Abstract
GPR54 is a G-protein-coupled receptor, which binds kisspeptins and is widely expressed throughout the brain. Kisspeptin–GPR54 signaling has been implicated in the regulation of pubertal and adulthood gonadotropin-releasing hormone (GnRH) secretion, and mutations or deletions of GPR54 cause hypogonadotropic hypogonadism in humans and mice. Other reproductive roles for kisspeptin–GPR54 signaling, including the regulation of developmental GnRH secretion or sexual behavior in adults, have not yet been explored. Using adult wild-type (WT) and GPR54 knock-out (KO) mice, we first tested whether kisspeptin–GPR54 signaling is necessary for male and female sexual behaviors. We found that hormone-replaced gonadectomized GPR54 KO males and females displayed appropriate gender-specific adult sexual behaviors. Next, we examined whether GPR54 signaling is required for proper display of olfactory-mediated partner preference behavior. Testosterone-treated WT males preferred stimulus females rather than males, whereas similarly treated WT females and GPR54 KO males showed no preference for either sex. Because olfactory preference is sexually dimorphic and organized during development by androgens, we assessed whether GPR54 signaling is essential for sexual differentiation of other sexually dimorphic traits. Interestingly, adult testosterone-treated GPR54 KO males displayed “female-like” numbers of tyrosine hydroxylase-immunoreactive and Kiss1 mRNA-containing neurons in the anteroventral periventricular nucleus and likewise possessed fewer motoneurons in the spino-bulbocavernosus nucleus than did WT males. Our findings indicate that kisspeptin–GPR54 signaling is not required for male or female copulatory behavior, provided there is appropriate adulthood hormone replacement. However, GPR54 is necessary for proper male-like development of several sexually dimorphic traits, likely by regulating GnRH-mediated androgen secretion during “critical windows” in perinatal development.
Keywords: metastin, Kiss-1, sexual behavior, olfactory partner preference, SNB motoneuron, AVPV, tyrosine hydroxylase, development, sex differences, hypogonadotropic hypogonadism, GnRH
Introduction
Many aspects of reproductive physiology and behavior, including the preovulatory luteinizing hormone (LH) surge, mate selection, and copulatory behavior, are sexually dimorphic. Many of these sexually dimorphic traits are differentiated early in perinatal development by the presence or absence of gonadal hormones (Phoenix et al., 1959; Baum, 1979; Bodo and Rissman, 2007). In males, but not females, perinatal androgens (and their estrogen metabolites) act to alter the developmental trajectory of sexually dimorphic neural substrates, resulting in adult neural circuitry, and the behaviors they control, which are masculinized (enhanced male-typical phenotypes) and defeminized (suppressed female-typical phenotypes). Thus, in addition to their adulthood activational effects on physiology and behavior, gonadal steroids also act early in development to organize the differentiation of sexually dimorphic neural substrates underlying behavior (Cooke et al., 1998).
GPR54 is a G-protein-coupled membrane receptor originally discovered in the rat and human genomes (Lee et al., 1999). GPR54 mRNA is expressed in the periphery (pancreas, placenta, and testes) and throughout the brain (hypothalamus, habenula, caudate nucleus, nucleus accumbens, and amygdala) (Lee et al., 1999; Kotani et al., 2001; Muir et al., 2001). GPR54 binds natural ligands called kisspeptins, which are encoded by the Kiss1 gene and synthesized in several brain regions, including the hypothalamic arcuate (ARC) and anteroventral periventricular (AVPV) nuclei (Kotani et al., 2001; Muir et al., 2001; Ohtaki et al., 2001; Gottsch et al., 2004; Shahab et al., 2005). GPR54 and kisspeptins are involved in the direct regulation of the reproductive neuroendocrine axis. In humans, cases of delayed puberty and hypogonadotropic hypogonadism have been linked to the absence of a functional GPR54 protein (de Roux et al., 2003; Seminara et al., 2003; Semple et al., 2005). Similarly, GPR54 knock-out (KO) mice display striking reproductive deficits, including underdeveloped gonads, diminished gonadotropins and steroid hormones, incomplete gametogenesis, and acyclicity (Funes et al., 2003; Seminara et al., 2003; Messager et al., 2005). The regulation of reproduction by GPR54 occurs at least at the level of gonadotropin-releasing hormone (GnRH) regulation, because GPR54 is expressed in GnRH neurons, and kisspeptin treatments stimulate GnRH and gonadotropin secretion, as well as Fos expression and electrical activity in GnRH cells (for review, see Dungan et al., 2006; Smith et al., 2006).
Despite evidence that kisspeptin–GPR54 signaling plays a critical role in the control of the neuroendocrine reproductive axis in puberty and adulthood, the functional significance of GPR54 in the regulation of perinatal androgen secretion (and hence, effects on sexual differentiation) has not yet been explored. In this report, we studied multiple sexually dimorphic traits in GPR54 KO mice to assess whether kisspeptin–GPR54 signaling during perinatal development is required for normal sex steroid-dependent sexual differentiation of the brain and behavior. Furthermore, although the expression of GPR54 in many non-GnRH neurons throughout the brain suggests that GPR54 signaling serves other functions in addition to regulating GnRH release, its role, if any, in other physiological or behavioral processes is unknown. We therefore also used our GPR54 KO model to test whether intact GPR54 signaling is necessary for proper adult display of male and female copulatory and sociosexual preference behaviors.
Materials and Methods
Animals.
GPR54 KO mice were produced by retroviral mutagenesis as described previously (Krasnow et al., 2004; Zeng et al., 2006). Briefly, an embryonic stem (ES) cell library was constructed by infecting 129/Sv ES cells with a retroviral vector that included a selection marker, termination codons in all reading frames, a splice adaptor, and a transcription terminator to ensure gene inactivation after insertion. Mutations in the GPR54 gene were found in the library by PCR analysis of genomic DNA by using vector-specific and gene-specific primers. Mutant clones isolated from the library were used for animal production using standard injection methods. Chimeric mice were bred with 129S1/SvImJ mice to generate heterozygotes in an inbred background. The resulting progeny were genotyped by PCR of tail DNA to identify homozygous male and female pups containing a disruption in the GPR54 gene, as well as their wild-type (WT) littermates. All pups were weaned at 18–20 d of age and were single- or group-housed on a 12 h light/dark cycle (lights off at 7:00 P.M. EDT) with mouse chow (7912; Harlan Teklad, Indianapolis, IN) and water available ad libitum. To verify knock-out status, a cohort of neonatal [day of birth designated postnatal day 1 (PND1)], prepubertal (21 d old), and adult (2.5–4 months old) male and female GPR54 KO and WT animals were studied for reproductive and somatic morphology and anatomy, including body weight, anogenital distance, gonad weights, and plasma gonadotropin levels (see Table 1 for animal numbers for each measure). In addition, paired GPR54 KO mice were confirmed to be infertile, although no other major behavioral impairments in locomotion, anxiety, depression, or pain responses were detected (H. Dungan, M. Gottsch, and R. Steiner, personal communication). All animal care and techniques were conducted in accordance with the National Institutes of Health Animal Care and Use Guidelines and with the approval of the Institutional Animal Care Committees of the University of Virginia and the University of Washington.
Table 1.
