Abstract
Pyrrolidine dithiocarbamate (PDTC) is a clinically tolerated inhibitor of nuclear factor-κB (NF-κB), antioxidant and antiinflammatory agent, which provides protection in brain ischemia models. In neonatal hypoxia–ischemia model, PDTC activates Akt and reduces activation of glycogen synthase kinase 3β (GSK-3β). Because chronic inflammation, oxidative stress, and increased GSK-3β activity are features of Alzheimer's disease (AD) pathology, we tested whether PDTC reduces brain pathology and improves cognitive function in a transgenic animal model of AD. A 7 month oral treatment with PDTC prevented the decline in cognition in AD mice without altering β-amyloid burden or gliosis. Moreover, marked oxidative stress and activation of NF-κB were not part of the brain pathology. Instead, the phosphorylated form of GSK-3β was decreased in the AD mouse brain, and PDTC treatment increased the phosphorylation of Akt and GSK-3β. Also, PDTC treatment increased the copper concentration in the brain. In addition, PDTC rescued cultured hippocampal neurons from the toxicity of oligomeric Aβ and reduced tau phosphorylation in the hippocampus of AD mice. Finally, astrocytic glutamate transporter GLT-1, known to be regulated by Akt pathway, was decreased in the transgenic AD mice but upregulated back to the wild-type levels by PDTC treatment. Thus, PDTC may improve spatial learning in AD by interfering with Akt–GSK pathway both in neurons and astrocytes. Because PDTC is capable of transferring external Cu2+ into a cell, and, in turn, Cu2+ is able to activate Akt, we hypothesize that PDTC provides the beneficial effect in transgenic AD mice through Cu2+-activated Akt pathway.
Keywords: Alzheimer's disease, inflammation, glycogen synthase, phosphorylation, β-amyloid, learning and memory
Introduction
There is compelling evidence that aggregation of amyloid-β (Aβ) peptides Aβ1–42 and Aβ1–40 is a key mediator of the pathogenesis of Alzheimer's disease (AD), a dementing neurodegenerative disease. Aβ peptides are directly neurotoxic by mechanisms involving oxidative stress, mitochondrial dysfunction, apoptosis, and hyperphosphorylation of tau, a microtubule-associated protein that becomes dysfunctional with hyperphosphorylation, causing thereby neurofibrillary tangle formation and abnormal neuronal functions (Behl et al., 1994; Goodman and Mattson, 1994; Busciglio et al., 1995; Meda et al., 1995; Anderson et al., 1996; London et al., 1996; Estus et al., 1997). Aβ also disturbs normal synaptic functions (Davies and Maloney, 1976; Beach et al., 2000) and increases brain susceptibility to injury (Nakagawa et al., 1999, 2000; Lauderback et al., 2001; Koistinaho et al., 2002). In addition to its direct harmful effect on neurons, aggregated Aβ activates microglia and astrocytes (Akiyama et al., 2000) to secrete proinflammatory molecules, reactive oxygen species, and other neurotoxins, causing indirect neurotoxicity. Moreover, expression of glutamate transporter 1 (GLT-1), an astrocytic glutamate transporter maintaining glutamate at nontoxic concentrations, is decreased in the AD brain and in Aβ-treated astrocytes (Harris et al., 1996; Masliah et al., 1996, 2000; Liang et al., 2002). There are several hypotheses for the molecular mechanisms for these pathogenic cascades triggered by Aβ in neurons and glia, including the pathway mediated by glycogen synthase kinase 3β (GSK-3β) (Grimes and Jope, 2001). GSK-3β can be dephosphorylated and activated by Aβ in vitro, and its levels are increased in AD brain (Takashima et al., 1996; Pei et al., 1997, 1999; Hye et al., 2005). Activation of GSK-3β can lead to apoptotic neuronal death, contribute to hyperphoshorylation of tau, and result in energy depletion with stress conditions. GSK-3β may also inhibit the expression of transcription factors that support cell survival (Grimes and Jope, 2001).
Dithiocarbamates are metal chelating compounds reported to be potent antioxidants and inhibitors of nuclear factor-κB (NF-κB), a transcription factor regulating expression of proinflammatory and proapoptotic genes (Schreck et al., 1992; Liu et al., 1999; Hayakawa et al., 2003). Dithiocarbamates have previously been used in the treatment of metal poisoning and fungal infections in humans, and their derivatives have been considered for use in treatment of patients with AIDS (Reisinger et al., 1990). Pyrrolidine dithiocarbamate (PDTC) is also protective in animal models of stroke, neonatal asphyxia, and spinal cord trauma (La Rosa et al., 2004; Nurmi et al., 2004, 2006) possibly because of its antiinflammatory or antioxidant properties. However, PDTC treatment activates Akt, inhibits GSK-3β, and reduces apoptosis in a neonatal asphyxia model (Nurmi et al., 2006).
Here, we report that oral PDTC treatment improves spatial learning of APP/PS1 transgenic mice without altering Aβ burden or glial activation. Whereas the brain pathology of APP/PS1 mice does not involve detectable increase in NF-κB activation or marked oxidative stress, we found that the active form of GSK-3β is increased in APP/PS1 transgenic mice and that PDTC activates Akt and downregulates the GSK-3β pathway.
Materials and Methods
Animals.
The APdE9 transgenic mice used in this study were generated by coinjection of chimeric mouse/human APP695 harboring the Swedish mutation and human PS1-dE9 vectors, both controlled by their own mouse prion protein promoter element (Jankowsky et al., 2004). The double transgenic mice were backcrossed to C57BL/6J strain for six generations to create APdE9 transgenic (APP/PS1, tg) mice in C57BL/6J background. Wild-type (wt) siblings were used as controls.
PDTC treatment.
Tg and their wt controls (n = 20) were treated with PDTC (20 mg/kg per day) in drinking water for 7 months, starting at the age of 9 months. The individual water consumption of the mice was assessed before starting the drug treatment and assumed to be constant throughout the experiment. The control groups received normal drinking water. No differences in body weights between PDTC- and H2O-treated mice were recorded. The mice were killed at the age of 16 months. In addition, separate groups of tg mice (n = 4–6) were given PDTC or normal drinking water for 4 months and analyzed by immunohistochemistry at the age of 9.5 months.
Morris water maze.
The effect of PDTC treatment on spatial learning and memory was assessed by Morris water maze at the age of 16 months. A black plastic pool with a diameter of 120 cm was used containing a submerged escape platform (black square; 14 × 14 cm) 1.0 cm below the water surface. The temperature of the water was kept constant throughout the experiment (20 ± 0.5°C), and a 10 min recovery period was allowed between the training trials.
The mice were first trained for 2 d to find the submerged platform with the help of an alley (1 m × 14 cm × 25 cm) leading to the platform. The task acquisition consisted of 4 consecutive days of testing, with five trials per day. If the mouse failed to find the escape platform within the maximum time (60 s), the animal was placed on the platform for 10 s by the experimenter. The platform location was kept constant, and the starting position varied between four constant locations at the pool rim. Mice were placed in the water with their nose pointing toward the wall at one of the starting points in a random manner. On the last trial of the fifth day, the platform was removed, and the mice were allowed to swim for 60 s to determine their search bias. Timing of the latency to find the submerged platform was started and ended by the experimenter. A computer connected to an image analyzer (HVS Image, Hampton, UK) monitored the swim path. Swimming speed was measured by dividing the path length by the time to find the platform. Only mice with a swimming speed >8 cm/s in all trials were included in the analysis to discard occasional floating mice. Search bias during the probe trial was measured by calculating the time the mice spent in the platform quadrant. The behavioral data were analyzed by using repeated-measures ANOVA (ANOVArm), and differences were accepted as significant at p < 0.05.
Electrophoretic mobility shift assay.
Electrophoretic mobility shift assay (EMSA) was performed as described previously in detail (Helenius et al., 1996). Nuclear proteins were isolated from fresh frozen cortical samples according to a modified protocol of Dignam et al. (1983). Double-stranded consensus and mutated oligonucleotides for NF-κB and activator protein 1 (AP-1) binding sites were from Santa Cruz Biotechnology (Santa Cruz, CA). Probes were labeled with T4 polynucleotide kinase (Promega, Madison, WI) and unspecific binding was blocked by 2 μg of poly(dI-dC):polyI(dI-dC) (Roche Applied Science, Basel, Switzerland) in an assay volume of 20 μl. The binding assays were performed as described previously (Helenius et al., 1996). In supershift assays, the specific antibodies (Santa Cruz Biotechnology) to p50 (sc-1192X), p65 (sc-372X), and YY1 (sc-281X) were added for 1 h after the binding reaction. Bound and free probes were separated in a native 4% polyacrylamide gel, and radioactive bands were visualized with STORM 860 imager (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Pixel volumes of specific bands were calculated with ImageQuaNT software (GE Healthcare). The EMSA data were analyzed by using ANOVA in SPSS software and statistical significance was assumed if p < 0.05. N = 8–10 per group.
