Abstract
The family of AMPA receptors is encoded by four genes that are differentially spliced to result in the flip or flop versions of the four subunits GluR1 to GluR4. GluR2 is further modified at the so-called Q/R site by posttranscriptional RNA editing. Delivery of AMPA receptors to the plasma membrane and synaptic trafficking are controlled by transmembrane AMPA receptor regulatory proteins (TARPs). Additionally, TARPs influence essential electrophysiological properties of AMPA receptor channels such as desensitization and agonist efficacies. Here, we compare the influence of all known TARPs (γ2, γ3, γ4, and γ8) on agonist-induced currents of the four AMPA receptor subunits, including flip and flop splice variants and editing variants. We show that, although agonist-induced currents of all homomeric AMPA receptor subunits as well as all heteromeric combinations tested are significantly potentiated when coexpressed with members of the TARP family in Xenopus laevis oocytes, the extent of TARP-mediated increase in agonist-induced responses is highly dependent on both the AMPA receptor subunit and the coexpressed TARP. Moreover, we demonstrate that the splice variant of the AMPA receptor plays a key role in determining the modulation of electrophysiological properties by associated TARPs. We furthermore present evidence that individual TARP–AMPA receptor interactions control the degree of desensitization of AMPA receptors. Consequently, because of their subunit-specific impact on the electrophysiological properties, TARPs play a major role as modulatory subunits of AMPA receptors and thus contribute to the functional diversity of AMPA receptors encountered in the CNS.
Keywords: glutamate receptor, stargazin, TARP, trafficking, voltage clamp, AMPA receptor
Introduction
AMPA receptors mediate most of the fast excitatory synaptic transmission in the vertebrate CNS and play an essential role in plasticity of excitatory synapses. The AMPA receptor family consists of four subunits GluR1 to GluR4 (Hollmann and Heinemann, 1994) forming functional homotetrameric and heterotetrameric receptor complexes (Rosenmund et al., 1998; Hollmann, 1999). Subunit diversity is increased by alternative splicing, leading to two splice variants for each AMPA receptor, named flip and flop (Sommer et al., 1990). Subunit heterogeneity is further increased by posttranscriptional RNA editing at the so-called Q/R site that determines electrophysiological properties and surface expression of the receptor complexes (Hollmann et al., 1991; Hume et al., 1991; Swanson et al., 1997; Greger et al., 2002, 2003).
In contrast to NMDA receptors, synaptic AMPA receptors undergo fast turnover (Sheng and Lee, 2003; Collingridge et al., 2004; Cognet et al., 2006). Stargazin (γ2) and three homologous transmembrane AMPA receptor regulatory proteins (TARPs) (γ3, γ4, and γ8) appear to control AMPA receptor trafficking (Tomita et al., 2003; Nicoll et al., 2006). Studies indicate that in vivo the majority of AMPA receptors is associated with some TARP (Nakagawa et al., 2005; Vandenberghe et al., 2005). This association initiates two independent events: an increase in AMPA receptor surface expression (Chen et al., 2000) and a modulation of their electrophysiological properties. The observed enlargement of agonist-evoked currents can be attributable to a decrease in the extent of receptor desensitization, to an increase in the relative efficacy of kainate (KA), and to a slowed rate of receptor desensitization and deactivation (Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005). EC50 values for glutamate and kainate shift toward lower concentrations in the presence of γ2 (Yamazaki et al., 2004; Turetsky et al., 2005). Additionally, it has been shown for GluR4 that coexpression of γ2 enlarges burst length and increases the probability of the channel to reach the highest conductance level (Tomita et al., 2005).
Up to now, modulatory effects of TARPs have almost exclusively been studied for γ2, whereas effects of other TARPs have not been described in detail. In this study, we investigate the influence of all known TARPs on agonist-induced currents of all AMPA receptors, including their flip/flop splice variants and editing forms, in Xenopus laevis oocytes. Although TARPs consistently increase current amplitudes, potentiation factors vary widely and depend strongly on the particular receptor–TARP combination. Coexpression of TARPs also causes an increase in the ratio of kainate- to glutamate-induced currents, a modulation strongly dependent on both the AMPA receptor and the associated TARP. We demonstrate that the extent of this increase is primarily a result of TARP-specific modulation of desensitization. We propose that the modulation of the extent of receptor desensitization is a major functional consequence of TARP association. Furthermore, we show that each TARP shifts the effective glutamate concentration to a different extent. In summary, these data suggest that electrophysiological properties of AMPA receptors are modulated by all four TARPs in a receptor subunit- and TARP-specific manner.
Materials and Methods
Cloning of rat calcium channel γ subunits.
cDNAs encoding the rat calcium channel γ subunits (γ2 is stargazin, γ3, γ4, and γ8) were cloned by reverse transcription-PCR. First-strand cDNA was synthesized from rat brain total RNA using a First-Strand cDNA Synthesis kit (Fermentas, St. Leon-Rot, Germany) or SuperScriptIII reverse transcriptase (Invitrogen, Karlsruhe, Germany). Oligonucleotide PCR primers were designed based on published rat γ subunit sequences (GenBank accession numbers AF361339 for γ2, AF361340 for γ3, AF361341 for γ4, and AF361346 for γ8) (Chu et al., 2001). The primers were tailed with either BamHI or XbaI restriction sites at the 5′ end and an EcoRI restriction site at the 3′ end to allow directed cloning into pSGEM, an expression vector for Xenopus oocytes (Villmann et al., 1997). To ensure full activity of the restriction sites, the palindromic sequences were capped by a random sequence of six nucleotides: γ2 sense, 5′-ATAGCGGGATCCATTATGGGGCTGTTTGATCGAGGT-3′; γ2 antisense, 5′-CGGCCGGAATTCTCATACGGGCGTGGTCCGGCGGTT-3′; γ3 sense, 5′-GGCGGCGGATCCATTATGAGGATGTGTGACAGAGGTAT-3′; γ3 antisense, 5′-CATCAGGAATTCTCAGACGGGCGTGGTGCG-3′; γ4 sense, 5′-CGCGACGGATCCACCATGGTGCGATGCGACCGC-3′; γ4 antisense, 5′-GGGCATGAATTCTCACACAGGGGTCGTCCGT-3′; γ8 sense, 5′-TTCGAGTCTAGAAAACTGGAGTCATTGAAACGCTGGA-3′; and γ8 antisense, 5′-GGGCGCGAATTCCTACACGGGCGTGGTTTTCCT-3′. After successful PCR, full-length cDNAs were cloned into pSGEM and verified by sequencing.
cRNA synthesis.
cRNA synthesis was performed as described previously (Villmann et al., 1999). Briefly, template DNA was linearized with a suitable restriction enzyme. cRNA was synthesized from 1 μg of linearized DNA using an in vitro transcription kit (Fermentas) with a modified protocol that uses 400 μm GpppG (GE Healthcare, Freiburg, Germany) for capping and an extended reaction time of 3 h with T7 polymerase. Trace labeling was performed with [α-32P]UTP to allow calculation of yields and evaluation of transcript quality by formaldehyde agarose gel electrophoresis.
Electrophysiological measurements in Xenopus laevis oocytes.