Comparison of somatic and reproductive characteristics of gonad-intact WT and GPR54 KO male and female mice
| Males |
Females |
|||
|---|---|---|---|---|
| Wild type | GPR54 KO | Wild type | GPR54 KO | |
| PND1 BW (g) | 1.53 ±0.05 | 1.57 ±0.05 | 1.45 ±0.04 | 1.48 ±0.03 |
| PND1 AGD (mm) | 1.2 ±0.05 | 1.3 ±0.06 | 0.9 ±0.06 | 1.0 ±0.04 |
| PND1 AGD ratio (mm/g) | 0.83 ±0.04 | 0.83 ±0.04 | 0.65 ±0.04 | 0.65 ±0.03 |
| Day 21 BW (g) | 10.2 ±0.4 | 9.8 ±0.4 | 10.0 ±0.5 | 9.4 ±0.4 |
| Day 21 AGD (mm) | 6.5 ±0.2 | 5.4 ±0.2* | 3.2 ±0.1 | 3.0 ±0.1 |
| Day 21 AGD ratio (mm/g) | 0.65 ±0.02 | 0.56 ±0.02* | 0.33 ±0.01 | 0.32 ±0.01 |
| Adult BW (g) | 28.0 ±1.0 | 25.1 ±1.0* | 21.3 ±0.7 | 22.2 ±0.8 |
| Ovaries (mg) | 19.9 ±0.9 | 7.5 ±0.7* | ||
| Testes (mg) | 243.6 ±9.7 | 9.0 ±1.7* | ||
| Seminal vesicles (mg) | 272.2 ±22.6 | 33.2 ±4.7* | ||
| Penis (mg) | 52.5 ±6.1 | 19.5 ±2.0* | ||
| Plasma LH (ng/ml) | 1.19 ±0.61 | 0.05 ±0.1* | ||
All measurements were taken in young adult mice (∼2.5–4 months old), except prepubertal body weight (BW) and anogenital distance (AGD), which were measured at PND1 and 3 weeks of age. GPR54 KO males differed from WT males in every adult measure as well as day 21 AGD. GPR54 KO females did not differ from WT females on any measure except adult ovarian weight. Animal numbers were 10 animals per group (testes, seminal vesicles, penis, LH), 10–13 animals/group (adult BW), and 15–26 animals per group (prepubertal BW, AGD). Asterisks indicate significant difference from WT animals of same sex.
Experiment 1: activation of GnRH neurons in WT and GPR54 KO mice.
To confirm disabling of the GPR54 gene in the GPR54 KO animals, we measured GnRH neuronal activation and LH release in GPR54 KO mice centrally infused with kisspeptin. Adult male GPR54 KO and WT littermates were briefly handled each day for ∼3 weeks to reduce stress of handling. All animals were then anesthetized with isoflurane and infused with ad libitum hand intracerebroventricular injections of either kisspeptin-54 [1 nmol (Phoenix Pharmaceuticals, Belmont, CA); n = 7 GPR54 KO and n = 8 WT mice] or vehicle (artificial CSF; n = 7 GPR54 KO and n = 8 WT mice) aimed at the lateral ventricle. The dose of kisspeptin used was previously shown to have a robust effect on LH release in WT mice when administered intracerebroventricularly (Gottsch et al., 2004). Thirty minutes after infusion, animals were briefly anesthetized with isoflurane vapors, and their blood was drawn by retro-orbital puncture. Ninety minutes after infusion, the animals were deeply anesthetized with ketamine–xylazine mixture and transcardially perfused with 0.9% saline followed by 10% formalin. Brains were collected and further postfixed in 10% formalin for 4–6 h. Fixed brains were soaked in 10% formalin:30% sucrose (1:1) overnight and then stored in 30% sucrose at 4°C. Brains were sectioned on a cryostat into four sets at 35 μm/section and stored in PBS plus NaAzide at 4°C. One set of sections was processed for double immunocytochemistry (ICC) for GnRH and Fos proteins. Plasma from the blood was assayed for LH concentrations using a sensitive mouse LH radioimmunoassay, performed by the University of Virginia Ligand Assay Laboratory as described previously (Kauffman and Rissman, 2004; Kauffman et al., 2005).
For double-label ICC, sections were first rinsed in PBS-Triton washes, blocked in normal serum, and then incubated in primary Fos antiserum (1:5000; s.c.-52; Santa Cruz Biotechnology, Santa Cruz, CA) for 48 h at 4°C. The tissue was then rinsed and incubated in biotinylated secondary antisera (1:500; horse anti-goat IgG; Vector Laboratories, Burlingame, CA) for 90 min. Next, the tissue was rinsed and treated with avidin–biotin complex (ABC; Vector Laboratories), after which immunoreactivity was visualized with nickel-intensified diaminobenzidine (DAB) activated by 0.1% hydrogen peroxide. After the DAB reaction was stopped, the tissue was incubated overnight in GnRH antiserum (1:5000; Affinity Bioreagents, Golden, CO) and then rinsed and exposed to biotinylated secondary antibody (1:500; goat anti-rabbit) and ABC for 90 min each. Immunoreactivity was then visualized with DAB (without nickel). This process results in GnRH cells that are labeled brown and Fos nuclei that are labeled black. Sections were mounted on gel-coated glass slides, coverslipped, and coded so that the investigator was blind to the treatment of each subject. The total number of GnRH cells exhibiting Fos-positive neuronal staining was counted in the medial septum, diagonal band of Broca, and the preoptic area (POA) [plates 22–32 of the mouse brain atlas of Franklin and Paxinos (1997)].
Experiment 2: sexual behavior of male WT and GPR54 KO mice.
GPR54 is expressed in many non-GnRH neurons throughout the brain, suggesting that kisspeptin–GPR54 signaling serves other functions in addition to regulating GnRH release. This experiment assessed whether male sexual behavior was impaired by the loss of GPR54 signaling. Intact adult male GPR54 KO (n = 10) and WT littermates (n = 10) received social exposure (Wersinger and Rissman, 2000) for 4 consecutive days, after which each animal was tested twice for sexual behavior (with an interval of 2–3 d between each test). In a follow-up experiment, adult GPR54 KO (n = 12) and WT mice (n = 14) were castrated and implanted with a SILASTIC implant containing testosterone [1 cm, 1.0 mm inner diameter (i.d.) × 2.2 mm outer diameter (o.d)]. This implant dose restores testosterone levels to within the normal physiological range in C57BL/6J male mice (Scordalakes and Rissman, 2003). Two to three weeks after receiving the implant, each animal was tested three times for sexual behavior (with an interval of 2–3 d between tests).
All tests of male sexual behavior were conducted in 18 × 38 cm Plexiglas testing boxes under red-light illumination during the dark phase of the light/dark cycle; boxes were placed on mirror stands to allow ventral viewing. Each subject was habituated to the testing box for 30 min before the introduction of a hormone-primed receptive stimulus female. Behavioral tests lasted for 45 min or until the male performed an ejaculation, whichever occurred first. Variables recorded included latencies to mount, thrust, and intromit; the number of thrusting and intromission bouts; and the occurrence of an ejaculation. Stimulus females (C57BL/6J) were ovariectomized in adulthood and implanted with a SILASTIC implant (1.96 mm i.d. × 3.18 mm o.d.) filled with estradiol benzoate (EB) (50 μg dissolved in 30 μl of sesame oil). Three hours before the tests, the females were injected subcutaneously with progesterone (P) (400 μg in 0.03 ml of sesame oil). To ensure maximal receptivity, all stimulus females received several rounds of sexual experience with a stud male in the weeks before experimental testing.
Experiment 3: sexual behavior of female WT and GPR54 KO mice.
The expression of GPR54 in many non-GnRH neurons throughout the brain suggests that kisspeptin–GPR54 signaling may affect other aspects of reproduction in addition to regulating GnRH release. This experiment assessed whether female sexual behavior was impaired by the loss of GPR54 signaling. Adult female GPR54 KO (n = 9) and WT (n = 12) littermates were ovariectomized and given 10–14 d to recover. Each animal was hormone-primed and tested four times for sexual behavior, with 4–5 d between trials. All tests of female sexual behavior were conducted starting 2 h after lights off, under red-light illumination, in 18 × 38 cm Plexiglas test chambers (as in experiment 2). Intact, sexually experienced C57BL/6J and DBA/2J males were used as testing partners (studs). Two days before testing, female subjects were injected subcutaneously with EB (0.5 μg dissolved in 0.05 ml of sesame oil). On the day of testing, progesterone (400 μg in 0.03 ml of sesame oil) was administered subcutaneously 3–4 h before the onset of the sexual behavior test. Each stud male was habituated for 30 min in the test box, before the introduction of the hormone-primed female. The behavior tests were terminated after the females received 20 mounts from the stud male (defined as both forepaws on the hind region), after an ejaculation, or after 25 min, whichever occurred first. For each subject, the onset to lordosis and the lordosis quotient (LQ; number of lordosis events per number of mounts) was scored. Lordosis was defined as the female placing all four paws on the ground when being mounted, with the subject's hind region elevated off the floor and back slightly arched.
