Abstract
Depolarization-induced suppression of inhibition (DSI) is an endocannabinoid-mediated short-term plasticity mechanism that couples postsynaptic Ca2+ rises to decreased presynaptic GABA release. Whether the gain of this retrograde synaptic mechanism is subject to long-term modulation by glutamatergic excitatory inputs is not known. Here, we demonstrate that activity-dependent long-term DSI potentiation takes place in hippocampal slices after tetanic stimulation of Schaffer collateral synapses. This activity-dependent, long-term plasticity of endocannabinoid signaling was specific to GABAergic synapses, as it occurred without increases in the depolarization-induced suppression of excitation. Induction of tetanus-induced DSI potentiation in vitro required a complex pathway involving AMPA/kainate and metabotropic glutamate receptor as well as CB1 receptor activation. Because DSI potentiation has been suggested to play a role in persistent limbic hyperexcitability after prolonged seizures in the developing brain, we used these mechanistic insights into activity-dependent DSI potentiation to test whether interference with the induction of DSI potentiation prevents seizure-induced long-term hyperexcitability. The results showed that the in vitro, tetanus-induced DSI potentiation was occluded by previous in vivo fever-induced (febrile) seizures, indicating a common pathway. Accordingly, application of CB1 receptor antagonists during febrile seizures in vivo blocked the seizure-induced persistent DSI potentiation, abolished the seizure-induced upregulation of CB1 receptors, and prevented the emergence of long-term limbic hyperexcitability. These results reveal a new form of activity-dependent, long-term plasticity of endocannabinoid signaling at perisomatic GABAergic synapses, and demonstrate that blocking the induction of this plasticity abolishes the long-term effects of prolonged febrile seizures in the developing brain.
Keywords: cannabinoid, inhibition, plasticity, seizure, development, hippocampus
Introduction
Febrile seizures are the most common form of childhood seizures, affecting 3–5% of infants (Shinnar and Glauser, 2002). Systematic prospective experimental studies found that prolonged (>15 min) febrile seizures in the developing rat brain persistently increased hippocampal excitability (Baram et al., 1997; Chen et al., 1999; Dube et al., 2000, 2006), in general agreement with a potential clinical association of prolonged, complex febrile seizures with temporal lobe epilepsy (Annegers et al., 1987; Cendes et al., 1993; French et al., 1993; Hesdorffer and Hauser, 2002). Therefore, uncovering the mechanisms that link prolonged febrile seizures to long-term decreases in seizure threshold remains an important task (Schuchmann et al., 2006).
After experimental febrile seizures, there is an enhancement of hippocampal GABA release without significant changes in excitatory transmission (Chen et al., 1999) or cell loss (Toth et al., 1998; Bender et al., 2003), but seizure threshold is decreased (Dube et al., 2000). Recently, a solution to this paradox has been suggested involving the long-lasting potentiation of depolarization-induced suppression of inhibition (DSI) by experimental febrile seizures (Chen et al., 2003). DSI is a form of short-term plasticity (Llano et al., 1991; Pitler and Alger, 1992) mediated by CB1 receptors (Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Wilson et al., 2001; Wilson and Nicoll, 2001), the most numerous G-protein-coupled receptors in the brain (Herkenham et al., 1990; Matsuda et al., 1990). Postsynaptic depolarization and subsequent Ca2+ rise triggers local endocannabinoid production, resulting in inhibition of GABA release from CB1-containing terminals. After febrile seizures, DSI amplitude and the number of presynaptic CB1 receptors are increased (Chen et al., 2003). DSI can occur after seizure-like discharges in pyramidal cells (Beau and Alger, 1998), and long-term increase in DSI is expected to facilitate seizures (Chen et al., 2003). Persistent DSI potentiation by febrile seizures is selective, because endocannabinoid-mediated, depolarization-induced suppression of excitation (DSE) (Ohno-Shosaku et al., 2002) remains unaffected (Chen et al., 2003).
The cannabinoid system is linked to epilepsy by additional evidence. High-frequency stimulation in slices or kainate-induced seizures in vivo leads to endocannabinoid production and release (Stella et al., 1997; Marsicano et al., 2003; Wettschureck et al., 2006), and CB1 receptors are upregulated after pilocarpine-induced status epilepticus (Wallace et al., 2003). Exogenously applied cannabinoid agonists are acutely anticonvulsant (Corcoran et al., 1973; Chesher and Jackson, 1974; Karler et al., 1974; Wada et al., 1975; Consroe and Wolkin, 1977; Chiu et al., 1979; Wallace et al., 2001, 2002; Shafaroodi et al., 2004; Blair et al., 2006), whereas cannabinoid antagonists are acutely proconvulsant (Wallace et al., 2002; Marsicano et al., 2003; Bernard et al., 2005). Therefore, endocannabinoid signaling significantly modulates seizures acutely (Monory et al., 2006) and, conversely, seizures persistently alter the endocannabinoid system.
Here, we show that induction of DSI potentiation is activity-dependent and requires CB1 receptors. When febrile seizures take place in the presence of CB1 antagonists, long-term enhancement of hippocampal excitability does not develop, indicating that the endocannabinoid system is a potential new target to prevent limbic hyperexcitability after prolonged developmental seizures.
Materials and Methods
Slice preparation.
Horizontal brain slices (450 μm; for paired recordings, 350 μm) were prepared as described previously (Chen et al., 1999, 2001; Foldy et al., 2006) from 16- to 18-d-old control or experimental littermate Sprague Dawley rats (Zivic-Miller, Zelienople, PA) 1 week after seizure induction. The animals were anesthetized with halothane, decapitated, and their brains were removed. The slices were incubated at 32°C in oxygenated (95% O2/5% CO2) artificial CSF [ACSF; containing the following (in mm): 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 1.25 NaH2PO4, and 10 glucose] in a holding chamber for at least 1 h before stimulating or recording. For visualized recordings, slices were sectioned and incubated in oxygenated, sucrose-containing ACSF [containing the following (in mm): 85 NaCl, 75 sucrose, 2.5 KCl, 25 glucose, 1.25 NaH2PO4, 4 MgCl2, 0.5 CaCl2, and 24 NaHCO3] for 1 h and then transferred to regular ACSF.
Hippocampal-entorhinal combined slices (Rafiq et al., 1995; Dube et al., 2000) were sectioned (450 μm) in cooled, oxygenated high-sucrose ACSF [containing the following (in mm): 200 sucrose, 3 KCl, 1.25 m NaH2PO4, 26 NaHCO3, 10 glucose, 0.9 MgCl2, and 2 CaCl2] along a 12° inclined transverse plane. Slices were then incubated at 32°C in an oxygenated low-Mg2+ ACSF [containing the following (in mm): 130 NaCl, 3 KCl, 26 NaHCO3, 2 CaCl2, 0.5 MgCl2, 1.25 NaH2PO4, and 10 mm glucose] for at least 1 h.
Electrophysiology.
For blind whole-cell recordings, individual slices were transferred to an interface-type recording chamber perfused with oxygenated ACSF at 35°C, which, depending on the experiment, contained some of the following drugs: 10 μm d-2-amino-5phosphovaleric acid (APV), 10 μm 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX), 5 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 200 μm LY341495, 10 μm bicuculline methochloride, or 1 μm SR141716A (SR). Tetanic stimulation (5 trains of 1 or 10 s stimuli at 100 Hz, with a stimulus width of 20 μs, and intensity of 2 mA, delivered at 30 s intervals) was applied through a bipolar 90 μm tungsten electrode placed in the stratum radiatum at the CA1–CA3 border. Sham control slices were handled the same way, but the stimulating current was not delivered. The slices were returned to the incubating chamber for washing in control ACSF, and kept for a minimum of 1 h before recording. Recordings were obtained using an Axopatch 200A amplifier (Molecular Devices, Union City, CA). Spontaneous IPSCs (sIPSCs) were recorded in ACSF containing APV, NBQX (or in some experiments, CNQX), and 5 μm carbachol. To evoke EPSCs or perisomatic IPSCs (latency, <3.1 ms), constant-current stimuli (20 μs) were applied through the tungsten electrode. The placement and distance of the recording and stimulating electrodes were kept constant as described previously (Chen et al., 1999). The recorded cells were from the middle third of the CA1 region (i.e., about half-way between the CA3 and the subiculum). Pipette solutions for DSI experiments consisted of the following (in mm): 140 CsCl, 2 MgCl2, 10 HEPES, 3 QX314, 0.2 EGTA, 1 MgATP, and 0.2 Na2GTP (for eIPSCs, no EGTA, ATP, or GTP was included in the pipette; for DSE, CsCl was replaced with K gluconate). Note that these pipette solutions were identical to those used previously to obtain data on the effects of febrile seizures on endocannabinoid signaling (Chen et al., 2003). The depolarizing pulse used to evoke DSI was a 100 or 500 ms step to 0 mV from a holding potential of −60 mV. For eEPSCs, recordings were in ACSF containing bicuculline, and DSE was induced by a depolarizing step of 10 s duration.
