Abstract
Because of their high regenerative potential, stem cells are an ideal resource for development of therapies that replace injured tissue mass and restore function in patients with end‐stage liver diseases. Using a rat model of bile duct ligation (BDL) and biliary fibrosis, we investigated cell engraftment, liver repopulation, and ectopic tissue formation after intrasplenic transplantation of epithelial stem/progenitor cells. Fetal liver cells were infused into the spleens of Fisher 344 rats with progressing biliary fibrosis induced by common BDL or rats without BDL. Cell delivery was well tolerated. After migration to the liver, donor‐derived stem/progenitor cells engrafted, differentiated into hepatocytes and cholangiocytes, and formed large cell clusters at 2 months in BDL rats but not controls. Substantial numbers of donor cells were also detected at the splenic injection site where they generated hepatic and nonhepatic tissue. Transplanted cells differentiated into phenotypes other than hepato/cholangiocytic cells only in rats that underwent BDL. Quantitative reverse‐transcription polymerase chain reaction analyses demonstrated marked up‐regulation of tissue‐specific genes of nonhepatic endodermal lineages (e.g., caudal type homeobox 2 [Cdx2], pancreatic and duodenal homeobox 1 [Pdx1], keratin 13 [CK‐13]), confirmed by immunohistochemistry. Conclusion: BDL and its induced fibrosis promote liver repopulation by ectopically transplanted fetal liver‐derived cells. These cell fractions contain multipotent stem cells that colonize the spleen of BDL rats and differentiate into multiple gastrointestinal tissues, including liver, pancreas, intestine, and esophagus. The splenic microenvironment, therefore, represents an ideal niche to assess the differentiation of these stem cells, while BDL provides a stimulus that induces their differentiation.
Abbreviations
- AFP
alpha‐fetoprotein
- BDL
bile duct ligation
- Cdx2
caudal type homeo box 2
- CK
cytokeratin
- Cldn
claudin
- Dlk
δ‐like
- DPPIV
dipeptidyl‐peptidase IV
- ED
embryonic day
- EGFP
enhanced green fluorescent protein
- F
Fisher
- FGF
fibroblast growth factor
- FLSPC
fetal liver stem/progenitor cell
- HNF
hepatocyte nuclear factor
- IHC
immunohistochemistry
- IL
interleukin
- iPSC
induced pluripotent stem cell
- mRNA
messenger RNA
- OV‐6
oval cell marker
- PAS
periodic acid–Schiff
- Pdx1
pancreatic and duodenal homeobox 1
- PH
partial hepatectomy
- qRT‐PCR
quantitative reverse‐transcription polymerase chain reaction
- Sox‐9
SRY‐related high‐mobility group box 9
- TAA
thioacetamide
- w/o
without
With their high proliferative capacity and differentiation potential, stem cells have great potential for regenerative therapy of liver disease.1, 2, 3 Our prior research demonstrated that fetal liver stem/progenitor cells (FLSPCs) can repopulate a cirrhotic liver and significantly reduce fibrosis.4 Such experiments not only show the therapeutic potential of fetal cells5 but also set a precedent for eventual therapy with pluripotent cells, such as reprogrammed adult cells (induced pluripotent stem cells [iPSCs]).6, 7, 8, 9
Cell transplantation is the standard procedure to evaluate the in vivo lineage potency of stem cells. Such studies are typically performed under selective (noncompetitive) conditions that are achieved by complete inhibition of host hepatocyte proliferation and that provide a growth advantage of infused cells, resulting in massive cell proliferation and liver replacement (see reviewed models5, 10). However, to determine the true biological capacity and therapeutic potential of stem cells, studies must be performed using competitive repopulation models that adequately reproduce human diseased microenvironments.
To date, the most favorable repopulation levels under nonselective conditions have been achieved with FLSPCs, isolated from timed‐pregnant dipeptidyl‐peptidase IV (DPPIV)+ Fisher (F)344 rats and infused through the portal vein into the liver of DPPIV– recipient rats.5 These immature cells differentiate into hepatocytes and cholangiocytes11 and significantly replace normal hepatic tissue,12, 13 which requires only a partial hepatectomy (PH) prior to cell infusion.12 Moreover, in rats with advanced fibrosis/cirrhosis induced by chronic thioacetamide (TAA) administration, intravenously infused FLSPCs replace severely injured parenchyma without a PH,4 demonstrating that the cirrhotic injury process substituted the effect of a PH in normal liver. These cells exhibit major characteristics of stem cells, e.g., extensive proliferation, differentiation into at least two lineages, liver‐specific function, and long‐term tissue replacement.14 We refer to these cells as stem/progenitor cells because they are a heterogeneous mixture that includes at least three different liver epithelial cell types15, 16 and may also contain undefined small populations with greater multipotency.
Chronic liver diseases that obstruct bile ducts lead to bile duct proliferation and alter the microenvironment to induce progressive biliary fibrosis.17 To study the influence of this liver injury on the engraftment, differentiation, and repopulation of high numbers of ectopically transplanted FLSPCs without an additional regenerative stimulus, we treated DPPIV– F344 rats with common bile duct ligation (BDL).18 BDL causes marked proliferation of small bile ducts along with activation of fibroblasts and stellate cells19 and is a milder injury than cirrhosis induced by TAA.4 Our experiments demonstrated that intrasplenically infused cells differentiated into hepatocytes and cholangiocytes in both liver and spleen. Surprisingly, we observed gastrointestinal and pancreatic DPPIV+ tissues that formed at the injection site of the spleen in BDL rats, which led us to study the multilineage differentiation potential of fetal liver‐derived, endodermal stem cells.
Materials and Methods
Animals and BDL
Timed‐pregnant wild‐type (DPPIV+) F344 rats were purchased from Charles River. F344‐Tg (enhanced green fluorescent protein [EGFP]) F455/Rrrc rats and mutant DPPIV– F344 rats (both originally obtained from the Rat Resource and Research Center, University of Missouri‐Columbia) were bred at the University of Pittsburgh and used to derive timed‐pregnant DPPIV+/EGFP+ and male DPPIV– F344 rats. For BDL, the common bile duct of 2‐3‐month‐old DPPIV– F344 rats was exposed and two ligatures placed at the proximal and distal duct ends, followed by excision of the central part. All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committees of the University of Pittsburgh in accordance with National Institutes of Health guidelines.
Isolation of Embryonic Day 15 Fetal Liver Cells
After microdissection of embryonic day (ED)15 fetal livers derived from pregnant DPPIV+ F344 or F344‐Tg (EGFP) F455/Rrrc rats, fetal liver cell suspensions were isolated using a two‐step digestion method, as detailed.20 Briefly, dissected fetal liver tissue was first minced and incubated with ethylene glycol‐bis(β‐aminoethyl ether)‐N,N,N′,N′‐tetraacetic acid, which was then followed by incubation with collagenase. The obtained unfractionated cell suspensions exhibited viabilities of at least 95%.