Experiment 4: olfactory sexual preference in male and female WT and GPR54 KO mice.
This experiment assessed whether functional kisspeptin–GPR54 signaling is necessary for the display of olfactory-based partner preference. Olfactory partner preference is a sexually dimorphic affiliative behavior that reflects the ability of males and females to choose sexual partners based on olfactory cues: male subjects prefer stimulus estrous females over intact males, whereas female subjects have either a small preference for males or no preference for either sex, depending on the experimental design (Rissman et al., 1999; Bakker et al., 2007; Bodo and Rissman, 2007). In the first study, gonad-intact GPR54 KO and WT males (n = 7 per genotype) from experiment 2 were tested for olfactory sexual preference (beginning 2 weeks after the conclusion of sexual behavior testing). In the second study, gonadectomized GPR54 KO and WT males and females from experiments 2 and 3 were tested (n = 9–13 animals per group). To control for the activational effects of steroid hormones, all subjects in the second study were given subcutaneous testosterone (T) implants: ovariectomized females received a T implant 2–3 weeks before olfactory testing; castrated males already had T implants (from the sexual behavior study) for 3–4 weeks at the time of olfactory testing. Several days before olfactory preference testing, all animals underwent daily social exposure to intact males and hormone-primed females, as described previously (Wersinger and Rissman, 2000).
Olfactory partner preference tests were conducted in a Y maze 1–3 h after lights out, under red-light illumination. Each animal was given two baseline habituation tests on 2 consecutive days (10 min each) followed by the experimental test on the third day. For each test, the subject had 1 min to habituate to the end of the Y maze and then 10 min with free access to all parts of maze. On the two habituation tests, the maze was empty (i.e., no stimulus animals). On the experimental test, the ends of the Y-maze arms contained a male or female stimulus animal retained behind a mesh wire screen; to facilitate transmission of olfactory signals, fans at the end of each arm lightly blew over the stimulus animals inward toward the body of the maze. In this design, the mesh wire barrier minimized but did not fully prevent direct nasal contact between subject and stimulus animals; thus, subjects were able to receive both volatile and nonvolatile olfactory odors from the stimulus animals. Stimulus males were gonad intact and stimulus females were ovariectomized and injected with EB and P (given 48 and 3 h before the test, respectively). During the test, the amount of time the subject animal spent in each specific arm of the Y maze (within the half proximal to the stimulus animal) was scored. An Olfactory Preference Index was calculated by subtracting the total time spent investigating the male stimulus from the total time spent investigating the female stimulus; a positive index value denoted a preference for females, whereas a negative value denoted a preference for males. In addition, the percentage of time spent investigating the male or female stimulus was determined (by dividing by total investigatory time).
Experiment 5: sexually dimorphic tyrosine hydroxylase neurons in the AVPV nucleus of WT and GPR54 KO mice.
Experiment 4 indicated that male GPR54 KO animals exhibit a “female-like” olfactory partner preference. Because mouse olfactory partner preference is sexually dimorphic and organized by perinatal exposure to gonadal steroids (Bodo and Rissman, 2007), one explanation for the preference behavior data is that, during development, the brains of GPR54 KO males experience female-like sexual differentiation. To test this hypothesis, we analyzed the classical sexually dimorphic population of tyrosine hydroxylase (TH)-immunoreactive (IR) neurons in the AVPV nucleus of adult male and female GPR54 KO and WT littermates (n = 6–8 per group). Adult mice of both genotypes and sexes were gonadectomized and implanted with a SILASTIC T implant as in experiment 2. Two weeks later, the animals were deeply anesthetized with ketamine mixture (10% xylazine, 20% ketamine, in 0.9% NaCl) and transcardially perfused with 0.9% NaCl and 10% formalin. Brains were collected, processed, and cut, as in experiment 1. One set of brain sections containing the AVPV nucleus was processed for single-label ICC for TH protein by using a protocol similar to that used for the double-label ICC in experiment 1 (stopping after the first nickel-intensified DAB reaction was completed). The primary antiserum for TH was used at 1:10,000 (rabbit polyclonal; Pel-Freez, Rogers, AR), and the secondary antiserum was used at 1:500 (goat anti-rabbit IgG; Vector Laboratories). Assayed tissue sections were mounted on gel-coated glass slides, coverslipped, and coded so that the investigator was blind to the sex/genotype of each subject. The total number of cell bodies exhibiting positive TH staining was counted bilaterally in the AVPV nucleus [plates 28–31 of the mouse brain atlas of Franklin and Paxinos (1997)].
Experiment 6: Kiss1 mRNA levels in the AVPV nucleus of WT and GPR54 KO males.
Experiment 5 indicated that adult GPR54 KO males have female-like numbers of TH neurons, significantly different from that of WT males. In this experiment, we assessed whether another sexually dimorphic neuronal population is also dependent on functional GPR54 signaling. The population of Kiss1 neurons in the AVPV nucleus is sexually dimorphic (Clarkson and Herbison, 2006; Kauffman et al., 2007). Similar to TH, the number of Kiss1 cells in the AVPV nucleus is higher in females than males, regardless of adulthood steroid levels, and this Kiss1 sex difference is organized by gonadal steroids during the critical postnatal period (Clarkson and Herbison, 2006; Kauffman et al., 2007). In this experiment, we tested whether functional GPR54 signaling is necessary to develop the normal adult male Kiss1 phenotype by comparing Kiss1 mRNA levels in the AVPV nucleus of GPR54 KO males and WT males. Five adult males of both genotypes were castrated and implanted with SILASTIC implants that were either empty or contained T. Animals were killed 1 week later, and their brains were collected and fresh-frozen on dry ice. Brains were cut on a cryostat into five sets, 20 μm per section, and the sections were mounted onto slides. Slides were stored at −80°C until processing for Kiss1 mRNA in situ hybridization.
In situ hybridization for Kiss1 mRNA was performed as described previously (Gottsch et al., 2004; Irwig et al., 2005). Briefly, radiolabeled (33P) antisense Kiss1 riboprobes were generated using the Kiss1-specific sequence spanning bases 76–486 of the mouse Kiss1 cDNA sequence (GenBank accession number AF472576). Slide-mounted brain sections were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× SSC, delipidated in chloroform, dehydrated in graded ethanols, and then allowed to air-dry before the hybridization procedure. A calculated volume of Kiss1 riboprobe (0.03 pm/slide) was combined with 1:20 vol yeast tRNA (Roche Diagnostics, Indianapolis, IN) in TE (0.1 m Tris/0.01 m EDTA, pH 8.0), heat-denatured for 3 min, iced for 5 min, added to prewarmed hybridization buffer at a ratio of 1:4, and added to each slide (100 μl/slide). Slides were then coverslipped and placed in humidity chambers at 55°C for 16 h. After hybridization, slides were washed in 4× SSC at room temperature and then placed into RNase [37 mg/ml RNase (Roche Diagnostics) in 0.15 m sodium chloride, 10 mm Tris, and 1 mm EDTA, pH 8.0] for 30 min at 37°C, followed by RNase buffer at 37°C for another 30 min. After a brief wash in 2× SSC at room temperature, slides were washed twice in 0.1× SSC at 62°C, then dehydrated in graded ethanols and air-dried. Slides were then dipped in Kodak NTB emulsion (VWR International, West Chester, PA), air-dried, and stored at 4°C for 4 d. Slides were then developed, dehydrated in graded ethanols, cleared in Citrasol (VWR International), and coverslipped with Permaslip (Sigma, St. Louis, MO). Slides were analyzed with an automated image processing system by a person unaware of the treatment group of each slide. The system consists of a Scion VG5 video acquisition board (Perceptics, Knoxville, TN) attached to a Power Macintosh G5 (Apple Computers, San Jose, CA) computer running custom grain-counting software was used to count the number of cells and the number of silver grains over each cell (a semiquantitative index of mRNA content per cell) (Gottsch et al., 2004; Irwig et al., 2005; Smith et al., 2005). Cells were considered Kiss1 positive when the number of silver grains in a cluster exceeded that of background by threefold.
