Abstract
The membrane phospholipid phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2 or PIP2] regulates many ion channels. There are conflicting reports on the effect of PtdIns(4,5)P2 on transient receptor potential vanilloid 1 (TRPV1) channels. We show that in excised patches PtdIns(4,5)P2 and other phosphoinositides activate and the PIP2 scavenger poly-Lys inhibits TRPV1. TRPV1 currents undergo desensitization on exposure to high concentrations of capsaicin in the presence of extracellular Ca2+. We show that in the presence of extracellular Ca2+, capsaicin activates phospholipase C (PLC) in TRPV1-expressing cells, inducing depletion of both PtdIns(4,5)P2 and its precursor PtdIns(4)P (PIP). The PLC inhibitor U73122 and dialysis of PtdIns(4,5)P2 or PtdIns(4)P through the patch pipette inhibited desensitization of TRPV1, indicating that Ca2+-induced activation of PLC contributes to desensitization of TRPV1 by depletion of PtdIns(4,5)P2 and PtdIns(4)P. Selective conversion of PtdIns(4,5)P2 to PtdIns(4)P by a rapamycin-inducible PIP2 5-phosphatase did not inhibit TRPV1 at high capsaicin concentrations, suggesting a significant role for PtdIns(4)P in maintaining channel activity. Currents induced by low concentrations of capsaicin and moderate heat, however, were potentiated by conversion of PtdIns(4,5)P2 to PtdIns(4)P. Increasing PtdIns(4,5)P2 levels by coexpressing phosphatidylinositol-4-phosphate 5-kinase inhibited TRPV1 at low but not at saturating capsaicin concentrations. These data show that at low capsaicin concentrations and other moderate stimuli, PtdIns(4,5)P2 partially inhibits TRPV1 in a cellular context, but this effect is likely to be indirect, because it is not detectable in excised patches. We conclude that phosphoinositides have both inhibitory and activating effects on TRPV1, resulting in complex and distinct regulation at various stimulation levels.
Keywords: TRPV1, TRP channel, phosphoinositides, PIP2, vanilloid, desensitization
Introduction
Transient receptor potential vanilloid 1 (TRPV1) channels are activated by warm temperatures, low pH, and capsaicin, the pungent compound of chili peppers (Caterina et al., 1997). In nociceptive neurons, activation of phospholipase C (PLC)-coupled receptors by pro-inflammatory agents such as bradykinin, NGF, ATP, or chemokines sensitizes TRPV1 to subsequent activation by moderately low pH, warm temperatures, and submaximal concentrations of capsaicin (Chuang et al., 2001; Tominaga et al., 2001; Moriyama et al., 2003; N. Zhang et al., 2005). This phenomenon underlies thermal hyperalgesia, the increased sensitivity to painful stimuli after tissue injury or inflammation. TRPV1 channels were reported to be inhibited by the phospholipid phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], and relief from this inhibition by PtdIns(4,5)P2 hydrolysis was proposed to mediate sensitization of TRPV1 by PLC-coupled agonists (Chuang et al., 2001). It is important to note that the potentiating effect of PtdIns(4,5)P2 hydrolysis was shown at low concentrations of capsaicin (10–100 nm) and moderately low pH. The idea that PtdIns(4,5)P2 inhibits TRPV1 was based on indirect experiments; the effects of phosphoinositides were not tested in excised patches directly (Chuang et al., 2001).
Most other mammalian TRP channels are activated by PtdIns(4,5)P2 (Hardie, 2007; Rohacs and Nilius, 2007; Voets and Nilius, 2007). The region that was proposed to be responsible for the inhibition of TRPV1 by PtdIns(4,5)P2 is in the distal C terminus of TRPV1 (Prescott and Julius, 2003), which is not conserved among TRPV or other TRP channels (amino acids 777–820). This raises the possibility that the inhibitory effect of PtdIns(4,5)P2 builds on a more conserved activating effect of PtdIns(4,5)P2 as proposed previously (Rohacs et al., 2005; Rohacs, 2007).
High concentrations of capsaicin desensitize TRPV1 currents in the presence of extracellular Ca2+ (Koplas et al., 1997; Mohapatra and Nau, 2003), an effect that may underlie the paradoxical use of capsaicin as an analgesic compound (Szallasi and Blumberg, 1999; Caterina and Julius, 2001). The calcium-dependent protein phosphatase calcineurin was proposed to mediate this desensitization (Docherty et al., 1996). The inhibition of desensitization by calcineurin inhibitors was partial (Mohapatra and Nau, 2005), suggesting that other pathways may also contribute to this phenomenon. Interestingly, PtdIns(4,5)P2 synthesis was shown to be important for the recovery of TRPV1 currents from desensitization (Liu et al., 2005), a finding that was difficult to fit with the originally published inhibition of TRPV1 by PtdIns(4,5)P2. The same report suggested that PtdIns(4,5)P2 is depleted during the desensitization process, based on the inhibition of the PtdIns(4,5)P2-sensitive Kir2.1 channel. The mechanism of PtdIns(4,5)P2 depletion was not examined; neither was the effect of interfering with PtdIns(4,5)P2 depletion on desensitization. A recent report showed that PtdIns(4,5)P2 activates TRPV1 in excised patches (Stein et al., 2006). This finding challenges the inhibitory role of PtdIns(4,5)P2 in the regulation of TRPV1, but it is compatible with the role of PtdIns(4,5)P2 depletion in desensitization of TRPV1 currents.
Here, we show that PtdIns(4,5)P2, its precursor PtdIns(4)P (PIP), and other phosphoinositides activate TRPV1 in excised patches. After exposure to high capsaicin concentrations, Ca2+ flowing through TRPV1 activates PLC, and the resulting depletion of PtdIns(4,5)P2 and PtdIns(4)P limits channel activity, leading to desensitization. We also conclude that at low capsaicin concentrations, in addition to its importance in maintaining channel activity, PtdIns(4,5)P2 also partially inhibits TRPV1 in a cellular context. Our data show that the balance between the inhibitory and activating effects of PtdIns(4,5)P2 depends on the stimulation level of the channel.
Materials and Methods
Cell culture.
Human embryonic kidney 293 (HEK293) cells were maintained in minimal essential medium solution (Invitrogen, San Diego, CA) supplemented with 10% fetal bovine serum (Invitrogen) and penicillin/streptomycin. The rat TRPV1 tagged with the myc epitope on the N terminus in pCDNA3 vector was transfected using the Effectene reagent (Qiagen, Chatsworth, CA). For the electrophysiology experiments in Figure 4, transfection was confirmed by measuring fluorescence of cotransfected green fluorescent protein (GFP). For experiments with rapalog (see Figs. 6, 7), the cells were transfected with the myc-tagged TRPV1, the plasma membrane-targeted cyan fluorescent protein (CFP)-tagged FRB fragment of the mammalian target of rapamycin (FRB) (T2098L) containing three copies of FRB, and the red fluorescent protein (RFP)-tagged FK506 binding protein 12 (FKBP12) linked to the phosphatase domain of PIP2 5-phosphatase (Varnai et al., 2006). For control experiments, the RFP–FKBP12–phosphatase domain was replaced with the RFP-tagged FKBP12 without the phosphatase domain.
Figure 4.
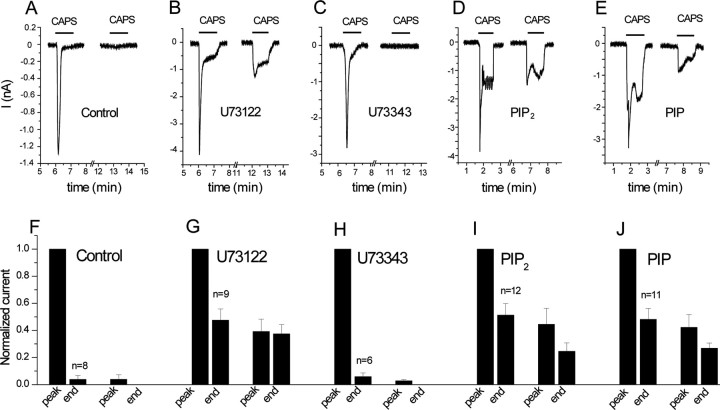
Desensitization of TRPV1 currents is inhibited by the PLC blocker U73122 and by PtdIns(4,5)P2 and PtdIns(4)P. Whole-cell patch-clamp measurements were performed at −60 mV on HEK cells expressing TRPV1, as described in Materials and Methods. A–E, Representative measurements. F–J, Statistics. A–C, F–H, Cells were preincubated with vehicle (A, F), 3 μm U73122 (B, G), and 3 μm U73343 (C, H), and 1 min capsaicin pulses were applied in the presence of 1 mm extracellular Ca2+. D, E, I, J, The pipette solution was supplemented with 100 μm diC8 PtdIns(4,5)P2 (D, I) or 100 μm DiC8 PtdIns(4)P (E, J). The first capsaicin pulse was applied in all experiments 5–10 min after the establishment of the whole-cell configuration. The data were normalized to the first peak current, and the current amplitudes were plotted for the end of the first capsaicin pulse, peak current of the second capsaicin pulse, and the end of the second capsaicin pulse (n = 6–12). CAPS, Capsaicin.
