Abstract
The master clock driving mammalian circadian rhythms is located in the suprachiasmatic nuclei (SCN) of the hypothalamus and entrained by daily light/dark cycles. SCN lesions abolish circadian rhythms of behavior and result in a loss of synchronized circadian rhythms of clock gene expression in peripheral organs (e.g., the liver) and of hormone secretion (e.g., corticosterone). We examined rhythms of behavior, hepatic clock gene expression, and corticosterone secretion in VPAC2 receptor-null (Vipr2−/−) mice, which lack a functional SCN clock. Unexpectedly, although Vipr2−/− mice lacked robust circadian rhythms of wheel-running activity and corticosterone secretion, hepatic clock gene expression was strongly rhythmic, but advanced in phase compared with that in wild-type mice. The timing of food availability is thought to be an important entrainment signal for circadian clocks outside the SCN. Vipr2−/− mice consumed food significantly earlier in the 24 h cycle than wild-type mice, consistent with the observed timing of peripheral rhythms of circadian gene expression. When restricted to feeding only during the daytime (RF), mice develop rhythms of activity and of corticosterone secretion in anticipation of feeding time, thought to be driven by a food-entrainable circadian oscillator, located outside the SCN. Under RF, mice of both genotypes developed food-anticipatory rhythms of activity and corticosterone secretion, and hepatic gene expression rhythms also became synchronized to the RF stimulus. Thus, food intake is an effective zeitgeber capable of coordinating circadian rhythms of behavior, peripheral clock gene expression, and hormone secretion, even in the absence of a functional SCN clock.
Keywords: circadian rhythms, corticosterone, entrainment, feeding, HPA axis, liver, VIP
Introduction
The master clock driving mammalian circadian rhythms resides within the suprachiasmatic nuclei (SCN) of the hypothalamus. However, many peripheral tissues also contain endogenous circadian clocks that are thought to play critical roles in the local control of physiology and metabolism (Balsalobre et al., 1998; Zylka et al., 1998; Damiola et al., 2000; Yamazaki et al., 2000; McNamara et al., 2001; Abe et al., 2002; Yoo et al., 2004). For example, in the liver, there are circadian rhythms in the expression of genes involved in nutrient metabolism, heme and glutamine biosynthesis, and drug detoxification (Kornmann et al., 2001; Reddy et al., 2006). The signals that entrain peripheral circadian clocks to the environment are unknown and are the subject of intense current investigation.
If rats and mice, which are nocturnal animals, are fed exclusively during the daytime [restricted feeding (RF)], the phase of circadian gene expression in some peripheral organs such as liver, kidney, heart, and pancreas becomes uncoupled from the SCN master pacemaker (Damiola et al., 2000; Stokkan et al., 2001; Horikawa et al., 2005). Animals then display two periods of activity in each 24 h, one during the subjective night and another during the day, in anticipation of feeding [food-anticipatory activity (FAA)] (Mistlberger, 1994; Davidson et al., 2003; Dudley et al., 2003; Horikawa et al., 2005). RF also induces food-anticipatory components of other daily rhythms, including body temperature, plasma corticosterone, and drinking. These feeding-entrained rhythms persist for several days if feedings are omitted. Daily rhythms of FAA and of gastrointestinal and metabolic functions can be entrained by RF in SCN-lesioned rodents. These studies (for review, see Stephan, 2002) have led to the proposal that there is a food-entrainable circadian oscillator (FEO) that drives the increase in locomotor activity in anticipation of scheduled feeding and is distinct from the light-entrainable oscillator in the SCN.
Vasoactive intestinal peptide (VIP), acting through the VPAC2 receptor (encoded by the Vipr2 gene), plays a pivotal role in the control of circadian activity in the SCN, promoting rhythmicity and synchronizing pacemaking neurons (Aton et al., 2005; Maywood et al., 2006). Both Vipr2−/− and VIP-deficient mice display a severely disrupted circadian phenotype (Harmar et al., 2002; Colwell et al., 2003), showing markedly attenuated or abolished behavioral rhythms in constant darkness and running wheel behavior that is determined predominantly by the prevailing lighting conditions (masking) rather than by the SCN clock. Unlike most other circadian mutant mice, which have alterations of the clock genes, Vipr2−/− mice have an intact molecular circadian oscillator and are therefore potentially able to sustain rhythms in peripheral organs. In this study, we provide the first characterization of the control of peripheral circadian rhythms in Vipr2−/− mice.
There were robust rhythms of clock gene expression in the liver of Vipr2−/− mice; these were phase advanced relative to wild type, consistent with an abnormal timing of food intake. Rhythms of hepatic clock gene expression and food-anticipatory rhythms of activity and corticosterone secretion could be entrained by RF, demonstrating their independence from the SCN.
Materials and Methods
Animals.
Adult male mice (wild type or Vipr2−/− on a C57BL/6J background: 4–12 months old at the start of study) were housed individually in polycarbonate cages and maintained under controlled conditions of lighting and temperature. For behavioral experiments (see below), the cages were also equipped with running wheels. Powdered diet was provided in custom 60 ml glass jars (Unifab, Kalamazoo, MI). Each jar was covered with a stainless steel lid containing a hole 22 mm in diameter. A stainless steel disc was placed under the lid on top of the diet; the disc contains six circular openings (each 11 mm in diameter) to allow food access while minimizing spillage. The design of the hoppers and the use of powdered diet allowed access to the food but prevented animals from removing and storing excess food in the cage. When the animals were housed in a light/dark (LD) cycle, the lighting schedule was 12 h of bright white light and 12 h of “darkness” of dim red light (<7 lux at cage level), lights on between 7:00 A.M. and 7:00 P.M. The dim red light was permanently on to facilitate animal care and to enable behavioral studies to be performed during the dark period. DD denotes constant lighting with dim red light. All experimentation was conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986.
