Abstract
The dynamic interplay between serotonin [5-hydroxytryptamine (5-HT)] neurotransmission and the hypothalamic–pituitary–adrenal (HPA) axis has been extensively studied over the past 30 years, but the underlying mechanism of this interaction has not been defined. A possibility receiving little attention is that 5-HT regulates upstream corticotropin-releasing hormone (CRH) signaling systems via activation of serotonin 2C receptors (5-HT2CRs) in the paraventricular nucleus of the hypothalamus (PVH). Through complementary approaches in wild-type rodents and 5-HT2CR-deficient mice, we determined that 5-HT2CRs are necessary for 5-HT-induced HPA axis activation. We used laser-capture PVH microdissection followed by microarray analysis to compare the expression of 13 5-HTRs. Only 5-HT2CR and 5-HT1DR transcripts were consistently identified as present in the PVH, and of these, the 5-HT2CR was expressed at a substantially higher level. The abundant expression of 5-HT2CRs in the PVH was confirmed with in situ hybridization histochemistry. Dual-neurohistochemical labeling revealed that approximately one-half of PVH CRH-containing neurons coexpressed 5-HT2CR mRNA. We observed that PVH CRH neurons consistently depolarized in the presence of a high-affinity 5-HT2CR agonist, an effect blocked by a 5-HT2CR antagonist. Supporting the importance of 5-HT2CRs in CRH neuronal activity, genetic inactivation of 5-HT2CRs produced a downregulation of CRH mRNA and blunted CRH and corticosterone release after 5-HT compound administration. These findings thus provide a mechanistic explanation for the longstanding observation of HPA axis stimulation in response to 5-HT and thereby give insight into the neural circuitry mediating the complex neuroendocrine responses to stress.
Keywords: serotonin, 5-HT2C receptor, mCPP, corticotropin-releasing hormone, paraventricular nucleus of the hypothalamus, corticosterone
Introduction
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis and 5-HT system has been implicated in the pathophysiology of disease states such as affective disorders, anxiety disorders, and obesity (Kelly et al., 1980; Heisler et al., 1998, 2003; Lucki, 1998; Porter et al., 2004). HPA axis activity is mediated by multiple central and peripheral inputs converging on the paraventricular nucleus of the hypothalamus (PVH), where corticotropin-releasing hormone (CRH) is synthesized before its regulated release from the median eminence into the hypophyseal portal circulation (Guillemin and Rosenberg, 1955; Spiess et al., 1981). This stimulates the release of adrenocorticotropin (ACTH) from the anterior lobe of the pituitary, which in turn acts on the adrenal cortex to trigger the release of glucocorticoids, such as corticosterone/cortisol (CORT), into the bloodstream. Enhanced 5-HT neurotransmission produces potent and consistent increases in plasma concentrations of CORT, whereas depletion of the 5-HT precursor or 5-HT transporter reduces CORT (Fuller and Snoddy, 1980, 1990; Silverstone et al., 1994; Vielhaber et al., 2005). The prevalent use of compounds augmenting the synaptic availability of 5-HT and the alterations in adiposity and mood induced by CORT excess or deficiency highlight the importance of understanding this cross talk (Kelly et al., 1980; Gold and Chrousos, 1985; Bagdy et al., 1989; Laferrere et al., 1994; Vielhaber et al., 2005).
We hypothesized that 5-HT stimulates the HPA axis via action at CRH-containing neurons in the brain, which subsequently augments CORT release. 5-HT-immunoreactive nerve terminals, although not densely evident within the PVH, are expressed in a pattern consistent with CRH-expressing cells (Sawchenko et al., 1983). Furthermore, light and electron microscopic immunocytochemistry has illustrated a synaptic interaction between 5-HT axons and CRH-containing neurons in the PVH (Liposits et al., 1987), and 5-HT compounds stimulate PVH CRH-expressing neurons (Gibbs and Vale, 1983; Brady et al., 1991; Hu et al., 1992; Laflamme et al., 1996; Javed et al., 1999; Jorgensen et al., 2002). Despite these findings, the relative importance of central 5-HT circuits and the involvement of specific 5-HTRs in HPA axis function remain unclear. Contributing to this uncertainty is the limited subtype specificity of the available 5-HTR agonists. These compounds have significant affinities for multiple 5-HTRs, which has complicated attempts to define the role of 5-HT and specific 5-HTRs in the regulation of the HPA axis via action at CRH neurons.
To clarify mechanisms underlying 5-HT-mediated activation of the HPA axis, we have established that the 5-HT2CR is the predominant 5-HTR subtype expressed in the PVH, that 5-HT2CR agonists and antagonists regulate CRH activity, and that genetic inactivation of 5-HT2CRs perturbs serotonergic regulation of the HPA axis. Altogether, these studies delineate a mechanism through which central 5-HT systems regulate neuroendocrine responses potentially relevant to a variety of neuropsychiatric disorders.
Materials and Methods
Animals.
Adult male C57BL/6 mice [2–3 months old; The Jackson Laboratory (Bar Harbor, ME), Charles River (Wilmington, MA), or CLEA (Tokyo, Japan)], hemizygous mice bearing a null mutation of the X-linked htr2c gene (5-HT2CR knock-out) congenic on a C57BL/6J background and age-matched wild-type littermates (2–4 months old) (Tecott et al., 1995), and Sprague Dawley rats [2–3 months old; Taconic Farms (Germantown, NY) or The Jackson Laboratory] were used. Rodents had water and laboratory chow pellets available ad libitum in a light (12 h on/12 h off) and temperature-controlled environment (21.5–22.5°C). All procedures used were approved by institutional Animal Care and Use committees or the United Kingdom Home Office.
Neurohistochemical studies.
Four experimental groups were used: (1) C57BL/6 mice treated at the onset of the light cycle with pyrogen-free 0.9% saline or the high-affinity 5-HT2CR agonist m-chlorophenyl-piperazine (mCPP) (2.5 or 5.0 mg/kg, i.p.; n = 3–4 per dose); (2) rats fitted with a catheter in the femoral vein as described previously (Elmquist et al., 1996; Elias et al., 1998) 5–7 d before treatment with saline, mCPP (0.5, 2.5, or 5.0 mg/kg, i.v.), or the 5-HT reuptake inhibitor/5-HT-stimulated release compound d-fenfluramine (d-fen; 0.1, 1.0, or 2.0 mg/kg, i.v.) at the onset of the light cycle (n = 3–5 per dose); (3) rats treated during the light cycle with 40 μg of colchicine (Sigma-Aldrich, St. Louis, MO) in 10 μl of pyrogen-free 0.9% saline infused into the lateral ventricle to enhance CRH visualization (n = 5); and (4) untreated 5-HT2CR knock-out and wild-type mice perfused during the light cycle for hypothalamic neuropeptide expression analysis (n = 8–9 per genotype). Using methods standard in our laboratory (Elmquist et al., 1996; Elias et al., 1998; Liu et al., 2003; Heisler et al., 2006), brain tissue was prepared through transcardial perfusion with 0.9% saline and then 10% neutral buffered formalin (Sigma-Aldrich) under chloral hydrate anesthesia (350 mg/kg, i.p.) 2 h after saline, mCPP, or d-fen treatment and 36–48 h after colchicine treatment. Brains were postfixed and sectioned coronally at a thickness of 25–30 μm on a freezing sliding microtome.
