Abstract
Ethanol and opiate self-administration are sensitive to manipulations of cannabinoid CB1 receptor function and, from this, a role for the endogenous cannabinoid system in the modulation of drug reward has been hypothesized. However, direct in vivo evidence of drug-induced alterations in brain endocannabinoid (eCB) formation has been lacking. To address this issue, we explored the effect of drug self-administration on interstitial eCB levels in the nucleus accumbens (NAc) shell using in vivo microdialysis. Ethanol, heroin, and cocaine were compared because the rewarding properties of ethanol and heroin are reduced by CB1 receptor inactivation, whereas cocaine reward is less sensitive to these manipulations. Ethanol self-administration significantly increased dialysate 2-arachidonoylglycerol (2-AG) levels with no concomitant change in dialysate anandamide (AEA) concentrations. Conversely, heroin self-administration significantly increased dialysate AEA levels, and induced a subtle but significant decrease in dialysate 2-AG levels. In each case, the relative change in dialysate eCB content was significantly correlated with the amount of drug consumed. In contrast, cocaine self-administration did not alter dialysate levels of either AEA or 2-AG. Local infusion of the CB1 antagonist SR 141716A into the NAc significantly reduced ethanol, but not cocaine, self-administration. Together with our previous observation that intra-NAc SR 141716A reduces heroin self-administration, these data provide novel in vivo support for an eCB involvement in the motivational properties of ethanol and heroin but not cocaine. Furthermore, the selective effects of ethanol and heroin on interstitial 2-AG and AEA provide new insight into the distinct neurochemical profiles produced by these two abused substances.
Keywords: anandamide, 2-AG, SR 141716A, CB1 receptor, microdialysis, addiction
Introduction
Converging evidence from human and animal studies implicates the endogenous cannabinoid system in the etiology of drug addiction. Endocannabinoids (eCBs) participate in long-term synaptic plasticity in several neural circuits that mediate the motivational effects of abused drugs, and it has been hypothesized that these neural adaptations participate in the development of compulsive drug use (Berke and Hyman, 2000; Gerdeman et al., 2002), as supported by the correlation between a genetic disruption of eCB clearance mechanisms and problem drug and alcohol use by humans (Sipe et al., 2002). Genetic deletion of cannabinoid CB1 receptors in mice results in reduced ethanol and morphine self-administration and attenuated ethanol- and opiate-induced place conditioning (Ledent et al., 1999; Hungund et al., 2003; Houchi et al., 2005). Similarly, in rats, the CB1 receptor antagonist SR 141716A (rimonabant) reduces ethanol and opiate self-administration (Caille and Parsons, 2003; Solinas et al., 2003; Colombo et al., 2005). Based on these observations, it has been suggested that drug-induced increases in eCB formation participate in the mediation of drug reward.
Consistent with this theory is evidence that repeated noncontingent drug administration results in altered levels of the endogenous cannabinoids N-arachidonoylethanolamide (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) in postmortem rat brain tissue (Gonzalez et al., 2004; Vigano et al., 2004). However, it is not clear whether these findings reflect sustained changes in brain eCB levels induced by chronic drug administration, or whether ongoing drug intake acutely alters eCB formation. Moreover, noncontingent drug administration can produce neurochemical, proteomic, and genomic effects that are substantially different from those induced by free-choice self-administration (Jacobs et al., 2003), and brain tissue eCB levels are robustly affected by rapid postmortem increases in eCB formation (Bazinet et al., 2005; Patel et al., 2005). Thus, although repeated noncontingent drug administration results in altered eCB levels in postmortem brain tissue, it remains unclear whether drug self-administration induces acute changes in interstitial eCB content that are consistent with an eCB involvement in mediating drug-related behaviors.
In this study, we explored the effect of operant drug self-administration on interstitial eCB levels in the shell subregion of the nucleus accumbens (NAc) using in vivo microdialysis in rats. The effects of ethanol, heroin, and cocaine self-administration were compared because the rewarding properties of ethanol and heroin are reduced by CB1 receptor inactivation, whereas cocaine reward is less sensitive to these manipulations (Martin et al., 2000; Cossu et al., 2001; Lesscher et al., 2005). The NAc shell was chosen because it is critically involved in mediating the rewarding properties of each of these drugs (Di Chiara, 2002, 2004). Previous work has shown that local CB1 antagonist administration into the NAc reduces heroin self-administration (Caille and Parsons, 2006). To extend these findings, we evaluated the effects of intra-NAc SR 141716A infusion on ethanol and cocaine self-administration. We report here that 2-AG and AEA levels are differentially altered in the NAc during ethanol and heroin self-administration, respectively, whereas neither eCB is altered during cocaine self-administration. Moreover, we found that intra-NAc CB1 antagonist administration alters ethanol and heroin self-administration but not cocaine self-administration.