Figure 6.
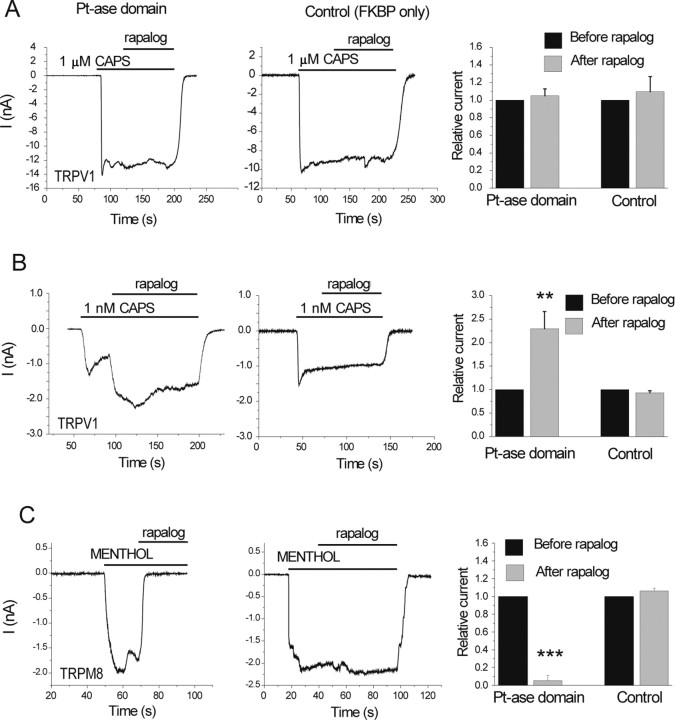
Translocation of the PIP2 5-phosphatase to the plasma membrane activates TRPV1 at low but not at high capsaicin (CAPS) concentrations. HEK cells were transfected with TRPV1, and the plasma membrane targeted CFP-tagged FRB (T2098L) and either the RFP-tagged FKBP12 (control) or FKBP12 fused to the phosphatase domain of the PIP2 5-phosphatase (Pt-ase domain) (Varnai et al., 2006). Measurements were performed at −60 mV in the whole-cell configuration, in nominally Ca2+-free solution, to avoid desensitization. Translocation of the Pt-ase domain was induced with 500 nm rapalog. A, B, Responses of cells to 1 μm (A) or 1 nm (B) capsaicin. The right panel in A shows the summary for n = 8–9 for 1 μm capsaicin. Current values 60 s after the application of rapalog were divided by the current level before the application of rapalog and plotted. B, Right, Summary for n = 16–18 for 1 nm capsaicin. Current values 30 s after the application of rapalog were divided by the current level before the application of rapalog and plotted. C, inhibition of currents induced by 500 μm menthol in cells expressing TRPM8 and the rapalog-inducible phosphatase (left) or the control construct FKBP only (middle).
Figure 7.
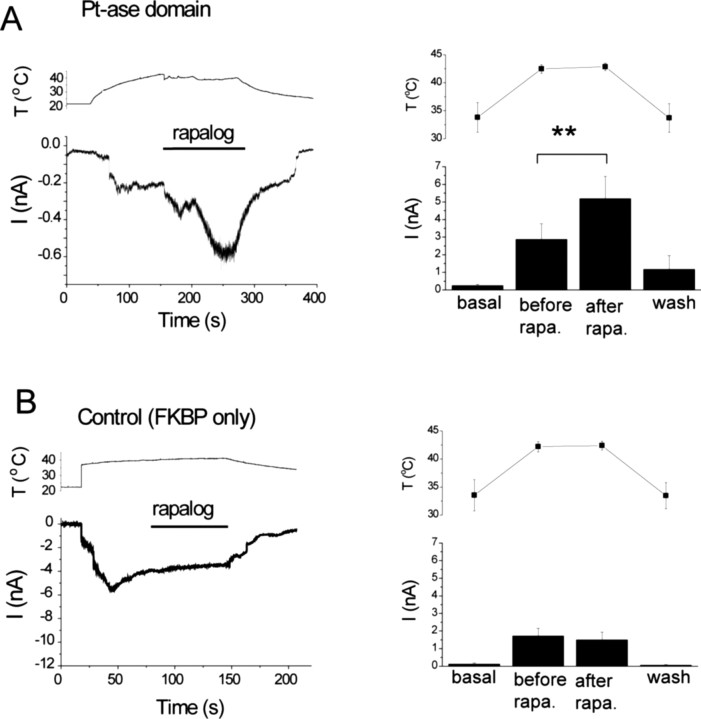
Conversion of PtdIns(4,5)P2 to PtdIns(4)P increases currents stimulated by moderately high temperatures. HEK cells were transfected with TRPV1 and the CFP-tagged FRB (T2098L) and either the RFP-tagged FKBP12 (control) or the FKBP12 fused to the phosphatase domain of the PIP2 5-phosphatase (Pt-ase domain). Measurements were performed at −60 mV in the whole-cell configuration. A, The effect of rapalog in the FKBP–phosphatase domain-expressing cells. Left, Representative trace for currents (I; bottom) and temperature (T; top) recorded in the same experiment. The horizontal bar shows the application of 500 nm rapalog. Right, Summary of the data (n = 9). Current and temperature values were measured just before the current started increasing (basal), before the application of rapalog (before rapa.), 20–30 s after the application of rapalog (after rapa.), and at the end of the decreasing phase of the temperature protocol after the washout of rapalog (wash). B, Similar experiments in control cells (n = 9).
Materials.
DiC8 phosphoinositides were purchased from Echelon (Salt Lake City, UT) or Cayman Chemical (Ann Arbor, MI), and arachidonyl-stearyl (AASt) PtdIns(4,5)P2 was purchased from Biomol (Plymouth Meeting, PA). Rapamycin, menthol, capsaicin, and most other chemicals were from Sigma (St. Louis, MO). U73122 and U73343 were purchased from Alexis Biochemicals/Axxora Platform (San Diego, CA). The rapamycin analog (AP21967, rapalog) was provided by the Regulation Kits section of Ariad Pharmaceutical (Cambridge, MA).
Mammalian electrophysiology.
Measurements were conducted 36–72 h after transfection in a solution containing (in mm) 137 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4. The same solution was used for the fluorescence measurements (see below). Borosilicate glass pipettes (World Precision Instruments, Sarasota, FL) of 2–4 MΩ resistance were filled with a solution containing (in mm) 135 K-gluconate, 5 KCl, 5 EGTA, 1 MgCl2, and 10 HEPES, pH adjusted to 7.2. For the experiments with rapalog (see Figs. 6, 7), the pipette solution was supplemented with 2 mm ATP. After formation of GΩ-resistance seals, whole-cell configuration was established, and currents were measured at −60 mV, using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Data were collected and analyzed with pClamp 9.0 software. Measurements were performed at room temperature (19–24°C).
Electrophysiology in oocytes.
Measurements were conducted on oocytes extracted from Xenopus laevis frogs using collagenase digestion. Oocytes were maintained in a solution containing (in mm) 87.5 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES. Expression of TRPV1, TRPV1Δ777–820, PLCδ3, and phosphatidylinositol-4-phosphate 5-kinase (PIP5K) and the components of the PIP2 5-phosphatase recruitment system was achieved by microinjection of linearized cRNA of these constructs in pGEMSH or pEXO vectors using a nanoliter-injector system (Warner Instruments, Hamden, CT). For two-electrode voltage-clamp (TEVC) measurements, thin-wall inner-filament-containing glass pipettes (World Precision Instruments) were filled with 3 m KCl in 1% agarose. Cells were perfused with a solution (ND96) containing (in mm) 96 NaCl, 2 KCl, 1 MgCl2, and 5 HEPES, pH adjusted to 7.4. In a subset of experiments (see Fig. 5), this solution was supplemented with 2 mm CaCl2 to measure desensitization. Currents were obtained using a GeneClamp 500B amplifier (Molecular Devices) either using a ramp protocol from −100 to +100 mV applied every second (0.25 mV/ms) or at constant holding at −60 mV. Measurements were performed at room temperature (19–21°C). Macropatch experiments were performed with borosilicate glass pipettes (World Precision Instruments) of 0.8–1.7 MΩ resistance filled with ND96. After establishing GΩ-resistance seals on devitellinized surfaces of oocytes, inside-out configuration was established, and currents were measured using a ramp protocol from −100 to +100 mV applied every second. The main perfusing solution contained (in mm) 96 KCl, 5 EGTA, and 10 HEPES, pH adjusted to 7.4. Currents were amplified with an Axopatch 200B unit (Molecular Devices) and analyzed with pClamp 9.0 software (Molecular Devices). To increase the stability of the excised patches, these measurements were performed at 17–18°C, controlled with an SC-20 inline heater/cooler (Warner Instruments).
