Abstract
An increasing number of studies indicate that leptin can regulate the activity of the mesolimbic dopamine system. The objective of this study was to examine the regulation of the activity of dopamine neurons by leptin. This was accomplished by examining the dopamine D2 receptor-mediated synaptic current that resulted from somatodendritic release of dopamine in brain slices taken from mice that lacked leptin (Lepob/ob mice). Under control conditions, the amplitude and kinetics of the IPSC in wild-type and Lepob/ob mice were not different. However, in the presence of forskolin or cocaine, the facilitation of the dopamine IPSC was significantly reduced in Lepob/ob mice. The application of l-3,4-dihydroxyphenylalanine (l-DOPA) increased the IPSC in Lepob/ob mice significantly more than in wild-type animals and fully restored the responses to both forskolin and cocaine. Treatment of Lepob/ob mice with leptin in vivo fully restored the cocaine-induced increase in the IPSC to wild-type levels. These results suggest that there is a decrease in the content of somatodendritic vesicular dopamine in the Lepob/ob mice. The release of dopamine from terminals may be less affected in the Lepob/ob mice, because the cocaine-induced rise in dopamine in the ventral striatum was not statistically different between wild-type and Lepob/ob mice. In addition, the relative increase in cocaine-induced locomotion was similar for wild-type and Lepob/ob mice. These results indicate that, although basal release is not altered, the amount of dopamine that can be released is reduced in Lepob/ob mice.
Keywords: dopamine, cocaine, Lepob/ob, leptin, obesity, locomotion
Introduction
The mesocorticolimbic dopamine (DA) system is involved in numerous physiological processes and disorders, including addiction, feeding, and motor function. A role for dopamine in the acquisition of natural rewards such as food, water, and sex is well established (Wise, 1996; Berridge and Robinson, 1998; Schultz, 1998; Kelley and Berridge, 2002; Levine et al., 2003). In addition to its role in signaling food reward, dopamine has also been implicated in the regulation of basal feeding, as dopamine receptor agonists and antagonists affect feeding (Phillips and Nikaido, 1975; Wise et al., 1978; Ettenberg and Camp, 1986), and dopamine-deficient mice become aphagic and will die without either forced feeding or daily administration of l-3,4-dihydroxyphenylalanine (l-DOPA) (Zhou and Palmiter, 1995; Szczypka et al., 2001). Furthermore, the anorectic effects of amphetamine are lost in dopamine-deficient mice, suggesting that amphetamine induced suppression of feeding is mediated through its effects on dopamine release (Cannon et al., 2004).
Leptin is an adipocyte-derived protein that was first identified as the product of the obese gene (Zhang et al., 1994). Mice lacking leptin (Lepob/ob) or its receptor are grossly obese, hyperphagic, and hypometabolic and show decreased activity (Coleman, 1978), and leptin administration to Lepob/ob or wild-type mice (WT) suppresses feeding and decreases body weight (Campfield et al., 1995; Halaas et al., 1995; Pelleymounter et al., 1995; Friedman and Halaas, 1998; Seeley and Woods, 2003). Leptin receptors are widely expressed in the CNS, with significant expression in hypothalamic and hindbrain nuclei (Mercer et al., 1996; Schwartz et al., 1996; Fei et al., 1997; Elmquist et al., 1998; Figlewicz et al., 2003; Hommel et al., 2006). Although leptin is known to affect hypothalamic and brainstem centers, it is clear that leptin acts at other central sites as well, including the mesocorticolimbic dopamine system. Leptin receptors have been identified in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) (Figlewicz et al., 2003; Hommel et al., 2006), and leptin regulates the expression of dopamine transporters (DATs) (Figlewicz et al., 1998). In addition, injection of leptin decreased both the basal and feeding-evoked levels of dopamine in the shell of the nucleus accumbens (NAc) (Krugel et al., 2003). Furthermore, leptin suppressed the rewarding effects of lateral hypothalamic self-stimulation (Fulton et al., 2000), reversed the conditioned place preference of rats to both sucrose and high-fat foods (Figlewicz et al., 2001, 2004), and blocked relapse to heroin seeking induced by food restriction (Shalev et al., 2001). Thus, leptin plays a clear role in the regulation of the mesocorticolimbic dopamine system.
This study examined the differences in the regulation of dopamine neurons in wild-type and Lepob/ob mice. The primary measure used was a dopamine, D2 receptor (D2R)-mediated synaptic current that resulted from the somatodendritic release of dopamine within the VTA and SNc (Beckstead et al., 2004). This D2R-mediated IPSC is calcium dependent, blocked by vesicle depletion, and enhanced by cocaine and l-DOPA, suggesting that somatodendritic dopamine release is vesicular in nature (Beckstead et al., 2004). The results of these studies suggest that dopamine function in Lepob/ob mice is relatively normal under basal conditions, but leptin deficiency may reduce dopamine-dependent inhibition when demand is increased.
Materials and Methods
Reagents.