Food restriction studies.
The RF regimen was introduced progressively: food availability was reduced over a 3 d period from 8 to 4 h/d (Marchant and Mistlberger, 1997). During RF, food hoppers were introduced into the cages daily at zeitgeber time or circadian time 4 (ZT4/CT4) and taken out at ZT8/CT8 (where, by convention, ZT0 is defined as the time of lights on in an LD cycle, and CT0 is time of lights on in the previous LD cycle). Mice had ad libitum access to water throughout all of the experiments. Body weight and food intake were closely monitored during periods of RF, and animals failing to feed or showing a >20% loss in weight were returned to normal feeding and excluded from the study or humanely killed.
Behavior and feeding studies.
Running wheel activity was recorded continuously throughout the experiment. During the ad libitum study, food intake was measured at intervals of 4 h over a 48 h period in both LD and DD conditions. For DD conditions, these measurements were commenced on the second complete circadian cycle (35 h) after transfer to DD, in accordance with our previous studies of activity (Harmar et al., 2002).
Data analyses.
Food intake in the ad libitum and RF studies was calculated from the weight of food hoppers on entering and directly after removing them from the cages. The mean and SEM of the weight of food consumed was calculated for every 4 h period for both wild-type (WT) and Vipr2−/− mice. In a few cases, the animals had spread large amounts of bedding material into the hoppers, which made accurate assessment of food intake impossible. In these cases, the individual animal's food intake during the 4 h period was excluded from calculations of mean and SEM for the period.
Clocklab software (Actimetrics, Wilmette, IL) was used to determine the time of activity onset, least-squares fit of activity onset, total activity indices, and χ2 periodograms of each of the animals in both the ad libitum and the RF studies. Circadian gene expression was determined using the cosine wave-fitting algorithm COSOPT (Straume, 2004), to identify statistically significant rhythms and corresponding phase optima.
Plasma hormone and liver gene expression studies.
In a second series of experiments, WT or Vipr2−/− mice were housed individually in LD or DD conditions and either fed ad libitum or entrained to a restricted feeding regimen as described above. Mice were killed rapidly by decapitation at 4 h intervals (at ZT/CT 4, 8, 12, 16, 20, and 24), and trunk blood was collected into heparinized tubes. The brains (for a separate study) and samples of liver tissue were removed, quickly frozen on dry ice, and subsequently stored at −80°C until required. Blood samples were centrifuged, and plasma was stored at −20°C before determination of plasma concentrations of corticosterone by radioimmunoassay (Holmes et al., 1997). Total RNA was extracted from the liver samples using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. TaqMan reverse transcriptase (RT)-PCR was performed using primers and probes for Per1 (Sangoram et al., 1998), Per2 (Panda et al., 2002), Bmal1, and Dbp (Bunger et al., 2000). Relative mRNA abundance was determined by use of the comparative δ-CT method using 18S ribosomal RNA as internal control. In situ hybridization was conducted and quantified as described previously (Akhtar et al., 2002), using tissues from mice killed at 6 h intervals in LD (at ZT 6, 12, 18, and 24) and at 5 h intervals in DD (at CT 0, 5, 10, 15, and 20).
Statistical analysis.
The significance of the differences between means was determined by two-way ANOVA followed by Bonferroni's post hoc test or by unpaired Student's t test, as appropriate. p < 0.05 was considered statistically significant.
Results
Timing of food intake
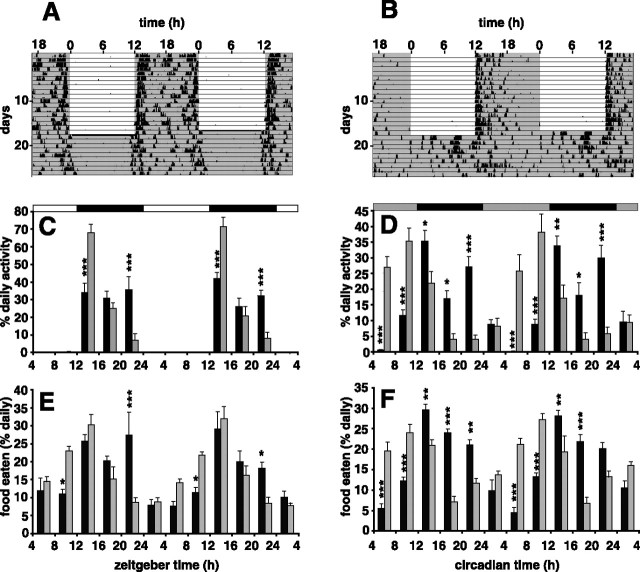
The wheel-running activity of both wild-type and Vipr2−/− mice housed in a 12 h LD cycle was almost entirely confined to the dark period (99% in wild type and 97% in knock-out), consistent with a strong masking effect of light (Fig. 1A–C). There was a diurnal rhythm of food intake (Fig. 1E), but this was much less tightly confined to the dark period (70% in wild type and 55% in knock-out), showing less evidence of masking by light. Vipr2−/− mice consumed food significantly earlier in the 24 h cycle than wild-type mice (Fig. 1E, Table 1). Two days after transfer into DD, the rhythm of food intake in wild-type animals was similar to that in LD, whereas the knock-out animals' rhythm was advanced by ∼7 h (Fig. 1F, Table 1).
Figure 1.