Figure 5.
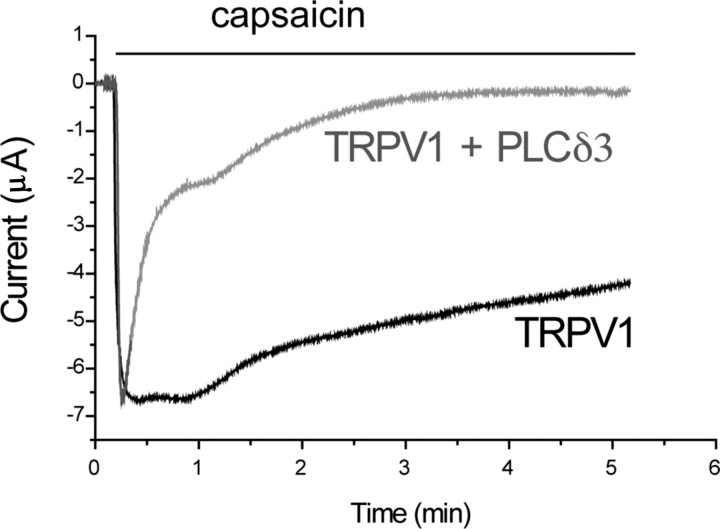
Coexpression of PLCδ3 accelerates desensitization of TRPV1 currents in oocytes. TEVC measurements were performed in Xenopus oocytes at a −60 mV holding potential, as described in Materials and Methods. Traces representing desensitization kinetics of oocytes expressing TRPV1 and those coexpressing PLCδ3 and the channel are shown; oocytes were stimulated with 5 μm capsaicin, in the presence of 2 mm calcium. See statistics in Results.
Fluorescence resonance energy transfer measurements.
HEK cells were transfected with the CFP- and yellow fluorescent protein (YFP)-tagged PH domains of PLCδ1 (van der Wal et al., 2001) and TRPV1. Measurements were performed using a photomultiplier-based system mounted on an IX-71 (Olympus, Tokyo, Japan) inverted microscope, equipped with a DeltaRAM excitation light source (Photon Technology International, Birmingham, NJ). For the fluorescence resonance energy transfer (FRET) measurements, excitation wavelength was 425 nm, and emission was detected parallel at 480 and 535 nm using two interference filters and a dichroic mirror to separate the two emission wavelengths. Data were collected using the Felix software (Photon Technology International), and the ratio of traces obtained at the two different wavelengths, correlating with FRET, were plotted (van der Wal et al., 2001). Measurements were performed at room temperature (19–24°C).
Confocal microscopy.
HEK cells were transfected with TRPV1 and the GFP-tagged OSH2–tandem PH domain that is targeted to the plasma membrane through binding to PtdIns(4)P (Roy and Levine, 2004). Experiments were performed 2 d after transfection. PtdIns(4)P depletion was assessed by the translocation of the PH-OSH2-GFP from the plasma membrane to the cytoplasm. The cells were observed with a Zeiss (Oberkochen, Germany) LSM-510 confocal microscope, equipped with an argon laser (488 nm), in the Confocal Imaging Facility of the New Jersey Medical School. Images were saved as TIFF files and were analyzed with the ImageJ software. The PH domain of OSH2 (256–424) was amplified from Saccharomyces cerevisiae DNA (American Type Culture Collection, Manassas, VA). Two separate PCRs were performed to achieve tandem PH domains with a linker between them. The OSH2–tandem PH domain was subcloned into pEGFP-C1 plasmid (Clontech, Moutain View, CA).
Measurement of inositol phosphates.
Inositol phosphates were measured as described previously (Nakanishi et al., 1995). Briefly, HEK cells transfected with TRPV1 were incubated with 3H-inositol for 24 h. The cells were washed and stimulated with 2 μm capsaicin for various time periods in the presence of 1.2 mm extracellular Ca2+, the reaction was terminated with the addition of ice-cold perchloric acid, and inositol phosphates were separated on HPLC, as described previously (Nakanishi et al., 1995).
Data analysis.
Dose–response relationships in response to diC8 PI(4,5)P2 were analyzed in the following way. A reference concentration of PI(4,5)P2 was applied repetitively throughout the measurement to correct for changes in the responsiveness of the patch. The data points were fitted with the Hill equation using the Microcal (Amherst, MA) Origin software to obtain the EC50 values, the Hill coefficients, and the maximal responses.
Data for all figures were expressed as mean ± SEM. Statistical significance was evaluated by t test.
Results
Phosphoinositides activate TRPV1 in excised patches
We studied the effects of phosphoinositides in excised patches on TRPV1 channels expressed in Xenopus oocytes. We found that poly-lysine, a poly-cation that chelates PtdIns(4,5)P2, inhibited the channels (Fig. 1), suggesting that endogenous negatively charged lipids keep the channels open after excision. After inhibition by poly-Lys, the channels were reactivated by both the long acyl-chain AASt PtdIns(4,5)P2 (Fig. 1A) and the short acyl-chain diC8 PtdIns(4,5)P2 (Fig. 1B,C). Other diC8 phosphoinositides [PtdIns(4)P, PtdIns(3,4,5)P3, PtdIns(3,4)P2, PtdIns(3,5)P2, and PtdIns(5)P] also activated TRPV1 (Fig. 1B). We also tested PtdIns, which at 100 μm made the patches unstable and the measurements noisy. The effect of PtdIns was <10% of that of PtdIns(4,5)P2 (data not shown).
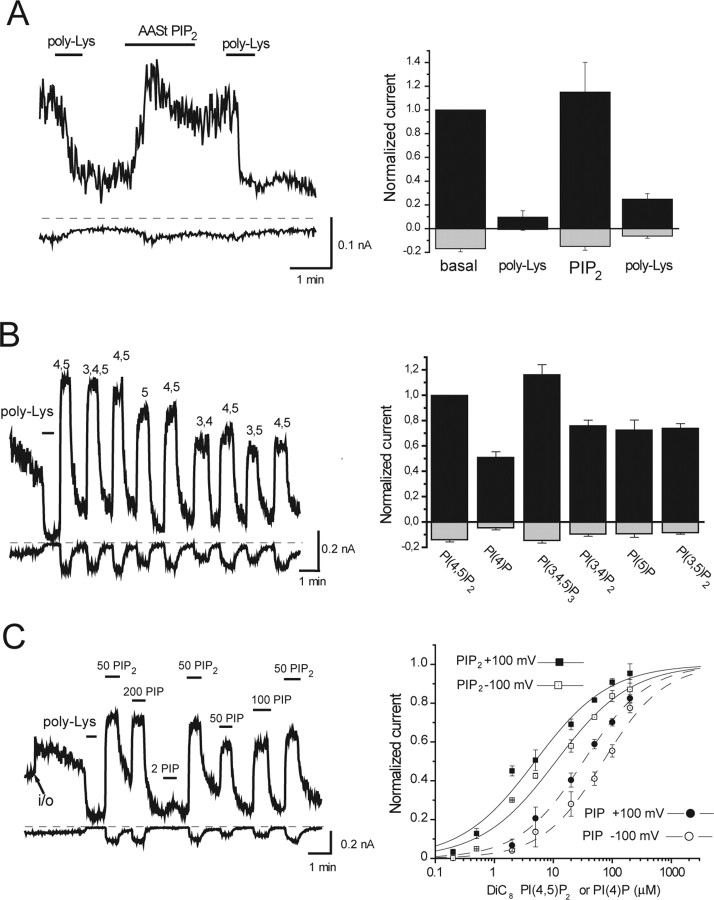
Figure 1.