Cocaine hydrochloride was obtained from the Research Triangle Institute under the National Institutes on Drug Abuse drug supply program. CGP56999a was a generous gift from Novartis Pharmaceuticals (Basel, Switzerland). Leptin was obtained from the National Hormone and Peptide Program (Torrance, CA) through the National Institute of Diabetes and Digestive and Kidney Diseases. The Alzet mini-osmotic pumps were obtained from Durect (Cupertino, CA). PBS was obtained from Mediatech (Herndon, VA). All other reagents were obtained from Sigma (St. Louis, MO).
Animals.
Wild-type C57Bl/6J and Lepob/ob mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or were bred on site. Animals were between the ages of 6 and 14 weeks for all experiments. All experiments were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University.
Slice preparation and electrophysiology.
Mice were deeply anesthetized with halothane before decapitation and removal of the entire brain. The brain was immediately submerged in ice-cold, carbogen (95% O2/5% CO2)-saturated artificial CSF (aCSF), and a brain block containing the ventral midbrain was made. The aCSF contained the following (in mm): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 21.4 NaHCO3, 11.1 glucose, and 0.4 ascorbic acid. Pseudohorizontal sections (220 μm) were cut with a Leica VT1000S vibratome, and the slices were incubated in aCSF containing 10 μm MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate] at 35°C for ∼30 min before transfer to the recording chamber. The slices were perfused with oxygen-saturated aCSF at a flow rate of ∼1.7 ml/min. Dopamine neurons were visualized using infrared optics and were identified by their location relative to the medial terminal nucleus of the accessory optic tract. Dopamine neurons were identified physiologically by the presence of spontaneous pacemaker firing (1–5 Hz), large hyperpolarization activated cation (Ih) currents, and sensitivity to dopamine. Recordings were made using a potassium methylsulfate (KMeSO4)-based solution containing the following (in mm): 115 KMeSO4, 10 NaCl, 1 MgCl2, 10 HEPES, 10 BAPTA (tetraK salt), 2 (Mg)ATP, and 0.3 (Na)GTP. Electrodes had resistances of ∼2.0–3.0 MΩ, and series resistance values were ∼5–20 MΩ. Trials were excluded if series resistance increased significantly during the experiment. The D2R IPSC was isolated pharmacologically by the inclusion of the antagonists of other evoked synaptic currents [AMPA receptors (DNQX, 10 μm), GABAA receptors (picrotoxin, 100 μm), and GABAB receptors (CGP56999a, 100 nm)] in the bath solution. Recordings were made using an Axopatch-1D amplifier and Axograph software. The D2R IPSC was evoked using a platinum bipolar stimulating electrode placed caudolaterally to the cells, and D2R IPSCs were evoked by injecting five stimuli (∼200 μA) at 20 Hz. Dopamine was applied iontophoretically through a ∼75–100 MΩ glass pipette held with negative backing current, and dopamine was ejected using a 5 nA pulse lasting 25 ms. Dopamine iontophoresis was used to isolate the postsynaptic dopamine response independent of dopamine release.
For the experiments examining paired-pulse depression of the D2R IPSC and the dopamine iontophoretic response, IPSCs and dopamine iontophoretic currents were elicited by the aforementioned methods. A total of five D2R IPSCs were stimulated either 5 or 7.5 s apart, followed by evoking five dopamine iontophoretic currents at the same interval as the D2R IPSC (5 or 7.5 s). The paired-pulse D2R IPSC followed by the paired-pulse dopamine iontophoretic currents were evoked three times for each cell, and the average was calculated for each cell.
Leptin treatment.
Leptin or PBS was administered to Lepob/ob mice via subcutaneous mini-osmotic pumps (Alzet model 2002). Mice were individually housed for 1–2 weeks before the experiment, and food intake and body weight were measured daily between 8:00 and 9:00 A.M. throughout the experiment. The Alzet mini-osmotic pumps were filled with either PBS or leptin (500 ng/μl) and were incubated overnight at 37°C in sterile saline before implantation. The mice were anesthetized with either isoflurane or 2,2,2-tribromoethanol (2%) followed by subcutaneous implantation of the pump, which was left in for 8 d. The flow rate of the mini-osmotic pumps was 0.5 μl/h, leading to the release of 250 ng leptin/h (6 μg/d). At the end of the treatment, the mice were killed, trunk blood was collected, and brain slices were prepared for electrophysiology.
Microdialysis measurement of dopamine in vivo.