Rhythms of food intake and wheel running in wild-type and Vipr2−/− mice fed ad libitum. A, B, Representative profiles of locomotor activity in wild-type (A) and Vipr2−/− (B) mice are presented in double-plotted format. Periods of darkness are shaded. For the first 16 d, animals were exposed to a 12 h LD cycle (darkness from 7:00 P.M. to 7:00 A.M.). From day 17, animals were maintained in constant darkness. C–F, Cumulative wheel running counts (C, D) and food intake (E, F) were monitored in wild-type (black bars) and Vipr2−/− (gray bars) mice over 4 h periods for 48 h in LD (C, E) or 2 d after transfer into DD (D, F). Values represent mean ± SEM; n = 8. The bars at the top indicate the dark period in black and the light period in white (C, E) or subjective night in black and subjective day in gray (D, F). *p < 0.05; **p < 0.01; ***p < 0.001 compared with Vipr2−/−.
Table 1.
Mean ± SEM acrophase and phase differences of running wheel activity and feeding behavior in wild-type and Vipr2−/− mice in LD and 2 d after transfer into DD conditions and with ad libitum access to food
| Running | Feeding | ||
|---|---|---|---|
| LD | |||
| Wild type | Acrophase | 0.48 ± 0.81 | 1.10 ± 1.40 |
| Vipr2−/− | Acrophase | 22.20 ± 1.10 | 19.75 ± 0.85 |
| Phase difference (h) | 2.28 | 5.35 | |
| DD | |||
| Wild type | Acrophase | 23.80 ± 1.20 | 23.85 ± 0.71 |
| Vipr2−/− | Acrophase | 16.20 ± 1.00 | 16.72 ± 0.96 |
| Phase difference (h) | 7.60 | 7.13 |
The phase difference is shown as the phase advance in Vipr2−/− mice.
Circadian rhythmicity of clock gene expression in Vipr2−/− mice
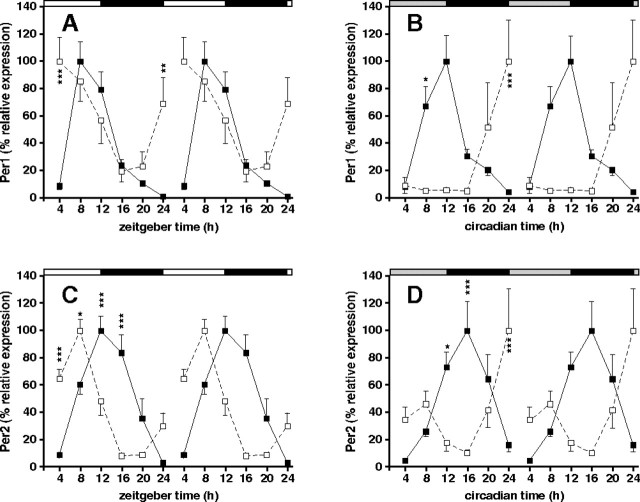
There were robust rhythms of clock gene expression in the liver of Vipr2−/− mice (p < 0.001) (Fig. 2). These rhythms were abnormal in phase, consistent with the abnormal phasing of food intake: in Vipr2−/− mice exposed to an LD cycle, the times of peak expression of the clock genes examined (Per1, Per2, Dbp, and Bmal1) were advanced (by 4–6 h) compared with wild-type mice (Fig. 2A,C, Table 2). Two days after transfer into DD, even greater phase advances (7–11 h) were seen (Fig. 2B,D, Table 2). To determine the generality and extent of this altered peripheral synchronization in Vipr2−/− mice, in situ hybridization was used to examine cycles of expression of the core clock genes Per2, Bmal1, Rev-erbα, and Cry1 and the clock-regulated genes Dbp, Wee1, Pnp, and Nfix in both liver and heart. In both organs, all of these circadian genes were phase advanced in mutant mice under a light/dark cycle (Fig. 3), and even more so 2 d after transfer into DD (Fig. 4). In the absence of a competent SCN, therefore, the circadian transcriptional program in the periphery remained functional but underwent a marked phase shift.
Figure 2.
Rhythms of clock gene expression in the liver of wild-type and Vipr2−/− mice. Values represent mean ± SEM; n = 5–8. A–D, Rhythms of Per1 (A, B) and Per2 (C, D) gene expression in the liver of wild-type (filled squares) and Vipr2−/− (open squares) mice in LD (A, C) or 2 d after transfer into DD (B, D) under conditions of ad libitum feeding are shown. Data were obtained by TaqMan RT-PCR. In each panel, one 24 h set of data has been plotted twice to better show the pattern of variation with time. The bars at the top indicate the dark period in black and the light period in white (A, C) or subjective night in black and subjective day in gray (B, D). *p < 0.05; **p < 0.01; ***p < 0.001 compared with Vipr2−/−.
Table 2.
Mean ± SEM acrophase and phase differences in hepatic gene expression rhythms of wild-type and Vipr2−/− mice in LD and 2 d after transfer into DD conditions and with ad libitum access to food
| Per1 | Per2 | Bmal1 | Dbp | ||
|---|---|---|---|---|---|
| LD | |||||
| Wild type | Acrophase | 16.56 ± 0.19 | 19.80 ± 1.20 | 4.34 ± 0.79 | 15.10 ± 1.1 |
| Vipr2−/− | Acrophase | 12.40 ± 1.70 | 14.13 ± 0.79 | 20.50 ± 1.40 | 8.66 ± 0.97 |
| Phase difference (h) | 4.26 | 5.67 | 7.84 | 6.44 | |
| DD | |||||
| Wild type | Acrophase | 17.35 ± 0.99 | 23.00 ± 1.60 | 5.60 ± 1.70 | 15.55 ± 0.82 |
| Vipr2−/− | Acrophase | 10.4 ± 3.40 | 12.10 ± 1.80 | 22.10 ± 3.40 | 5.90 ± 2.10 |
| Phase difference (h) | 6.95 | 10.90 | 7.50 | 9.65 |
The phase difference is shown as the phase advance in Vipr2−/−mice.
