Abstract
The recently identified Mas-related gene (Mrg) family of G-protein-coupled receptors is expressed almost exclusively in dorsal root ganglion (DRG) neurons. The expression of one family member, MrgD, is even further confined to IB4+, nonpeptidergic, small-diameter nociceptors. Although the functional consequences of MrgD activation are not known, this expression profile provides intriguing potential for a role in pain sensation or modulation. In a recombinant cell line, we first assessed the functional significance of MrgD activation by coexpressing MrgD with the KCNQ2/3 potassium channel, a channel implicated in pain. Whole-cell voltage-clamp recordings revealed that bath application of the ligand for MrgD, β-alanine, resulted in robust inhibition of KCNQ2/3 activity. Pharmacological blockade of Gi/o and phospholipase C signaling revealed a partial and complete block of the response, respectively. We extended these observations to dissociated DRG neuron cultures by examining MrgD modulation of M-currents (carried primarily by KCNQ2/3). Here too, β-alanine-induced activation of endogenous MrgD inhibited M-currents, but primarily via a pertussis toxin-sensitive pathway. Finally, we assessed the consequence of β-alanine-induced activation of MrgD in phasic neurons. Phasic neurons that fired a single action potential (AP) before β-alanine application fired multiple APs during β-alanine exposure. In sum, we provide evidence for a novel interaction between MrgD and KCNQ/M-type potassium channels that contributes to an increase in excitability of DRG neurons and thus may enhance the signaling of primary afferent nociceptive neurons.
Keywords: nociceptor, GPCR, β-alanine, M-current, DRG, excitability
Introduction
The Mas-related genes (Mrgs), also termed sensory neuron-specific G-protein-coupled receptors (SNSRs), comprise a recently identified family of G-protein-coupled receptors (GPCRs) that are implicated in sensory perception (Dong et al., 2001; Lembo et al., 2002). The Mrg family consists of MrgA–MrgH, found in rodents, and MrgX, found in humans. However, of particular note is MrgD. MrgD is expressed across species from rodents to nonhuman primates and humans (Zhang et al., 2005). In addition, its expression is restricted to dorsal root ganglion (DRG) neurons (Dong et al., 2001; Shinohara et al., 2004; Milasta et al., 2006), specifically, nonpeptidergic, small-diameter, IB4+ C-fiber neurons (Dong et al., 2001; Zylka et al., 2005). Together, these properties of MrgD provide for an experimentally tractable therapeutic target for pain.
The ability to probe for effects of MrgD activation was made possible by the identification of the putative ligand for MrgD, β-alanine (Shinohara et al., 2004). In recombinant cell lines, β-alanine-induced activation of MrgD resulted in elevated intracellular calcium and also suppression of forskolin-induced cAMP production, an effect that was inhibited by pertussis toxin (PTX) treatment, thus suggesting coupling to Gq and Gi proteins (Shinohara et al., 2004). Axonal tracer experiments revealed that MrgD-containing DRG neurons terminate in the skin but not blood vessels, muscle, or other major internal organs, thus suggesting discrete levels of organization for nociceptive afferents (Zylka et al., 2005). However, the effect of MrgD activation on nociceptor function remains an open issue.
Nociceptors in the DRG transmit information from the periphery to the spinal cord and CNS and can be classified biophysically based on their action potential (AP) properties. Tonic neurons fire multiple APs in response to depolarizing current injection. Phasic neurons, however, fire only a single AP even in response to increasing amounts of depolarizing current injection. This property of phasic neurons is attributable in large part to M-currents (Wang and McKinnon, 1995), which are low-threshold, slowly activating, non-inactivating potassium channels (Brown and Adams, 1980). M-channels consist of KCNQ2/3 subunits (Wang et al., 1998), are blocked by linopirdine and XE991 (Lamas et al., 1997; Schnee and Brown, 1998), and are found in the DRG (Passmore et al., 2003). In addition, modulation of M-currents has been implicated in pain sensation (Blackburn-Munro and Jensen, 2003; Passmore et al., 2003).