Immunohistochemistry.
The pentobarbital-anesthetized mice were transcardially perfused with heparinized saline. The brains were dissected out and the left hemisphere was further dissected into hippocampal and cortical samples and snap frozen in liquid nitrogen. The right hemisphere was immersion-fixed with 4% paraformaldehyde for 21 h and cryoprotected in 30% sucrose in phosphate buffer for 48 h. The hemibrains were frozen on liquid nitrogen and cut in 20-μm-thick cryosections.
Activation and proliferation of microglia and astrocytes were assessed using immunostaining for CD45 and CD11b (1:500 dilution; Serotec, Oxford, UK), and glial fibrillary acidic protein (GFAP) (1:500 dilution; DakoCytomation, Glostrup, Denmark). Phospho-tau was analyzed using AT8 (Innogenetics, Gent, Belgium; 1:100 dilution) and AT100 (Innogenetics; 1:100 dilution) antibodies. Primary antibody binding was detected using either biotinylated secondary antibodies (1:200 dilution; Vector Laboratories, Burlingame, CA), avidin–biotin complex (Vectastain Elite kit; Vector Laboratories), and by reacting NiDAB (nickel diaminobenzidine) with H2O2 or by using Alexa Fluor 568-conjugated secondary antibody (1:200 dilution; Invitrogen, Eugene, OR). Human Aβ was detected with pan-Aβ antibody (1:200 dilution; BioSource, Nivelles, Belgium) followed with Alexa Fluor 568-conjugated secondary antibody (1:200 dilution; Invitrogen). The compact, fibrillar form of Aβ was assessed by incubating the sections with 1% thioflavin in distilled H2O for 20 min, followed by a quick rinse with water and dehydration through ascending series of alcohol before dipping in xylene and coverslipping.
Frontal cortical area from four to six sections in 200 μm intervals through the hippocampi was evaluated per animal. For quantification of immunoreactive areas, the sections were imaged with an Olympus AX70 microscope (Olympus, Melville, NY) with an attached digital camera (Color View 12 or F-View; Soft Imaging System, Munster, Germany) running an Analysis Software (Soft Imaging System). All immunoreactive areas were quantified using ImagePro Plus software (Media Cybernetics, Silver Spring, MD). Data are expressed by area of hippocampi occupied by immunoreactivity and represented as the mean ± SEM. The data were analyzed using Student's t test, ANOVA, or a nonparametric test when appropriate in SPSS software, and statistical significance was assumed if p < 0.05.
Aβ1–40 and Aβ1–42 ELISAs.
The levels of Aβ1–40 and Aβ1–42 were analyzed by ELISA from freshly frozen hippocampal samples. For the analysis of soluble fraction of Aβ species, the brain samples were homogenized in 7× volume of 20 mm Tris, pH 8.5, containing complete inhibitory mixture (Roche Diagnostics, Mannheim, Germany). Samples were centrifuged for 1 h at 23,000 rpm at 4°C. Supernatant was taken for analysis of soluble fraction of Aβ species. To analyze insoluble fraction of Aβ, the remaining pellet was resolved in guanidine buffer (5.0 m guanidine-HCl/50 mm Tris-HCl, pH 8.0). The levels of Aβ40 and Aβ42 were quantified using Signal Select Beta Amyloid ELISA kits (BioSource) according to the manufacturer's protocol. The level of total Aβ1–40 and Aβ1–42 was standardized to brain tissue weight and expressed as nanograms of Aβ per gram (brain tissue). The data were analyzed using Student's t test in SPSS software, and statistical significance was assumed if p < 0.05.
Measurement of copper concentration and markers of oxidative stress.
Copper content of the freshly frozen cortical samples was detected from pyrolyzed samples by graphite furnace atomic absorption mass spectroscopy (Z-8100 Polarized Zeeman; Hitachi, Tokyo, Japan) at the City of Kuopio Environmental Health Laboratory.
Reduced glutathione (GSH) concentration in the tissue extracts was measured with 2,3-naphthalenedicarboxaldehyde derivatization as described previously (Orwar et al., 1995). Superoxide dismutase 1 (SOD1) activity was measured from tissue homogenates by a previously described method (Beauchamp and Fridovich, 1971). Shortly, tissues were homogenized in a 10× volume of 20 mm Tris buffer, pH 7.4. After centrifugation for 5 min at 20,000 × g, SOD1 activity was assayed from the supernatant. For detection of carbonylated proteins, cytosolic protein fractions separated from APP/PS1 and wt mouse brain samples were derivatized with 2 mm DNPH (2,4-dinitrophenylhydrazine) (Sigma, St. Louis, MO), precipitated, and dissolved as described previously (Korolainen et al., 2002) before running in SDS-PAGE with Mini Protean II to separate proteins for blotting them onto Hybond-P (APBiotech, Leicester, UK) polyvinylidene difluoride (PVDF) membranes and staining with anti-DNP (2,4-dinitrophenol) primary antibody (DakoCytomation) (Korolainen et al., 2002). The data were analyzed using Student's t test or ANOVA when appropriate in SPSS software, and statistical significance was assumed if p < 0.05.
Western blotting.
The phosphorylation state of GSK-3β and Akt was detected by Western blotting using the following antibodies: anti-phospho-Akt (pAkt-Ser473), anti-Akt, anti-phospho-GSK-3β (pGSK-3β-Ser9), and anti-GSK-3β, all from Cell Signaling (Beverly, MA). GLT-1 antibody (Calbiochem, La Jolla, CA) was used to detect the possible upregulation of GLT-1 transporter. Briefly, the freshly frozen hippocampal brain samples were homogenized in 50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EGTA, 1 mm EDTA, 1% NP-40, 1 mm Na3NO4, and 20 mm NaF containing complete protease inhibitor mixture (Roche Diagnostics). The samples were centrifuged for 20 min at 13,000 rpm, and the supernatant was mixed with standard Laemmli buffer and separated in 10% SDS-PAGE on Mini-Protean III electrophoresis device (Bio-Rad, Hercules, CA). After electrophoresis, proteins were transferred to a PVDF membrane (GE Healthcare) with a Mini-Protean II blotting cell (Bio-Rad) according to the manufacturer's instructions, and immunostained with the primary antibodies. This was followed by incubation with HRP-conjugated secondary antibodies and detection using ECL Plus kit (GE Healthcare). The bands were visualized using STORM 860 imager (GE Healthcare) and quantified with ImageQuant software (GE Healthcare). As a loading control, the membranes were blotted against actin (Sigma) and visualized by using Cy5-conjugated secondary antibody. The data were analyzed using Student's t test in SPSS software, and statistical significance was assumed if p < 0.05.
Hippocampal neuronal cultures and exposures to Aβ.
Primary neuronal hippocampal cultures were made by dissecting hippocampi from embryonic day 18 mouse fetal brains. The cultures were prepared as described previously (Brewer et al., 1993). After dissociation, the cells were suspended in DMEM/10% fetal bovine serum with penicillin–streptomycin (Invitrogen) and plated on a poly-ornithine-precoated (0.5 μg/μl; Sigma) 48-well culture plates. A total of 150,000 cells was plated per well, and the medium was changed to serum-free Neurobasal culture medium supplemented with 2% B27, 500 μm glutamine, 25 μm glutamate, and penicillin–streptomycin (Invitrogen). To obtain ∼90% pure neuronal culture, cells were treated with AraC (Sigma) at days 2–4 to prevent proliferation of other cell types. Three days later, one-third of the medium was changed.
Aβ1–42 was purchased from American Peptide (Sunnyvale, CA) and dissolved to a concentration of 1 mg/ml in sterile water. To obtain fibrillar Aβ preparation (fAβ1–42), the dissolved peptide was incubated at 37°C for 7 d. For Aβ oligomer-rich preparation (oAβ1–42), the dissolved peptide was used immediately. Neurons were cotreated with freshly prepared oAβ1–42 or fAβ1–42 at a concentration of 10 μm and PDTC at a concentration of 1 μm for 24 h.
