Abstract
The persistent activity of protein kinase Mζ (PKMζ) maintains synaptic long-term potentiation (LTP) and spatial memory, but the interactions between PKMζ and the other protein kinases implicated in synaptic plasticity are unknown. During LTP, PKMζ is rapidly synthesized from a PKMζ mRNA that encodes a protein kinase Cζ (PKCζ) catalytic domain without a regulatory domain; thus, second messengers that activate full-length PKC isoforms are not required to stimulate PKMζ. Like other PKCs, however, PKMζ must be phosphorylated on its activation loop by phosphoinositide-dependent protein kinase-1 (PDK1) for optimal catalytic activity. Thus, two sequential steps are required for the persistent increased PKMζ activity that maintains LTP: de novo synthesis of PKMζ and phosphorylation of its activation loop. Here, using a panel of antisera to phosphorylated and nonphosphorylated sites on PKMζ, we show that PI3-kinase (phosphoinositide 3-kinase), CaMKII (Ca2+/calmodulin-dependent protein kinase II), MAPK (mitogen-activated protein kinase), PKA (protein kinase A), mTOR (mammalian target of rapamycin), all important for LTP induction, as well as preexisting PKMζ, regulate the new synthesis of PKMζ during LTP. In contrast, PDK1 forms a complex with PKMζ and maintains maximal phosphorylation of its activation loop. Thus, the two steps of PKMζ formation serve separate functions in LTP: the initial regulated synthesis of PKMζ is the site of convergence and integration for multiple kinases of induction, whereas the constitutive phosphorylation of PKMζ by PDK1 initiates the persistent autonomous activity that sustains maintenance.
Keywords: CaMKII, ERK, learning and memory, LTP, long-term potentiation, phosphoinositide 3-kinase, protein synthesis
Introduction
Multiple protein kinases are important for long-term potentiation (LTP), an activity-dependent, persistent increase in synaptic transmission critical for learning and memory storage (Bliss and Collingridge, 1993; Sweatt, 1999; Bliss et al., 2006; Pastalkova et al., 2006; Whitlock et al., 2006). Most of these kinases function in the transient induction phase of LTP that triggers synaptic potentiation and during initial learning and memory consolidation. In contrast, only a single protein kinase, the atypical protein kinase C (aPKC) isoform, protein kinase Mζ (PKMζ), has been shown to be critical during the maintenance of LTP that sustains potentiation and for the persistence of spatial memory storage in the hippocampus (Sacktor et al., 1993; Ling et al., 2002; Bliss et al., 2006; Pastalkova et al., 2006).
PKMζ can maintain LTP because the kinase is autonomously active in neural tissue (Sacktor et al., 1993) and, thus, able to persistently enhance synaptic strength (Ling et al., 2002, 2006; Serrano et al., 2005). This autonomous activity is due, in part, to the unique structure of PKMζ (Hernandez et al., 2003). Most PKC isoforms consist of an N-terminal regulatory domain, containing an autoinhibitory pseudosubstrate sequence and second messenger-binding sites, and a C-terminal catalytic domain (Dekker et al., 1995; Newton, 2001). Under basal conditions, full-length PKC isoforms are inactive because the pseudosubstrate interacts with and inhibits the catalytic domain; second messengers, such as Ca2+ and diacylglycerol, stimulate PKCs by binding to the regulatory domain and causing a conformational change that releases this autoinhibition. PKMζ, in contrast, is generated by synthesis from a brain-specific PKMζ mRNA that encodes a ζ catalytic domain without a regulatory domain (Hernandez et al., 2003; Muslimov et al., 2004). Thus, the second messengers that activate full-length PKC isoforms are not required to stimulate PKMζ.