Frog oocytes of stages V or VI were surgically removed from the ovaries of Xenopus laevis (Nasco, Fort Atkinson, WI) anesthetized with 3-aminobenzoic acid ethylester (1.5 g/L; Sigma-Aldrich, Taufkirchen, Germany). Lumps of ∼20 oocytes were incubated for 1.5 h with 784 U/ml (4 mg/ml) collagenase type I (Worthington, Lakewood, NJ) in Ca2+-free Barth's solution (in mm: 88 NaCl, 1.1 KCl, 2.4 NaHCO3, 0.8 MgSO4, and 15 HEPES-NaOH, pH 7.6) with slow agitation to remove the follicular cell layer and then washed extensively with Barth's solution [in mm: 88 NaCl, 1.1 KCl, 2.4 NaHCO3, 0.3 Ca(NO3)2, 0.4 CaCl2, 0.8 MgSO4, and 15 HEPES-NaOH, pH 7.6]. Oocytes were maintained in Barth's solution supplemented with 100 μg/ml gentamicin, 40 μg/ml streptomycin, and 63 μg/ml penicillin. Defolliculated oocytes were injected with 2 ng of cRNA when receptor subunits were expressed alone and with 2.2 ng for coexpression experiments with TARPs (2 ng of AMPAR-cRNA and 0.2 ng of TARP-cRNA) using a nanoliter injector (World Precision Instruments, Sarasota, FL). In case of expression of heteromeric AMPA receptors, both cRNAs were mixed before injection and 2 ng of the mixture were injected per oocyte. Four to 5 d after injection, oocyte current responses were recorded in magnesium frog Ringer's solution (MgR) (in mm: 115 NaCl, 2.5 KCl, 1.8 MgCl2, and 10 HEPES-NaOH, pH 7.2) under voltage clamp at −70 mV holding potential with a TurboTec 10CX amplifier (npi Electronic, Tamm, Germany) controlled by Pulse software (HEKA Elektronik, Lambrecht, Germany). Recording pipettes were pulled from borosilicate glass (Hilgenberg, Malsfeld, Germany) using a PIP5 pipette vertical puller (HEKA Elektronik). Voltage electrodes had resistances of 1–4 MΩ and were filled with 3 m KCl; current electrodes had resistances of 0.5–1.5 MΩ and were filled with 3 m CsCl. Compounds were applied for 20 s by superfusion at a flow rate of ∼5 ml/min. Glutamatergic agonists (300 μm for Glu and 150 μm for KA) were prepared in MgR. To determine EC50 values for glutamate, 10–12 different agonist concentrations were applied to the same oocyte, and steady-state values of the evoked currents were measured. Data from each oocyte were fitted separately, and EC50 values obtained this way from four to six oocytes were averaged. Data presented here are reported as mean ± SEM. Statistical significance was determined with an unpaired Student's t test.
Labeling of cell surface proteins using biotinylated concanavalin A.
To identify the fraction of receptor protein inserted in the plasma membrane, glycosylated surface proteins were labeled with biotinylated concanavalin A (ConA) and isolated by streptavidin/agarose-mediated precipitation of the biotinyl-ConA–protein complex. Briefly, intact oocytes were incubated in 10 μm biotinyl-ConA (Sigma-Aldrich, Munich, Germany) for 30 min at room temperature. After five 10 min washes in normal frog Ringer's solution, intact oocytes were homogenized in H buffer [20 μl/oocyte; 100 mm NaCl, 20 mm Tris-HCl, pH 7.4, 1% Triton X-100, plus a mixture of proteinase inhibitors (Complete tablets; Roche Molecular Biochemicals, Mannheim, Germany)] and were kept at 4°C for 1 h on a rotating rod. After centrifugation at 16,000 × g for 15 min, the supernatants were supplemented with 20 μl of streptavidin/agarose beads (Fluka, Steinheim, Germany) and incubated at 4°C for 3 h on the rotating rod. The streptavidin/agarose beads were then pelleted by a 5 min spin at 16,000 × g and washed three times in H buffer. The final pellets were boiled in 20 μl of SDS-PAGE loading buffer (6 m urea, 0.8 m β-mercaptoethanol, 6% SDS, 20% glycerol, 25 mm Tris-HCl, pH 6.8, and 0.1% bromophenol blue).
Gel electrophoresis and Western blotting.
Proteins were separated by SDS-PAGE using the Mini-Protean 3 system (Bio-Rad, Munich, Germany) and then electroblotted onto Hybond ECL nitrocellulose membranes (GE Healthcare). The nitrocellulose membranes were blocked with 1× Roti-Block (Roth, Karlsruhe, Germany) in TBS-T (140 mm NaCl, 20 mm Tris-HCl, pH 7.6, and 0.1% Tween 20), and detection of proteins was performed using rabbit anti-GluR1, which was a kind gift from Richard L. Huganir (Department of Neuroscience, Howard Hughes Medical Institute, The Johns Hopkins University School of Medicine, Baltimore, MD), and HRP-conjugated goat anti-rabbit (Sigma-Aldrich, Munich, Germany) antibodies. Blots were developed using ECL solutions (Pierce, Rockford, IL).
Electrophysiological measurements in human embryonic kidney 293 cells.
Exponentially growing human embryonic kidney 293 (HEK293) cells were grown in Minimum Essential Medium Eagle, Joklik Modification (JMEM) (Sigma-Aldrich, Taufkirchen, Germany) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Paisley, UK) at 37°C, 8% CO2 in polyornithine-coated 35 mm dishes. At 1 h before transfection, the cell culture medium was changed to DMEM (Invitrogen) supplemented with 10% FBS. Transfections were done using a modified calcium phosphate precipitation technique (Chen and Okayama, 1987). Briefly, cells were transfected with 3.0 μg of DNA, with an equimolar ratio of AMPA receptor cDNA cloned into pcDNA3.0 and either γ2–enhanced cyan fluorescent protein (ECFP)/pECFP-N1 or pECFP-N1. Transfections were performed for 8 h at 37°C with 3% CO2. After incubation, the cells were washed with PBS before changing back to JMEM. After 48–72 h past transfection, whole-cell recordings were performed using an EPC-9 amplifier (HEKA Elektronik) controlled by Pulse 8.70 software (HEKA Elektronik). Currents were digitized with a sampling rate of 10 kHz and filtered at 3 kHz. Recording pipettes were pulled from borosilicate glass (GC150TL-10; Harvard Apparatus, Edenbridge, UK) using a PIP5 pipette vertical puller (HEKA Elektronik) and had resistances of 4–8 MΩ. Agonists (3 mm glutamate or 600 μm kainate) were prepared in extracellular solution and applied using a theta glass capillary (Hilgenberg) mounted on a piezo electric controller (Physik Instrumente, Karlsruhe, Germany) that bathed the suspended cell in a laminar flow for 200 ms. The extracellular solution consisted of 140 mm NaCl, 4 mm KCl, 2 mm CaCl2, 1 mm MgCl2, and 10 mm HEPES-NaOH, pH 7.3; the pipette solution consisted of 130 mm CsF, 4 mm NaCl, 1 mm MgCl2, 0.5 mm CaCl2, 11 mm EGTA, and 10 mm HEPES-KOH, pH 7.3. The current responses were measured at room temperature at a holding potential of −60 mV. Current responses were filtered at 1–2 kHz after recording.
Results
TARP-specific modulation of GluR1(Q)flip is caused by differential changes in electrophysiological properties and not receptor trafficking
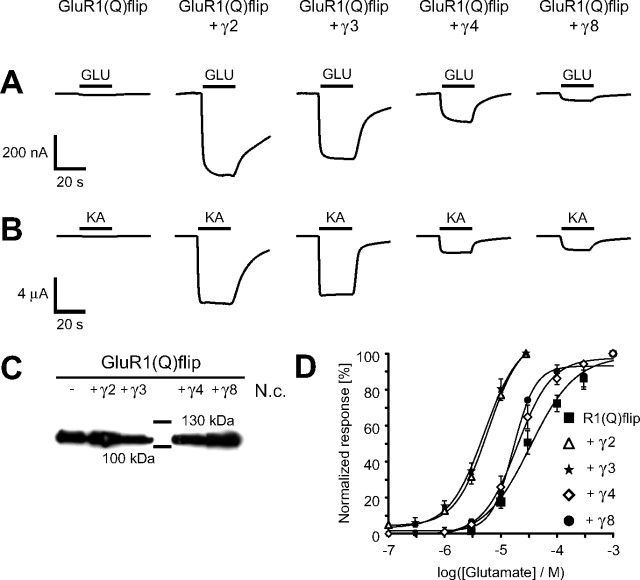
Coexpression of GluR1(Q)flip with each of the four TARPs resulted in a robust potentiation of glutamate- and kainate-evoked currents (Fig. 1A,B). Remarkably, this potentiation was strongly dependent on the particular TARP. γ2 caused the strongest potentiation of glutamate- and kainate-induced steady-state currents (23.1 ± 11.3-fold, n = 6, for glutamate and 71.8 ± 13.2-fold, n = 6, for kainate), whereas γ8 had the weakest effect (2.2 ± 0.4-fold for glutamate and 14.5 ± 3.2-fold for kainate, both n = 8) (Fig. 2B). To clarify whether the different potentiation factors caused by each TARP were a consequence of differences in surface expression of GluR1(Q)flip subunits, we analyzed the amount of plasma membrane-resident GluR1(Q)flip. Western blots revealed that γ2 and γ8 led to an equally modest increase of approximately twofold in GluR1(Q)flip protein in the plasma membrane of Xenopus laevis oocytes, whereas coexpression of γ4 had no effect on the amount of surface-incorporated GluR1 and coexpression of γ3 even led to a small decrease of surface-incorporated GluR1(Q)flip (Fig. 1C). These findings were confirmed in three independent experiments, so that random oocyte variability can be excluded as the reason for the observed expression levels.