The form of Aβ preparations was analyzed by running 0.5 μg of cross-linked [adapted from Levine (1995)] samples on a 18% multiphasic buffer system SDS-PAGE gel as described previously (Wiltfang et al., 1997). After the run, the separated proteins were transferred onto PVDF membrane (GE Healthcare). Aβ was detected using an antibody recognizing human Aβ (clone 6E10; 1:3000 dilution; Signet, Dedham, MA) followed by secondary HRP-labeled anti-mouse antibody (GE Healthcare; 1:5000 dilution). The detection was done by ECL Plus kit (GE Healthcare), and the membranes were scanned on STORM 860 imager (GE Healthcare). Neurons exposed to Aβ1–42 and PDTC (n = 3–4 per group) were stained with Hoechst 33342, and viable neurons were counted under fluorescent microscope based on the absence of condensed chromatin.
Results
PDTC treatment markedly attenuates cognitive deficits of transgenic APP/PS1 mice
We first investigated whether a long-term, oral PDTC treatment reduces spatial learning deficits in transgenic APP/PS1 mice. The treatment was started at the age of 9 months when the first Aβ deposits had already developed. Tests for acquisition and a probe trial were performed after 7 months of treatment.
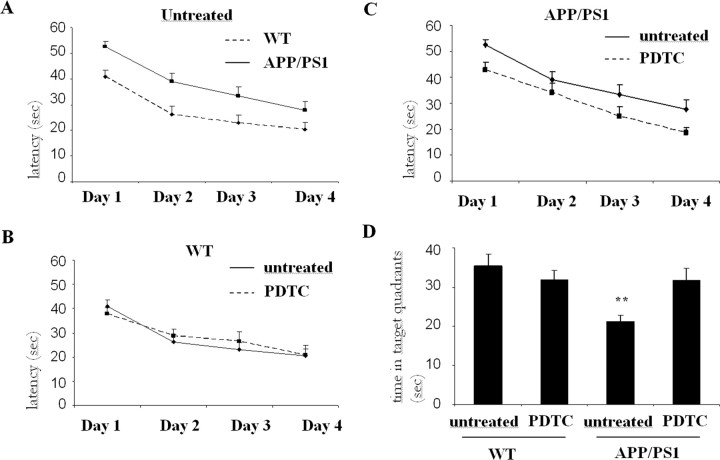
The ability of the mice to learn and process spatial information was tested by the Morris water maze. At the age of 16 months, tg mice demonstrated increased latency to locate the hidden platform (ANOVA with repeated measures; F(1,68) = 7.0; p = 0.01) compared with wt mice (Fig. 1A). Because the effect of PDTC was dependent on the genotype (interaction nearly significant; p = 0.055), wt and tg mice were evaluated separately. In wt mice, PDTC treatment had no effect (Fig. 1B); however, PDTC significantly improved learning of tg mice (ANOVArm; F(1,31) = 6.6; p = 0.015) as depicted in Figure 1C. The swimming speed did not differ between the groups (ANOVArm; F(3,68) = 2.0; p = 0.12).
Figure 1.
Long-term PDTC treatment prevented the cognitive decline of transgenic APP/PS1 mice. Test for acquisition was performed for 16-month-old transgenic APP/PSI mice and their wt controls after 7 months of PDTC treatment. Sixteen-month-old APP/PS1 mice were significantly slower in finding the platform when compared with wt controls as shown in A (ANOVA with repeated measures; F(1,68) = 7.0; p = 0.01). B, C, In wt mice, PDTC had no effect (B), whereas in transgenic mice, PDTC significantly improved the ability to locate the platform (C) (ANOVArm; F(1,31) = 6.6; p = 0.015). D, In the probe trial on day 4, PDTC did not affect the search bias of wt mice, whereas it significantly increased the time spent in the former platform quadrant of tg mice. Two tg mice were excluded as statistical outliers, one in the control and one in the PDTC group. **Significantly different from the wt control (p = 0.01; Dunnett's post hoc test). The final numbers of the animals per each group were as follows: wt, 17; wt PDTC, 19; tg, 15; and tg PDTC, 13. Error bars indicate SEM.
The last trial of day 4 was the probe trial. The platform was removed to test the tendency of an animal to search the platform from its previous correct location during a 60 s probe trial. Again, the ANOVA revealed impaired spatial memory of tg mice compared with their wt controls (F(1,66) = 7.5; p = 0.008). Similarly to the acquisition phase, the effect of PDTC was dependent on the genotype (genotype by drug interaction, p = 0.009). No PDTC effect was observed in wt mice (t(37) = 0.9; p = 0.38), whereas it significantly enhanced search bias in tg mice (t(29) = 3.3; p = 0.003) (Fig. 1D).
NF-κB and AP-1 DNA binding activities are not changed in APP/PS1 mice and not affected by PDTC treatment
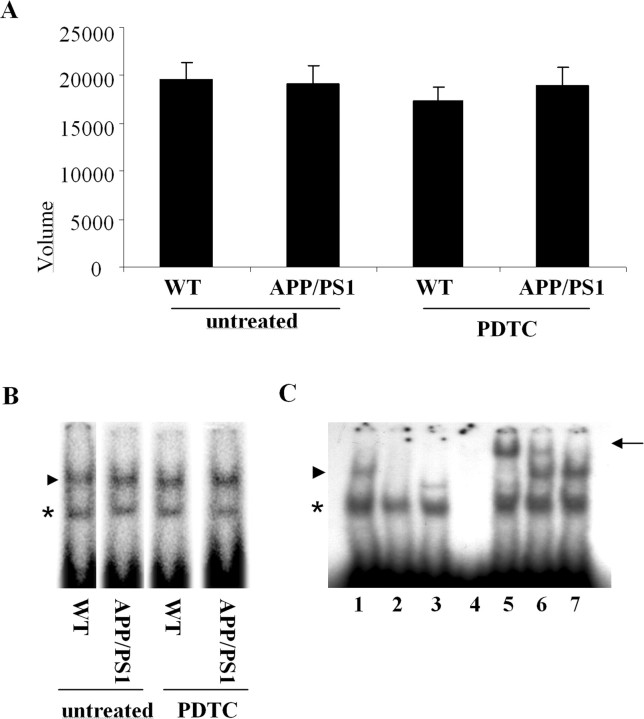
Because PDTC is known to be able to inhibit NF-κB, we run EMSA to detect the effect of PDTC on DNA binding activity of NF-κB in APP/PS1 tg and wt mice. EMSA revealed no differences in the DNA binding activity of NF-κB (Fig. 2) or AP-1 (data not shown) between untreated tg mice and their wt controls. PDTC treatment did not significantly alter DNA binding activities of NF-κB or AP-1 in either wt or tg mice.
Figure 2.
DNA binding activity of NF-κB was not altered in APP/PS1 mice nor affected by PDTC treatment. A, B, Quantitative analysis (A) and a typical autoradiograph (B) of electrophoretic mobility shift assay of the DNA binding activity of NF-κB in wt and APP/PS1 transgenic mouse brain. The assay did not reveal any alterations in the DNA binding activity of NF-κB in APP/PS1 mice compared with their water-treated controls. PDTC treatment did not have any effect on the DNA binding activities (n = 8–10 per group). Error bars indicate SEM. C shows the specificity of the NF-κB complex formation (arrowhead) in control mice. The lanes are as follows: (1) normal assay, (2) 100× unlabeled competitor (cold consensus probe), (3) labeled mutated NF-κB binding probe, (4) consensus probe without protein, (5) supershift assay with specific anti-p50, (6) supershift assay with anti-p65, and (7) supershift assay with anti-YY1. The arrowheads show the specific NF-κB complex, the arrow shows the supershifted complex, and the asterisk shows the unspecific complex. The specific NF-κB complex contains both p50 and p65 proteins.