Brain tissue was processed for single-label free-floating in situ hybridization histochemistry (ISHH), single-label immunohistochemistry (IHC) (Elmquist et al., 1996; Elias et al., 1998; Heisler et al., 2002; Liu et al., 2003; Yamamoto et al., 2003), or dual-label ISHH and IHC using methods detailed previously (Liu et al., 2003; Heisler et al., 2006). ISHH was performed using an antisense 35S-labeled CRH (Liu et al., 2003), 35S-labeled 5-HT2CR (Molineaux et al., 1989), 35S-labeled cocaine- and amphetamine-regulated transcript (CART) (Couceyro et al., 1997), 35S-labeled pro-opiomelanocortin (POMC) (Cheung et al., 1997), or 35S-labeled melanin-concentrating hormone (MCH) (Qu et al., 1996) riboprobe generated from cDNA templates by in vitro transcription with a T3 (5-HT2CR and CART), T7 (CRH), or SP6 (POMC and MCH) polymerase, according to the manufacturer's protocol (Promega, Madison, WI). Sections processed for single-label ISHH were then mounted onto SuperFrost slides (Fisher Scientific, Hampton, NH), exposed to Biomax MR film (Kodak, Rochester, NY), and then dipped in NTB 2 photographic emulsion (Kodak). After 0.5–4 weeks, slides were developed with Kodak Fixer and D-19 Developer (Kodak). Dual-label ISHH and IHC or single-label IHC sections were processed using c-fos rabbit primary antiserum (Ab-5; 1:50,000; Oncogene, San Diego, CA) or rabbit CRH primary antiserum (1:10,000; Phoenix Pharmaceuticals, Belmont, CA) and biotinylated donkey anti-rabbit IgG secondary antibody (1:1000; Jackson ImmunoResearch, West Grove, PA) in PBS and 0.25% Triton X (Sigma-Aldrich).
Single and dual labeling was assessed throughout the rostral–caudal axis of the PVH, the arcuate nucleus of the hypothalamus (ARC) for POMC mRNA analysis, or the lateral hypothalamic area (LHA) for MCH mRNA analysis (Paxinos and Franklin, 2001). For single-label IHC analysis, a threshold of size and intensity of immunoreactive-positive neurons was set. For dual-label analysis, clusters of grains of 35S-labeled CRH overlying c-fos immunoreactivity (FOS-IR)-positive neurons or 35S-labeled 5-HT2CR grains overlying CRH-IR cell bodies that were three times greater than background hybridization levels and conformed to the immunoreactive cell body were counted as coexpressed. Basal CRH, CART, POMC, and MCH mRNA expression in 5-HT2CR knock-out and wild-type mice was examined in adjacent sections of brain tissue by determining the intensity of autoradiographic images of the 35S-labeled neuropeptides on Biomax MR film as measured with a light box, a digital camera interface, and NIH Image software. PVH 35S-labeled CRH, PVH 35S-labeled CART, ARC 35S-labeled POMC, and LHA 35S-labeled MCH signal density was analyzed by computing the integrated density (the sum of the gray values minus background). This was performed in four sections in PVH (0.70, 0.82, 0.94, and 1.06 mm caudal to bregma), ARC (1.46, 1.70, 1.94, and 2.18 mm caudal to bregma), or LHA (1.22, 1.58, 1.94, and 2.18 mm caudal to bregma), and the average integrated density of neuropeptide hybridization signal was calculated.
Laser-capture microdissection and Affymetrix GeneChip analysis.
C57BL/6 mice (n = 3) were rapidly decapitated, and brains were extracted, snap frozen, and sectioned coronally at a thickness of 14 μm on a cryostat. Brain sections were mounted onto RNase-free membrane-coated glass slides (PALM MembraneSlides; P.A.L.M. Microlaser Technologies, Bernried, Germany), fixed in 95% ethanol, rehydrated in 75 and 50% ethanol, stained with 1% cresyl violet, dehydrated in a graded ethanol series, and immersed in Histoclear. The extent of the PVH (0.70–1.22 mm caudal to bregma, yielding ∼35 sections) was microdissected using a PALM Microlaser System (P.A.L.M. Microlaser Technologies). Total RNA was isolated (typical yield, 20 ng) in accord with the manufacturer's protocol (RNAqueous-Micro Kit; Ambion, Austin, TX). RNA was amplified and biotin labeled using two cycles of in vitro transcription (GeneChip IVT labeling kit; Affymetrix, Santa Clara, CA) before hybridization onto Affymetrix Murine Expression Arrays 430 2.0 using an Affymetrix fluidics station according to the manufacturer's protocol. Chips were scanned using an Affymetrix GS3000 scanner, and data were extracted using Affymetrix GCOS software. Downstream analysis was performed using GeneSpring 7.2 (Agilent Technologies, Palo Alto, CA). Data regarding the presence/absence and normalized expression values for transcripts of 13 5-HTR genes in the PVH were obtained.
Real-time quantitative PCR.
C57BL/6 mice (n = 4–5 per treatment) were pretreated with 0.9% saline or the selective blood–brain barrier-penetrating 5-HT2CR antagonist SB242084 dihydrochloride (1.0 mg/kg, i.p.) (Kennet et al., 1997) and 30 min later were treated with 0.9% saline or d-fen (3.0 mg/kg, i.p.). Sixty minutes after this, mice were decapitated, and the hypothalamus was removed for RNA extraction using methods described previously (Nonogaki et al., 2006). Briefly, total RNA was isolated using the RNeasy Midi kit (Qiagen, Hilden, Germany) according to the manufacturer's directions, and cDNA synthesis was performed using a Super Script III First-Strand Synthesis System for reverse transcription (RT)-PCR kit (Invitrogen, Rockville, MD) using 1 μg of total RNA. cDNA synthesized from total RNA was evaluated in a real-time PCR quantitative system (Light Cycler Quick System 350S; Roche Diagnostics, Mannheim, Germany). The primers used were mouse CRH (sense, 5′-CCG GGC AGA GCA GTT AGC-3′; antisense, 5′-CAA CAT TTC ATT TCC CGA TAA TCT C-3′) and mouse β-actin (sense, 5′-TTG TAA CCA ACT GGG ACG ATA TGG-3′; antisense, 5′-GAT CTT GAT CTT CAT GGT GCT AGG-3′). The relative amount of mRNA was calculated with β-actin mRNA as the invariant control. The data are presented as percentage change of the mean value of the saline-treated control group.
Electrophysiology studies.
Using standard electrophysiological methods (Cowley et al., 1999a; Pronchuk et al., 2002), recordings in the presence of mCPP (4–6 μm), a control solution, and/or RS102221 (500 nm to 1 μm) were made from 35 medial parvocellular PVH (mpPVH) neurons from 250 μm coronal brain slices of male Sprague Dawley rats. Briefly, glass patch micropipettes (5–7 MΩ) were filled with pipette solution for perforated-patch recordings (Cowley et al., 2003). Once stable perforated-patch recording conditions were established, GABAA receptor-mediated synaptic responses were evoked with the cell held in voltage clamp at −40 mV. Membrane potential was assessed in current clamp. Neurons served as their own controls; drugs were applied and washed out via bath perfusion (Cowley et al., 1999b, 2003). After this, Neurobiotin was electroporated into the cell, and the slice tissue was fixed overnight in a 4% paraformaldehyde in PBS solution at 4°C and dehydrated overnight at 4°C in 20% sucrose in PBS. Tissue was then cut (25 μm) with a cryostat (Jung Frigocut 2800E; Leica Microsystems, Bannockburn, IL) and processed for IHC for CRH (1:3000; Peninsula Laboratories, Belmont, CA) and thyrotropin-releasing hormone (TRH; 1:3000; Dr. Eduardo Nillni, Brown University, Providence, RI).
CRH ex vivo release.