Cell Transplantation and Liver Repopulation
ED15 fetal liver cells (1.5 ± 0.1 × 108) were transplanted without a PH into the spleens of DPPIV– F344 rats at 2 or 4 weeks after BDL or age‐matched untreated normal recipients. Rats were killed at different time points following cell transplantation. For engraftment studies, transplanted fetal liver cells were detected by EGFP (see below). Liver replacement was determined by enzyme histochemistry for DPPIV, as described.21
Histochemistry and Immunohistochemistry
Detailed information regarding immunohistochemical analyses and the periodic acid–Schiff (PAS) detection of glycogen with or without pretreatment with 0.5% α‐amylase is described in the Supporting Materials and Methods.
Microscopy and Imaging
Tissue sections were assessed using an AxioObserver Z1 microscope (Carl Zeiss). Microscopic images were obtained with an AxioCam HRc camera and processed with ZEN pro 2012 imaging software (Carl Zeiss MicroImaging).
Quantitative Reverse‐Transcription Polymerase Chain Reaction Analysis
Total RNA was extracted from snap‐frozen tissue samples using the Trizol reagent (Life Technologies) and treated with deoxyribonuclease I (New England Biolabs), followed by cleanup using the RNeasy Plus Mini/Micro Kit (Qiagen). Quantitative reverse‐transcription polymerase chain reaction (qRT‐PCR) was performed with at least two independent experiments, each with duplicate assays, using the StepOnePlus Real Time PCR System (Applied Biosystems). Samples were analyzed using Power SYBR Green Master Mix (Applied Biosystems). Messenger RNA (mRNA) abundance was determined by normalization of the data to the expression levels of glyceraldehyde 3‐phosphate dehydrogenase (Gapdh) mRNA. A complete list of primers is shown in Supporting Table S1. Data were analyzed using Excel (Microsoft Office 2010) software and are reported as mean ± SEM.
Results
Intrasplenic Infusion of Fetal Liver Cells and their Engraftment in the Liver After BDL
To test the feasibility of ED15 fetal liver cells for repopulation of the liver damaged by BDL, ~1.5 × 108 unfractionated cells were infused into the spleen at 4 weeks (n = 4). All studies were performed without 2/3 PH, a proliferative stimulus that would have been required for fetal liver stem/progenitor cells to engraft and repopulate under nonselective normal liver conditions.12 Fetal liver cells were isolated from transgenic EGFP+ F344 rats. EGFP is expressed throughout the cell cytoplasm and nucleus on ED15 and therefore can be readily used as a marker to trace individual embryonic donor cells early after cell transplantation.4, 20
Despite the large number of infused cells, the treatment was well tolerated, and tissue samples were analyzed at day 1 and 7 after cell transplantation. At day 1, large areas of engrafted donor‐derived EGFP+ cells were observed in the injection site of recipient spleen (Fig. 1A). Some of the transplanted stem/progenitor cells migrated to the recipient liver and were detected in close proximity to expanding portal areas (Fig. 1B, left panel). At day 7, small groups of EGFP+ hepatocyte‐like cells were observed (Fig. 1B, right panel), indicating proliferation of engrafted stem/progenitor cells.
Figure 1.
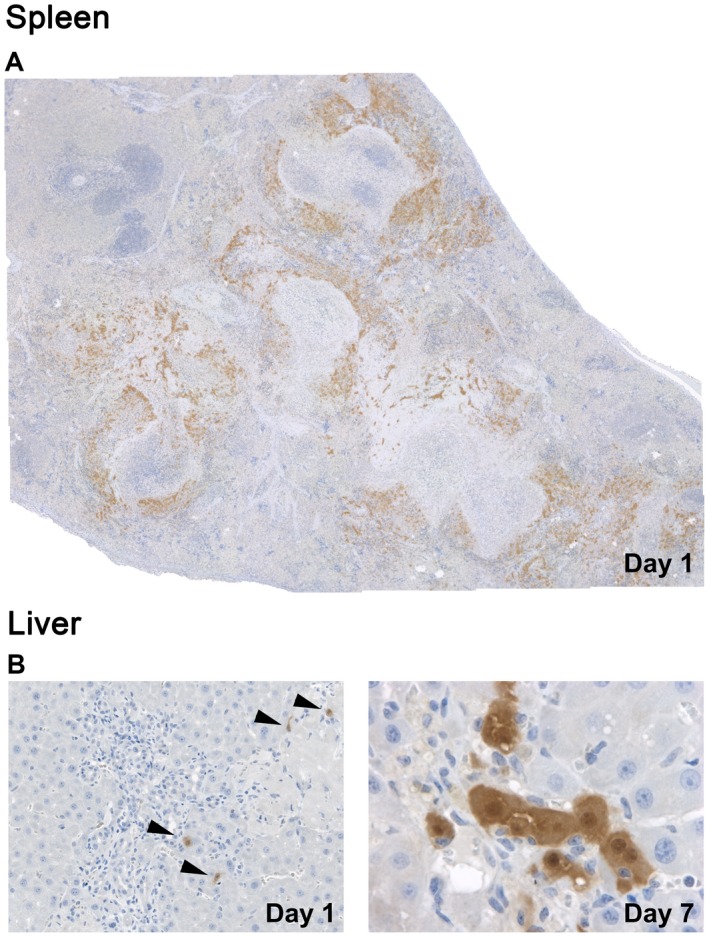
Engraftment of ectopically transplanted stem/progenitor cells in bile duct‐ligated rats. Bile duct proliferation and fibrosis was induced by ligation of the common bile duct, and ~1.5 × 108 EGFP+/DPPIV+ ED15 fetal liver cells were infused without PH into the spleen of DPPIV– F344 rats at 2/4 weeks after BDL. IHC for EGFP (brown) shows (A) engraftment of EGFP+ cells in the splenic injection site and (B, left) after migration into the liver at day 1 (arrowheads). (B, right) Proliferating EGFP+ cells in the recipient liver at day 7. Original magnification (A) ×100, (B, left) ×200, (B, right) ×640. Image in panel A contains 36 merged microscopic fields.