PDTC treatment has no effect on brain Aβ burden, gliosis, or markers of oxidative stress in APP/PS1 mice
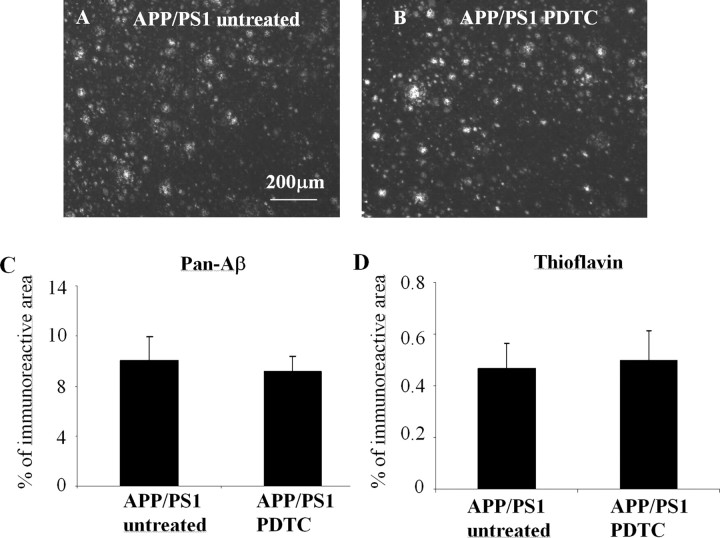
Antioxidants and antiinflammatories have been reported to reduce Aβ deposits or inhibit Aβ1–42 production (Cole et al., 2004; Lim et al., 2005; Townsend et al., 2005). We analyzed the brain Aβ burden by both immunohistochemistry and Aβ ELISA from the cortex and hippocampus. PDTC treatment did not decrease the cortical area covered by pan-Aβ immunoreactivity or the thioflavin-positive plaque load (Fig. 3). Also, PDTC had no effect on the hippocampal soluble or insoluble Aβ1–40 or Aβ1–42 levels as analyzed by ELISA (Table 1).
Figure 3.
PDTC treatment had no effect on the brain Aβ burden. The effect of PDTC treatment on the brain Aβ burden was analyzed immunohistochemically by detecting diffuse Aβ deposition using pan-Aβ antibody staining and compact fibrillar plaques using thioflavin staining. The 16-month-old APP/PS1 mice exhibited extensive deposition of Aβ in the cortex detected by pan-Aβ antibody (A); however, quantification revealed that PDTC treatment had no effect either on the pan-Aβ immunoreactive areas (B, C) or thioflavin-stained area (D). Error bars indicate SEM.
Table 1.
Effect of PDTC on the brain Aβ levels as analyzed by ELISA
| APP/PSI untreated | APP/PSI PDTC | |
|---|---|---|
| Aβ1–42 soluble | 207.28 ± 144.22 | 236.64 ± 154.72 |
| Aβ1–42 insoluble | 16,318 ± 3754.70 | 13,588.75 ± 1859.30 |
| Aβ1–40 soluble | 85.67 ± 32.16 | 111.76 ± 15.98 |
| Aβ1–40 insoluble | 7235 ± 2359.26 | 7001.5 ± 2211.15 |
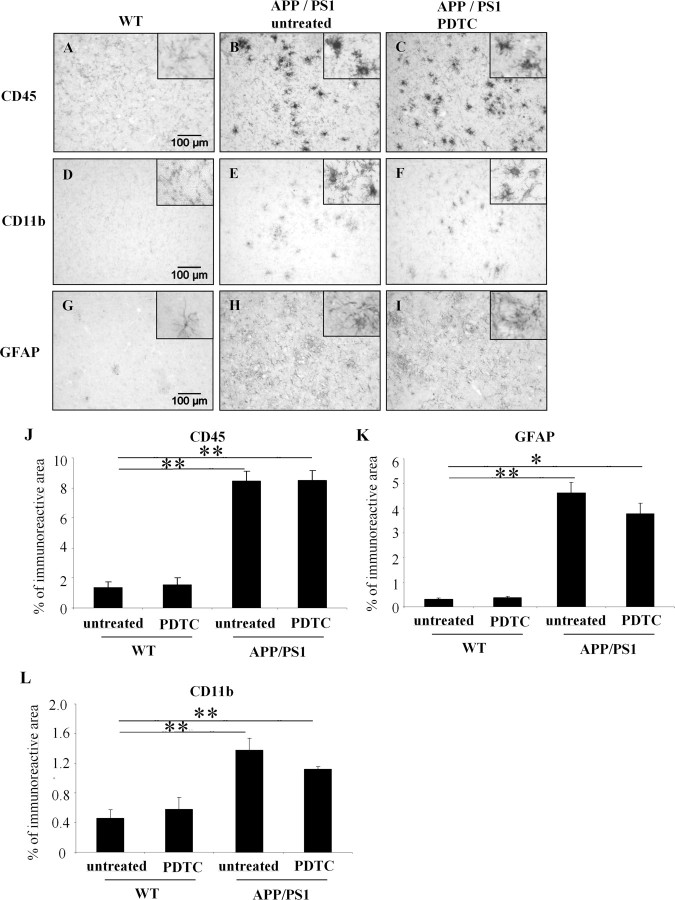
To investigate whether PDTC treatment has antiinflammatory effects by reducing gliosis in APP/PS1 mice, we assessed astrogliosis and microgliosis using GFAP as an astrocytic marker and CD45 and CD11b as microglia markers, respectively. All markers showed a clear increase of gliosis in untreated APP/PS1 mouse brain when compared with untreated wt controls (Fig. 4). However, PDTC treatment did not significantly alter GFAP, CD11b, or CD45 immunoreactivity in APP/PS1 mice or in wt controls as shown in Figure 4. Investigation of GFAP and CD45 immunoreactivities at 4 months of the PDTC treatment confirmed that no significant reductions in these general inflammatory markers (percentage of immunoreactive area: GFAP, tg untreated, 2.16 ± 0.40%, and tg PDTC treated, 1.94 ± 0.12%, p > 0.05, Student's t test; CD45, tg untreated, 4.12 ± 0.71%, and tg PDTC treated, 3.67 ± 0.44%, p > 0.05, Student's t test; N = 4–6 per group) were evident even at an earlier time point and before the observed changes in cognitive functions.
Figure 4.
APP/PS1 mice exhibited markedly elevated gliosis which was not altered by PDTC treatment. Immunohistochemistry revealed significant gliosis as analyzed by microglial markers CD11b, CD45, and an astrocytic marker GFAP. A, D, and G show CD45, CD11b, and GFAP staining in frontal cortical area in wt mice, respectively. APP/PS1 mice had significant increased intensity of staining in all markers as shown in B, E, and H. PDTC treatment did not alter any of the glial markers analyzed (C, F, I). The high-power insets show typical morphology of stained glial cells. The quantification of each of the staining is shown in graphs J–L. Error bars indicate SEM. *p < 0.05; **p < 0.01.
Oxidative stress is implicated in AD pathology, and several AD models display alterations in markers of oxidative stress, such as GSH, SOD1, and carbonylated proteins (Cole et al., 2004). However, we did not detect changes in these markers in APP/PS1 mice when compared with wt control mice, or with PDTC treatment (data not shown), suggesting that brain pathology in APP/PS1 mice does not involve marked oxidative damage.