Materials and Methods
Subjects
Fifty-two male Wistar rats (225–250 g; Charles River Laboratories, Wilmington, MA) were used. For the microdialysis tests, the group sizes for each self-administered drug were as follows: ethanol (n = 9), heroin (n = 7), and cocaine (n = 8). For the intra-NAc drug infusion tests, the group sizes for each self-administered drug were as follows: ethanol (n = 11) and cocaine (n = 10). Microdialysates were collected from seven drug-naive control rats for evaluation of potential alterations in baseline eCB levels induced by drug self-administration training. All animals were housed in groups of three in a temperature-controlled vivarium (22°C) with a 12 h light/dark cycle (lights off at 6 A.M.) and given ad libitum access to food and water. The studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals provided by the National Institutes of Health.
Drugs and reagents
Heroin hydrochloride and cocaine hydrochloride were obtained from the National Institute on Drug Abuse (Bethesda, MD) and were dissolved in a vehicle of sterile 0.9% saline. Ethanol (10% w/v) was prepared with 95% ethyl alcohol and water. Saccharin (Sigma, St. Louis, MO) was dissolved in water and used for the sweet solution fading procedure used to establish operant ethanol self-administration (Samson, 1986). SR 141716A was generously provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (Washington, DC) and was dissolved in a vehicle of ethanol:emulphor:saline (1:1:18). AEA, 2-AG, 1(3)-arachidonoylglycerol (1-AG), and (S)-(+)-arachidonyl-2′-hydroxy-1′-propylamide (S-2 methanandamide) were used as chromatographic standards and were from Cayman Chemical (Ann Arbor, MI). All other reagents were of the highest obtainable grade from Sigma-Aldrich (St. Louis, MO).
Surgery
Intravenous catheterization.
Rats to be trained to self-administer heroin or cocaine were prepared with chronic indwelling SILASTIC jugular catheters under isoflurane anesthesia (1.5–2.0%) as described previously (Caille and Parsons, 2004). Catheters were flushed daily with sterile heparinized saline (30 USP units/ml), and the animals were allowed a 7 d minimum of postoperative recovery before the initiation of self-administration training.
Intracerebral microdialysis cannulas
After operant self-administration training (see below), animals in the ethanol and cocaine groups were anesthetized (isoflurane 1.5–2.0% vapor) and implanted with a microdialysis guide cannula (SciPro, Sanborn, NY) aimed at the NAc shell [from bregma: anteroposterior (AP), +1.6 mm; mediolateral (ML), ±0.8 mm; and dorsoventral (DV), −6.0 mm from dura] (Paxinos and Watson, 1998). Because animals in the heroin self-administration group had skull-mounted catheter ports to minimize chewing (Caille and Parsons, 2004), these animals received NAc shell microdialysis guide cannulas at the time of the jugular catheter implantation. All animals received a minimum 5 d of postoperative recovery before experimentation.
Intracerebral infusion cannulas
Animals previously trained to self-administer either ethanol or cocaine were anesthetized (isoflurane 1.5–2.0% vapor) and implanted with a bilateral microinfusion guide cannula (22 gauge, 12 mm length, stainless steel) that terminated 3 mm above the ventral surface of the NAc (from bregma: AP, +1.6 mm; ML, ±2.0 mm; and DV, −5.0 mm from dura) (Paxinos and Watson, 1998). These coordinates are the same as those used previously to evaluate the effects of intra-NAc SR 141716A on heroin self-administration (Caille and Parsons, 2006) and provide injector placements at the interface between the shell and core (“shore”) of the NAc thereby allowing drug delivery without producing substantial damage to the region of interest after repeated infusions. A minimum 5 d of postoperative recovery were allowed before experimentation.
Drug self-administration training
Self-administration training and testing was performed in standard operant chambers housed in sound-attenuated and ventilated cubicles (Coulbourn Instruments, Allentown, PA) (for an additional description, see Caille and Parsons, 2006). Training sessions were conducted 5 d/week during the dark phase of the light cycle. For each drug, training continued until the total number of reinforcers per session stabilized to within ± 10% of the mean for 3 consecutive days (baseline criterion).
Operant ethanol self-administration was established under a fixed-ratio 1 (FR-1) timeout 20 s (TO-20 s) schedule of reinforcement using a sweet solution fading procedure slightly modified from Samson (1986). Lever pressing behavior was reinforced by delivery of 0.1 ml aliquots of liquid to a sipper cup for oral consumption, and the final ethanol reinforcer concentration was 10% (w/v). Ethanol self-administration sessions were 30 min in duration.
Animals in the heroin self-administration group were trained to intravenously self-administer unit doses of 20 μg/0.1 ml heroin under an FR-1 TO-20 s schedule of reinforcement. Animals in the cocaine group were trained to self-administer cocaine (0.25 mg/0.1 ml) under an FR-5 TO-20 s schedule of reinforcement. The heroin and cocaine self-administration sessions were each 2 h in duration.
In vivo microdialysis
On the morning of the microdialysis experiments, each animal was lightly anesthetized (1–2% isofluorane), and a microdialysis probe (2 mm polyethyl sulfone membrane, 15 kDa MW cutoff; SciPro) was inserted and secured to the previously implanted guide cannula. The probes were perfused with artificial CSF (0.6 μl/min) composed of the following (in mm): 149 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 0.25 ascorbic acid, 5.4 d-glucose, and with 30% (w/v) hydroxypropyl-β-cyclodextrin (HP-β-CD). Inclusion of HP-β-CD in the perfusate provides a substantial increase in the dialysis recovery of eCBs [similar to findings by Walker et al. (1999)]. Approximately 5 h after probe implantation, dialysate samples were collected at 10 min intervals over a 60 min baseline period and during subsequent operant drug self-administration.