In the current study, we explored the possibility that an interaction exists between MrgD and M-currents (KCNQ2/3) in the DRG. We find, in a recombinant system, that β-alanine-induced activation of MrgD results in robust inhibition of KCNQ2/3 currents. This effect is extended to a more physiologically relevant context in dissociated cultures of DRG neurons, in which activation of endogenous MrgD results in inhibition of M-currents. Gi proteins likely mediate these effects, because they are prevented by pertussis toxin treatment. Finally, we show that β-alanine-induced activation of MrgD results in an increase in phasic neuron excitability.
Some of these data were published previously for the Society for Neuroscience conference (Crozier et al., 2006).
Materials and Methods
Cell culture preparation.
Chinese hamster ovary (CHO) cells stably expressing rat KCNQ2/3 subunits (Wang et al., 2004) were transiently cotransfected with rat MrgD and enhanced green fluorescent protein (EGFP) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions with slight modifications. Cells were transfected with MrgD at 75% of the manufacturer's recommended DNA concentration (6 μg/5 ml of plating medium). After 4–5 h of transfection incubation at 37°C, the cells were rinsed with HBSS, and the media were replaced with preequilibrated DMEM without antibiotics and transferred to 26°C, at which they remained until use (48–72 h after transfection). We observed that the reduced DNA concentration and lower temperature after transfection assisted in promoting functional (assessed by β-alanine-induced suppression of KCNQ2/3) surface expression of MrgD. CHO cell media consisted of DMEM, 10% fetal bovine serum (FBS), G418 (800 μg/ml), hygromycin (800 μg/ml), MEM nonessential amino acids (0.1 mm), and 1% Pen/Strep.
DRG cultures were prepared from ∼6-week-old male Sprague Dawley or Long–Evans rats in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Wyeth Institutional Animal Care and Use Committee. No experimentally detectable difference was observed between the two strains, so the data were pooled. After CO2 asphyxiation, the rats were cervically dislocated, and the fur was sprayed liberally with 70% ethanol to reduce contamination. DRGs from all spinal levels were harvested, nerve roots were trimmed, and the DRGs were subjected to collagenase (2 mg/ml) treatment for ∼15 min at 37°C, followed by 0.05% trypsin-EDTA for ∼30 min at 37°C. The enzymatic reaction was stopped by washing the cells with DMEM containing 10% FBS. The cells were spun at 800 rpm for 4 min, the supernatant was removed, and fresh growth medium was added. The cells were then mechanically triturated through a 1000 μl pipette tip, passed through a 40 μm cell strainer, and plated on poly-d-lysine (0.1 mg/ml; Sigma, St. Louis, MO)-treated glass coverslips (Assistent, Sondheim, Germany). DRG media consisted of DMEM, 10% FBS, 1% Pen/Strep, and 50 ng/ml GDNF (glial cell line-derived neurotrophic factor) (Molliver et al., 1997) to promote the survival of MrgD-containing neurons. Recordings were performed 24–48 h after plating.
Electrophysiology.
Whole-cell voltage-clamp recordings were performed (Hamill et al., 1981) in external solution consisting of HBSS (#14025; Invitrogen) supplemented with 10 mm HEPES and 10 mm glucose. Pipettes with filament (1.5 × 0.86 mm; 10 cm length; Sutter Instrument, Novato, CA) had resistances of ∼2–5 MΩ when filled with internal solution containing the following (in mm): 140 KCl, 2 MgCl2, 4 EGTA, 10 HEPES, and 0.14 CaCl2, pH 7.2 with either KOH or Tris-OH. All recordings were performed at room temperature. For whole-cell voltage-clamp recordings, series resistance was compensated 70–90%.
For CHO cell recordings, GFP-containing cells were identified by fluorescence and considered for additional analysis. β-alanine was bath applied using a perfusion control system (VC-6; Warner Instruments, Hamden, CT) at 100 μm, which is approximately sevenfold higher than the published EC50 (Shinohara et al., 2004). KCNQ2/3 currents were evoked every 10 s by delivering a 1 s depolarizing voltage pulse from −80 mV to −20 mV (see Fig. 1 A).