Repopulation of Fetal Stem/Progenitor Cells in BDL Rats
To investigate whether stem/progenitor cells are capable of repopulating the liver after BDL without the additional stimulus provided by a PH that is required in normal liver, we performed cell transplantation experiments using DPPIV to follow the fate and expansion of donor cells. DPPIV is a marker of differentiated hepatic lineage cells that first appears at ED17/18 and then gradually changes to different patterns characteristic of mature hepatocytes and cholangiocytes. Unfractionated fetal liver cells were isolated from wild‐type DPPIV+ F344 rats and infused into the spleen of DPPIV– F344 rats at 2 or 4 weeks after BDL (n = 8). As controls, we included age‐matched normal rats that received intrasplenic cell infusion without BDL (n = 4).
In bile duct‐ligated rats, ectopically infused fetal liver cells migrated and engrafted in the recipient liver to form small and large DPPIV+ periportal hepatocyte clusters that replaced up to 2% of the liver at 2 months after cell infusion (Fig. 2A, left). In contrast, cells transplanted into control rats without BDL showed minimal repopulation by DPPIV+ cells at 2 months, with only single cells or very small clusters apparent (Fig. 2A, right). In BDL rats, donor‐derived stem/progenitor cells differentiated into clusters of hepatocytes with canalicular DPPIV and also formed bile ducts with cytoplasmic DPPIV (Fig. 2B, left). Although the clusters were far smaller in sham‐operated rats, they also showed hepatocyte and bile duct expression patterns (Fig. 2B, right). Finally, dual immunohistochemistry (IHC) for DPPIV with hepatocyte nuclear factor (HNF)4α or claudin (Cldn)‐7, performed on the large donor‐derived cell clusters that were observed only in BDL rats, confirmed differentiation of stem/progenitor cells into both hepatic lineages (Fig. 2C). We did not observe other types of DPPIV+ cells in the liver.
Figure 2.
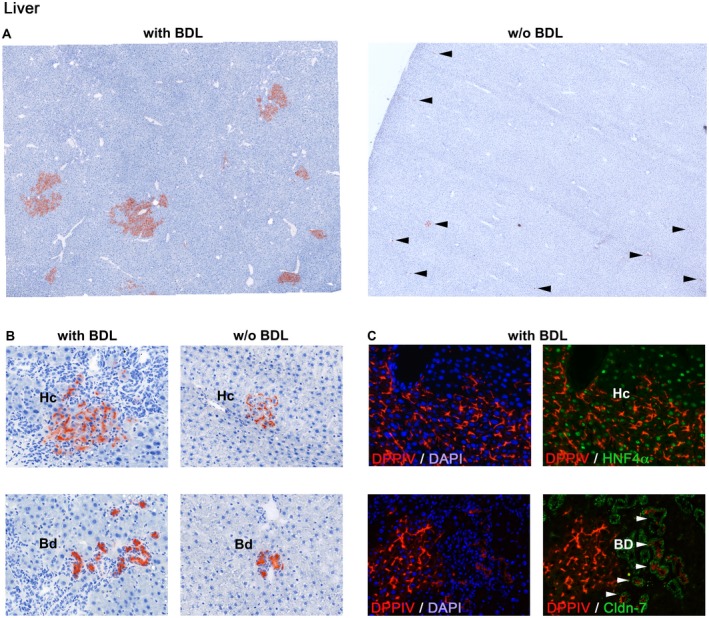
Tissue repopulation by transplanted fetal liver cells in bile duct‐ligated and control rats. ED15 fetal liver cells derived from DPPIV+ donors were transplanted without PH into the spleen of DPPIV– F344 rats with BDL or untreated rats. (A) In BDL rats, enzyme histochemistry for DPPIV (red) shows that transplanted stem/progenitor cells engrafted in the liver and formed hepatic cell clusters of various sizes at 2 months (A, left). However, only single cells or individual very small cell clusters (arrowheads) were observed in the liver of control rats (A, right). (B) Cells differentiated into hepatocytes expressing DPPIV in a bile canaliculi or bile duct cells expressing DPPIV in the cytoplasm. (B, right panels) Atypical larger cell clusters were used to show DPPIV expression patterns in the control group. (C) Differentiation into both hepatic lineages was confirmed by dual IHC for DPPIV/HNF4α (C, above) or DPPIV/Cldn‐7 (C, below), performed on tissue sections derived from BDL rats. Original magnification (A) ×100, (B,C) ×200. Images in A contain 36 merged microscopic fields. Abbreviations: Bd, bile duct cells; DAPI, 4′,6‐diamidino‐2‐phenylindole; Hc, hepatocytes.
BDL therefore stimulates tissue replacement by transplanted fetal liver cells, although engraftment and repopulation levels were lower than that achieved by intraportal infusion of fetal liver cells in TAA‐induced cirrhosis.4 Nevertheless, even the moderate injury and fibrosis of BDL promotes repopulation by maturing fetal liver cells.
Multilineage Differentiation of Fetal Liver‐Derived Stem/Progenitor Cells in the Spleen
Despite the lack of liver repopulation in recipient rats without BDL, DPPIV+ fetal liver cells engrafted at the injection site of the spleen and formed large regions of extrahepatic epithelial tissues at 2 months after cell infusion, small ducts lined by simple cuboidal epithelium, and two hepatocyte patterns, acini with central canalicular lumens and cords with small bile canaliculi. These structures recapitulate the liver parenchyma and bile ductules of both immature and mature liver. Ducts and hepatocytes were dispersed within connective tissue that displaced the normal splenic architecture (Supporting Fig. S1).
Spleens in recipient rats with BDL also showed large regions of engrafted and differentiated donor cells (Fig. 3A), but these contained a wide variety of DPPIV+ epithelial structures (Fig. 3B) in addition to hepatocytes (Fig. 3C,a) and small ducts (Fig. 3C,b). Some larger ducts and cysts were lined with flattened epithelium and others with “hobnail” epithelium (Fig. 3C,c). Organized structures included branching intestinal segments lined by columnar epithelium with interspersed goblet cells that had lumina filled with deteriorating cells and mucin (Fig. 3C,d,e). Nonluminal structures included round masses of cuboidal cell groups that recapitulated pancreatic islets (Fig. 3C,f).
Figure 3.

Differentiation of fetal liver cells into nonhepatic tissues in the recipient spleen of BDL rats. (A,B) Panels show histochemical detection of DPPIV in tissue formed at the intrasplenic injection site 2 months after ED15 fetal liver cell infusion. Note the variety of donor‐derived DPPIV+ cell structures with nonhepatic phenotypes in rats that underwent BDL prior to cell transplantation, mature hepatic cords with small central DPPIV+ and hepatic acini with a central DPPIV+ lumens, hepatic cords and cholangiolar ducts, and an intestinal tube with goblet cells. (C) Hematoxylin and eosin staining of spleen tissue in BDL rats at 2 months after cell infusion. The panels show (a) hepatic cords, (b) cholangiolar ducts, (c) simple cysts lined with flattened (arrows) or “hobnail” epithelium (arrowheads), (d) an intestinal cyst, (e) an intestinal tube with goblet cells surrounded by a circumferential stroma, (f) and a pancreatic islet with adjacent acini. Original magnification (A; C,a‐c,e,f) ×200, (B) ×400, (C,d) ×80. Each image in panel A contains 20 merged microscopic fields.
