Abstract
Long-term potentiation (LTP) is an activity-dependent strengthening of synapses that is thought to underlie memory storage. Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been a leading candidate as a memory molecule because it is persistently activated after LTP induction and can enhance transmission. Furthermore, a mutation that blocks persistent activation blocks LTP and forms of learning. However, direct evidence for a role of the kinase in maintaining synaptic strength has been lacking. Here, we show that a newly developed noncompetitive inhibitor of CaMKII strongly reduces synaptic transmission in the CA1 region of the hippocampal slice. This occurs through both presynaptic and postsynaptic action. To study the role of CaMKII in the maintenance of LTP, inhibitor was applied after LTP induction and then removed. Inhibition occurred in both LTP and control pathways but only partially recovered. The nonrecovering component was attributable primarily to a postsynaptic change. To test whether nonrecovery was attributable to a persistent reversal of LTP, we first saturated LTP and then transiently applied inhibitor. This procedure allowed additional LTP to be induced, indicating a reversal of an LTP maintenance mechanism. This is the first procedure that can reverse LTP by chemical means and suggests that a component of synaptic memory is attributable to CaMKII. The procedure also enhanced the LTP that could be induced in the control pathway, consistent with the idea that CaMKII is involved in controlling basal synaptic strength, perhaps as a result of LTP that occurred in vivo.
Keywords: CaMKII, depotentiation, hippocampus, LTP, maintenance, memory
Introduction
Progress has been made in elucidating the processes involved in the induction and expression of LTP (Bliss and Collingridge, 1993; Malenka and Nicoll, 1999; Lee et al., 2004). In contrast, relatively little is known about the LTP maintenance processes that underlie the persistence of synaptic memory (Hrabetova and Sacktor, 1996; Bhalla et al., 2002; Lisman et al., 2002; Si et al., 2003; Levenson and Sweatt, 2005). Ca2+/calmodulin-dependent protein kinase II (CaMKII) has an important role in LTP. Enhancing postsynaptic kinase activity increases transmission, an enhancement that occludes LTP (Lledo et al., 1995). Conversely, blocking the kinase by pharmacological (Malinow et al., 1989) or genetic (Silva et al., 1992; Giese et al., 1998) methods blocks or reduces LTP (Hinds et al., 1998). Several lines of information have pointed to a role of CaMKII in LTP maintenance. Calcium-dependent autophosphorylation of the enzyme makes it active in the absence of Ca2+ (Miller and Kennedy, 1986), giving the enzyme switch-like properties (Lisman, 1994; Lisman and Zhabotinsky, 2001; Miller et al., 2005). Consistent with this, LTP increases CaMKII autophosphorylation for hours (Fukunaga et al., 1993; Barria et al., 1997; Ahmed and Frey, 2005) and produces a persistent translocation of the kinase to the postsynaptic density (PSD) (Otmakhov et al., 2004).
Despite this evidence for long-lasting changes in CaMKII, the role of the kinase in LTP maintenance remains unclear. In most experiments showing an effect of CaMKII inhibitor, the inhibitor was applied before induction; the resulting inhibition of LTP could therefore be caused by effects on induction. To test specifically for a role in maintenance, inhibitor must be applied after induction. Studies examining the effects of postsynaptic application of CaMKII inhibitors or mixtures of CaMKII and PKC inhibitors have given variable results; some report reductions of LTP (but not basal transmission), even when inhibitor is applied at long times after induction (Feng, 1995), whereas most studies, including those from our laboratory, found no effect (Malinow et al., 1989; Malgaroli et al., 1992; Otmakhov et al., 1997; Chen et al., 2001). These negative results are puzzling; if active CaMKII is present under basal conditions and persistently increased by LTP, one would expect the kinase to contribute to basal transmission and LTP maintenance.
We have reexamined the role of CaMKII in maintenance using the first available noncompetitive inhibitor of CaMKII [CaMKIINtide (Chang et al., 1998, 2001)]. A noncompetitive inhibitor may be particularly potent under conditions in which substrate concentration is high, as in the PSD. We have used a membrane-permeant form of this inhibitor and find that it produces powerful inhibition of basal transmission and LTP. Experiments were conducted to determine whether maintenance or expression processes were affected. The results indicate that CaMKIINtide can produce depotentiation and therefore acts on a maintenance process. This is the first demonstration that maintenance can be reversed biochemically and strongly suggests that a component of synaptic memory is attributable to CaMKII.
Materials and Methods
The animals' care was in accordance with institutional guidelines.
Slice electrophysiology.
Transverse hippocampal slices (350 μm) were prepared from 17- to 23-d-old Long–Evans rats. After preparation, slices were allowed to recover for a minimum of 2 h in an inverted interface chamber held in an atmosphere of 95% O2 and 5% CO2. For the experiments, slices were transferred to a submersion-type recording chamber mounted on an Olympus (Tokyo, Japan) BX51WI microscope and were continuously superfused (2–4 ml/min) with artificial CSF (ACSF) containing the following (in mm): 120 NaCl, 26 NaHCO4, 1 NaH2PO4, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, and 10 d-glucose. Before entering the chamber, ACSF was continuously bubbled with 95% O2 and 5% CO2 in a 20 ml syringe. A total volume of 10 ml of ACSF was recirculated during each experiment (including experiments with and without drug application; see below, Extracellular drug application) using a two-way pump. Recirculation was used to conserve peptide, which was obtainable only in limited quantity. Experiments were conducted at 30–31°C. Extracellular field potential recordings were made with a glass recording electrode (0.2–0.3 MΩ) filled with ACSF and placed in stratum radiatum of the CA1 region. To stimulate independent inputs, two monopolar stimulating electrodes (glass pipettes filled with ACSF; 0.25–0.35 MΩ resistance) were positioned 100–150 μm to each side of the recording electrode. Stimulus strength was adjusted to have a field EPSP (fEPSP) amplitude of 50–60% of the population spike threshold. LTP was induced by tetanic stimulation (one or four series of stimuli delivered at 100 Hz, 1 s and spaced 2 min apart). Stimulus strength was the same for both test and tetanic stimulation. Test stimulation alternated between the two stimulation electrodes. The interstimulus interval was 6 s in most experiments, 2 min for alternated synaptic/AMPA stimulation, and 30 s for LTP saturation experiments. Data were acquired by a personal computer with a Digidata-1200 interface (Molecular Devices, Foster City, CA) by a custom-made Axobasic 2.1 program. An fEPSP baseline of 10–20 min was recorded before LTP induction or drug application.
