Abstract
Striatal cholinergic interneurons are tonically active neurons and respond to sensory stimuli by transiently suppressing firing that is associated with sensorimotor learning. The pause in tonic firing is dependent on dopaminergic activity; however, its cellular mechanisms remain unclear. Here, we report evidence that dopaminergic inhibition of hyperpolarization-activated cation current (Ih) is involved in this process. In neurons exhibiting regular firing in vitro, exogenous application of dopamine caused a prolongation of the depolarization-induced pause and an increase in the duration of slow afterhyperpolarization (sAHP) after depolarization. Partially blocking Ih with specific blocker ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride) reduced firing and mimicked the effects of dopamine on sAHP. The Ih, being active at membrane potentials negative than −50 mV, was inhibited by dopamine via activation of the D2-like receptor, but not D1-like receptor. The inhibitory effects of the D2 receptor activation on Ih were mediated through a protein kinase A-independent cyclic AMP pathway. Consistently, D2-like receptor agonist quinpirole showed comparable effects on sAHP and firing rate as those induced by Ih channel blocker. Moreover, dopamine was unable to further affect the sAHP duration in neurons when Ih was blocked. These findings indicate that D2 receptor-dependent inhibition of Ih may be a novel mechanism for modulating the pause response in tonic firing in cholinergic interneurons.
Keywords: hyperpolarization-activated cation current, afterhyperpolarization, pause, dopamine, ZD7288, basal ganglia
Introduction
Cholinergic interneurons are fundamental to striatal functions, including movement control and sensorimotor learning (Graybiel et al., 1994). Recordings of cholinergic interneurons in vivo (Wilson et al., 1990; Reynolds et al., 2004) and in vitro (Jiang and North, 1991; Bennett and Wilson, 1999; Goldberg and Wilson, 2005; Wilson, 2005) have revealed that these cells correspond to the tonically active neurons (TANs) in the striatum. The TANs exhibit pauses in tonic firing in response to salient stimuli during associative learning (Aosaki et al., 1994a,b; Apicella, 2002; Morris et al., 2004), which are dependent on dopaminergic inputs from the substantia nigra (Aosaki et al., 1994a; Watanabe and Kimura, 1998). However, the cellular mechanisms underlying the pause response are still unclear. Previous studies suggest that activity-dependent changes in both synaptic efficacy and intrinsic membrane property are involved in the occurrence of firing pause (Bennett et al., 2000; Suzuki et al., 2001; Maurice et al., 2004; Reynolds et al., 2004; Wilson, 2005; Wilson and Goldberg, 2006). In addition, the time course of the pause response may be determined by intrinsic membrane properties, because cholinergic interneurons possess ionic conductances that are capable of maintaining tonic spiking and reshaping synaptic inputs (Wilson, 2005; Wilson and Goldberg, 2006).
The majority of cholinergic interneurons in vitro exhibit a regular single-spiking pattern, independent of any synaptic input and apparently controlled by apamin-sensitive calcium-dependent potassium currents (Bennett and Wilson, 1999; Wilson, 2005; Wilson and Goldberg, 2006). After bursts or subthreshold depolarizing pulses, these neurons are able to develop a slow afterhyperpolarization (sAHP) lasting several seconds, which in turn results in a decrease in firing rate or a pause in tonic spiking (Bennett et al., 2000; Reynolds et al., 2004; Wilson, 2005). Indeed, it has been shown that dopaminergic augmentation of sAHP through enhancing excitatory synaptic inputs induces pause responses in cholinergic interneurons in vivo (Reynolds et al., 2004). Several ionic conductances contribute to the sAHP in cholinergic interneurons (Bennett et al., 2000; Wilson, 2005; Wilson and Goldberg, 2006). The onset of sAHP is primarily mediated by an apamin-insensitive calcium-dependent potassium current (IsAHP), and can be amplified regeneratively by a rapidly activating hyperpolarization-activated potassium current. The recovery from sAHP is dependent on a hyperpolarization-activated cation current (Ih). Thus, modulations on these currents may cause changes in the onset and time course of sAHP. Considering the slow kinetics in the decay of IsAHP (Wilson and Goldberg, 2006), it is possible that the sAHP duration is mainly controlled by Ih activation. The present study was therefore designed to examine the impacts of Ih activation on sAHP and to determine whether Ih is involved in the dopamine-dependent pause response in cholinergic interneurons.
Materials and Methods
Male Wistar rats (100–180 g; Charles River Laboratories, Wilmington, MA) were used in the present study. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Brain slice preparation.