For extracellular recordings in hippocampal-entorhinal combined slices (Rafiq et al., 1995; Dube et al., 2000) performed in low-Mg2+ ACSF, recording pipettes were filled with normal ACSF and placed in the CA1 pyramidal cell layer, and the tungsten stimulating electrode was positioned in the CA1 stratum radiatum. The stimulation protocol consisted of a 2 s, 60 Hz train of stimuli, and stimulus intensities were adjusted to four times the minimal intensity required to evoke a population spike of 0.5 mV in amplitude, usually 3 to 4 mA. Six stimulating trains with a 10 min interval were applied to each slice, unless sustained epileptiform activity developed. Recording was terminated if sustained seizure-like activity lasted for >35 min. After the recording of the responses to the stimulating trains, the recording pipette was placed at four additional recording sites in CA1, to ascertain the network-wide nature of the recorded population activity. Duration of epileptiform discharges was determined by considering episodes with repetitive spikes, with amplitudes >2 times the background and frequency >1 Hz, lasting for >3 s (Dube et al., 2000).
For visualized paired whole-cell recordings, slices were transferred to a submerged-type recording chamber perfused with oxygenated ACSF at 33°C. Slices were visualized with an upright microscope (BX-50; Olympus, Tokyo, Japan) with infrared differential interference contrast optics. Whole-cell recordings were obtained from interneurons with patch pipettes (3–5 MΩ) filled with internal solution containing the following (in mm): 126 K-gluconate, 4 KCl, 10 HEPES, 4 MgATP, 0.3 Na2GTP, 10 phosphocreatine, and 0.2% biocytin, pH 7.2, 270–290 mOsm. The interneurons were all DSI-sensitive [hence CB1 receptor expressing, cholecystokinin (CCK) positive] interneurons that evoked fast unitary IPSCs (Foldy et al., 2006). Pyramidal cells were recorded with internal solution containing the following (in mm): 40 KCl, 90 K-gluconate, 1.8 NaCl, 1.7 MgCl2, 3.5 KCl, 0.05 EGTA, 10 HEPES, 2 Mg-ATP, 0.4 Na2-GTP, and 10 phosphocreatine, pH 7.2, 270–290mOsm. DSI was elicited with a 500 ms depolarizing pulse to 0 mV from a holding potential of −70 mV. Recordings were made using MultiClamp 700A and MultiClamp 700B amplifiers (Molecular Devices). Immunoreactivity for CCK and parvalbumin was determined as described by Foldy et al. (2006). For CCK, we used a mouse monoclonal antibody [mAb 9303; diluted 1:1000; generously provided by the Antibody/Radioimmunoassay Core of the CURE/Digestive Diseases Research Center (University of California, Los Angeles, Los Angeles, CA), and National Institutes of Health Grant DK41301]. For parvalbumin, a rabbit polyclonal antibody (PV-28; Swant, Bellinzona, Switzlerland) was used. For further details, see Foldy et al. (2006).
All drugs were obtained from Tocris, (Ellisville, MO) except for carbachol, which was obtained from Sigma (St. Louis, MO), and SR141716A, which was obtained from the National Institute of Mental Health Chemical Synthesis Program at either SRI International (Palo Alto, CA) or Research Triangle Institute (Research Triangle Park, NC).
Hyperthermia-induced experimental febrile seizures.
The hyperthermia-induced experimental prolonged febrile seizure paradigm has been described previously (Baram et al., 1997; Dube et al., 2000). Briefly, on postnatal day 10 (P10), the core temperature of pups was raised using a regulated stream of moderately heated air. Rectal temperatures were measured at baseline, at 2 min intervals, and at the onset of hyperthermic seizures, which occur in virtually all rats. Hyperthermia (defined as core temperature >39.5°C) was maintained for 30 min, aiming for a core temperature of 41–42°C, and the presence and duration of seizures for each pup were noted at 2 min intervals. The number of seizure sessions was defined as the number of 2 min intervals in which the rat was seizing. After the hyperthermia period, rats were placed on a cool surface, monitored for 15 min, and then returned to home cages. For the animals that received an injection of the CB1 receptor antagonist SR141716A systemically, SR141716A was first dissolved in alcohol and then normal saline was added (alcohol to saline ratio, 1:20). The total volume injected intraperitoneally to each pup was 70 μl. The composition and volume of the control vehicle solution were the same as in the case of the SR141716A-containing solution, but no SR141716A was added. The injection was administered 1 h before hyperthermia was performed (SR141716A dose was 10 mg/kg or 1 mg/kg, as specified in the text).
Western blots.
Hippocampi from either controls or animals with hypothermia-induced seizures (HT), which had been preinjected with either SR or vehicle, were prepared for Western blots as described previously (Chen et al., 2003). Briefly, hippocampi were homogenized in fivefold (volume/weight) excess buffer containing 25 mm HEPES, 10 mm EDTA, 6 mm MgCl, 100 μm PMSF, 10 μm leupeptin, 100 μm benzamidine, and 10 μm aprotenin, pH 7.4. Homogenates were centrifuged for 5 min at 1000 × g, the supernatants saved, and the pellets were rehomogenized in buffer and centrifuged as above. The protein concentrations of the combined supernatants were determined using a Bio-Rad (Hercules, CA) assay. Protein (75 μg) from each animal were separated by SDS-PAGE and transferred to nitrocellulose. Western blot analysis was performed blind with respect to experimental treatment using an antibody raised against the last 15 amino acids of rat CB1 receptor fused to glutathione S-transferase (CB1 L15; 1:250). The antibody was purified and specificity verified as described previously (Bodor et al., 2005). The top portion of the blot containing the higher molecular weight proteins was probed with antibodies (Sigma) to neurofilament polypeptides with apparent molecular weight of 160 kDa and 200 kDa. The CB1 band density was normalized to the neurofilament band density to control for small variations in protein loading. Immunoreactivity was visualized using HRP-conjugated secondary antibodies and DAB/Ni2+ staining (Vector Laboratories, Burlingame, CA). The intensities of the immunoreactive bands were quantified using NIH Image software (http://rsb.info.nih.gov/nih-image/).
EEG recordings.
For implantation of hippocampal depth electrodes (single twisted-wire electrode; Plastics One, Roanoke, VA) rats were anesthetized with ketamine/xylazine and placed in a stereotaxic frame. The scalp was incised, and a small hole was drilled into the right parietal bone. The electrode was placed into the brain stereotaxically and fixed in place with cyanoacrylate adhesive, steel screws, and dental cement. The wound was closed around the electrode with sutures and the animals were placed on a heating pad for recovery and returned to their cage after awakening. For adult rats (6 weeks after HT), the coordinates of the electrodes were as follows: anteroposterior (AP), 3.3 mm from bregma, lateral (L), 2.2 mm; ventral (V), 3.0 mm. For juvenile rats (P8), the coordinates were as follows: AP, 2.0 mm from bregma; L, 2.0 mm; V, 2.5 mm vertical. Implantation was performed 2 to 3 d before EEG recording. Electrode position was subsequently verified from histology. On the day of the recording, animals were placed into the recording chamber and the implanted electrodes were attached to a flexible cable. The signal was amplified at a gain of 1000 on a Brownlee Precision (Santa Clara, CA) 210A amplifier. Baseline EEG activity was recorded for 10 min, and then a single dose of kainate (5 mg/kg, dissolved in saline to 5 mg/ml) was given intraperitoneally. EEGs were then recorded for 60 min continuously. The criteria for an EEG seizure were the following: (1) the presence of repetitive spike and sharp-wave discharges, with a frequency >1 Hz, (2) amplitude of activity >2 times baseline activity, and (3) a duration of >5 s. A seizure was considered to terminate when this activity stopped for >3 s.
Data analysis.
Recordings were filtered at 4 or 5 kHz using a Bessel filter and digitized at 10 kHz with a Digidata 1322A analog–digital interface (Molecular Devices). Statistical analyses were performed with SigmaPlot or SPSS software (SPSS, Chicago, IL) using a t test (for data containing two groups) or an f test (for more than two groups), or the nonparametric Kruskal–Wallis test (for the hippocampal–entorhinal cortex combined slice data) with a level of significance of p < 0.05. Data are presented as mean ± SEM, and n is the number of recorded cells, slices, or animals.
Results
Strategy for the prevention of the long-term consequences of febrile seizures through modulating plasticity of endocannabinoid signaling
How do hyperthermia-induced experimental febrile seizures (HTs) lead to persistent potentiation of endocannabinoid signaling at perisomatic GABAergic synapses in the hippocampus (Chen et al., 2003), and does prevention of DSI potentiation block the HT-induced long-term enhancement of limbic network excitability (Dube et al., 2000)? To answer these important questions, a multistep strategy was implemented. First, to circumvent difficulties associated with in vivo testing of drug effects during HT induction, we identified an in vitro paradigm of DSI potentiation. Second, using blockers for various neurotransmitters and neuromodulators in the in vitro paradigm, we delineated key steps involved in the induction of DSI potentiation. Third, we determined that the receptor blockers that were effective in the in vitro paradigm prevented the in vivo, HT-induced DSI potentiation. Finally, we showed that approaches effective at blocking DSI potentiation also prevented the long-term enhancement of limbic network hyperexcitability after HT in vivo.
Increased neuronal activity results in potentiation of DSI in control slices
We reasoned that if in vivo febrile seizures enhance DSI in CA1 pyramidal cells through increased neuronal activity alone, then electrical stimulation of afferent fibers in acute in vitro slices may be able to enhance DSI. However, such an effect has not been described previously. In fact, there is evidence that certain stimulation paradigms can depress inhibitory transmission from specific populations of CB1-expressing fibers [resulting in inhibitory long-term depression (iLTD)] (Chevaleyre and Castillo, 2003, 2004). Therefore, we tested whether increased neuronal activity in hippocampal slices results in potentiation of DSI in CA1 pyramidal cells.