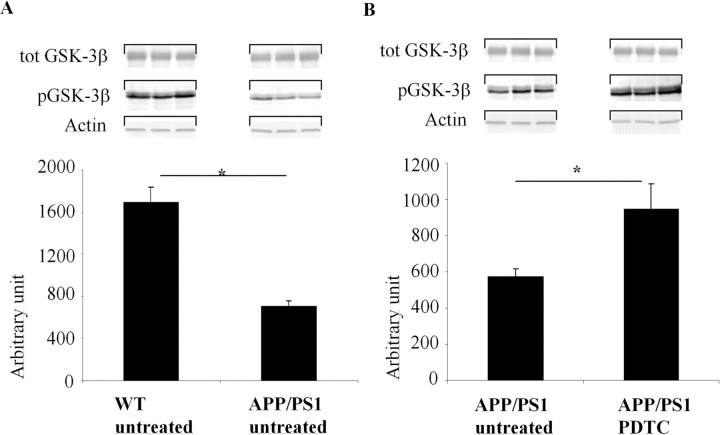
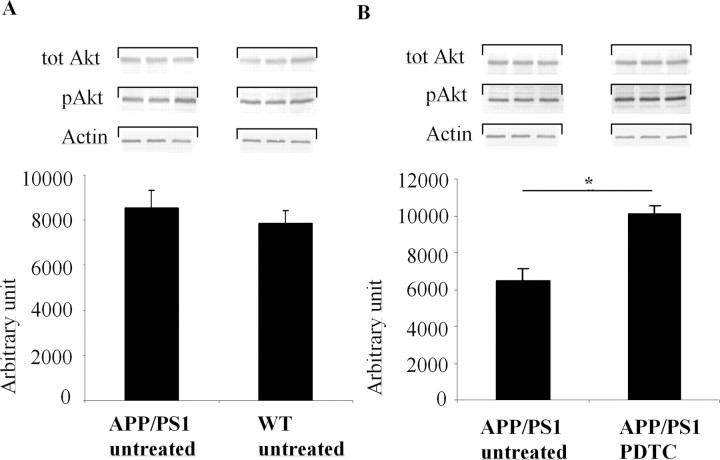
Inactive pGSK-3β-Ser9 is decreased in APP/PS1 mouse brain and PDTC treatment increases pGSK-3β-Ser9 and active pAkt-Ser473
Because GSK-3β activity may play a role in AD and PDTC has been reported to interfere with Akt GSK-3β pathway, we determined the levels of inactive GSK-3β and active Akt in the mouse brains. Increased phosphorylation of Ser9 in GSK-3β reflects decreased activity of GSK-3β, whereas phosphorylation of Akt in Ser473 reflects increased activity of Akt. Quantification of Western blots revealed that the amount of pGSK-3β-Ser9 (Fig. 5A) but not pAkt-Ser473 (Fig. 6A) was significantly decreased in untreated APP/PS1 mice in the brain samples when compared with untreated wt animals. However, PDTC treatment significantly increased pAkt-Ser473 in APP/PS1 mice (by 60%; p < 0.01) (Fig. 6B) but not in wt mice (data not shown). In addition, pGSK-3β-Ser9 levels in APP/PS1 mouse brain were significantly increased after PDTC treatment (Fig. 5B). These results indicate that the level of the active GSK-3β is increased in APP/PS1 mouse brains and that PDTC treatment increases the activity of protective Akt pathway. The increased levels of pGSK-3β-Ser9 and pAkt-Ser473 were not attributable to an increase in total GSK-3β or total Akt, because the amount of total GSK-3β and total Akt was unchanged by PDTC treatment (Figs. 5, 6, respectively).
Figure 5.
PDTC treatment increased phosphorylation of GSK-3β-Ser9. An illustration of typical immunoblots with antibodies against pGSK-3β-Ser9 and total GSK-3β and their subsequent quantification showed that the level of pGSK-3β-Ser9 was decreased in APP/PS1 mice compared with wt mice (A) and that PDTC treatment significantly increased the levels of pGSK-3β-Ser9 in APP/PS1 mouse brain (B). The differences in the level of pGSK-3β-Ser9 were not attributable to changes in total pGSK-3β, because the amount of total GSK-3β remained unchanged. Membranes were blotted against actin as a loading control. Error bars indicate SEM. *p < 0.05.
Figure 6.
PDTC treatment increased phosphorylation of pAkt-Ser473. A, APP/PS1 mice had similar levels of pAkt-Ser473 compared with untreated wt mice. B, However, PDTC treatment significantly increased the levels of pAkt-Ser473. This increase was independent of the amount of total Akt, which remained unchanged in all animals. The membranes were blotted against actin as a loading control. Error bars indicate SEM. *p < 0.05.
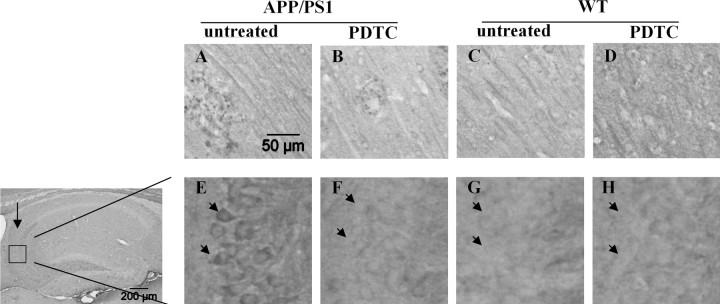
PDTC treatment reduces phosphorylated tau in the hippocampus of APP/PS1 mice
Because tau is a direct and relevant target of GSK-3β in AD, we determined the effect of PDTC on tau phosphorylation by using AT8 antibody, which detects the tau phosphorylated at Ser202, a target of many tau kinases including GSK-3β; and AT100 antibody, which detects epitopes phospho-Ser212, a target of many tau kinases including GSK-3β, and phospho-Thr214. AT8 stained strongly some short neurites that were associated with putative Aβ deposits in APP/PS1 mice (Fig. 7A–D). PDTC treatment had no clear effect on these AT8-immunoreactive, presumably dystrophic neurites. AT8 immunoreactivity was also observed in region-specific neuronal cell bodies, especially in the CA3 pyramidal cells (Fig. 7E–H), in which quantification revealed that cytosolic AT8 immunoreactivity was 66% greater in untreated APP/PS1 mice (8.3 ± 1.9% area covered by immunoreactivity) than in untreated wt mice (5.0 ± 1.0%). PDTC treatment had no effect on the AT8 immunoreactivity of these cells in wt mice, but significantly decreased the AT8 immunoreactivity in APP/PS1 mice by 80% (to 1.7 ± 0.4%; p < 0.01). AT100 antibody stained neuronal cell bodies in the cortex and CA3 neurons, as well as dystrophic neurites around the plaques. Quantitative analysis of AT100 immunostaining in the CA3 pyramidal cells showed a trend but not a statistically significant reduction in PDTC-treated versus untreated in APP/PS1 mice (percentage of immunoreactive area: 2.56 ± 0.48 and 3.87 ± 1.58% in PDTC-treated and untreated tg mice, respectively; N = 5 per group; p = 0.07, Student's t test), possibly because only one epitope of the AT100 antibody may be a target of GSK-3β. Overall, these results suggest, although do not prove, that the PDTC-induced increase in the active Akt and reduction in the active GSK-3β may occur at least partially in neurons.
Figure 7.
PDTC treatment reduced the amount of phosphorylated tau in the hippocampus CA3 region of APP/PS1 mice. Photomicrographs showing AT8 immunoreactivity in the cortex (A–D) and CA3 pyramical cells (E–H) of wt and APP/PS1 mouse brains. A, AT8 immunoreactivity was observed surrounding putative Aβ deposits in the cortical areas of APP/PS1 mice, presumably reflecting dystrophic neurites. B–D, This staining was not clearly altered by PDTC treatment (B) and was absent in wt mice (C, D). E–H, High-power insets show AT8 immunoreactivity observed in the hippocampal CA3 region (arrow) pyramidal cells (arrowheads), in which APP/PS1 mice (E) exhibited more staining compared with wt mice (G). PDTC treatment did not alter the AT8 staining in wt mice (G, H); however, it decreased AT8 immunoreactivity in hippocampal CA3 region (F). The quantitative data are shown in Results. Scale bar, 50 μm.
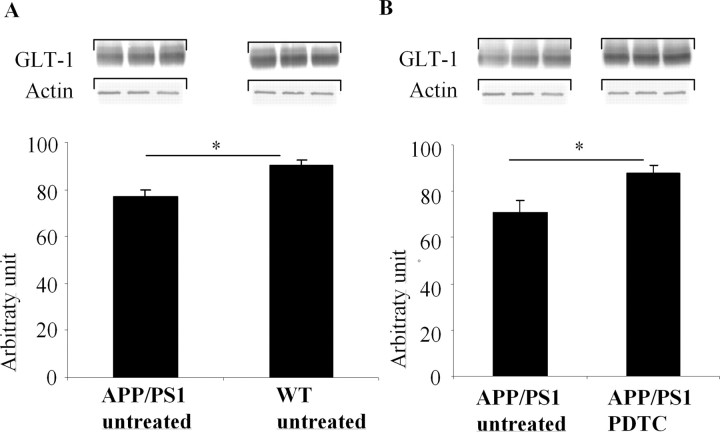
PDTC treatment upregulates the reduced GLT-1 levels in APP/PS1 mouse brain
Because defects in uptake of glutamate through glial glutamate transporter GLT-1 have been reported in AD and in Aβ-treated cultured astrocytes (Harris et al., 1996; Masliah et al., 2000; Liang et al., 2002) and the expression of GLT-1 is regulated by Akt pathway (Li et al., 2006), we determined the changes in GLT-1 in our experimental settings by Western blotting. The cortical GLT-1 levels were 18% lower in untreated APP/PS1 mice compared with untreated wt mice (Fig. 8A). PDTC treatment increased GLT-1 levels by 24% (p < 0.05) in APP/PS1 mice (Fig. 8B) but had no effect on GLT-1 in wt mice (data not shown). These results suggest that PDTC treatment increases the amount of active Akt in astrocytes and thereby increases GLT-1 levels.