Figure 3.
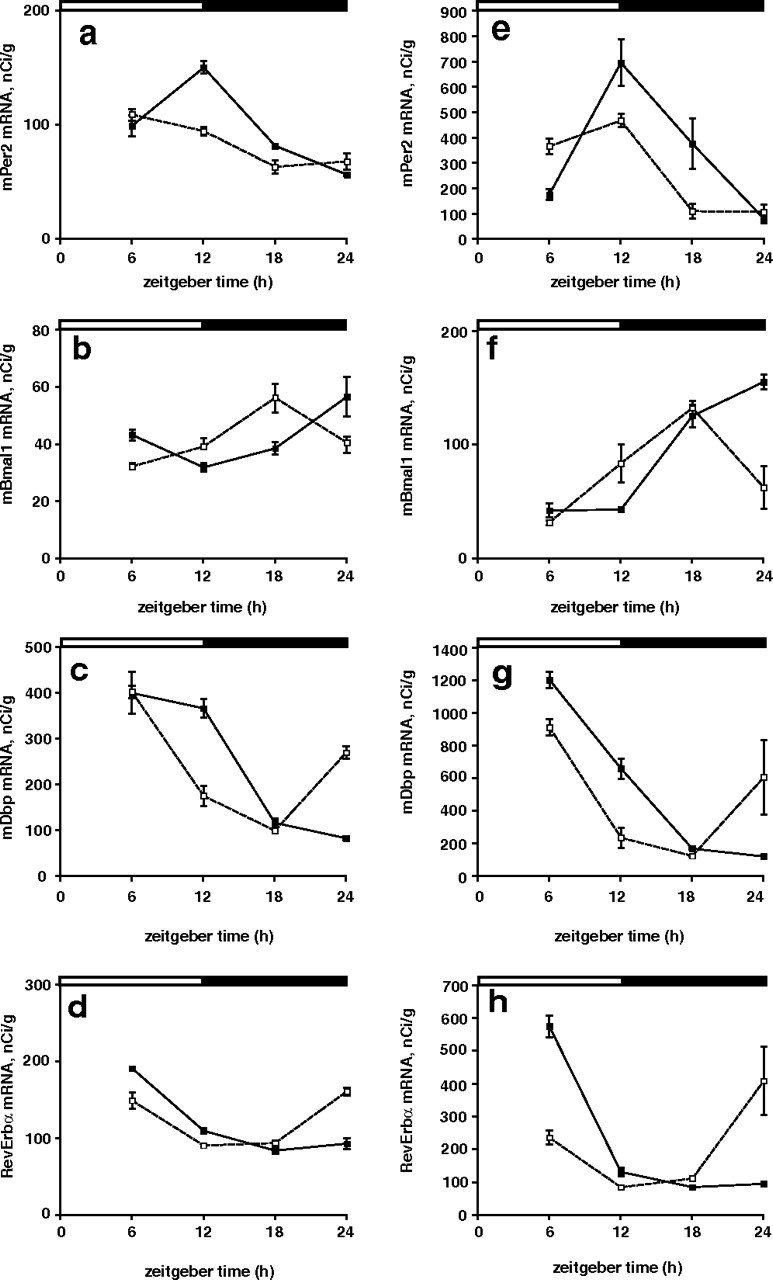
a–h, Rhythms of clock gene expression in the heart (a–d) and liver (e–h) of wild-type and Vipr2−/− mice in LD. Rhythms of Per2 (a, e), Bmal1 (b, f), Dbp (c, g), and RevErbα (d, h) gene expression in wild-type (filled squares) and Vipr2−/− (open squares) mice are shown. Data were obtained by in situ hybridization. The bars at the top indicate the dark period in black and the light period in white. Values represent mean ± SEM.
Figure 4.