Figure 1.
Coexpression of TARPs differentially enlarges agonist-induced currents of GluR1(Q)flip. A, B, Glutamate-induced (A) and kainate-induced (B) responses of GluR1(Q)flip alone and in combination with each of the four TARPs were recorded from Xenopus oocytes in magnesium Ringer's solution. The application of agonists (300 μm Glu or 150 μm KA) is indicated by black bars. C, Western Blot analysis of surface membrane preparations (for details, see Methods and Materials) from Xenopus laevis oocytes expressing homomeric GluR1(Q)flip alone and in coexpression with each of the four members of the TARP family. Uninjected oocytes (N.c.) served as control for cross-reactivity of the antibodies. D, Dose–response curves for the agonist l-glutamate were determined from oocytes injected with cRNAs of GluR1(Q)flip alone and in combination with each of the four TARPs. Values are means ± SEM (n = 4–6), normalized to the maximal responses. The EC50 values are given in Results.
Figure 2.
Increase in agonist-induced currents of AMPA receptors during coexpression of TARPs. Glutamate-induced (300 μm; black columns) and kainate-induced (150 μm; gray columns) responses of AMPA receptors alone and in combination with each of the four TARPs were recorded from Xenopus oocytes in magnesium Ringer's solution. In the top row, GluR1 (A, B) and GluR2 (C, D) are depicted compared with GluR3 (E, F) and GluR4 (G, H) in the bottom row. For quantification, agonist-induced currents of all eight AMPA receptors in coexpression with a TARP were normalized to the responses mediated by the respective AMPA receptor subunit expressed without TARP. Data are shown ± SEM (n = 5–12). *p < 0.05; **p < 0.01; ***p < 0.005 (Student's t test) compared with the respective AMPA receptor subunit expressed without TARP.
The limited alteration in plasma membrane-incorporated GluR1(Q)flip cannot explain some of the larger potentiation factors of agonist-induced currents. Moreover, the increase in current amplitudes differed strongly between the tested agonists glutamate and kainate. Each of the four TARPs potentiated kainate-induced currents much stronger than glutamate-induced currents, resulting in an increase in the ratio of kainate- to glutamate-induced currents (IKA/IGlu ratio) (Table 1). This shift in the IKA/IGlu ratio was dependent on the coexpressed TARP, leading to IKA/IGlu ratios between 3.2 ± 0.6 (n = 6, +γ4) and 15.0 ± 5.1 (n = 6, +γ2) compared with 1.9 ± 0.3 (n = 5) determined for GluR1(Q)flip expressed in the absence of any TARP. It has been described previously that γ2 shifts the EC50 values for glutamate and kainate to lower concentrations (Yamazaki et al., 2004; Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005). Here, we determined that all four TARPs reduce EC50 values for glutamate and to different extents (Fig. 1D). γ2 and γ3 behave similarly, shifting the EC50 for glutamate from 37.2 ± 9.0 μm (n = 4) determined for GluR1(Q)flip alone to 6.0 ± 0.6 μm (n = 5; p < 0.01) and 5.3 ± 0.7 μm (n = 4; p < 0.01), respectively. Coexpressions of γ8 or γ4 led to a considerably less pronounced shift of the EC50 for glutamate compared with γ2 or γ3, with 20.3 ± 3.3 μm [n = 4; p < 0.05 compared with GluR1(Q)flip and p < 0.005 compared with GluR1(Q)flip + γ2] for γ4 and 16.8 ± 0.4 μm [n = 3; p < 0.05 compared with GluR1(Q)flip and p < 0.005 compared with GluR1(Q)flip + γ2] for γ8.
Table 1.
Coexpression of TARPs leads to a shift of IKA/IGlu ratios of homomerically expressed AMPA receptor subunits
| GluR1(Q)o | GluR1(Q)i | GluR2(Q)o | GluR2(Q)i | GluR2(R)i | GluR3(Q)o | GluR3(Q)i | GluR4(Q)o | GluR4(Q)i | |
|---|---|---|---|---|---|---|---|---|---|
| — | 4.2 ± 0.6 | 1.9 ± 0.3 | 5.2 ± 1.4 | 0.6 ± 0.1*** | 0.7 ± 0.1 | 1.3 ± 0.3 | n.d.a | 17.8 ± 5.1 | 3.7 ± 0.8 |
| γ2 | 15.7 ± 4.0** | 15.0 ± 5.1* | 28.3 ± 6.5** | 1.4 ± 0.1*** | 1.3 ± 0.1*** | 33.3 ± 8.4* | 16.4 ± 4.3 | 215 ± 26*** | 22.6 ± 2.5*** |
| γ3 | 17.2 ± 7.1* | 8.8 ± 2.2* | 18.0 ± 4.4* | 2.0 ± 0.1*** | 1.3 ± 0.0*** | 17.6 ± 8.6* | 15.5 ± 6.0 | 180 ± 22*** | 29.4 ± 3.8*** |
| γ4 | 2.0 ± 0.3* | 3.2 ± 0.6 | 4.9 ± 0.8 | 1.2 ± 0.1*** | 1.7 ± 0.3* | 7.8 ± 3.2* | 4.9 ± 0.3 | 27.8 ± 3.1 | 4.7 ± 0.5 |
| γ8 | 13.7 ± 2.8*** | 10.0 ± 1.3** | 12.7 ± 2.6* | 1.4 ± 0.1*** | 2.7 ± 0.2*** | 5.5 ± 0.2*** | 5.7 ± 0.5 | 69.6 ± 11.9*** | 7.4 ± 0.8** |
The ratios of kainate- to glutamate-induced currents (IKA/IGlu ratio) were calculated for each oocyte and averaged (±SEM). Concentrations of the applied agonists were 150 μm for kainate and 300 μm for glutamate. GluR1(Q)o, GluR1(Q)flop; GluR2(R)i, GluR2(R)flip. *p < 0.05; **p < 0.01; ***p < 0.005. n.d., Not determined.
aBecause of small agonist-induced currents, IKA/IGlu ratios could not be calculated; therefore, no significances could be determined for the ratios in this column.
Differential impact of the flip/flop domains of AMPA receptors on the TARP-mediated increase in agonist-induced currents
Next, we analyzed whether the TARP-specific impact on agonist-induced currents may depend on the splice variant of the associated AMPA receptor. Similar to GluR1(Q)flip, GluR1(Q)flop-mediated currents were increased by all members of the TARP family and, as observed for GluR1(Q)flip, to different extents (Fig. 2A). γ2, γ3, and γ4 boosted glutamate-induced currents at the GluR1(Q)flop splice variant most effectively, whereas coexpression of γ8 resulted in a relatively small current potentiation. Kainate-induced currents were affected maximally by γ2 and γ3 (∼220-fold for γ2 and 300-fold for γ3). Coexpression with γ4 or γ8 resulted in a less potent increase in kainate-induced currents but still produced potentiation factors of ∼100-fold. In contrast to the flip variant, glutamate-induced responses of GluR1(Q)flop were more strongly potentiated by γ2 and γ4 than kainate-induced currents (Fig. 2A). In general, the increase of agonist-induced currents by TARPs was larger for GluR1(Q)flop than for GluR1(Q)flip. Depending on the coexpressed TARP, the difference between potentiation factors of GluR1(Q)flop compared with GluR1(Q)flip varied from 3-fold to 10-fold for kainate-induced currents and from ∼2-fold to 30-fold for glutamate-induced currents.