Liquid chromatography/mass spectrometry analysis of dialysate eCB content
Dialysate levels of AEA, 2-AG, and 1-AG were determined using liquid chromatography coupled with electrospray ionization mass spectrometry. 2-AG is relatively unstable and readily converts to 1-AG during sample analysis (Rouzer et al., 2002). This was evident by a progressive decline in 2-AG signal accompanied by a progressive increase in 1-AG signal over time during the repeated analysis of 2-AG standards during method development (data not shown). To control for this, 2-AG and 1-AG peak areas were summed for all analyses reported here. Five microliter microdialysate aliquots were spiked with 5 μl of 100 nm of S-2 methanandamide and loaded onto a precolumn [0.5 × 2.5 mm, Haisil high load C18 column (HL C18), 5 μm; Higgins Analytical, Mountain View, CA) using a 30% MeOH (v/v) mobile phase delivered at 70 μl/min. After a 2 min wash period, mobile phase flow through the precolumn was reversed via a switching valve, and the eCBs were delivered to a 0.3 × 50 mm microbore analytical column (Haisil HL C18, 3 μm; Higgins Analytical) using an isocratic mobile phase consisting of 70% MeOH (v/v) delivered at 5 μl/min. The analytical column eluent was delivered via a nanoelectrospray interface into the mass spectrometer (1100MSD; Agilent Technologies, Santa Clara, CA) that was run in positive selected ion monitoring mode to maximize sensitivity. Similar to findings by others (Giuffrida et al., 2000), we find that sodium adducts of these molecules provide greater sensitivity than do their protonated forms. The following mass/charge ratios were used: AEA, 370.3 [molecular ion (M) + 1 Na]; 2-AG and 1-AG, 401.3 (M + 1 Na); S-2 methanandamide, 384.3 (M + 1 Na). External calibration curves were constructed from a minimum of three standard concentrations (each run in duplicate) and were generated daily. Under these conditions, the limits of quantitation were ∼0.1 nm for each analyte.
Intra-NAc SR 141716A testing
The effects of local administration of SR 141716A on ethanol and cocaine self-administration were evaluated in separate groups of animals. After establishment of stable self-administration behavior, the animals received an initial microinjector insertion (no liquid infusion) immediately before self-administration to acclimate them to the procedure and to produce the initial tissue damage from injector insertion. Subsequently, vehicle and SR 141716A infusions were made via bilateral 33 gauge microinjectors that extended 2 mm beyond the tip of the guide cannulas. Infusions of 1 μl per side were made over a 2 min period, followed by an additional 1 min to allow drug diffusion before injector removal. Stylets were then replaced in the guide cannulas, and the rats were allowed immediate access to drug self-administration. The effects produced by vehicle, 1.0, or 3.0 μg per side SR 141716A were evaluated, and the dose presentation was randomized between animals. Drug tests were performed at weekly intervals, and each animal was tested once with each dose.
Statistical analyses
Group differences in baseline dialysate AEA and 2-AG concentrations were evaluated by ANOVA with drug history as the between-subjects factor. Subsequently, dialysate AEA and 2-AG levels were transformed to percentages of average baseline dialysate concentration for evaluation of changes in dialysate eCB content during drug self-administration as performed by ANOVA with repeated measures over time. To investigate correlations between total drug intake and relative change in dialysate eCB content, the area under the curve (AUC) for AEA and 2-AG was calculated for each animal by subtracting 100 from each percentage of baseline data points and summing all data points collected during the experimental period (t = 0–120 min) (see Fig. 1). The relationship between self-administered drug intake and the AUC value for AEA or 2-AG was then determined using Pearson's parametric correlation. The effects of intra-NAc SR 141716A administration on ethanol and cocaine self-administration were evaluated using a within-subjects design with repeated measures ANOVA.
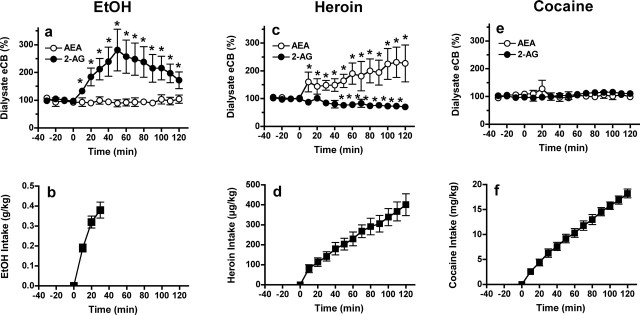
Figure 1.
Selective effects of ethanol, heroin, and cocaine self-administration on eCB levels in NAc shell microdialysates. Top, The effects of ethanol (EtOH; a), heroin (c), and cocaine (e) self-administration on dialysate AEA (open circles) and 2-AG (filled circles) levels expressed as the percentage of change from predrug baseline. Bottom, Cumulative drug intake during the self-administration sessions (b, ethanol 10% (w/v) oral intake; n = 9) (d, heroin 20 μg per infusion, i.v.; n = 7) (f, cocaine 0.25 mg per infusion, i.v.; n = 8). Error bars indicate SEM. *p < 0.05; relative to baseline.