Whole-cell patch-clamp recordings were conducted under visual guidance by an upright microscope equipped with infrared differential interference contrast optics (E600FN; Nikon, Tokyo, Japan). Voltage-clamp recordings were made with an EPC-10 patch-clamp amplifier (HEKA Elektronik, Heidelberg, Germany), and data were digitized at 12 kHz using the HEKA Elektronik software Pulse. Patch pipettes (3–5 MΩ) were filled with internal solution containing the following (in mm): 130 K-gluconate, 5 KCl, 2 MgCl2, 0.6 EGTA, 10 HEPES, 4 Mg-ATP, and 0.3 Na3-GTP, pH 7.3.
Data analysis and statistics.
Axobasic 2.1 program (custom version) was used for on-line and initial off-line analysis. Data were then imported to Excel (Microsoft, Seattle, WA) for additional analysis and plotting. Summary graphs were generated by normalizing the 10–20 min baseline preceding LTP induction or drug application and averaging data across experiments. Error bars correspond to SEM. Igor program (WaveMetrics, Lake Oswego, OR) was used for Student's t tests, one-way ANOVA post-test, and post hoc Tukey's tests. p < 0.05 was used as criterion of significance. Average numbers in text correspond to mean ± SD.
CaMKII inhibitors and control drugs.
Antennapedia-CaMKIINtide (Ant-CaMKIINtide; RQIKIWFQNRRMKWK-KRPPKLGQIGRSKRV-VIEDDRIDDVLK) was obtained from the Biopolymer Synthesis Center (California Institute of Technology, Pasadena, CA). This peptide is a component of the α form of an endogenous inhibitor of CaMKII called CaMKIIN (Chang et al., 1998). The inhibitory peptide itself is referred to as CaMKIINtide by the Soderling group (Chang et al., 1998) and as CaNtide by the Vitale group (Illario et al., 2003). Because of the possible confusion with CaN (calcineurin), we chose to use the name CaMKIINtide. (antennapedia sequence, RQIKIWFQNRRMKWKK).
Antennapedia-autocamtide-2-related inhibitor peptide II (Ant-AIP-II; RQIKIWFQNRRMKW-KKKKKLRRQEAFDAL) and reversed Ant-Tirap138–151 [Ant-Tirap-R, control peptide for the NF-κB (nuclear factor κB) inhibitor peptide Ant-Tirap138–151; RQIKIWFQNRRMKW-KKSVIAGGPAADRLQL], were purchased from EMD Biosciences (La Jolla, CA). Stock solutions (2 mm) were divided into aliquots and maintained at −20°C until the time of experiment.
Extracellular drug application.
During the experiments, stock solutions (2.5–35 μl) were added to the syringe with ACSF to achieve the desired final concentration (0.5–7 μm). We observed that foam formation during excessive bubbling in the syringe produced a decrease in the Ant-CaMKIINtide effect; possibly, inhibitor that stuck to the foam surface was removed from circulation. When bubbling was decreased to a level at which foam formation was avoided (but enough slice oxygenation was provided, as indicated by stable fEPSP recording and an fEPSP/fiber volley relationship of at least 2–3), highly reproducible inhibitor effects were observed. Drugs were washed out by changing syringe solution: recirculation was stopped and the syringe was refilled with fresh, oxygenated ACSF. After changing at least four times the original solution volume, the final 10 ml of recirculating volume was reestablished. As indicated by control experiments with ACSF recirculation (no peptide) and solution change at similar time as in test experiments, this procedure by itself caused a small effect on fEPSP (see Fig. 1E,F), probably because of small changes in oxygenation.
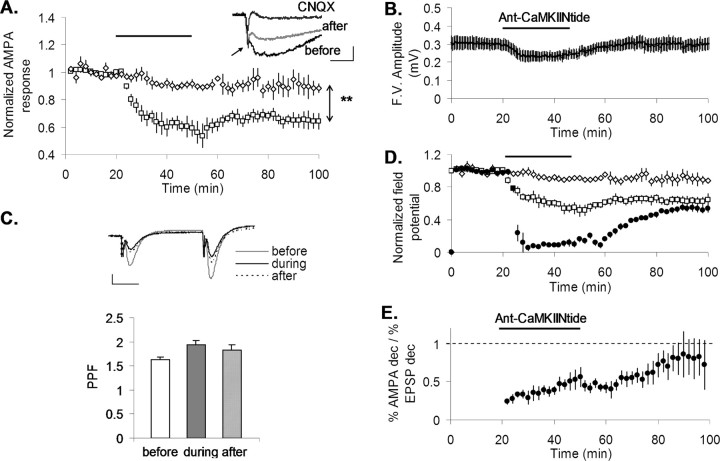
Figure 1.
Inhibition of CaMKII depresses potentiated and basal transmission in a dose-dependent manner. A, B, Single experiment (A) and summary graph (B; n = 5) showing the effect of Ant-CaMKIINtide (bath application; 3 μm) on basal (filled diamonds) and potentiated (open circles; arrow, 100 Hz, 1 s) transmission. A, Inset, Averaged (n = 10) basal fEPSP before and after 30 min drug application. Calibration: 5 ms, 0.2 mV. C, Percentage decrease in transmission after 30 min inhibitor applications at different concentrations is the same in LTP and naive pathways (diagonal line represents the ratio if the values of percentage inhibition of naive and percentage inhibition of potentiated responses are equal). D, Percentage decrease in basal transmission after 30 min as a function of inhibitor concentration (n = 5 for 0.5–5 μm; n = 2 for 7 μm). E, Superimposed results of the effect on basal transmission from interleaved experiments in which 5 μm different peptides containing the Ant segment was applied. Ant-CaMKIINtide (open squares; n = 5) and Ant-AIP (horizontal lines; n = 7) are CaMKII inhibitors. Ant (gray circles; n = 5) is the antennapedia segment alone, and ACSF is a control experiment (no peptide) in which the recirculating solution was changed at times similar to those used in test experiments (black circles; n = 4). F, Summary plot showing the percentage decrease in fEPSP (measured during the last 5 min of peptide application) from the same experiments as in E. The results from application of Ant- Tirap-R (n = 5), a peptide unrelated to CaMKII, were also included. Levels of significance in the post hoc pairwise Tukey's test, *p < 0.01; **p < 0.0005; NS, p > 0.5. Norm., Normalized.
Pressure application of AMPA.
AMPA was locally applied by pressure injection through the recording electrode (Fedorov and Reymann, 1993). AMPA concentration inside the pipette was 100–150 μm. Pressure pulses of 7–10 ms and 3–5 psi were applied every 2 min using a Picrospritzer III (Parker Instrumentation, Cleveland, Ohio) and were alternated with electrical stimulation of Schaffer collaterals (see above). A short-lasting (∼10 ms) pressure artifact was observed at the beginning of the recorded AMPA responses. At the end of the experiment, CNQX was added to remove the AMPA response, and the isolated artifact was directly measured. The artifact was then subtracted from all of the responses.