Figure 8.
PDTC treatment increased the levels of astrocytic GLT-1. APP/PS1 mice exhibited a significant decrease in the level of astrocytic receptor GLT-1 (A). PDTC treatment significantly increased the levels of GLT-1, bringing it back to normal as depicted in B. The membranes were blotted against actin as a loading control. Error bars indicate SEM. *p < 0.05.
PDTC increases brain copper concentration in APP/PS1 mice
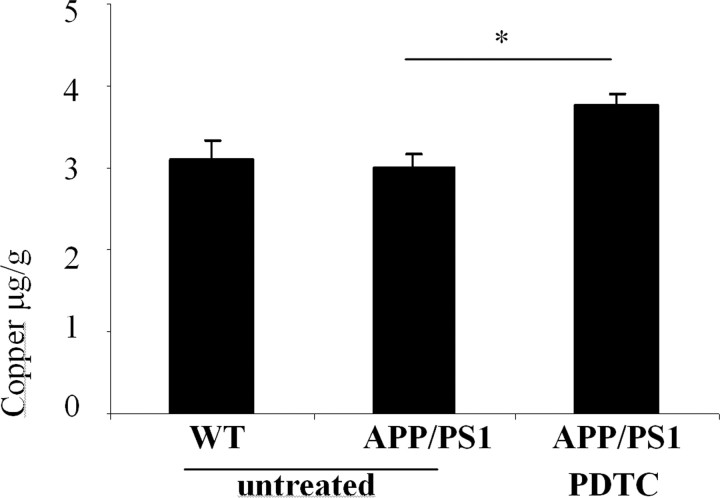
Because Cu2+ has been reported to activate Akt (Ostrakhovitch et al., 2002), and PDTC is a metal chelator capable of transferring external Cu2+ into a cell (Verhaegh et al., 1997), we measured the brain copper concentrations from the cortical samples by atomic absorption spectrophotometer. Whereas no difference in the copper concentration between the untreated APP/PS1 and wt mice was observed, PDTC treatment increased copper concentration in APP/PS1 by 26% (p < 0.05) (Fig. 9).
Figure 9.
PDTC treatment increased the cortical copper concentration. Untreated APP/PS1 and wt mice exhibited a similar amount of copper in the cortex as measured by atomic absorption spectophotometer. Long-term PDTC treatment significantly increased the copper concentration in APP/PS1 mice. Error bars indicate SEM. *p < 0.05.
PDTC protects hippocampal neurons against oligomer-rich Aβ but not fibrillar Aβ
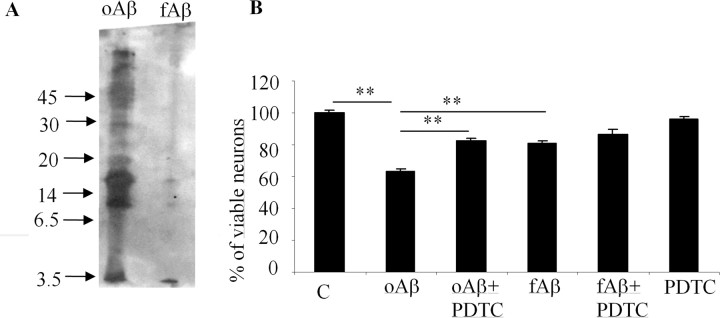
To test whether PDTC protects directly neurons against Aβ1–42-induced injury and whether PDTC has differential effect on neurotoxicity induced by Aβ oligomers and fibrillar Aβ, we run cell culture experiments on primary hippocampal neurons. The neuronal purity of these cultures is 94%. Western blot analysis revealed that the oAβ1–42 preparation contained monomeric, various forms of oligomeric and small amount of high-molecular-weight forms of Aβ that moved very little on the gel. Fibrillization of Aβ (fAβ1–42) resulted in high-molecular-weight aggregates that did not move on gel at all (Fig. 10A).
Figure 10.
PDTC protected primary hippocampal neurons against oligomer-rich Aβ induced toxicity. A, Freshly dissolved Aβ preparation contained mostly oligomers, ranging from monomeric Aβ to high-molecular-weight forms. Fibrillization of Aβ resulted predominantly in high-molecular-weight aggregates that did not penetrate into the SDS-PAGE gel used in the current study. B, Exposure of primary hippocampal neurons to the freshly dissolved, oligomer-rich Aβ preparation caused ∼40% cell death as analyzed by the appearance of condensed chromatin. The cell death was significantly diminished by cotreatment with 1 μm PDTC. Fibrillar Aβ was also toxic causing ∼20% cell death, although the effect was significantly smaller compared with oligomer-rich preparation. PDTC failed to protect the neurons against fibrillar Aβ toxicity. PDTC alone did not affect the cell viability. Error bars indicate SEM. *p < 0.01.
oAβ1–42 caused ∼40% cell death after 24 h exposure (Fig. 10B). Cotreatment of neurons with 1 μm PDTC significantly, although not completely, protected the cells against Aβ toxicity. oAβ1–42 was significantly more toxic compared with fAβ1–42, which caused only ∼20% neuron death. PDTC failed to prevent any toxicity caused by fibrillar Aβ. PDTC alone did affect the neuronal viability.
Discussion
Our results showed that PDTC, a clinically tolerated dithiocarbamate capable of crossing the blood–brain barrier (Frank et al., 1995; Nurmi, 2004), markedly prevented or cured the cognitive deficits in aged APP/PS1 tg mice. This beneficial effect of PDTC was seen when the treatment was started after the first Aβ deposits had already developed. Because brain Aβ burden and gliosis were unaltered by the treatment and the cell culture experiments showed that PDTC provides protection against oligomer-rich Aβ1–42 preparation but not fibrillar Aβ1–42 preparation, it is likely that the effect of PDTC was based on prevention of the Aβ oligomer-mediated neuronal dysfunction, which involves activation of GSK-3β. Considering that antiinflammatories and antioxidants typically reduce both the Aβ burden and gliosis in animal models of AD (Jantzen et al., 2002; Cole et al., 2004; Lim et al., 2005; Townsend and Pratico, 2005), and that NF-κB activity and markers of oxidative stress were not induced in our APP/PS1 mice at the age 16 months, it is very likely that the beneficial effect of PDTC is only marginally if at all based on its potential antioxidative and antiinflammatory effects. Instead, in agreement with previous studies on human AD and AD mouse models, we found that the inactive form of GSK-3β was decreased in APP/PS1 mouse brain and that PDTC treatment increased the active form of Akt and upregulated the inactive pGSK-3β-Ser9. Although the methodology we used to determine the GSK-3β activation has certain limitations, it is conceivable that the improved spatial learning can be attributed to the PDTC-induced activation of Akt pathway (Pei et al., 1997, 1999). This suggestion is in line with the previous studies showing (1) that spatial learning deficits are regulated by conditionally overexpressed GSK-3β in the mouse brain (Hernandez et al., 2002; Engel et al., 2006), (2) that mutant presenilins can activate GSK-3β (Weihl et al., 1999), (3) that activation of Akt pathway is necessary for the expression of long-term potentiation (Sanna et al., 2002; Karpova et al., 2006), and (4) that inhibitors of Akt pathway impair long-term consolidation and recognition memory in rats (Horwood et al., 2006). Moreover, cerebrolysin (a mixture of peptides and amino acids obtained from porcine brain tissue), PPARγ (peroxisome proliferator-activated receptor-γ) agonists, and the Aβ antibodies that ameliorate behavioral deficits in transgenic AD mice or AD have also potential to decrease GSK-3β activation (Inestrosa et al., 2005; Watson et al., 2005; Ma et al., 2006; Rockenstein et al., 2006; Sastre et al., 2006).