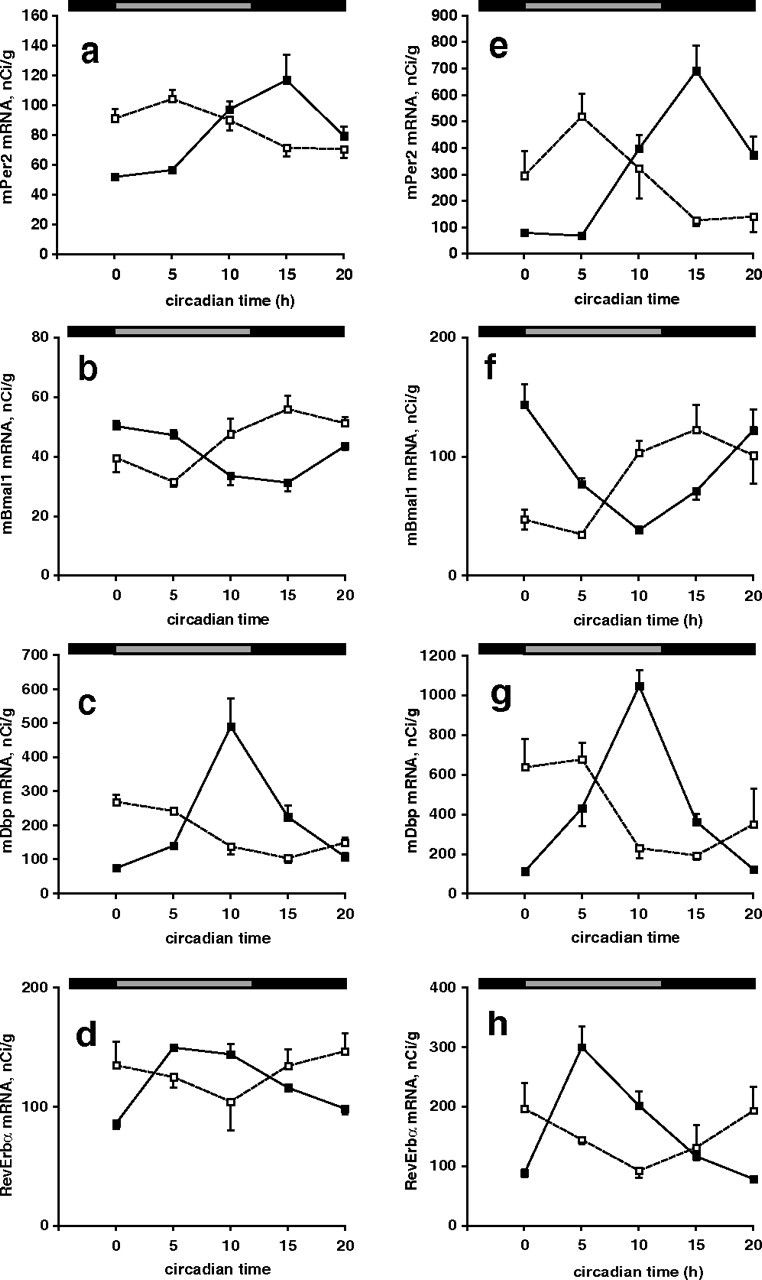
a–h, Rhythms of clock gene expression in the heart (a–d) and liver (e–h) of wild-type and Vipr2−/− mice 2 d after transfer into DD. Rhythms of Per2 (a, e), Bmal1 (b, f), Dbp (c, g), and RevErbα (d, h) gene expression in wild-type (filled squares) and Vipr2−/− (open squares) mice are shown. Data were obtained by in situ hybridization. The bars at the top indicate subjective night in black and subjective day in gray. Values represent mean ± SEM.
The temporal distribution of circadian gene expression was therefore advanced in direct correspondence to the altered rhythms of food intake in Vipr2−/− mice. This showed that Vipr2−/− mice retain some capacity for circadian coordination in the periphery and that feeding-related cues may be important in internal coordination.
Effects of RF on wheel-running activity
To test whether an FEO was functional in Vipr2−/− mice, we compared the ability of wild-type and Vipr2−/− mice to entrain to an RF schedule (Fig. 5). When subjected to 4 h of RF during the light phase of an LD cycle, both wild-type and Vipr2−/− mice developed two daily peaks of wheel-running activity, the first (food-anticipatory activity) during the daytime immediately preceding food presentation and the second at the onset of darkness (Fig. 5A–C). When transferred to DD, both wild-type and Vipr2−/− mice continued to display daily bouts of food-anticipatory wheel running of increased intensity (Fig. 5A,B,D) (p < 0.05; unpaired Student's t test). There was, however, a marked difference in behavior between the genotypes. Whereas wild-type mice continued to display a second daily bout of activity at the onset of subjective night, consistent with the activity of the SCN circadian clock, Vipr2−/− mice displayed only a single daily peak of activity in anticipation of food presentation (i.e., the SCN-regulated activity bout was not evident). In mice of both genotypes, daily bouts of food-anticipatory activity did not persist when ad libitum access to food was restored. In wild-type animals, circadian bouts of activity at the onset of subjective night continued, again consistent with a functional SCN, whereas Vipr2−/− mice displayed a broad peak of weakly rhythmic activity, with no clear time of onset and with a maximum in the light phase of the previous LD cycle. The timing of this activity was similar to that seen in Vipr2−/− mice that had not been subjected to food restriction. These data show that a functional FEO was retained in the SCN-compromised Vipr2−/− mice.
Figure 5.