LTP saturation/depotentiation experiments.
To compute the “additional” potentiation produced by drug application after LTP saturation (see Fig. 6), the percentage LTP in these experiments was corrected for the potentiation that occurred in the absence of drug. Because the latter varied somewhat, it was important to measure in each set of experiments as interleaved controls.
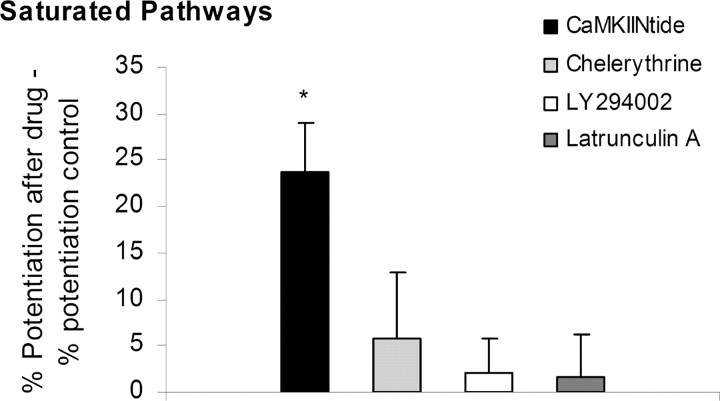
Figure 6.
PKC, PI3-K, or F-actin inhibition does not reverse saturated LTP. The same protocol was used as for CaMKII inhibitor (see Fig. 5A) with chelerythrine (an inhibitor of PKC), LY294002 (an inhibitor of PI3-K), and latrunculin A (an inhibitor of actin polymerization). Each inhibitor reduced the maintained potentiation during application [chelerythrine (8 μm), 48.6 ± 3.5% (n = 8); LY294002 (45 μm), 17.9 ± 6.2% (n = 5); latrunculin A (2 μm), 21.2 ± 4.7% (n = 5); data not shown]. After inhibitor washout, reversal of saturation was tested by applying a series of tetani. Subtracting the percentage potentiation in controls from the percentage potentiation after drug application gives the percentage additional LTP attributable to drug. Only Ant-CaMKIINtide (5 μm) allowed significant additional LTP.
Results
CaMKIINtide is a peptide derived from a recently discovered endogenous inhibitor of CaMKII (Chang et al., 1998, 2001; Lepicard et al., 2006). It inhibits both Ca-dependent and Ca-independent activity. The inhibitor is the most specific known; it inhibits both α and β CaMKII, but not CaMKI, CaMKIV, PKC, or PKA. We used a form of CaMKIINtide that was made membrane permeant by the addition of an antennapedia sequence (Ant-CaMKIINtide) (Fink et al., 2003; Illario et al., 2003; Meffert et al., 2003; Schmitt et al., 2004; Illario et al., 2005). Because this inhibitor does not have to be injected intracellularly, we were able to use field-recording methods on hippocampal slices, allowing the long-duration recordings that were crucial for our experiments. Biochemical experiments on isolated neurons (Schmitt et al., 2004) show that nearly complete inhibition of CaMKII is achieved by 5 μm bath concentration of Ant-CaMKIINtide. Similar concentrations inhibit the CaMKII T286 autophosphorylation (measured on Western blots) produced by 25 μm NMDA (5 min with 1 μm glycine) in hippocampal slices [concentration/percentage inhibition, 0.5 μm/17%; 2 μm/66%; 5 μm/83%; n = 4 (E. Guire and T. Soderling, personal communication)].
Inhibitor was applied after inducing LTP in one pathway but not in another (the “naive pathway”). Figure 1A,B shows that 3 μm Ant-CaMKIINtide strongly reduced the fEPSP in both the potentiated and naive pathways. The maximum inhibition during inhibitor application (30 min) at a range of concentrations was comparable in both pathways (Fig. 1C). Control experiments for LTP stability in the absence of inhibitor application are shown in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). The concentration dependence was determined and found to be half-maximal at ∼3 μm (Fig. 1D), consistent with the range that produces CaMKII inhibition. We also observed a similar but smaller effect on basal transmission using a second inhibitor of CaMKII (Fig. 1E,F) (p < 0.0005). This inhibitor is Ant-AIP-II. It is competitive with respect to many substrates (Ishida et al., 1994), perhaps accounting for its weaker action. Control experiments with antennapedia alone (Ant) showed no significant effect on the fEPSP (Fig. 1E,F) (p > 0.5) and no effect on holding current or the EPSC in whole-cell recordings (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Additional controls using antennapedia attached to a peptide not related to CaMKII (TirapR) show no effect (Fig. 1F) (p > 0.5). Note that perfusion of these control substances produces no effect only when compared with that produced by solution change itself (ACSF), which causes a small effect because of the use of recirculating solution (one-way ANOVA test and post hoc Tukey's honestly significant difference test for pairwise comparisons). Together, these results indicate that inhibition of CaMKII produces a decrement in basal and potentiated transmission. The effects on basal transmission are consistent with a previous study showing that bath application of CaMKII peptide inhibitor strongly reduces basal transmission through presynaptic action (Waxham et al., 1993).
In the experiments of Fig. 1, synaptic stimulation was given repetitively during application of the inhibitor. Thus one possible interpretation is that the inhibitor enhanced a normal activity-dependent process that weakens synapses. To test this possibility, we compared the effect of inhibitor on two pathways, turning off test pulses in one pathway during almost the entire period of inhibitor application. In the other pathway, tests pulses were continuously applied, as before. Supplemental Figure 3A (available at www.jneurosci.org as supplemental material) shows that when tested 20 min after inhibitor application, the effect was quite similar in both pathways. Direct measurements of spiking during inhibitor application indicated no increase in the rate of spontaneous spikes (supplemental Fig. 3B, available at www.jneurosci.org as supplemental material). As an additional test, we asked whether the effects of inhibitor could be blocked by an NMDA receptor antagonist, which is known to block LTD (Mulkey and Malenka, 1992) and depotentiation (O'Dell and Kandel, 1994). We found that inhibitor reduced basal transmission in the presence of APV (supplemental Fig. 4, available at www.jneurosci.org as supplemental material), whereas application of APV alone did not significantly affect transmission (Otmakhova and Lisman, 2004). These results argue that the weakening produced by the peptide is unlikely to involve a normal activity-dependent process.