Phosphoinositides activate TRPV1 channels. Currents were measured in large membrane patches excised from oocytes expressing TRPV1 using the ramp protocol described in Materials and Methods. Representative traces show currents at +100 mV (top trace) and −100 mV (bottom trace); dashed lines show zero current. Phosphoinositides were applied directly to the intracellular surface of the patch in the presence of 1 μm capsaicin (A, B) and 10 μm capsaicin (C) in the patch pipette. A, Effect of the long-chain AASt PtdIns(4,5)P2 (5 μm) and poly-l-lysine (1 and 30 μg/ml) for the first and second applications, respectively (n = 4). B, The effects of various phosphoinositides. The left panel is a representative trace, and the right panel shows statistics for +100 and −100 mV [n = 7–12, except PtdIns(5)P2, which is n = 3] for the effects of different phosphoinositides compared with PtdIns(4,5)P2. All phosphoinositides were short acyl-chain (DiC8) applied at 50 μm. C, Dose–response measurement with diC8 PIP2 and PIP. poly-Lys, Poly-l-lysine.
PtdIns(3,4,5)P3 and PtdIns(3,4)P2, the products of phosphoinositide 3′-kinases, as well as PtdIns(5)P and PtdIns(3,5)P2 are found in the plasma membrane at con-centrations much lower than those of PtdIns(4,5)P2 (Fruman et al., 1998); thus, their role in maintaining TRPV1 activity is unlikely under physiological conditions. PtdIns(4)P, the precursor of PtdIns(4,5)P2, in contrast, is found in the plasma membrane in quantities similar to that of PtdIns(4,5)P2 (Fruman et al., 1998). To better characterize the effects of PtdIns(4,5)P2 and PtdIns(4)P, we performed dose–response measurements with these two lipids (Fig. 1C). PtdIns(4)P had a very similar maximal effect to PtdIns(4,5)P2, but its EC50 was shifted to the right. Also, the apparent affinity of TRPV1 for PtdIns(4)P and PtdIns(4,5)P2 was slightly lower at negative than at positive voltages, similarly to TRPM8 (Rohacs et al., 2005). PtdIns(4,5)P2 at +100 mV had an EC50 of 4.9 ± 1.3 μm and a Hill coefficient of 0.69 ± 0.09. At −100 mV, the same values were 11.1 ± 3.5 μm and 0.69 ± 0.10. For PtdIns(4)P at +100 mV, the EC50 was 32.4 ± 13.1 μm and the Hill coefficient was 0.80 ± 0.13. At −100 mV, the same values were 70.2 ± 7.1 μm and 0.84 ± 0.11.
The experiments in Figure 1 were performed in the presence of high concentrations of capsaicin in the patch pipette [1 μm (A, B) and 10 μm (C)]. Because the proposed inhibitory effect of PtdIns(4,5)P2 is observed at low agonist concentrations (Chuang et al., 2001), we also tested the effect of PtdIns(4,5)P2 in the presence of 100 nm capsaicin, a concentration corresponding to low stimulus strength in oocytes. Under these conditions, diC8 PtdIns(4,5)P2 still activated and poly-Lys inhibited TRPV1 (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). We also tried various concentrations of poly-Lys (0.1–30 μg/ml) and an antibody against PtdIns(4,5)P2; none of these activated TRPV1 currents in excised patches in the presence of low- or high-capsaicin stimuli (data not shown). We conclude that in excised patches, PtdIns(4,5)P2 activates TRPV1 regardless of the agonist concentration, confirming the results of Stein et al. (2006).
Capsaicin activates PLC in cells expressing TRPV1
First, we used a FRET-based method (van der Wal et al., 2001; Rohacs et al., 2005), to show that capsaicin induces depletion of PtdIns(4,5)P2 in the presence of extracellular Ca2+ in TRPV1-expressing cells (Fig. 2A). This method is based on the translocation of the CFP/YFP-tagged PLCδ1 PH domain from the plasma membrane to the cytoplasm after PtdIns(4,5)P2 depletion, which is shown in the figure as downward deflection of the FRET ratio traces. Although this method has been claimed to also monitor changes in inositol-1,4,5-trisphosphate (IP3 or InsP3) levels (Hirose et al., 1999), it is clear that the translocation of these fluorescent reporter proteins may happen after PtdIns(4,5)P2 depletion without formation of IP3 (Suh et al., 2006; Varnai et al., 2006).
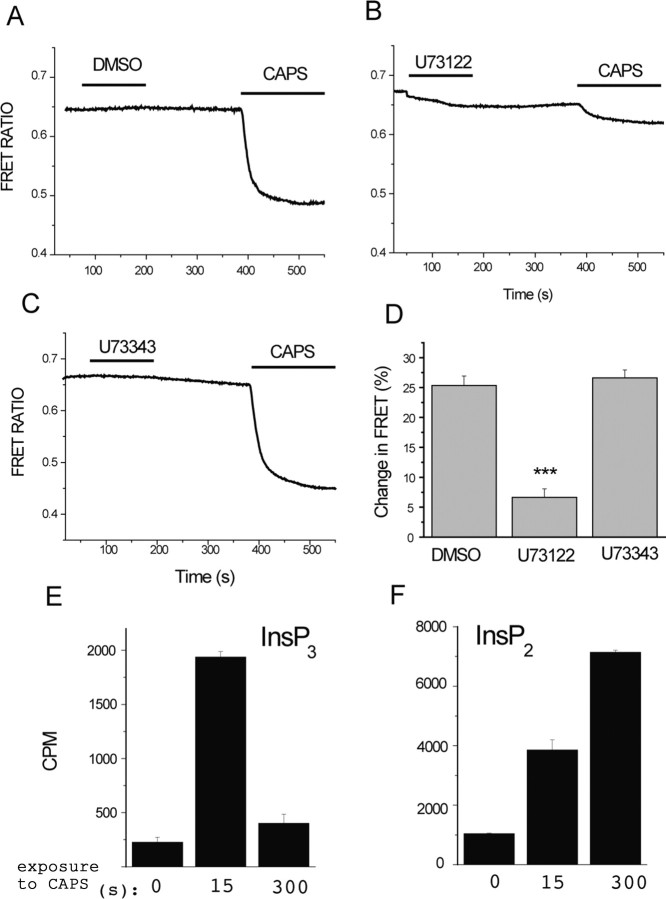
Figure 2.
Capsaicin activates PLC in TRPV1-expressing cells. A–D, Fluorescence was measured in HEK 293 cells expressing TRPV1 and the CFP- and YFP-tagged PLCδ1 PH domains, as described in Materials and Methods. Cells were pretreated with DMSO (A), 3 μm U73122 (B), or 3 μm U73343 (C) for 2 min, and then 1 μm capsaicin (CAPS) was applied, as indicated by the horizontal lines. The extracellular medium contained 1 mm Ca2+. The change in FRET ratio (D) was measured by dividing the value 2 min after the application of capsaicin with the FRET ratio value before the application of capsaicin to result Q, and then 100 − (100 × Q) was plotted (n = 14–17). ***p < 0.005. E, F, HEK cells expressing TRPV1 were incubated with 3H-inositol, as described in Materials and Methods, and stimulated with 2 μm capsaicin for the time periods indicated. Inositol phosphates were extracted and separated on HPLC, and the radioactivity was plotted for IP3 (InsP3; E) and IP2 (InsP2; F).
Depletion of PtdIns(4,5)P2 thus can happen either by dephosphorylation by phosphatase enzymes (Suh et al., 2006; Varnai et al., 2006) or via degradation by PLC (Varnai and Balla, 1998). To differentiate between these two possibilities, we examined the effect of U73122, an inhibitor of PLC, on the PtdIns(4,5)P2 hydrolysis induced by capsaicin. We used 3 μm U73122 for 2 min, followed by a 3 min wash, a similar protocol that was reported to minimize the direct effect of U73122 on PtdIns(4,5)P2 levels monitored with the PLCδ1 PH domain (Horowitz et al., 2005). In our hands, even in this protocol U73122 induced a small decrease in the FRET signal. This could be attributable to the reported variability between U73122 batches (Balla, 2001). More importantly, capsaicin applied after pretreatment with U73122 induced only a negligible PtdIns(4,5)P2 hydrolysis (Fig. 2B,D), whereas the inactive analog U73343 had no effect (Fig. 2C,D).
We also measured IP3 production in response to capsaicin. Increased IP3 is only expected if PtdIns(4,5)P2 is broken down by PLC, but not if it is dephosphorylated by phosphatases. We found that capsaicin increased IP3 and IP2 production (Fig. 2E,F), confirming PLC activation. Fifteen seconds after the application of capsaicin, both IP3 and its degradation product IP2 were elevated. Five minutes after the application of capsaicin, IP2 levels further increased, whereas IP3 returned to levels slightly above control (Fig. 2E,F), which is compatible with dephosphorylation of IP3 to IP2 in the cytoplasm.