Experiment 7: sexually dimorphic SNB motoneurons in WT and GPR54 KO mice.
The spinal nucleus of the bulbocavernosus (SNB) is a sexually dimorphic population of motoneurons that innervates the perineal musculature. Adult males possess greater numbers of SNB motoneurons than adult females, and this sex difference is organized developmentally by perinatal androgens (Breedlove and Arnold, 1980; Wagner and Clemens, 1989; Forger, 2006). To determine whether GPR54 signaling is required for proper male-like development and sexual differentiation of spinal cord motoneurons, we analyzed the number of SNB cells in the spinal cords of adult WT and GPR54 KO males and females (n = 4–6 per group). All animals were gonadectomized and implanted with a T implant 4–6 weeks before being killed. After the animals were killed, spinal cords were rapidly dissected out and postfixed by immersion in 10% formalin for 48 h at 4°C. Fixed spinal tissue was then embedded in paraffin and sectioned on a sliding microtome at 10 μm. Cut spinal sections were mounted on slides and stained with cresyl violet (Winans and Powers, 1977). SNB motoneurons were counted bilaterally by an investigator blind to the genotype. Only those motoneurons in which the nucleus was visible were included in the counts. As reported previously in mice (Wagner and Clemens, 1989; Forger et al., 1997; Zuloaga et al., 2007), SNB motoneurons had a slightly more dispersed distribution than in rats, and many SNB cells extended ventrally and ventrolaterally along the gray-white border of the medial ventral horn.
Statistical analysis.
In each experiment, significant differences in group means were assessed via ANOVA, with post hoc analysis determined by Fisher's PLSD. The percentage of animals in each group that displayed sexual behavior (experiments 2 and 3) was compared by using a χ2 test. The sexual behavior of females in experiment 3 over the four behavior trials was analyzed with repeated-measures ANOVA. For all comparisons, statistical significance was set at p < 0.05.
Results
Somatic and reproductive anatomy of GPR54 KO mice
Gross morphological and anatomical analysis of GPR54 KO mice revealed a general phenotype similar to previously reported findings of impaired sexual maturation and diminished functioning of the neuroendocrine reproductive axis in other GPR54 KO mice (Funes et al., 2003; Seminara et al., 2003; Messager et al., 2005). We found that intact adult GPR54 KO male mice weighed less than WT males (p < 0.05), whereas mean body weights of adult GPR54 KO females did not differ from WT females (Table 1). Testes weights, seminal vesicle weights, and penis weights, as well as plasma LH concentrations, were dramatically lower in adult GPR54 KO males than in adult WT males (p < 0.01) (Table 1). Likewise, ovarian size was severely reduced in adult GPR54 KO females relative to WT females (p < 0.05) (Table 1). Anogenital distances, measured in mice on the day of birth (PND1) and at 3 weeks of age, were significantly smaller in females of both genotypes compared with males at each time point (Table 1). In males, anogenital distances were not significantly different between genotypes on PND1 but were significantly smaller in GPR54 KOs than WTs at 3 weeks of age (p < 0.01) (Table 1). Mean prepubertal body weights of all four groups did not significantly differ from each other at either PND1 or 3 weeks of age (Table 1).
Experiment 1: GnRH neurons and plasma LH in GPR54 KO mice do not respond to kisspeptin infusions
Kisspeptin had a strong inductive effect on the reproductive axis in adult WT males but not GPR54 KO males. Brains from WT males infused intracerebroventricularly with kisspeptin contained many GnRH neurons colabeled with Fos (Fig. 1 A). More than 85% of GnRH neurons of WT males contained nuclear Fos after kisspeptin treatment, whereas <1% of GnRH cells were Fos positive after vehicle treatment (p < 0.05) (Fig. 1 C). In contrast, virtually no GnRH neurons (< 2%) of GPR54 KO males contained nuclear Fos after kisspeptin treatment (Fig. 1 B). The percentage of GnRH neurons colabeling with Fos was not different between GPR54 KO males receiving kisspeptin or vehicle and was significantly lower in both of these groups compared with that of WT males receiving kisspeptin (p < 0.05) (Fig. 1 C). In WT males receiving kisspeptin, there were no regional differences in the induction of Fos in GnRH neurons present in the medial septum, diagonal band of Broca, and POA regions (∼86% for each region; data not shown). Corresponding with the high level of activation of GnRH neurons, WT males infused with kisspeptin had elevated plasma levels of LH compared with all other groups (p < 0.05 for all comparisons); in contrast, GPR54 KO males did not show any significant increase in LH after kisspeptin treatment (relative to vehicle) (Fig. 1 D).
Figure 1.
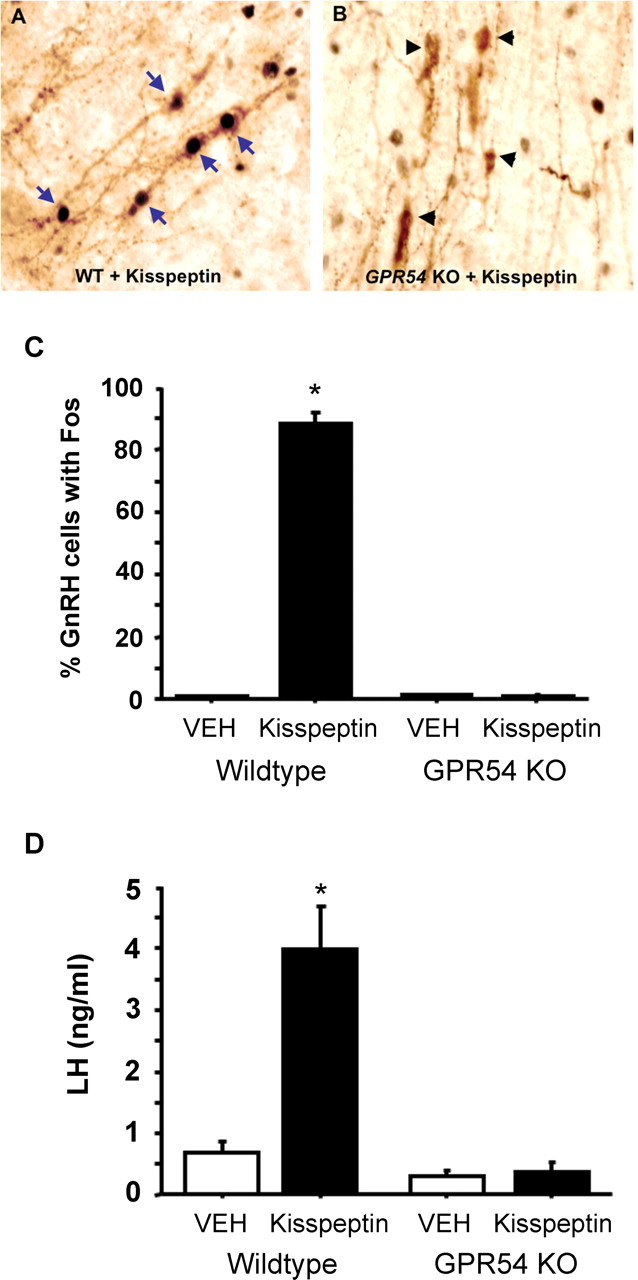
Representative photomicrographs of GnRH-immunoreactive neurons in the forebrain of gonad-intact WT (A) and GPR54 KO (B) male mice infused intracerebroventricularly with 1 nmol of kisspeptin. Fos immunoreactivity (labeled black) is visible in the nucleus of GnRH cells (labeled brown) of WT but not GPR54 KO mice. Blue arrows denote example GnRH neurons colabeled with Fos; black arrowheads denote GnRH neurons lacking Fos. C, Mean (±SEM) percentage of GnRH neurons colabeled with Fos in WT and GPR54 KO males treated with kisspeptin or vehicle (VEH). *p < 0.05, significantly different from vehicle-treated WT mice. D, Mean (±SEM) concentration of plasma LH in WT and GPR54 KO males 30 min after treatment with kisspeptin or vehicle (VEH). *p < 0.05, significantly different from vehicle-treated WT mice.