To further characterize intrasplenic formation of (non)hepatic tissues, we examined tissue samples for specific gene expression. We used RT‐PCR analyses, controlled by analysis of ED15 fetal liver, to survey endoderm‐derived tissues (Fig. 4; Supporting Fig. S2). The relatively low mRNA expression values of progenitor markers (Fig. 4), which are normally highly expressed on ED155 (Supporting Fig. S2A), and abundant expression of hepatocyte‐ and bile duct‐related genes (Fig. 4) indicated maturation and liver lineage differentiation of donor cells at 2 months after cell infusion in both control and BDL spleens. Subsequent immunohistochemical analyses for alpha‐fetoprotein (AFP) and δ‐like (Dlk)‐1 showed that the majority of transplanted cells differentiated into mature cells because these progenitor markers were rarely detected (Supporting Fig. S3). The expression patterns of hepatocyte phenotypes were confirmed by IHC for HNF4α (Fig. 5A). Furthermore, glycogen detection by the PAS reaction demonstrated hepatocyte maturation and hepatocyte‐specific metabolic activity in the spleens of both control and BDL rats (Fig. 5B). Other markers, comprising SRY‐related high‐mobility group box 9 (Sox‐9), Cldn‐7, cytokeratin (CK)‐7, oval cell marker (OV‐6), and CK‐19, showed patterns characteristic of bile ducts (Fig. 5C,D; Supporting Fig. S4A‐C).
Figure 4.
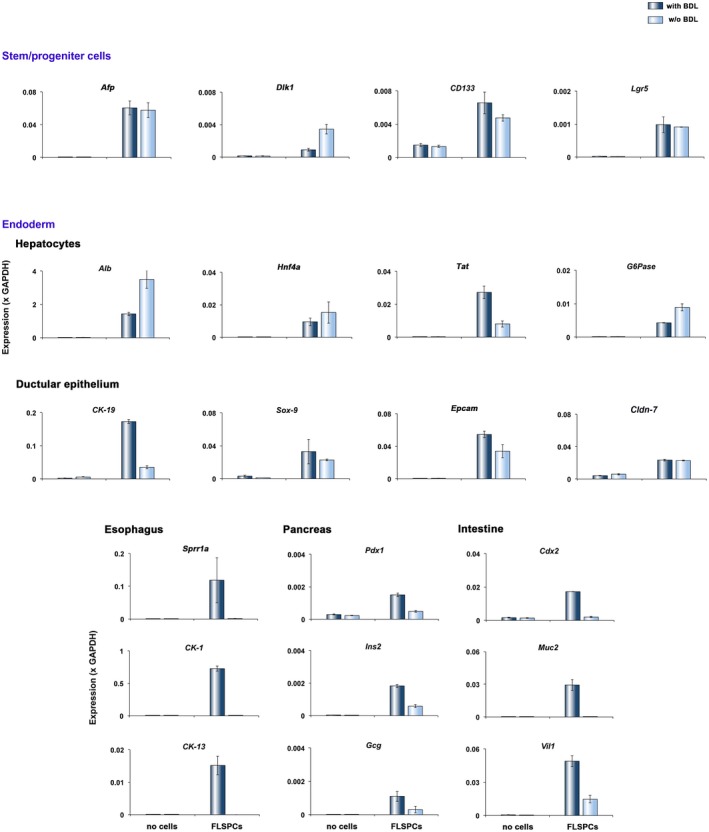
Gene expression of tissue outgrowth in spleens 2 months after cell infusion. RNA was extracted from control spleens and cell‐infused spleens from rats with or without BDL (n = 3/group), pooled, and analyzed by qRT‐PCR. Plots show mean ± SEM values of two replicates for each analysis. Abbreviations: Alb, albumin; CD133, clusters of differentiation 133; Epcam, epithelial cell adhesion molecule; Gcg, glucagon; G6Pase, glucose‐6‐phosphatase; Ins2, insulin 2; Lgr5, leucine‐rich repeat containing G protein‐coupled receptor 5; Muc2, mucin 2 oligomeric mucus/gel‐forming; Sprr1a, small proline‐rich protein 1A; Tat, tyrosine aminotransferase; Vil1, villin 1.
Figure 5.
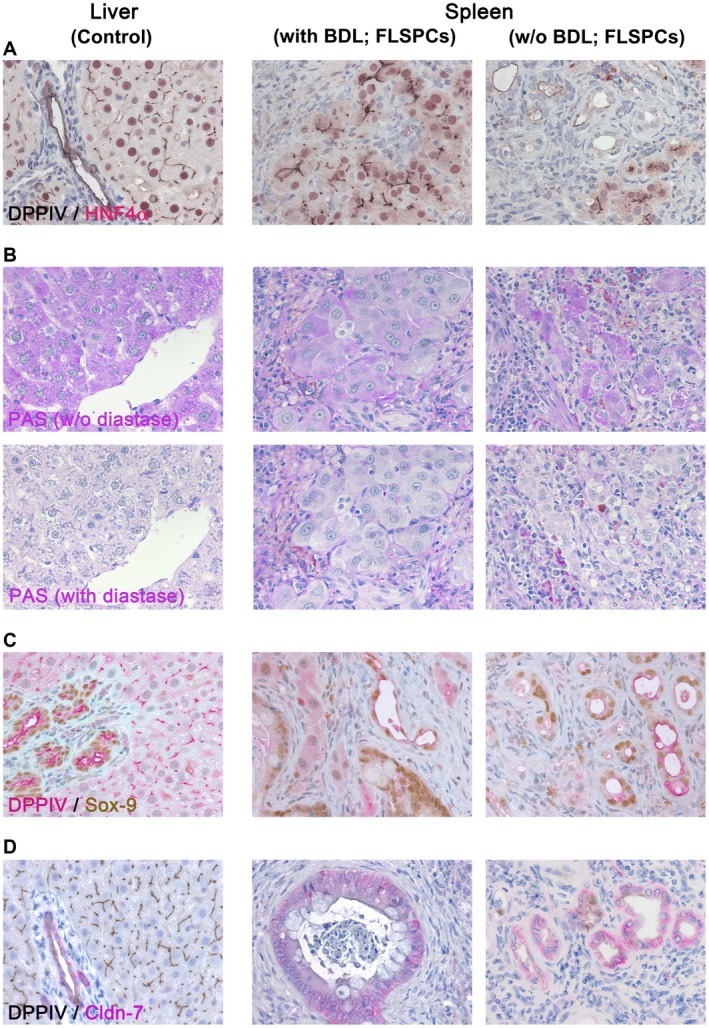
Characterization of DPPIV+ donor cell‐derived tissue structures in the spleens of BDL or untreated DPPIV– recipient rats at 2 months after cell infusion. (A) Tissue sections from spleen regions at the injection site of fetal liver cells were immunohistochemically analyzed for hepatocyte‐specific HNF4α. (B) Panels illustrate glycogen storage detected by PAS staining in donor‐derived mature hepatocytes (B, upper panels). Pretreatment with diastase confirmed hepatocyte‐specific glycogen in transplanted cells (B, lower panels). (C,D) Tissue sections were immunohistochemically analyzed for bile duct‐specific Sox‐9 and Cldn‐7 expression. Liver sections were used as controls. DPPIV detection gives central linear staining of bile canaliculi in hepatic cords, the luminal surface of cholangiocytes in bile ducts, and cytoplasmic staining of scattered intestinal cells, vascular, and undifferentiated ducts. Original magnification ×400 (additional analyses in Supporting Fig. S4).