Results
Drug history before microdialysis
Animals in the ethanol group received 65 ± 0.9 30 min self-administration training sessions to establish stable intake patterns before dialysis testing. The average daily ethanol intake during this training period was 0.40 ± 0.03 mg/kg, resulting in an average blood alcohol concentration of 28.6 ± 4.9 mg/dl, as determined during the self-administration training period. The average total ethanol intake for animals in this group was 26.3 ± 2.2 mg/kg before the dialysis test.
Animals in the heroin group received 22 ± 1.0 2 h training sessions with an average daily intake of 715 ± 79 μg/kg heroin and a total intake of 16 ± 1.6 mg/kg before the dialysis test. Animals in the cocaine group received 21 ± 0.2 2 h training sessions with an average daily intake of 19.5 ± 1.7 mg/kg and a total cocaine intake of 447 ± 18 mg/kg before the dialysis test.
Baseline dialysate AEA and 2-AG levels are unaltered as a function of previous drug self-administration history
Baseline AEA levels were 2.36 ± 0.5, 2.03 ± 0.4, and 2.6 ± 0.5 nm, and 2-AG levels in these same samples were 5.41 ± 1.0, 5.02 ± 0.9, and 6.02 ± 1.0 nm for the ethanol, heroin, and cocaine groups, respectively. In drug-naive animals, baseline AEA levels were 1.83 ± 0.22 nm, and 2-AG levels were 4.82 ± 0.51 nm. Baseline levels of either anandamide (F(3,27) = 0.613) or 2-AG (F(3,26) = 0.821) did not differ between any of the groups (including drug-naive controls) despite substantial drug exposure during self-administration training. This contrasts with reports of relatively sustained changes in brain tissue eCB content induced by repeated ethanol, morphine, and cocaine administration (Gonzalez et al., 2002, 2004; Vigano et al., 2004). These differential findings suggest either that repeated noncontingent bolus drug administration induces effects on brain eCB levels that are distinct from those induced by daily limited-access drug self-administration or that chronic drug exposure affects processes influencing postmortem eCB accumulation inherently reflected in analyses of brain tissue eCB content (Bazinet et al., 2005; Patel et al., 2005).
Ethanol, heroin, and cocaine self-administration induce specific alterations in NAc shell eCB levels
Animals in the ethanol group consumed 0.39 ± 0.03 g/kg ethanol during the microdialysis test session (Fig. 1b), and this produced a significant increase in dialysate 2-AG levels (F(8,16) = 4.836; p < 0.0001) with no concomitant alteration in AEA (F(8,16) = 1.129; NS) (Fig. 1a). The rise and fall of 2-AG levels followed a temporal pattern similar to the profile of blood alcohol concentrations after oral administration (Pastino and Conolly, 2000). The relative change in dialysate 2-AG levels was positively correlated with the amount of ethanol consumed (Fig. 2A).
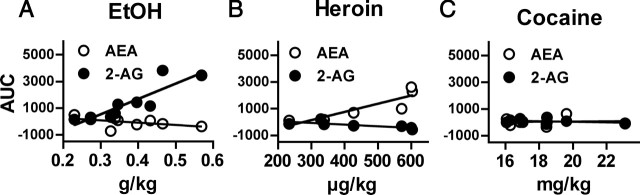
Figure 2.
Correlation between total drug intake and the relative change in dialysate AEA (open circles) and 2-AG (filled circles) during self-administration. Relative change in eCB levels calculated as the total AUC of the percentage of baseline data shown in Figure 1a, c, and e. A, Relative increases in dialysate 2-AG were significantly correlated with ethanol intake (r2 = 0.7683; p < 0.005) whereas changes in dialysate AEA were not (r2 = 0.2499). B, Relative increases in dialysate AEA (r2 = 0.7870; p < 0.01) and decreases in dialysate 2-AG (r2 = 0.6317; p < 0.05) were significantly correlated with heroin intake. C, There were no correlations between changes in either AEA (r2 = 0.0042) or 2-AG (r2 = 0.1166) and cocaine intake.
In contrast to ethanol, heroin self-administration (443 ± 61 μg/kg total intake) (Fig. 1d) was associated with a significant increase in dialysate AEA levels (F(6,15) = 3.465; p < 0.0001) and a slight but significant decrease in dialysate 2-AG levels (F(6,15) = 3.042; p < 0.0005) (Fig. 1c). The relative change in dialysate AEA levels was positively correlated with the amount of heroin consumed, and a significant negative correlation was found between heroin intake and changes in dialysate 2-AG (Fig. 2B).
There was no significant effect of cocaine self-administration (18 ± 0.9 mg/kg total intake) (Fig. 1f) on dialysate levels of either AEA (F(7,15) = 0.649; NS) or 2-AG (F(7,15) = 1.201; NS) (Fig. 1e) and no significant correlations between cocaine intake and relative changes in either AEA or 2-AG (Fig. 2C). Dialysis probe placements for each drug group are shown in Figure 3.