Brain slices were prepared using procedures similar to those described previously (Pang et al., 2002; Deng et al., 2005). Briefly, the animals were anesthetized with ketamine-HCl (1 mg/kg, i.p.) and perfused transcardially with an ice-cold (4°C) sucrose solution containing (in mm) 230 sucrose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 10 MgSO4, and 10 glucose, pH 7.4, 290–305 mOsm/L, equilibrated with 95% O2 and 5% CO2. The brains were quickly removed, and transverse striatal slices (300 μm) were cut using a vibratome (VT1000S; Leica, Nussloch, Germany) in the sucrose solution. The slices were maintained in an artificial CSF (ACSF) containing (in mm) 130 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, pH 7.4, 295–305 mOsm/L. The ACSF was continuously equilibrated with 95% O2 and 5% CO2, and slices were incubated for >1 h before recording.
Electrophysiological recording.
Recording electrodes were prepared from borosilicate glass (World Precision Instruments, Sarasota, FL) using a horizontal electrode puller (P-97; Sutter Instruments, Novato, CA). Electrodes had resistances of 2–4 MΩ when filled with an intracellular solution containing (in mm) 120 KMeSO4, 12 KCl, 1 MgCl2, 1 EGTA, 0.2 CaCl2, 10 HEPES, 2 Mg-ATP, and 0.4 Na-GTP, pH 7.4, 295–300 mOsm/L. Oxygenated ACSF was used as bath solution, and the flow rate was adjusted to 2–3 ml/min. Cholinergic interneurons were visualized with an infrared-differential interference contrast microscope (BX50WI; Olympus Optical, Tokyo, Japan) and a CCD camera. All recordings were performed at 32 ± 1°C with an Axopatch 200B amplifier (Molecular Devices, Foster City, CA). After tight-seal (>1 GΩ) formation, the electrode capacitance was compensated. For cell-attached recordings, voltage-clamp mode was used and data acquisition was terminated if the seal resistance fell <1 GΩ. During whole-cell recordings, series resistance (8–15 MΩ) was monitored periodically, and cells with >15% change were excluded from the analysis. For current-clamp recordings, fast I-clamp mode was used. Signals were filtered at 2 kHz and digitized at a sampling rate of 5 kHz using a data-acquisition program (Axograph 4.6; Molecular Devices).
Histochemical staining.
To identify the recorded cells morphologically, 2% neurobiotin (Vector Laboratories, Burlingame, CA) was included in intracellular solution in some experiments and iontophoresed into the cell by passing depolarizing current pulses after successful recording. Slices were fixed overnight with 4% paraformaldehyde at 4°C and then incubated in 0.1% horseradish peroxidase-conjugated avidin D (Vector Laboratories) in 0.01 m potassium PBS (pH 7.4) with 0.5% Triton X-100 for 24 h at room temperature (24°C). After the detection of peroxidase activity with 3,3′-diaminobenzidine as chromogen, slices containing labeled cells were mounted on gelatin-coated slides and processed for histological examination.
Drug application.
All drugs were purchased from Sigma (St. Louis, MO) unless otherwise noted and were bath applied. Drugs were dissolved as concentrated stocks in either water or DMSO and stored at −20°C. Sodium metabisulfite (50 μm) was used as an antioxidant. Working solutions were prepared immediately before use. When DMSO or sodium metabisulfite was used to prepare the drug solution, equivalent amounts were added to ACSF as controls. To examine the dopaminergic modulation in cholinergic interneurons, dopamine (5–100 μm) was used as the agonist for dopamine receptors. (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride (SKF38393) (20 μm) and R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) (20 μm) were used as the selective D1-like receptor agonist and antagonist, respectively. Quinpirole (20 μm) and (±)sulpiride (20 μm) were used as the selective D2-like receptor agonist and antagonist, respectively. Membrane permeable 8-bromo-cAMP (100 μm) and Rp-cAMP (50 μm) were used as the analog and inhibitor of cAMP signaling pathway, respectively. H-89 (5 μm) was used as the selective inhibitor of protein kinase A (PKA). To characterize the Ih in cholinergic interneurons, 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288; 5 or 30 μm; Tocris Bioscience, Ellisville, MO) and CsCl (2 mm) were used as blockers of Ih channels. Tetrodotoxin (TTX; 1 μm) was used to block Na+ channels. In experiments for recording Ih, CdCl2+ (300 μm), 4-aminopyridine (2 mm), and tetraethylammonium (5 mm) were also added to bath solution to block voltage-dependent Ca2+ and K+ channels. In the present study, synaptic blockers were not used because the spontaneous activity of cholinergic interneurons is independent of synaptic input (Bennett and Wilson, 1999).