For surgery, mice were anesthetized with intraperitoneal 2,2,2-tribromoethanol (2%). A microdialysis guide shaft made of 21-gauge stainless steel was placed 3 mm above the ventral striatum using the following coordinates: anteroposterior, 1.1 mm; lateral, 0.8 mm; and ventral, 1.5 mm, with reference to bregma, midsagittal sinus, and surface of leveled skull, respectively. Microdialysis probes were inserted 1 week later and were constructed to extend 3 mm beyond the guide shaft and thus lodge in the medial posterior NAc. Microdialysis probes were constructed of concentric 26 gauge stainless steel and fused silica capillary tubing with a tip of cellulose membrane 1 mm long. Probes were implanted into the NAc at least 18 h before experimentation and were perfused with a modified Ringer's solution (142 mm NaCl, 3.9 mm KCl, 1.2 mm CaCl2, 1.0 mm MgCl2, 1.35 mm Na2HPO4, 0.3 mm NaH2PO4, pH 7.3) at a flow rate of 1.0 μl/min. Samples were collected every 15 min for 60 min before and after intraperitoneal injection of cocaine. Dialysates were analyzed for DA by reverse-phase HPLC with electrochemical detection (BAS Epsilon amperometric detector). DA was separated on a phase II column (Brownlee; PerkinElmer, Waltham, MA) with 3.2 mm bore and 3 μm, C-18 packing. The mobile phase contained 100 mm monochloracetic acid, 500 μm EDTA, 600 μm octanesulfonic acid, and 8.4% v/v acetonitrile, pH 3.4. The mobile phase flow rate was 0.9 ml/min.
Confirmation of probe placement.
After microdialysis, brains were prepared to confirm placement of the microdialysis probe. Animals were overdosed with halothane anesthesia. The brains were removed and transferred to a solution containing 20% sucrose and 10% formalin in PBS for a minimum of 7 d followed by emersion in 30% sucrose in PBS for 48 h before slicing. Slices (30 μm) were cut on a Leica cryostat and placed on poly-l-lysine-coated coverslips and were dried for a minimum of 1 week. Slices were then stained with thionin (1% in dH2O), and the probe placements were confirmed. Locations of the probe placements are indicated by asterisks in supplemental Figure 1 (available at www.jneurosci.org as supplemental material).
Locomotor assays.
The age of the mice used in locomotor assays was limited to adult mice 11–14 weeks of age. Locomotor activity was monitored using Med Associates locomotor cages (Med Associates, Georgia, VT) and Activity Monitor software. Horizontal distance was measured by the sequential breaking of infrared beams. Mice were acclimated to the test procedure for 3 d, followed by alternate testing on days 4 and 5. For the acclimatization, mice were injected with saline, and activity was monitored for 60 min. On the test days, one half of the wild-type and one half of the Lepob/ob mice were injected with cocaine on day 4 and saline on day 5, whereas the other half were injected with saline on day 4 and cocaine on day 5. Therefore, half of the animals received saline followed by cocaine, and the other half received cocaine first, followed by saline. Activity was monitored for 60 min and was collected in 5 min bins. All solutions were administered by intraperitoneal injections in a volume of 10 ml/kg.
Data analysis.
The electrophysiology data were analyzed using Axograph, Excel (Microsoft, Redmond, CA), and IgorPro software. Student's t tests or ANOVA was used to determine statistically significant differences. Analysis of the cocaine-induced locomotion was performed by the Oregon Health & Science University Center for Biostatistics, Computing, and Informatics.
Results
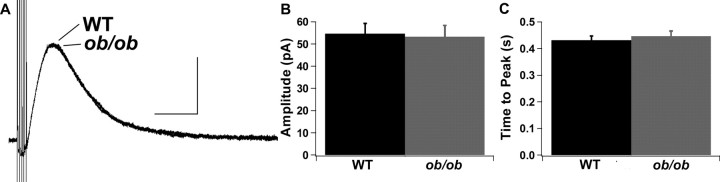
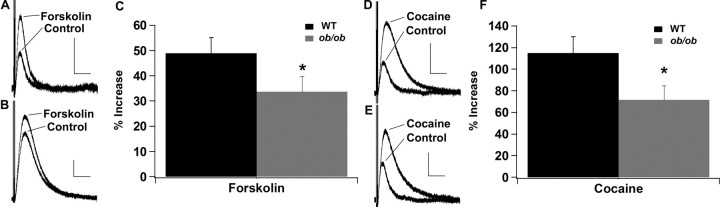
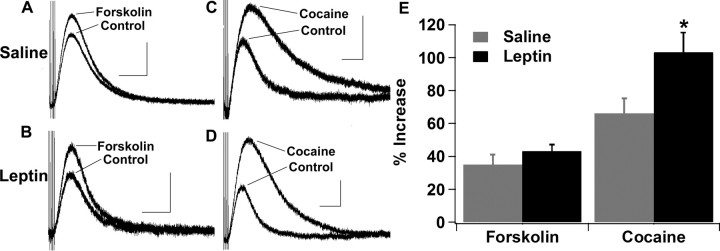
To determine whether leptin can regulate the activity of dopamine neurons in the VTA and SNc, the D2R IPSC was examined in wild-type and leptin-deficient Lepob/ob mice. When baseline D2R IPSCs from wild-type and Lepob/ob mice were averaged, there was no difference in the basal D2R IPSC between wild-type and Lepob/ob mice for either the mean amplitude or time to peak of the D2R IPSC (amplitude: WT, 54.7 ± 4.6 pA; Lepob/ob, 53.3 ± 5.0 pA; time to peak: WT, 0.43 ± 0.02 s; Lepob/ob, 0.45 ± 0.02 s) (Fig. 1). However, when different treatments were used to increase the size of the D2R IPSC, it became evident that the D2R IPSCs were not identical between wild-type and Lepob/ob mice (Figs. 2, 3). Application of forskolin (10 μm), which increases the D2R IPSC by increasing the probability of vesicle release, increased the amplitude of the D2R IPSC in both wild-type and Lepob/ob mice (Fig. 2A–C). However, the extent of the increase induced by forskolin was significantly reduced in slices from Lepob/ob mice (Fig. 2A–C). Similarly, the acute application of cocaine (1 μm), which increases the amplitude of the D2R IPSC through the inhibition of dopamine reuptake, increased the amplitude of the D2R IPSC in slices from both wild-type and Lepob/ob mice (Fig. 2D–F). The extent of the increase induced by cocaine was also significantly reduced in Lepob/ob mice (Fig. 2D–F). Thus, although the baseline D2R IPSC was not altered in Lepob/ob mice, under conditions in which dopamine release is increased, the enhancement of the D2R IPSC was smaller in the Lepob/ob mice.