Tetanic stimulation (5× 10 s at 100 Hz) in the CA1 stratum radiatum layer in acute hippocampal slices from control animals that did not experience previous febrile seizures resulted in a significant potentiation of DSI (Fig. 1 A,B) (age, P18; tetanization was performed in control ACSF, followed by at least 1 h incubation in control ACSF before DSI was measured). Specifically, compared with sham-stimulated controls, tetanic stimulation significantly increased the peak amplitude of DSI of sIPSCs (recorded in the presence of the ionotropic glutamate receptor blockers 5 μm CNQX, 10 μm APV, and the muscarinic agonist 5 μm carbachol to enhance sIPSC frequency) (Martin et al., 2001; Kim et al., 2002) (Fig. 1 A,B,D, left pair of bar graphs) [DSI amplitude, expressed as percent decrease in sIPSC charge transfer after the depolarizing pulse, with respect to the prepulse control period, in cells from sham-stimulated slices (“sham-tet”), 18.9 ± 4.0%, n = 11 cells; DSI in cells from tetanically stimulated slices (“tetanus”), 37.8 ± 4.2%, n = 10].
Figure 1.
Increased neuronal activity leads to potentiation of DSI. A, B, Strong (5 × 10 s at 100 Hz) tetanic stimulation in the stratum radiatum (including the Schaffer collateral pathway) in slices from control animals increased DSI amplitude and prolonged the DSI decay in CA1 pyramidal cells. Example traces are shown in A; summary data are shown in B (based on 3 DSI episodes per cell separated by 1 min). Square waves above traces and summary graph indicate time and duration of depolarization. C, Even after tetanic stimulation, DSI is completely blocked in the presence of the CB1 receptor antagonist SR141716 (1 μm). D, Tetanic stimulation and hyperthermic seizures (performed 1 week before recording), both potentiate DSI to a similar extent. Additionally, tetanic stimulation cannot further potentiate DSI in slices from HT animals, suggesting a common mechanism of potentiation. E, The potentiation of DSI requires a strong tetanic stimulation, as a weaker protocol (5 × 1 s at 100 Hz) does not increase DSI. For all experiments in this figure, DSI was induced by a 500 ms depolarization to 0 mV and recorded in the presence of carbachol and ionotropic glutamate receptor blockers (see Materials and Methods). Recordings were made at least 1 h after sham stimulation (sham-tet) or tetanus (tetanus). In this and following figures, asterisks indicate significant differences (p < 0.05).
In addition to enhancing the peak DSI, tetanization also significantly prolonged the duration of DSI (Fig. 1 A,B) (DSI single exponential decay time constants in cells from sham-tetanized slices, τdecay = 2.06 ± 0.32 s; tetanized, τdecay = 9.19 ± 2.17 s). Therefore, tetanization of afferents in acute slices from control animals was able to reproduce the two major, characteristic changes in DSI that were reported to take place after in vivo febrile seizures, namely, the significant potentiation of DSI peak amplitude and the prolongation of DSI decay.
In vitro, tetanus-induced DSI potentiation was long-lasting, persisting as long as slices could be kept viable (up to 5 h), and the DSI potentiation was evident in virtually all CA1 pyramidal cells. The potentiated DSI after the tetanization, similar to DSI in sham-stimulated control slices, could be abolished with 1 μm of the CB1 antagonist SR141716A [rimonabant (Rinaldi-Carmona et al., 1994)] (Fig. 1 C) (DSI in the presence of SR141716A, in cells from sham-stimulated slices, 4.5 ± 5.0%, n = 5; in tetanized slices, −0.4 ± 7.9%, n = 5), indicating that the potentiated DSI is mediated by CB1 receptors. Furthermore, the DSI potentiation took place without significant changes in the baseline sIPSC charge transfer (measured before the depolarizing steps used to evoke DSI; cells from sham-stimulated slices, 64.7 ± 6.9 pA*ms; in tetanized slices, 63.8 ± 6.1 pA*ms). To our knowledge, this is the first demonstration of DSI potentiation induced by increased neuronal activity in vitro.
Occlusion of tetanus-induced DSI potentiation by previous febrile seizures
The data presented above showed that seizure-like afferent activity in vitro could potentiate DSI amplitude and duration, similar to what takes place after in vivo febrile seizures (Chen et al., 2003). The similarity between in vitro tetanus- and in vivo HT-induced DSI potentiation was tested further by determining whether the in vivo febrile seizures could occlude DSI potentiation caused by in vitro tetanization. In contrast to the potentiation of DSI of sIPSCs after tetanization of slices from control animals (Fig. 1 D, left pair of bar graphs), tetanization in slices from age-matched (P18), littermate animals that had experienced HT 1 week before the recording session failed to enhance DSI, compared with sham-stimulated slices from HT animals (Fig. 1 D, right pair of bar graphs). Specifically, in slices from animals that had febrile seizures, the peak amplitude of DSI was already as large as in tetanized slices from control animals, and could not be increased further with tetanization (DSI amplitude, in cells from sham-stimulated control slices from HT animals, 32.4 ± 4.4%, n = 10; in cells from tetanized slices from HT animals, 38.1 ± 6.3%, n = 5). Therefore, previous in vivo HT occluded the DSI potentiating effects of in vitro tetanization, suggesting a common underlying mechanism.
Requirement for strongly enhanced afferent activity for DSI potentiation
How sensitive is the DSI amplitude to changes in neuronal activity? Can smaller increases in afferent inputs potentiate DSI, or does long-term DSI potentiation take place only after seizure-like events? To answer these questions, sIPSCs were recorded in sham-control slices, and in slices that were stimulated with either weaker or stronger tetanus (stimulation was in control ACSF, the recordings in 10 μm NBQX, 10 μm APV and 5 μm carbachol). As in the previous experiments, the stronger (5× 10 s at 100 Hz) tetanus caused a significant potentiation of DSI (Fig. 1 E) (DSI in cells from sham-stimulated slices, 29.0 ± 3.8%, n = 11; from tetanized slices, 44.7 ± 3.4%, n = 11). In contrast, a weaker (5× 1 s at 100 Hz) tetanus did not result in increased DSI (Fig. 1 E) (DSI in cells from sham-stimulated slices, 29.5 ± 4.9%, n = 10; in tetanized slices, 27.9 ± 4.6%, n = 10). Therefore, DSI potentiation requires strongly enhanced inputs, such as those that may take place with seizure-like network hyperactivity.
Robustness of tetanus-induced DSI potentiation
The similarity of the effects on DSI amplitude and duration after tetanization of afferent fibers in vitro and HT in vivo suggested that the activity-induced potentiation of DSI is a robust phenomenon that may take place under a variety of experimental conditions. Therefore, additional experiments were performed to test the robustness of DSI potentiation. Tetanic stimulation, similar to its enhancement of the DSI of sIPSCs, also increased the DSI of multifiber, electrical stimulation evoked IPSCs (eIPSCs) (Fig. 2 A,B) (DSI in cells from sham-tetanized slices, 15.1 ± 2.3%, n = 11; from tetanized slices, 29.6 ± 3.2%, n = 11). Because these experiments involving eIPSCs were conducted in the absence of carbachol, these results also indicated that the tetanus-induced DSI potentiation could be observed both in the presence and absence of cholinergic stimulation. The tetanus-induced DSI potentiation took place without a significant change in eIPSC amplitudes (Fig. 2 C) (for description of positioning of stimulation electrodes, see Materials and Methods). The lack of a change in eIPSC amplitude after tetanization was in agreement with the lack of a tetanus-induced alteration in sIPSC charge transfer (see above), further indicating that no significant iLTD (Chevaleyre and Castillo, 2003, 2004) took place under these conditions (for paired recording data, see below).
Figure 2.
Tetanic stimulation rapidly potentiates DSI of eIPSCs, increases the sensitivity of eIPSCs to CB1 receptor antagonists/inverse agonists, and does not affect the magnitude of DSE. A, B, Tetanic stimulation potentiates DSI of eIPSCs, and the full effect of potentiation is present within 15 min after stimulation. Representative traces are shown in A, summary data are shown in B. Square wave in B represents time and duration of depolarizing pulse. IPSCs were evoked with stimulation at the border of stratum radiatum and stratum pyramidale. DSI was induced by a 500 ms depolarization to 0 mV and recorded in the presence of ionotropic glutamate receptor blockers, but without carbachol. C, In agreement with the lack of change in sIPSC charge transfer after tetanus, the amplitude of evoked IPSCs is not changed by tetanic stimulation for any stimulation intensity tested (note that the stimulating and recording electrodes were carefully positioned in a reproducible manner) (Chen et al., 1999, 2001) (see Materials and Methods). D, Tetanic stimulation potentiates DSI evoked by either 100 or 500 ms depolarizing pulses. E, Tetanic stimulation causes eIPSCs to become sensitive to the CB1 antagonist SR141716. The inset shows representative traces. Calibration: 50 ms, 100 pA. Symbols refer to E and F. F, The potentiation of endocannabinoid signaling caused by tetanic stimulation is specific to inhibitory synapses, as the amplitude and decay of DSE are not affected by tetanus. DSE was evoked by a 10 s depolarizing pulse and was recorded in the presence of bicuculline (10 μm). The square wave represents the time and duration of the depolarizing pulse. The inset shows representative traces. Calibration: 80 ms, 400 pA.