A second mechanism, however, is also important for PKC activation. Each PKC has little or no activity without phosphorylation of the “activation loop” of its catalytic domain by phosphoinositide-dependent protein kinase-1 (PDK1) (Newton, 2003). This phosphorylation, which is important for the activity of most members of the AGC [protein kinase A (PKA)/PKG/PKC] superfamily of protein kinases, converts the kinase catalytic domain from an inactive conformation to an active conformation that can efficiently bind substrates (Biondi, 2004). The function of PDK1 phosphorylation, however, varies for the different AGC kinases. Phosphorylation of the full-length aPKCs by PDK1, for example, is important in their regulation by extracellular signals (Chou et al., 1998; Le Good et al., 1998). These signals activate aPKCs by first stimulating receptor-linked phosphoinositide 3 (PI3)-kinase, which produces the lipid second messenger, phosphatidylinositol-3,4,5-trisphosphate (Ptdins-3,4,5-P3). Ptdins-3,4,5-P3 can then bind to both PDK1 and the regulatory domain of aPKC. A PDK1-docking site in the C-terminal of the aPKC catalytic domain may also facilitate interactions between the two kinases (Balendran et al., 2000a). Positioned together, PDK1 can then phosphorylate the aPKC catalytic domain on its activation loop (T410 in PKCζ), converting it from inactive to active conformation.
Because nonphosphorylated PKMζ, like PKCζ, is inactive (Balendran et al., 2000b; Smith and Smith, 2002) [or minimally active (Le Good and Brindley, 2004)], PKMζ synthesized after tetanic stimulation must also undergo activation loop phosphorylation to acquire the autonomous activity that maintains LTP. The mechanism for this critical phosphorylation step is unknown. Because PI3-kinase is important for LTP induction (Kelly and Lynch, 2000; Sanna et al., 2002; Opazo et al., 2003), PDK1 phosphorylation of PKMζ may be regulated in LTP, as has been shown for the phosphorylation of the full-length aPKCs, PKCζ and PKCι/λ, in other cellular contexts (Chou et al., 1998; Le Good et al., 1998). Alternatively, because PDK1 could directly bind PKMζ (Balendran et al., 2000a), PDK1 phosphorylation of PKMζ might be constitutive in the brain and, thus, part of the maintenance mechanism that sustains LTP (Ling et al., 2002; Serrano et al., 2005; Pastalkova et al., 2006).
Here, using a panel of antisera to phosphorylated and nonphosphorylated sites on PKMζ, we measured both the new synthesis and activation loop phosphorylation of PKMζ during LTP. We found that the initial synthesis of PKMζ is regulated by multiple kinases important for LTP induction, and is thus the convergent target of many signaling pathways in LTP. In contrast, the phosphorylation of PKMζ by PDK1 is constitutive, ensuring optimal PKMζ activity to maintain LTP.
Materials and Methods
Animals.
For all experiments, hippocampi of 18- to 30-d-old, male Sprague Dawley rats were removed after decapitation under halothane-induced anesthesia, according to State University of New York Downstate Medical Center Animal Use and Care Committee standards.
Preparation of antisera and immunoblotting.
Antisera to the phosphorylated state of T410 (pT410) was from Cell Signaling (Danvers, MA) (1:1000); C-terminal ζ antisera (1: 100) was used as described previously (Sacktor et al., 1993). Phosphorylated T560 antiserum (pT560, 1:100) was generated by immunizing with a phosphopeptide (EPVQLpTDDC) conjugated to keyhole limpet hemocyanin (Lampire Biological Laboratories, Pipersville, PA). The phosphopeptide was injected intramuscularly into New Zealand rabbits and serum collected after 1–3 boosts at 1 month intervals. Antiserum to PDK1 (1:100) was a generous gift from Alex Toker (Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA).
Immunoblots were performed as described previously (Sacktor et al., 1993). Briefly, purified PKMζ (∼100 ng) or total hippocampal protein (10–15 μg) were subjected to SDS-PAGE. The proteins were transferred to nitrocellulose and blocked for 90 min in 1% bovine serum albumin and 1% hemoglobin in Tris-HCl, pH 7.5 (10 mm), NaCl (150 mm), and Nonidet (0.2%) (TBSN) (unless otherwise stated, reagents were from Sigma, St. Louis, MO). The nitrocellulose membrane was incubated in primary antisera overnight at 4°C. The membrane was washed in TBSN and then incubated for 90 min with secondary antibody coupled to alkaline-phosphatase (1:2000). The blots were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium, scanned, and analyzed with NIH Image.