Figure 1.
There is no difference in the basal D2R IPSC between wild-type and Lepob/ob mice. A, Average baseline D2R IPSCs from wild-type (n = 28) and Lepob/ob (n = 28) mice. Calibration: 30 pA, 0.5 s. B, Mean amplitude of baseline D2R IPSCs from wild-type and Lepob/ob mice. C, Mean time to peak of baseline D2R IPSCs from wild-type and Lepob/ob mice.
Figure 2.
The increase in the D2R IPSC elicited by forskolin and cocaine is significantly reduced in Lepob/ob mice. A, Sample D2R IPSCs from wild-type mice in response to forskolin (10 μm). B, Sample D2R IPSCs from Lepob/ob mice in response to forskolin. C, Mean increase in the D2R IPSC after forskolin treatment (WT, n = 15; Lepob/ob, n = 13; *p < 0.05). D, Sample D2R IPSCs from wild-type mice in response to cocaine (1 μm). E, Sample D2R IPSCs from Lepob/ob mice in response to cocaine. F, Mean increase in the D2R IPSC after cocaine treatment (WT, n = 19; Lepob/ob, n = 15; *p < 0.05). Calibration: 50 pA, 1 s.
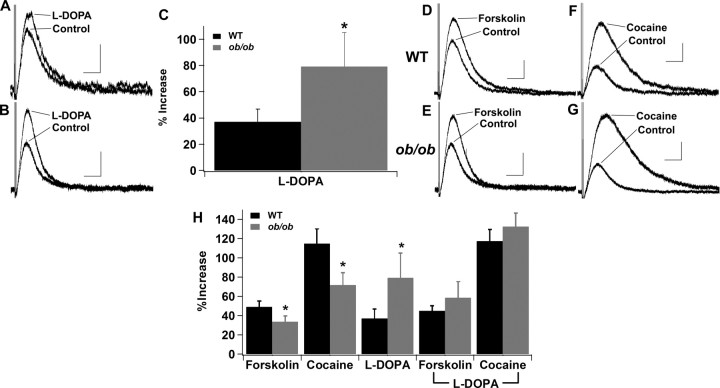
Figure 3.
l-DOPA has a significantly larger effect on the D2R IPSC in Lepob/ob mice and fully restores the response to cocaine. A, Sample D2R IPSCs from wild-type mice in response to l-DOPA (10 μm). B, Sample D2R IPSCs from Lepob/ob mice in response to l-DOPA. C, Mean increase in the D2R IPSC in response to l-DOPA (WT, n = 14; Lepob/ob, n = 9; *p < 0.05). D–H, Effects of forskolin (10 μm) and cocaine (1 μm) on the D2R IPSC after pretreatment with l-DOPA (10 μm). D, Sample D2R IPSCs from wild-type mice in response to forskolin after pretreatment with l-DOPA. E, Sample D2R IPSCs from Lepob/ob mice in response to forskolin after pretreatment with l-DOPA. F, Sample D2R IPSCs from wild-type mice in response to cocaine after pretreatment with l-DOPA. G, Sample D2R IPSCs from Lepob/ob mice in response to cocaine after pretreatment with l-DOPA. H, Mean increase in the D2R IPSC in response to each of the treatments described in this figure and in Figure 2 (l-DOPA–forskolin: WT, n = 13, Lepob/ob, n = 7; l-DOPA–cocaine: WT, n = 12, Lepob/ob, n = 7; *p < 0.05). Calibration: 50 pA, 0.5 s.