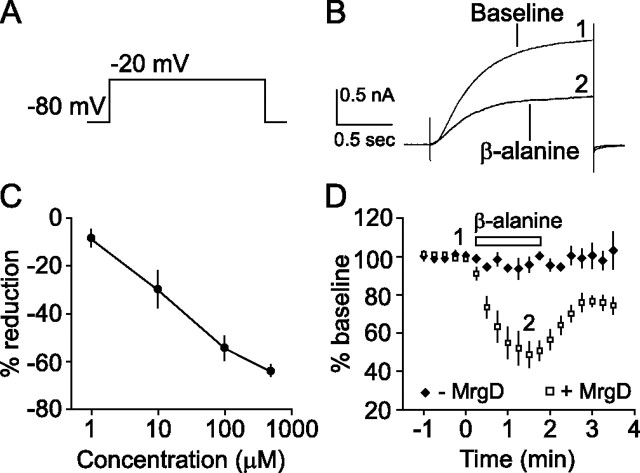
Figure 1.
β-Alanine-induced activation of MrgD strongly inhibits KCNQ2/3 currents in CHO cells. A, Typical step depolarization voltage protocol used to elicit activation of KCNQ2/3 currents. B, Sample sweeps before (1) and ∼1 min into (2) β-alanine (100 μm) exposure. C, Concentration–response relationship for 1, 10, 100, and 500 μm β-alanine. D, Time course of MrgD modulation of KCNQ2/3 currents. Currents were maximally inhibited within ∼1 min of exposure to β-alanine (100 μm) but only when MrgD was present. 1 and 2 refer to times when currents were averaged and displayed in B. Open symbols, Plus MrgD; closed symbols, minus MrgD; open horizontal bar, duration of β-alanine exposure.
For DRG recordings, IB4+ cells that express MrgD (Dong et al., 2001) were prelabeled with IB4-conjugated Alexa Fluor 594 (Invitrogen) at 1.5 μg/ml for 10 min and then rinsed for 5 min before recording. Voltage-clamp recordings were obtained, and M-currents, which consist of KCNQ2/3 (Wang et al., 1998), were measured every 10 s, typically from −30 mV to −50 mV. M-currents were assessed by taking the difference of the means between the start (∼10 ms) and end of the hyperpolarizing pulse (see Fig. 3 A). Whole-cell current-clamp recordings were obtained to examine the effect of MrgD activation on action potential properties of phasic neurons. Depolarizing current injections in 50 pA steps were delivered to discriminate between phasic and tonic neurons. β-Alanine was applied at 500 μm for the neuronal recordings. Tetrodotoxin (1 μm) was present for recordings of M-currents.
Figure 3.
Activation of endogenous MrgD suppresses M-currents and enhances phasic DRG neuron excitability. A, Top depicts the voltage protocol used to monitor M-currents. Sample sweeps during baseline and β-alanine exposure (500 μm) illustrate MrgD-induced suppression of M-currents. For summary data and effects of G-protein signaling inhibitors, see Figure 2 B. The amplitude of M-currents was measured as the difference in mean current indicated by the brackets between the onset (∼10 ms) and end of the hyperpolarizing pulse. B, A consequence of β-alanine exposure is enhanced neuronal excitability reflected as an increase in action potential firing rate.
Reagents.
All cell culture reagents were purchased from Invitrogen. Tetrodotoxin (Sigma) and SCH50911 (Tocris Bioscience, Ellisville, MO) were prepared in water at 1000×, stored at −20°C, and diluted on the day of use. β-Alanine (Sigma) was prepared fresh daily. U73122, edelfosine, and linopirdine (Sigma) were dissolved in ethanol, XE991 (Tocris Bioscience) was dissolved in water, and all were stored at −80°C. PTX (0.2 mg/ml; Sigma) was used at 100 ng/ml. All dilutions were made using external solution. Pharmacological recordings were typically interleaved with control recordings on a daily basis (e.g., PTX experiments one day and control recordings the next) to ensure that the cultures were responsive to β-alanine.