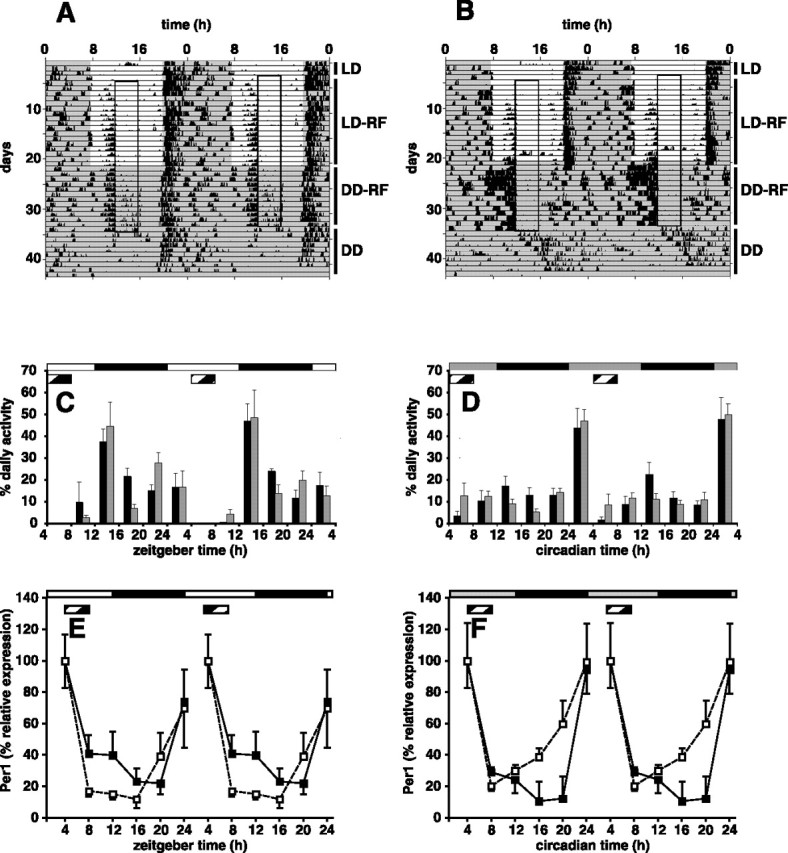
Entrainment of behavior and clock gene expression to an RF schedule in Vipr2−/− mice. A, B, Representative profiles of locomotor activity in wild-type (A) and Vipr2−/− (B) mice are presented in double-plotted format. After an initial period of entrainment to an LD cycle with ad libitum access to food (LD; days 1–4), food availability was reduced to 4 h/d (A, B, boxed regions; from 11:00 A.M. to 3:00 P.M.), initially in an LD cycle (LD-RF; days 5–21) and subsequently in DD (DD-RF; days 22–34). Ad libitum access to food was then restored (DD; days 35–43). C, D, Cumulative wheel running counts were monitored under an RF schedule in wild-type (black bars) and Vipr2−/− (gray bars) mice over 4 h periods for 48 h in LD (C) or 2 d after transfer into DD (D). E, F, Rhythms of Per1 gene expression in the liver of wild-type (filled squares) and Vipr2−/− (open squares) mice under an RF schedule were measured by TaqMan RT-PCR in LD (E) or 2 d after transfer into DD (F). One 24 h set of data has been plotted twice to better show the pattern of variation with time. C–F, The bars at the top indicate the dark period in black and the light period in white (C, E) or subjective night in black and subjective day in gray (D, F). The period of food availability in C–F is indicated by a striped bar. Values represent mean ± SEM.
Effects of RF on hepatic gene expression rhythms
To test the impact of RF on peripheral gene expression, Vipr2−/− and wild-type mice were entrained to an RF schedule, and liver gene expression rhythms were examined. In WT mice, the time of peak expression of the Per1 gene was coincident with the onset of food presentation (at ZT/CT 4) under both LD and DD conditions (Fig. 5E,F). This same pattern was evident in Vipr2−/− mice: there was no difference between genotypes in the effect of RF on Per1 rhythms in the liver. Dbp showed a similar pattern to Per1, whereas maximal expression of Per2 and Bmal1 occurred 2 and 12 h later, respectively (data not shown). In contrast to the phase differences seen in mice fed ad libitum, there were no significant differences in the profiles of gene expression of any of the clock genes measured between wild-type and Vipr2−/− mice under a restricted feeding regimen in either LD or DD conditions. It is therefore evident that the RF was able to coordinate circadian gene expression in the periphery as effectively in Vipr2−/− mice as it does in WT mice and that it can therefore act independently of the SCN.
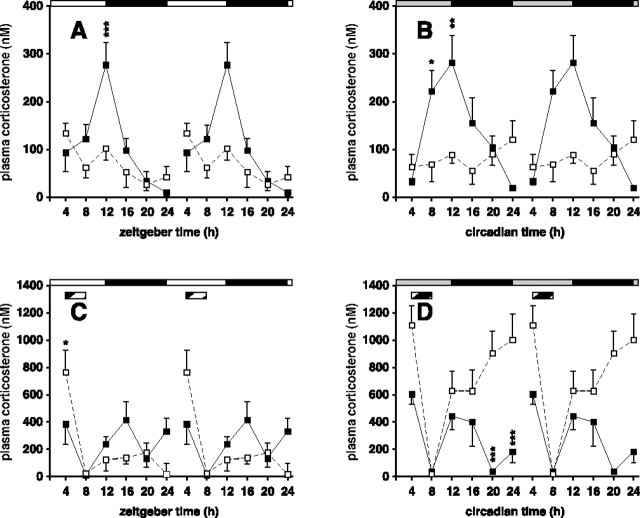
Circadian rhythms of corticosterone secretion
To obtain a second, independent report on the competence of the FEO, plasma corticosterone levels were assayed in both genotypes under ad libitum and restricted feeding. In agreement with previous reports, there was a significant circadian rhythm of plasma corticosterone (p < 0.001) in wild-type mice, with a peak coincident with the onset of the activity period (Fig. 6A,B). Unexpectedly, there was no significant rhythmicity in corticosterone secretion in ad libitum fed Vipr2−/− mice, even in conditions (under an LD cycle) in which both behavior and peripheral circadian gene expression were strongly rhythmic, albeit phase advanced in the latter case (Fig. 6A). This demonstrates that rhythmic corticosterone secretion is regulated independently of activity/rest cycles: it is not a passive consequence of behavioral activity. Circadian gene expression in the liver (and heart) is not dependent on rhythmic corticosterone secretion.
Figure 6.
Rhythms of corticosterone secretion in wild-type and Vipr2−/− mice. Values represent mean ± SEM; n = 5–8. A–D, Peripheral blood was sampled at 4 h intervals from wild-type (filled squares) and Vipr2−/− (open squares) mice housed in a 12/12 h LD cycle (A, C) or 2 d after transfer into constant darkness (B, D) under conditions of ad libitum feeding (A, B) or with RF (C, D). In each panel, one 24 h set of data has been plotted twice to better show the pattern of variation with time. The bars at the top indicate the dark period in black and the light period in white (A, C) or subjective night in black and subjective day in gray (B, D). C, D, The period of food availability is indicated by a striped bar. *p < 0.05; **p < 0.01; ***p < 0.001 compared with Vipr2−/−.