An even more pronounced difference in the TARP influence on agonist-induced currents can be seen between the flop and flip splice variants of GluR2. The impact of TARPs on kainate-induced currents of GluR2(Q)flop was ∼30-fold larger than for the flip isoform coexpressed with the same TARP (Fig. 2C,D). For glutamate-induced currents, the difference between potentiation factors for the flop and flip isoforms was even larger, at values ∼100-fold.
However, despite the larger impact of TARPs on the flop isoforms of GluR1 and GluR2, steady-state agonist-induced currents of the flop isoforms still remained smaller than those of the respective flip isoforms when coexpressed with the same TARP. Consequently, coexpression of a TARP decreases the difference between the steady-state amplitudes of agonist-induced currents of flop and flip isoforms.
The differences in modulation of GluR1(Q) and GluR2(Q) by TARPs suggested an AMPA receptor subunit-dependent mechanism of modulation. Therefore, we extended our investigation to the other unedited AMPA receptor subunits, GluR3 and GluR4, analyzing flip and flop splice variants of each subunit.
In contrast to the results for GluR1 and GluR2, TARPs enhanced currents mediated by the flip variant of GluR3 more strongly than currents of the flop variant (Fig. 2E,F). A second difference from GluR1 and GluR2 was that glutamate-induced currents mediated by GluR3(Q)flop were potentiated very weakly compared with kainate-induced currents (Fig. 2E). In contrast, glutamate-induced currents of GluR3(Q)flip were potentiated higher or at least to the same extent as kainate-induced currents (Fig. 2F). Also, potentiation factors of kainate-induced currents were approximately fivefold and those of glutamate-induced currents up to 30-fold larger for GluR3(Q)flip than for GluR3(Q)flop. Larger potentiation factors for the flip variants than for the flop variants were not observed for any other AMPA receptor subunit, which indicates to be a unique property of GluR3. Another factor distinguishing GluR3 from GluR1 and GluR2 is the impact of γ3. This TARP increases current amplitudes of GluR1 and GluR2 strongly, to an extent comparable with γ2, whereas it enlarges agonist-induced currents of GluR3 to a lesser extent, comparable with the potentiation by γ4. For GluR3, as shown before for GluR1 and GluR2, γ8 was the least efficient of all TARPs with respect to the potentiation of agonist-induced currents.
Glutamate-induced currents of GluR4(Q)flop were enhanced very weakly by coexpression of any TARP, similar to what was observed for GluR3(Q)flop (Fig. 2G). Kainate-induced currents, however, were increased efficiently, and, in contrast to GluR3(Q)flop, γ3 had a similar potency as γ2 to enlarge kainate-induced currents of GluR4(Q)flop. This preferential enhancement of kainate-induced currents seen for GluR4(Q)flop resulted in a dramatic increase of the IKA/IGlu ratios, which to this extent was not seen with any other homomerically expressed AMPA receptor (Table 1). The largest shift in the IKA/IGlu ratio was observed for the combination of GluR4(Q)flop and γ2, as evidenced by an increase from 17.8 ± 5.1 (n = 10) determined for GluR4(Q)flop expressed without any TARP to 215.3 ± 26.7 (n = 12). For the GluR4(Q)flip subunit, IKA/IGlu ratios were also increased during coexpression with a TARP but to a lesser extent than for the flop isoform. The largest shift was seen for the combination with γ3, which led to a value of 29.4 ± 3.8 (n = 10) compared with 3.7 ± 0.8 (n = 10) determined for GluR4(Q)flip expressed without any TARP (Table 1). Although impacts of TARPs on glutamate-induced currents (very weakly enhanced) and kainate-induced currents (strongly enhanced) were similar for GluR4(Q)flop and GluR3(Q)flop, we did not observe a comparable impact of TARPs on the flip splice variants of GluR4(Q) and GluR3(Q) (Fig. 2F,H). TARPs are known to decrease the extent of receptor desensitization (Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005), thus increasing glutamate-induced steady-state currents of AMPA receptors. Therefore, we investigated whether the very weak enhancement of glutamate-induced steady-state currents of GluR3(Q)flop and GluR4(Q)flop by γ2 is correlated to a similarly weak inhibition of receptor desensitization. Therefore, we analyzed current responses of GluR3(Q)flop and GluR4(Q)flop in the presence or absence of γ2 by whole-cell patch-clamp recordings in HEK293 cells (Fig. 3A). We found a clear increase of ∼80-fold (n = 3–5) in the kainate-induced peak currents of GluR3(Q)flop coexpressed with γ2 compared with GluR3(Q)flop expressed alone. Additionally, the extent of desensitization of GluR3(Q)flop was not significantly altered by coexpression of γ2 (Fig. 3B). For the other examined subunit GluR4(Q)flop, we observed similar results: kainate-induced peak currents were potentiated ∼140-fold (n = 6), and the extent of desensitization was not significantly altered by coexpression of γ2 (Fig. 3B). Thus, the results of patch-clamp recordings from HEK293 cells confirmed the weak potentiation of glutamate-induced and the large potentiation of kainate-induced steady-state currents of GluR3(Q)flop and GluR4(Q)flop by coexpression of TARPs observed in Xenopus oocytes.
Figure 3.
Patch-clamp analysis of desensitization of GluR3(Q)flop and GluR4(Q)flop. A, Glutamate-induced whole-cell responses of GluR3(Q)flop in the presence and absence of γ2 were recorded from HEK293 cells. The application of agonist (3 mm glutamate) is indicated by black bars. B, Quantification of the extent of desensitization [100% − (Isteady state/Ipeak) × 100%] of GluR3(Q)flop and GluR4(Q)flop in both the presence and absence of γ2. Data are shown ± SEM (n = 3–6).
Influence of the Q/R site amino acid on the TARP-mediated enhancement of agonist-induced currents
Next, we focused on the potential influence of the amino acid at the Q/R editing site on TARP-mediated current modulation. Therefore, we compared the influence of TARPs on the physiologically edited AMPA receptor subunit GluR2(R)flip and the engineered, unedited subunit GluR2(Q)flip. Although agonist-induced currents of both isoforms were increased by each TARP, responses of GluR2(R)flip showed a much stronger potentiation than those of GluR2(Q)flip (Figs. 2D, 4A). For GluR2(R)flip, the ability of the TARPs to enhance agonist-induced currents was pronounced for γ2 and γ3 and weak for γ8 and γ4. Potentiation factors for glutamate-induced currents ranged from 22.7 ± 10.7 (n = 8) for coexpression with γ4 to 227.6 ± 65.7 (n = 7) for coexpression with γ2 (Fig. 4A). For kainate-induced currents the potentiation factors ranged from 34.6 ± 14.4 (n = 8) when GluR2(R)flip was coexpressed with γ4 to 306.5 ± 84.1 (n = 7) during coexpression with γ2. Compared with GluR2(R)flip, the potentiation factors for GluR2(Q)flip were ∼20-fold smaller (Fig. 2D). TARPs had only little influence on the IKA/IGlu ratios of both unedited (Q) and edited (R) GluR2flip, leading to a small shift from 0.7 ± 0.1 determined for GluR2(R)flip (n = 5) to 2.7 ± 0.2 determined for GluR2(R)flip in coexpression with γ8 (n = 8) (Table 1).
Figure 4.
