Dam TV. Evidence for the existence of serotonin type-2 receptors on cholinergic terminals in rat cortex. Brain Res. 1985;333:345–349. doi: 10.1016/0006-8993(85)91590-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res. 2000;115:205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Roerig B, Katz LC. Modulation of intrinsic circuits by serotonin 5-HT3 receptors in developing ferret visual cortex. J Neurosci. 1997;17:8324–8338. doi: 10.1523/JNEUROSCI.17-21-08324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Liu R, Dave KD, Schwab D, Alexander G, Aloyo VJ, Harvey JA. Effect of serotonin depletion on 5-HT2A-mediated learning in the rabbit: evidence for constitutive activity of the 5-HT2A receptor in vivo. Psychopharmacology (Berl) 2006;184:173–181. doi: 10.1007/s00213-005-0245-7. [DOI] [PubMed] [Google Scholar]
- Stark H, Scheich H. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: a long-term microdialysis study of metabolites. J Neurochem. 1997;68:691–697. doi: 10.1046/j.1471-4159.1997.68020691.x. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19(RC8):1–4. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Interaction of serotonin with somatosensory cortical neuronal responses to afferent synaptic inputs and putative neurotransmitters. Brain Res Bull. 1986;17:507–518. doi: 10.1016/0361-9230(86)90218-2. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Reorganization of the cochleotopic map in the bat's auditory system by inhibition. Proc Natl Acad Sci USA. 2002;99:15743–15748. doi: 10.1073/pnas.242606699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Suga N. Corticofugal amplification of facilitative auditory responses of subcortical combination-sensitive neurons in the mustached bat. J Neurophysiol. 1999;8:817–824. doi: 10.1152/jn.1999.81.2.817. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Corticofugal amplification of subcortical responses to single tone stimuli in the mustached bat. J Neurophysiol. 1997;78:3489–3492. doi: 10.1152/jn.1997.78.6.3489. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]