An important aspect of Ant-CaMKIINtide action concerns its reversibility. When the inhibitor was removed from the bath, a component of transmission recovered, reaching a steady value within 30 min. However, this steady value did not correspond to full recovery (Fig. 1A,B; supplemental Fig. 5, available at www.jneurosci.org as supplemental material). The nonrecovering or persistent component of the effect, defined as the difference between transmission before inhibitor application and the steady state reached after its removal, was always evident (n = 156). We will return later to the basis of this component.
As an additional test that Ant-CaMKIINtide is working as an effective CaMKII inhibitor, we checked whether it can block LTP induction, as do other inhibitors of CaMKII (Malinow et al., 1989). We used a moderate concentration (3 μm), because partial transmission had to be maintained. Under these conditions, application of a 100 Hz, 1 s tetanus produced very small potentiation (Fig. 2A). After removal of the inhibitor and partial recovery of transmission, we reduced stimulation to make the response comparable with that during inhibitor application. Much larger potentiation could now be induced (Fig. 2A). The summary data (Fig. 2B) show 17.5 ± 7.4% potentiation when the tetanus was given during inhibitor application and 56.7 ± 6.5% potentiation when the tetanus was given after removal of inhibitor (n = 7; p < 0.001). Thus, partial inhibition of CaMKII produces a partial inhibition of LTP induction, as expected.
Figure 2.
Ant-CaMKIINtide reversibly interferes with LTP induction. The experiment was done with two pathways; in one, a tetanus was given during inhibitor application and after washout (filled diamonds), whereas the other pathway served as a control (open circles) A, Representative experiment in which a tetanus (100 Hz, 1 s) was applied to one synaptic pathway in the presence of Ant-CaMKIINtide (3 μm), inducing almost no potentiation. After peptide washout, the amplitude of stimulation was decreased so that transmission equaled that during peptide application. A tetanus under these conditions induced substantial LTP in the same pathway. B, Summary of all experiments (n = 7) during the application of Ant-CaMKIINtide (B1) and after washout of the inhibitor (B2).
Previous work with bath application of CaMKII inhibitory peptide showed dramatic inhibition of transmission and established that it was caused by presynaptic action (Waxham et al., 1993). However, it is unclear how this peptide entered cells, and so it may not have gained access to the postsynaptic cell. The antennapedia method we have used should make the inhibitor enter both presynaptic and postsynaptic cells, and the observed reduction of LTP is consistent with postsynaptic action. To test directly for postsynaptic action, we applied brief pulses of AMPA by pressure injection through the extracellular recording electrode and measured the resulting field potential (Fedorov and Reymann, 1993). Figure 3A shows that Ant-CaMKIINtide significantly decreased the AMPA response relative to that in control experiments in which AMPA pulses were applied continuously and no inhibitor was perfused [45.4 ± 11.3% (inhibitor) vs 9.8 ± 3.6% (ACSF); n = 5; p < 0.0005]. These results thus directly demonstrate that there is postsynaptic action of the inhibitor on the AMPA-mediated response. We tested whether this postsynaptic effect of bath-applied permeant CaMKIINtide could be reproduced by postsynaptic application of impermeant inhibitor (50 μm) and found that the effect was always smaller (average reduction at 30 min, 20%; n = 5). This could be because of failure to achieve sufficient concentration in the dendrites or because synaptic strength is governed by a transsynaptic process that requires simultaneous inhibition of kinase on both sides of the membrane (see Discussion).
Figure 3.
The effect of CaMKII inhibitor on transmission has a nonrecovering component that is primarily postsynaptic. A, Presynaptic stimulation was alternated with local application of AMPA by pressure injection through the recording electrode (open squares; n = 5). Ant-CaMKIINtide (5 μm) caused a decrease in the AMPA-induced response that showed a large nonrecovering component after drug washout (45.4 ± 11.3%). In control experiments for AMPA-response stability, the solution was changed, but no inhibitor was included (open diamonds; n = 4). Inset, AMPA-induced response before inhibitor and after washout. AMPA response was completely inhibited by bath application of CNQX (40 μm). The arrow indicates the pressure artifact. Calibration: 0.2 mV, 20 ms. B, Summary plot showing reversible decrease in fiber volley (F.V.; n = 15). C, Averaged traces showing PPF before (t = 0–20 min), during (t = 45–55 min), and after (t = 90–100 min) inhibitor (top). Summary plot showing the average PPF values (bottom) at the three different stages (1.64 ± 0.11, 1.93 ± 0.22, and 1.82 ± 0.25, respectively; n = 5; p > 0.1; one-way ANOVA test). D, Inhibitor effect on AMPA response compared with inhibitor effect of simultaneously measured EPSP (filled circles). The nonrecovering component of the EPSP was 46.5 ± 8.9% and was not statistically different from the nonrecovering component of the AMPA response (37.3 ± 13.1%; p > 0.1; n = 5). The preceding calculations were done with reference to the original baseline, but a similar conclusion is reached if the nonrecovering component is measured with reference to control experiments (at t = 90–100 min) in which AMPA responses (see Fig. 3A) and EPSPs (data not shown) were recorded during a similar experimental protocol without inhibitor application (35.3 ± 8.9 and 26.1 ± 13.1%; n = 5; p > 0.1; paired t test). E, Ratio of percentage decrease in AMPA response to percentage decrease in EPSP as a function of time during and after inhibitor application.
To determine whether presynaptic function is affected by inhibitor, we examined the fiber volley, an early component of the field potential that reflects the number of axons excited by the stimulating electrode. Figure 3B shows that the fiber volley was decreased by inhibitor application (n = 15). This effect was completely reversed after inhibitor was removed. The observed effect on axon excitability is not surprising, because it has been shown that some voltage-gated currents can be modulated by CaMKII (Lu et al., 1994; Robinson et al., 2004; Varga et al., 2004). There are reasons to suspect that CaMKII is also involved in presynaptic release processes (Llinas et al., 1991; Waxham et al., 1993; Chi et al., 2001). We therefore measured paired-pulse facilitation (PPF), a form of short-term plasticity that varies inversely with release probability. PPF ratio varied from 1.64 ± 0.11 (before inhibitor application) to 1.93 ± 0.22 (during application), and after drug washout, it decreased to 1.83 ± 0.25. These values were not statistically different (one-way ANOVA test, F = 2.635; p > 0.1) and indicate that either there is no change in the probability that an action potential produces release or that such a change is not accompanied by a substantial change in PPF.