Specificity of the phosphorylation state antisera was determined by both immunoblotting of phosphorylated and dephosphorylated PKMζ (see Fig. 1B) and peptide competition. Peptide competition was performed by preincubating the primary antisera for 2 h at room temperature with 10 μg/ml of either phosphorylated or nonphosphorylated versions of the immunizing peptide. Immunostaining of hippocampal PKMζ on immunoblot was blocked by the phosphorylated peptide but not by the nonphosphorylated peptide (data not shown).
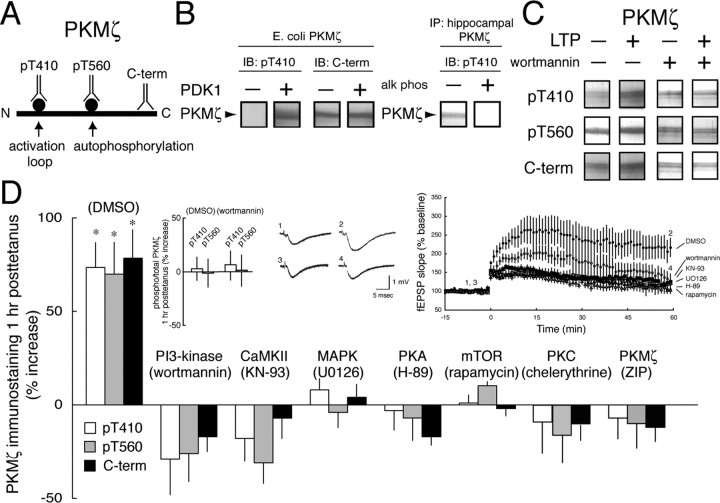
Figure 1.
Synthesis, not phosphorylation of PKMζ is the common target of multiple protein kinases in LTP induction. A, Illustration of the phosphorylation sites and C-terminal (C-term) epitope used for antisera production. B, Specificity of the pT410 antiserum. Left, pT410 antiserum recognizes E. coli-expressed PKMζ after phosphorylation by PDK1; C-terminal antiserum recognizes both phosphorylated and nonphosphorylated PKMζ. Right, Exposure of hippocampal PKMζ to calf intestinal phosphatase eliminates pT410 immunostaining. C, Representative experiment showing PKMζ immunostaining with all three antisera increases after LTP and the blockade of the increases by application of the PI3-kinase inhibitor wortmannin. D, PKMζ immunostaining with all three antisera increases in parallel 1 h after tetanization in the carrier DMSO (0.01%). Asterisks denote p < 0.05. Inhibitors of PI3-kinase, CaMKII, MAPK, PKA, mTOR, PKC, and PKMζ (in 0.01% DMSO) block the synthesis of PKMζ (p > 0.5 between LTP and control slices for each inhibitor; n = 4), but do not affect the relative amounts of phospho-PKMζ and total PKMζ. Insets, Left, Ratios of pT410/C-terminal and pT560/C-terminal immunostaining show no change in the proportion of phosphorylated PKMζ after LTP and blockade of LTP by wortmannin. Middle, Representative fEPSPs for time points shown at the right (DMSO, 1, 2; wortmannin, 3, 4). Right, Time courses of experiments showing LTP in DMSO and blockade of LTP by kinase inhibitors (p > 0.5 between baseline responses and responses 1 h after tetanization for each inhibitor; n = 8).
Immunoprecipitation of PKMζ and PDK1.