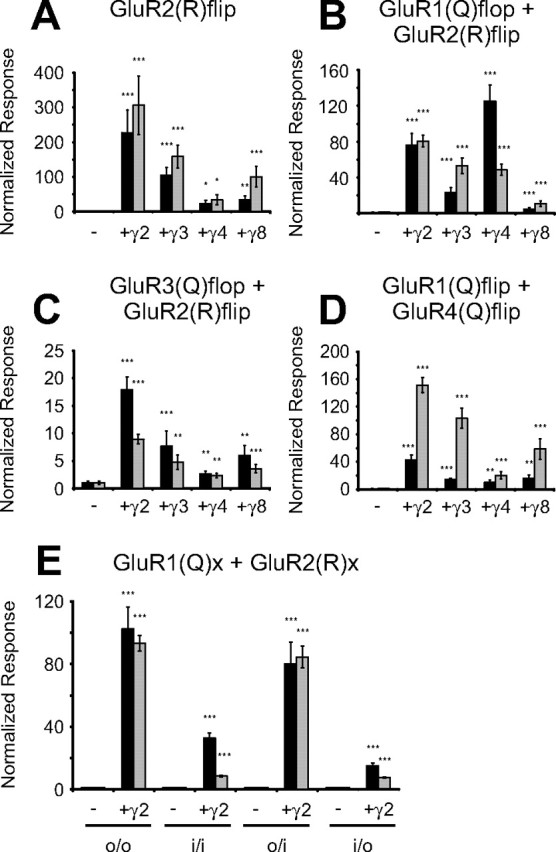
Comparison of the current amplitude-modulating effects of TARPs on GluR2(R)flip and several heteromeric AMPA receptors. A–D, Identical amounts of GluR2(R)flip (A), GluR1(Q)flop/GluR2(R)flip (B), GluR3(Q)flop/GluR2(R)flip (C), and GluR1(Q)flip/GluR4(Q)flip (D) cRNAs were coinjected with each TARP, and agonist-induced currents were recorded 4–6 d after injection. Glutamate-induced (300 μm; black columns) and kainate-induced (150 μm; gray columns) responses of AMPA receptors alone and in combination with each of the four TARPs were recorded from Xenopus oocytes in magnesium Ringer's solution. Normalization was performed as described in Figure 2 (*p < 0.05; **p < 0.01; ***p < 0.005). E, Glutamate-induced (300 μm; black columns) and kainate-induced (150 μm; gray columns) responses of heteromeric GluR1(Q)/GluR2(R) combinations in the absence and presence of γ2 were recorded from Xenopus oocytes. Normalization was performed as described in Figure 2 (*p < 0.05; **p < 0.01; ***p < 0.005 compared with the respective heteromeric AMPA receptor expressed without γ2). The given combination of contributing splice variants is indicated by the abbreviations listed below the x-axis: o/o, GluR1(Q)flop/GluR2(R)flop; i/o, GluR1(Q)flip/GluR2(R)flop.
Modulation of heteromeric AMPA receptor assemblies by TARPs depends on the subunit composition
In the CNS of vertebrates, AMPA receptors are generally expressed as heteromers mainly built from combinations of GluR1 or GluR3 with an edited GluR2 variant (Wenthold et al., 1996). In addition, functional receptor complexes in most cases contain both splice isoforms (Mansour et al., 2001; Brorson et al., 2004). Therefore, we examined three physiologically relevant combinations of AMPA receptor subunits, GluR1(Q)flop with GluR2(R)flip, GluR3(Q)flop with GluR2(R)flip, and GluR1(Q)flip with GluR4(Q)flip, in coexpression with each of the four TARPs (Fig. 4B–D). The assembly of heteromeric receptor complexes containing both unedited (Q) and edited (R) receptor variants was verified by analysis of current–voltage relationships. Only oocytes that showed a linear current–voltage relationship with reversal potentials ranging from 0 to −10 mV were included in the statistics. Oocytes showing glutamate-induced currents below 20 nA were excluded from the statistics because of potential expression of homomeric GluR2(R)flip. The rectifying current–voltage relationships of GluR1(Q)flip/GluR4(Q)flip could not be used to prove heteromeric assembly. Therefore, the assembly of GluR1(Q)flip with GluR4(Q)flip was confirmed by comparing the amplitudes of agonist-induced responses of coexpressed subunits to responses measured for homomerically expressed GluR1(Q)flip and GluR4(Q)flip receptors in the same preparation of oocytes.
A clear enhancement of agonist-induced currents by TARPs was observed for the coexpression of GluR1(Q)flop with GluR2(R)flip (Fig. 4B). To our surprise, γ4 increased glutamate-induced currents stronger than all other TARPs (125 ± 18-fold; n = 10). The increase of glutamate-induced currents was weaker for γ2 (76 ± 13-fold; n = 14; p < 0.05 compared with γ4) and weakest for γ3 and γ8, with potentiation factors of 23 ± 5 (n = 10) for γ3 and 5 ± 1 (n = 18) for γ8. Kainate-induced currents of GluR1(Q)flop/GluR2(R)flip were potentiated strongest by γ2 (80 ± 7-fold; n = 11) and to a slightly smaller extent by γ3 and γ4 (53 ± 9-fold for γ3 and 49 ± 6-fold for γ4; both n = 10; p < 0.01 compared with γ2). Coexpression of γ8 had the weakest influence on kainate-induced currents (11 ± 3-fold; n = 18). Simultaneously, the influence of TARPs on the IKA/IGlu ratios of GluR1(Q)flop/GluR2(R)flip varied from a decrease observed for the coexpression with γ4 to a small increase observed for γ3 and γ8 (Table 2). Next, we investigated to which degree the impact of TARPs on heteromeric receptors such as GluR1(Q)/GluR2(R) is dependent on the splice variants of the contributing subunits. To this end, all possible combinations that can arise from GluR1(Q) and GluR2(R) in either the flop or the flip splice forms were tested in the presence or absence of γ2. We detected a clear dependence of the impact of γ2 on the splice variant composition of receptor complexes. As observed before for the homomerically expressed GluR1(Q) and GluR2(Q) subunits, agonist-induced currents of receptors composed of only flop-spliced subunits were more strongly potentiated by γ2 than the responses of flip-spliced receptors (Fig. 4E). The impact of γ2 on heteromeric receptors containing both splice variants depended on which AMPA receptor subunit was flip spliced (Fig. 4E). In contrast to the nearly equal enlargement of glutamate- and kainate-induced currents of GluR1(Q)flop/GluR2(R)flip by coexpression of γ2, glutamate-induced currents of the combination GluR1(Q)flip/GluR2(R)flop were more strongly potentiated than kainate-induced currents (15 ± 2-fold for glutamate and 8 ± 0-fold for kainate; both n = 6). Not only the potentiation of agonist-induced currents but also the direction and the extent of the shift in the ratio between kainate- and glutamate-induced currents caused by coexpression of γ2 were dependent on the splice variant composition of heteromeric receptors. For GluR1(Q)/GluR2(R) heteromers composed of only flop variants, as described previously for GluR1(Q)flop/GluR2(R)flip, we detected no significant change in the IKA/IGlu ratios when γ2 was coexpressed (Table 3). When GluR1(Q)flip contributed to the heteromeric complex, we observed a decrease of the IKA/IGlu ratios during coexpression of γ2. Thus, our data are in line with a previous report by Turetsky et al. (2005), who also showed a reduction of IKA/IGlu ratios by γ2 (if steady-state currents are compared) of selected splice variant combinations of heteromeric receptors built from GluR1(Q) and GluR2(R). However, that study did not include the combination of GluR1(Q)flop/GluR2(R)flip investigated here, which showed no reduction of the IKA/IGlu ratio by γ2 but, as demonstrated previously, showed a strong dependence on the coexpressed TARP in the shift of IKA/IGlu ratios.
Table 2.
Effects of TARPs on IKA/IGlu ratios of heteromeric AMPA receptors
Table 3.
The splice variant combination in heteromeric receptor complexes determines the impact of γ2 on IKA/IGlu ratios
For the heteromeric combination of GluR3(Q)flop/GluR2(R)flip, we detected a clear potentiation by all four TARPs (Fig. 4C). γ2 enhanced glutamate-induced currents 17.9 ± 2.3-fold (n = 6) and kainate-induced currents 8.9 ± 0.9-fold (n = 6). γ3, γ4, and γ8 potentiated agonist-induced currents of GluR3(Q)flop/GluR2(R)flip heteromers weaker than γ2 [p < 0.05 compared with GluR3(Q)flop/GluR2(R)flip + γ2]. Interestingly, for the combination GluR3(Q)flop/GluR2(R)flip, TARPs caused equal or larger potentiation of glutamate-induced currents than of kainate-induced currents, similar to what we had observed previously for GluR3(Q)flip. Strikingly, both contributing subunits, GluR2(R)flip and GluR3(Q)flop, did not show such a behavior when they were investigated individually as homomerically expressed receptors (Figs. 2E, 4A). Coexpression of TARPs dropped the IKA/IGlu ratio of GluR3(Q)flop/GluR2(R)flip from 13.1 ± 1.2 (n = 9) (without TARPs) to values between 11.4 ± 0.7 (γ4; n = 8) and 6.7 ± 0.4 (γ2; n = 6) (Table 2).