Tetanus-induced DSI potentiation was significant not only when assessed with 500 ms depolarizing pulses to 0 mV, because the DSI enhancing effect of tetanization could also be observed with 100 ms depolarizing pulses (Fig. 2 D) (DSI, with a 100 ms depolarizing pulse, in cells from sham-stimulated slices, 11.5 ± 3.0%, n = 10; in tetanized slices, 20.1 ± 2.7%, n = 10; for data on DSI potentiation measured with 500 ms depolarizing pulse, see above).
In addition, DSI potentiation after tetanization of Schaffer collaterals in vitro had a rapid onset, as DSI of eIPSCs in pyramidal cells was already significantly potentiated 15 min after tetanization (Fig. 2 A, bottom row, B, open triangles) (mean time after tetanus, 15.5 ± 1.1 min; range, 10–20 min; DSI in cells from slices 15 min after tetanus, 31.7 ± 4.8%, n = 8). These data indicate the robustness, as well as the rapid onset, of the DSI potentiation resulting from a period of strongly increased afferent activity to CA1.
Tetanic stimulation of Schaffer collaterals enhances tonic inhibition of GABA release by CB1 receptors
The above data show that in vitro stimulation of Schaffer collaterals, similar to in vivo febrile seizures, results in increases in the amplitude and duration of DSI. Febrile seizures, in addition to potentiating DSI amplitude and duration, have been shown to increase the CB1-dependent tonic inhibition of GABA release (Chen et al., 2003). Therefore, we performed experiments to determine whether in vitro tetanization enhances the tonic inhibition of GABA release by CB1 receptors. As shown in Figure 2 E, perfusion of the CB1 antagonist (and inverse agonist) SR141716A (Rinaldi-Carmona et al., 1994; Bouaboula et al., 1997; Landsman et al., 1997; Pan et al., 1998; Vasquez and Lewis, 1999; Hurst et al., 2002) in CA1 pyramidal cells in nontetanized slices (from control animals) did not cause a significant change in the peak amplitude of eIPSCs, in agreement with previous reports (Hoffman and Lupica, 2000; Wilson and Nicoll, 2001; Chen et al., 2003). However, in cells from tetanized slices (from control animals), perfusion of SR141716A resulted in a significant increase in eIPSC amplitude (Fig. 2 E) (recordings made >1 h after tetanus; peak eIPSC amplitude in the presence of SR141716A, with respect to pre-SR141716A baseline, in cells from sham-stimulated control slices, 95.5 ± 1.8%, n = 4; from tetanized slices, 129.1 ± 9.1%, n = 5). The potentiating effect of SR141716A on eIPSC peak amplitudes after tetanization was remarkably similar to its effect on eIPSCs after in vivo HT [Chen et al. (2003), their Fig. 3 A,B].
Figure 3.
Paired recordings show that DSI at CB1 expressing interneuron to pyramidal cell synapses is potentiated by tetanic stimulation. A, Biocytin fill from a representative interneuron demonstrates the presence of CCK immunostaining and the absence of PV immunostaining (all cells that were successfully recovered were CCK positive and PV negative; n = 9 of 14 recordings). Traces on the right demonstrate the characteristic firing pattern and hyperpolarizing response of CCK-positive interneurons after the injection of +140 pA and − 80pA current pulses. These cells are similar to those examined and illustrated by Foldy et al. (2006). B, DSI of uIPSCs is significantly increased by tetanic stimulation. Top traces show action potentials evoked in the presynaptic basket cell; bottom traces show 20 individual uIPSCs in the postsynaptic pyramidal cell from each time point (gray) as well the averaged uIPSC (black). The time points were as follows, with the zero time point being the start of the depolarizing pulse: baseline or pre-DSI period, −2 s to 0 s; DSI, 0.5 to 2.5 s; recovery, 4 to 6 s. The illustrated traces are consecutive responses obtained by triggering action potentials in the presynaptic interneuron at 10 Hz; as described previously in detail (Foldy et al. 2006), this stimulation frequency resulted in stable responses over time and also allowed us to obtain a sufficiently large number of events for reliable analysis. Calibration: B, presynaptic, 25 mV, 5 ms; postsynaptic, 25 pA, 5 ms. All experiments in this figure were performed in control ACSF and DSI was evoked by 500 ms pulses.
Tetanization does not change DSE
The effects of febrile seizures on cannabinoid signaling have been shown to be specific to GABAergic synapses, as HT caused DSI potentiation without changes in DSE (Chen et al., 2003). As shown in Figure 2 F, the potentiation in CB1-mediated inhibition of transmitter release after tetanic stimulation in vitro was also selective to DSI, because it failed to cause any significant alteration in DSE (DSE in cells from sham-tetanized slices, 17.4 ± 3.7%, n = 6; tetanized, 18.5 ± 4.1%, n = 6; note that the depolarizing pulse needs to be longer for evoking DSE, 10 s) (Ohno-Shosaku et al., 2002; Chen et al., 2003, see also Straiker and Mackie, 2005). Together, the experiments presented so far indicate that the in vitro tetanus-induced DSI potentiation replicates several key features of DSI potentiation observed after in vivo febrile seizures, including the selectivity of the modulation of DSI but not DSE.
Tetanus-induced DSI potentiation occurs at synapses between endocannabinoid-sensitive interneurons and pyramidal cells
The data presented above show that tetanic stimulation significantly enhances DSI of both sIPSCs and eIPSCs. Because sIPSCs and eIPSCs originate from a mixed population of GABAergic fibers (containing both CB1-expressing, cannabinoid-sensitive and CB1-negative, cannabinoid-insensitive axons), it is possible that tetanic stimulation increased the relative contribution of CB1-expressing axons to the recorded events, leading to an apparent potentiation of DSI, without an actual increase in endocannabinoid signaling at axons between CB1-containing terminals and pyramidal cells. To investigate this question, we performed paired whole-cell patch-clamp recordings between DSI sensitive presynaptic interneurons evoking fast unitary IPSCs (uIPSCs; 10–90% rise-times of uIPSCs, 0.62 ± 0.05 ms; n = 14) and their pyramidal cell postsynaptic targets. The DSI sensitivity identified these presynaptic cells as CB1 receptor expressing interneurons, and the fast uIPSC rise times indicated that they were most likely basket cells (Katona et al., 1999; Wilson et al., 2001; Foldy et al., 2006; Glickfeld and Scanziani, 2006). Synaptically connected pairs were obtained from slices that were either sham-stimulated or tetanized before the beginning of the recording session (the time between the stimulation and the establishment of the paired recordings was 27.7 ± 2.4 min; the extracellular medium was control ACSF during both the tetanization and the paired recordings).
As illustrated in Figure 3, tetanization significantly potentiated DSI of the uIPSCs recorded between the interneurons and the CA1 pyramidal cells. Specifically, DSI in sham-stimulated control slices was 55.7 ± 11.7% (n = 8 pairs), and it was significantly increased in pairs recorded from slices that were tetanically stimulated (87.3 ± 4.1%; n = 6). Therefore, data from these paired-recording experiments confirmed the results from the above-described sIPSC and eIPSC DSI experiments indicating that tetanization potentiates DSI. In addition, in agreement with the lack of iLTD of the sIPSCs and perisomatic eIPSCs (Fig. 2 C), the amplitude of the uIPSCs in these paired recordings was also not decreased after the tetanic stimulation (if anything, there was a nonsignificant trend for increased uIPSC amplitude after tetanization; uIPSC amplitudes, including failures, in pairs from sham-stimulated slices, 70.1 ± 13.9 pA; in tetanized slices, 108.0 ± 53.0 pA).
Mechanisms of induction of DSI potentiation after tetanic stimulation in vitro: dual requirement for non-NMDA ionotropic and metabotropic glutamate receptors
Based on the similarities established by the previous experiments between the DSI potentiation after tetanic stimulation in vitro and DSI potentiation after HT in vivo, we took advantage of the accessibility of the in vitro tetanization paradigm to pharmacological manipulations to investigate the mechanisms underlying the induction phase of DSI potentiation. In contrast to the potentiation of DSI when tetanization was performed in ACSF (as in all previous experiments), tetanization in the presence of 10 μm NBQX did not increase DSI (Fig. 4 A, NBQX) (DSI of eIPSCs after sham-stimulation in NBQX, 19.9 ± 2.6%, n = 9; after tetanization in NBQX, 16.3 ± 3.2%, n = 10; note that in these and subsequent experiments on induction mechanisms, the slices were incubated in control ACSF during the >1 h time between the tetanization and the beginning of patch-clamp recording for the DSI assessment, to wash out the particular drug that was being tested for its effect on induction). Therefore, AMPA/kainate glutamate receptors are critical for the induction of DSI potentiation. In contrast, significant DSI potentiation took place when tetanization was performed in the presence of 10 μm APV (Fig. 4 A, APV) (DSI after sham-stimulation in APV, 18.2 ± 2.5%, n = 10; after tetanization in APV, 32.0 ± 2.7%, n = 11), indicating that NMDA receptors are not necessary for the induction of plasticity of endocannabinoid signaling (these results were confirmed with 50 μm APV in a separate series of experiments; DSI after sham-stimulation in APV, 17.0 ± 1.8%, n = 6; after tetanization in APV, 30.8 ± 2.9%, n = 6). Interestingly, the broad-spectrum metabotropic glutamate receptor antagonist LY341495 (200 μm) was also able to completely abolish the induction of DSI potentiation (Fig. 4 A, LY341495) (DSI after sham-stimulation in LY341495, 20.5 ± 2.7%, n = 10; after tetanization in LY341495, 18.5 ± 1.9%, n = 9). Therefore, both non-NMDA ionotropic as well as metabotropic glutamate receptors are necessary for the afferent stimulation-induced potentiation of DSI. However, GABAA receptor activity is not required for this plasticity to occur, as the GABAA receptor blocker bicuculline (10 μm) failed to inhibit the tetanus-induced potentiation of DSI (Fig. 4 A, bicuculline) (DSI after sham-stimulation in bicuculline, 18.6 ± 3.3%, n = 9; after tetanization in bicuculline, 31.7 ± 2.6%; n = 10). Note also that the sensitivity of the tetanus-induced potentiation of DSI to AMPA/kainate receptor blockade further distinguished this form of plasticity of endocannabinoid signaling from iLTD, which could be induced both in the presence or absence of NBQX (Chevaleyre and Castillo, 2003).