Hippocampal tissue was homogenized at 4°C in 1 μl per hippocampus (or 100 μl per CA1 region for physiology experiments) of buffer containing the following: 50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 1 mm EGTA, 0.1 mm phenylmethylsulfonyl fluoride, 5 mm benzamidine, aprotinin (17 kallikrein U/ml), 0.1 mm leupeptin, 1% phosphatase inhibitor cocktails 1 and 2, and 1% NP-40. Homogenization was with a #19 Kontes glass tube and a Talboy 102 homogenizer, using 20 strokes at 60 rpm. Ten microliters of the homogenate was removed for determination of total protein by bicinchoninic acid assay (Pierce, Rockford, IL). The remaining homogenate was centrifuged at 13,000 × g for 1 h. The supernatant (S1) was precleared by adding normal goat serum conjugated to agarose beads (30 μl per 200 μl S1), nutating for 1 h, and centrifuging at 13,000 × g for 2 min. Beads conjugated to a C-terminal aPKC antibody that recognizes both ζ and PKCι/λ (Hernandez et al., 2003) were added to the cleared supernatant (20 μl/200 μl), nutated overnight, and centrifuged at 13,000 × g for 2 min. For PDK1 immunoprecipitation, anti-PDK1 antisera was used at 1:200 dilution. The pelleted beads were washed three times in 200 μl homogenization buffer, centrifuging at 13,000 × g for 2 min between washes. The immunoprecipitated protein was then resuspended in 25 μl of sample buffer, heated for 10 min at 90°C, and assayed by immunoblot, as described above.
PKMζ dephosphorylation.
PKMζ immunoprecipitated from one hippocampus was treated with 10 U of calf alkaline intestinal phosphatase (New England Biolabs, Ipswich, MA) in a final volume of 50 μl, containing the immunoprecipitated protein in homogenization buffer, 100 mm NaCl, and 10 mm MgCl2. After 1 h at 37°C, the reaction was stopped by the addition of sample buffer and heated for 10 min at 90°C.
PDK1 phosphorylation of PKMζ.
Hemagglutinin (HA)-tagged PKMζ generated from Escherichia coli, which does not express PDK1, or PKMζ generated from baculovirus/Sf9 cells (Ling et al., 2002), which was dephosphorylated (at 4°C to preserve its native conformation), were used as substrates for PDK1. The reaction was for 30 min at 30°C with 0–15 U of PDK1 (Calbiochem, La Jolla, CA), 10 mm MgCl2, 1 mm β-mercaptoethanol, and 350 μm Na-ATP (in 30 μl final volume). The reaction was stopped by the addition of sample buffer and heated for 10 min at 90°C. At the higher concentrations of PDK1 tested, the T410 phosphorylation of PKMζ at 10 and 30 min was equivalent, indicating saturation of the reaction (data not shown).
By comparing immunostaining with the C-terminal antiserum of a known quantity of recombinant PKMζ, we calculated the amount of PKMζ in the hippocampus as 0.019 ± 0.003% of total protein (n = 3), a level of expression comparable with a conventional PKC isoform in brain (Huang et al., 1987).
Hippocampal slice preparation, stimulation, and recording.
Transverse hippocampal slices (450 μm) were prepared as described previously (Hrabetova and Sacktor, 1996). Hippocampi were dissected, bathed in cold dissecting buffer, and sliced with a McIlwain tissue slicer. The dissecting buffer contained the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 11 glucose, 10 MgCl2, and 0.5 CaCl2, bubbled with 95% O2/5% CO2, bringing the solution to pH 7.4. After 30 min the slices were placed in an interface recording chamber (2.1 ml total volume; Fine Science Tools, Foster City, CA), prewarmed to 32.0 ± 0.5°C, and perfused at 0.5 ml/min with the saline solution containing 1.2 mm MgCl2 and 1.7 mm CaCl2.
A bipolar platinum/iridium (80/20%) electrode (Frederick Haer, Bowdoin, ME) was placed in CA1 stratum radiatum to evoke field EPSPs (fEPSPs) from Schaffer collateral-commissural/CA1 synapses. Field EPSPs were recorded using glass microelectrodes with a resistance of 3–8 MΩ, filled with the recording saline, and positioned 200 μm from the stimulating electrodes. Current intensity of test stimuli (20–40 μA, 0.1 ms duration) was set to produce one-third maximal fEPSPs (1.5–2.0 mV). The frequency of test stimulation was every 15 s. Baseline fEPSPs were recorded for at least 10 min to ensure stability of the responses. Data were collected and analyzed using Sigma 60 (GW Instruments, Somerville, MA). The slope of the fEPSP was measured between 10 and 50% of the initial phase of the fEPSP response. The tetanization protocol was four 100 Hz, 1 s trains, set at 75% of the maximal fEPSP response (15–30 μA above the test stimuli current intensity), delivered at 20 s intertrain intervals (Scharf et al., 2002). Data were analyzed with Excel (Microsoft, Redmond, WA) and pClamp (version 9.0; Molecular Devices, Union City, CA). Test stimulation alone did not affect phospho-PKMζ or total PKMζ levels, compared with slices receiving no stimulation (n = 6; p > 0.05) (data not shown). Therefore, controls for LTP experiments were adjacent hippocampal slices bathed in the drug without test stimulation. Values are presented as mean ± SEM.