CRH release was measured in 5-HT2CR knock-out and wild-type mice (n = 29 per genotype) 3–4 h after the onset of the light cycle using methods described previously (Raber and Bloom, 1994; Raber et al., 1995). Briefly, a 1.5 mm coronal section of the hypothalamus beginning 0.7 mm caudal to bregma was dissected and placed onto a Brinkmann (Westbury, NY) tissue chopper for the preparation of 300 μm slices. Slices representing one entire hypothalamus were placed into individual chambers and superfused using an in vitro superfusion system (Brandel, Gaithersburg, MD). CRH release was determined 100 min later for a basal CRH measure and in 15 min intervals (collections 1 and 2) after mCPP (1 μm), 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI) (10 μm), 8-hydroxy-2-(dipropylamino)tetralin (8-OH-DPAT) (1 μm), or d-fen (1 μm) infusion. To confirm tissue viability at the end of the experiment, slices were exposed to KCl (60 mm) for 30 min; data from any tissue that did not increase release at least 50% over baseline were discarded. The concentration of CRH in the superfusates was determined by radioimmunoassay (RIA) as described previously (Raber and Bloom, 1994; Raber et al., 1995).
CORT RIA.
Plasma CORT was measured in 5-HT2CR knock-out and wild-type mice both basally and after mCPP or d-fen (n = 6–10 per genotype and treatment). Specifically, basal CORT was assessed 1 h after the onset of the light and dark cycle in untreated mice using a rat corticosterone RIA kit according to the manufacturer's protocol (MP Biomedicals, Irvine, CA). In another group of mice, plasma CORT was assessed 1 h after treatment with saline, mCPP (2.5 or 5.0 mg/kg, i.p.), or d-fen (3.0 mg/kg, i.p.), which were administered 2–4 h after the onset of the light cycle.
Drugs.
The high-affinity 5-HT2CR agonists mCPP (Sigma-Aldrich) and DOI (Sigma-Aldrich), the high-affinity 5-HT1R agonist 8-OH-DPAT (Sigma-Aldrich), and the 5-HT reuptake inhibitor/5-HT release stimulator d-fen (Sigma-Aldrich) were dissolved in 0.9% sterile saline and administered either intraperitoneally or intravenously for in vivo studies. The 5-HT2CR antagonist SB242084 dihydrochloride (Sigma-Aldrich) was prepared as a stock solution by dissolving 5.0 mg in 1.0 ml of distilled water. Immediately before use, 0.1 ml of this stock solution was suspended in 4.9 ml of 0.9% sterile saline. For electrophysiological experiments, mCPP was prepared as 100 μm stock solution in distilled water and diluted to a final concentration of between 2 and 6 μm immediately before use; the high-affinity 5-HT2CR antagonist RS102221 (Tocris Bioscience, Ellisville, MO) was prepared as a stock solution of 10 mm and diluted to 500 nm in artificial CSF immediately before use.
Data analysis.
Before performing statistical tests, data were assessed for normality with a Shapiro-Wilk's test to determine whether parametric or nonparametric tests were warranted using SPSS PC (Chicago, IL) Advanced Statistics (version 11.5) software. Neurohistochemical, endocrine, and behavioral data were analyzed with ANOVA followed by Tukey's post hoc comparisons, independent-samples t tests, or paired t tests. Electrophysiological data and hypothalamic CRH release data were analyzed with paired t tests. For all analyses, significance was assigned at p ≤ 0.05.
Results
5-HT agonists activate PVH CRH-containing neurons in vivo
We hypothesized that 5-HT-induced CORT release is predominantly achieved via action at CRH-containing neurons in the PVH. The most commonly used 5-HT compounds enhancing CORT release are mCPP and fenfluramine (Fuller and Snoddy, 1980, 1990; Sevy et al., 1994; Silverstone et al., 1994; Broocks et al., 2000; Vielhaber et al., 2005). In addition to 5-HT2CRs, mCPP also has high binding affinity for the 5-HT2AR, 5-HT1AR, 5-HT1BR, and 5-HT3R subtypes (Koe et al., 1992). We investigated whether mCPP or d-fen activate PVH neurons in vivo using FOS-IR as a marker of neuronal activation. Consistent with previous reports, we observed that both mCPP (2.5 or 5.0 mg/kg, i.v.) and d-fen (1.0 or 2.0 mg/kg, i.v.) induced robust and dose-related increases in total FOS-IR expression compared with saline (p < 0.001; n = 4–5 per dose) in the rat PVH (Li and Rowland, 1993; Singewald et al., 2003).
Previous reports indicate that dl-fen (25.0 mg/kg, i.p.) and d-fen (5.0–10.0 mg/kg, i.p.) induce c-fos in CRH-IR perikarya in the rat PVH (Richard et al., 1992; Laflamme et al., 1996; Javed et al., 1999). Using adjacent sections of brain tissue from rats treated with saline (Fig. 1a), mCPP (2.5 mg/kg, i.v.) (Fig. 1b), or d-fen (1.0 mg/kg, i.v.), we next examined whether these compounds increase FOS in CRH mRNA-containing cells (n = 4–5 per dose). Using IHC to identify FOS-IR-positive cells and ISHH to label CRH mRNA-containing neurons (Fig. 1c), we observed that mCPP induced FOS-IR in 72 ± 5% of 35S-labeled CRH-expressing neurons (Fig. 1d,e), and d-fen induced FOS-IR in 88 ± 1% of 35S-labeled CRH-expressing neurons, whereas saline induced FOS-IR in only 14 ± 1% of 35S-labeled CRH-expressing neurons. Therefore, the 5-HT compounds were highly effective in inducing FOS-IR in CRH neurons. Conversely, not all FOS-IR neurons were positive for CRH. As may be noted in Figure 1d, approximately one-half of FOS-IR-positive neurons (mCPP, 57 ± 3%; d-fen, 55 ± 2%) did not meet the criteria for coexpression with CRH-containing neurons. This is comparable with the expression rate observed after saline treatment (43 ± 2%). Together, these findings indicate that 5-HT compounds substantially activate PVH CRH neurons and suggest that 5-HT compounds (and saline) also activate non-CRH-containing PVH neurons in vivo.
Figure 1.
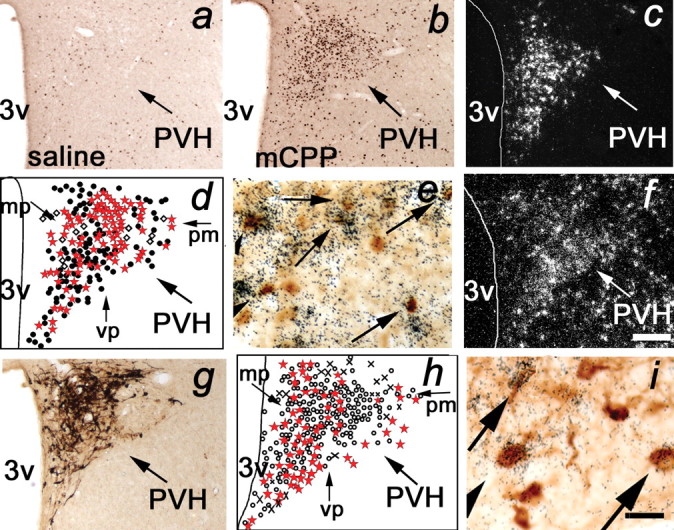
mCPP and d-fen activated PVH CRH-containing neurons expressing 5-HT2CRs in vivo. a, b, Compared with saline (a), mCPP (b; 2.5 mg/kg, i.v.) induced a significant increase in FOS-IR (brown nuclear stain) in parvocellular PVH neurons. c–e, mCPP induced FOS-IR in PVH CRH mRNA-containing neurons. c, CRH mRNA (cluster of white grains) is expressed in parvocellular PVH neurons. d, Schematic representation of PVH CRH mRNA and FOS-IR coexpression. Open diamonds, CRH mRNA; filled circles, FOS-IR; red stars, coexpression of CRH mRNA and FOS-IR. e, Arrows identify coexpression of CRH mRNA (cluster of black grains) and FOS-IR (brown nuclear stain). f, 5-HT2CR mRNA (cluster of white grains) is expressed in parvocellular PVH neurons. g–i, 5-HT2CR mRNA is coexpressed with PVH CRH-IR-containing neurons. g, PVH CRH-IR (brown cytoplasm stain) expression. h, Schematic of PVH 5-HT2CR mRNA and CRH-IR coexpression. Circles, CRH-IR; crosses, 5-HT2CR mRNA; red stars, coexpression of CRH-IR and 5-HT2CR mRNA. i, Arrows identify coexpression of 5-HT2CR mRNA (cluster of black grains) and CRH-IR (brown cytoplasm stain). 3v, Third ventricle; mp, medial parvocellular division; pm, posterior magnocellular division; vp, ventral parvocellular division. Scale bars: (in f) a–d, f–h, 200 μm; (in f) e, 20 μm; i, 30 μm.