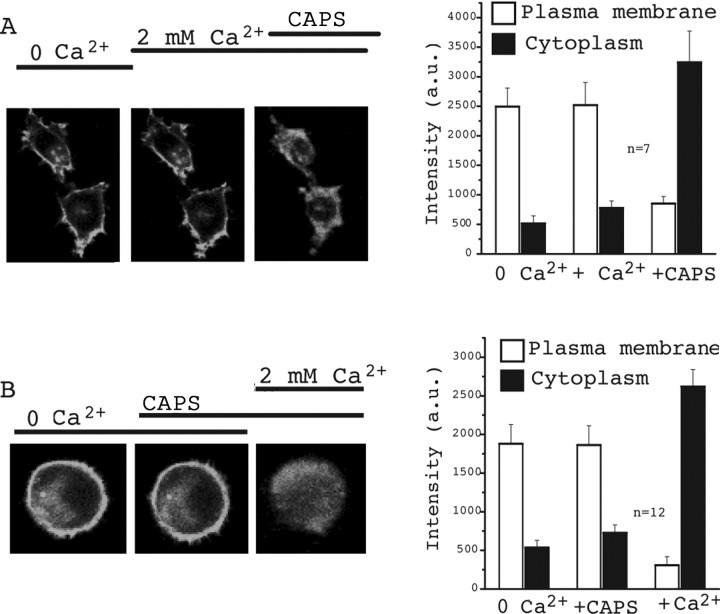
The dimerized, GFP-tagged PH domain of OSH2, a yeast homolog of the oxysterol-binding protein, has been used to monitor PtdIns(4)P levels (Roy and Levine, 2004). We used this technique to examine whether activation of TRPV1 by capsaicin also leads to PtdIns(4)P depletion. Figure 3 shows that this construct localizes mainly to the plasma membrane in resting cells. Application of capsaicin in the presence of Ca2+ induced a marked translocation of GFP fluorescence to the cytoplasm (Fig. 3A). Stimulation of TRPV1-expressing HEK cells with 1 μm capsaicin in the absence of extracellular Ca2+ did not change the localization of GFP fluorescence (Fig. 3B). Increasing extracellular Ca2+ to 2 mm, in the continued presence of capsaicin, induced a marked translocation of the GFP fluorescence to the cytoplasm (Fig. 3B). These data show that capsaicin, in addition to PtdIns(4,5)P2 breakdown, also induces PtdIns(4)P depletion in the plasma membrane, and this effect depends on the presence of extracellular Ca2+.
Figure 3.
Capsaicin (CAPS) induces PtdIns(4)P depletion in the presence of extracellular Ca2+. HEK cells were transfected with TRPV1 and the GFP-tagged OSH2–tandem PH domain that is targeted to the plasma membrane through binding to PtdIns(4)P. PtdIns(4)P depletion was assessed by the translocation of the PH-OSH2-GFP from the plasma membrane to the cytoplasm. A, The images show fluorescence of two representative cells in control (0 Ca2+), 2 min after the addition of 2 mm Ca2+, and 2 min after application of 1 μm capsaicin. The right panel shows summary of the data. An area was selected on the plasma membrane (more intense GFP) as well as in the cytoplasm (less intense), and the average pixel intensity in the area of selection was represented in arbitrary units (a.u.). B is the same as A, but the order of application of capsaicin and Ca2+ is reversed.
Interfering with phosphoinositide depletion inhibits desensitization of TRPV1 currents
Next, we performed whole-cell patch-clamp experiments in HEK cells expressing TRPV1, to test the effects of PLC inhibition and phosphoinositides on desensitization of TRPV1. We have measured TRPV1 currents at −60 mV in response to two subsequent 1-min-long capsaicin pulses in the presence of 1 mm extracellular Ca2+. By the end of the first pulse, currents decreased to almost zero, and by the end of the second pulse, there was invariably no current in control cells (Fig. 4A,F). This measurement was performed without added ATP in the internal solution in the patch pipette; the lack of recovery between the two capsaicin pulses is consistent with previous reports showing requirement for ATP for the recovery from desensitization (Liu et al., 2005). After pretreatment with U73122, TRPV1 currents desensitized much less; there was ∼40% of the peak current left at the end of the second capsaicin pulse (Fig. 4B,G). The inactive analog U73343 did not have any effect on the desensitization (Fig. 4C,H). We used the same protocol for application of U73122 for the current measurements as for the FRET experiments. U73122 itself induced a small transient inward current in 6 of 12 cells (data not shown).
Inhibition of PLC interferes with various downstream processes in addition to depleting phosphoinositides. To examine the involvement of phosphoinositide depletion more directly, we included either PtdIns(4,5)P2 (Fig. 4D,I) or its precursor PtdIns(4)P (Fig. 4E,J) in the whole-cell patch pipette and tested their effects on desensitization. Both phosphoinositides inhibited desensitization of TRPV1 currents to a similar extent to U73122. The initial rapid decline of the current was not affected by any of these manipulations, which is compatible with the involvement of other mechanisms, such as calcineurin (Mohapatra and Nau, 2005) in desensitization of TRPV1 currents.
As a negative control, we included PtdIns in the pipette, because this compound had minimal effect on TRPV1 in excised patches. In all four measurements, in which diC8 PtdIns (100 μm) was included in the patch pipette, the capsaicin-induced current decreased to zero by the end of the first 60 s capsaicin pulse (data not shown). Similarly to excised patches, however, the whole-cell seals were unstable, preventing the application of multiple capsaicin pulses with PtdIns in the pipette. The level of desensitization correlates with current amplitudes; higher-amplitude currents tend to desensitize faster and more completely, presumably because of the larger Ca2+ influx. The amplitude of the capsaicin-induced currents was 2.12 ± 0.47 nA (n = 8) in control measurements, 3.48 ± 0.75 (n = 9) in U73122-treated cells, and 2.42 ± 0.49 (n = 6) in U73343-treated cells. The amplitude of the capsaicin-induced currents was 3.32 ± 0.49 (n = 12) in cells dialyzed with PtdIns(4,5)P2, and 3.32 ± 0.49 nA (n = 11) in cells dialyzed with PtdIns(4)P. Because both the U73122-treated and PtdIns(4,5)P2 or PtdIns(4)P dialyzed cells had, on average, larger amplitudes than controls, the differences in current amplitudes cannot account for the inhibition of desensitization.
We also attempted to reconstitute desensitization of TRPV1 currents in Xenopus oocytes. TRPV1 currents in oocytes showed slow and incomplete desensitization (Fig. 5), making this model system suitable for testing the effect of heterologously expressed PLC isoforms. When we coexpressed PLCδ3, a highly Ca2+ sensitive PLC isoform, desensitization became faster and more complete (Fig. 5). The residual current after 5 min of capsaicin application was 38.0 ± 4.4% of the peak current for control, whereas the same value was 9.9 ± 2.1% for the PLCδ3-expressing oocytes (n = 10 for both groups). This measurement was performed at a constant −60 mV holding potential. The contribution of the endogenous Ca2+-activated Cl− current was minimal, because ionomycin did not induce any current at this voltage (supplemental Fig. 2A, available at www.jneurosci.org as supplemental material). Because activation of PLC produces IP3 that releases Ca2+ from intracellular stores, the measurements shown in Figure 5 were conducted on cells also expressing the type-1 IP3 5-phosphatase. This enzyme inactivates IP3, preventing the further rise in cytoplasmic calcium caused by Ca2+ release, which is presumably larger in PLCδ3-expressing cells, and thus could cause differences in desensitization. In control experiments, the IP3 phosphatase completely eliminated the Ca2+-induced Cl− current in response to carbachol, in oocytes expressing the M1 muscarinic receptor and this enzyme (supplemental Fig. 2B, available at www.jneurosci.org as supplemental material). PLCδ3 coexpression also accelerated desensitization of TRPV1 in oocytes not expressing the IP3 phosphatase (data not shown).
Similarly to TRPM8 (Rohacs et al., 2005), the kinetics of desensitization of TRPV1 in oocytes is slower than that observed in mammalian cells. There are several differences that may account for such discrepancies; oocytes, for example, have a much lower surface-to-volume ratio, therefore the overall Ca2+ increase in the cytoplasm maybe smaller than in mammalian cells. It is also possible that oocytes have lower levels of PLCδ isoforms than mammalian cells. Also, the experiments shown in Figure 4 for HEK cells were performed without added ATP in the pipette solution that accelerates desensitization (Liu et al., 2005). In the oocyte experiments, the agarose in the recording electrodes prevents loss of intracellular ATP, which may also contribute to the slower desensitization.