The above results indicate that during the application of Ant-CaMKIINtide, the decrement in transmission is attributable to both presynaptic and postsynaptic effects. One can ask whether this is similarly true for the nonrecovering component that is evident after removal of inhibitor. In our AMPA application experiments (Fig. 3A), there was a significant difference between the decrement in transmission after inhibitor washout compared with control experiments without peptide application (37.3 ± 13.1% vs 11.2 ± 9.3%, respectively; p < 0.01; t = 90–100 min). This shows that the nonrecovering component is at least in part attributable to a postsynaptic effect. To address the localization issue more quantitatively, we compared the effect of inhibitor on the response to applied AMPA (which reflects only postsynaptic processes) to the effect on the synaptically evoked EPSP recorded during the same experiments (Fig. 3D). During the period of peptide application (30 min), the reduction of the EPSP was considerably larger (85.6 ± 6.1%, relative to the initial 20 min baseline) than the reduction in the response to applied AMPA (45.4 ± 11.3%), as expected if there is both presynaptic action and postsynaptic action. However, after removal of inhibitor (at t = 90–100 min), the nonrecovering component of the synaptic response and the nonrecovering component of the response to applied AMPA were not statistically different (see Fig. 3D legend). By comparing the effect of inhibitor on total transmission to the isolated postsynaptic effect, we determined the fraction of the effect that is postsynaptic (Fig. 3E). It can be seen that the acute effect is both presynaptic and postsynaptic but that the effect becomes mainly postsynaptic within 30 min after removal of inhibitor. We conclude that the nonrecovering component is primarily expressed postsynaptically (but induction may depend on both presynaptic and postsynaptic effects; see Discussion).
Several additional properties of the nonrecovering (persistent) component of the effect are noteworthy. This component was observed in both the potentiated and naive pathways, as shown in supplemental Figure 5 (available at www.jneurosci.org as supplemental material), in which the magnitude of the nonrecovering effect on potentiated pathway is plotted versus that on naive pathway (experiments were done at different inhibitor concentrations). Furthermore, the magnitude of the nonrecovering component was found to be graded (Fig. 4) with inhibitor concentration in the 3–5 μm range (30 min application, basal conditions). To test whether the nonrecovering component could lead to complete silencing of synapses, we increased the duration (2 h) and concentration (10 μm) of Ant-CaMKIINtide application. This did not increase the amplitude of the nonrecovering component (56.1 ± 6.1 vs 54.3 ± 13.8%). These results indicate that only a fraction of transmission can be persistently turned off by CaMKII inhibitor (i.e., the nonrecovering component does not approach 100%).
Figure 4.
The nonrecovering component of transmission is saturable. The magnitude of the nonrecovering component is graded at lower concentrations but is saturable at higher concentrations and longer duration of application [3 μm/30 min (n = 4); 5 μm/30 min (n = 6); 10 μm/120 min (n = 4)].
We next focused on the possible mechanism of the nonrecovering component. The simplest explanation would be that inhibitor entered the cell and could not exit, perhaps because of cleavage of antennapedia from the peptide. To test this possibility, inhibitor was applied by local pressure injection through the recording electrode. In this case, even inhibitor that had become impermeant would have a reversible effect, because it could diffuse intracellularly away from the site of application. We found, however, that local inhibitor application still produced a nonrecovering component (supplemental Fig. 6, available at www.jneurosci.org as supplemental material). An additional indication that the inhibitor could be effectively removed, either by leaving the cell or by intracellular proteolysis (Fernandez et al., 1991), was the reversal of the inhibition of LTP induction (Fig. 2) and the reversal of the inhibition in the fiber volley (Fig. 3B). Together, these results make it difficult to explain the nonrecovering component by the residual presence of Ant-CaMKIINtide.
Another possible explanation of the nonrecovering component is that a process that controls the strength of transmission is turned off by the transient application of inhibitor. According to this interpretation, the effect is a biochemical form of depotentiation that reverses a process responsible for maintaining synaptic strength. The standard test for activity-driven depotentiation (Dudek and Bear, 1993) is that saturated LTP can be reversed, as indicated by the ability to subsequently induce additional LTP. Importantly, a recent use of this test showed that an agent that reduced LTP (when applied after induction) did not allow additional LTP to be induced, indicating that in this case an expression process rather than a maintenance process was affected (Delgado and O'Dell, 2005). This test can therefore distinguish between changes in expression and maintenance processes.
A schematic of the experimental protocol used to test the possibility that Ant-CaMKIINtide is producing a chemical form of depotentiation is shown in Figure 5A. We first saturated LTP in one pathway with four tetani and examined the stability of the saturation. If we waited 90–100 min (the “intervening period”) and then gave an additional series of four tetani, short-term potentiation was induced, but there was no additional LTP (Fig. 5B, open circles) (1 ± 2%; n = 9). In interleaved experiments, we transiently (30 min) applied Ant-CaMKIINtide during the intervening period. A second group of tetani was then given after inhibitor was removed (and recovery was stable). In this case, substantial LTP could be induced (Fig. 5B, filled circles) (24 ± 5%; n = 9). The difference (additional potentiation) made possible by transient application of inhibitor was significant at the 0.0005 level. This difference cannot be attributed to differences in the level of synaptic transmission at the time of LTP induction; in experiments without inhibitor, the stimulus strength was reduced at t = 110 min to make transmission strength equal to that in experiments in which peptide was applied. The changes in fEPSP caused by LTP induction, drug, and stimulus intensity change can all be seen in the representative experiments shown in supplemental Figure 7A,B (available at www.jneurosci.org as supplemental material). Supplemental Figure 7C (available at www.jneurosci.org as supplemental material) compares the average fEPSP for the tetanized pathway in the cases in which inhibitor was or was not applied and shows that the equalization procedure was successful (p > 0.5). We conclude that Ant-CaMKIINtide can reverse LTP saturation, allowing additional LTP to be induced.
Figure 5.
CaMKII inhibition reverses saturated LTP and allows for subsequent potentiation. We conducted two-pathway experiments (see supplemental Fig. 5, available at www.jneurosci.org as supplemental material), but for clarity, the data are presented here separately for potentiated and naive pathways. A, A schematic of the experimental protocol used to test reversal of LTP saturation. B, Average of nine experiments showing the effect of the second series of tetani on the previously saturated pathway, with and without inhibitor application. C, A schematic of the experimental protocol used to test for the effect of inhibitor on the magnitude of LTP than can be induced by a first set of tetani in the naive pathway. D, Average of nine experiments showing the effect of the first series of tetani on the naive pathways, with and without previous inhibitor application. Norm., Normalized.
It should be noted (supplemental Fig. 7B, available at www.jneurosci.org as supplemental material) that although substantial LTP could be restored, even multiple tetani never fully brought potentiation back to the level before application of inhibitor. It is perhaps relevant that experiments that induce LTP after depotentiation also cannot fully restore the initial LTP (Dudek and Bear, 1993). The reason for incomplete restoration is unclear but may be related to slow transitions in the state of the synapse that govern bidirectional plasticity (Montgomery and Madison, 2002).