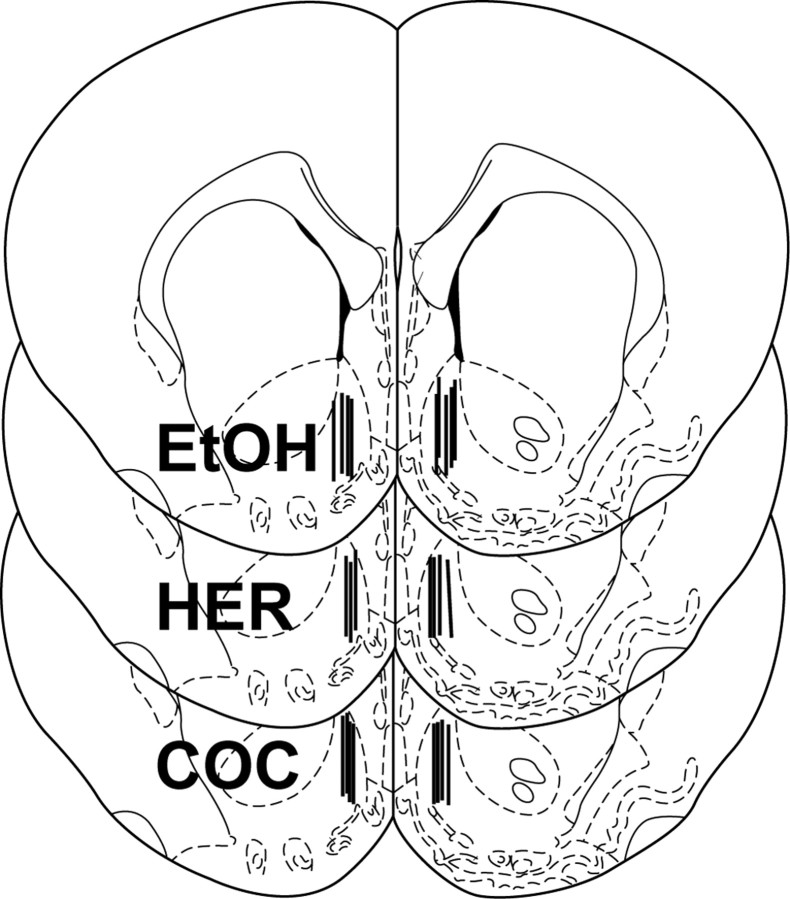
Figure 3.
Schematic representation of the dialysis probe placements for each of the drug self-administration tests shown in Figure 1. Each section represents a coronal brain slice 1.6 mm anterior to bregma (Paxinos and Watson, 1998), and each vertical bar corresponds to the location of the active microdialysis membrane area within the medial NAc shell. Separate sections are used to display the probe placements in the ethanol (EtOH; n = 9), heroin (HER; n = 7), and cocaine (COC; n = 8) self-administration groups, respectively.
Intra-NAc infusion of the CB1 antagonist SR 141716A reduces ethanol self-administration but not cocaine self-administration
During the final three self-administration sessions before the pretreatment tests, animals in the ethanol group obtained an average of 27.6 ± 2.9 ethanol reinforcers per session (corresponding to 0.38 ± 0.04 g/kg and resulting in 29.9 ± 4.6 mg/dl blood alcohol), and there was no significant effect of intra-NAc vehicle administration on ethanol intake. Subsequently, it was found that intra-NAc SR 141716A administration significantly decreased ethanol self-administration relative to the vehicle condition (F(2,20) = 6.409; p < 0.01) with significant decreases observed after administration of both 1 and 3 μg per side of SR 141716A (Fig. 4a).
Figure 4.