Data analysis.
Data were acquired and digitized using a Multiclamp 700B amplifier and Digidata 1440 analog-to-digital converter (Molecular Devices, Sunnyvale, CA) and analyzed on a PC running pClamp 9.2. Statistical comparisons (ANOVA) between groups were performed using Microsoft (Seattle, WA) Excel with p < 0.05 followed by Fisher's least-squared difference test with Bonferroni adjustments for multiple comparisons. Error bars indicate SEM.
Results
To ascertain the functional significance of MrgD activation by its putative ligand β-alanine (Shinohara et al., 2004), we first determined whether an interaction could be observed between MrgD and KCNQ2/3, the molecular correlate of the M-type potassium channel. CHO cells stably expressing KCNQ2/3 were transiently cotransfected with MrgD and EGFP. Whole-cell voltage-clamp recordings were obtained from EGFP-expressing neurons, and a +60 mV depolarizing pulse from a holding potential of −80 mV (Fig. 1 A) was delivered to activate KCNQ2/3 channels. Stable baseline responses were collected for 1 min in the presence of bath-applied control external solution, and then β-alanine (100 μm) was bath applied. Within ∼1 min of application, KCNQ2/3 currents were robustly suppressed by β-alanine (50.5 ± 4.8% inhibition; n = 14) (Figs. 1 D, 2 A). In addition, β-alanine had no effect on cells without MrgD, indicating that the response was mediated by MrgD.
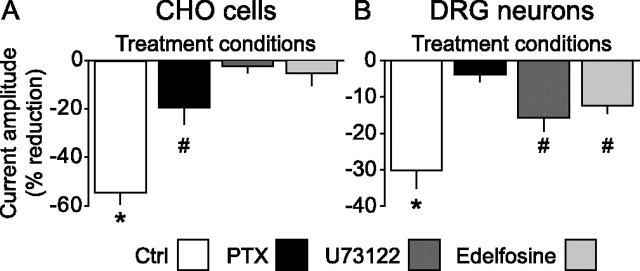
Figure 2.
Differential coupling of KCNQ2/3 and M-channels to MrgD-activated G-proteins in CHO cells and DRG neurons, respectively. A, Whole-cell voltage-clamp experiments revealed that MrgD activation by β-alanine (100 μm) in CHO cells containing KCNQ2/3 resulted in strong suppression of evoked currents. This effect was partially blocked by PTX treatment (100 ng/ml; overnight) and completely prevented by acute inhibition of phospholipase C (U73122, 1 μm; edelfosine, 10 μm). *Group is statistically larger than all other groups (p < 0.001). #Statistically smaller than β-alanine control response and larger than the PLC inhibitor groups (p < 0.017). B, In contrast, M-currents in DRG neurons were only partially blocked by PLC inhibition, but responses to β-alanine were completely prevented by PTX treatment (100 ng/ml; overnight). *Group is statistically larger than all other groups (p < 0.001). #Statistically smaller than β-alanine control response and larger than PTX group (p < 0.017). Ctrl, Control.
We next sought to assess the involvement of G-protein signaling pathways that are initiated after β-alanine binding to MrgD. Pharmacological blockade of Gi coupling with PTX (100 ng/ml; overnight) resulted in an incomplete effect (19.6 ± 6.7% inhibition; n = 10) (Fig. 2 A). However, acute blockade of phospholipase C (PLC) with U73122 (1 μm) or edelfosine (10 μm) completely prevented the effect of β-alanine-induced suppression of KCNQ2/3 currents (U73122, 2.5 ± 2.6% inhibition, n = 7; edelfosine, 5.3 ± 5.0% inhibition, n = 4) (Fig. 2 A). These data suggest that in CHO cells, MrgD modulation of KCNQ2/3 occurs predominantly via Gq and the PLC pathway.