We have shown that PtdIns(4)P activates TRPV1 in excised patches (Fig. 1B,C) and it is similarly effective to PtdIns(4,5)P2 in inhibiting desensitization (Fig. 4D,E, I, J). We also have shown that capsaicin-induced activation of TRPV1 depleted PtdIns(4)P in the presence of extracellular Ca2+ (Fig. 3). PtdIns(4)P is also a substrate for PLC (Rebecchi and Pentyala, 2000), and it is known to be depleted during G-protein-coupled receptor- or calcium-mediated (ionomycin-induced) activation of PLC (Balla et al., 2005; Horowitz et al., 2005). PtdIns(4)P is found in quantities comparable to PtdIns(4,5)P2 in the plasma membrane (Fruman et al., 1998), raising the possibility that PtdIns(4)P, in addition to PtdIns(4,5)P2, also plays a role in maintaining TRPV1 activity. To test this possibility, we used the recently reported rapamycin-inducible PIP2 5-phosphatase recruitment system to deplete PtdIns(4,5)P2 by converting it to PtdIns(4)P (Varnai et al., 2006). Briefly, this technique is based on the rapamycin-inducible translocation of the phosphatase domain of the type IV PIP2 5-phosphatase to the plasma membrane, where it rapidly depletes PtdIns(4,5)P2 and inhibits the PIP2-sensitive TRPM8 (Varnai et al., 2006) and KCNQ2/3 (Suh et al., 2006) channels. The rapamycin analog (AP21967, rapalog) did not inhibit currents elicited by 1 μm capsaicin (Fig. 6A) in HEK cells expressing TRPV1 and the rapamycin-inducible PIP2 5-phosphatase constructs. In these experiments, we used rapalog instead of rapamycin, because rapamycin induced a transient inhibition of TRPV1 currents in control experiments (FKBP12 expressed without the phosphatase domain), an effect that was not seen with rapalog. These experiments were performed using a mutant form of FRB (T2098L) as recommended for rapalog (Varnai et al., 2006) and in the absence of extracellular Ca2+ to prevent desensitization. Rapalog promptly and completely inhibited TRPM8 in control measurements (Fig. 6C), similarly to rapamycin (Varnai et al., 2006). These data are compatible with the notion that in addition to PtdIns(4,5)P2, its precursor PtdIns(4)P also plays a role in maintaining TRPV1 channel activity. Other explanations for the lack of effect of rapalog-induced PIP2 depletion, such as inability of the phosphatase to access PtdIns(4,5)P2 molecules bound to the channel, however, cannot be completely excluded.
PtdIns(4,5)P2 partially inhibits TRPV1 at low stimulation strength
We also tested the effect of rapalog on currents induced by low concentrations of capsaicin. Figure 6B shows that rapalog further activated currents elicited by 1 nm capsaicin. Rapalog had no significant effect in cells expressing TRPV1 and the control constructs (Fig. 6B) for the 5-phosphatase translocation system: FRB in the plasma membrane and FKBP12 in the cytoplasm, lacking the 5-phosphatase domain (Varnai et al., 2006). To achieve low stimulation strength, we used 1 nm capsaicin in these experiments, which is lower than the usual “low” capsaicin concentration used in HEK cells (∼10 nm), because the capsaicin dose response in HEK cells expressing TRPV1 and the rapamycin-inducible phosphatase recruitment system was shifted to the left. This left shift was also observed in the control cells, expressing FRB and FKBP, without the phosphatase (supplemental Fig. 3, available at www.jneurosci.org as supplemental material), indicating that the dose–response shift was not attributable to altered PtdIns(4,5)P2 levels but rather the presence of other components of the recruitment system. Although these data have to be interpreted with some caution because of the altered sensitivity of TRPV1, they confirm the inhibitory effect of PtdIns(4,5)P2 at low stimulation strength that was suggested previously (Chuang et al., 2001).
We also performed similar experiments in Xenopus oocytes expressing TRPV1 and the phosphatase recruitment system. In oocytes, capsaicin sensitivity of TRPV1 was not significantly altered by these constructs, yet rapamycin further increased currents evoked by low capsaicin concentration (100 nm) and had no effect on currents induced by high capsaicin (1 μm) (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). Note that the dose response of TRPV1 to capsaicin is shifted to the right in Xenopus oocytes (Caterina et al., 1997) when compared with TRPV1 expressed in mammalian cells (Tominaga et al., 1998); thus, to obtain low stimulation strength, we applied 100 nm capsaicin in the oocyte experiments.
Heat is probably the most important physiological stimulus of TRPV1, and PtdIns(4,5)P2 depletion was also reported to sensitize these channels to activation by heat (Chuang et al., 2001). Thus, we examined the effect of rapalog-induced PtdIns(4,5)P2 depletion on TRPV1 currents elicited by moderate heating. Currents elicited by increasing the temperature to ∼42°C were further increased by rapalog in HEK cells expressing the phosphatase recruitment system (Fig. 7A), but not in control cells (Fig. 7B). This temperature increase constitutes a moderate activation level, because the currents were similar in amplitude to those elicited by 1 nm capsaicin, and were much smaller than those induced by 1 μm capsaicin.
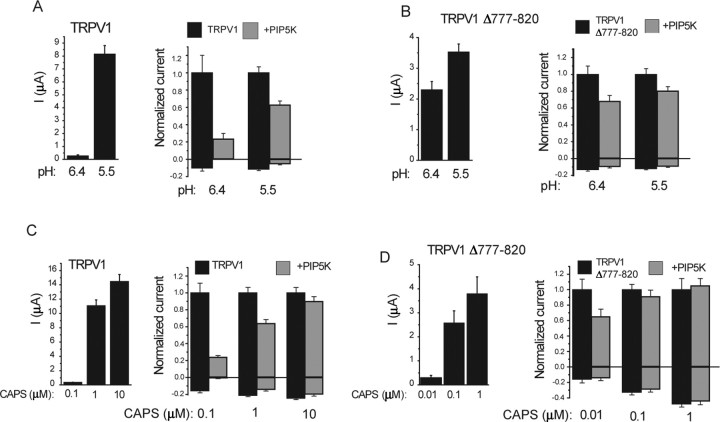
Next, we examined the effect of coexpressing PIP5K type Iβ, an enzyme that was shown to increase PtdIns(4,5)P2 levels (Lin et al., 2005) and affect PtdIns(4,5)P2-sensitive ion channels (Shyng et al., 2000). PIP5K inhibited TRPV1 channel activity induced by decreasing pH to 6.4 and 5.5 in Xenopus oocytes (Fig. 8A). The inhibition was more prevalent at the more moderate stimulus (pH 6.4). This observation is also in concert with the published inhibitory effect of PtdIns(4,5)P2 on TRPV1 (Chuang et al., 2001). The inhibition was reduced but not eliminated by the deletion of the C-terminal 777–820 region (Fig. 8B) that was implicated by Chuang et al. (2001) in the inhibition by PtdIns(4,5)P2.
Figure 8.
The effect of coexpression of PIP5K on TRPV1 currents in Xenopus oocytes. TRPV1 currents were measured with TEVC, as described in Materials and Methods. Currents at +100 and −100 mV are shown. Left panels show average current amplitudes, and right panels show currents normalized to current amplitudes elicited by the actual stimulus without PIP5K coexpression. A, B, The effect of PIP5K on low pH (6.5 and 5.5)-induced currents is shown for the wild type (A) and the Δ777–820 mutant (B) of TRPV1. C, D, The effect of PIP5K on capsaicin (CAPS)-induced currents is shown for the wild type (C) and the Δ777–820 mutant (D) of TRPV1.
When we used capsaicin as a stimulus, coexpression of PIP5K inhibited the currents at 100 nm capsaicin (Fig. 8C). At 1 μm capsaicin, there was a moderate inhibition by PIP5K, and no inhibition was observed at 10 μm capsaicin. The lack of inhibition at 10 μm capsaicin shows that the effect of PIP5K is unlikely to be mediated by reduced surface expression. The TRPV1Δ777–820 mutant was not inhibited significantly by PIP5K at 100 nm and 1 μm capsaicin. This mutant, however, has a higher sensitivity to capsaicin (Prescott and Julius, 2003). When we tested the effect of PIP5K at 10 nm capsaicin, which corresponds roughly to the relative effect of 100 nm capsaicin on the wild-type TRPV1, we saw an inhibition that was less than that observed on the wild type (Fig. 8D).