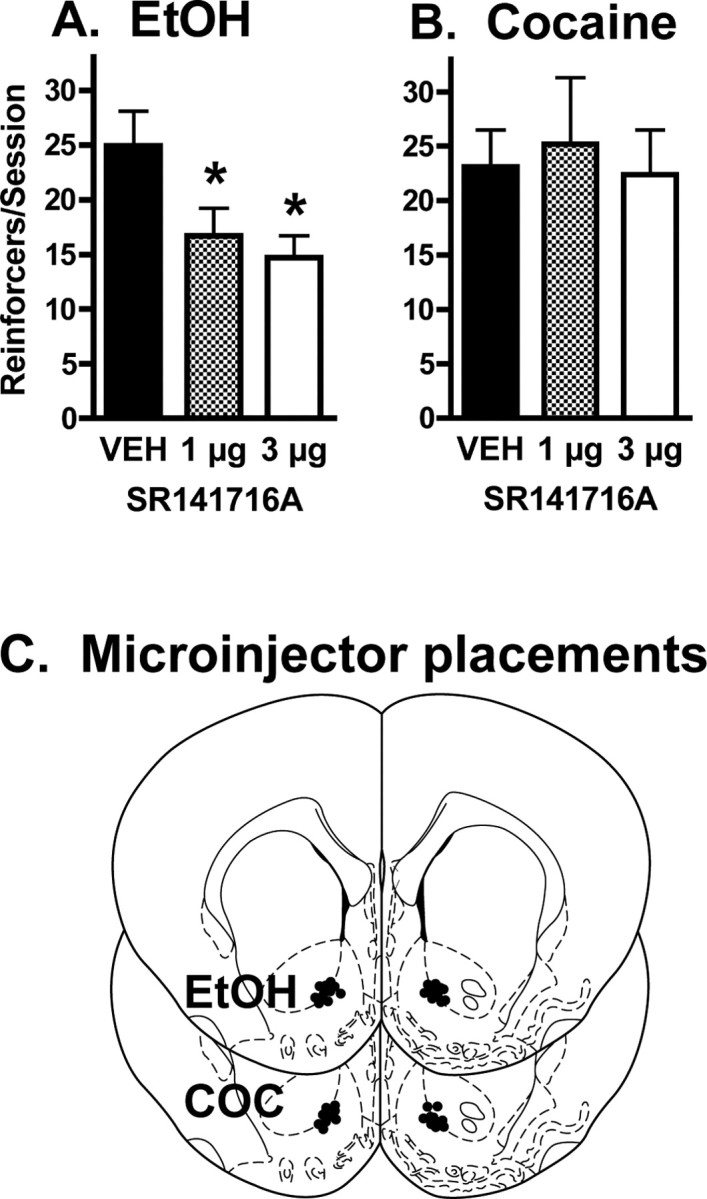
Effect of intra-NAc infusion of the CB1 receptor antagonist SR 141716A on ethanol and cocaine self-administration. A, Bilateral SR 141716A infusions into the NAc significantly reduced ethanol self-administration relative to vehicle infusions (F(2,20) = 6.409; p < 0.01), and significant reductions were produced by both 1 and 3 μg per side SR 141716A (n = 11). These results are comparable to the previously reported reduction in heroin self-administration induced by intra-NAc SR 141716A (Caille and Parsons, 2006). Error bars indicate SEM. *p < 0.05. B, In contrast, intra-NAc administration of these same SR 141716A doses produced no effect on cocaine self-administration (F(2,18) = 1.314; n = 10). Error bars indicate SEM. C, Placements of each NAc drug microinjector in the NAc. Each brain section represents a coronal slice 1.6 mm anterior to bregma (Paxinos and Watson, 1998), and each circle corresponds to the location of the microinjector tip within the NAc. Separate slices are shown for placements in the ethanol and cocaine (COC) self-administration experiments, respectively.
In contrast, intra-NAc SR 141716A administration did not alter cocaine self-administration. Under baseline conditions, these animals obtained an average of 26.5 ± 2.5 cocaine reinforcers per session (corresponding to 16.3 ± 2.0 mg/kg per session). There was no significant effect of intra-NAc vehicle administration on cocaine intake, and intra-NAc administration of 1 and 3 μg per side SR 141716A did not alter cocaine self-administration relative to the vehicle condition (F(2,18) = 1.314; NS) (Fig. 4b).
Discussion
Interstitial eCB levels in the NAc shell are altered by ethanol and heroin but not cocaine self-administration
The present data demonstrate that ethanol and heroin self-administration each induce transient alterations in interstitial eCB levels in the NAc shell, whereas cocaine self-administration does not. The increase in NAc eCB levels during ethanol and heroin self-administration suggests a role for NAc eCB transmission in the reinforcing effects produced by these drugs. This is also supported by the observation that intra-NAc CB1 antagonist administration decreases both ethanol and heroin self-administration (Caille and Parsons, 2006). These findings are consistent with reports that CB1 receptor-deficient mice display reduced ethanol and morphine self-administration compared with wild types (Ledent et al., 1999; Cossu et al., 2001; Hungund et al., 2003; Naassila et al., 2004) and that pharmacologic blockade of CB1 receptors reduces both ethanol and opiate self-administration by rats (Gallate and McGregor, 1999; Caille and Parsons, 2003; De Vries et al., 2003; Solinas et al., 2003; Colombo et al., 2005). CB1 receptor inactivation also attenuates ethanol- and opiate-induced neurochemical events involved in the mediation of drug reward (Hungund et al., 2003; Caille and Parsons, 2006). Moreover, high alcohol preference and excessive alcohol intake are associated with altered expression and function of CB1 receptors and fatty acid amidohydrolase, a primary enzyme responsible for eCB degradation (Basavarajappa and Hungund, 2001; Hansson et al., 2007). Collectively, these observations provide strong evidence for eCB involvement in the rewarding effects of ethanol and opiate self-administration.
In contrast, there has been little evidence to suggest an influence of eCB neurotransmission in the mediation of psychostimulant reward. CB1 receptor deletion in mice does not alter cocaine-induced place conditioning (Martin et al., 2000) and produces equivocal effects on psychostimulant self-administration (Cossu et al., 2001; Soria et al., 2005). CB1 antagonist pretreatment does not interfere with cocaine self-administration by rats (De Vries et al., 2001; Lesscher et al., 2005; Caille and Parsons, 2006) and does not alter cocaine-induced increases in NAc dopamine (Caille and Parsons, 2006; Xi et al., 2006). Thus, our observations that cocaine intake does not alter eCB levels in the NAc shell and that intra-NAc CB1 antagonist administration does not affect cocaine self-administration are consistent with the extant literature. Moreover, these findings provide an important negative control for the effects observed with ethanol and heroin self-administration.