PVH CRH-containing neurons express 5-HT2CRs
We hypothesized that 5-HT directly activates CRH-containing neurons via action at 5-HTRs expressed by these cells. To investigate which 5-HTRs are expressed in the PVH, we used laser-capture microdissection to remove the rostral–caudal extent of the PVH. We compared levels of 5-HTR expression in the PVH using Affymetrix 430 2.0 Murine Expression Arrays. Probe sets representing 5-HT1ARs, 5-HT1BRs, 5-HT1DRs, 5-HT1FRs, 5-HT2BRs, 5-HT2CRs, 5-HT3ARs, 5-HT3BRs, 5-HT4Rs, 5-HT5ARs, 5-HT5BRs, 5-HT6Rs, and 5-HT7Rs were present on the array. Of these, the Gi-coupled 5-HT1DR (Htr1d BB829587; expression level mean ± SEM, 60 ± 16.0) and Gq-coupled 5-HT2CR (Htr2c BQ174268; expression level mean ± SEM, 340 ± 37.8) were most highly expressed and were the only 5-HTRs consistently identified as present in the PVH of mice (n = 3). If 5-HT directly activates CRH-containing neurons to affect the HPA axis, as we hypothesize, then these data suggest that the 5-HT2CR is a strong candidate receptor through which this effect may be achieved. This would be consistent with the mCPP and d-fen PVH-induced FOS-IR data described above; both mCPP and the metabolite of d-fen, norfenfluramine, have high binding affinity for 5-HT2CRs (Porter et al., 1999).
5-HT2CRs are widely expressed in the rodent brain (Molineaux et al., 1989). We further assessed the distribution pattern and chemical phenotype of PVH 5-HT2CR-expressing neurons in the rat using ISHH with a 35S-labeled 5-HT2CR riboprobe applied to the hypothalamus. 5-HT2CR mRNA (n = 5) (Fig. 1f) is diffusely expressed in the parvocellular PVH, where CRH-containing neurons reside (Fig. 1c,g). Using dual-labeling techniques, we observed that 48 ± 9% of total PVH CRH-IR neurons express 35S-labeled 5-HT2CR mRNA (n = 5) (Fig. 1h,i). These findings were consistent throughout the rostral–caudal extent of the PVH and suggest that 5-HT2CRs are positioned to regulate PVH CRH-containing neurons.
5-HT agonist activates PVH CRH-containing neurons
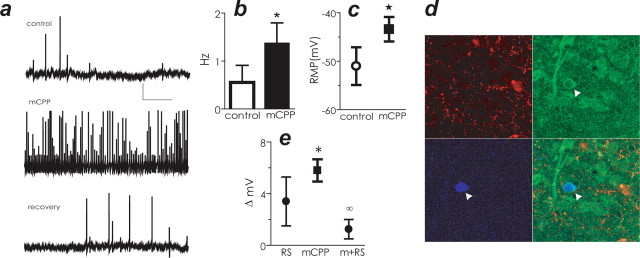
Using electrophysiological techniques, we next recorded from neurons in coronal rat hypothalamic slices containing the mpPVH in the presence of the high-affinity 5-HT2CR agonist mCPP to assess the direct effect of this compound on CRH activity (n = 35). Using a perforated-patch version of whole-cell intracellular recording technique (Cowley et al., 1999b; Pronchuk et al., 2002), we observed that mCPP (4–6 μm) depolarized and induced more than a twofold increase in spontaneous firing rates in five of 14 neurons; an effect reversed by drug washout (Fig. 2a–c). Nine neurons did not respond to mCPP (data not shown). These 14 mpPVH neurons were subsequently stained for the presence of biocytin and CRH. Figure 2d illustrates a multipolar CRH-positive, mCPP-sensitive neuron in mpPVH. We observed that 100% (five of the five) of neurons that responded to mCPP were CRH-positive, and 100% of the neurons that did not respond to mCPP (nine of nine) were not.
Figure 2.
mCPP depolarized and increased the firing rate of CRH-containing neurons in the PVH. a, mCPP depolarized mpPVH neurons. Membrane potential of mpPVH neuron recorded in control saline (top), in the presence of mCPP (middle), and after a 15 min washout (bottom). Calibration bars: 20 mV, 10 s. b, mCPP increased the firing rate of mpPVH CRH-containing neurons compared with control conditions. c, mCPP depolarized mpPVH CRH-containing neurons compared with control conditions. RMP, Resting membrane potential. d, Confirmation that recordings were made from CRH-containing neurons (arrowhead indicates recorded cell). Top left, TRH immunoreactivity; top right, CRH immunoreactivity; bottom left, biocytin histochemistry; bottom right, three images merged. e, 5-HT2CR antagonist RS102221 (RS) did not significantly change membrane potential compared with control. In contrast, mCPP significantly changed membrane potential, and this effect was blocked by treatment with RS. Data are expressed as mean ± SEM. *p < 0.05 compared with control; ∞p < 0.05 compared with mCPP.
We next examined whether the effects of mCPP on CRH neuronal activity could be blocked with a 5-HT2CR antagonist. The 5-HT2CR antagonist RS102221 (500 nm to 1 μm) did not induce alterations in membrane potential compared with control treatment (mean ± SEM control = −60.4 ± 3.5, n = 5; RS102221 = −54.60 ± 2.9, n = 5), whereas mCPP produced a significant depolarization compared with control in four of six mpPVH cells (Fig. 2e). RS102221 attenuated mCPP-induced depolarization in five of five cells (Fig. 2e). Together, these data suggest that 5-HT2CR stimulation activates PVH CRH neurons.