Data analysis.
Recording data were analyzed with Axograph 4.6. The firing rate was obtained from a 2 min sample of spontaneous spike. The coefficient of variation (CV), a measure of irregularity in interspike intervals, was calculated for cells with a firing rate of >1 Hz. The depolarization-induced pause was defined as the time difference between the termination of the depolarizing current pulse and the peak of the first subsequent spike. The sAHP amplitude was measured as the difference in voltage between the mean subthreshold membrane potential and the maximum hyperpolarization. The time-to-peak of sAHP was calculated as the time between the onset of sAHP and the sAHP peak. The sAHP duration was defined as the time between the onset of sAHP and return of the membrane potential to half sAHP amplitude. For cells in which drug application caused depolarization or hyperpolarization, current injection was used to reset the preapplication membrane potentials.
The Ih in cholinergic interneurons was evoked by a series of hyperpolarizing voltage commands (from −60 to −150 in 10 mV steps, 2 s) from a holding potential of −50 mV. The Ih amplitude was calculated as the current difference between the instantaneous current (measured just after the decay of the capacitive transient) and the steady-state current (mean current of 50 ms before the termination of each voltage step). Voltage-dependent activation of Ih was determined by normalizing the peak amplitudes of tail currents at −50 mV after various hyperpolarizing voltage commands, and then by fitting the current–voltage relationship with a Boltzmann function. The time constants of activation and deactivation were estimated by fitting Ih traces evoked by various hyperpolarizing steps and tail currents at various potentials after a −150 mV step, respectively, with single exponential functions.
The data are presented as mean ± SEM. Statistical difference was detected using paired or unpaired Student's t test (StatView 5.0; Abacus Concepts, Berkeley, CA). Changes were considered significant when p < 0.05.
Results
Cholinergic interneurons in the neostriatum were identified based on their morphological and electrophysiological features (Kawaguchi, 1993; Bennett and Wilson, 1998). As shown in Figure 1A, these cells had large somata with thick primary dendrites and smaller higher-order dendrites bearing few spines. Whereas depolarization induced repetitive spiking followed by a large-amplitude and long-duration afterhyperpolarization, negative current injection produced an initial hyperpolarization followed by a depolarizing sag in the membrane potential, indicating the presence of a cation current Ih. In slices, cholinergic interneurons were tonically active, and most neurons exhibited a single-spiking pattern (Bennett and Wilson, 1999; Wilson, 2005). On average, these neurons discharged spontaneously at a firing rate of 3.19 ± 0.32 Hz, with a CV of 0.22 in the interspike interval (n = 36) (Fig. 1B).
Figure 1.
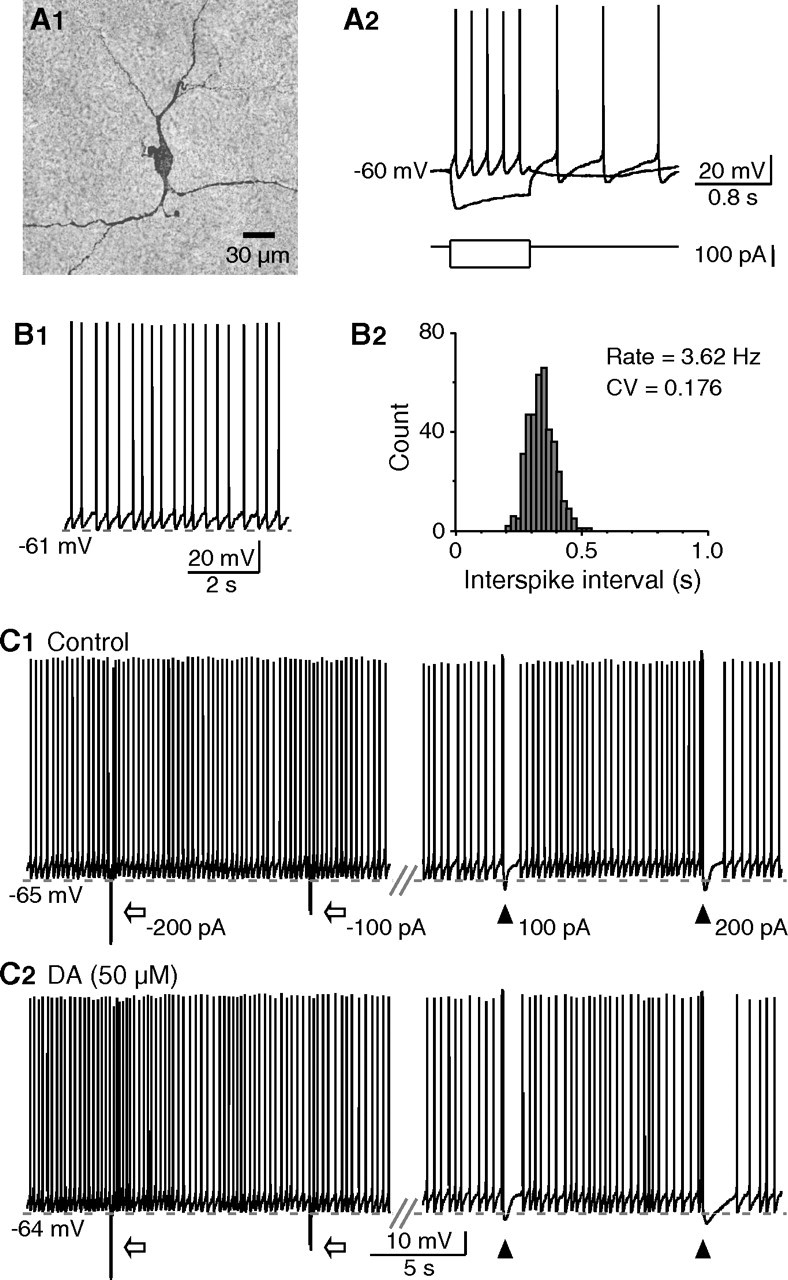