For the heteromeric combination GluR1(Q)flip/GluR4(Q)flip, we observed strongly enlarged kainate-induced currents during coexpression with a TARP, with potentiation factors ranging from 20.2 ± 5.1 (γ4; n = 10) to 151.4 ± 11.3-fold (γ2; n = 9). The enlargement of glutamate-induced currents of GluR1(Q)flip/GluR4(Q)flip was most pronounced for γ2 (42.4 ± 7.3-fold; n = 9), whereas the other TARPs caused a smaller potentiation of glutamate-induced currents (Fig. 4D). We saw a TARP-dependent increase in the IKA/IGlu ratios for this combination (Table 2). The strongest shift was induced by coexpression with γ3, which increased the ratio from 2.6 ± 0.8 (n = 11) to 17.8 ± 1.9 (n = 9).
The extent of TARP-mediated reduction of AMPA receptor desensitization is primarily determined by the interacting TARP
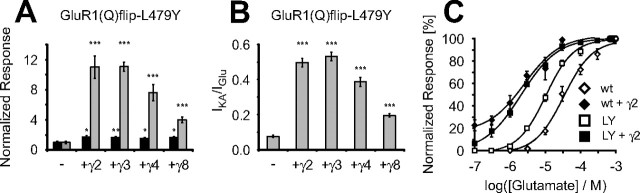
Coexpression of γ2 (and also γ3) has been reported to result in altered electrophysiological properties of AMPA receptors, predominantly by decreasing receptor desensitization (Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005), and by increasing the relative efficacy for the partial agonist kainate (Tomita et al., 2005). To allow for a quantitative estimation of these two effects, we analyzed the action of γ2 on the virtually nondesensitizing L479Y mutant of GluR1(Q)flip (Stern-Bach et al., 1998; Sun et al., 2002). In a recent publication, it was stated that γ2 led to an increase in surface-expressed receptor protein for the flip-spliced variant of GluR1–L479Y but, at the same time, did not increase glutamate-induced currents (Tomita et al., 2007). However, this conclusion was derived from measurements performed with a nonsaturating concentration of glutamate, and glutamate-induced currents of the mutant construct had smaller amplitudes than the investigated wild-type subunit, a finding that cannot be reconciled with the well known characteristics of the LY mutant. In our hands, GluR1(Q)flip–L479Y evoked larger glutamate-induced responses than the wild type. Furthermore, agonist-induced currents of this receptor mutant are actually enlarged by coexpression with γ2, because glutamate-induced currents were increased by a factor of 1.7 ± 0.2 (n = 6) and kainate-induced currents by a factor of 11.0 ± 1.5 (n = 5). Therefore, we conclude from our data that the L479Y mutation in GluR1 does not lead to an alternative mechanism of TARP interaction as postulated by Tomita et al. (2007). To support this conclusion, we analyzed the three other TARPs in combination with GluR1(Q)flip–L479Y. Glutamate-induced currents were enlarged by all TARPs to an equal extent, whereas kainate-induced currents were enlarged strongly by γ2 and γ3, less by γ4 (p < 0.05 compared with coexpression with γ2), and only weakly by γ8 (p < 0.01 compared with coexpression with γ4) (Fig. 5A). The observed proportionally stronger enhancement of kainate- versus glutamate-induced currents of GluR1(Q)flip–L479Y led to an increase in the IKA/IGlu ratio from 0.08 ± 0.01 to 0.50 ± 0.02 for γ2 and to 0.53 ± 0.02 for γ3 (both n = 5) (Fig. 5B). Correlating with their weaker impact on kainate-induced currents, γ4 shifted the IKA/IGlu ratio to a value of 0.39 ± 0.03 (n = 6; p < 0.05 compared with coexpression with γ2) and γ8, 0.20 ± 0.01 (n = 5; p < 0.005 compared with coexpression with γ4) (Fig. 5B). Because receptor desensitization is virtually abolished by the introduction of the L479Y mutation (Stern-Bach et al., 1998; Sun et al., 2002) and because all TARPs lead to a similar increase in glutamate-induced currents of GluR1(Q)flip–L479Y, our observations indicate that the TARP-dependent differences in potentiation of glutamate-induced currents for the wild-type subunit must be attributable to different levels of TARP-mediated inhibition of receptor desensitization. Additionally, the increase of kainate-induced currents was slightly dependent on the coexpressed TARP, even in the nondesensitizing mutant, which emphasizes again that TARP modulation is an agonist-dependent mechanism. The increase of kainate-induced currents is one of the prominent effects of the electrophysiological modulation of AMPA receptors by TARPs. However, the diverse IKA/IGlu ratios of GluR1(Q)flip–L479Y in coexpression with the different TARPs indicates that the so-called apparent efficacy for the partial agonist kainate is not enlarged to a degree comparable with the full agonist glutamate, because the IKA/IGlu ratios have values below one.
Figure 5.
Influence of TARPs on the desensitization properties of AMPA receptors. A, Xenopus laevis oocytes injected with 2 ng of GluR1(Q)flip–L479Y cRNA, and 0.2 ng of TARP cRNA were recorded in magnesium Ringer's solution 4–5 d after injection. Glutamate-induced (black columns) and kainate-induced (gray columns) responses of GluR1(Q)flip–L479Y alone and in combination with each of the four TARPs were recorded. To quantify the increase in agonist-induced currents, agonist-induced currents without coexpression of a TARP were set to 1 for GluR1(Q)flip–L479Y (mean absolute current responses, 4270 ± 644 nA for glutamate and 341 ± 53 nA for kainate). Data are shown ± SEM (n = 5–7). B, Ratios of kainate- to glutamate-induced currents were calculated for five to seven oocytes and averaged. Data are shown ± SEM. C, Dose–response curves for the agonist l-glutamate were determined from oocytes expressing GluR1(Q)flip or GluR1(Q)flip-L479Y in the presence or absence of γ2. Values are means ± SEM (n = 4–6), normalized to the maximal responses. The EC50 values are given in Results.
Previous publications (Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005) correlated the leftward shift in the dose–response relationship of AMPA receptors by γ2 with the inhibition of desensitization. We determined the EC50 values for glutamate for GluR1(Q)flip and GluR1(Q)flip–L479Y expressed alone and in combination with γ2. As described previously (Stern-Bach et al., 1998), introduction of the L479Y mutation led to a reduction of the EC50 value for glutamate compared with the wild-type subunit from 37.2 ± 9.0 μm (n = 4) to 10.4 ± 0.9 μm [n = 3; p < 0.05 compared with GluR1(Q)flip] (Fig. 5C). Contrary to Priel et al. (2005), we found an additional decrease of the EC50 value for glutamate from 10.4 ± 0.9 to 2.2 ± 0.7 μm [both n = 4; p < 0.005 compared with GluR1(Q)flip–L479Y] when γ2 was coexpressed with GluR1(Q)flip–L479Y. Notably, this EC50 value was virtually identical to the one obtained for the simultaneously investigated wild-type subunit in coexpression with γ2 (Fig. 5C).