To better understand the role of MrgD in pain-sensing neurons, we assessed the effect of activating endogenous MrgD on the M-current in dissociated cultures of DRG neurons. Before recording, neurons were treated with IB4-conjugated Alexa Fluor 594 to increase the likelihood of examining MrgD-containing neurons [∼75% of IB4+ neurons are MrgD-containing (Zylka et al., 2005)]. This treatment has no detectable effect on the biophysical properties of DRG neurons (Wu and Pan, 2004). Small-diameter, IB4+ DRG neurons were chosen, and M-currents were assayed by a deactivation protocol (Fig. 3 A). After collecting 1 min of stable baseline responses, β-alanine was bath applied (500 μm). Similar to the recordings from CHO cells, β-alanine-induced activation of MrgD resulted in inhibition of M-currents (30.2 ± 4.8% inhibition; n = 9) (Figs. 2 B, 3). After β-alanine exposure, application of linopirdine (20 μm), a selective M-current antagonist (Lamas et al., 1997; Schnee and Brown, 1998), blocked the remaining current, thus confirming that we were studying M-currents (Fig. 3 A). Because of the relatively high concentration of β-alanine used and its structural similarity to GABA, we also performed experiments in the presence of the GABAB antagonist SCH50911 (20 μm). These experiments were nearly identical to those performed in the absence of the GABAB antagonist (26.5 ± 2.8% inhibition; n = 6; data not shown) and indicate that the response to β-alanine is not mediated by GABAB receptors. Finally, MrgD-induced suppression of KCNQ/M-currents exhibited selectively because GIRKs (G-protein-activated inward rectifying K+ channels) were not affected by β-alanine exposure (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
We next assessed the effects of pharmacological intervention of MrgD-induced modulation of M-currents in DRG neurons. In contrast to the experiments in CHO cells, blockade of PLC activity by U73122 (1 μm) or a second PLC inhibitor edelfosine (10 μm) had a modest but significant effect (U73122, 15.8 ± 3.7% inhibition, n = 8; edelfosine, 12.5 ± 2.1% inhibition, n = 7). However, PTX treatment completely prevented the response (3.9 ± 1.8% inhibition; n = 6) (Fig. 2 B). Together, these data suggest a differential sensitivity between CHO cells and DRG neurons to the pharmacological reagents used and are consistent with MrgD-induced modulation of KCNQ/M-channels primarily by Gi/o proteins in DRG neurons.
The limiting of phasic neurons to a single action potential has been attributed to M-current activation (Wang and McKinnon, 1995). We therefore hypothesized that if MrgD activation resulted in suppression of the M-current, an increase in firing rate should be observed in these neurons. To address this issue, whole-cell current-clamp recordings were obtained from small-diameter, IB4+, phasic DRG neurons (for details on determinations of phasic vs tonic firing neurons, see Materials and Methods). The data confirm our hypothesis, because application of β-alanine (500 μm) contributed to an increase in neuronal excitability such that the firing rate of five of seven neurons increased nearly twofold (baseline, 0.82 Hz; β-alanine, 1.64 Hz; n = 7) (Fig. 3 B). Some neurons, after β-alanine exposure, were also exposed to linopirdine (20 μm; data not shown), which further augmented the firing rate, thus suggesting that MrgD activation inhibits a fraction of the total available M-current.
Discussion
The recently identified Mrg family of GPCRs exhibits highly concentrated expression in sensory neurons (Dong et al., 2001; Lembo et al., 2002). One family member, MrgD, is found in rodents and humans, and its expression is restricted to nonpeptidergic, small-diameter, IB4+ DRG neurons (Dong et al., 2001; Zylka et al., 2003; Zylka et al., 2005). These attributes provide for an intriguing therapeutic utility and, with the recent identification of the ligand for MrgD, β-alanine (Shinohara et al., 2004), have allowed researchers to probe for the functional significance of MrgD activation. Here, we report the first evidence of an interaction of MrgD with a potassium channel, KCNQ2/3, that modulates the properties of phasic DRG neurons. Whole-cell voltage-clamp recordings from CHO cells, which exogenously expressed both KCNQ2/3 and MrgD, revealed a >50% inhibition of KCNQ2/3 currents in the presence of β-alanine. Furthermore, we provide evidence that a similar interaction exists natively between MrgD and M-currents in DRG neurons and that MrgD couples predominantly to Gi/o proteins. Finally, we show that phasic neurons, which fire a single AP before MrgD activation, fire repeatedly during β-alanine application, and thus the excitability of DRG neurons that contain MrgD is enhanced.