Potentiation of depolarization-induced pause in tonic firing by dopamine. A, Morphological and physiological characterization of striatal cholinergic interneurons. A1, This neuron had large soma with dendrites bearing no spines. A2, During whole-cell recording, negative current injection produced an initial hyperpolarization followed by a depolarizing sag in the membrane potential, whereas depolarizing current induced repetitive spiking followed by a large-amplitude and long-duration afterhyperpolarization. B, A cholinergic interneuron exhibited regular single-spike pattern spontaneously. B1, B2, The firing rate and CV in interspike interval of tonic firing (B1) were shown in the histogram (B2). C, Brief depolarizations, but not hyperpolarizations, produced transient suppression (pauses) in tonic firing. The pauses were associated with the sAHP after depolarizing pulses (100 ms, 100 or 200 pA). Hyperpolarizing pulses (100 ms, −100, or −200 pA) were followed by increased firing caused by rebound depolarization. C1, C2, Compared with that of control (C1), the time course of pauses induced with the same current pulse was prolonged in the presence of dopamine (C2).
Dopaminergic potentiation of depolarization-induced suppression in tonic firing
Previous studies have shown that, in cholinergic interneurons in vivo, both subthreshold and suprathreshold depolarizations produce an sAHP associated with a pause in tonic firing (Reynolds et al., 2004). Similar responses were observed in our in vitro recordings. Brief depolarizations with sufficient current injection (≥100 pA, 80–100 ms), but not hyperpolarizing current pulses, induced an sAHP that resulted in a transient suppression (or pause) in tonic firing (Fig. 1C). The duration of induced pauses was positively related to the extent of depolarization that has profound effects on the amplitude and time course of sAHP (Reynolds et al., 2004). Depolarizing pulses of 100 and 200 pA elicited pauses of 1.61 ± 0.33 and 2.35 ± 0.56 s (n = 8), respectively. These data further support that the sAHP after driven activity may underlie the pause responses in cholinergic interneurons (Reynolds et al., 2004; Wilson and Goldberg, 2006).
Dopaminergic activity is involved in the pause response (Aosaki et al., 1994a,b; Watanabe and Kimura, 1998), presumably by potentiating excitatory synaptic inputs that increase sAHP amplitude (Reynolds et al., 2004). However, synaptic inputs can be amplified by intrinsic membrane conductances (Wilson, 2005), raising a possibility that dopaminergic modulation of these conductances may also contribute to the pause response. We therefore examined whether activation of dopamine receptor has effects on the depolarization-induced pause by exogenous application of dopamine. Hyperpolarizing current was used to maintain membrane potentials at the predrug levels, because dopamine caused a baseline depolarization (Aosaki et al., 1998) that might affect sAHP. In the presence of dopamine (50 μm), the pause was prolonged significantly by 33.7 ± 10.7 and 41.8 ± 9.4% when induced with depolarizing pulses of 100 and 200 pA, respectively (n = 8; p < 0.01), whereas the sAHP amplitude remained about the same (99.6 ± 3.7% of control at 100 pA; 101.8 ± 2.0% of control at 200 pA) (Fig. 1C). These results demonstrate that dopamine is effective to prolong pauses in tonic firing.