Results
Multiple kinases converge to regulate PKMζ synthesis in LTP induction
We used three antisera to assay PKMζ (Fig. 1A): a phosphospecific antiserum to its activation loop to assay its high activity conformation (pT410; for clarity, the amino-acid numbers of phosphorylation sites in PKMζ refer to the sequence of the full-length PKCζ), a second phosphospecific antiserum to the ζ autophosphorylation site used to assay catalytic competence (pT560) (Le Good et al., 1998), and an antiserum to the PKMζ C-terminal to measure total PKMζ. To confirm the phosphorylation-state specificity of the pT410 antisera, PKMζ produced from E. coli, which does not express PDK1, was immunostained with pT410 and C-terminal antisera (Fig. 1B, left). As expected, the C-terminal antiserum recognized the E. coli-expressed PKMζ, whereas the pT410 did not. After phosphorylation by exogenous PDK1, both antisera recognized the bacterially expressed PKMζ. Conversely, PKMζ immunoprecipitated from rat hippocampus with the C-terminal antiserum was recognized by the pT410 antiserum, and incubation with calf alkaline intestinal phosphatase eliminated pT410 immunoreactivity (Fig. 1B, right). Similar results were obtained with the pT560 antiserum (data not shown).
We then examined the changes in phospho-PKMζ (measured by either pT410 or pT560 antisera immunostaining) and total PKMζ (measured by C-terminal antiserum immunostaining) during LTP (Fig. 1D, middle and right insets) (p < 0.01, paired t test between fEPSP responses, before and 1 h after tetanization in DMSO; n = 8). The immunostaining for all three antisera increased in parallel, indicating that LTP caused an increase in total PKMζ, but no change in the proportion of PKMζ that was phosphorylated (Fig. 1C,D) [two-way ANOVA showed a main effect of treatment (F(7,72) = 16.892, p < 0.0001; Tukey's post hoc test, p < 0.0001) whereas the main effect of antisera, comparing the increases in pT410, pT560, and C-terminal immunostaining to each other was not significant (F(2,72) = 0.59; p > 0.05); left inset shows no change in the ratio of phospho-PKMζ to PKMζ during LTP].
We determined whether PI3-kinase, which activates full-length aPKCs by PDK1 phosphorylation of T410 (Chou et al., 1998; Le Good et al., 1998), increased the phosphorylation of PKMζ during LTP. As expected, the PI3-kinase inhibitor, wortmannin (400 nm), blocked the induction of LTP (Fig. 1D, middle and right insets). We found that wortmannin prevented the synthesis of PKMζ during LTP, but did not affect the relative amounts of phospho-PKMζ (either pT410 or pT560) and total PKMζ (Fig. 1C,D, left insert) (p > 0.05).
Because PI3-kinase was critical for the synthesis of PKMζ, we examined other pathways regulating translation in LTP. Mitogen-activated protein kinase (MAPK), which is in the same signaling pathway as calmodulin-dependent protein kinase II (CaMKII) (Giovannini et al., 2001) and PKA (Blitzer et al., 1998) in LTP induction, is critical for new, activity-dependent protein synthesis (Kelleher et al., 2004). Inhibitors of MEK (MAPK-extracellular signal-related kinase kinase) that activates MAPK (U0126, 20 μm), CaMKII (KN-93, 20 μm), and PKA (H-89, 20 μm), all blocked LTP induction and PKMζ synthesis (Fig. 1D). Because both PI3-kinase and MAPK can regulate the growth-related increase in protein synthesis mediated by the mammalian target of rapamycin kinase (mTOR) (Gingras et al., 2001), we examined the effect of the mTOR inhibitor rapamycin (200 nm) and found that the agent also blocked PKMζ synthesis during LTP (Fig. 1D). Finally, in addition to its role in LTP maintenance, basal PKMζ has been implicated in LTP induction (Sajikumar et al., 2005). Both the PKC inhibitor chelerythrine [2 μm, a low dose that may be selective toward PKMζ (Ling et al., 2002)] and the PKMζ inhibitor ζ-inhibitory peptide (ZIP; 1 μm) blocked the increase of PKMζ in LTP (Fig. 1D). Although all of the inhibitors blocked PKMζ synthesis and LTP induction, none affected the relative amounts of phospho-PKMζ and total PKMζ (Fig. 1D) (p > 0.5).