5-HT agonists require 5-HT2CRs to stimulate CRH release and regulate CRH mRNA
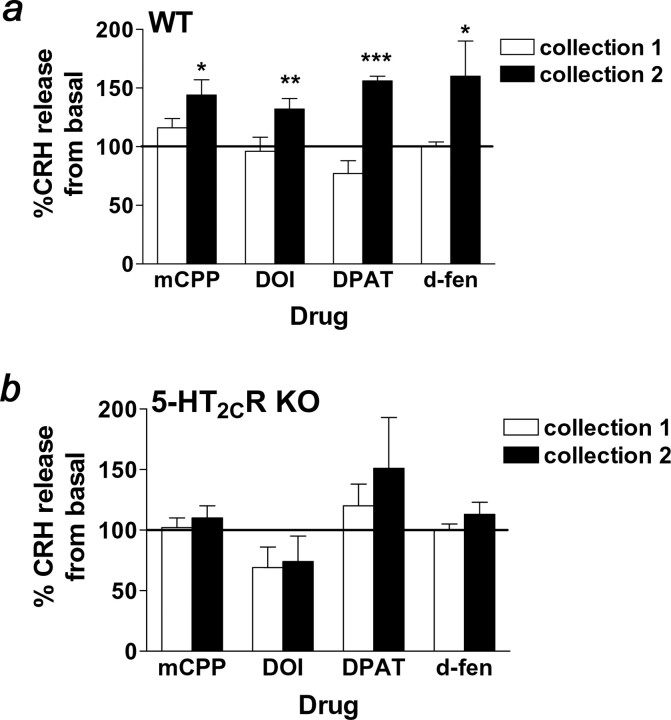
To examine whether 5-HT-induced activation of CRH neurons also impacts on CRH release via action at 5-HT2CRs, hypothalamic slices containing the PVH from 5-HT2CR knock-out and wild-type mice were superfused with mCPP (1.0 μm), DOI (10.0 μm), or 8-OH-DPAT (1.0 μm) (n = 19 per genotype). Despite no differences in basal CRH release, fractions collected after drug administration revealed significant phenotypic differences in CRH release in response to mCPP, DOI, and 8-OH-DPAT. Specifically, mCPP (Fig. 3a) (p < 0.05), DOI (Fig. 3a) (p < 0.01), and 8-OH-DPAT (Fig. 3a) (p < 0.001) significantly increased the percentage of CRH release in wild-type mice. Supporting the notion that the complete effect of 5-HT agonist-induced CRH release requires functional 5-HT2CRs, these compounds were ineffective in enhancing the percentage of CRH release in 5-HT2CR-deficient mice (Fig. 3b).
Figure 3.
Genetic 5-HT2CR inactivation abolished 5-HT compound-stimulated CRH release. a, b, mCPP (1.0 μm), DOI (10.0 μm), 8-OH-DPAT (1.0 μm), and d-fen (1.0 μm) stimulated the in vitro release of CRH 15–45 min after drug infusion (black bars; collection 2) in wild-type mice (WT; a) but not 5-HT2CR knock-out littermates (5-HT2CR KO; b) relative to the basal collection period in hypothalamic extracts. Zero to fifteen minutes after drug infusion (white bars; collection 1), CRH release was not different from basal release in either genotype. Data are expressed as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 compared with basal CRH release using paired t test.
We next investigated the effect of d-fen (1.0 μm; n = 10 per genotype) on CRH release. d-Fen stimulates the release of endogenous 5-HT and blocks its reuptake, thereby facilitating 5-HT action at all receptors. This provides a more physiologically relevant assessment of 5-HT-induced stimulation of CRH release than that obtained from 5-HT agonist treatment. We observed that d-fen significantly increased the percentage of CRH release in wild-type mice (Fig. 3a) (p < 0.05), but this effect was abolished in 5-HT2CR knock-out mice (Fig. 3b). These data provide compelling support for a critical role for 5-HT2CRs in 5-HT-induced CRH release.
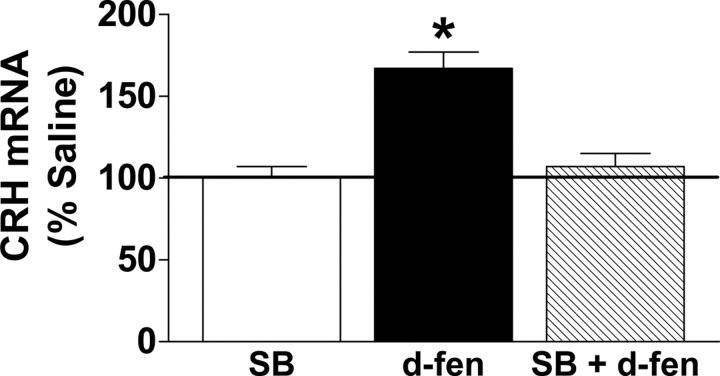
To extend these findings, we then examined whether d-fen alters CRH mRNA in vivo and whether acute pharmacological blockade of 5-HT2CRs would attenuate this effect. C57BL/6 mice were pretreated with 0.9% saline or the 5-HT2CR antagonist SB242084 (1.0 mg/kg, i.p.) and were treated with 0.9% saline or d-fen (3.0 mg/kg, i.p.) 30 min later (n = 4–5 per treatment). No differences were observed between SB242084 and 0.9% saline treatment. However, relative to SB242084, d-fen significantly increased hypothalamic CRH mRNA expression, and pretreatment with SB242084 abolished this effect (Fig. 4) (p < 0.05). These findings demonstrate that acute 5-HT2CR blockade is sufficient to render ineffective d-fen-induced increased CRH mRNA.
Figure 4.
Pharmacological 5-HT2CR blockade abolished d-fen-induced enhancement of CRH mRNA expression. Acute treatment with the 5-HT2CR antagonist SB242084 (SB; 1.0 mg/kg, i.p.; white bar) produced no effect on CRH mRNA compared with 0.9% saline treatment. Relative to SB treatment, d-fen (3.0 mg/kg, i.p.; black bar) significantly increased the percentage of CRH mRNA expression in the hypothalamus as measured by real-time quantitative PCR, and pretreatment with SB (1.0 mg/kg, i.p.; striped bar) abolished this effect. Data are expressed as mean ± SEM. *p < 0.05, d-fen compared with all other conditions.
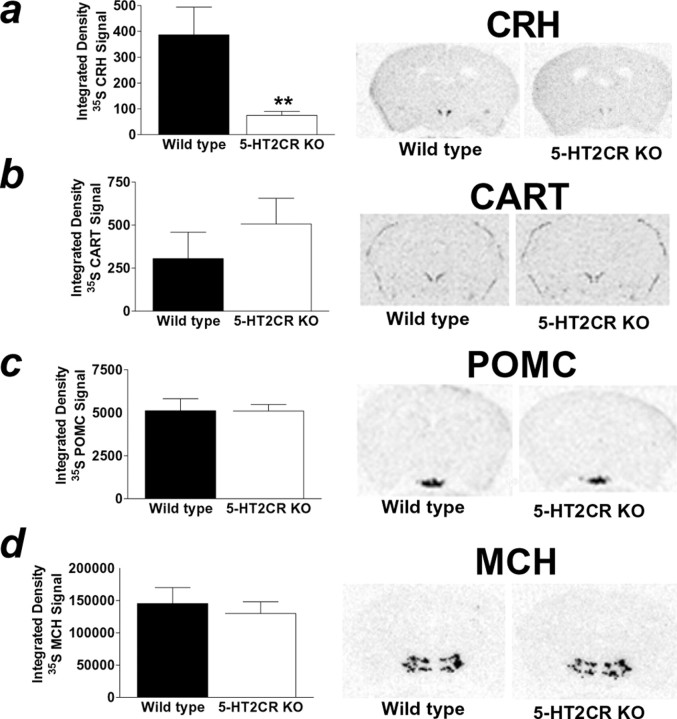
If 5-HT action at 5-HT2CRs is critical to 5-HT regulation of CRH release, then it may be hypothesized that mice lacking functional 5-HT2CRs will display a downregulation of CRH mRNA. We investigated this hypothesis by comparing basal CRH mRNA expression in 5-HT2CR-deficient and wild-type mice using ISHH (n = 8–9 per genotype). Densitometric analysis of CRH hybridization in the PVH revealed that 5-HT2CR knock-out mice displayed significantly reduced CRH mRNA expression compared with their wild-type littermates (Fig. 5a) (p < 0.01). We investigated the specificity of this effect by assessing expression of other hypothalamic neuropeptides in adjacent sections of tissue. Unlike CRH, 5-HT2CR knock-out mice exhibited levels of PVH CART mRNA similar to wild-type littermates (Fig. 5b). Similarly, 5-HT2CR knock-out and wild-type mice displayed similar levels of ARC POMC mRNA (Fig. 5c) and LHA MCH mRNA (Fig. 5d). Together, these data strongly support an integral role for the 5-HT2CRs in the regulation of CRH expression and in the subsequent release of CRH into the hypophyseal portal system.