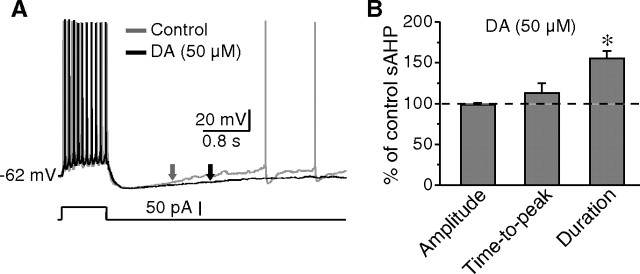
The dopamine-mediated prolongation of pauses might be attributable to regulations on the recovery of sAHP. To test this possibility, the effects of dopamine on the time course of sAHP after depolarizing pulses (50–150 pA, 1 s) were examined. Application of dopamine led to an increase of 55.1 ± 8.9% (n = 6; p < 0.01) in the duration (measured at half amplitude) of sAHP elicited by the same current pulse. However, neither the amplitude nor the time-to-peak of sAHP was altered (Fig. 2). These findings indicate that dopamine causes a deceleration in the recovery from sAHP. Importantly, this effect occurs at membrane potentials more negative than the half sAHP amplitude (−67.2 ± 1.3 mV; n = 6), a voltage range that unlikely activates persistent sodium currents. Together with previous reports (Wilson, 2005), it is strongly suggested that dopaminergic modulation of Ih, at least partially, is responsible for the slowdown of recovery from sAHP.
Figure 2.
Dopamine-mediated increase in sAHP duration. A, The typical responses of a cholinergic interneuron to depolarizing pulses before and after dopamine application. Note that the sAHP duration was prolonged by dopamine (arrows). B, Histogram showing that the sAHP duration was increased in the presence of dopamine. The amplitude and time-to-peak of sAHP remained unchanged. *p < 0.01.
Characterization of Ih in cholinergic interneurons
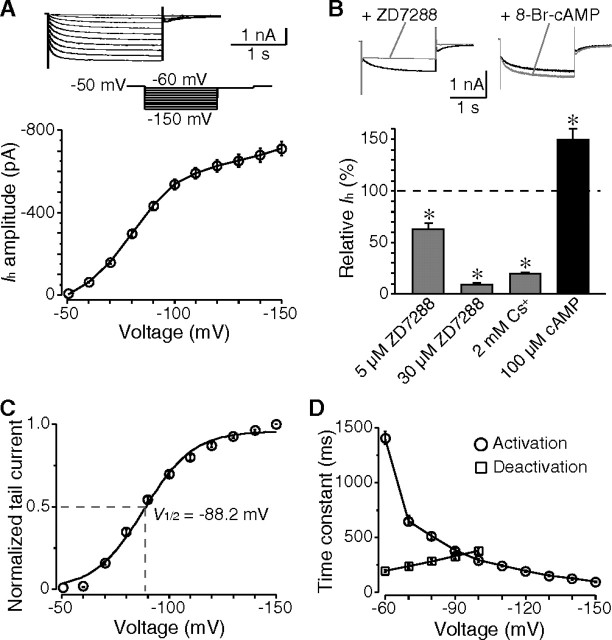
To test whether Ih plays roles in determining the time course of sAHP, we first examined the characteristics of Ih in cholinergic interneurons. A time- and voltage-dependent inward current was activated by hyperpolarizing steps more negative than −50 mV (Fig. 3A). This low activation threshold (−50 to −60 mV) indicates that Ih channels are active during sAHP. The current amplitude was 409.3 ± 15.1 pA (n = 29) when evoked with a command voltage of −90 mV. Application of ZD7288, a specific Ih channel blocker, blocked the current by 37.7 ± 8.1% (n = 6) and 90.6 ± 3.2% (n = 5) at concentrations of 5 and 30 μm, respectively. The Ih was also blocked by 2 mm Cs+ (80.1 ± 3.4%; n = 7), but not by Ba2+ (200 μm) (data not shown). Intracellular cAMP modulates Ih in neurons independent of PKA activity (Pedarzani and Storm, 1995). In the presence of 8-bromo-cAMP (100 μm), the Ih in cholinergic interneurons was dramatically enhanced by 49.9 ± 18.3% (n = 5; p < 0.05) (Fig. 3B).