Figure 4.
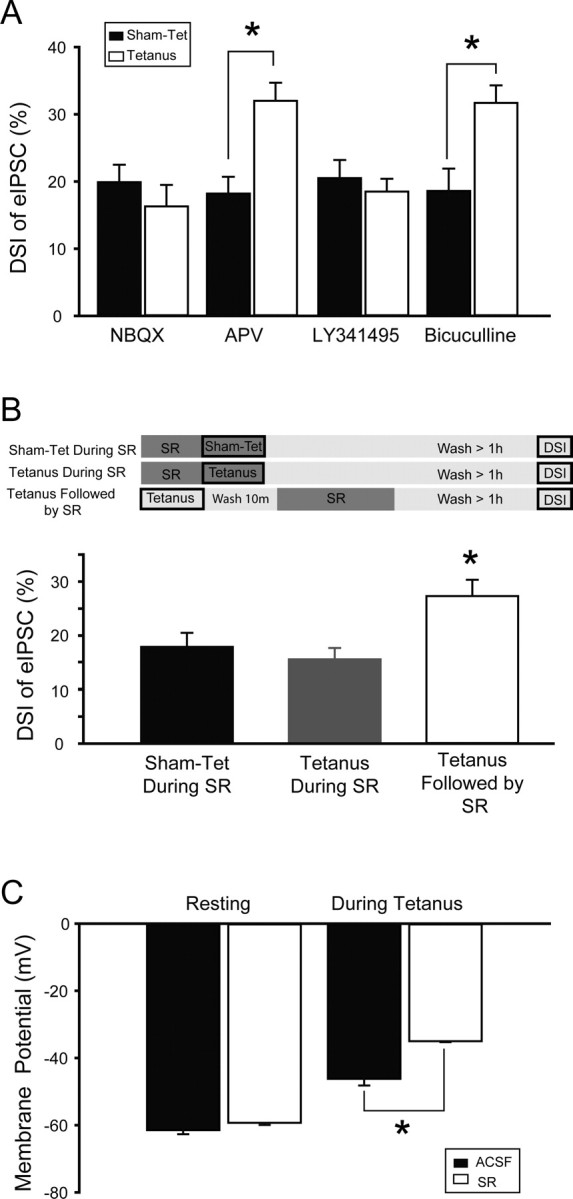
Activation of AMPA/kainate, mGluR, and CB1 receptors is required during tetanus for potentiation of DSI. A, Tetanus-induced potentiation of DSI is blocked when NBQX (5 μm), an AMPA/kainate receptor antagonist, or LY341495 (200 μm), a broad spectrum mGluR antagonist, is present during tetanization. Blockade of NMDARs with APV (10 μm), or blockade of GABAA receptors with bicuculline (10 μm) during tetanization does not prevent potentiation of DSI. B, Potentiation of DSI is prevented when SR141716, a CB1R antagonist, is present during tetanus (SR was applied for 6 min, which was followed by a >60 min wash in ACSF). To exclude the possibility that this effect was caused by an incomplete wash out of SR from the slice, a control experiment was performed in which tetanus was applied in the absence of SR, followed by a 6 min perfusion of the slice with SR, followed by a 60 min wash with ACSF (tetanus followed by SR). The presence of potentiated DSI in this experiment indicates that SR can be completely eliminated from the slice with a 60 min wash. The top panel demonstrates experimental protocol, where dark boxes indicate presence of SR in extracellular medium. C, The presence of SR during tetanus does not decrease the effective activation of excitatory afferents. In fact, SR did not change the pretetanus resting membrane potential, but the average membrane potential during tetanic stimulation was depolarized in the presence of SR and, as detailed in the text, the number of action potentials elicited by each tetanic train was also increased in the presence of SR.
Requirement of CB1 receptors for induction of tetanus-induced DSI potentiation in vitro
Because high-frequency stimulation of Schaffer collaterals in hippocampal slices is known to transiently increase endocannabinoid levels (Stella et al., 1997), we next tested whether CB1 receptor activation is involved in DSI potentiation (same experimental paradigm as in the experiments of Fig. 4 A). Tetanic stimulation, or sham stimulation, was applied in the presence of the CB1 antagonist SR141716A (Fig. 4 A, sham-tet/tetanus during SR) (3 min perfusion with SR141716A was followed by tetanic- or sham stimulation, during which SR141716A perfusion was continued; total SR141716A perfusion, 6 min), which in turn was followed by >60 min wash in control ACSF and then DSI was assessed. Importantly, when tetanic stimulation was performed in the presence of SR141716A, DSI potentiation did not occur (Fig. 4 B, left and middle bar graphs) (DSI in cells from sham-tetanized slices, 17.9 ± 2.6%, n = 10; in cells from slices tetanized in SR141716A, 15.6 ± 2.1%, n = 10). Therefore, a major conclusion from these observations, with significant relevance for the design of new strategies to oppose the long-term consequences of febrile seizures (see below), is that CB1 receptor activation is an absolute requirement for the induction of this plasticity in endocannabinoid signaling.
A number of control experiments were performed to exclude alternative interpretations of the preceding tetanization experiments performed in the presence of SR141716A. First, the lack of DSI potentiation after tetanic stimulation in SR141716A was not attributable to incomplete wash-out of the drug (note that this is a potentially significant concern, because SR141716A blocks DSI), because DSI potentiation did take place when SR141716A was applied 10 min after the tetanus (Fig. 4 B, tetanus followed by SR) (DSI in cells from slices that were tetanized in ACSF, then perfused with ACSF containing SR141716A for 6 min, followed by >60 min wash in ACSF, 27.3 ± 3.0%, n = 10).
Second, the blocking effect of SR141716A also could not be explained by a decreased amount of neuronal activity in SR141716A, because tetanic stimulation in the presence of SR141716A actually resulted in a larger average depolarization during tetanization, compared with tetanic stimulation in ACSF (Fig. 4 C) (average membrane potential during the 100 Hz tetanus, calculated from 5-ms-long samples collected at 10 kHz, taken 2.5 ms after each stimulus during the tetanic train, when tetanization was performed in ACSF, −46.2 ± 2.0 mV, n = 4; when tetanization was performed in SR141716A, −35.0 ± 0.3 mV, n = 5; note that the resting membrane potential, measured before the tetanic stimulation was applied, was not altered by the presence of SR141716A, resting membrane potential in ACSF, −61.5 ± 1.2 mV, n = 4; in SR141716A, −59.3 ± 0.6 mV, n = 5). Furthermore, tetanization in the presence of SR141716A also increased the number of action potentials that the CA1 pyramidal cells fired during the stimulation (number of action potentials per tetanic train in ACSF, 28.8 ± 7.2, n = 4; in SR141716A, 50.0 ± 7.4, n = 5). Note that the higher number of action potentials in CA1 pyramidal cells during the tetanic stimulation in the presence of SR141716A compared with ACSF is in agreement with previous observations on the acute seizure-enhancing effects of SR141716A application in vivo (Wallace et al., 2002; Marsicano et al., 2003; Bernard et al., 2005).
Together, the results of these experiments demonstrated that both glutamate and CB1 receptors are involved in the mechanisms underlying DSI potentiation. Furthermore, these experiments also showed that application of the CB1 antagonist after the tetanus was not effective in preventing activity dependent DSI potentiation, indicating that activation of CB1 receptors is critical only during, but not after the induction period.
CB1 receptor blockade during seizures inhibits seizure-induced DSI potentiation
Can we use the previous insights into the in vitro induction mechanisms of activity-dependent DSI potentiation to prevent the DSI potentiation that takes place after HT in vivo? In agreement with previous reports (Chen et al., 2003), DSI was significantly increased in animals that were preinjected with vehicle (5% alcohol, total injected volume, 70 μl) (see Materials and Methods) and subjected to HT 1 week before the recording session at age P10 (HT plus vehicle), compared with vehicle-injected, age-matched littermate normothermic controls (Fig. 5 A, vehicle, left pair of bar graphs) (DSI in cells from slices from control, vehicle-injected animals, 19.9 ± 2.4%, n = 9; from HT vehicle-injected animals, 30.2 ± 3.3%, n = 10), indicating that the vehicle used to dissolve SR141716A in the subsequent experiments did not interfere with DSI potentiation. However, when HT was induced in animals that were preinjected with SR141716A (in the same vehicle) at either high (10 mg/kg) or low (1 mg/kg) concentration 1 h before the seizure induction, the potentiation of DSI did not take place (Fig. 5 A, right pair of bar graphs, HT+SR) (DSI in cells from slices from HT animals preinjected with 1 mg/kg SR141716A before seizure induction, 16.8 ± 2.7%, n = 7; from HT animals preinjected with 10 mg/kg SR141716A, 16.5 ± 3.3%, n = 8). Therefore, as predicted by the in vitro experiments, blockade of CB1 receptors prevents the upregulation of DSI by the experimental febrile seizures.