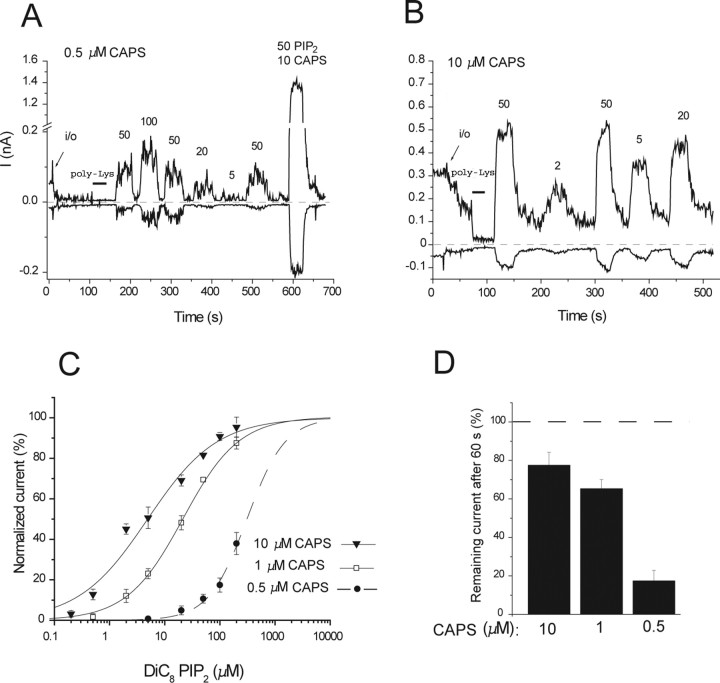
Capsaicin changes the apparent affinity of the activating PtdIns(4,5)P2 effect
Menthol, the specific activator of TRPM8, sensitizes that channel to activation by PtdIns(4,5)P2 [i.e., shifts the PtdIns(4,5)P2 dose response to the left] (Rohacs et al., 2005). To test the effect of the stimulation strength on the apparent affinity of the activating effect of PtdIns(4,5)P2 on TRPV1, we performed dose–response measurements with diC8 PtdIns(4,5)P2 in the presence of different capsaicin concentrations (Fig. 9). The EC50 for PtdIns(4,5)P2 at +100 mV was 4.9 ± 2.7, 20.3 ± 2.5, and 309.7 + 54.6 μm in the presence of 10, 1, and 0.5 μm capsaicin, respectively. The Hill coefficients were 0.69 ± 0.09, 0.88 ± 0.06, and 1.25 ± 0.22 in the presence of 10, 1, and 0.5 μm capsaicin, respectively. Note that in the presence of 0.5 μm capsaicin, diC8 PtdIns(4,5)P2 elicited very small responses that showed no signs of saturation at higher PtdIns(4,5)P2 concentrations; therefore, we were unable to fit the data with a Hill function. In each of those experiments, we applied a pulse of 50 μm diC8 PtdIns(4,5)P2 together with 10 μm capsaicin and calculated the expected maximum response from that value. Using the calculated Imax, we could fit the points with a Hill function. It is not possible to tell from the data whether in the presence of 0.5 μm capsaicin the Imax evoked by PtdIns(4,5)P2 is the same or different from that in the presence of 10 μm capsaicin; therefore, the given values for the EC50 and Hill coefficient should only be treated as approximation in the presence of 0.5 μm capsaicin. Nevertheless, it is clear from the data that the EC50 for PtdIns(4,5)P2 is shifted to the right at lower capsaicin concentrations.
Figure 9.
High capsaicin (CAPS) concentrations increase the apparent affinity of TRPV1 for PtdIns(4,5)P2. A, B, Representative traces in the presence of 0.5 μm capsaicin (A) and 10 μm capsaicin (B) for dose responses with diC8 PtdIns(4,5)P2. C, Hill fits of the PtdIns(4,5)P2 dose responses at various capsaicin concentrations (+100 mV). D, Statistics of the current rundown in the presence of various capsaicin concentrations (n = 8, 44, and 11 for 10, 1, and 0.5 μm capsaicin, respectively). In the presence of 1 and 10 μm capsaicin, there was an immediate change in current after excision, which was quite variable: sometimes an increase, sometimes a decrease or no change; therefore, the values given are normalized to the current immediately after excision. In the presence of 0.5 μm capsaicin in many patches, there was a fast increase, but it disappeared so rapidly that its amplitude was not always possible to determine; thus, the values were normalized to the cell-attached level.
Rundown of current activity in ATP-free excised patches is thought to be attributable to dephosphorylation of PtdIns(4,5)P2 by endogenous lipid phosphatases for many PtdIns(4,5)P2-sensitive channels (Hilgemann and Ball, 1996). The velocity of rundown shows correlation with the apparent affinity for PtdIns(4,5)P2; channels with lower PtdIns(4,5)P2 affinity run down faster (Zhang et al., 1999). In the presence of 0.5 μm capsaicin, current activity ran down faster than in the presence of 1 or 10 μm capsaicin (Fig. 9D). This is again consistent with the lower apparent affinity of TRPV1 for PtdIns(4,5)P2 in the presence of low capsaicin concentrations.
Discussion
The role of phosphoinositides in the desensitization of TRPV1
High capsaicin concentrations in the presence of extracellular Ca2+ lead to transient activation of TRPV1 currents, a phenomenon called desensitization. Here, we show that PtdIns(4,5)P2, PtdIns(4)P, and other phosphoinositides activate TRPV1 in excised patches. We also demonstrate that activation of TRPV1 in the presence of extracellular Ca2+ leads to the activation of PLC and the depletion of PtdIns(4,5)P2 and PtdIns(4)P. The PLC inhibitor U73122 and inclusion of PtdIns(4,5)P2 or PtdIns(4)P in the patch pipette during whole-cell measurements inhibited desensitization. The likely interpretation of our data is that after activation of PLC, the ensuing phosphoinositide depletion removes PtdIns(4,5)P2 and PtdIns(4)P from their activating site(s), leading to desensitization of TRPV1 currents.
Locally applied capsaicin is widely used as an analgesic. The rationale behind this is that after an initial phase, when it elicits an intense burning sensation, capsaicin receptors become desensitized, and this provides pain relief (Szallasi and Blumberg, 1999). The initial burning sensation, however, can be very intense, especially when capsaicin is used in higher concentrations, which leads to low patient compliance (Sawynok, 2003). The involvement of phosphoinositide depletion in the desensitization of TRPV1 raises the interesting possibility that altering PtdIns(4,5)P2 metabolism locally may allow better local pain control, with less side effects.
The effect of PtdIns(4)P
PtdIns(4)P, the precursor of PtdIns(4,5)P2, activated TRPV1 in excised patches (Fig. 1B,C) and inhibited de-sensitization similarly to PtdIns(4,5)P2 (Fig. 4E,J). In the plasma membrane, PtdIns(4)P is found in quantities comparable to that of PtdIns(4,5)P2 (Fruman et al., 1998), thus it is likely that PtdIns(4)P plays a role in keeping TRPV1 open in a cellular context. Consistent with this, conversion of PtdIns(4,5)P2 to PtdIns(4)P did not inhibit TRPV1 currents at saturating capsaicin concentrations (Fig. 6A). Although PtdIns(4,5)P2 has received a lot of attention recently (Hilgemann et al., 2001; Suh and Hille, 2005), much less is known about the role of PtdIns(4)P as a regulator of ion channels. It was shown that PtdIns(4)P activates some members of the inwardly rectifying K+ channel family in excised patches (Fan and Makielski, 1997; Baukrowitz et al., 1998; Rohacs et al., 1999), but its role in the regulation of these channels in a cellular context is not established. PtdIns(4)P also activates TRPM4 (Z. Zhang et al., 2005; Nilius et al., 2006) and TRPM8 (Rohacs et al., 2005), but its effect is much smaller than that of PtdIns(4,5)P2. Our data suggest that PtdIns(4)P plays an important role in maintaining the activity of TRPV1, which, as we discuss below, is distinct from that of PtdIns(4,5)P2 in certain aspects.
The inhibitory effect of PtdIns(4,5)P2
We found that PtdIns(4,5)P2 partially inhibits TRPV1 in intact cells, an effect that is only prevalent at low stimulation strength. This is based on the following findings. Depletion of PtdIns(4,5)P2 with a rapamycin-inducible PIP2 5-phosphatase potentiated TRPV1 currents at low capsaicin concentration (Fig. 6B and supplemental Fig. 4, available at www.jneurosci.org as supplemental material) and when activated by moderate heating (Fig. 7). Increasing PtdIns(4,5)P2 levels by coexpressing PIP5K inhibited TRPV1 currents that were evoked by low capsaicin concentrations or a moderate drop in pH (Fig. 8.). These data are consistent with the previous report (Chuang et al., 2001) suggesting an inhibitory effect of PtdIns(4,5)P2, especially considering that PLC-mediated potentiation is also mainly prevalent at low stimulus strength. Our data also suggest that the inhibitory effect is specific for PtdIns(4,5)P2 over PtdIns(4)P, raising the interesting possibility that specific changes in levels of PtdIns(4,5)P2 or PtdIns(4)P by lipid kinases and phosphatases may have distinct effects on ion channels. It is likely that the inhibitory effect of PtdIns(4,5)P2 is indirect, because we and others (Stein et al., 2006) could not detect it in excised patches. It is possible, for example, that it is mediated by a cytoplasmic PtdIns(4,5)P2-binding protein or some other factor that is quickly lost in excised patches. It will require additional studies to identify the additional factor(s) that is required for the inhibitory effect of PtdIns(4,5)P2.