M-channels are so named because they were originally identified by modulation mediated by M1 muscarinic acetylcholine receptor (mAChR) activation (Brown and Adams, 1980; Marrion et al., 1989), and details of this mechanism have emerged. For example, in superior cervical ganglion neurons (SCGs), activation of mAChR stimulates release of Gαq, activation of PLC, and subsequent conversion of phosphatidylinositol bisphosphate (PIP2) to IP3. Because PIP2 is thought necessary to keep the channel in the open state (Zhang et al., 2003), depletion of PIP2 can result in suppression of the M-current (Suh and Hille, 2002; Zhang et al., 2003; Winks et al., 2005). In addition to Gq coupling, mAChR-induced modulation of the M-current may also have a PTX-sensitive Gi/o component (Haley et al., 2000). Our results indicate that both mechanisms may be in play in MrgD regulation of M-currents. MrgD-induced suppression of KCNQ2/3 is completely prevented by blocking PLC activity in CHO cells, but the β-alanine effect is also partially PTX sensitive. In DRG neurons, partial prevention of the MrgD-induced suppression of M-currents was achieved by PLC inhibitors, although a more robust blockade of the response was produced by PTX. Together, these results implicate both PTX-sensitive and PTX-insensitive G-proteins in M-current regulation and are consistent with the reported capacity of MrgD to couple via both Gq and Gi/o proteins (Shinohara et al., 2004). The manner in which dual activation of these pathways may be interacting in the various cells may be revealed by subsequent research. One simple possibility is that the cell types (CHO cells and SCG and DRG neurons) express varying types or levels of G-proteins that may contribute to differential modulation of the M-current.
Although we report here on the interaction of MrgD with KCNQ/M-currents, other receptors or ion channels may be potential effectors that may have important consequences for pain. In rodents, MrgD mRNA colocalizes with the ATP-gated channel P2X3 receptor and in rats, but not mice, with vanilloid receptor 1 (Dong et al., 2001; Zylka et al., 2003), and both receptor types play important roles in pain sensation. In addition, sodium, calcium, and other potassium channels may be modulated by MrgD. For instance, we have observed MrgD-induced modulation of N-type calcium channels (Crozier et al., 2006). Thus, the potential targets and modes of action of MrgD in pain may be quite varied. In addition, that rodents (MrgA–H) and humans (MrgD, MrgX) contain more than one Mrg, which may form heterodimers, suggests that the readouts from painful stimuli mediated by Mrgs may be complex. In vivo evidence for Mrgs in pain is limited, but one report found that activation of SNSR1 (MrgC) by its ligand, γ2-MSH, was pronociceptive (Grazzini et al., 2004). Finally, a change in MrgD levels in response to chronic-constriction injury, a model of inflammatory and neuropathic pain, was noted by Shinohara et al. (2004), but an increase or decrease in MrgD levels was not reported. To date, the precise cellular localization (e.g., nerve terminals, cell body, or primary afferents) of MrgD remains unknown; thus, critical questions relating to how and where MrgD participates in pain remain open.
Alterations in M-current activity have been linked to neuronal excitability in conditions such as seizures, cognition, and pain (Surti and Jan, 2005). Mutations in KCNQ2 or KCNQ3 can cause epilepsy and were correlated with benign familial neonatal convulsions (Biervert et al., 1998; Singh et al., 1998). Blockers of KCNQ channels such as linopirdine or XE991 have been investigated as “cognitive enhancers” for the treatment of memory disorders such as Alzheimer's disease (Surti and Jan, 2005). Conversely, retigabine, a KCNQ channel opener, can suppress seizures in animal models (Dailey et al., 1995; Rostock et al., 1996) and can also reduce neuropathic pain (Blackburn-Munro and Jensen, 2003; Passmore et al., 2003).