Constitutive phosphorylation of the PKMζ activation loop
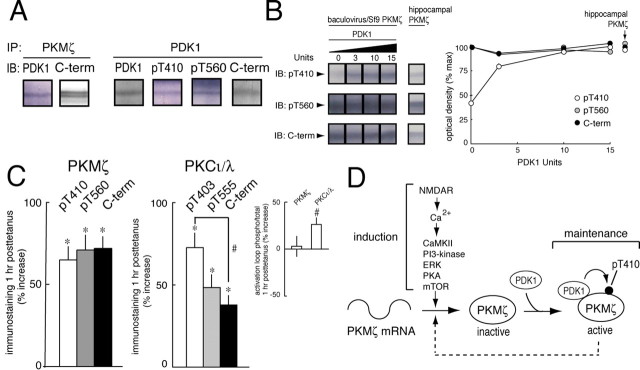
These results indicate that, although multiple signaling pathways of LTP induction regulate the synthesis of PKMζ, the phosphorylation state of PKMζ before and after LTP is equivalent and not affected by any of the inhibitors. This suggests that activation loop phosphorylation of PKMζ might be constitutive, perhaps through a direct interaction between PKMζ and PDK1, as has been described in vitro (Balendran et al., 2000a). Consistent with this notion, we found that immunoprecipitation of PKMζ from hippocampus coprecipitated PDK1 (Fig. 2A, left), and, conversely, immunoprecipitation of PDK1 coprecipitated PKMζ (Fig. 2A, right). Because PDK1 activity does not require second messenger stimulation (Biondi, 2004), the interaction between the two kinases in brain may result in maximal phosphorylation of PKMζ. To quantify the phosphorylation of PKMζ, we compared the phospho/total immunostaining of recombinant PKMζ that had been maximally phosphorylated by PDK1 to that of hippocampal PKMζ (Fig. 2B). We found that the phosphorylation states of maximally phosphorylated recombinant PKMζ and hippocampal PKMζ were equivalent.
Figure 2.
Constitutive phosphorylation of PKMζ by PDK1. A, PKMζ and PDK1 form a complex in hippocampal tissue. Left, Immunoprecipitation of PKMζ from hippocampus coprecipitates PDK1; right, immunoprecipitation of PDK1 from hippocampus coprecipitates PKMζ, as detected by all three antisera. B, Hippocampal PKMζ is maximally phosphorylated on its activation loop. Left, Representative experiment showing increasing amounts of PDK1 saturate phosphorylation of PKMζ at levels that are equivalent to the phosphorylation state of endogenous hippocampal PKMζ (shown at a lower concentration). Right, Mean ± SEM of four PDK1 phosphorylation experiments (the SEM is smaller than the symbols for each data point; pT410 immunostaining at 15 PDK1 U is set at 100%; C-terminal (C-term) immunostaining of hippocampal PKMζ is set at 100%; hippocampal PKMζ, n = 7). C, Left, PKMζ immunostaining 1 h after tetanization shows all three antisera increase in parallel, relative to nontetanized control slices. Right, PKCι/λ immunostaining 1 h after tetanization shows increases in activation loop (pT403) phosphorylation relative to total PKCι/λ in the tetanized slices, as well as increases in immunostaining for all antisera relative to nontetanized slices. Inset, Increase in the proportion of activation loop phosphorylated PKCι/λ, but not PKMζ, after LTP. Asterisks and hash mark denote p < 0.05. D, Illustration of the biochemical pathways of LTP induction and maintenance. In induction, postsynaptic NMDAR activation, critical for PKMζ synthesis (Sacktor et al., 1993; Osten et al., 1996), leads to increases in Ca2+, which stimulates multiple kinases that are critical for PKMζ synthesis from PKMζ mRNA. The newly synthesized, nonphosphorylated PKMζ binds to the constitutively active PDK1 and is maximally phosphorylated on T410 to generate the autonomous activity maintaining LTP. PKMζ may form a positive feedback loop to sustain increases in its synthesis during maintenance (dashed line).