Ethanol and heroin self-administration produce distinct effects on NAc AEA and 2-AG levels
Although cannabinoids potently modulate neural transmission in several brain regions involved in drug reward (Riegel and Lupica, 2004; Perra et al., 2005; Xi et al., 2006), it has been unclear which eCB mediators may be involved in modulating drug self-administration behavior. The present data reveal that ethanol self-administration increases interstitial 2-AG levels with no concurrent change in AEA levels, whereas heroin self-administration increases AEA levels and simultaneously decreases 2-AG levels. In each case, the relative change in dialysate eCB content was significantly correlated with the amount of drug consumed during self-administration.
The importance of these drug-specific effects on 2-AG and AEA formation remains to be elucidated. These two signaling molecules are characterized by somewhat distinct pharmacologic profiles at cannabinoid and noncannabinoid receptors and some ion channels (Sugiura et al., 2002; van der Stelt and Di Marzo, 2005). It is noteworthy that 2-AG is a full CB1 agonist, whereas AEA is a partial agonist at these receptors (Hillard, 2000). Accordingly, it is conceivable that the differential effects of ethanol and heroin on 2-AG and AEA formation underlie recent observations that ethanol self-administration is relatively more sensitive to manipulations of eCB clearance than is heroin self-administration (Solinas et al., 2005; Hansson et al., 2007).
Potential mechanisms for an eCB involvement in addiction
The observation that intra-NAc SR 141716A infusion reduces ethanol and heroin self-administration (Caille and Parsons, 2006) suggests that drug-induced increases in NAc eCB levels participate in ethanol and opiate reward through a CB1 receptor-mediated process. These receptors provide an inhibitory influence on GABAergic and glutamatergic neurotransmission in the NAc (Hoffman and Lupica, 2001; Manzoni and Bockaert, 2001; Robbe et al., 2001), and their activation is thought to reduce the excitability of efferent GABAergic medium spiny neurons. As such, drug-induced eCB formation in the NAc may decrease GABA release in regions innervated by the NAc such as the ventral tegmental area (VTA) and ventral pallidum. Decreased GABAergic input to the VTA likely disinhibits mesolimbic dopamine neurons, and thus activation of NAc CB1 receptors may contribute to ethanol reward by facilitating increases in mesolimbic dopamine release (Hungund et al., 2003; Perra et al., 2005). However, CB1 modulation of opiate reward appears to occur through dopamine-independent mechanisms (Caille and Parsons, 2003, 2006). Rather, opiate-induced decreases in ventral pallidal GABA levels are thought to contribute to the reinforcing effects of heroin (Bardo, 1998; Xi and Stein, 2000, 2002; Caille and Parsons, 2004), and recent evidence suggests that this effect is mediated in part through NAc CB1 receptors (Caille and Parsons, 2006). Interestingly, CB1 receptor antagonism does not alter cocaine-induced increases in NAc dopamine or cocaine-induced decreases in ventral pallidal GABA (Caille and Parsons, 2006), consistent with the presently reported lack of cocaine-induced alterations in NAc eCB levels.
eCBs may also participate in the development of habitual behaviors that are supplementary to actual drug-taking behavior. Several studies have reported a role for eCB signaling in activity-dependent long-term synaptic plasticity in several brain regions including the NAc (Robbe et al., 2002; Hoffman et al., 2003; Fourgeaud et al., 2004), and it has been hypothesized that this type of cellular adaptation contributes to the transition from casual drug use to the compulsive behavior that characterizes addiction (Berke and Hyman, 2000; Gerdeman et al., 2002). Given the proposed modulatory influence of eCBs on cortical input to the NAc (Robbe et al., 2001), it is possible that ethanol- and opiate-induced alterations in eCB formation contribute to this aspect of drug dependence. In this regard, it is now recognized that the core and shell subregions of the NAc participate in specific aspects of the addiction process, with the shell playing a greater role in the mediation of drug reward and the core participating in the conditioning and compulsive aspects of addiction (Cardinal et al., 2002; Di Chiara, 2002; Kalivas and McFarland, 2003; Koya et al., 2006). It is presently unknown whether drug intake induces a differential eCB response between the core and shell, although the widely reported influence of CB1 receptors in drug- and cue-induced drug-seeking behavior (Fattore et al., 2005; Xi et al., 2006) suggests possible eCB effects in the core subregion.
Finally, a recent report from Hansson et al. (2007) demonstrates a potential influence of frontal cortical eCB signaling in the phenotype of high voluntary ethanol consumption. Moreover, eCB mechanisms in the VTA may play a modulatory role in drug-induced activation of the mesolimbic reward circuit (Lupica and Riegel, 2005; Perra et al., 2005). Thus, it is likely that eCB mechanisms in a variety of brain regions influence the behavioral effects produced by ethanol and opiates.