Figure 5.
5-HT2CR knock-out mice displayed downregulated PVH CRH mRNA but similar PVH CART, ARC POMC, and LHA MCH mRNA expression compared with wild-type littermates. In situ hybridization of 35S-labeled CRH, CART, POMC, and MCH antisense probes was performed in adjacent coronal sections of wild-type (black bar) and 5-HT2CR knock-out (KO; white bar) littermate mouse brains. a, 5-HT2CR KO mice displayed reduced PVH CRH mRNA compared with wild-type littermates as determined by integrated density analysis of 35S-labeled CRH. The autoradiogram illustrates representative sections 0.82 caudal to bregma. b–d, In contrast, 5-HT2CR KO and wild-type mice exhibited comparable levels of PVH 35S-labeled CART (b; autoradiogram illustrates representative sections 0.82 caudal to bregma), similar levels of ARC 35S-labeled POMC (c; autoradiogram illustrates representative sections 1.70 caudal to bregma), and comparable levels of LHA 35S-labeled MCH (d; autoradiogram illustrates representative sections 1.94 caudal to bregma). Data are expressed as mean ± SEM. **p < 0.01, wild-type versus 5-HT2CR KO mice.
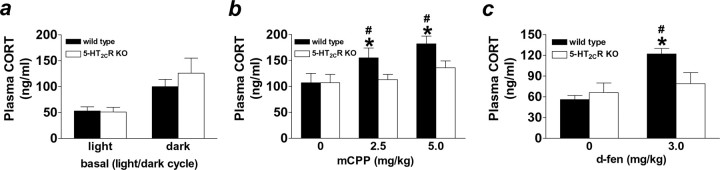
5-HT agonists require 5-HT2CRs to stimulate glucocorticoid release
CRH release from the PVH begins a cascade of endocrine events in the HPA axis. Consistent with their effects on CRH release, mCPP and d-fen significantly increase levels of plasma CORT (Sevy et al., 1994; Silverstone et al., 1994; Broocks et al., 2000). To determine whether 5-HT2CRs contribute to these actions, we compared 5-HT agonist-induced CORT release in 5-HT2CR knock-out and wild-type mice. We first assessed basal CORT in 5-HT2CR-deficient mice and wild-type littermates at the onset of the light and dark cycle. As is well established in rodents, mice displayed higher plasma CORT during the dark compared with light cycle. No significant phenotypic differences were found in basal plasma CORT (Fig. 6a). However, after 5-HT-induced HPA axis stimulation, a significant phenotypic difference in plasma CORT was observed. Of note, saline-treated mice displayed higher CORT in the mCPP study (Fig. 6b) compared with the d-fen study (Fig. 6c), and we attribute this variation to the use of different procedure rooms. Despite this, both mCPP (2.5 and 5.0 mg/kg) and d-fen (3.0 mg/kg) produced consistent and significant increases in CORT levels compared with saline treatment in wild-type mice (Fig. 6b,c) (p < 0.05). In contrast, 5-HT2CR knock-out mice did not alter CORT in response to mCPP or d-fen (Fig. 6b,c). These data indicate that action at 5-HT2CRs contributes to 5-HT-induced activation of the HPA axis, affecting both the release of CRH and the subsequent release of CORT.
Figure 6.
5-HT2CR knock-out mice displayed normal circadian CORT but did not respond to mCPP- or d-fen-stimulated CORT release. a, Wild-type (black bars) and 5-HT2CR knock-out (KO; white bars) mice displayed comparable levels of CORT measured in the plasma 1 h after the onset of the light and dark cycles. In contrast, a significant genotypic difference in CORT release was observed after HPA axis stimulation. b, c, Both mCPP (b) and d-fen (c) significantly increased in vivo release of CORT in wild-type mice but were ineffective in 5-HT2CR KO littermates. Data are expressed as mean ± SEM. #p < 0.05, drug versus 0.9% saline treatment; *p < 0.05, wild-type versus 5-HT2CR KO mice.
Discussion
A reciprocal functional interaction between the central 5-HT system and HPA axis has been shown to exist under normal physiological conditions and has been postulated to be of particular relevance in pathological states. Compounds used to increase 5-HT neurotransmission have been widely prescribed for the treatment of depression, panic disorder, and obesity, and some evidence suggests that the efficacy of these compounds may be related to their ability to correct concomitant dysregulation of the HPA axis (Linkowski et al., 1987; Brady et al., 1991). Indeed, compounds such as mCPP and d-fen both potently reduce food intake and enhance CRH and CORT release. Although not assessed here, it is intriguing to speculate that a mechanism through which 5-HT affects both feeding behavior and HPA axis activity is via regulation of CRH activity in the PVH.
Using complementary approaches in wild-type rats, wild-type mice, and mice with a 5-HTR gene disruption, here we demonstrate that 5-HT affects the HPA axis via regulation of CRH activity and expression through action at 5-HT2CRs. Specifically, we observed that 5-HT-mimetic agents commonly used to stimulate glucocorticoid release significantly activated CRH-expressing neurons, both in vivo and using electrophysiological techniques. Therefore, it is possible that although 5-HT-immunoreactive nerve terminals are not densely evident within the PVH (Sawchenko et al., 1983), the release of 5-HT is sufficient to substantially affect the activity of CRH-containing neurons.
To determine which receptor(s) contribute to 5-HT activation of CRH neurons, we extracted the rostral to caudal extent of the PVH and compared expression of 5-HTR genes using microarray transcriptomics. The preponderance of research thus far using compounds such as 5-HT2C/2AR agonist DOI and the high-affinity 5-HT1AR agonist 8-OH-DPAT has implicated 5-HT2ARs and 5-HT1ARs in 5-HT-induced ACTH and CORT release (Gilbert et al., 1988; Calogero et al., 1989, 1993; Pan and Gilbert, 1992; Zhang et al., 2004). We were therefore surprised that the 5-HTR gene transcript most highly expressed encoded the Gq-coupled 5-HT2CR, a receptor through which direct activation of CRH-containing neurons could be achieved. We confirmed the abundant expression of 5-HT2CRs in the rat PVH using ISHH. The diffuse expression of 5-HT2CRs in the PVH of rats and mice implicates this receptor in multiple PVH-regulated functions and behaviors. Of particular interest was the overlapping 5-HT2CR mRNA distribution with CRH-containing neurons. Using dual-neurohistochemical labeling, we observed that approximately one-half of CRH-expressing neurons coexpressed 5-HT2CRs. We speculate that the stringency of our dual-labeling criteria produced an underestimate of the actual endogenous coexpression rate predicted by the in vivo and electrophysiological studies. Alternatively, the consistent mCPP-induced activation of CRH-containing neurons may involve action at another 5-HTR coexpressed with CRH, such as the 5-HT2ARs, which were not assessed by the GeneChip. However, a recent report suggests that 5-HT2ARs are not critically involved in HPA axis activity, because the complete abolition of the 5-HT2AR gene does not impair or alter HPA axis function (Weisstaub et al., 2006).