Figure 3.
Expression of Ih in cholinergic interneurons. A, The time- and voltage-dependent currents evoked by a series of hyperpolarizing voltage steps from a holding potential of −50 mV. These currents were activated at membrane potentials negative than −50 mV. B, Pharmacological properties of Ih. The Ih was blocked by ZD7288 in a dose-dependent manner and by Cs+. Additionally, application of 8-bromo-cAMP dramatically enhanced the current amplitude. Data were measured at −90 mV voltage steps. C, Plot of normalized tail current showing the voltage-dependent activation of Ih, with a half-activation voltage of −88 mV. D, Both activation and deactivation rate of Ih were voltage dependent. *p < 0.01.
The voltage-dependent activation of Ih was analyzed based on the tail current, and showed a half-activation voltage (V1/2) of −88.2 ± 1.9 mV (n = 18) (Fig. 3C). The rate of both activation and deactivation of Ih was voltage dependent. Whereas the time constant of activation decreased sharply with more hyperpolarization, the deactivation became faster with increasing depolarization (Fig. 3D). The Ih displayed an activation time constant of 386.3 ± 23.6 ms when evoked with a hyperpolarizing step of −90 mV (n = 12), and a deactivation time constant of 237.3 ± 17.3 ms when measured at −70 mV after a hyperpolarization of −150 mV (n = 10). These measures are consistent with previous observations that cholinergic interneurons express Ih channel subtypes that exhibit slow activating kinetics (Santoro et al., 2000; Robinson and Siegelbaum, 2003; Notomi and Shigemoto, 2004).
Contribution of Ih to sAHP duration
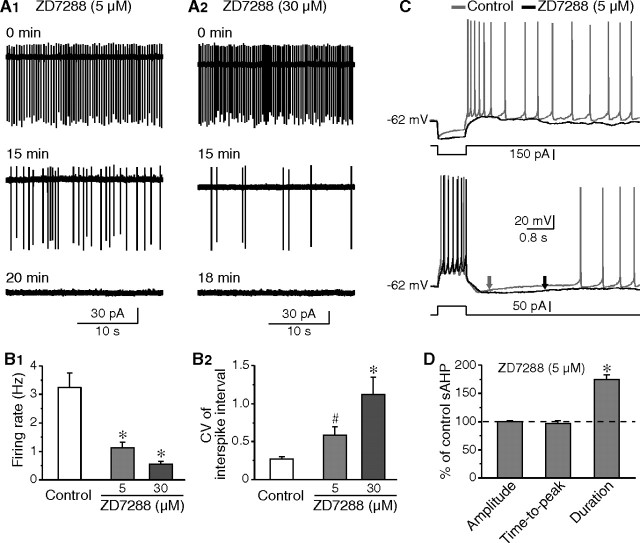
The Ih is critical to the pacemaking activity in various neuron types. In cholinergic interneurons, this current provides depolarizing influence at subthreshold potentials, and together with persistent sodium current, ensures tonic firing (Bennett et al., 2000). Application of ZD7288 produced a progressive reduction in firing rate and regularity in all neurons tested (Fig. 4A,B). Notably, the effects of ZD7288 on tonic firing were concentration-dependent, correlating with the blockade of Ih (Fig. 3B). At ∼15 min in the presence of ZD7288, the firing rate decreased from 3.22 ± 0.51 Hz (control; n = 16) to 1.24 ± 0.21 Hz (5 μm ZD7288; n = 7; p < 0.05) and 0.55 ± 0.11 Hz (30 μm ZD7288; n = 9; p < 0.01); meanwhile, the CV in interspike interval increased from 0.26 (control; n = 16) to 0.58 (5 μm ZD7288; n = 7; p < 0.01) and 1.12 (30 μm ZD7288; n = 9; p < 0.01). Blockade of Ih caused quiescence in spontaneous firing in most neurons (five of seven with 5 μm ZD7288, and nine of nine with 30 μm ZD7288) within 20 min. These results confirm that the firing rate and regularity in spontaneous activity are mostly controlled by Ih in cholinergic interneurons.
Figure 4.