To relate these expressions to induced nonhepatic tissue structures, we performed immunohistochemical analyses for pancreatic and duodenal homeobox 1 (Pdx1), insulin, caudal type homeo box 2 (Cdx2), villin, CK‐1, and CK‐13, which characterize mature pancreatic tissue, intestinal epithelium, and esophagus (Fig. 6) and are not expressed in fetal (Supporting Fig. S5) or adult livers (Fig. 6). We observed ductular cell structures with nuclear Pdx1 expression and islets of Langerhans with scattered Pdx1‐positive and insulin‐positive cells in spleens of BDL rats, indicating differentiation into multiple pancreatic lineages (Fig. 6A,B). Cdx2 and villin were expressed in donor cell‐derived intestinal epithelium in BDL rats but not in normal recipient rats (Fig. 6C,D). Moreover, various cytokeratins (CK‐7/14[OV‐6]/19) normally expressed in bile ducts and intestine were also expressed in the induced nonhepatic tissues (Supporting Fig. S4, middle panels). Further immunohistochemical analyses for CK‐1/13 demonstrated esophageal tissue structures within the spleen of cell‐transplanted BDL rats (Fig. 6E,F). Moreover, simultaneous detection of DPPIV/Pdx1, DPPIV/villin, and villin/Cdx2 confirmed that nonhepatic tissues observed in the spleen of BDL rats were derived from transplanted stem/progenitor cells (Fig. 6G).
Figure 6.
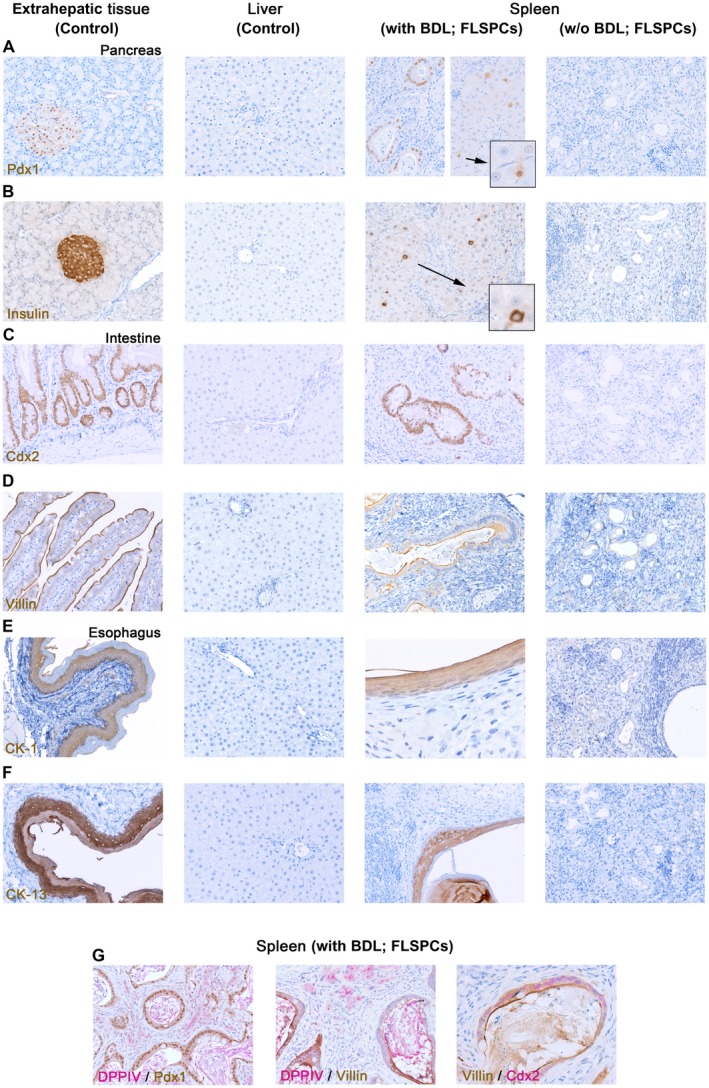
Multilineage differentiation of transplanted fetal liver cells within the splenic microenvironment of BDL rats. (A‐F) Immunohistochemical analyses of donor cell‐derived tissue structures in the spleen of recipient rats with or without BDL prior to cell infusion. Tissue‐specific markers were used to characterize differentiated fetal liver cells. Sections from pancreas, intestine, esophagus, or liver were used as positive or negative controls (first or second column). Antibody‐stained sections demonstrated that intrasplenically engrafted donor cells differentiated into various nonhepatic tissues in BDL rats (third column) but not in normal recipient rats (fourth column). (G) Simultaneous IHC confirmed that newly generated tissue structures derived from differentiated donor cells. Original magnification (A‐G) ×200, (G, right panel) ×400, (E, second panel from right) ×640; additional analyses in Supporting Fig. S5.
Taken together, these studies clearly demonstrate that cells from fetal liver show multilineage endodermal differentiation and maturation in the spleen when stimulated by BDL.
Discussion
Growing evidence that fibrosis and cirrhosis can be reversible in humans raises hope that patients with chronic liver diseases can be treated with an appropriate antifibrotic therapy in the future.22 Numerous antifibrotic strategies have been evaluated to date in rodents; however, therapeutic options are still limited in humans. Therefore, alternative strategies are desperately needed to compensate for the loss of functional tissue mass and fibrosis in diseased livers.