The only other 5-HTR transcript identified as “present” in our sample was the Gi-coupled 5-HT1DRs. Of note, the 5-HT1D/1BR agonist CP94253 significantly induced FOS-IR in the PVH (Lee et al., 2004). Although not investigated here, it is possible that mCPP may also influence CRH activity indirectly through action at PVH 5-HT1DRs. For example, 5-HT1DRs may be expressed in PVH neuronal cell bodies or processes, providing a local inhibitory input onto CRH-containing neurons, and thereby mCPP action at PVH 5-HT1DRs may indirectly facilitate CRH activity. This possibility warrants additional consideration.
Contrary to expectation, we did not observe consistent or high 5-HT1AR transcript expression in the PVH. It is possible that 8-OH-DPAT may affect PVH CRH activity by reducing an inhibitory input arising from other brain regions via action at terminal 5-HT1ARs. Alternatively or additionally, 8-OH-DPAT may reduce the activity of the 5-HT transporter, thereby facilitating the action of 5-HT at PVH CRH 5-HT2CR-expressing neurons. 8-OH-DPAT also has some affinity for the 5-HT2CRs (pKi 5.2), in addition to the 5-HT1ARs (pKi 8.7) (Hoyer 1989), and it is therefore conceivable that 8-OH-DPAT may affect CRH release directly through action at PVH 5-HT2CRs.
To discern the selective effect of action at 5-HT2CR in CRH activity, we recorded from neurons in the presence of mCPP and the 5-HT2CR antagonist RS102221. We observed that mCPP reliably depolarized the mpPVH neurons, an effect blocked by RS102221. These data suggest that 5-HT enhances the activity of PVH CRH neurons via activation of 5-HT2CRs. Moreover, 5-HT2CR knock-out mice do not increase CRH release or CRH mRNA in response to mCPP and/or d-fen. Given that mCPP has affinity for multiple 5-HT receptors, and d-fen increases 5-HT availability at all receptors, these data strongly argue for the selective role of 5-HT2CRs in CRH release and expression. These findings do not appear to be a consequence of developmental compensatory changes in central 5-HT circuits, because 5-HT2CR knock-out mice display levels of many 5-HT system-related genes similar to those of wild-type littermates (Lopez-Gimenez et al., 2002). However, the possibility remains that our results may be influenced by a compensatory alteration in neurocircuitry affecting CRH neurons arising from the constitutive genetic ablation of the 5-HT2CRs in the knock-out mice. For this reason, complementary studies were performed in wild-type rodents, and the data obtained support an integral role for 5-HT2CRs in 5-HT activation of CRH neurons.
The dramatic basal downregulation of CRH mRNA in the 5-HT2CR knock-out mice indicates that 5-HT tonically regulates the expression of PVH CRH mRNA via action at these receptors. The normal basal CORT exhibited by the 5-HT2CR knock-out mice in the face of PVH CRH mRNA suppression was surprising (Nonogaki et al., 1998), given that glucocorticoid feedback regulates PVH CRH mRNA (Herman et al., 1990). A possible explanation for this is that the combined activity of PVH arginine vasopressin (AVP) and CRH neurons is sufficient to induce adequate basal ACTH release to promote wild-type-like basal CORT in the 5-HT2CRs knock-out mice, whereas under HPA axis stimulation, the deficiency in CRH is evident. Interestingly, genetic inactivation of 5-HT3ARs has been demonstrated to reduce PVH AVP mRNA (Bhatnagar et al., 2004), suggesting that 5-HT may regulate multiple PVH neuropeptides. These data are consistent with our findings that both mCPP and d-fen induced FOS-IR in non-CRH-labeled PVH neurons. Future studies are required to investigate whether 5-HT2CRs knock-out mice also display altered HPA axis activation after behavioral stress stimuli and the extent to which the downregulated CRH mRNA affects HPA axis function under varying physiological conditions of HPA axis activity.
Altered HPA axis function in 5-HT2CR knock-out mice may be relevant to the enhanced antidepressant-like effect of selective serotonin reuptake inhibitors observed in 5-HT2CR knock-out mice (Cremers et al., 2004) and the hyperphagia and obesity phenotype (Tecott et al., 1995; Nonogaki et al., 1998). In light of these phenotypic results, it is possible that 5-HT2CR-induced CRH activity may be particularly germane in pathological states such as depression and obesity.
In summary, we show for the first time that 5-HT2CRs are not only expressed in CRH-containing neurons in the PVH but that 5-HT circuits regulate the expression of PVH CRH mRNA. We further demonstrate that 5-HT2CR-deficient mice exhibit blunted CRH and CORT release after 5-HT-induced HPA axis stimulation. The data presented define a mechanism underlying the longstanding observation of 5-HT-regulated glucocorticoid release and provide insight into upstream circuits affecting the neuroendocrine response to stress. These findings provide a new model to investigate the concomitant dysregulation of central 5-HT circuits and the HPA axis in the etiology and treatment of pathological states related to mood and energy homeostasis.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01DK65171, the National Alliance for Research on Schizophrenia and Depression, and the Bank of America–Giannini Foundation (L.K.H.); Scientific Research (C2) and Takeda Research Foundation (K.N.); the Wellcome Trust (G.S.H.Y., S.O'R.); the Medical Research Council (L.T., S.O'R.); National Institute of Mental Health (NIMH) Grant R01MH061583 and NIDDK Grant P01DK056116 (J.K.E.); NIMH Grant R01MH61624 and the EJLB Foundation (L.H.T.); National Institutes of Health Grant R01AG20904 and Ellison Medical Foundation Grant AG-NS-0201 (J.R.); and Canadian Institutes of Health Research Grant MT 10520 and Merck Research Laboratories (W.F.C.).