Figure 5.

Blocking CB1 receptors in vivo during experimental febrile seizures prevents both potentiation of DSI and increase in CB1 receptor number. A, The potentiation of DSI observed after HT is prevented when SR is injected in vivo before HT induction at a dose of either 1 or 10 mg/kg. B, Typical western blots of hippocampal tissue (top) and their quantitative analysis (bottom) demonstrate a significant (35.9%) increase in the amount of CB1 receptor protein after HT (HT+vehicle) as compared with littermate controls (either vehicle- or SR-injected controls). The injection of SR in vivo (1 mg/kg) before HT induction (HT+SR) prevents the increased expression of CB1 protein.
CB1 receptor blockade during febrile seizures abolishes the seizure-induced increase in CB1 receptor numbers
Previous results have shown that febrile seizures increase the number of CB1 receptors expressed in the hippocampus (as assessed with Western blotting) without altering the cell type-specificity of the CB1 receptor expression and without the sprouting of the perisomatic CB1-expressing axons in the CA1 layer (Chen et al., 2003). Interestingly, other forms of seizures (e.g., pilocaprine-induced seizures) have also been shown to result in a persistent increase in CB1 receptors (Wallace et al., 2003), indicating that upregulation of CB1 receptors may be a robust consequence of seizures. Because the increase in CB1 receptor numbers has been suggested to be a mechanism associated with the DSI potentiation resulting from febrile seizures (Chen et al., 2003), in the next series of experiments we determined whether the presence of CB1 antagonists during HT abolishes the long-term increase in CB1 receptors.
As illustrated in Figure 5 B, CB1 receptor protein levels were significantly increased in Western blots in hippocampi from vehicle-injected animals that experienced febrile seizures (Fig. 5 B, HT+vehicle) (percent CB1 levels in HT+vehicle animals, 135.9 ± 6.0%; n = 4 animals) compared with vehicle-injected, sham-treated littermate controls (Fig. 5 B, control+vehicle) (100.0 ± 5.9%; n = 4 animals) one week after the febrile seizures. Injection of SR141716A (1 mg/kg, i.p.) to control animals did not change the CB1 receptor expression levels (Fig. 5 B, control+SR) (CB1 levels in control+SR animals, 89.7 ± 4.1%; n = 4 animals). In contrast to the HT+vehicle animals, however, CB1 levels were not persistently elevated after the febrile seizures in animals that were preinjected with SR141716A (1 mg/kg) (Fig. 5 B, HT+SR) (CB1 levels in HT+SR animals, 101.6 ± 9.5%; n = 5 animals). These data show that CB1 receptor blockade during seizure induction prevents the long-term elevation of CB1 receptor expression after febrile seizures.
CB1 receptor antagonism during seizure induction prevents the long-term increase in hippocampal network excitability after febrile seizures: in vitro and in vivo assessment
Febrile seizures are known to cause a long-lasting increase in hippocampal network excitability, as demonstrated by a decrease in threshold for self-sustaining, recurrent seizure-like electrical discharges after the application of brief high-frequency test stimuli in acute combined entorhino-hippocampal slices in vitro and by the decreased in vivo seizure threshold for the limbic convulsant kainate (Dube et al., 2000). Because CB1 receptor blockade prevented the in vivo HT-induced upregulation of DSI as well as the HT-induced increase in CB1 receptor numbers, in the next series of experiments we determined whether CB1 receptor blockade during seizure induction also prevents the development of long-term hyperexcitability induced by febrile seizures (Dube et al., 2000).
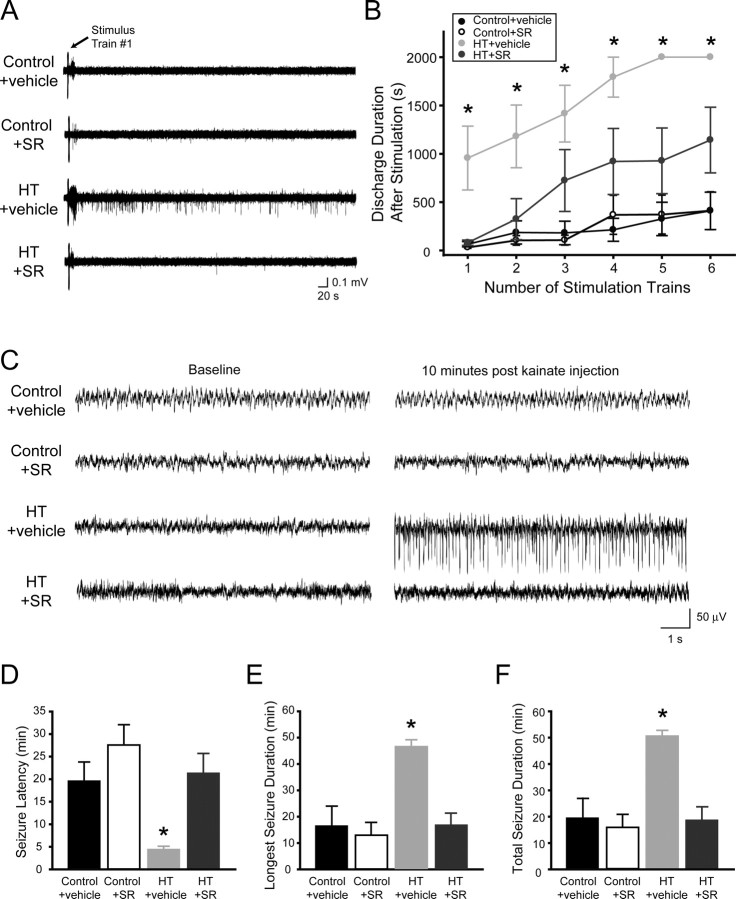
First, we measured the effect of CB1 receptor antagonism on limbic hyperexcitability using short trains of electrical stimuli to the Schaffer collaterals to measure seizure threshold, in combined hippocampal-entorhinal slices prepared 1 week after the experimental febrile seizure induction (note that the stimulus trains were used to probe the excitability of the network, not to induce changes in DSI; accordingly, these test stimuli were considerably shorter than what is required for DSI potentiation). As shown in Figure 6 A, the first stimulus train caused only a few, short after-discharges without sustained field activity in combined hippocampal-entorhinal slices from control animals, or from control animals injected with SR141716A (1 mg/kg, i.p.) (Fig. 6 A, control+vehicle, control+SR). In contrast, even the first stimulus train evoked the characteristic, previously described (Dube et al., 2000), self-sustaining population activity in slices from animals that experienced experimental febrile seizures 1 week before the recording and were injected with vehicle before the febrile seizures (Fig. 6 A, HT+vehicle), demonstrating that febrile seizures prominently increased hippocampal network excitability in a long-term manner.
Figure 6.
Blocking CB1 receptors in vivo during febrile seizures prevents the long-lasting decrease in seizure threshold. A–F, Seizure thresholds were tested both with electrical stimulation in vitro at 1 week after febrile seizures (A, B) and with kainate in vivo 6 weeks after febrile seizures (C–F). A, One train of brief high-frequency test stimulation of the Schaffer collaterals (2 s at 60 Hz) in hippocampal-entorhinal slices leads to self-sustaining epileptiform discharges in vehicle-injected HT animals, but not in SR-injected HT animals or in controls. Recordings were performed 1 week after HT induction. Note that the high-frequency stimulus train is used here to test the excitability of the network, not to induce changes in DSI (the test stimulation is considerably shorter than what is required for DSI potentiation). B, Plot of stimulation train number versus the duration of spontaneous field discharges shows that the injection of SR, at 1 mg/kg, prevents the increase in epileptiform activity seen after HT. C, Representative traces from hippocampal depth EEG recordings demonstrate that, 10 min after injection of kainate (5 mg/kg), seizures have initiated in HT+vehicle animals but not in HT + SR (1 mg/kg) animals or control animals. D, The latency to seizure initiation is significantly decreased by HT (HT+vehicle), but this decrease is prevented by injection of SR (HT+SR). E, F, SR injection prevents the increase seen after HT in both the duration of the longest single seizure and the cumulative duration of all seizures.
However, when the animals were preinjected with SR141716A and then subjected to experimental febrile seizures, the network hyperexcitability was decreased compared with the HT plus vehicle animals (Fig. 6 A, HT+SR). Population data for these experiments, for consecutive test stimuli (delivered every 10 min) is shown in Figure 6 B (number of slices: control+vehicle, n = 8, from n = 4 animals; control+SR, n = 8, from n = 4 animals; HT+vehicle, n = 9, from n = 4 animals; HT+SR, n = 9, from n = 4 animals; note that, because the clinical definition of status epilepticus is 30 min of seizures, the recordings were terminated if the self-sustaining activity lasted longer than 35 min, resulting in the apparent plateau observable after five stimulation trains for the activity from HT+vehicle slices in Fig. 6 B). Therefore, these data demonstrate that the presence of SR141716A during HT induction significantly limited the development of network hyperexcitability resulting from the febrile seizures.