These results indicate that brain PKMζ is constitutively phosphorylated by PDK1. To determine whether this mechanism is specific to PKMζ, we compared the phosphorylation of PKMζ during LTP with that of the other aPKC isoform expressed in the hippocampus, the full-length PKCι/λ (Hernandez et al., 2003), which is also activated after tetanization (Sacktor et al., 1993; Osten et al., 1996). Whereas again we found that the relative amounts of phosphorylated and total PKMζ remained constant after LTP (Fig. 2C, left and inset) (p > 0.05), the relative amount of activation loop phosphorylation to total level of PKCι/λ increased, indicating regulated phosphorylation (Fig. 2C, right and inset) (p < 0.05). Immunostaining for total PKCι/λ also increased after LTP, relative to control (p < 0.005), consistent with previous results (Osten et al., 1996).
Discussion
Here, we found that the two sequential steps for the formation of autonomously active PKMζ, protein synthesis and activation loop phosphorylation, serve distinct functions in the transition from the induction to maintenance of LTP (Fig. 2D). The primary site of the regulation of PKMζ formation in LTP induction is its initial, presumably rate-limiting step, as is commonly observed in the regulatory control of metabolic pathways. In contrast, the subsequent reaction, activation loop phosphorylation, is constitutive, thus ensuring that the newly synthesized kinase is fully converted into its maximally active state to maintain LTP.
Combined with the previous finding that actin filament formation also regulates PKMζ synthesis in LTP induction (Kelly et al., 2007), our results indicate that PKMζ synthesis is the convergent target of many signaling pathways in LTP. These pathways likely regulate the increased translation of PKMζ from pre-existing PKMζ mRNA, rather than increased transcription, because PKMζ message levels do not change 1 h posttetanization (S. Kremlev and M. Kelly, personal communication) and metabolic labeling of PKMζ protein increases within minutes after tetanization, too rapid for new transcription of the 100 kb PKMζ pre-RNA (Hernandez et al., 2003). Our results do not exclude the possibility that other proteins that function either in parallel with or downstream of PKMζ may also be sites of convergent translational regulation (Kelleher et al., 2004). A trend toward a decrease in PKMζ after LTP with some of the inhibitors (Fig. 1D) suggests that proteolytic degradation of the kinase may also be increased after strong afferent synaptic stimulation (Fonseca et al., 2006; Hou et al., 2006; Karpova et al., 2006).
The requirement of basal PKMζ activity for the synthesis of new PKMζ during LTP suggests a positive feedback loop (Fig. 2D, dashed line) that may be important for the persistent increased PKMζ activity that maintains in vivo LTP for at least 1 d and sustains spatial memory for up to 1 month (Pastalkova et al., 2006). Additional work will be required to determine whether this positive feedback loop is necessary and sufficient for these sustained effects of PKMζ.
Whereas PKMζ synthesis is the final common target of many signaling pathways in LTP induction, the constitutive activation loop phosphorylation of PKMζ represents the beginning of the biochemical mechanism of LTP maintenance (Fig. 2D). This constitutive phosphorylation of PKMζ by PDK1 during LTP is in contrast to the regulated phosphorylation of the full-length aPKC isoforms, which is important for their stimulation by growth factors (Chou et al., 1998; Le Good et al., 1998) and hormones, particularly insulin (Standaert et al., 1997). Indeed, the constitutive activation loop phosphorylation of PKMζ appears similar to the PDK1 phosphorylation of the conventional/novel PKC isoforms (cPKC/nPKCs), which occurs immediately after their synthesis (Newton, 2003; Biondi, 2004). The interaction between PDK1 and c/nPKCs, however, is only transient and serves to make the c/nPKCs competent for subsequent regulation by second messengers. In contrast, the interaction between PDK1 and PKMζ in brain is stable and results in maximal phosphorylation and activity of PKMζ for its persistent function. Although it is possible that nonphosphorylated PKMζ may be rapidly degraded and therefore undetected by our immunoblot assay, this may not be the case because full-length PKCζ appears to be stable without activation-loop phosphorylation (Balendran et al., 2000b; Le Good and Brindley, 2004).