The D2R IPSC is increased in wild-type animals with the application of the dopamine precursor l-DOPA (Beckstead et al., 2004). This result suggests that the vesicular stores of dopamine can be increased to facilitate the amplitude of the D2R IPSC. Here, slices from wild-type and Lepob/ob mice were treated with l-DOPA, and the increase in amplitude of the IPSC was compared. l-DOPA (10 μm) increased the amplitude of the D2R IPSC in slices from both wild-type and Lepob/ob mice (Fig. 3A–C). However, unlike the results obtained with forskolin and cocaine, treatment with l-DOPA resulted in a significantly larger increase in the D2R IPSC in slices from Lepob/ob mice. Furthermore, after bath application of l-DOPA, application of forskolin (Fig. 3D,E,H) and cocaine (Fig. 3F–H) led to increases in the D2R IPSC in Lepob/ob mice that were identical to those observed in slices from wild-type mice (compare values in Fig. 3H). These data suggest that the amount of dopamine packaged per vesicle was reduced in Lepob/ob mice, and that increasing the amount of dopamine per vesicle with l-DOPA treatment led to equal responsiveness of the D2R IPSC in wild-type and Lepob/ob mice.
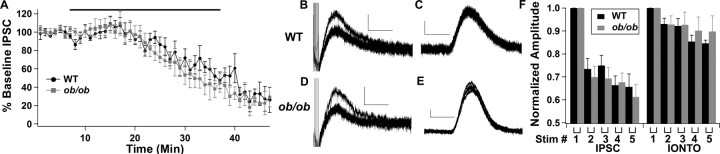
Although these results suggest that there was a reduced vesicular dopamine content, it is also possible that deficits in the vesicle pool size or probability of vesicle release in Lepob/ob mice may contribute to the alterations in the D2R IPSC. To examine vesicle pool size, the vesicular monoamine transporter inhibitor, reserpine, was used to deplete dopamine from vesicles. If there were differences in the vesicle pool size between wild-type and Lepob/ob mice, the rate of decrease in the D2R IPSC may differ as vesicles are depleted. Perfusion with reserpine (1 μm) led to a progressive decrease in the amplitude of the D2R IPSC at a rate that was similar for slices from wild-type and Lepob/ob mice (Fig. 4A), suggesting that there was no difference in the vesicle pool size between wild-type and Lepob/ob mice. Potential differences in the probability of release of dopamine between wild-type and Lepob/ob mice were tested next by evoking repeated D2R IPSCs. It was necessary to separate the stimuli by 5 or 7.5 s so that the IPSC would decline to baseline before the next IPSC. With this protocol, the second IPSC was smaller than the initial IPSC in both wild-type and Lepob/ob mice, with little further reduction observed on subsequent stimulation (Fig. 4). The amount of depression observed was not different between slices from wild-type and Lepob/ob mice at either the 5 s (Fig. 5B–F) or 7.5 s (data not shown) intervals. In addition, repeated iontophoretic applications of dopamine were used to control for postsynaptic desensitization, and there was no difference in the small amount of desensitization observed with the repeated iontophoretic applications of dopamine (Fig. 4C,E,F). Thus, it appears that there was no difference in the probability of somatodendritic release of dopamine within the VTA/SNc between wild-type and Lepob/ob mice.
Figure 4.
The alterations in the D2R IPSC in Lepob/ob mice are not attributable to decreased vesicle pool size or altered probability of release. A, Perfusion of reserpine (1 μm) reduced the D2R IPSC equally in wild-type and Lepob/ob mice. The black bar denotes the time of perfusion of reserpine (WT, n = 5; Lepob/ob, n = 5). B–F, The D2R IPSCs from wild-type and Lepob/ob mice show similar levels of paired-pulse depression. B, Sample traces of five repeated D2R IPSCs from wild-type mice. C, Sample traces of five repeated dopamine iontophoretic currents from wild-type mice. D, Sample traces of five repeated D2R IPSCs from Lepob/ob mice. E, Sample traces of five dopamine iontophoretic currents from Lepob/ob mice. F, Mean amplitude of the D2R IPSCs and dopamine iontophoretic currents during the repeated pulses (WT, n = 5; Lepob/ob, n = 6). Calibration: 20 pA, 1 s.
Figure 5.
Treatment of Lepob/ob mice with leptin in vivo restores the full response of the D2R IPSC to cocaine. A, Sample traces of the effects of forskolin on the D2R IPSC from Lepob/ob mice treated with saline in vivo. B, Sample traces of the effects of forskolin on the D2R IPSC from Lepob/ob mice treated with leptin in vivo. C, Sample traces of the effects of cocaine on the D2R IPSC from Lepob/ob mice treated with saline in vivo. D, Sample traces of the effects of cocaine on the D2R IPSC from Lepob/ob mice treated with leptin in vivo. E, Mean increase in the D2R IPSC after treatment with forskolin and cocaine in Lepob/ob mice treated in vivo with saline or leptin (saline–forskolin, n = 17; leptin–forskolin, n = 14; saline–cocaine, n = 18; leptin–cocaine, n = 14). Calibration: 50 pA, 0.5 s.