Experiment 2: testosterone-treated GPR54 KO males exhibit male copulatory behavior
Gonad-intact GPR54 KO males, unlike WT males, did not display male sexual behavior. Most gonad-intact WT males displayed mounts, thrusts, and intromissions, whereas none of the gonad-intact GPR54 KO males exhibited sexual behavior (p < 0.05) (Table 2). ∼40% of the intact WT males exhibited an ejaculation within the 45 min testing period whereas no gonad-intact GPR54 KO males ejaculated (p < 0.05). In contrast to gonad-intact animals, T-treated GPR54 KO males exhibited robust male sexual behavior. More than 80% of T-treated GPR54 KO males displayed mounting and thrusting, similar to the percentage of T-treated WT males displaying mounts with thrusts (Table 2). Similarly, the percentage of T-treated animals displaying intromissions was not different between the two genotypes (Table 2). The total number of thrusting bouts, but not intromission bouts, was higher in T-treated GPR54 KO than T-treated WT males (p < 0.01; Table 2), and the percentage of males ejaculating in the testing period was significantly lower in the KO than WT genotype (p < 0.05; Table 2). There were no significant differences between the two genotypes in the mean latencies to begin displaying mounts, thrusts, or intromissions (p > 0.45; Table 2).
Table 2.
Male sexual behavior of gonad-intact and T-treated WT and GPR54 KO mice
| % Males mounting and thrusting | % Malesintromitting | % Males ejaculating | Mean latency to begin mounting (min) | Mean number of thrusting bouts | Mean number of intromission bouts | |
|---|---|---|---|---|---|---|
| Gonad-intact | ||||||
| Wild-type | 80 | 70 | 40 | 10.7 ± 1.5 | 6.1 ± 1.2 | 9.9 ± 1.7 |
| GPR54 KO | 0* | 0* | 0* | na | na | na |
| T-treated | ||||||
| Wild-type | 71 | 71 | 64 | 7.1 ± 1.8 | 4.9 ± 1.0 | 9.7 ± 2.7 |
| GPR54 KO | 83 | 67 | 17* | 8.8 ± 1.6 | 15.7 ± 3.4* | 9.3 ± 2.2 |
All male mice were paired with a hormone-primed sexually receptive female for 45 min. Gonad-intact GPR54 KO males did not display any sexual behavior. In contrast, castrated T-treated GPR54 KO males displayed mounting, thrusting, intromissions, and in the case of two animals, an ejaculation. Animal numbers were 10 animals per group (gonad-intact) and 12–14 animals per group (T-treated). *p < 0.05, significantly different from similarly treated WT males.na, Not applicable because gonad-intact KO males did not display any sexual behavior.
Experiment 3: hormone-primed GPR54 KO mice exhibit normal female sexual behavior
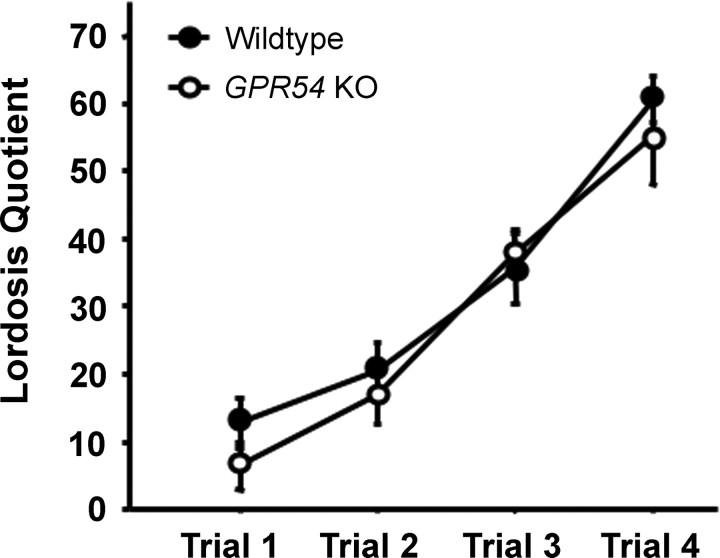
The majority of ovariectomized GPR54 KO and WT females that were primed with EB and P showed full female sexual behavior when tested with a stud male. The percentage of females exhibiting lordosis did not differ between genotypes (83% vs 78% of WT and KO females, respectively). The LQ of GPR54 KO and WT mice also did not significantly differ from each other over the course of four testing trials, with both genotypes showing LQ levels of ∼60 by the last trial (Fig. 2). The latency to begin showing lordosis on the last behavioral trial also did not differ between groups (data not shown).
Figure 2.
Mean (±SEM) LQs of adult female WT and GPR54 KO mice over four sexual behavior trials. All females were ovariectomized in adulthood and hormone primed with EB and P before testing with a stud male. There were no statistical differences in the degree of female sexual behavior displayed between the two genotypes.
Experiment 4: GPR54 KO males do not exhibit an olfactory partner preference for estrous females
Gonad-intact WT males displayed a significant olfactory partner preference for females over males, spending >70% of their investigatory time with female stimulus animals. In contrast, gonad-intact GPR54 KO males exhibited no preference for either sex, spending a similar proportion of their investigatory time with the male and female stimulus animals (48% vs 52%, respectively). The Olfactory Preference Index (a measure of preference for the female stimulus) was significantly lower in gonad-intact GPR54 KO males than WT males (12.9 ± 8.3 s vs 111.4 ± 21.7 s; p < 0.01).
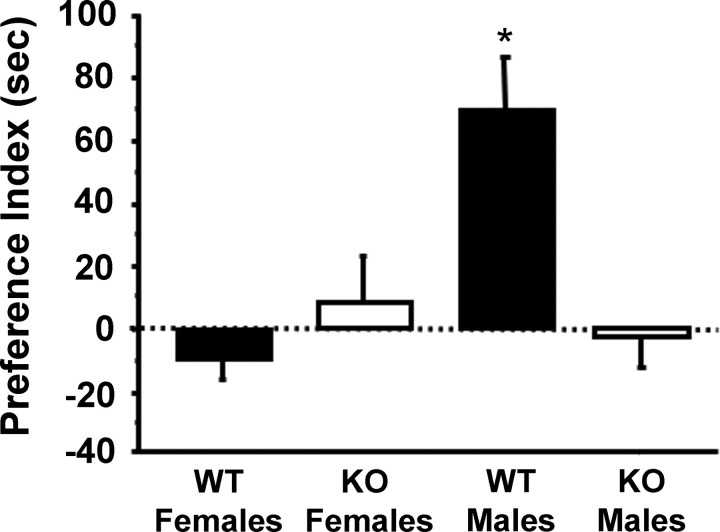
In contrast to male sexual behavior of GPR54 KO mice, which was reinstated by T treatment, olfactory partner preference of GPR54 KO males was not altered by T treatment. Similar to gonad-intact WT males, T-treated WT males exhibited a strong preference for females (Fig. 3). Conversely, T-treated GPR54 KO males, like T-treated WT and GPR54 KO females, showed no olfactory preference for either sex (Fig. 3). All three of these groups displayed Preference Indexes that were significantly lower than those of WT males (p < 0.05 for each comparison) (Fig. 3). Similarly, T-treated GPR54 KO males and females, along with T-treated WT females, spent approximately equal time (∼50%) investigating male and female stimuli, whereas T-treated WT males spent a significantly greater fraction of their total time (∼70%) investigating stimulus females (p < 0.05 for all groups compared with WT males).
Figure 3.
Mean (±SEM) Olfactory Preference Index of testosterone-treated WT and GPR54 KO males and females. Positive values of the Preference Index reflect an olfactory preference for stimulus females; negative values reflect a preference for stimulus males. No preference for either sex would produce a Preference Index of 0. Testosterone-treated WT males had a higher Preference Index than all of the other testosterone-treated groups; the Preference Indices of testosterone-treated WT females, KO females, and KO males did not differ from each other. *p < 0.05, significantly different from all other groups.