In agreement with the hypothesis that Akt pathway is induced by PDTC treatment, we observed decreased immunoreactivity for phosphorylated tau in the CA3 pyramidal neurons and overall increase in GLT-1, a major astrocytic glutamate transporter that has previously been reported to be reduced in AD models (Harris et al., 1996; Masliah et al., 2000; Liang et al., 2002). Although GSK-3β is responsible for phosphorylating Ser202 (Mandelkow et al., 1992; Ishiguro et al., 1993), the major site for abnormal phosphorylation of tau resulting in formation of paired helical filaments (PHFs) in AD (Ikura et al., 1998), it is uncertain whether the PDTC-induced reduction we observed in Ser202 phosphorylation (AT8 immunoreactivity) contributes to the improved cognitive functions, because PHFs cannot be found in transgenic APP or APP/PS1 mice and we could not detect reduction in AT8-immunoreactive dystrophic neurites around Aβ deposits. We also observed a tendency for decreased tau phosphorylation in PDTC-treated APP/PS1 mice by using AT100 antibody, which detects phosphorylation of Ser212 (a target of several kinases, including GSK-3β) and Thr214, supporting the notion that PDTC affects GSK-3β activity in neurons of APP/PS1 mice. Similarly, although Akt pathway is known to control the expression of GLT-1 (Li et al., 2006), which is necessary for normal LTP and maintaining glutamate at nontoxic concentration (Hatten et al., 1991; Sanna et al., 2002), it is uncertain whether the increased GLT-1 levels we observed after PDTC treatment in APP/PS1 mice is sufficient or needed for the recovery of cognitive functions. Nevertheless, our findings perfectly support the hypothesis that PDTC treatment activated Akt–GSK-3β pathway both in neurons and astrocytes with functional consequences that have relevance in AD pathology. Furthermore, although not relevant for cognitive functions in this AD mouse model, these effects of PDTC are potentially beneficial in human AD, in which PHFs correlate with cognitive deficits (Giannakopoulos et al., 2003).
Several studies have linked copper ions with AD. Serum Cu2+ levels in AD patients are increased (Squitti et al., 2002), senile plaques in AD brain are enriched with copper ions (Lovell et al., 1998), Cu2+ may increase β-sheet formation of Aβ (Miura et al., 2004), and Cu2+ enhances Aβ neurotoxicity in some cell culture studies (Huang et al., 1999; Opazo et al., 2002). Moreover, some copper chelators, such as clioquinol, partially dissemble Aβ deposits in transgenic AD models (Cherny et al., 2001; Lee et al., 2004). However, APP and Aβ overproduction enables intracellular copper to be transported out of the cell, leading to cellular copper insufficiency, and under certain conditions, Aβ–copper complexes are neuroprotective (Maynard et al., 2002; Bayer et al., 2003; Phinney et al., 2003). Moreover, copper deficiency may lead to reduced activity of Cu, Zn-SOD1 (Bayer et al., 2003), a major cytoplasmic antioxidant, and thereby alter endogenous antioxidant defense system, including possible SOD1-induced activation of Akt (Noshita et al., 2003). Overall, copper homeostasis may be disturbed in AD, and the functions of Cu2+ in AD pathogenesis appear to be complicated. Whereas the brain copper concentrations in APP/PS1 and wt mice were statistically the same in our study, PDTC treatment significantly increased copper levels in APP/PS1 mouse brains. PDTC is a Cu2+ chelator capable of transferring external Cu2+ into a cell (Verhaegh et al., 1997), suggesting that PDTC-increased concentration of copper may be attributable to increased intracellular Cu2+. PDTC treatment could thus alter the ratio of extracellular and intracellular copper, and compensate the Aβ-induced reduction in intracellular Cu2+. Because Cu2+ can also activate Akt (Ostrakhovitch et al., 2002), we hypothesize that PDTC-induced increase in intracellular Cu2+ triggers Akt phosphorylation, leading to decreased activity of GSK-3β, reduced tau phosphorylation, increased GLT-1 expression, and activation of cell survival supporting functions of Akt pathway. This hypothesis does not exclude the possibility that other copper-dependent functions may also contribute to the improved cognitive functions in PDTC-treated APP/PS1 mice. An interesting finding is that Aβ peptides may bind copper (Curtain et al., 2001; Zou et al., 2002), thereby possibly causing some pathological effects. Whether PDTC binds and prevents metal binding of Aβ peptides is not known.
It is unclear why PDTC treatment caused changes in Akt pathway in APP/PS1 but not in wt mice. Several changes in cellular functions are triggered by mutant PS1 and APP that may alter the response of neurons and astrocytes to PDTC, including mutant PS1 and intracellular Aβ themselves, which are able to inhibit activation of Akt pathway (Baki et al., 2004; Magrane et al., 2005). In addition, inflammatory mediators induced in APP/PS1 mouse brain may regulate Akt (Grzelkowska-Kowalczyk and Wieteska-Skrzeczynska, 2006). It may be possible that APP/PS1 pathology results in compensatory promotion of Akt pathway to maintain it at normal levels, and that an additional Akt activating signal, such as PDTC or Cu2+, then results in additional activation of Akt. One may also speculate that PDTC or Cu2+ form such complexes with Aβ that have high impact on Akt pathway. However, there is no experimental evidence for this hypothesis. Nevertheless, the fact that Akt, GSK-3β, tau, or GLT-1 were not significantly altered in wt mouse brain by PDTC treatment suggests that the overall effects of PDTC may be milder in healthy brain and that PDTC treatment, at the low doses used in the present study, may affect only disturbed cellular functions and cause relatively few adverse effects. In agreement with this idea, we did not observe any side effects or changes in the body weight in our PDTC-treated mice. Because diothiocarbamates are relatively well tolerated in humans, and PDTC has several potentially beneficial effects on neurodegeneration processes, our observation of improved cognitive function in a relevant animal model of AD warrants additional studies on PDTC and its analogs toward clinical trials.
Footnotes
This work was supported by the Sigrid Juselius Foundation, Academy of Finland, and the Nordic Centre of Excellence Program in Molecular Medicine 2004–2009 entitled “Neurodegeneration.” We thank Dr. David Borchelt (University of South Florida, Gainesville, FL) and Dr. J. Jankowsky (California Institute of Technology, Pasadena, CA) for providing the breeder mice for the study.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Schafer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schussel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA. 2003;100:14187–14192. doi: 10.1073/pnas.2332818100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Kuo YM, Spiegel K, Emmerling MR, Sue LI, Kokjohn K, Roher AE. The cholinergic deficit coincides with Abeta deposition at the earliest histopathologic stages of Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:308–313. doi: 10.1093/jnen/59.4.308. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Lorenzo A, Yeh J, Yankner BA. Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA. NSAID and antioxidant prevention of Alzheimer's disease: lessons from in vitro and animal models. Ann NY Acad Sci. 2004;1035:68–84. doi: 10.1196/annals.1332.005. [DOI] [PubMed] [Google Scholar]
- Curtain CC, Ali F, Volitakis I, Cherny RA, Norton RS, Beyreuther K, Barrow CJ, Masters CL, Bush AI, Barnham KJ. Alzheimer's disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J Biol Chem. 2001;276:20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel T, Hernandez F, Avila J, Lucas JJ. Full reversal of Alzheimer's disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S, Tucker HM, van Rooyen C, Wright S, Brigham EF, Wogulis M, Rydel RE. Aggregated amyloid-β protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J Neurosci. 1997;17:7736–7745. doi: 10.1523/JNEUROSCI.17-20-07736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank N, Christmann A, Frei E. Comparative studies on the pharmacokinetics of hydrophilic prolinedithiocarbamate, sarcosinedithiocarbamate and the less hydrophilic diethyldithiocarbamate. Toxicology. 1995;95:113–122. doi: 10.1016/0300-483x(94)02890-7. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Grzelkowska-Kowalczyk K, Wieteska-Skrzeczynska W. Exposure to TNF-alpha but not IL-1beta impairs insulin-dependent phosphorylation of protein kinase B and p70S6k in mouse C2C12 myogenic cells. Pol J Vet Sci. 2006;9:1–10. [PubMed] [Google Scholar]