Another approach to block desensitization in AMPA receptors is the application of 100 μm cyclothiazide (CTZ) in addition to the agonist glutamate. Cyclothiazide is known to virtually completely block desensitization of flip variants of AMPA receptors (Partin et al., 1993, 1994). We detected that the potency of cyclothiazide to increase glutamate-induced currents of GluR1(Q)flip is reduced when a TARP is coexpressed, and this influence was specific for each TARP (Fig. 6A,B). This finding was surprising because Tomita et al. (2006) observed no γ2-induced alteration of the potency of CTZ for the flip-spliced variant of GluR1. In our hands, CTZ potentiated glutamate-induced currents 57 ± 15-fold (n = 5) when GluR1(Q)flip was expressed without any TARP. Coexpression with γ8 reduced this potentiation by CTZ to 32 ± 7-fold (n = 5), which was the weakest impact of TARPs on the potency of CTZ (Fig. 6B). Coexpression of γ4 resulted in the largest reduction of potency of CTZ, reducing the potentiation factor to 7.1 ± 0.6-fold (n = 7). γ2 and γ3 had intermediate and comparable effects on the potency of CTZ (19 ± 2-fold for γ2 and γ3; both n = 7). It should be noted that CTZ potentiated glutamate-induced responses to the same absolute amplitudes regardless of the coexpressed TARP, which themselves caused different basal current levels in the absence of CTZ. Additionally, coapplication of cyclothiazide and glutamate resulted in smaller IKA/IGlu+CTZ ratios for GluR1(Q)flip and also for each coexpression with a TARP compared with the respective IKA/IGlu ratios (Fig. 6C). The largest reduction was seen for the IKA/IGlu ratio determined for the combination of GluR1(Q)flip and γ2, which was reduced from 14.5 ± 1.4 to 0.8 ± 0.0 in presence of cyclothiazide (both n = 7), whereas the smallest reduction was seen for the combination with γ4 because the IKA/IGlu ratio was reduced from 2.4 ± 0.2 to 0.3 ± 0.0 (both n = 7) (Fig. 6C). The ranking and values of the IKA/IGlu+CTZ ratios are nearly identical to the values of the IKA/IGlu ratio determined for the GluR1(Q)flip–L479Y mutant in coexpression with each of the four TARPs, which indicates that both the introduction of the LY mutation and the application of CTZ have equal impacts on the interaction of TARPs with AMPA receptors.
Figure 6.
The impact of cyclothiazide on GluR1(Q)flip depends on the associated TARP. A, B, Glutamate-induced responses of AMPA receptors alone and in combination with each of the four TARPs were recorded in Xenopus laevis oocytes in the presence or absence of 100 μm CTZ. For GluR1(Q)flip and GluR1(Q)flip + γ2, responses are illustrated in the presence (gray trace; bottom scale on the y-coordinate) and in the absence (black trace; top scale on the y-coordinate) of CTZ (A). IGlu+CTZ/IGlu ratios were calculated for each oocyte and averaged [± SEM; **p < 0.01; ***p < 0.005 compared with GluR1(Q)flip; B]. C, Ratios of kainate- to glutamate-induced currents were calculated for five to eight oocytes and averaged. Gray columns indicate IKA/IGlu ratios, and white columns show IKA/IGlu ratios determined with 300 μm glutamate in the presence of 100 μm CTZ. Data are shown ± SEM. Note the different scales on the y-axis and the break in y-axis and columns.
In summary, our data imply that the modulation of electrophysiological properties of AMPA receptors critically depends on both the coexpressed receptor subunit and the TARP. Furthermore, agonist-induced currents of AMPA receptors of the flop isoform are more strongly increased than currents of the flip isoform (except for GluR3). Additionally, we observed a stronger enlargement of agonist-induced currents by TARPs for the edited R isoform compared with the unedited Q isoform. Heteromeric AMPA receptor combinations were found to be modulated by TARPs in an individual manner, because the impact of TARPs on heteromeric receptors does not reflect their impact on the contributing individual homomeric subunits. Coexpression of TARPs with AMPA receptors shifted the ratio of kainate- to glutamate-induced currents to higher values but to different extents depending on both the coexpressed AMPA receptor and TARP. The shift of the IKA/IGlu ratio was strongest in the case of coexpression with γ2 or γ3 and weakest for coexpression with γ4. γ8 had an intermediate effect on this ratio. We demonstrated that the ability to shift the IKA/IGlu ratio correlates mainly with the degree of inhibition of AMPA receptor desensitization by coexpressed TARPs and to a much lesser extent with the increase of the effectiveness of the partial agonist kainate. Also, we found a pronounced decrease in the EC50 for the physiological agonist glutamate, the degree of which was dependent on the coexpressed TARP.
Discussion
We establish that each of the four members of the TARP family is capable of potentiating agonist-induced responses at all four AMPA receptors, flip as well as flop splice variants, and Q as well as R editing versions. We show that TARP-mediated increase of cell surface receptor expression in Xenopus oocytes does not cause the observed large increase in currents, implying that most of the increase results from TARP-specific modulation of electrophysiological receptor properties. The extent of that modulation primarily depends on the individual receptor/TARP combination. For most homomerically expressed AMPA receptor subunits, γ2, γ3, and γ4 were the most effective TARPs in increasing glutamate-induced currents. For kainate-induced currents, γ2 and γ3 were most effective, whereas γ8 had the weakest effect with both agonists. Thus, TARP efficiencies are essentially independent of the associated AMPA receptor subunits and reflect mainly TARP properties. AMPA receptors and TARPs share a restricted regional and developmental distribution pattern throughout the CNS (Tomita et al., 2003; Beneyto and Meador-Woodruff, 2004). However, observed electrophysiological properties of distinct AMPA receptor/TARP combinations could not be correlated with a known codistribution pattern of AMPA receptors and TARPs. AMPA receptors underlie developmental modifications such as Q/R editing and alternative splicing. During embryogenesis, low levels of unedited GluR2 can be detected, and GluR1 is mostly spliced to the flip variant (Burnashev et al., 1992; Nutt and Kamboj, 1994; Jakowec et al., 1995). However, we could not detect any striking differences in the modulation of these receptor variants in coexpression with γ4, the predominant TARP in this developmental period (Tomita et al., 2003), compared with the modulation of subunits predominantly expressed in the adult.
The flip/flop domain: a structural determinant for TARP efficacy
Each given TARP led to distinct current potentiation factors, varying with the receptor subunits (Fig. 2). For kainate, and more strongly glutamate, currents of flop-spliced receptor variants were increased more than those of the corresponding flip variants. This observation extends previous reports for γ2 (Priel et al., 2005; Tomita et al., 2005) and γ3 (Turetsky et al., 2005) that these two TARPs decrease AMPA receptor desensitization. Our data imply that all TARPs decrease receptor desensitization and show a generally larger impact on the faster desensitizing (Mosbacher et al., 1994) flop-spliced receptors, with the notable exception of GluR3. Although GluR3(Q)flop desensitizes fivefold faster than GluR1(Q)flop, this cannot explain the unique behavior of GluR3(Q)flop because GluR4(Q)flop possesses comparable desensitization properties (Mosbacher et al., 1994). R/G editing (Lomeli et al., 1994) can also be ruled out because both variants of GluR2 contain the same amino acid (glycine) at this position as GluR3. Differences between GluR3 and other AMPA receptors have been reported for the flop-specific desensitization inhibitor PEPA (2,6-difluoro-4-[2-(phenylsulfonylamino)ethylthio]-phenoxyacetamide) (Sekiguchi et al., 2002). Because interacting molecules can distinguish between AMPA receptors, TARPs could potentially exploit those same molecular differences. These findings emphasize the importance of specific AMPA receptor subunits for TARP-mediated modulation.
Q/R site amino acid and subunit composition of heteromeric receptors influence TARP-dependent modulation
Because the edited GluR2(R)flip is potentiated much stronger than the unedited GluR2(Q)flip, this may suggest that TARPs lead to an inherently more pronounced modification of electrophysiological properties of R-edited versus unedited (Q) subunits, possibly a result of more efficient protein–protein interactions.
Our analysis of heteromeric AMPA receptors revealed that TARP effects on heteromers cannot be predicted from effects on homomers. In particular, TARP effects on heteromeric receptors are neither the sum nor the mean of their effects on homomeric receptors but differ individually. This dramatically increases the complexity of receptor modulation by TARPs and AMPA receptor functional diversity.
New insights into the interplay between TARPs and the modulatory effects of CTZ and the L479Y mutation
We demonstrated that the L479Y mutation in GluR1(Q) does not alter effects of γ2 on glutamate-induced currents, a finding contradicting Tomita et al. (2007). For this virtually nondesensitizing mutant, we determined an approximately twofold potentiation of glutamate-induced currents by γ2. This potentiation is fully explained by increased surface receptor expression and possibly a small inhibition of residual receptor desensitization.