Experiment 5: the sexually dimorphic TH population in the AVPV nucleus is female-like in GPR54 KO males
The number of TH neurons in the AVPV nucleus is sexually dimorphic, with females typically possessing more TH cells than males in this region (Simerly, 1989). Using single-label ICC, we identified TH-IR neurons in the AVPV nucleus of T-treated WT and GPR54 KO male and female mice. WT female possessed significantly more TH-IR neurons than WT males (p < 0.001) (Fig. 4). GPR54 KO males also possessed significantly more TH-IR cells in the AVPV nucleus than WT males (p < 0.01) (Fig. 4). There was a trend for the number of TH-IR neurons to be lower in GPR54 KO males than WT females, but this did not reach statistical significance (p < 0.07) (Fig. 4). GPR54 KO females had TH-IR cell numbers similar to those of WT females and GPR54 KO males but significantly higher than WT males (p < 0.01) (Fig. 4).
Figure 4.
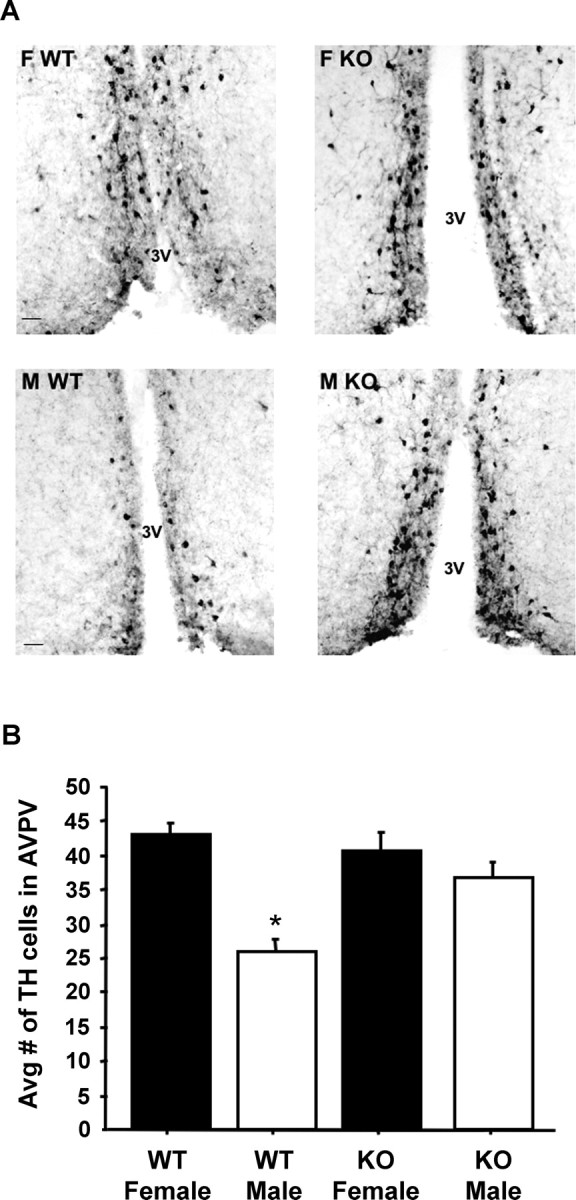
A, Representative photomicrographs of TH neurons in the AVPV nucleus of testosterone-treated WT and GPR54 KO mice of both sexes. F, Female; M, male; 3V, third ventricle. Scale bar, 50 μm. B, Mean (±SEM) number of TH neurons in the AVPV nucleus of testosterone-treated WT and GPR54 KO male and female mice. *p < 0.05, significantly different from all other groups.
Experiment 6: GPR54 KO males have more Kiss1 neurons in the AVPV nucleus than WT males
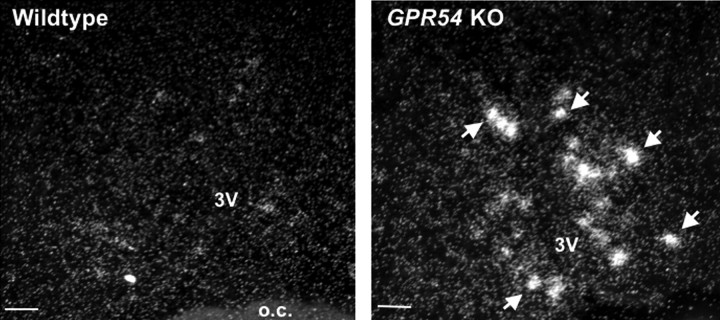
In rodents, levels of Kiss1 mRNA are sexually dimorphic in the AVPV nucleus, with females possessing many more Kiss1 neurons than males, regardless of adulthood steroid levels (Clarkson and Herbison, 2006; Kauffman et al., 2007). Using in situ hybridization, we found that adult GPR54 KO males treated with T had significantly greater numbers of Kiss1 neurons in the AVPV nucleus than did similarly treated WT males (26.0 ± 9.1 neurons vs 2.0 ± 2.0 neurons; p < 0.05) (Fig. 5). Likewise, T-treated GPR54 KO males had significantly higher levels of Kiss1 mRNA content per cell in the AVPV nucleus than WT males (119.3 ± 22.4 silver grains per cell vs 13.3 ± 13.0 silver grains per cell; p < 0.01) (Fig. 5). Castrated males of both genotypes contained virtually no Kiss1 neurons (data not shown), as expected from lack of steroid hormone stimulation. No group differences in Kiss1 neuron number were observed in WT and GPR54 KO males in the ARC (data not shown), a region in which Kiss1 is not sexually dimorphic in rodents (Kauffman et al., 2007).
Figure 5.
Photomicrographs of Kiss1 mRNA-containing neurons in the AVPV nucleus of representative testosterone-treated WT and GPR54 KO male mice. GPR54 KO males had significantly more Kiss1 neurons in the AVPV nucleus than WT males, as well as greater numbers of silver grains per Kiss1 cell (see Results for numerical values). White arrows denote sample Kiss1 neurons. 3V, Third ventricle; o.c., optic chiasm. Scale bar, 50 μm.
Experiment 7: male GPR54 KO mice exhibit female-like SNB motoneuron numbers
The number of SNB motoneurons is sexually dimorphic, with male rodents possessing more SNB cells than females in adulthood (Breedlove and Arnold, 1980; Forger et al., 1997; Park et al., 2002). Analysis of the SNB cell number in our mice indicated that T-treated WT males had significantly more SNB motoneurons than mice in any of the other groups (p < 0.05 for each group relative to WT males) (Fig. 6). T-treated GPR54 KO males had ∼50% fewer SNB neurons than WT males, similar in number to that of T-treated WT and GPR54 KO females (Fig. 6).
Figure 6.