Second, we performed experiments to measure the effect of CB1 antagonism on seizure-induced long-term changes in seizure thresholds, by assessing seizure threshold from in vivo hippocampal EEG recordings after the application of the prototypic limbic convulsant kainate (5 mg/kg, i.p.), 6 weeks after the ex-perimental febrile seizures. In agreement with previous reports (Dube et al., 2000), animals that experienced HT exhibited a significant decrease in threshold for kainate-induced seizures (Fig. 6 C) compared with controls. Specifically, in animals that experienced HT after vehicle injection, application of kainate resulted in seizures that appeared significantly faster and lasted significantly longer (Fig. 6 D–F) (seizure latency: control+vehicle, 19.6 ± 4.2 min, n = 7; HT+vehicle, 4.5 ± 0.7 min, n = 8; duration of longest seizure episode: control+vehicle, 16.0 ± 7.7 min; HT+vehicle, 46.1 ± 3.0 min; total seizure duration: control+vehicle, 19.5 ± 7.5 min; HT+vehicle, 50.5 ± 2.3 min). In contrast, animals that experienced febrile seizures in the presence of SR141716A showed no evidence of significantly decreased seizure thresholds after the kainate challenge (Fig. 6 C–F) (seizure latency: control+SR, 27.6 ± 4.5 min, n = 6; HT+SR, 21.4 ± 4.3 min, n = 8; duration of longest seizure episode: control+SR, 12.9 ± 4.6 min; HT+SR, 16.7 ± 4.8 min; total seizure duration: control+SR, 15.9 ± 5.0 min; HT+SR, 18.7 ± 4.9 min). These data demonstrate that CB1 antagonism during febrile seizures can significantly counteract and even prevent the long-term, seizure-induced decrease in seizure threshold in the hippocampus.
In principle, SR141716A may have decreased long-term hyperexcitability by decreasing febrile seizures. However, this was highly unlikely, because SR141716A actually enhanced neuronal depolarization and firing during tetanization (Fig. 4 C), and SR141716A has been shown to act as a proconvulsant in vivo (Wallace et al., 2002; Marsicano et al., 2003; Bernard et al., 2005). Indeed, we found no alterations in parameters of HT induction in the animals involved in the in vitro and in vivo assessment experiments. First, application of SR141716A did not affect the baseline body temperature (HT+vehicle, 32.9 ± 0.4°C, n = 21; HT+SR, 33.0 ± 0.3°C, n = 22). Second, the number of seizure sessions during the ∼30-min-long experimental febrile seizure-induction procedure (see Materials and Methods) was also unchanged (HT+vehicle, 5.8 ± 0.3; HT+SR, 6.5 ± 0.3; note that, if anything, there was a nonsignificant trend toward more seizure sessions in SR141716A, which obviously could not explain the protection against long-term hyperexcitability provided by SR141716A). Third, the threshold temperature for febrile seizures was also unaltered by SR141716A (HT+vehicle, 40.5 ± 0.3°C; HT+SR, 39.8 ± 0.2°C).
We also performed experiments to determine whether SR141716A was also effective in blocking the long-term effects of febrile seizures on seizure thresholds if applied after the start of the febrile seizure induction period. Therefore, the experiments described above in connection with Figure 6 C–F were repeated with SR141716A injected (1 mg/kg, i.p.) 2 min after the start of the febrile seizures (as above, the assessment of seizure thresholds with kainate was performed 6 weeks after the febrile seizures). The data showed that the animals that experienced febrile seizures with SR141716A injected 2 min after the start of the seizure induction period showed no evidence of decreased seizure thresholds after the kainate challenge (seizure latency: control+vehicle, 20.1 ± 4.6 min, n = 9; HT+vehicle, 4.8 ± 0.6 min, n = 8; HT+SR, 16.0 ± 3.0 min; n = 8; duration of longest seizure episode: control+vehicle, 18.0 ± 4.3 min; HT+vehicle, 48.6 ± 1.7 min; HT+SR, 9.9 ± 2.9 min; total seizure duration: control+vehicle, 25.4 ± 3.8 min; HT+vehicle, 51.7 ± 1.4 min; HT+SR, 18.9 ± 2.2 min). Thus, CB1 antagonism can significantly counteract and prevent the long-term, seizure-induced decrease in seizure threshold in the hippocampus not only when SR141716A is preinjected before the onset of the seizures, but also when it is injected 2 min after the start of febrile seizure induction.
Finally, we sought to provide additional evidence for the acute proconvulsant action of SR141716A. For technical reasons, we could not reliably record EEG seizures during febrile seizure induction, therefore, we measured the effect of SR141716A (1 mg/kg) on acute in vivo kainate-induced seizures in P10 animals. As expected, the data showed that SR141716A had a significant proconvulsant effect (seizure latency after kainate injection in vehicle injected controls, 17.3 ± 6.6 min, n = 5; in SR141716A injected animals, 6.5 ± 1.8 min, n = 5). The acute proconvulsant action of CB1 receptor blockade is in full agreement with our in vitro data described above indicating more depolarized membrane potentials and more action potential discharges in response to tetanic stimulation of afferents in the presence of SR141716A (Fig. 4 C and related data), and it also agrees with previous reports from different epilepsy models on the acute seizure enhancing effects of SR141716A (Wallace et al., 2002; Marsicano et al., 2003; Bernard et al., 2005). Therefore, CB1 receptor blockade during experimental febrile seizures has a significant inhibitory effect on the long-term reduction in seizure threshold despite its acute proconvulsant actions.
Discussion
Role for CB1 receptors in inducing plasticity of endocannabinoid signaling and limbic hyperexcitability
CB1 receptors are the most numerous G-protein-coupled receptors in the brain (Herkenham et al., 1990; Matsuda et al., 1990), strategically positioned at both GABAergic and glutamatergic synapses to regulate neurotransmitter release (Katona et al., 1999, 2006; Kawamura et al., 2006; Monory et al., 2006). As indicated by their numerical abundance and specific expression patterns, CB1 receptors modulate hippocampal excitability in both healthy and pathological states (Hajos et al., 2000; Wilson et al., 2001; Carlson et al., 2002; Chen et al., 2003; Wallace et al., 2003; Monory et al., 2006). Here, we showed that prevention of endocannabinoid signaling plasticity decreases the persistent effects of prolonged febrile seizures on limbic excitability.
To determine key steps in the induction pathway for DSI plasticity, we first developed an in vitro model of DSI potentiation allowing rapid testing of pharmacological agents. Strong tetanic stimulation of excitatory afferents in slices replicated a number of effects of in vivo febrile seizures. Namely, tetanic stimulation enhanced DSI amplitude and duration, increased CB1-mediated tonic inhibition of GABA release, and did not significantly modify DSE. Activity-dependent DSI potentiation in slices was rapid and occluded by previous febrile seizures, and the induction mechanism required non-NMDA ionotropic and metabotropic glutamate receptors, and CB1 receptors.
Taking advantage of these mechanistic insights, we returned to the in vivo febrile seizure paradigm to demonstrate that CB1 receptor antagonism during HT induction prevents three major consequences of febrile seizures, namely, the persistent DSI potentiation and increase in CB1 receptors, and the development of long-lasting limbic hyperexcitability. It is interesting to note that the key finding of the present paper (i.e., that CB1 receptors are required for the induction of both seizure-induced endocannabinoid plasticity and long-term changes in seizure thresholds), could not have been predicted from our previous observation that febrile seizures persistently modify endocannabinoid signaling pathways (Chen et al., 2003). The present results may provide novel therapeutic avenues for infants who are most at risk of developing long-term limbic hyperexcitability from prolonged febrile seizures (Annegers et al., 1987; Cendes et al., 1993; French et al., 1993; Hesdorffer and Hauser, 2002).
DSI potentiation and iLTD: two distinct forms of endocannabinoid plasticity modulating GABAergic synaptic transmission
Persistent DSI potentiation indicates that neuronal activity can modify CB1 receptor-dependent short-term plasticity of GABAergic transmission in a long-term manner. Therefore, activity-dependent DSI potentiation first described in this paper joins the recently discovered iLTD (Chevaleyre and Castillo, 2003, 2004) as two mechanisms by which glutamatergic afferent activity can result in long-term depression of GABA-release from CB1 receptor expressing GABAergic axons. DSI potentiation is clearly distinct from iLTD, because tetanic stimulation that resulted in DSI potentiation did not depress sIPSCs, eIPSCs, or uIPSCs. A major reason why tetanic stimulation of Schaffer collaterals did not result in iLTD is likely to be that iLTD is a property of CB1 expressing interneuronal inputs to pyramidal cell dendrites (Chevaleyre and Castillo, 2003, 2004), but not of perisomatic inputs studied in this paper. Furthermore, in contrast to iLTD induction, DSI potentiation requires AMPA/kainate receptors. In contrast, metabotropic glutamate receptor antagonists block the induction of both DSI potentiation (present study) and iLTD (Chevaleyre and Castillo, 2003, 2004), indicating shared molecular steps, likely related to the production of endocannabinoids (Maejima et al., 2005).