Mechanisms for drug-specific alterations in NAc eCB formation
Several notable differences in the cellular effects produced by ethanol, heroin, and cocaine may contribute to the presently observed drug-specific alterations in NAc eCB formation. Although all three drugs increase NAc dopamine levels, they do so through distinct mechanisms. Ethanol and opiates potentiate dopamine release by increasing mesolimbic dopamine neuronal firing (Gysling and Wang, 1983; Matthews and German, 1984; Brodie et al., 1999), whereas cocaine inhibits dopamine reuptake mechanisms resulting in a prolonged increase in extracellular dopamine that decreases dopamine cell firing rates (Einhorn et al., 1988; Brodie and Dunwiddie, 1990; Lacey et al., 1990). It is possible that these distinct effects of ethanol and heroin versus cocaine on mesolimbic dopamine cell firing contribute to the drug-specific effects on NAc eCB formation. In addition, cocaine self-administration produces greater decreases in the firing rates of NAc medium spiny neurons than does heroin self-administration (Chang et al., 1998), and it is possible this effect of cocaine reduces depolarization-induced calcium currents necessary for eCB formation (Zhuang et al., 2005). It is notable, however, that NAc cell firing is increased during drug-free cocaine-seeking behavior (Peoples et al., 2004), and this may result in increased eCB formation that participates in the reinstatement of drug-seeking behavior (relapse) for a number of abused substances including cocaine (Fattore et al., 2005; Le Foll and Golberg, 2005; Xi et al., 2006). Finally, in light of the proposed involvement of metabotropic glutamate receptors in eCB formation (Maejima et al., 2001; Narushima et al., 2006), it is conceivable that cocaine-induced decreases in cell surface mGluR5 (metabotropic glutamate receptor-5) receptor expression (Swanson et al., 2001; Fourgeaud et al., 2004) precludes an effect of cocaine self-administration on NAc eCB formation.
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA014619 and National Institute on Drug Abuse Grant DA019962. We gratefully acknowledge the generous gift of SR 141716A provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program. This is publication 18171 from The Scripps Research Institute. We thank Michael Arends for editorial assistance.
References
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Cannabinoid receptor agonist-stimulated [35S]guanosine triphosphate gammaS binding in the brain of C57BL/6 and DBA/2 mice. J Neurosci Res. 2001;64:429–436. doi: 10.1002/jnr.1094. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem Res. 2005;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. Cocaine effects in the ventral tegmental area: evidence for an indirect dopaminergic mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:660–665. doi: 10.1007/BF00175709. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Caille S, Parsons LH. SR141716A reduces the reinforcing properties of heroin but not heroin-induced increases in nucleus accumbens dopamine in rats. Eur J Neurosci. 2003;18:3145–3149. doi: 10.1111/j.1460-9568.2003.02961.x. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. Eur J Neurosci. 2004;20:593–596. doi: 10.1111/j.1460-9568.2004.03497.x. [DOI] [PubMed] [Google Scholar]
- Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol Biochem Behav. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W. Cannabinoid CB(1) antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology. 2005;48:1097–1104. doi: 10.1016/j.neuropharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl) 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Valenti M, de Miguel R, Fezza F, Fernandez-Ruiz J, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in reward-related brain regions of alcohol-exposed rats, and their possible relevance to alcohol relapse. Br J Pharmacol. 2004;143:455–464. doi: 10.1038/sj.bjp.0705963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Actions of cocaine on rat dopaminergic neurones in vitro. Br J Pharmacol. 1990;99:731–735. doi: 10.1111/j.1476-5381.1990.tb12998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Narushima M, Hashimoto K, Kano M. Endocannabinoid-mediated short-term suppression of excitatory synaptic transmission to medium spiny neurons in the striatum. Neurosci Res. 2006;54:159–164. doi: 10.1016/j.neures.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Pastino GM, Conolly RB. Application of a physiologically based pharmacokinetic model to estimate the bioavailability of ethanol in male rats: distinction between gastric and hepatic pathways of metabolic clearance. Toxicol Sci. 2000;55:256–265. doi: 10.1093/toxsci/55.2.256. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Lynch KG, Lesnock J, Gangadhar N. Accumbal neural responses during the initiation and maintenance of intravenous cocaine self-administration. J Neurophysiol. 2004;91:314–323. doi: 10.1152/jn.00638.2003. [DOI] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer CA, Ghebreselasie K, Marnett LJ. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem Phys Lipids. 2002;119:69–82. doi: 10.1016/s0009-3084(02)00068-3. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat. 2005;77:111–122. doi: 10.1016/j.prostaglandins.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Vigano D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–1857. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Increased mesolimbic GABA concentration blocks heroin self-administration in the rat. J Pharmacol Exp Ther. 2000;294:613–619. [PubMed] [Google Scholar]
- Xi ZX, Stein EA. GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol. 2002;37:485–494. doi: 10.1093/alcalc/37.5.485. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Hampson RE, Deadwyler SA. Behaviorally relevant endocannabinoid action in hippocampus: dependence on temporal summation of multiple inputs. Behav Pharmacol. 2005;16:463–471. doi: 10.1097/00008877-200509000-00020. [DOI] [PubMed] [Google Scholar]