References
- Bagdy G, Calogero AE, Aulakh CS, Szemeredi K, Murphy DL. Long-term cortisol treatment impairs behavioral and neuroendocrine responses to 5-HT1 agonists in the rat. Neuroendocrinology. 1989;50:241–247. doi: 10.1159/000125248. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Sun LM, Raber J, Maren S, Julius D, Dallman MF. Changes in anxiety-related behaviors and hypothalamic-pituitary-adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav. 2004;81:545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Brady LS, Whitfield HJ, Jr, Fox RJ, Gold PW, Herkenham M. Long-term antidepressant administration alters corticotropin-releasing hormone, tyrosine hydroxylase, and mineralocorticoid receptor gene expression in rat brain. Therapeutic implications. J Clin Invest. 1991;87:831–837. doi: 10.1172/JCI115086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, George A, Jestrabeck C, Opitz M, Bartmann U, Gleiter CH, Meineke I, Roed IS, Ruther E, Hajak G. Increased psychological responses and divergent neuroendocrine responses to m-CPP and ipsapirone in patients with panic disorder. Int Clin Psychopharmacol. 2000;15:153–161. doi: 10.1097/00004850-200015030-00004. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bernardini R, Margioris AN, Bagdy G, Gallucci WT, Munson PJ, Tamarkin L, Tomai TP, Brady L, Gold PW, Chrousos GP. Effects of serotonergic agonists and antagonists on corticotropin-releasing hormone secretion by explanted rat hypothalami. Peptides. 1989;10:189–200. doi: 10.1016/0196-9781(89)90096-x. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bagdy G, Moncada ML, D'Agata R. Effect of selective serotonin agonists on basal, corticotrophin-releasing hormone- and vasopressin-induced ACTH release in vitro from rat pituitary cells. J Endocrinol. 1993;136:381–387. doi: 10.1677/joe.0.1360381. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Chen C, Clarke IJ. Estrogen transiently increases delayed rectifier, voltage-dependent potassium currents in ovine gonadotropes. Neuroendocrinology. 1999a;69:254–260. doi: 10.1159/000054426. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999b;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, Honig G, Bogeso KP, Westerink BH, den Boer H, Wikstrom HV, Tecott LH. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD. Effect of serotonin-releasing drugs on serum corticosterone concentration in rats. Neuroendocrinology. 1980;31:96–100. doi: 10.1159/000123057. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD. Serotonin receptor subtypes involved in the elevation of serum corticosterone concentration in rats by direct- and indirect-acting serotonin agonists. Neuroendocrinology. 1990;52:206–211. doi: 10.1159/000125586. [DOI] [PubMed] [Google Scholar]
- Gibbs DM, Vale W. Effect of the serotonin reuptake inhibitor fluoxetine on corticotropin-releasing factor and vasopressin secretion into hypophysial portal blood. Brain Res. 1983;280:176–179. doi: 10.1016/0006-8993(83)91189-7. [DOI] [PubMed] [Google Scholar]
- Gilbert F, Brazell C, Tricklebank MD, Stahl SM. Activation of the 5-HT1A receptor subtype increases rat plasma ACTH concentration. Eur J Pharmacol. 1988;147:431–439. doi: 10.1016/0014-2999(88)90178-1. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Clinical studies with corticotropin releasing factor: implications for the diagnosis and pathophysiology of depression, Cushing's disease, and adrenal insufficiency. Psychoneuroendocrinology. 1985;10:401–419. doi: 10.1016/0306-4530(85)90080-0. [DOI] [PubMed] [Google Scholar]
- Guillemin R, Rosenberg B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955;57:599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, Cone RD, Elmquist JK. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann NY Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang C-Y, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Herman JP, Wiegand SJ, Watson SJ. Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations. Endocrinology. 1990;127:2408–2417. doi: 10.1210/endo-127-5-2408. [DOI] [PubMed] [Google Scholar]
- Hoyer D. 5-Hydroxytryptamine receptors and effector coupling mechanisms in peripheral tissues. In: Fozard J, editor. Peripheral actions of 5-HT. Oxford: Oxford UP; 1989. pp. 72–99. [Google Scholar]
- Hu SB, Lightman SL, Tannahill LA. 5-Hydroxytryptamine stimulates corticosteroid-sensitive CRF release from cultured foetal hypothalamic cells. Role of protein kinases. Brain Res. 1992;574:266–270. doi: 10.1016/0006-8993(92)90826-u. [DOI] [PubMed] [Google Scholar]
- Javed A, Kamradt MC, Van de Kar LD, Gray TS. D-Fenfluramine induces serotonin-mediated Fos expression in corticotropin-releasing factor and oxytocin neurons of the hypothalamus, and serotonin-independent Fos expression in enkephalin and neurotensin neurons of the amygdala. Neuroscience. 1999;90:851–858. doi: 10.1016/s0306-4522(98)00523-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Muller M, Warberg J. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J Neuroendocrinol. 2002;14:788–795. doi: 10.1046/j.1365-2826.2002.00839.x. [DOI] [PubMed] [Google Scholar]
- Kelly WF, Checkley SA, Bender DA. Cushing's syndrome, tryptophan and depression. Br J Psychiatry. 1980;136:125–132. doi: 10.1192/bjp.136.2.125. [DOI] [PubMed] [Google Scholar]
- Kennet GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Koe KB, Nielsen JA, Macor JE, Heym J. Biochemical and behavioural studies of the 5-HT1B receptor agonist, CP-94,253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- Laferrere B, Lahlou N, Saltiel H, Roger M, Basdevant A, Oppert JM, Guy-Grand B. Hypersensitivity of the corticotropic axis to the serotoninergic agent clomipramine in obese women. Obes Res. 1994;2:328–336. doi: 10.1002/j.1550-8528.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Bovetto S, Richard D, Rivest S. Effect of dexfenfluramine on the transcriptional activation of CRF and its type 1 receptor within the paraventricular nucleus of the rat hypothalamus. Br J Pharmacol. 1996;117:1021–1034. doi: 10.1111/j.1476-5381.1996.tb16692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG. Tonic regulation of satiety by 5-HT receptors in the mouse: converging evidence from behavioural and c-fos immunoreactivity studies. Eur J Neurosci. 2004;19:3017–3025. doi: 10.1111/j.0953-816X.2004.03406.x. [DOI] [PubMed] [Google Scholar]
- Li BH, Rowland NE. Dexfenfluramine induces Fos-like immunoreactivity in discrete brain regions in rats. Brain Res Bull. 1993;31:43–48. doi: 10.1016/0361-9230(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, Copinschi G, Van Cauter E. 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab. 1987;65:141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Tecott LH, Palacios JM, Mengod G, Vilaro MT. Serotonin 5- HT (2C) receptor knockout mice: autoradiographic analysis of multiple serotonin receptors. J Neurosci Res. 2002;67:69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Molineaux SM, Jessell TM, Axel R, Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Ohashi-Nozue K, Oka Y. A negative feedback system between brain serotonin systems and plasma active ghrelin levels in mice. Biochem Biophys Res Commun. 2006;341:703–707. doi: 10.1016/j.bbrc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Pan L, Gilbert F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology. 1992;56:797–802. doi: 10.1159/000126332. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain, in stereotaxic coordinates. Ed 2. San Diego: Academic; 2001. [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology (Berl) 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- Pronchuk N, Beck-Sickinger AG, Colmers WF. Multiple NPY receptors inhibit GABA(A) synaptic responses of rat medial parvocellular effector neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2002;143:535–543. doi: 10.1210/endo.143.2.8655. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Raber J, Bloom FE. IL-2 induces vasopressin release from the hypothalamus and the amygdala: role of nitric oxide-mediated signaling. J Neurosci. 1994;14:6187–6195. doi: 10.1523/JNEUROSCI.14-10-06187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Koob GF, Bloom FE. Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling; comparison with the hypothalamic response. J Pharmacol Exp Ther. 1995;272:815–824. [PubMed] [Google Scholar]
- Richard D, Rivest S, Rivier C. The 5-hydroxytryptamine agonist fenfluramine increases Fos-like immunoreactivity in the brain. Brain Res. 1992;594:131–137. doi: 10.1016/0006-8993(92)91037-f. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Sevy S, Brown SL, Wetzler S, Kotler M, Molcho A, Plutchik R, van Praag HM. Effects of alprazolam on increases in hormonal and anxiety levels induced by meta-chlorophenylpiperazine. Psychiatry Res. 1994;53:219–229. doi: 10.1016/0165-1781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Rue JE, Franklin M, Hallis K, Camplin G, Laver D, Cowen PJ. The effects of administration of mCPP on psychological, cognitive, cardiovascular, hormonal and MHPG measurements in human volunteers. Int Clin Psychopharmacol. 1994;9:173–178. doi: 10.1097/00004850-199409000-00005. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Vielhaber K, Riemann D, Feige B, Kuelz A, Kirschbaum C, Voderholzer U. Impact of experimentally induced serotonin deficiency by tryptophan depletion on saliva cortisol concentrations. Pharmacopsychiatry. 2005;38:87–94. doi: 10.1055/s-2005-837808. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung J-P, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gray TS, D'Souza DN, Carrasco GA, Damjanoska KJ, Dudas B, Garcia F, Zainelli GM, Sullivan Hanley NR, Battaglia G, Muma NA, Van de Kar LD. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J Pharmacol Exp Ther. 2004;310:59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]