Mechanisms of activity-dependent plasticity of DSI
The key events resulting in DSI potentiation can be delineated as follows. The initial steps involve glutamate release and the activation of both AMPA/kainate ionotropic and metabotropic glutamate receptors, which would be expected to result in the postsynaptic synthesis and release of endocannabinoids. The endocannabinoid is likely to be 2-AG, because Schaffer collateral stimulation has been shown to transiently increase 2-AG but not anandamide levels (Stella et al., 1997), although kainate-induced seizures are accompanied by rapid increases in both 2-AG and anandamide (Wettschureck et al., 2006) or anandamide alone (Marsicano et al., 2003). Exactly how tetanus-induced spike in endocannabinoid levels leads to DSI potentiation is not fully understood. On the presynaptic side, elevation of CB1 receptor levels after febrile seizures has been suggested to be a mechanism contributing to enhanced DSI amplitude (Chen et al., 2003). Increased CB1 receptor levels are expected to also prolong DSI and enhance tonic inhibition of GABA release (for detailed discussion, see Chen et al., 2003), which are two additional consequences of both tetanic stimulation and febrile seizures. Whether upregulation of CB1 receptors occurs after tetanic stimulation in slices is unclear, but it could take place through several potential mechanisms, including the rapid movement of CB1 receptors closer to the release sites from large receptor pools (Nyiri et al., 2005). On the postsynaptic side, one possibility is that episodes of strong glutamate receptor activation result in long-lasting priming of the activity of certain Ca2+-sensitive endocannabinoid synthetic enzymes (Bisogno et al., 2003; Hashimotodani et al., 2005; Katona et al., 2006).
Although details will need to be investigated further, a critical insight provided by the present study is that CB1 receptor activation is a key step in DSI potentiation, both after tetanic stimulation in vitro and after febrile seizures in vivo. Application of SR141716A prevents CB1 receptor activation at the terminals of CCK+ basket cells, which may be necessary for the downstream molecular steps leading to long-term presynaptic alterations in the endocannabinoid sensitivity of GABA release. Alternatively, it is possible that CB1 receptor blockade acted through the prevention of CB1 receptor activation on glutamatergic terminals, although it is difficult to see how that would interfere with DSI potentiation, especially because blockade of CB1 receptors on glutamatergic terminals would be expected to abolish DSE during tetanus and febrile seizures, resulting in even larger increases in glutamate release. It is interesting to note also that neuronal activity potentiated both the CB1-dependent phasic (DSI) and tonic inhibition of GABA release, without altering DSE in a long-term manner. As with the potentiation of DSI, the enhancement in the tonic inhibition of GABA release by CB1 receptors may have resulted from a variety of presynaptic and/or postsynaptic processes, including increases in the number or constitutive activity of presynaptic CB1 receptors, or through augmentation of the tonic synthesis and release of endocannabinoid ligands from postsynaptic cells. It will also be interesting to establish in future studies whether CB1 receptor blockade during febrile seizures also prevents other known consequences of these seizures, including potentiation of GABA release and enhancement of I h (Chen et al., 1999, 2001).
CB1 receptor blockade is acutely proconvulsant but prevents chronic increases in limbic hyperexcitability caused by seizures
Paradoxically, CB1 receptor blockade inhibited development of long-term decreases in seizure thresholds despite acute proconvulsant actions of SR141716A. That a proconvulsant drug can exert beneficial chronic effects on seizure thresholds is a novel finding with major potential implications for the ongoing search for new antiepileptic compounds. The proconvulsant nature of SR141716A is well established, because it can promote seizure-like discharges both in vitro and in vivo in different seizure paradigms and in different age groups (Wallace et al., 2002; Marsicano et al., 2003; Bernard et al., 2005; present study). Although the exact reason for the excitability-promoting actions of acute CB1 receptor blockade is not fully established, the most likely mechanism is blockade of activity-dependent depression of glutamate release (Marsicano et al., 2003; Monory et al., 2006) (i.e., antagonism of DSE). That SR141716A significantly decreased the long-term effects of febrile seizures on seizure thresholds despite its acute proconvulsant actions indicates that CB1 receptors play a critical role in inducing persistent changes in limbic networks during seizures, which should inspire future research for therapeutic applications. Some caveats will need to be carefully considered in this regard, including the acute proconvulsant actions of SR141716A that would have to be counteracted with anticonvulsant medications. In contrast, SR141716A shows a promising safety record in clinical trials as a potent antiobesity (and antismoking) agent (Despres et al., 2005; Pi-Sunyer et al., 2006), indicating that its long-term administration may be a possible method to prevent the plasticity of endocannabinoid signaling and long-term limbic network hyperexcitability after prolonged childhood seizures.
Future projects will need to establish exactly what kinds of manipulations of CB1 receptor-dependent signaling systems are the most beneficial under different conditions. A recent study showed that CB1 receptors on glutamate terminals are particularly important in regulating kainate-induced seizures, suggesting that boosting CB1 receptor-mediated control of glutamate release is the logical strategy for acute seizure control (Monory et al., 2006). In contrast, our results indicate that the opposite strategy (i.e., the blockade of CB1 receptors during seizures) may be the most beneficial intervention to counteract the long-term effects of childhood seizures, even if the blockade of CB1 receptors worsens the seizures acutely. These results indicate that the endocannabinoid system is likely to provide exciting new opportunities to develop fundamentally novel therapeutical strategies to prevent the long-term effects of prolonged seizures in infancy.
Footnotes
This work was supported by National Institutes of Health Grants NS38580 (I.S.), NS33213 (M.S.), and DA00286 and DA11322 (K.M.), and the Deutsche Forschungsgemeinschaft NE1185/1-1 (A.N.). We thank Dr. D. Piomelli for discussions and Ms. R. Zhu for expert technical assistance.
References
- Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Gerth A, Schultz L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Res Dev Brain Res. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beau FE, Alger BE. Transient suppression of GABAA-receptor-mediated IPSPs after epileptiform burst discharges in CA1 pyramidal cells. J Neurophysiol. 1998;79:659–669. doi: 10.1152/jn.1998.79.2.659. [DOI] [PubMed] [Google Scholar]
- Bender RA, Dube C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci USA. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Andermann E, Robitaille Y, Lopes-Cendes I, Peters T, Melanson D. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Ratzliff A, Hilgenberg L, Gulyas A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- Chesher GB, Jackson DM. Anticonvulsant effects of cannabinoids in mice: drug interactions within cannabinoids and cannabinoid interactions with phenytoin. Psychopharmacologia. 1974;37:255–264. doi: 10.1007/BF00421539. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chiu P, Olsen DM, Borys HK, Karler R, Turkanis SA. The influence of cannabidiol and delta 9-tetrahydrocannabinol on cobalt epilepsy in rats. Epilepsia. 1979;20:365–375. doi: 10.1111/j.1528-1157.1979.tb04816.x. [DOI] [PubMed] [Google Scholar]
- Consroe P, Wolkin A. Cannabidiol-antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201:26–32. [PubMed] [Google Scholar]
- Corcoran ME, McCaughran JA, Jr, Wada JA. Acute antiepileptic effects of 9-tetrahydrocannabinol in rats with kindled seizures. Exp Neurol. 1973;40:471–483. doi: 10.1016/0014-4886(73)90088-5. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA. Febrile seizures and the risk of epilepsy. In: Baram TZ, Shinnar S, editors. Febrile seizures. San Diego: Academic; 2002. pp. 63–76. [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song ZH, Nie J, Lewis D, Reggio PH. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol Pharmacol. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- Karler R, Cely W, Turkanis SA. Anticonvulsant properties of delta 9-tetrahydrocannabinol and other cannabinoids. Life Sci. 1974;15:931–947. doi: 10.1016/0024-3205(74)90009-5. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Martin LA, Wei DS, Alger BE. Heterogeneous susceptibility of GABA(A) receptor-mediated IPSCs to depolarization-induced suppression of inhibition in rat hippocampus. J Physiol (Lond) 2001;532:685–700. doi: 10.1111/j.1469-7793.2001.0685e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq A, Zhang YF, DeLorenzo RJ, Coulter DA. Long-duration self-sustained epileptiform activity in the hippocampal-parahippocampal slice: a model of status epilepticus. J Neurophysiol. 1995;74:2028–2042. doi: 10.1152/jn.1995.74.5.2028. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Sipila ST, Voipio J, Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaroodi H, Samini M, Moezi L, Homayoun H, Sadeghipour H, Tavakoli S, Hajrasouliha AR, Dehpour AR. The interaction of cannabinoids and opioids on pentylenetetrazole-induced seizure threshold in mice. Neuropharmacology. 2004;47:390–400. doi: 10.1016/j.neuropharm.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17(Suppl 1):S44–S52. doi: 10.1177/08830738020170010601. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol (Lond) 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada JA, Osawa T, Corcoran ME. Effects of tetrahydrocannabinols on kindled amygdaloid seizures and photogenic seizures in Senegalese baboons, Papio papio . Epilepsia. 1975;16:439–448. doi: 10.1111/j.1528-1157.1975.tb06071.x. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428:51–57. doi: 10.1016/s0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295–301. doi: 10.1016/s0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, van der Stelt M, Tsubokawa H, Krestel H, Moers A, Petrosino S, Schutz G, Di Marzo V, Offermanns S. Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol Cell Biol. 2006;26(15):5888–5894. doi: 10.1128/MCB.00397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]