Treatment of Lepob/ob mice with leptin in vivo suppresses food intake, decreases body weight, and reverses many of the defects resulting from leptin deficiency (Campfield et al., 1995; Halaas et al., 1995; Pelleymounter et al., 1995). To determine whether the alterations in the somatodendritic release of dopamine within the VTA/SNc observed in Lepob/ob mice could be reversed by leptin restoration, Lepob/ob mice were treated with leptin (250 ng/h) or saline for 8 d through subcutaneous mini-osmotic pumps, and the effects of forskolin and cocaine on the D2R IPSC were examined. Leptin administration suppressed feeding and decreased body weight over the course of the treatment period (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Although forskolin (1 μm) treatment led to a small, nonsignificant enhancement of the D2R IPSC in leptin-treated Lepob/ob mice compared with saline-treated Lepob/ob mice (Fig. 5A,B,E), the cocaine-induced increase in the D2R IPSC from leptin-treated Lepob/ob mice was significantly enhanced (Fig. 5C–E) and was nearly restored to wild-type levels (compare with Figs. 2, 3). Thus, it appears that in vivo leptin treatment can reverse the alterations in the local release of dopamine within the VTA/SNc.
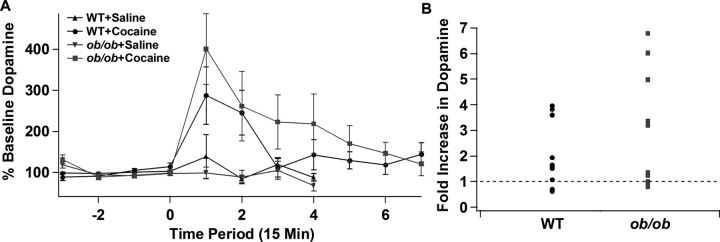
We next determined whether there were alterations in extracellular dopamine at projection sites in Lepob/ob mice by performing microdialysis measurements of dopamine in the ventral striatum. Although there were slightly higher dopamine levels in wild-type mice, baseline levels of dopamine were not significantly different between wild-type and Lepob/ob mice (data not shown). Saline injection did not change dopamine output in the ventral striatum in either genotype (Fig. 6A). Cocaine (20 mg/kg, i.p.) increased extracellular dopamine in both wild-type and Lepob/ob mice, and the resulting increases in extracellular dopamine were quite variable (ranges: WT, 0.626- to 3.965-fold; Lepob/ob, 0.798- to 6.781-fold) (Fig. 6B). When the dopamine output was examined over time, there appeared to be mildly prolonged release in Lepob/ob mice that did not reach statistical significance (Fig. 6A). Although ANOVA indicated a significant main effect of time (F(10,191) = 5.77; p < 0.0001) in the cocaine-injected mice, there was no significant main effect of genotype (F(1,191) = 3.17; p = 0.0764) or a sig-nificant genotype × time interaction (F(10,191) = 0.58; p = 0.8299). Thus, although cocaine had a significantly smaller effect on the D2R IPSC within the VTA/SNc of Lepob/ob mice, the cocaine-induced increase in dopamine in the ventral striatum was not significantly different.
Figure 6.
The cocaine-stimulated release of dopamine in the ventral striatum is increased in Lepob/ob mice. Microdialysis measurement of dopamine levels in the ventral striatum in wild-type and Lepob/ob mice in response to saline or cocaine (20 mg/kg) injection. Each time point represents a 15 min period. A, Mean dopamine levels (expressed as percentage baseline dopamine levels) in wild-type and Lepob/ob mice in response to saline or cocaine injection. Saline or cocaine was injected at time 0. B, Fold increase in dopamine in response to cocaine injection for each individual animal (WT, n = 12; Lepob/ob, n = 10).
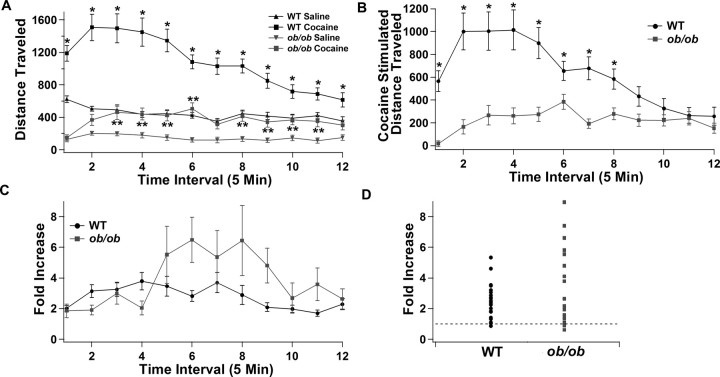
Because the locomotor response to cocaine is related to increases in dopamine within the ventral striatum, we next determined whether the locomotor response to cocaine was altered in Lepob/ob mice. As has been observed previously (Dauncey, 1986; Calcagnetti et al., 1987; Dauncey and Brown, 1987; Ahima et al., 1999; Collin et al., 2000), Lepob/ob mice showed significantly lower locomotor activity under baseline conditions compared with wild-type mice (Fig. 7A, saline). Although both wild-type and Lepob/ob mice showed significant increases in locomotor activity after cocaine injection (20 mg/kg) (Fig. 7A,B), the increase in locomotion induced by cocaine was significantly smaller in Lepob/ob mice (Fig. 7B). To control for the reduced baseline activity in Lepob/ob mice, the increases in locomotor activity were normalized to baseline levels. When the locomotor activity was normalized to the levels observed after saline injection (baseline), there was a trend for a delayed increase in locomotion induced by cocaine in Lepob/ob mice compared with wild-type animals (Fig. 7C). This trend was similar to the longer-lasting increase in dopamine output in the ventral striatum observed in the microdialysis studies. Although neither of these trends was statistically significant, the two observations may be linked.