- Harris ME, Wang Y, Pedigo NW, Jr, Hensley K, Butterfield DA, Carney JM. Amyloid beta peptide (25–35) inhibits Na+-dependent glutamate uptake in rat hippocampal astrocyte cultures. J Neurochem. 1996;67:277–286. doi: 10.1046/j.1471-4159.1996.67010277.x. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Liem RK, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius M, Hanninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Huang X, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JD, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, Saunders AJ, Lim J, Moir RD, Glabe C, Bowden EF, Masters CL, Fairlie DP, Tanzi RE, Bush AI. Cu(II) potentiation of alzheimer abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J Biol Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- Hye A, Kerr F, Archer N, Foy C, Poppe M, Brown R, Hamilton G, Powell J, Anderton B, Lovestone S. Glycogen synthase kinase-3 is increased in white cells early in Alzheimer's disease. Neurosci Lett. 2005;373:1–4. doi: 10.1016/j.neulet.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Ikura Y, Kudo T, Tanaka T, Tanii H, Grundke-Iqbal I, Iqbal K, Takeda M. Levels of tau phosphorylation at different sites in Alzheimer disease brain. NeuroReport. 1998;9:2375–2379. doi: 10.1097/00001756-199807130-00041. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res. 2005;304:91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, Uchida T, Imahori K. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325:167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova A, Sanna PP, Behnisch T. Involvement of multiple phosphatidylinositol 3-kinase-dependent pathways in the persistence of late-phase long term potentiation expression. Neuroscience. 2006;137:833–841. doi: 10.1016/j.neuroscience.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Kettunen MI, Goldsteins G, Keinanen R, Salminen A, Ort M, Bures J, Liu D, Kauppinen RA, Higgins LS, Koistinaho J. Beta-amyloid precursor protein transgenic mice that harbor diffuse A beta deposits but do not form plaques show increased ischemic vulnerability: role of inflammation. Proc Natl Acad Sci USA. 2002;99:1610–1615. doi: 10.1073/pnas.032670899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolainen MA, Goldsteins G, Alafuzoff I, Koistinaho J, Pirttila T. Proteomic analysis of protein oxidation in Alzheimer's disease brain. Electrophoresis. 2002;23:3428–3433. doi: 10.1002/1522-2683(200210)23:19<3428::AID-ELPS3428>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- La Rosa G, Cardali S, Genovese T, Conti A, Di Paola R, La Torre D, Cacciola F, Cuzzocrea S. Inhibition of the nuclear factor-kappaB activation with pyrrolidine dithiocarbamate attenuating inflammation and oxidative stress after experimental spinal cord trauma in rats. J Neurosurg Spine. 2004;1:311–321. doi: 10.3171/spi.2004.1.3.0311. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Friedman JE, Angel I, Kozak A, Koh JY. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging. 2004;25:1315–1321. doi: 10.1016/j.neurobiolaging.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Levine H., III Soluble multimeric Alzheimer beta(1–40) pre-amyloid complexes in dilute solution. Neurobiol Aging. 1995;16:755–764. doi: 10.1016/0197-4580(95)00052-g. [DOI] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J Neurochem. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- London JA, Biegel D, Pachter JS. Neurocytopathic effects of beta-amyloid-stimulated monocytes: a potential mechanism for central nervous system damage in Alzheimer disease. Proc Natl Acad Sci USA. 1996;93:4147–4152. doi: 10.1073/pnas.93.9.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Ma QL, Lim GP, Harris-White ME, Yang F, Ambegaokar SS, Ubeda OJ, Glabe CG, Teter B, Frautschy SA, Cole GM. Antibodies against beta-amyloid reduce Abeta oligomers, glycogen synthase kinase-3beta activation and tau phosphorylation in vivo and in vitro. J Neurosci Res. 2006;83:374–384. doi: 10.1002/jnr.20734. [DOI] [PubMed] [Google Scholar]
- Magrane J, Rosen KM, Smith RC, Walsh K, Gouras GK, Querfurth HW. Intraneuronal β-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J Neurosci. 2005;25:10960–10969. doi: 10.1523/JNEUROSCI.1723-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Drewes G, Biernat J, Gustke N, Van Lint J, Vandenheede JR, Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992;314:315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 2000;163:381–387. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Miura T, Mitani S, Takanashi C, Mochizuki N. Copper selectively triggers beta-sheet assembly of an N-terminally truncated amyloid beta-peptide beginning with Glu3. J Inorg Biochem. 2004;98:10–14. doi: 10.1016/j.jinorgbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nakamura M, McIntosh TK, Rodriguez A, Berlin JA, Smith DH, Saatman KE, Raghupathi R, Clemens J, Saido TC, Schmidt ML, Lee VM, Trojanowski JQ. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J Comp Neurol. 1999;411:390–398. [PubMed] [Google Scholar]
- Nakagawa Y, Reed L, Nakamura M, McIntosh TK, Smith DH, Saatman KE, Raghupathi R, Clemens J, Saido TC, Lee VM, Trojanowski JQ. Brain trauma in aged transgenic mice induces regression of established abeta deposits. Exp Neurol. 2000;163:244–252. doi: 10.1006/exnr.2000.7375. [DOI] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Lewen A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke. 2003;34:1513–1518. doi: 10.1161/01.STR.0000072986.46924.F4. [DOI] [PubMed] [Google Scholar]
- Nurmi A. Kuopio University Publications; 2004. The role of nuclear factor kappa-B in models of adult and neonatal cerebral ischemia. The effects of pyrrolidine dithiocarbamate. Doctoral dissertation. [Google Scholar]
- Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjanheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004;91:755–765. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40:1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Opazo C, Huang X, Cherny RA, Moir RD, Roher AE, White AR, Cappai R, Masters CL, Tanzi RE, Inestrosa NC, Bush AI. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H2O2. J Biol Chem. 2002;277:40302–40308. doi: 10.1074/jbc.M206428200. [DOI] [PubMed] [Google Scholar]
- Orwar O, Fishman HA, Ziv NE, Scheller RH, Zare RN. Use of 2,3-naphthalenedicarboxaldehyde derivatization for single-cell analysis of glutathione by capillary electrophoresis and histochemical localization by fluorescence microscopy. Anal Chem. 1995;67:4261–4268. doi: 10.1021/ac00119a010. [DOI] [PubMed] [Google Scholar]
- Ostrakhovitch EA, Lordnejad MR, Schliess F, Sies H, Klotz LO. Copper ions strongly activate the phosphoinositide-3-kinase/Akt pathway independent of the generation of reactive oxygen species. Arch Biochem Biophys. 2002;397:232–239. doi: 10.1006/abbi.2001.2559. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, Horne P, Yang J, Sekoulidis J, Coomaraswamy J, Chishti MA, Cox DW, Mathews PM, Nixon RA, Carlson GA, St George-Hyslop P, Westaway D. In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci USA. 2003;100:14193–14198. doi: 10.1073/pnas.2332851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger EC, Kern P, Ernst M, Bock P, Flad HD, Dietrich M. Inhibition of HIV progression by dithiocarb. German DTC Study Group. Lancet. 1990;335:679–682. doi: 10.1016/0140-6736(90)90802-c. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. J Neurosci Res. 2006;83:1252–1261. doi: 10.1002/jnr.20818. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci USA. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitti R, Lupoi D, Pasqualetti P, Dal Forno G, Vernieri F, Chiovenda P, Rossi L, Cortesi M, Cassetta E, Rossini PM. Elevation of serum copper levels in Alzheimer's disease. Neurology. 2002;59:1153–1161. doi: 10.1212/wnl.59.8.1153. [DOI] [PubMed] [Google Scholar]
- Takashima A, Noguchi K, Michel G, Mercken M, Hoshi M, Ishiguro K, Imahori K. Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci Lett. 1996;203:33–36. doi: 10.1016/0304-3940(95)12257-5. [DOI] [PubMed] [Google Scholar]
- Townsend KP, Pratico D. Novel therapeutic opportunities for Alzheimer's disease: focus on nonsteroidal anti-inflammatory drugs. FASEB J. 2005;19:1592–1601. doi: 10.1096/fj.04-3620rev. [DOI] [PubMed] [Google Scholar]
- Verhaegh GW, Richard MJ, Hainaut P. Regulation of p53 by metal ions and by antioxidants: dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol Cell Biol. 1997;17:5699–5706. doi: 10.1128/mcb.17.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Weihl CC, Ghadge GD, Kennedy SG, Hay N, Miller RJ, Roos RP. Mutant presenilin-1 induces apoptosis and downregulates Akt/PKB. J Neurosci. 1999;19:5360–5369. doi: 10.1523/JNEUROSCI.19-13-05360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltfang J, Smirnov A, Schnierstein B, Kelemen G, Matthies U, Klafki HW, Staufenbiel M, Huther G, Ruther E, Kornhuber J. Improved electrophoretic separation and immunoblotting of beta-amyloid (A beta) peptides 1–40, 1–42, and 1–43. Electrophoresis. 1997;18:527–532. doi: 10.1002/elps.1150180332. [DOI] [PubMed] [Google Scholar]
- Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid β-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22:4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]