For a better understanding of key factors and conditions stimulating engraftment and expansion of stem cells in the host microenvironment that augment hepatic tissue replacement, we established two hepatic fibrosis models in mutant DPPIV– F344 rats.4, 19 Using BDL, a preclinical model of biliary fibrosis with a milder liver injury than TAA‐induced cirrhosis, we made important observations with potential for future clinical and research applications. First, our studies demonstrated that high numbers of fetal liver cells can be infused into the recipient spleen without adverse side effects. Ectopically transplanted epithelial stem/progenitor cells migrated to the fibrotic liver where they engrafted, differentiated into both hepatic cell lineages, and formed cell clusters. Second, we showed that fetal liver cells exhibit multilineage differentiation potential into intestine, pancreas, and esophagus that form at the injection site of the spleen in BDL rats. Thus, rat fetal livers harbor endodermal multipotent stem cells while BDL provides a stimulus that causes them to differentiate into nonhepatic endodermal tissues. Although fetal liver is a potential source of therapeutic endodermal stem cells, the human counterpart is not likely to be used for therapeutic liver repopulation. Nevertheless, the stem cells represent an excellent tool to identify the essential properties that enable fetal cells to effectively engraft and repopulate the liver, with the ultimate goal of engineering human cells to reproduce these properties. Moreover, BDL/splenic injection is an experimental system that can be applied to differentiation of both endogenous and iPSC‐derived stem cells (Fig. 7).
Figure 7.
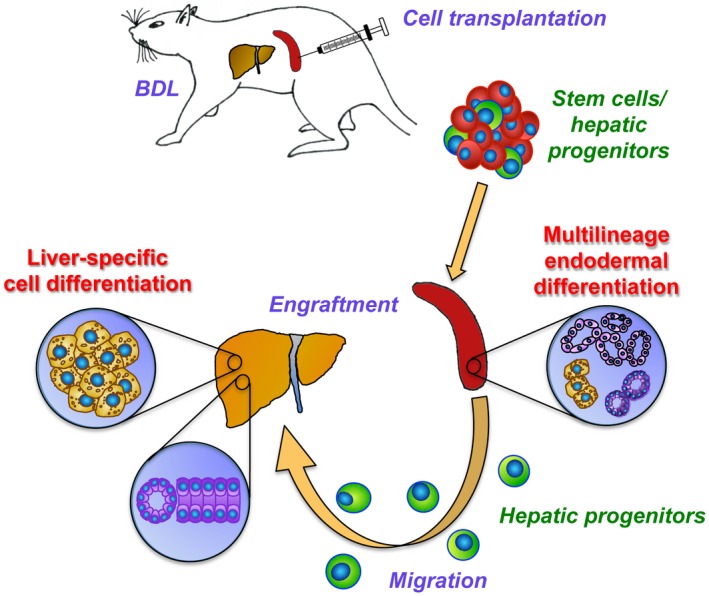
Experimental summary.
Cell delivery through the portal circulation is the preferred route for clinical cell transplantation. However, ectopic infusion will be necessary if the portal vein is inaccessible and can reduce the risk of portal hypertension and liver infarction after transplantation of high cell numbers.23, 24 Using a preclinical swine model, Raschzok et al.25 demonstrated that hepatocytes that were infused intra‐arterially into the spleen migrated and engrafted in the recipient liver with significantly decreased microembolization compared to intraportal cell infusion whereas cells directly injected into the splenic parenchyma were retained. In a recent phase I‐II, matched, case‐control study, preliminary results suggest that infusion of human unfractionated fetal liver cells through the splenic artery could be a well‐tolerated procedure in patients with advanced cirrhosis.26 Because the authors transplanted unlabeled cells of low quantity, subsequent studies exploring engraftment and differentiation behavior of immature donor cells are missing. In the present study, we transplanted high numbers of fetal liver cells into the spleen of BDL rats and showed that this delivery route was well tolerated. Our analyses provided clear documentation that BDL stimulates differentiation and expansion of engrafted cells in the liver. Because engraftment/repopulation levels at 2 months were only a few percent, considerably lower than observed in advanced fibrosis/cirrhosis,4 we did not expect an effect of stem/progenitor cells on fibrogenesis and therefore did not characterize liver function or fibrosis levels. Nevertheless, the results show that even mild injury drives expansion of ectopically transplanted stem/progenitor cells.
Liver cells originate from the foregut endoderm and start expressing hepatic lineage genes with the formation of the hepatic bud on ~ED9.27 At ED14, three types of hepatic lineage epithelial cells were identified, bipotential AFP+/albumin+/CK‐19+ cells, hepatocytic AFP+/albumin+/CK‐19¯ cells, and biliary AFP¯/albumin¯/CK‐19+ cells.15, 16 However, the majority of fetal liver cells are hematopoietic. Early lineage cells without these markers are also likely. Both AFP+ populations express Dlk‐1, another progenitor marker that is absent from mature liver but reactivated in adult progenitor cells.11, 28 Under nonselective conditions, intraportally infused Dlk‐1+ fetal cells engraft in the liver.11 We now show that following intrasplenic injection, epithelial fetal liver cells also engraft in the spleen and differentiate into more mature hepatic lineages.