As shown above (Figs. 1, 4), inhibitor affected not only the LTP pathway, but also the naive pathway. One possibility is that such basal transmission reflects LTP that occurred naturally during the life of the animal. Recent work demonstrates that learning potentiates synapses in the CA1 region and that this occludes with LTP (Gruart et al., 2006; Whitlock et al., 2006). Furthermore, consistent with this, the stronger the basal strength of synapses, the smaller the LTP that can be experimentally induced (Liao et al., 1992; Debanne et al., 1999; Montgomery et al., 2001). These findings have implications for CaMKII, because, with the exception of LTP that occurs in very young animals (Yasuda et al., 2003), all forms of LTP in the CA1 region are dependent on CaMKII. Indeed, even the first steps of AMPA channel insertion into synapses (i.e., into silent synapses) can be driven by CaMKII (Wu et al., 1996; Poncer et al., 2002). Importantly, under basal conditions, CaMKII is present at synapses (Otmakhov et al., 2004). Thus, inhibition of the kinase at naive synapses might affect basal transmission by reversing LTP that occurred in vivo. A critical test of this hypothesis is that application and removal of inhibitor should make it possible to induce larger LTP in the naive pathway compared with the case in which the inhibitor was not applied. Figure 5C,D and supplemental Figure 7A,B (naive; available at www.jneurosci.org as supplemental material) indicate that this is the case. Without application of inhibitor, 35 ± 7% LTP could be induced; if inhibitor was transiently applied, leading to a persistent reduction in transmission, 113 ± 10% LTP could be induced (n = 9). This difference was significant at the 0.0001 level. Supplemental Figure 7C (naive; available at www.jneurosci.org as supplemental material) shows that this difference cannot be attributed to differences in transmission at the time of LTP induction (p > 0.05). We conclude that the reversal of basal transmission allows greater LTP to be induced.
In the next series of experiments, we investigated whether other agents might act similarly to CaMKII antagonists in reversing LTP. Several agents have been reported to reduce transmission when applied after LTP induction (Krucker et al., 2000; Ling et al., 2002; Sanna et al., 2002; Opazo et al., 2003); these include chelerythrine (an inhibitor of PKC), 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride [LY294002; an inhibitor of phosphoinositide 3-kinase (PI3-K)], and latrunculin A (an inhibitor of actin polymerization). To study these inhibitors, we used the same LTP saturation protocol and timing as for CaMKII inhibitor. The percentage additional potentiation that could be induced after transient drug application compared with interleaved controls without drug application is shown in Figure 6. Although each of these inhibitors reduced transmission (see Fig. 6 legend), none of these drugs allowed additional LTP to be induced. Thus, by these tests, CaMKII inhibitor has a unique ability to reverse a maintenance process.
Discussion
Our results show that a noncompetitive CaMKII inhibitor, Ant-CaMKIINtide, can reverse an LTP maintenance mechanism. Application of inhibitor after LTP induction reduced potentiation. When the inhibitor was removed, there was partial but incomplete recovery. The nonrecovering component is primarily postsynaptic. To test whether the nonrecovering component resulted from the reversal of a maintenance process, we applied a standard test for depotentiation: the ability to reverse saturated LTP. We found that reversal occurred, as demonstrated by the ability to induce additional LTP. This is the first report of a biochemical process that reverses LTP and implicates CaMKII in the maintenance of LTP.
The reversal caused by CaMKII inhibitor also occurred in the nontetanized naive pathway. After such reversal, larger LTP could be induced, indicating that the peptide weakened a process that maintained basal transmission. A simple but speculative interpretation is that the naive (basal) pathway had undergone an LTP-like process in vivo (Gruart et al., 2006; Whitlock et al., 2006). Consistent with this is the finding of occlusion: the stronger basal transmission is, the less LTP can be induced (Liao et al., 1992; Debanne et al., 1999; Montgomery et al., 2001). Thus the inhibitor might be acting on a process similar to the LTP that is induced experimentally. Support for such similarity comes from the study of CaMKII involvement in LTP and basal transmission. During LTP, CaMKII becomes persistently autophosphorylated (Barria et al., 1997; Lengyel et al., 2004; Ahmed and Frey, 2005) and persistently translocated to the PSD (Otmakhov et al., 2004). However, even under basal conditions, CaMKII can be found in isolated PSDs (Strack et al., 1997b; Petersen et al., 2003) and in synaptic puncta (Bayer et al., 2006). Moreover, a fraction of this kinase is in the autophosphorylated state (Strack et al., 1997a; Trinidad et al., 2005). Thus, basal and potentiated synapses differ only quantitatively. Activated CaMKII, when introduced into cells, enhances AMPA-mediated transmission (Pettit et al., 1994; Lledo et al., 1995; Shirke and Malinow, 1997; Poncer et al., 2002). From this perspective, it is not surprising that CaMKII inhibitor should affect both potentiated and naive pathways.
The ability to reverse a component of transmission using Ant-CaMKIINtide, a noncompetitive inhibitor, is complemented by similar but smaller effects using Ant-AIP-II (Fig. 1E,F). The latter inhibitor is based on the autoinhibitory domain of CaMKII and may be competitive with respect to some substrates. The small effect of AIP might explain why similar effects were not noted with other related inhibitors (AC3) (Otmakhov et al., 1997; Chen et al., 2001). This and other possible explanations for the different results obtained with different CaMKII inhibitors are discussed in detail below.
Although we made attempts to maximize the dose and duration of inhibitor application, the nonrecovering component after removal of inhibitor remained only a fraction of total transmission: the maximal nonrecovering component was ∼55% (Fig. 4). This finding suggests that the processes irreversibly affected by the inhibitor are responsible for only a component of transmission. The insensitive component could be related to CaMKII-independent forms of potentiation. Such forms account for all of LTP in young animals (Yasuda et al., 2003). Another possibility is that the insensitive component is affected by CaMKII inhibitor but in a reversible manner.
During the application of inhibitor, synaptic transmission is reduced to ∼20% of maximal (Fig. 1D). This can be understood as the sum of three effects: a reduction to 50% as a result of a postsynaptic change (Fig. 3A), a 25% reduction as a result of a presynaptic decrease in excitability (Fig. 3B), and an additional small effect caused by a reduction in the probability of release (Waxham et al., 1993).
Reconciliation with previous experiments
Previous studies have shown that postsynaptic application of pseudo-substrate inhibitors of CaMKII inhibitors can block LTP but not decrease basal transmission or reverse established LTP (Malinow et al., 1989; Malgaroli et al., 1992; Otmakhov et al., 1997; Chen et al., 2001). Why then does Ant-CaMKIINtide have all three effects? We cannot give a definite answer, but we point to several possible explanations.