The development of pharmacological reagents for KCNQ2/3 was driven by efforts to modulate aberrant neuronal excitability. In the experiments presented here, MrgD activation inhibits a fraction of the total M-current; thus, the consequence of MrgD activation may be akin to a rheostat rather than an ON/OFF switch in modulating M-currents and neuronal excitability. Because KCNQ2/3 subunits may be expressed primarily in axons (Chung et al., 2006), a speculative mode of action of β-alanine-induced activation of MrgD would be to promote the excitability of primary nociceptive afferents.
Footnotes
We thank Ru Shen for providing the CHO cell line containing KCNQ2/3, Dr. Mary Lynn Mercado for assistance with DRG cultures, Flora Jow for helpful discussions, Youping Huang for assistance with statistical comparisons, and Dr. Mark Bowlby for helpful discussions and a critical reading of this manuscript.
References
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol. 2003;460:109–116. doi: 10.1016/s0014-2999(02)02924-2. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA. 2006;103:8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Ajit SK, Kaftan EK, Pausch MH. MrgD activation modulates N-type (Cav2.2) calcium channel activity. Soc Neurosci Abstr. 2006;32:802–18. [Google Scholar]
- Dailey JW, Cheong JH, Ko KH, Adams-Curtis LE, Jobe PC. Anticonvulsant properties of D-20443 in genetically epilepsy-prone rats: prediction of clinical response. Neurosci Lett. 1995;195:77–80. doi: 10.1016/0304-3940(95)11783-s. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Puma C, Roy MO, Yu XH, O'Donnell D, Schmidt R, Dautrey S, Ducharme J, Perkins M, Panetta R, Laird JM, Ahmad S, Lembo PM. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci USA. 2004;101:7175–7180. doi: 10.1073/pnas.0307185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley JE, Delmas P, Offermanns S, Abogadie FC, Simon MI, Buckley NJ, Brown DA. Muscarinic inhibition of calcium current and M current in Gαq-deficient mice. J Neurosci. 2000;20:3973–3979. doi: 10.1523/JNEUROSCI.20-11-03973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Smart TG, Marsh SJ, Brown DA. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br J Pharmacol. 1989;98:557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasta S, Pediani J, Appelbe S, Trim S, Wyatt M, Cox P, Fidock M, Milligan G. Interactions between the Mas-related receptors MrgD and MrgE alter signalling and trafficking of MrgD. Mol Pharmacol. 2006;69:479–491. doi: 10.1124/mol.105.018788. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostock A, Tober C, Rundfeldt C, Bartsch R, Engel J, Polymeropoulos EE, Kutscher B, Loscher W, Honack D, White HS, Wolf HH. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23:211–223. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther. 1998;286:709–717. [PubMed] [Google Scholar]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Suh B-C, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Surti TS, Jan LY. A potassium channel, the M-channel, as a therapeutic target. Curr Opin Investig Drugs. 2005;6:704–711. [PubMed] [Google Scholar]
- Wang HS, McKinnon D. Potassium currents in rat prevertebral and paravertebral sympathetic neurones: control of firing properties. J Physiol (Lond) 1995;485:319–335. doi: 10.1113/jphysiol.1995.sp020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-S, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang K, McIlvain B, Tseng E, Kowal D, Jow F, Shen R, Zhang H, Shan QJ, He L, Chen D, Lu Q, Dunlop J. Validation of an atomic absorption rubidium ion efflux assay for KCNQ/M-channels using the ion Channel Reader 8000. Assay Drug Dev Technol. 2004;2:525–534. doi: 10.1089/adt.2004.2.525. [DOI] [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZZ, Pan HL. Tetrodotoxin-sensitive and -resistant Na+ channel currents in subsets of small sensory neurons of rats. Brain Res. 2004;1029:251–258. doi: 10.1016/j.brainres.2004.09.051. [DOI] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Taylor N, Xie Y, Ford R, Johnson J, Paulsen JE, Bates B. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res Mol Brain Res. 2005;133:187–197. doi: 10.1016/j.molbrainres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]