Our previous reports showed that unfractionated or Dlk‐1‐enriched cell suspensions derived from ED14/15 rat fetal livers differentiated predominantly into hepatocytes and cholangiocytes in normal and in TAA‐induced cirrhotic liver. Repopulated DPPIV+ cells expressed albumin, asialoglycoprotein receptor, uridine 5'‐diphospho‐glucuronosyl transferase, and glutamine synthetase. They also showed glucose‐6‐phosphatase expression and glycogen storage. All are characteristic markers of mature functional hepatocytes.4, 11, 12 Moreover, transplanted fetal liver cells formed ducts and expressed CK‐19, Cldn‐7, OV‐6, epithelial cell adhesion molecule (EpCAM), clusters of differentiation (CD)44, and connexin 43, indicating differentiation to cholangiocytes.4, 11 The present study also showed differentiation to hepatocyte and cholangiocyte lineages in the liver as there were characteristic DPPIV expression patterns and HNF4α or Cldn‐7 expression for hepatocytes and cholangiocytes, respectively. However, growth in the spleen showed different processes. Without BDL, the cells made a network of small ducts and hepatic structures showing Sox‐9, Cldn‐7, CK‐7, OV‐6, CK‐19, HNF4α, and hepatocyte‐specific glycogen. Hepatocytes and bile ducts were still present after BDL but were now accompanied by multipotent formation of intestine, pancreas, and esophagus. This multiorgan differentiation suggests stimulation of prehepatic endodermal stem cells within the injected cell mixture29; such stem cells have been characterized in the fetal intrahepatic biliary tree.30 The high expression of Sox‐9, which marks the differentiation of stem cells into a variety of tissues and organs,31 shows a critical feature of this multilineage differentiation. In addition, prominent expression of Cdx2 and Pdx1 reveals the cell fate specification into intestinal and pancreatic structures.32, 33, 34
The present study uncovered the remarkable differentiation potential of fetal liver. The newly generated nonhepatic tissues (mainly intestinal structures and to a lesser extent pancreatic islets and esophagus) were observed after BDL, indicating the presence of specific systemic factors or cytokines that stimulate stem cell differentiation. It is also important to consider how BDL stimulates this differentiation of nonhepatic endoderm. Because the initiating process is in the liver, stimulation of differentiation in the spleen must act through an endocrine mechanism. Bile acids, cytokines, and other disease‐specific factors are all possibilities. High serum concentrations of bile acids are characteristic of BDL rats35, 36 and have also been reported in humans with obstructive cholestasis, advanced cirrhosis, and end‐stage cholestatic liver diseases.37 Moreover, bile acids can induce ectopic expression of Cdx2 and mucin 2 oligomeric mucus/gel‐forming (Muc2)38 and can initiate hepatic differentiation of stem cells39 through activation of the farnesoid X receptor (FXR).40 However, our analyses detected very low levels of Fxr mRNA in both freshly isolated and differentiating fetal liver cells (Supporting Fig. S6A). A direct effect of bile acids during the observed multilineage differentiation process therefore seems unlikely. Three other factors are prominent. We recently demonstrated marked up‐regulation of interleukin (IL)‐6, fibroblast growth factor (FGF)18, and osteopontin following BDL.19 Osteopontin, a pleiotropic cytokine and downstream target of Sox‐9, is a major regulator of liver fibrosis,41 and FGF18 stimulates proliferation in liver and small intestine as a growth factor.42 However, we found no evidence that previous studies investigated the effect of osteopontin or FGF18 on Cdx2 or Pdx1 expression. High serum concentrations of IL‐6 were reported in experimental fibrosis/cirrhosis models43, 44 and in patients with liver cirrhosis of different etiologies.45 IL‐6, a multifunctional cytokine synthesized by fibroblasts, monocytes, Kupffer cells, T cells, and endothelial cells,46 can induce up‐regulation of Cdx2 in epithelial cells.47, 48 Because differentiating fetal liver cells express high mRNA levels of the essential IL‐6 signal transducer glycoprotein 13046 (Supporting Fig. S6B), IL‐6‐mediated signaling could be involved in the discovered multilineage differentiation. Nevertheless, all three of these factors warrant further investigation.
Our observations could have a significant impact on the field of regenerative medicine. Cell‐based therapies have aimed to restore normal liver function, and numerous studies have characterized the liver repopulation capacity of various candidate cell populations. Different protocols have been established to induce hepatocyte‐like cells in vitro from immature cells, e.g., iPSCs.49, 50 However, these are not the same as the stem cells of normal development or the specialized stem cells that arise in injured livers. Specific injury provides new stimuli for differentiation that might refine the programming of donor stem cells. Identifying these stimuli is therefore an important goal. Moreover, the splenic microenvironment provides an excellent platform for studying the differentiation potential of populations designed for cell‐based therapies.
Supporting information
Supported by the National Institutes of Health (grant R01 DK090325 to M.O.).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Tewary M, Shakiba N, Zandstra PW. Stem cell bioengineering: building from stem cell biology. Nat Rev Genet 2018;19:595‐614. [DOI] [PubMed] [Google Scholar]
- 2. Shiota G, Itaba N. Progress in stem cell‐based therapy for liver disease. Hepatol Res 2017;47:127‐141. [DOI] [PubMed] [Google Scholar]
- 3. Iansante V, Chandrashekran A, Dhawan A. Cell‐based liver therapies: past, present and future. Philos Trans R Soc Lond B Biol Sci 2018;373:pii.20170229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yovchev MI, Xue Y, Shafritz DA, Locker J, Oertel M. Repopulation of the fibrotic/cirrhotic rat liver by transplanted hepatic stem/progenitor cells and mature hepatocytes. Hepatology 2014;59:284‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oertel M. Fetal liver cell transplantation as a potential alternative to whole liver transplantation? J Gastroenterol 2011;46:953‐965. [DOI] [PubMed] [Google Scholar]
- 6. Asgari S, Moslem M, Bagheri‐Lankarani K, Pournasr B, Miryounesi M, Baharvand H. Differentiation and transplantation of human induced pluripotent stem cell‐derived hepatocyte‐like cells. Stem Cell Rev 2013;9:493‐504. [DOI] [PubMed] [Google Scholar]
- 7. Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, Thorgeirsson SS, et al. Engrafted human stem cell‐derived hepatocytes establish an infectious HCV murine model. J Clin Invest 2014;124:4953‐4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Li Y, Wang X, Zhang W, Sauer V, Chang CJ, et al. Amelioration of hyperbilirubinemia in gunn rats after transplantation of human induced pluripotent stem cell‐derived hepatocytes. Stem Cell Reports 2015;5:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hannoun Z, Steichen C, Dianat N, Weber A, Dubart‐Kupperschmitt A. The potential of induced pluripotent stem cell derived hepatocytes. J Hepatol 2016;65:182‐199. [DOI] [PubMed] [Google Scholar]
- 10. Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol 2015;17:971‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oertel M, Menthena A, Chen YQ, Teisner B, Jensen CH, Shafritz DA. Purification of fetal stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology 2008;134:823‐832. [DOI] [PubMed] [Google Scholar]