The first has to do with the properties of the inhibitor used. It should be emphasized that the KN62/KN93 class of CaMKII inhibitor interferes with calmodulin binding to CaMKII (Tokumitsu et al., 1990; Sumi et al., 1991); this class of drugs would thus be expected to interfere with the Ca2+-dependent activation of CaMKII during LTP but not interfere with the Ca2+-independent activity that may underlie basal or potentiated transmission. The peptide pseudo-substrate inhibitors that selectively block LTP induction do however block the Ca2+-independent form of CaMKII, so some other explanation is required to explain their selectivity. The fact that these inhibitors are competitive with some substrates may provide an explanation. They may be effective at blocking the induction processes, which is probably initiated in the cytoplasm, where the substrate concentrations are low, but be less effective at blocking maintenance processes, which may depend on events in the PSD, where the substrate concentrations are high. A related issue has to do with the nature of the substrates involved in induction and maintenance. It is known that there are multiple substrate binding sites on CaMKII and that different peptide inhibitors interact with different binding sites (Ishida et al., 1995). A given inhibitor can thus affect some CaMKII targets much more strongly than others. For instance, the inhibitor AC3 has an IC50 of 0.1 μm for Ca2+-independent autophosphorylation of soluble CaMKII, whereas the IC50 is 10 μm for Ca2+-dependent and -independent PSD-CaMKII phosphorylation of a 180 kDa PSD substrate (Chen et al., 2001). Thus, an inhibitor could differentially affect induction and maintenance because different sites on CaMKII were involved. Finally, it is possible that CaMKII contributes to maintenance through a structural rather than enzymatic process (Lisman and Zhabotinsky, 2001) and that Ant-CaMKIINtide, but not other inhibitors, interferes with a binding reaction important for structural assembly. Recent work shows that the binding of CaMKII to the NMDA channel is necessary for LTP induction (Barria and Malinow, 2005) and that this binding occurs in a two-step process (Bayer et al., 2006). Importantly, the first step can be blocked by AC3, whereas once tight binding occurs, the complex is no longer dissociated by AC3. This might explain why AC3 and related peptides can block LTP induction but have little or no effect on maintenance (Otmakhov et al., 1997; Chen et al., 2001). It would thus be of importance to determine whether CaMKIINtide interferes with the binding of CaMKII to the NMDA channel.
The second class of explanations has to do with the fact that the use of a permeant inhibitor means that in our experiments, inhibitor was applied both presynaptically and postsynaptically. This cannot be the only explanation, because we found that classic pseudo-substrate inhibitors are less effective than CaMKIINtide, even when applied to both sides of the synapse (Fig. 1E). However, the two classes of explanation are not mutually contradictory and could both apply. Recent work has demonstrated that LTP is produced by an interaction between presynaptic and postsynaptic CaMKII (Ninan and Arancio, 2004; Lu and Hawkins, 2006). It is thus similarly possible that the reversal of LTP that we observed, although postsynaptic in expression, is dependent on an interaction between presynaptic and postsynaptic CaMKII. Consistent with this possibility, postsynaptic application of impermeant CaMKIINtide did not produce an decrement in the synaptic response as large as the inhibition of the postsynaptic response to AMPA (Fig. 3D) produced by bath application of permeant inhibitor.
Evidence for role of PKM-ζ in LTP maintenance
Recent work provides evidence that PKM-ζ has a critical role in the maintenance of late-phase LTP (Hrabetova and Sacktor, 1996; Serrano et al., 2005). The kinase is synthesized within the dendrites after LTP induction (Cracco et al., 2005). Directly adding kinase to the postsynaptic cell leads to enhancement of transmission, whereas blocking the kinase leads to inhibition of late-phase LTP (Ling et al., 2002). Importantly, inhibition of the kinase in the hippocampus of rats leads to erasure of memory, consistent with a role in memory maintenance (Pastalkova et al., 2006). Application of inhibitor after LTP induction reduces transmission in the LTP pathway. This might be because of an effect on expression processes; such effects do not lead to a reversal of LTP (Delgado and O'Dell, 2005). The critical test that distinguishes between these possibilities is whether inhibitor brings LTP out of saturation. Experiments of this kind have not been previously reported. Our own experiments did not provide any indication that PKM-ζ inhibitor can bring LTP out of saturation (Fig. 6); however, our inhibitor application times were chosen to be the same as used in our CaMKII inhibitor experiments (30 min) and are shorter than the many hours of inhibitor application used to reverse behavioral memory with PKM-ζ inhibitor (Pastalkova et al., 2006). Thus, different results might have been obtained had we used much longer applications.
Summary of evidence regarding a role of CaMKII in maintenance
Our finding that CaMKII inhibitors can reverse LTP is of particular interest given the other lines of evidence that CaMKII could be important in the maintenance of synaptic strength. Measurements indicate that changes in CaMKII persist, at least through the early stages of LTP (hours): there is a persistent increase in CaMKII activity (Fukunaga et al., 1993), persistent autophosphorylation of Thr286 up to 8 h (Ahmed and Frey, 2005), persistent phosphorylation of a CaMKII substrate (Barria et al., 1997; Lee et al., 2000), and persistent translocation of the kinase from the cytoplasm to the synapse (Strack et al., 1997b; Otmakhov et al., 2004; Bayer et al., 2006). Importantly, the autophosphorylation and structural properties of the enzyme provide a basis for understanding how persistent autophosphorylation of the kinase occurs (Lisman and Zhabotinsky, 2001; Miller et al., 2005). If persistent activation of CaMKII is blocked by mutation of Thr286, then LTP is prevented (Giese et al., 1998). These observations, together with the ability of CaMKII to enhance transmission (Lledo et al., 1995), suggest that CaMKII should contribute to the maintenance of LTP. Our results provide evidence that this is the case.
Footnotes
This work was supported by National Institutes of Health Grants R01 NS050944 and R01 NS027337 and the Pew Latin American Fellows Program for the Biomedical Sciences (M.S.). We thank Neal Waxham, Nick Otmakhov, and Leslie Griffith for helpful comments on this manuscript as well as Nonna Otmakhova and Ed Richard for support and suggestions. We thank Dr. Suzanna Horvath for providing us with Ant-CaMKIINtide and for helpful advice on its handling and storage and Eric Guire and Tom Soderling for useful discussions about this inhibitor. We thank German Fernandez for his help with spiking measurements.