Figure 7.
Cocaine-induced locomotion is altered in Lepob/ob mice. A, Total distance traveled in wild-type and Lepob/ob mice in response to injections of saline and cocaine (20 mg/kg). Each time point represents a 5 min interval (*p < 0.05 WT saline vs cocaine; **p < 0.05 Lepob/ob saline vs cocaine; n = 22 for both WT and Lepob/ob mice for all panels). B, Increase in distance traveled in response to cocaine (relative to saline) in wild-type and Lepob/ob mice (*p < 0.05). C, Fold increase in distance traveled in response to cocaine in wild-type and Lepob/ob mice. D, Scatter plot of the fold increase in the total distance traveled in response to cocaine over the entire 60 min session for each individual mouse.
Discussion
This study demonstrates an interaction between leptin and the mesolimbic dopamine system within the dopamine cell body region. The D2R IPSC mediates inhibitory feedback within the VTA/SNc, and under conditions in which dopamine release was increased, the regulation of that local inhibition was significantly reduced in Lepob/ob mice. The enhancement of the D2R IPSC induced by cocaine was significantly reduced in Lepob/ob mice, and the ability of l-DOPA to reverse the deficit in the D2R IPSC suggests that the alterations in the D2R IPSC resulted from a decreased vesicular filling of dopamine within the VTA/SNc. Furthermore, treatment of Lepob/ob mice with leptin in vivo fully reversed the inability of cocaine to facilitate the D2R IPSC. Interestingly, neither the cocaine-induced increase in dopamine release measured in the ventral striatum, nor the relative increase in cocaine-induced locomotion was significantly different in Lepob/ob mice. As will be discussed below, this result is not consistent with the results obtained in brain slices containing the cell bodies (our study) or in brain slices measuring dopamine release in the nucleus accumbens (Fulton et al., 2006).
Somatodendritic and terminal release of dopamine
The present results suggest that there may be some disconnect between the somatodendritic release of dopamine within the VTA/SNc and the release of dopamine in projection areas, such as the ventral striatum. It has been demonstrated previously that the vesicular monoamine transporter 2 (VMAT2) is regulated differently between somatodendritic and axonal locations (Li et al., 2005). VMAT2 is the protein responsible for vesicular loading of dopamine, and somatodendritic VMAT2 is targeted to a regulated secretory pathway for somatodendritic release (Li et al., 2005). Thus, alterations in the somatodendritic VMAT2 pathway could potentially alter dopamine release from the somatodendritic and axonal sites by independent mechanisms. In our experiments, the decrease in cocaine-induced dopamine levels within the VTA/SNc in Lepob/ob mice was not reflected in a decrease in dopamine in the ventral striatum. A possible explanation for this finding is that the reduction in the cocaine-induced dopamine levels within the VTA/SNc of Lepob/ob mice led to increased activity of dopamine neurons and resulted in prolonged dopamine levels in the ventral striatum.
Fulton et al. (2006) recently reported that the baseline and electrically evoked release of dopamine in the ventral striatum was significantly reduced in Lepob/ob mice. This is in contrast to the present in vivo observations that neither basal nor cocaine-induced dopamine levels in the ventral striatum were decreased in Lepob/ob mice when measured via microdialysis. The reasons for the differences between our findings (R) and those of Fulton et al. (F) are most likely technical, such as the use of adult (R) versus neonatal (F) animals, cocaine (R) versus electrical stimulation (F), differences between microdialysis (R) and amperometry (F), and perhaps most importantly, the measurement of dopamine levels in vivo in this study versus analysis of dopamine in brain slices in the previous work.
Locomotor activity
Locomotor activity was significantly different between wild-type and Lepob/ob mice (Fig. 7). As has been observed previously (Dauncey, 1986; Calcagnetti et al., 1987; Dauncey and Brown, 1987; Ahima et al., 1999; Collin et al., 2000), Lepob/ob mice showed significantly reduced locomotion under baseline conditions, and although activity of Lepob/ob mice increased significantly after cocaine injection, the absolute magnitude of this effect was decreased relative to wild-type mice. However, when locomotor activity was normalized to the baseline activity, Lepob/ob mice were not statistically different from wild-type mice. In addition, the baseline levels of dopamine measured in these experiments were not significantly different between wild-type and Lepob/ob mice, which suggests that the decreased baseline activity of Lepob/ob mice was not a result of decreased baseline dopamine levels within the ventral striatum. Thus, it appears that the reduced baseline activity of Lepob/ob mice is a consequence of either their obesity or their lack of leptin but is not attributable to altered basal extracellular dopamine levels.