- 12. Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 2006;130:507‐520. [DOI] [PubMed] [Google Scholar]
- 13. Menthena A, Koehler C, Sandhu JS, Yovchev MI, Hurston E, Shafritz DA, et al. Activin A, p15INK4b signaling, and cell competition promote stem/progenitor cell repopulation of livers in aging rats. Gastroenterology 2011;140:1009‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology 2006;43(Suppl. 1):S89‐S98. [DOI] [PubMed] [Google Scholar]
- 15. Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, et al. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol 2000;156:2017‐2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oertel M, Menthena A, Chen YQ, Shafritz DA. Properties of cryopreserved fetal liver stem/progenitor cells that exhibit long‐term repopulation of the normal rat liver. Stem Cells 2006;24:2244‐2251. [DOI] [PubMed] [Google Scholar]
- 17. Fabris L, Spirli C, Cadamuro M, Fiorotto R, Strazzabosco M. Emerging concepts in biliary repair and fibrosis. Am J Physiol Gastrointest Liver Physiol 2017;313:G102‐G116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol 1984;65:305‐311. [PMC free article] [PubMed] [Google Scholar]
- 19. Yovchev MI, Locker J, Oertel M. Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes. J Hepatol 2016;64:1348‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yovchev MI, Oertel M. Fetal liver stem/progenitor cell transplantation: a model to study tissue mass replacement and cell‐based therapies. Methods Mol Biol 2017;1506:101‐115. [DOI] [PubMed] [Google Scholar]
- 21. Yovchev MI, Dabeva MD, Oertel M. Isolation, characterization, and transplantation of adult liver progenitor cells. Methods Mol Biol 2013;976:37‐51. [DOI] [PubMed] [Google Scholar]
- 22. Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut 2015;64:830‐841. Erratum in: Gut 2015;64:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res 2015;54:162‐177. [DOI] [PubMed] [Google Scholar]
- 24. DeWard AD, Komori J, Lagasse E. Ectopic transplantation sites for cell‐based therapy. Curr Opin Organ Transplant 2014;19:169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raschzok N, Teichgräber U, Billecke N, Zielinski A, Steinz K, Kammer NN, et al. Monitoring of liver cell transplantation in a preclinical swine model using magnetic resonance imaging. Cell Med 2010;1:123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pietrosi G, Vizzini G, Gerlach J, Chinnici C, Luca A, Amico G, et al. Phases I‐II matched case‐control study of human fetal liver cell transplantation for treatment of chronic liver disease. Cell Transplant 2015;24:1627‐1638. [DOI] [PubMed] [Google Scholar]
- 27. Zhao R, Duncan SA. Embryonic development of the liver. Hepatology 2005;41:956‐967. [DOI] [PubMed] [Google Scholar]
- 28. Tanimizu N, Tsujimura T, Takahide K, Kodama T, Nakamura K, Miyajima A. Expression of Dlk/Pref‐1 defines a subpopulation in the oval cell compartment of rat liver. Gene Expr Patterns 2004;5:209‐218. [DOI] [PubMed] [Google Scholar]
- 29. Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 2009;25:221‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Semeraro R, Carpino G, Cardinale V, Onori P, Gentile R, Cantafora A, et al. Multipotent stem/progenitor cells in the human foetal biliary tree. J Hepatol 2012;57:987‐994. [DOI] [PubMed] [Google Scholar]
- 31. Jo A, Denduluri S, Zhang B, Wang Z, Yin L, Yan Z, et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis 2014;1:149‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stringer EJ, Duluc I, Saandi T, Davidson I, Bialecka M, Sato T, et al. Cdx2 determines the fate of postnatal intestinal endoderm. Development 2012;139:465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hryniuk A, Grainger S, Savory JG, Lohnes D. Cdx function is required for maintenance of intestinal identity in the adult. Dev Biol 2012;363:426‐437. [DOI] [PubMed] [Google Scholar]
- 34. Shih HP, Seymour PA, Patel NA, Xie R, Wang A, Liu PP, et al. A gene regulatory network cooperatively controlled by Pdx1 and Sox9 governs lineage allocation of foregut progenitor cells. Cell Rep 2015;13:326‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ennis M, Clements B, Campbell GR, Halliday MI, Barclay RG, Rowlands BJ. The effect of obstructive jaundice on systemic concentrations of bile acids, histamine and antibodies to the core region of endotoxin glycolipid. Agents Actions 1993;38:C283‐C285. [Google Scholar]
- 36. Lozano E, Sanchez‐Vicente L, Monte MJ, Herraez E, Briz O, Banales JM, et al. Cocarcinogenic effects of intrahepatic bile acid accumulation in cholangiocarcinoma development. Mol Cancer Res 2014;12:91‐100. [DOI] [PubMed] [Google Scholar]
- 37. Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury ‐ what is the link? J Hepatol 2017;67:619‐631. [DOI] [PubMed] [Google Scholar]
- 38. Xu Y, Watanabe T, Tanigawa T, Machida H, Okazaki H, Yamagami H, et al. Bile acids induce cdx2 expression through the farnesoid x receptor in gastric epithelial cells. J Clin Biochem Nutr 2010;46:81‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sawitza I, Kordes C, Götze S, Herebian D, Häussinger D. Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci Rep 2015;5:13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999;3:543‐553. [DOI] [PubMed] [Google Scholar]
- 41. Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, et al. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology 2012;56:1108‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu MC, Qiu WR, Wang YP, Hill D, Ring BD, Scully S, et al. FGF‐18, a novel member of the fibroblast growth factor family, stimulates hepatic and intestinal proliferation. Mol Cell Biol 1998;18:6063‐6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tahan G, Akin H, Aydogan F, Ramadan SS, Yapicier O, Tarcin O, et al. Melatonin ameliorates liver fibrosis induced by bile‐duct ligation in rats. Can J Surg 2010;53:313‐318. [PMC free article] [PubMed] [Google Scholar]
- 44. Yang YY, Hsieh SL, Lee PC, Yeh YC, Lee KC, Hsieh YC, et al. Long‐term cannabinoid type 2 receptor agonist therapy decreases bacterial translocation in rats with cirrhosis and ascites. J Hepatol 2014;61:1004‐1013. [DOI] [PubMed] [Google Scholar]
- 45. Gudowska‐Sawczuk M, Wrona A, Gruszewska E, Cylwik B, Panasiuk A, Flisiak R, et al. Serum level of interleukin‐6 (IL‐6) and N‐terminal propeptide of procollagen type I (PINP) in patients with liver diseases. Scand J Clin Lab Invest 2018;78:125‐130. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt‐Arras D, Rose‐John S. IL‐6 pathway in the liver: from physiopathology to therapy. J Hepatol 2016;64:1403‐1415. [DOI] [PubMed] [Google Scholar]
- 47. Ikeda H, Sasaki M, Ohira S, Ishikawa A, Sato Y, Harada K, et al. Tumor necrosis factor‐alpha induces the aberrant expression of mucus core protein‐2 in non‐neoplastic biliary epithelial cells via the upregulation of CDX2 in chronic cholangitis. Hepatol Res 2008;38:1006‐1017. [DOI] [PubMed] [Google Scholar]
- 48. Cobler L, Pera M, Garrido M, Iglesias M, de Bolós C. CDX2 can be regulated through the signalling pathways activated by IL‐6 in gastric cells. Biochim Biophys Acta 2014;1839:785‐792. [DOI] [PubMed] [Google Scholar]
- 49. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663‐676. [DOI] [PubMed] [Google Scholar]
- 50. Si‐Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte‐like cells from induced pluripotent stem cells. Hepatology 2010;51:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