In RF conditions, the profile of corticosterone secretion was altered in both wild-type and Vipr2−/− mice. In wild-type mice entrained to an RF schedule, in both LD and DD conditions, two daily peaks of glucocorticoid secretion were observed, one resembling that seen in animals fed ad libitum, reaching maximal values just before the day–night transition (or subjective night for DD mice) and the second occurring at the beginning of the period of food availability (Fig. 6C,D). In contrast, in Vipr2−/− mice in LD, there was just a single peak of corticosterone secretion coincident with the onset of food availability. Moreover, in DD conditions, the plasma corticosterone concentrations of Vipr2−/− mice were high for most of the 24 h period, again reaching a peak immediately before feeding and declining to a minimum at the end of feeding before showing a progressive increase to peak before the next period of food presentation. These data independently confirm the existence of a competent FEO in the Vipr2−/− mice and demonstrate the potency of food-related cues in establishing internal circadian synchrony.
Discussion
Our data show that there is a functional FEO in Vipr2−/− mice. Moreover, they clearly show that under a variety of experimental conditions, the entrainment of the liver circadian clock and the secretion of corticosterone are linked to the timing of feeding and support the hypothesis (Stokkan et al., 2001) that the liver and other peripheral oscillators are not directly coupled to the SCN but controlled indirectly, through rhythmic feeding behavior.
In contrast to our findings with Vipr2−/− mice, previous studies in groups of SCN lesioned mice (Hara et al., 2001; Akhtar et al., 2002; Iijima et al., 2002; Terazono et al., 2003; Kudo et al., 2004; Guo et al., 2005), including two studies performed with a similar lighting protocol to ours (Akhtar et al., 2002; Guo et al., 2005), have reported a loss of circadian rhythms of clock gene expression in the liver and other peripheral organs. It is not clear that ability of light to suppress activity (masking) was intact after SCN lesion in any of these studies, and in some, the absence of masking by light was used as a criterion for the success of SCN lesion. The role of the SCN in masking of locomotor activity by light is controversial (Redlin and Mrosovsky, 1999; Li et al., 2005), but it seems that masking is lost in most SCN lesioned mice, either because of collateral damage to extra-SCN pathways required for masking or because an intact (albeit arrhythmic) SCN may be required for the masking response (Hara et al., 2001; Iijima et al., 2002; Terazono et al., 2003; Kudo et al., 2004). Masking responses to light are preserved when circadian rhythmicity is lost in mice with mutations in core clock genes (Vitaterna et al., 1999; Bunger et al., 2000; Zheng et al., 2001). In SCN lesioned animals, we would predict that feeding behavior would be arrhythmic, leading to a loss of synchronized rhythms of clock gene expression in peripheral tissues.
A masking response to light was intact in Vipr2−/− mice, so that 97% of the wheel-running activity of Vipr2−/− mice housed in an LD cycle took place in the dark period. Food intake was also rhythmic, although less tightly confined to the dark period. Wheel running and food intake were also significantly rhythmic in the mutant animals 2–3 d after transfer from an LD cycle into DD. In both LD and DD, Vipr2−/− mice consumed food significantly earlier in the 24 h cycle than wild-type mice, and there were corresponding differences in the timing of clock gene expression in the liver. We conclude that there is a functional hierarchy in the control of peripheral circadian rhythms, in which, even in the absence of a functional SCN clock, the daily patterns of activity imposed by an LD cycle are able to maintain feeding cycles and thereby entrain an FEO and rhythms of gene expression in liver and elsewhere. This rhythm persists, at least for a few days, when animals are transferred into constant conditions. It may not be appropriate to use wheel-running behavior as an index of the activity of the FEO (as has been done in many previous studies), because under some conditions, there is dissociation between the timing of feeding and of wheel-running behavior.
Vipr2−/− mice developed food-anticipatory behavior when subjected to a daily RF schedule, further consistent with the presence of an FEO independent of the SCN. SCN lesioned mice (Marchant and Mistlberger, 1997; Hara et al., 2001) and homozygous Clock mutant (Clk/Clk) mice (Pitts et al., 2003; Horikawa et al., 2005) also display food-anticipatory behavior that persists after temporal feeding cues are removed for several cycles, indicating that the FEO is a circadian timer located outside the SCN and not dependent on a functional Clock gene.
In nocturnal rodents, there is a circadian rhythm of corticosterone secretion from the adrenal cortex, which peaks during the late subjective day. The SCN is thought to drive this rhythm, which is abolished by SCN lesions (Moore and Eichler, 1972; Abe et al., 1979; Filipski et al., 2004), through neural connections to the corticotrophin-releasing factor and arginine–vasopressin neurons of the paraventricular nuclei of the hypothalamus (Buijs et al., 1993) and probably also through the sympathetic innervation of the adrenal gland (Jasper and Engeland, 1994). Although the activity of the SCN is the predominant pathway influencing the hypothalamo–pituitary–adrenal (HPA) axis under conditions of ad libitum feeding, which occurs mainly at night, restricted daytime feeding establishes a bimodal temporal pattern of corticosterone secretion in rodents (Krieger, 1974; Honma et al., 1984; Holmes et al., 1997; Balsalobre et al., 2000; Le Minh et al., 2001). One peak of glucocorticoid secretion resembles that seen in animals fed ad libitum, reaching maximal values just before the light/dark transition and the period of activity. A second peak of glucocorticoid secretion, seen only in animals fed during the day, occurs just before the period of food availability, when the HPA axis is normally quiescent. It is possible that the same mechanism responsible for FAA (the putative FEO) may underlie the anticipatory rise in plasma corticosterone observed in animals entrained to an RF schedule, because this is suppressed by ad libitum feeding but reappears at the phase predicted by the previous RF schedule when animals are fasted (Honma et al., 1996) and can only be entrained by feeding at intervals within the circadian range (Honma et al., 1984).