Thus, the persistent activity of PKMζ that maintains LTP is generated by the elimination of the two essential mechanisms of PKC regulation. The standard regulation by second messengers is eliminated by the deletion of the ζ regulatory domain from the translation product of PKMζ mRNA (Hernandez et al., 2003). Here, we show that regulated activation loop phosphorylation, critical for the stimulation of other aPKC isoforms, is eliminated by the constitutive binding and phosphorylation of PDK1. These unique properties allow PKMζ to be the specific protein kinase maintaining memory storage (Bliss et al., 2006; Pastalkova et al., 2006).
Footnotes
This work was supported by National Institutes of Health Grants MH53576 and MH57068 to T.C.S.
References
- Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Cζ (PKCζ) and PKC-related kinase 2 by PDK1. J Biol Chem. 2000a;275:20806–20813. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000b;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- Biondi RM. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem Sci. 2004;29:136–142. doi: 10.1016/j.tibs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Laroche S. Neuroscience. ZAP and ZIP, a story to forget. Science. 2006;313:1058–1059. doi: 10.1126/science.1132538. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- Dekker LV, Palmer RH, Parker PJ. The protein kinase C and protein kinase C related gene families. Current Opinion Structural Biol. 1995;5:396–402. doi: 10.1016/0959-440x(95)80103-0. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Control of translation by the target of rapamycin proteins. Prog Mol Subcell Biol. 2001;27:143–174. doi: 10.1007/978-3-662-09889-9_6. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Morrison JH, Iyengar R, Landau EM. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J Neurosci. 2001;21:7053–7062. doi: 10.1523/JNEUROSCI.21-18-07053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Sacktor TC. Bidirectional regulation of protein kinase Mζ in the maintenance of long-term potentiation and long-term depression. J Neurosci. 1996;16:5324–5333. doi: 10.1523/JNEUROSCI.16-17-05324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FL, Yoshida Y, Nakabayashi H, Huang K-P. Differential distribution of protein kinase C isozymes in the various regions of brain. J Biol Chem. 1987;262:15714–15720. [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Thomas U, Knopfel T, Behnisch T. Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J Neurosci. 2006;26:4949–4955. doi: 10.1523/JNEUROSCI.4573-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung HY, Kang H, T S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelly A, Lynch MA. Long-term potentiation in dentate gyrus of the rat is inhibited by the phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology. 2000;39:643–651. doi: 10.1016/s0028-3908(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Kelly MT, Yao Y, Sondhi R, Sacktor TC. Actin polymerization regulates the synthesis of PKMζ in LTP. Neuropharmacology. 2007;52:41–45. doi: 10.1016/j.neuropharm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Brindley DN. Molecular mechanisms regulating protein kinase Cζ turnover and cellular transformation. Biochem J. 2004;378:83–92. doi: 10.1042/BJ20031194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mζ enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Muslimov IA, Nimmrich V, Hernandez AI, Tcherepanov A, Sacktor TC, Tiedge H. Dendritic transport and localization of protein kinase Mζ mRNA: Implications for molecular memory consolidation. J Biol Chem. 2004;279:52613–52622. doi: 10.1074/jbc.M409240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O'Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mζ in LTP. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mζ in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Smith JB. Lack of constitutive activity of the free kinase domain of protein kinase C zeta. Dependence on transphosphorylation of the activation loop. J Biol Chem. 2002;277:45866–45873. doi: 10.1074/jbc.M206420200. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Toward a molecular explanation for long-term potentiation. Learn Mem. 1999;6:399–416. doi: 10.1101/lm.6.5.399. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]