Contribution of Ih to tonic firing and sAHP. A, Cell-attached recordings demonstrating that, in the presence of ZD7288 at concentrations of either 5 μm (A1) or 30 μm (A2), both of the firing rate and regularity were gradually decreased. B, Group data showing the effects of ZD7288 on firing rate (B1) and CV in interspike interval (B2). ZD7288 of higher concentration was more effective. C, Partial blockade of Ih with 5 μm ZD7288 reduced the depolarizing sag during hyperpolarizing pulses and the subsequent rebound depolarization (top). Meanwhile, the sAHP duration was increased (arrows, bottom). D, Group data showing that blockade of Ih significantly prolonged sAHP, but had no effect on sAHP amplitude and time-to-peak. #p < 0.05; *p < 0.01.
The effects of ZD7288 on sAHP were then examined because Ih is active during sAHP and is responsible for the recovery from sAHP. When ZD7288 (5 μm) was applied, the voltage sag observed during a hyperpolarizing pulse and the subsequent rebound depolarization were remarkably reduced (Fig. 4C), indicative of the partial blockade of Ih. Moreover, application of ZD7288 resulted in a moderate hyperpolarization (−5.9 ± 2.1 mV; n = 8), and a decrease in sAHP amplitude (4.0 ± 1.6 mV; n = 5). Current injection was thus used to maintain membrane potentials at preapplication levels. Under this condition, blockade of Ih with 5 μm ZD7288 caused a significant increase in sAHP duration (by 74.0 ± 8.4%; n = 13; p < 0.01), but no detectable change was obtained in both sAHP amplitude and time-to-peak (Fig. 4C,D). These results are comparable with that of dopamine application, and indicate that partially blocking Ih is sufficient to prolong sAHP duration.
Dopaminergic modulation of Ih in cholinergic interneurons
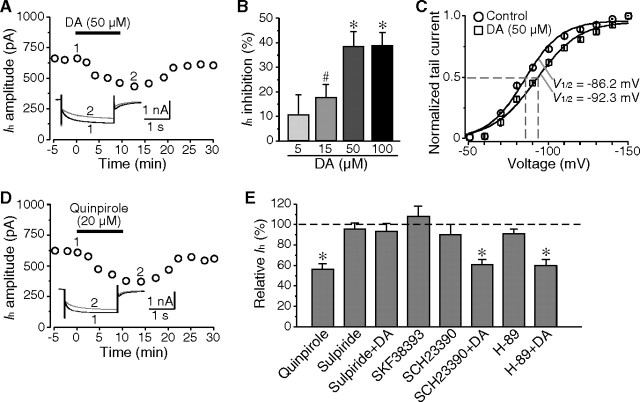
If Ih was involved in the dopamine-induced prolongation of sAHP, it should be regulated by dopamine. As expected, application of dopamine reversibly inhibited Ih in a dose-dependent manner, reaching a maximal effect at a concentration of 50 μm (by 38.2 ± 6.3%; n = 6; p < 0.01) (Fig. 5A,B). In addition, dopamine (50 μm) caused a hyperpolarizing shift in the voltage-dependent activation of Ih (V1/2 in control, −86.2 ± 1.3 mV; V1/2 in dopamine, −92.3 ± 2.0 mV; n = 5; p < 0.05) (Fig. 5C).
Figure 5.
Inhibition of Ih by dopamine through D2 receptor activation. A, Dopamine reversibly inhibited Ih in a cholinergic interneuron. B, Histogram showing the dose-dependent effect of dopamine on Ih. C, Application of dopamine caused a shift of voltage-dependent activation of Ih in the hyperpolarizing direction. D, Quinpirole, the D2-like receptor agonist, showed similar effects on Ih at a concentration of 20 μm as dopamine. E, The inhibitory effect of dopamine on Ih was mediated by D2 receptor activation. D2-like receptor antagonist (sulpiride, 20 μm), but not D1-like receptor antagonist (SCH23390, 20 μm) blocked the inhibition of Ih by dopamine. Both D1-receptor agonist (SKF38393, 20 μm) and antagonist (SCH23390, 20 μm) had no discernable effect on Ih. The Ih amplitude was not altered in the presence of PKA inhibitor (H-89, 5 μm). In addition, coapplication of H-89 and dopamine (50 μm) still significantly reduced Ih. #p < 0.05; *p < 0.01.