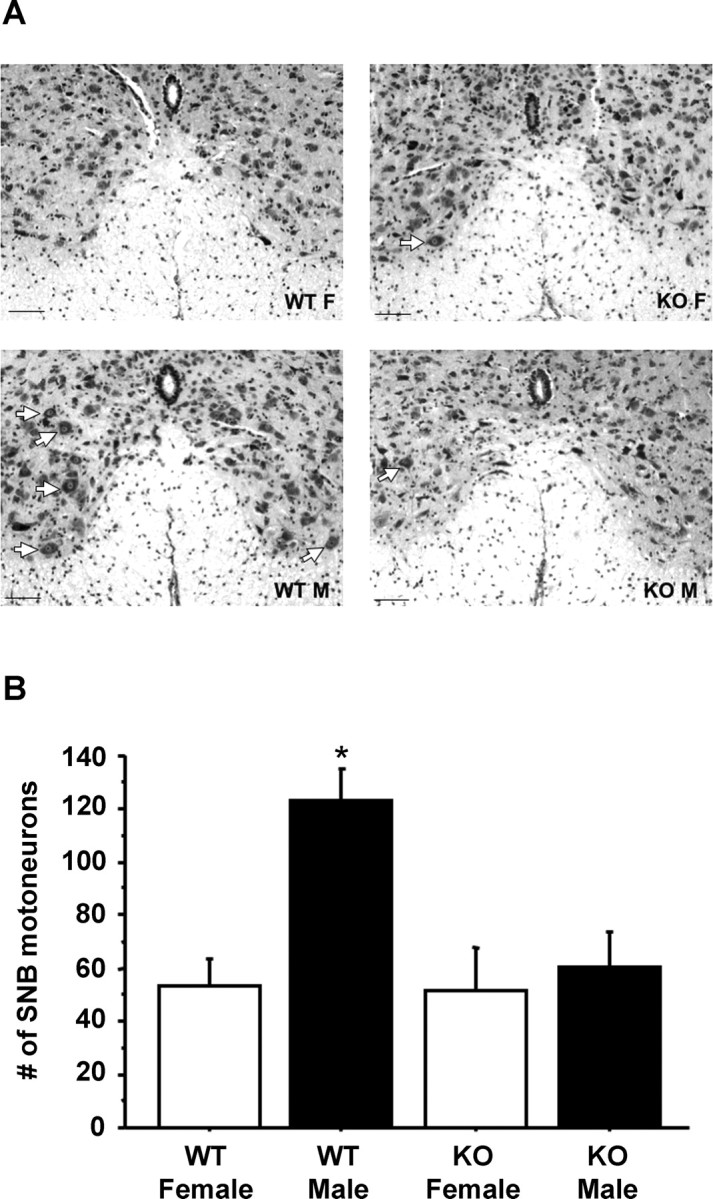
A, Photomicrographs of the ventral horn of the spinal cord of representative male (M) and female (F) WT and GPR54 KO mice. Photomicrographs were taken at 20× magnification. White arrows denote example SNB motoneurons, noticeable primarily in the WT males. Scale bar, 50 μm. B, Mean (±SEM) SNB motoneuron counts in adult WT and GPR54 KO mice of both sexes. *p < 0.01, significantly different from all other groups.
Discussion
The present study confirms previous findings of impaired reproduction in adult GPR54 KO mice (Seminara et al., 2003) but further indicates that the display of sexual behavior does not require intact kisspeptin–GPR54 signaling, provided steroid hormones are present in adulthood. Our findings also indicate that kisspeptin–GPR54 signaling is necessary for the proper differentiation of a number of sexually dimorphic phenotypes. Specifically, adult GPR54 KO males exhibit incomplete masculinization/defeminization of several sexually dimorphic measures, including olfactory sexual preference, TH neuron number and Kiss1 gene expression in the AVPV nucleus, and SNB motoneuron number in the spinal cord. Although adult GPR54 KO animals of both sexes have markedly diminished reproductive axis (present study and Funes et al., 2003; Seminara et al., 2003), the inability of GPR54 KO males to exhibit normal male-like parameters in the sexually dimorphic measures is not caused by a lack of activational adult testosterone, because all animals were testosterone-replaced before testing. Thus, the female-like phenotypes in brain and behavior of adult GPR54 KO males are likely attributable to impairments in the organizational actions of gonadal steroids during early development rather than their activational effects in adulthood. We conclude that kisspeptin–GPR54 signaling is necessary for proper gonadal steroid secretion (or action) during the perinatal critical period and hence for complete sexual differentiation.
Previous studies in mice and humans indicated that GPR54 signaling is critical for sexual maturation and reproduction (Funes et al., 2003; Seminara et al., 2003). Our results corroborate these previous findings of severe reductions in reproductive anatomical measures in both male and female GPR54 KO mice. In males, testes, seminal vesicle, and penis weights, along with plasma LH levels, were markedly lower in adult GPR54 KO mice compared with WT males. Likewise, in females, ovarian weights were significantly reduced in GPR54 KO animals. Anogenital distance (at 3 weeks of age) and adult body weights were also reduced in GPR54 KO males compared with WT males, likely reflecting diminished androgen production in the absence of kisspeptin–GPR54 signaling. Confirming the absence of functional GPR54 signaling in our mice, kisspeptin-treated GPR54 KO males did not display increased plasma LH or Fos induction in GnRH neurons as did WT males.
Although adult GPR54 KO mice lack sufficient gonadal hormones to activate mating behavior, their ability to display sexual behavior when provided with sex steroids had not been determined. In our study, gonad-intact GPR54 KO males did not exhibit sexual behavior when paired with hormone-primed females; however, male copulatory behavior (mounting, thrusting, etc.) was rescued with adult testosterone treatment. Although fewer GPR54 KO males ejaculated than WT males, this is likely attributable to incomplete penile development (micropenis) in the GPR54 KOs. During testing, GPR54 KO males often mixed complete intromissions with missed/partial intromissions (scored as thrusts), thereby reducing penile-vaginal contact and the likelihood of ejaculation. Like testosterone-treated GPR54 KO males, GPR54 KO females displayed normal sexual behavior (lordosis) when hormone-primed in adulthood. Thus, the mechanisms underlying sexual behavior are preserved and intact in the absence of developmental and adulthood GPR54 signaling, but these behaviors still require gonadal steroids in adulthood to be activated. Interestingly, preliminary data indicates that kisspeptin injections increase sexual behavior in female but not male mice (Keller and Bakker, 2006). Because mating is maximal in hormone-primed GPR54 KO females, kisspeptin–GPR54 signaling is apparently sufficient, but not necessary, for female sexual behavior. However, the stimulatory effects of kisspeptin injections on lordosis may be attributable to their stimulation of GnRH neurons, because GnRH itself can increase mating in female rodents (Sakuma and Pfaff, 1980; Kauffman and Rissman, 2004).
Mammalian mate selection and sexual partner preference are sexually dimorphic and influenced by social odor cues processed by the main and accessory olfactory systems (Vandenbergh, 2006). Both olfactory systems are sexually dimorphic and organized by developmental gonadal steroids (Guillamon and Segovia, 1997; Weruaga et al., 2001; Baum and Keverne, 2002; Bodo and Rissman, 2007). In our study, GPR54 KO males failed to display typical male-like olfactory partner preferences, despite testosterone treatment, suggesting an essential role for GPR54 signaling in the development and/or display of this sexually dimorphic behavior. Although volatile and nonvolatile cues can influence partner preference, our testing design does not discriminate between the involvement of each cue type; thus, it remains to be determined which olfactory system(s) is feminized/demasculinized in the absence of GPR54 signaling. However, the female-like preference behavior of GPR54 KO males was not caused by anosmia, because GPR54 KO and WT males performed similarly in a “hidden cookie test” (A. S. Kauffman, unpublished observations).
Sexual differentiation of the male brain occurs during critical developmental windows during which testosterone (or its metabolite estradiol) organizes sexually dimorphic neural populations. In the AVPV nucleus, both TH and Kiss1 are sexually differentiated in response to organizational effects of steroids, with females having more TH and Kiss1 neurons than males (Simerly, 1989; Kauffman et al., 2007). We observed altered sexual differentiation of these two forebrain systems in the absence of GPR54 signaling. GPR54 KO males had greater numbers of TH and Kiss1 neurons than WT males, indicating demasculinization and/or feminization of these neuronal systems. These neuroanatomical findings mirror our olfactory preference behavior data, supporting the conjecture that developmental kisspeptin–GPR54 signaling is required for proper male-like sexual differentiation. We note that the ability of testosterone-treated GPR54 KO males to display masculine copulatory behavior does not preclude their having undergone female-like sexual differentiation: WT female mice, without any prenatal hormone treatments, display “normal” male sexual behavior (mounting and thrusting) when given steroids in adulthood, as do aromatase KO and Tfm male mice (Wersinger and Rissman, 2000; Bakker et al., 2004; Sato et al., 2004; Bodo and Rissman, 2007). However, androgen receptor KO mice did not display high levels of male-typical mating behavior after E treatment (Sato et al., 2004), suggesting critical (and unknown) organizational and/or activational differences between Tfm and ARKO mice.