We further demonstrated a TARP-dependent, variable decrease in the potency of the desensitization inhibitor CTZ. These findings contradict previous reports that γ2 does not decrease the potency of CTZ for GluR1flip (Tomita et al., 2006). The decrease observed here could be attributable to a TARP dependency of CTZ efficacy or to a TARP-dependent decrease in receptor desensitization, because a strong inhibition of receptor desensitization would result in a reduced apparent potency of CTZ. We favor the second explanation but cannot rule out the first or a combination of both.
TARP-induced reduction of receptor desensitization depends on TARPs as well as on AMPA receptors
TARP-mediated modulation of electrophysiological properties has been attributed to two distinct mechanisms: reduction of desensitization and increase in apparent kainate efficacy (Tomita et al., 2005; Turetsky et al., 2005). Both mechanisms influence the ratio of kainate- to glutamate-induced currents. This ratio increases when the relative kainate efficacy increases but decreases when receptor desensitization is reduced, which enlarges glutamate-induced currents. We observed a distinctly TARP-specific increase in IKA/IGlu ratios, implying that the increase in apparent kainate efficacy outweighs any TARP-mediated reduction in receptor desensitization (Table 1). We tested whether high IKA/IGlu ratios were correlated with strong TARP-dependent increases in kainate-induced currents. For γ2 and γ3, this correlation mostly held up but not for γ4 and γ8 (Fig. 2, Table 1). Despite causing the smallest increase in kainate-induced responses for most AMPA receptors, γ8 robustly increased IKA/IGlu ratios. Thus, there is no correlation between a high IKA/IGlu ratio and the TARP-dependent increase in kainate-induced currents. The second possible factor, a TARP-dependent decrease in receptor desensitization, was investigated by expressing the virtually nondesensitizing GluR1(Q)flip–L479Y mutant with each of the four TARPs (Fig. 5A). Additionally, we coapplied the desensitization inhibitor cyclothiazide plus glutamate to all four GluR1(Q)flip/TARP combinations (Fig. 6). Both approaches yielded identical results, because glutamate-induced currents were increased to the same level independently of the TARP. The increase in kainate-induced currents, however, showed a distinct dependency on the coexpressed TARP (Fig. 5A).
We conclude that each TARP decreases wild-type receptor desensitization to a different extent, thus explaining the observed diverse TARP-mediated increases in IKA/IGlu ratios. For example, γ4 in coexpression with almost all AMPA receptors led to the smallest increase in the IKA/IGlu ratios of all TARPs (Table 1). However, γ4 does not cause the weakest increase in kainate efficacy among TARPs (Fig. 5A); a weak increase in kainate efficacy can be ruled out as an explanation. The small increases in wild-type IKA/IGlu ratios therefore imply a counteracting strong decrease in receptor desensitization. This may constitute a functional correlation with the peculiar expression of γ4 during development that differs from all other TARPs (Tomita et al., 2003). Our findings also suggest that TARP-mediated modulation of AMPA receptors is strongly dependent on the applied agonist. It might be speculated that the agonist-dependent modulation of AMPA receptors is based on multiple interaction sites between AMPA receptors and TARPs, which contribute differentially in any given AMPA receptor/TARP combination. Additionally, it is possible that, in certain combinations, not all potential interaction sites contribute to the subunit interface.
The largest TARP-mediated increases in IKA/IGlu ratios were seen for the fast-desensitizing receptor subunits GluR3(Q)flop and GluR4(Q)flop (Fig. 3, Table 1) because of a very weak potentiation of glutamate-induced currents coinciding with a large increase in kainate-induced currents (Fig. 2E,G). Thus, the desensitization properties of AMPA receptors may also influence the effectiveness of TARPs as ion channel modulators.
TARP modulation of agonist EC50 values suggests a mechanism for TARP/AMPA receptor interaction
We showed that all TARPs cause different reductions of the EC50 for glutamate (Fig. 1D). It had been stated that γ2 does not decrease the low (compared with wild type) EC50 for glutamate at the GluR1–L479Y mutant and that therefore the γ2-dependent decrease in the EC50 of wild-type GluR1 must be exclusively based on reduced receptor desensitization (Priel et al., 2005). To our surprise, we found a distinct additional decrease in the EC50 for glutamate of GluR1–L479Y when this mutant was coexpressed with γ2 (Fig. 5C). We conclude that the reduction of receptor desensitization by TARPs cannot be the exclusive reason for the leftward shift in the dose–response relationships. We postulate that the association of a TARP directly or indirectly causes an efficiency-enhancing conformational change of either the ligand binding domain of the AMPA receptor or its gating domains.
This study provides additional insight into the complex modulation of electrophysiological properties of AMPA receptors, particularly desensitization and ligand efficacies, by TARPs. The degree of modulation is crucially dependent on both interaction partners and especially on key structural properties of the receptor subunit such as the flip/flop domain and the amino acid located at the Q/R editing site. Importantly, heteromeric AMPA receptor complexes are TARP-specifically modulated according to their subunit and also splice variant composition. This illuminates the importance of TARPs for the complexity of AMPA receptor-mediated synaptic transmission in the CNS.
Footnotes
We thank Petra Wahle (Department of General Zoology and Neurobiology, Developmental Neurobiology Group, Ruhr University Bochum) for providing rat brain tissue. We also thank Daniel Tapken for critical reading of this manuscript and Björn Peters for expert oocyte preparations.
References
- Beneyto M, Meador-Woodruff JH. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol. 2004;468:530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Li D, Suzuki T. Selective expression of heteromeric AMPA receptors driven by flip-flop differences. J Neurosci. 2004;24:3461–3470. doi: 10.1523/JNEUROSCI.5023-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chu PJ, Robertson HM, Best PM. Calcium channel gamma subunits provide insights into the evolution of this gene family. Gene. 2001;280:37–48. doi: 10.1016/s0378-1119(01)00738-7. [DOI] [PubMed] [Google Scholar]
- Cognet L, Groc L, Lounis B, Choquet D. Multiple routes for glutamate receptor trafficking: surface diffusion and membrane traffic cooperate to bring receptors to synapses. Sci STKE. 2006;327:pe13. doi: 10.1126/stke.3272006pe13. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Hollmann M. Structure of ionotropic glutamate receptors. In: Jonas P, Monyer H, editors. Handbook of experimental pharmacology, Vol 141, Ionotropic glutamate receptors in the CNS. Berlin: Springer; 1999. pp. 3–98. [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Calcium permeability of KA-AMPA gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann S. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Yen L, Kalb RG. In situ hybridization analysis of AMPA receptor subunit gene expression in the developing rat spinal cord. Neuroscience. 1995;67:909–920. doi: 10.1016/0306-4522(95)00094-y. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring RB, Clements JD, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Kamboj RK. Differential RNA editing efficiency of AMPA receptor subunit GluR2 in human brain. NeuroReport. 1994;5:1679–1683. doi: 10.1097/00001756-199408150-00034. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Mayer ML. Cyclothiazide differentially modulates desensitization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol. 1994;46:129–138. [PubMed] [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate-receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Nishikawa K, Aoki S, Wada K. A desensitization-selective potentiator of AMPA-type glutamate receptors. Br J Pharmacol. 2002;136:1033–1041. doi: 10.1038/sj.bjp.0704804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–134. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Tomita S, Sekiguchi M, Wada K, Nicoll RA, Bredt DS. Stargazin controls the pharmacology of AMPA receptor potentiators. Proc Natl Acad Sci USA. 2006;103:10064–10067. doi: 10.1073/pnas.0603128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci USA. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villmann C, Bull L, Hollmann M. Kainate binding proteins possess functional ion channel domains. J Neurosci. 1997;17:7634–7643. doi: 10.1523/JNEUROSCI.17-20-07634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villmann C, Strutz N, Morth T, Hollmann M. Investigation by ion channel domain transplantation of rat glutamate receptor subunits, orphan receptors and a putative NMDA receptor subunit. Eur. J Neurosci. 1999;11:1765–1778. doi: 10.1046/j.1460-9568.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]