References
- Ahmed T, Frey JU. Plasticity-specific phosphorylation of CaMKII, MAP-kinases and CREB during late-LTP in rat hippocampal slices in vitro. Neuropharmacology. 2005;49:477–492. doi: 10.1016/j.neuropharm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O'Leary H, Schulman H, De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–1023. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Calcium/calmodulin-dependent protein kinase II inhibitor protein: localization of isoforms in rat brain. Neuroscience. 2001;102:767–777. doi: 10.1016/s0306-4522(00)00520-0. [DOI] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Strack S, Colbran RJ, Lisman JE. Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J Neurophysiol. 2001;85:1368–1376. doi: 10.1152/jn.2001.85.4.1368. [DOI] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus. 2005;15:551–556. doi: 10.1002/hipo.20078. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Heterogeneity of synaptic plasticity at unitary CA3–CA1 and CA3–CA3 connections in rat hippocampal slice cultures. J Neurosci. 1999;19:10664–10671. doi: 10.1523/JNEUROSCI.19-24-10664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JY, O'Dell TJ. Long-term potentiation persists in an occult state following mGluR-dependent depotentiation. Neuropharmacology. 2005;48:936–948. doi: 10.1016/j.neuropharm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dudek S, Bear M. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov NB, Reymann KG. Simultaneous local pressure microejection of excitatory amino acids and field potential recording with a single micropipette in the hippocampal slice. J Neurosci Methods. 1993;50:83–90. doi: 10.1016/0165-0270(93)90058-y. [DOI] [PubMed] [Google Scholar]
- Feng TP. The involvement of PKC and multifunctional CaM kinase II of the postsynaptic neuron in induction and maintenance of long-term potentiation. Prog Brain Res. 1995;105:55–63. doi: 10.1016/s0079-6123(08)63283-5. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Mery J, Vandromme M, Basset M, Cavadore JC, Lamb NJ. Effective intracellular inhibition of the cAMP-dependent protein kinase by microinjection of a modified form of the specific inhibitor peptide PKi in living fibroblasts. Exp Cell Res. 1991;195:468–477. doi: 10.1016/0014-4827(91)90398-e. [DOI] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of the CA3–CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, Tonegawa S, Malinow R. CA1 long-term potentiation is diminished but present in hippocampal slices from alpha-CaMKII mutant mice. Learn Mem. 1998;5:344–354. [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S, Sacktor TC. Bidirectional regulation of protein kinase M ζ in the maintenance of long-term potentiation and long-term depression. J Neurosci. 1996;16:5324–5333. doi: 10.1523/JNEUROSCI.16-17-05324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illario M, Cavallo AL, Bayer KU, Di Matola T, Fenzi G, Rossi G, Vitale M. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J Biol Chem. 2003;278:45101–45108. doi: 10.1074/jbc.M305355200. [DOI] [PubMed] [Google Scholar]
- Illario M, Cavallo AL, Monaco S, Di Vito E, Mueller F, Marzano LA, Troncone G, Fenzi G, Rossi G, Vitale M. Fibronectin-induced proliferation in thyroid cells is mediated by αvβ3 integrin through Ras/Raf-1/MEK/ERK and calcium/CaMKII signals. J Clin Endocrinol Metab. 2005;90:2865–2873. doi: 10.1210/jc.2004-1520. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kitani T, Okuno S, Fujisawa H. Inactivation of Ca2+/calmodulin-dependent protein kinase II by Ca2+/calmodulin. J Biochem (Tokyo) 1994;115:1075–1082. doi: 10.1093/oxfordjournals.jbchem.a124460. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lengyel I, Voss K, Cammarota M, Bradshaw K, Brent V, Murphy KP, Giese KP, Rostas JA, Bliss TV. Autonomous activity of CaMKII is only transiently increased following the induction of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2004;20:3063–3072. doi: 10.1111/j.1460-9568.2004.03748.x. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Mizuno K, Antunes-Martins A, von Hertzen LS, Giese KP. An endogenous inhibitor of calcium/calmodulin-dependent kinase II is up-regulated during consolidation of fear memory. Eur J Neurosci. 2006;23:3063–3070. doi: 10.1111/j.1460-9568.2006.04830.x. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Liao D, Jones A, Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992;9:1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994;17:406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci USA. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Gruner JA, Sugimori M, McGuinness TL, Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol (Lond) 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FM, Hawkins RD. Presynaptic and postsynaptic Ca(2+) and CaMKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc Natl Acad Sci USA. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HK, Fern RJ, Nee JJ, Barrett PQ. Ca(2+)-dependent activation of T-type Ca2+ channels by calmodulin-dependent protein kinase II. Am J Physiol. 1994;267:F183–F189. doi: 10.1152/ajprenal.1994.267.1.F183. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malgaroli A, Malinow R, Schulman H, Tsien RW. Persistent signalling and changes in presynaptic function in long-term potentiation. Ciba Found Symp. 1992;164:176–191. doi: 10.1002/9780470514207.ch12. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Miller P, Zhabotinsky AM, Lisman JE, Wang X-J. The stability of a stochastic CaMKII switch: dependence on the number of enzyme molecules and protein turnover. PLoS. 2005 doi: 10.1371/journal.pbio.0030107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron. 2002;33:765–777. doi: 10.1016/s0896-6273(02)00606-2. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Pavlidis P, Madison DV. Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron. 2001;29:691–701. doi: 10.1016/s0896-6273(01)00244-6. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Ninan I, Arancio O. Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron. 2004;42:129–141. doi: 10.1016/s0896-6273(04)00143-6. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O'Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Griffith LC, Lisman JE. Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation. J Neurosci. 1997;17:5357–5365. doi: 10.1523/JNEUROSCI.17-14-05357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol. 2004;92:2027–2039. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Perlman S, Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994;266:1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by α-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol (Lond) 2004;556:353–368. doi: 10.1113/jphysiol.2003.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JM, Wayman GA, Nozaki N, Soderling TR. Calcium activation of ERK mediated by calmodulin kinase I. J Biol Chem. 2004;279:24064–24072. doi: 10.1074/jbc.M401501200. [DOI] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirke AM, Malinow R. Mechanisms of potentiation by calcium-calmodulin kinase II of postsynaptic sensitivity in rat hippocampal CA1 neurons. J Neurophysiol. 1997;78:2682–2692. doi: 10.1152/jn.1997.78.5.2682. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the Aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem. 1997a;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997b;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- Trinidad JC, Thalhammer A, Specht CG, Schoepfer R, Burlingame AL. Phosphorylation state of postsynaptic density proteins. J Neurochem. 2005;92:1306–1316. doi: 10.1111/j.1471-4159.2004.02943.x. [DOI] [PubMed] [Google Scholar]
- Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham MN, Malenka RC, Kelly PT, Mauk MD. Calcium/calmodulin-dependent protein kinase II regulates hippocampal synaptic transmission. Brain Res. 1993;609:1–8. doi: 10.1016/0006-8993(93)90847-g. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]