The inhibitory effect of PtdIns(4,5)P2 on TRPV1 was proposed to play a role in sensitization of TRPV1 to moderate stimuli by PLC-coupled agonists such as bradykinin and NGF (Chuang et al., 2001). Various other mechanisms have also been proposed to underlie this phenomenon. For bradykinin (Premkumar et al., 2005), extracellular ATP (Tominaga et al., 2001), and CCL3 (N. Zhang et al., 2005), the involvement of protein kinase C (PKC) was also demonstrated. It was shown that NGF induces translocation of TRPV1 to the plasma membrane, thus increasing the number of active channels (X. Zhang et al., 2005). This finding was later confirmed, and it was also demonstrated that NGF treatment increases the maximal response to capsaicin, with minimal effect on EC50 (Stein et al., 2006). In contrast, agonists of G-protein-coupled receptors, such as ATP, sensitize mainly by shifting the capsaicin dose response to the left (Tominaga et al., 2001), similarly to PKC (Vellani et al., 2001). It is clear that multiple pathways contribute to sensitization of TRPV1 currents and that various agonists may use different mechanisms (Zhang and McNaughton, 2006). It will require future studies to understand the relative contributions of PtdIns(4,5)P2 depletion and other pathways to sensitization of TRPV1.
How to reconcile the opposing effects of PtdIns(4,5)P2 on TRPV1?
In the presence of saturating capsaicin concentrations, PtdIns(4,5)P2 and/or PtdIns(4)P are needed for channel activity, and no inhibitory effect is present. In the presence of moderate stimuli, such as low capsaicin concentrations or moderate increases of temperature, the situation is more complicated, because both inhibition and activation are present. A concurrent presence of an activating and inhibitory effect is likely to result in a bell-shaped dependence of channel activity on PtdIns(4,5)P2 levels. If resting PtdIns(4,5)P2 levels are to the right of the peak of such a bell-shaped dose response, a moderate PtdIns(4,5)P2 depletion would result in channel activation, whereas increased PtdIns(4,5)P2 levels would result in channel inhibition, which is consistent with our data. Similar opposing effects of PtdIns(4,5)P2 were shown for voltage-dependent Ca2+ channels (Wu et al., 2002), thus dual regulation by phosphoinositides may be a general phenomenon among ion channels.
Our model implies that during the phenomenon of sensitization, PLC-coupled agonists induce a moderate depletion of PtdIns(4,5)P2, removing the inhibitory effect of PtdIns(4,5)P2 but not reaching low enough lipid levels to inhibit channel activity. In contrast, we assume that high capsaicin concentrations induce a severe depletion of PtdIns(4,5)P2 that limits channel activity during desensitization. When discussing severe and moderate PtdIns(4,5)P2 depletion, we have to keep in mind that it is the local phosphoinositide concentration that determines channel activity, which is difficult to measure. It is also possible that the inhibitory and activating PtdIns(4,5)P2 molecules are in different pools. PLC-coupled agonists mainly activate PLCβ and PLCγ isoforms, as opposed to capsaicin, which is likely to activate PLCδ isoforms. The different PLC isoforms may have different access to the PtdIns(4,5)P2 molecule that exerts the inhibitory effect and the one that exerts the activating effect. Finally, it is also possible that different PLC isoforms deplete PtdIns(4)P versus PtdIns(4,5)P2 to different extents, which may also contribute to differential effects on the channel.
Our study mainly focused on capsaicin, but it is likely that phosphoinositides exert dual effects on TRPV1 currents activated by protons and heat also. PLC-coupled agonists do not only sensitize TRPV1 to capsaicin but also to protons and heat (Chuang et al., 2001). We have shown that H+-induced currents were inhibited by PIP5K and that currents evoked by moderate heating were potentiated by rapalog-induced PtdIns(4,5)P2 depletion. TRPV1 currents also desensitize when activated by a large drop in pH (Bhave et al., 2002). Although the role of phosphoinositides in the desensitization of TRPV1 induced by protons is feasible, it needs to be addressed experimentally in the future. TRPV1 is also activated by depolarization; both heat and capsaicin induce large shifts in the voltage dependence of TRPV1, and lower capsaicin concentrations induce smaller shifts in voltage dependence than higher capsaicin concentrations (Voets et al., 2004). Several of our measurements were performed at various voltages, and generally the effects of manipulating phosphoinositide levels were similar at positive and negative voltages (Figs. 1, 8, 9 and supplemental Fig. 4, available at www.jneurosci.org as supplemental material). Thus, it is unlikely that the differential effects of phosphoinositides are attributable to the different voltage shifts at low and high capsaicin concentrations. The dose–response relationships of both PtdIns(4,5)P2 and PtdIns(4)P were slightly leftshifted at positive voltages (Fig. 1C), therefore voltage also modulates the effect of phosphoinositides on TRPV1, similarly to TRPM8 (Rohacs et al., 2005).
Summary
Our data show that phosphoinositides have dual effects on TRPV1 and the balance between the inhibitory and activating effects depend on the strength of stimulation. The activating effect of phosphoinositides is likely to be direct, because it prevails in excised patches. We show that activation of TRPV1 by high capsaicin concentrations in the presence of extracellular Ca2+ leads to activation of PLC and the ensuing depletion of PtdIns(4,5)P2 and PtdIns(4)P contributes to the desensitization of these channels. Our data also demonstrate the existence of an inhibitory effect of PtdIns(4,5)P2 on TRPV1 at low stimulation levels in intact cells. Obviously, the regulation of TRPV1 by phosphoinositides is highly complex. Additional studies are required to fully elucidate the mechanism and the interplay between the activating and inhibitory phosphoinositide effects.
Footnotes
T.B., P.V., and A.B. were supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development–National Institutes of Health. T.R. was supported by the American Heart Association, the Alexander and Alexandrine Sinsheimer Foundation, and the University of Medicine and Dentistry of New Jersey Foundation. The insightful comments of Dr. John Reeves are highly appreciated. The clones for the phosphatase recruitment system in the oocyte vector pEXO were kindly provided by Drs. Peter Enyedi and Gabor Czirjak (Semmelweis University, Budapest, Hungary).
References
- Balla A, Tuymetova G, Tsiomenko A, Varnai P, Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol Biol Cell. 2005;16:1282–1295. doi: 10.1091/mbc.E04-07-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T. Pharmacology of phosphoinositides, regulators of multiple cellular functions. Curr Pharm Des. 2001;7:475–507. doi: 10.2174/1381612013397906. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2 mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol (Lond) 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R. Regulation of cardiac Na+/Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Yan F, Shimamura S, Barg S, Shyng SL. Membrane phosphoinositides control insulin secretion through their effects on ATP-sensitive K+ channel activity. Diabetes. 2005;54:2852–2858. doi: 10.2337/diabetes.54.10.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositol phospholipids. Proc Natl Acad Sci USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Rohacs T. Regulation of TRP channels by PIP2. Pflügers Arch. 2007;453:753–762. doi: 10.1007/s00424-006-0153-7. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflügers Arch. 2007 doi: 10.1007/s00424-007-0275-6. in press. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Chen J, Prestwich GD, Logothetis DE. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J Biol Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Lopes CMB, Michailidis I, Logothetis DE. PI(4,5)2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Barbieri A, Gumusboga A, Cukras C, Pike L, Davis JN, Stahl PD, Nichols CG. Modulation of nucleotide sensitivity of ATP-sensitive potassium channels by phosphatidylinositol-4-phosphate 5-kinase. Proc Natl Acad Sci USA. 2000;97:937–941. doi: 10.1073/pnas.97.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal J, Habets R, Varnai P, Balla T, Jalink K. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J Biol Chem. 2001;276:15337–15344. doi: 10.1074/jbc.M007194200. [DOI] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-3Hinositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol (Lond) 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Nilius B. Modulation of TRPs by PIPs. J Physiol (Lond) 2007 doi: 10.1113/jphysiol.2007.132522. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci USA. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005;280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]