In rodents, the development and differentiation of SNB motoneurons and anogenital distance are, like olfactory preference, androgen dependent (Breedlove et al., 1982; Bodo and Rissman, 2007). In GPR54 KO males, both the number of SNB motoneurons (in adults) and anogenital distance (at 3 weeks of age) were significantly reduced relative to WT males, suggesting reduced androgen activity sometime in perinatal development. In mice, castration in adulthood moderately decreases SNB cell size and dendrite length (Zuloaga et al., 2007) and, in some strains, cell number (Wee and Clemens, 1987). However, differences in adult androgen levels cannot account for our genotypic differences in SNB number, because all animals were testosterone-replaced in adulthood for at least 4–6 weeks before SNB analysis. Thus, insufficient activational effects of androgens are unlikely to account for the female-like SNB pattern in GPR54 KO males. Instead, developmental GPR54 signaling is likely required for proper male-like differentiation of SNB motoneurons.
In mice, there are two well established periods of androgen exposure experienced by perinatal males: one occurs during the last few days of gestation and another on PND1 (Pang and Tang, 1984; Motelica-Heino et al., 1988; Corbier et al., 1992). Gestational androgen secretion appears to be intact in GPR54 KO males, as indicated by the presence of male genitalia and accessory sex organs, along with male-like anogenital distance on PND1 (which are all androgen dependent). In contrast, genotypic differences in anogenital distance by day 21 suggest that postnatal androgen secretion, or its actions, is deficient in GPR54 KO males. Likewise, the lack of masculinization (and/or defeminization) of the AVPV Kiss1 and TH systems, SNB motoneurons, and behavioral partner preference implies that GPR54 KO males lack sufficient androgen exposure during early postnatal life.
The mechanism of kisspeptin–GPR54 signaling in the postnatal differentiation process is unclear. Given the established role of GPR54 in controlling GnRH secretion in adulthood, it seems most likely that neural kisspeptin–GPR54 signaling is required for GnRH-mediated androgen secretion that occurs in males in early postnatal development; however, we cannot rule out a role for kisspeptin–GPR54 signaling “downstream” of androgen secretion at the level of developing neurons. We also note that GPR54 is also expressed in the testis (Kotani et al., 2001; Ohtaki et al., 2001); it is therefore conceivable that postnatal androgen production depends on peripheral GPR54 signaling in the testis, independent of the central regulation of GnRH secretion by GPR54.
In normal males, perinatal gonadal steroids alter the development of sexually dimorphic neural circuitry, producing adult neural circuits (and their physiological/behavioral outputs) that are both masculinized and defeminized. Evidence suggests that the masculinization and defeminization processes, although complementary, are mediated by independent hormonal and neural mechanisms (Kudwa et al., 2005; Todd et al., 2005). It is unknown whether the female-like sexual differentiation of brain and behavior observed in GPR54 KO males represents a complete sex reversal of sexually dimorphic phenotypes (impaired masculinization and defeminization) or only a partial effect (impaired masculinization but not defeminization, or vice versa). Last, although GPR54 signaling is critical for puberty and fertility in humans, its role in human development and sexual differentiation of the brain/behavior is unclear, especially because most male patients with GPR54 mutations are not evaluated until puberty. Recently, however, one infant carrying a mutated GPR54 was identified at birth with a micropenis and cryptorchidism, suggesting reduced androgen exposure during development (Semple et al., 2005). Additional clinical studies will be important for determining whether developmental GPR54 signaling is required for proper differentiation of sexually dimorphic phenotypes in humans, as is the case in mice.
Footnotes
This work was supported by National Institute of Mental Health Grants F32 MH070084 (A.S.K.) and RO1 MH57759 (E.F.R.) and National Institutes of Health (NIH) Grants R01 HD27142 (R.A.S.), T32 HD07382 (J.H.P.), and T32 DK07646 (A.A.M.). This work was also supported by the NIH–National Institute of Child Health and Human Development through cooperative agreements U54 HD28934 (University of Virginia Histology and Ligand Assay Cores) and U54 HD12629 (University of Washington) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. We thank Jessie Gatewood, Savera Shetty, Hallie Gardener, and Aileen Wills for excellent laboratory assistance. We also thank Heather Dungan and Dr. Victor Navarro for helpful comments on this manuscript.
References
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Szpirer J, Szpirer C, Balthazart J. Exposure to oestrogen prenatally does not interfere with the normal female-typical development of odour preferences. J Neuroendocrinol. 2007;19:329–334. doi: 10.1111/j.1365-2826.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–181. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG, Howell ML, Bengston L, MacKenzie L, DeChiara TM, Yancopoulos GD. Sexual dimorphism in the spinal cord is absent in mice lacking the ciliary neurotrophic factor receptor. J Neurosci. 1997;17:9605–9612. doi: 10.1523/JNEUROSCI.17-24-09605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. New York: Academic; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44:377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2005;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Rissman EF. A critical role for the evolutionarily conserved gonadotropin-releasing hormone II: mediation of energy status and female sexual behavior. Endocrinology. 2004;145:3639–3646. doi: 10.1210/en.2004-0148. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Buenzle J, Fraley GS, Rissman EF. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav. 2005;48:141–151. doi: 10.1016/j.yhbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Keller M, Bakker J. Kisspeptin-10 stimulates female sexual behavior in mice. Soc Neurosci Abstr. 2006;32:259–10. [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Krasnow SM, Hohmann JG, Gragerov A, Clifton DK, Steiner RA. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology. 2004;79:268–277. doi: 10.1159/000079632. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci USA. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O'Dowd BF. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelica-Heino I, Castanier M, Corbier P, Edwards DA, Roffi J. Testosterone levels in plasma and testes of neonatal mice. J Steroid Biochem. 1988;31:283–286. doi: 10.1016/0022-4731(88)90351-2. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Pang SF, Tang F. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. J Endocrinol. 1984;100:7–11. doi: 10.1677/joe.0.1000007. [DOI] [PubMed] [Google Scholar]
- Park JJ, Zup SL, Verhovshek T, Sengelaub DR, Forger NG. Castration reduces motoneuron soma size but not dendritic length in the spinal nucleus of the bulbocavernosus of wild-type and BCL-2 overexpressing mice. J Neurobiol. 2002;53:403–412. doi: 10.1002/neu.10103. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerell AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. LH-RH in the mesencephalic central grey can potentiate lordosis reflex of female rats. Nature. 1980;283:566–567. doi: 10.1038/283566a0. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor alpha. Behav Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Pheromones and mammalian reproduction. In: Neill JD, editor. Knobil and Neill's physiology of reproduction. Ed 3. Boston: Elsevier; 2006. pp. 2041–2058. [Google Scholar]
- Wagner CK, Clemens LG. Anatomical organization of the sexually dimorphic perineal neuromuscular system in the house mouse. Brain Res. 1989;499:93–100. doi: 10.1016/0006-8993(89)91138-4. [DOI] [PubMed] [Google Scholar]
- Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424:305–310. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Dopamine activates masculine sexual behavior independent of the estrogen receptor α. J Neurosci. 2000;20:4248–4254. doi: 10.1523/JNEUROSCI.20-11-04248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weruaga E, Brinon JG, Porteros A, Arevalo R, Aijon J, Alonso JR. A sexually dimorphic group of atypical glomeruli in the mouse olfactory bulb. Chem Senses. 2001;26:7–15. doi: 10.1093/chemse/26.1.7. [DOI] [PubMed] [Google Scholar]
- Winans SS, Powers JB. Olfactory and vomeronasal deafferentation of male hamsters: histological and behavioral analyses. Brain Res. 1977;126:325–344. doi: 10.1016/0006-8993(77)90729-6. [DOI] [PubMed] [Google Scholar]
- Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, Wu LJ, Toyoda H, Zhao MG, Rohde AD, Gragerova G, Onrust R, Bergmann JE, Zhuo M, Gaitanaris GA. Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol. 2006;26:9352–9363. doi: 10.1128/MCB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Monks DA, Breedlove SM, Jordan CL. Androgen-sensitivity of somata and dendrites of spinal nucleus of the bulbocavernosus (SNB) motoneurons in male C57BL6J mice. Horm Behav. 2007;51:207–212. doi: 10.1016/j.yhbeh.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]