Amphetamine caused a similar differential effect on locomotion in Lepob/ob mice with saline-treated Lepob/ob mice showing reduced amphetamine-induced locomotion relative to saline-treated wild-type mice (Fulton et al., 2006). In addition, amphetamine-induced locomotion was increased in Lepob/ob mice that were treated for 10 d with leptin (Fulton et al., 2006). Likewise, the present study demonstrated that treatment of Lepob/ob mice with leptin increased the cocaine-induced facilitation of the dopamine IPSC. Thus, there is a common regulation of psychostimulant-induced release of dopamine in Lepob/ob mice after treatment with leptin.
Potential mechanisms
How the congenital lack of leptin leads to the deficits in dopamine vesicular filling within the VTA/SNc and the potential changes in the regulation of dopamine release in terminal areas is not known. One possibility is that leptin may function as a tonic inhibitory signal to dopamine neurons within the VTA/SNc. This is supported by the recent finding that leptin caused a modest decrease in the firing of VTA dopamine neurons (Hommel et al., 2006). The prolonged lack of a leptin-mediated inhibitory signal may result in a small, tonic increase in basal activity of dopamine neurons. Over time, this small tonic activation could alter the regulation of somatodendritic vesicular filling within the VTA/SNc without significantly altering basal levels of dopamine in terminal areas. It is also possible that the prolonged lack of leptin affects protein levels through altered leptin-dependent gene expression. This is supported by the recent findings that leptin activates signal transducer and activator of transcription 3 (STAT3) in VTA dopamine neurons, and that the levels of tyrosine hydroxylase are decreased in Lepob/ob mice (Fulton et al., 2006; Hommel et al., 2006). Thus, it is possible that the congenital lack of leptin caused alterations in the D2R IPSC through dysregulated expression of proteins such as tyrosine hydroxylase or VMAT2. A third possibility is that secondary effects of the lack of leptin mediate the alterations observed in Lepob/ob mice. However, because leptin receptors are expressed in dopamine neurons, leptin injection stimulates phosphorylation of STAT3 within dopamine neurons, and injection of leptin directly into the VTA affects feeding (Figlewicz et al., 2003; Fulton et al., 2006; Hommel et al., 2006), it is likely that the at least some of the alterations are a result of the lack of leptin action directly within the VTA/SNc rather than secondary effects resulting from the lack of leptin. Other leptin-resistant models have been demonstrated to contain altered levels of DATs (Figlewicz et al., 1998). Thus, altered DAT levels or altered responsiveness of D2Rs could have potentially played a role in the results observed here. However, because the baseline D2R IPSC was not different between wild-type and Lepob/ob mice, and acute treatment with l-DOPA fully restored the responses to both forskolin and cocaine, alterations in DAT or D2Rs do not appear to contribute to the deficits observed here.
The interesting question of whether “reward” is altered in Lepob/ob mice remains unresolved. Leptin has been demonstrated to alter dopamine output in response to refeeding (Krugel et al., 2003) and affects the relapse to drug seeking (Shalev et al., 2001) in wild-type animals. However, because the results presented here indicate that both basal and cocaine-stimulated extracellular dopamine levels are normal in Lepob/ob mice, it seems unlikely that changes in extracellular dopamine are significantly responsible for the hyperphagia and obesity of Lepob/ob mice.
Footnotes
This work was supported by National Institutes of Health Grants K01DK070931 (A.G.R.), R01DA04523 (J.T.W.), and R01DA14639 (G.P.M.). We thank Dr. Malcolm Low for the use of the Med Associates locomotor chambers. We also thank Caroline Koudelka (Oregon Health & Science University Center for Biostatistics, Computing, and Informatics) for help with statistics on the locomotor activity experiments. Finally, we also thank members of the Williams laboratory for helpful discussions.
References
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Flynn JJ, Margules DL. Opioid-induced linear running in obese (ob/ob) and lean mice. Pharmacol Biochem Behav. 1987;26:743–747. doi: 10.1016/0091-3057(87)90606-x. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cannon CM, Abdallah L, Tecott LH, During MJ, Palmiter RD. Dysregulation of striatal dopamine signaling by amphetamine inhibits feeding by hungry mice. Neuron. 2004;44:509–520. doi: 10.1016/j.neuron.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Collin M, Hakansson-Ovesjo ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res Mol Brain Res. 2000;81:51–61. doi: 10.1016/s0169-328x(00)00167-4. [DOI] [PubMed] [Google Scholar]
- Dauncey MJ. Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia. 1986;42:547–549. doi: 10.1007/BF01946696. [DOI] [PubMed] [Google Scholar]
- Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1987;72:549–559. doi: 10.1113/expphysiol.1987.sp003096. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. Haloperidol induces a partial reinforcement extinction effect in rats: implications for a dopamine involvement in food reward. Pharmacol Biochem Behav. 1986;25:813–821. doi: 10.1016/0091-3057(86)90392-8. [DOI] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Res Bull. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73:229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78:834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–633. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Nikaido RS. Disruption of brain stimulation-induced feeding by dopamine receptor blockade. Nature. 1975;258:750–751. doi: 10.1038/258750a0. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]