Our data on corticosterone are consistent with a model in which the circadian activity of both the SCN and of a separate FEO influences rhythms of hormone secretion. There was no significant rhythmicity in corticosterone secretion in Vipr2−/− mice fed ad libitum, suggesting that circadian rhythms of corticosterone secretion are “hard wired” to the SCN clock and are directly controlled by events downstream of oscillations of clock gene expression in the SCN. Our findings accord with an earlier study (Scarbrough et al., 1996) showing that antisense antagonism of VIP mRNA in the SCN temporarily abolished the circadian rhythm of corticosterone secretion. In both wild-type and Vipr2−/− mice entrained to an RF schedule, a peak of corticosterone secretion preceding the period of food presentation was observed. Thus, Vipr2−/− mice develop anticipatory rhythms of glucocorticoid secretion when entrained to an RF schedule. In Vipr2−/− mice entrained to an RF schedule in DD conditions, plasma concentrations of corticosterone were low immediately after feeding and then increased progressively to reach maximal levels before feeding. The reasons for the hypersecretion of corticosterone under these conditions is not known; additional studies are necessary to examine possible differences in the HPA axis and in stress responses between wild-type and Vipr2−/− mice.
We conclude from these studies that (1) the SCN clock is not required for the expression of circadian rhythms in peripheral tissues; (2) Vipr2−/− mice, which lack a robust SCN circadian clock, nevertheless possess food-anticipatory rhythms of behavior and corticosterone secretion, consistent with the presence of an intact FEO; (3) the phasing of the circadian clock in the liver is abnormal in Vipr2−/− mice, probably because their feeding behavior is timed differently from wild-type animals as a consequence of the defective SCN clock; (4) the SCN may form part of an anatomical pathway essential for the masking of behavior by light; and (5) the circadian rhythm of corticosterone secretion seen in ad libitum fed animals is driven by the SCN clock. These studies establish that the Vipr2−/− mouse is likely to be an important animal model in which to dissect the mechanisms by which peripheral circadian rhythms are controlled.
Footnotes
This work was supported by the Medical Research Council. We thank Dr. Marty Straume for assistance with the use of COSOPT software.
References
- Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29:119–131. doi: 10.1159/000122913. [DOI] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Filipski E, King VM, Etienne MC, Li X, Claustrat B, Granda TG, Milano G, Hastings MH, Levi F. Persistent twenty-four hour changes in liver and bone marrow despite suprachiasmatic nuclei ablation in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R844–R851. doi: 10.1152/ajpregu.00085.2004. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi J-C, Kelly JS, Maywood ES, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J Neurosci. 1997;17:4056–4065. doi: 10.1523/JNEUROSCI.17-11-04056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma KI, Honma S, Hiroshige T. Feeding-associated corticosterone peak in rats under various feeding cycles. Am J Physiol. 1984;246:R721–R726. doi: 10.1152/ajpregu.1984.246.5.R721. [DOI] [PubMed] [Google Scholar]
- Honma S, Katsuno Y, Abe H, Honma K. Aging affects development and persistence of feeding-associated circadian rhythm in rat plasma corticosterone. Am J Physiol. 1996;271:R1514–R1520. doi: 10.1152/ajpregu.1996.271.6.R1514. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Minami Y, Iijima M, Akiyama M, Shibata S. Rapid damping of food-entrained circadian rhythm of clock gene expression in clock-defective peripheral tissues under fasting conditions. Neuroscience. 2005;134:335–343. doi: 10.1016/j.neuroscience.2005.03.057. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology. 1994;59:97–109. doi: 10.1159/000126645. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Preitner N, Rifat D, Fleury-Olela F, Schibler U. Analysis of circadian liver gene expression by ADDER, a highly sensitive method for the display of differentially expressed mRNAs. Nucleic Acids Res. 2001;29:E51–E51. doi: 10.1093/nar/29.11.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Kudo T, Nakayama E, Suzuki S, Akiyama M, Shibata S. Cholesterol diet enhances daily rhythm of Pai-1 mRNA in the mouse liver. Am J Physiol Endocrinol Metab. 2004;287:E644–E651. doi: 10.1152/ajpendo.00095.2004. [DOI] [PubMed] [Google Scholar]
- Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gilbert J, Davis FC. Disruption of masking by hypothalamic lesions in Syrian hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:23–30. doi: 10.1007/s00359-004-0569-5. [DOI] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997;765:273–282. doi: 10.1016/s0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R57–R67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O'Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Masking by light in hamsters with SCN lesions. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;184:439–448. doi: 10.1007/s003590050343. [DOI] [PubMed] [Google Scholar]
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, Young MW, Weitz CJ, Takahashi JS. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Harney JP, Rosewell KL, Wise PM. Acute effects of antisense antagonism of a single peptide neurotransmitter in the circadian clock. Am J Physiol. 1996;270:R283–R288. doi: 10.1152/ajpregu.1996.270.1.R283. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Straume M. DNA microarray time series analysis: automated statistical assessment of circadian rhythms in gene expression patterning. Methods Enzymol. 2004;383:149–166. doi: 10.1016/S0076-6879(04)83007-6. [DOI] [PubMed] [Google Scholar]
- Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]