Cholinergic interneurons express both D2 and D5 dopamine receptors (Yan et al., 1997). D1- and D2-like receptor agonists and antagonists were used to determine which subtype of dopamine receptor contributed to the dopamine-mediated inhibition of Ih. As shown in Figure 5D, application of D2-like receptor agonist quinpirole (20 μm) reversibly reduced Ih in all neurons tested (by 43.3 ± 5.6%; n = 6; p < 0.01), which mimicked the effects of dopamine. However, the D1-like receptor agonist SKF38393 (20 μm) showed inconsistent effects on Ih. Application of SKF38393 caused an increase of Ih in three of six neurons (by 22.4 ± 5.5%; n = 3; p < 0.01), but had no obvious effect on the other three cells. Overall, no significant change of Ih was detected in the presence of SKF38393 (7.8 ± 10.2%; n = 6; p > 0.05). These results support that the inhibitory effects of dopamine are mainly mediated by D2 receptors (Fig. 5E). Both D1-like receptor antagonist SCH23390 (20 μm) and D2-like receptor antagonist sulpiride (20 μm) failed to alter Ih, indicating that there is no tonic modulation of Ih by dopamine. Our data argue against a recent report that D2 receptor stimulation had no obvious effect on Ih (Maurice et al., 2004). This apparent discrepancy may simply stem from the different D2 receptor agonists applied at different concentrations [20 μm quinpirole vs 10 μm R(−)-propylnorapomorphine], because dopaminergic modulation of Ih is dose-dependent. At higher concentrations, quinpirole may activate 5-hydroxytrypt-amine (5-HT) receptors and, thus, cause nonspecific acts; however, activation of 5-HT receptors has no effect on Ih in cholinergic interneurons (Blomeley and Bracci, 2005). In supporting the above data, application of sulpiride (20 μm), but not SCH23390 (20 μm), completely blocked the inhibitory effects of dopamine on Ih (Fig. 5E).
One of the signaling pathways for the D2 receptor is inhibition of intracellular cAMP accumulation (Neve et al., 2004). We tested whether the same pathway mediated the Ih modulation. Application of Rp-cAMP (50 μm), an inhibitor of the cAMP signaling pathway, resulted in a significant reduction of Ih (33.1 ± 4.0%; n = 7; p < 0.01). In agreement with that the signaling was mediated by cAMP, activation of D2 receptor by quinpirole (20 μm) showed no further inhibition on Ih in the presence of Rp-cAMP (4.1 ± 1.3%; n = 5; p > 0.1). To test whether the cAMP pathway was PKA-dependent, we applied a selective PKA inhibitor H-89 that has been shown to be effective on potassium currents (Deng et al., 2005). The Ih was not changed by H-89 (5 μm) application (92.7 ± 3.8% of control; n = 5; p > 0.1). In addition, dopamine (50 μm) was still effective to inhibit Ih when coapplied with H-89 (by 40.2 ± 6.1%; n = 7; p < 0.01) (Fig. 5E). These data support the conclusion that D2 receptor activation mediates the dopaminergic inhibition of Ih through a PKA-independent cAMP pathway.
Involvement of Ih in dopamine-induced prolongation of sAHP
The preceding data are consistent with the idea that D2 receptor activation reduces tonic firing and prolongs sAHP duration, which resemble the effects of Ih channel blocker ZD7288. In fact, application of quinpirole (20 μm) led to a significant decrease in firing rate (from 3.15 ± 0.77 to 0.84 ± 0.17 Hz; n = 6; p < 0.01) (Fig. 6A), and an increase in CV in interspike interval (from 0.30 to 1.06; n = 6; p < 0.01). We then examined the effects of quinpirole on sAHP duration. Sodium channels were blocked with TTX (1 μm) before quinpirole application, to eliminate the concern of persistent sodium current. As shown in Figure 6B, the depolarizing sag during hyperpolarizing current pulses and the subsequent rebound depolarization were reduced by quinpirole (20 μm). Furthermore, quinpirole application produced a prolongation in sAHP duration (by 44.2 ± 8.1%; n = 6; p < 0.01), without affecting the amplitude and time-to-peak (Fig. 6B,C).
Figure 6.

Involvement of Ih in dopaminergic modulation of sAHP. A, Cell-attached recordings showing that D2 receptor activation with quinpirole (20 μm) reduced spontaneous firing, accompanied by an increase in irregularity. B, After TTX treatment (1 μm), application of quinpirole reduced the depolarizing sag during hyperpolarizing pulses and the subsequent rebound depolarization (top), and prolonged sAHP (arrows, bottom). C, Group data showing that, in the presence of TTX, quinpirole significantly increased sAHP duration, but was ineffective on sAHP amplitude and time-to-peak. D, A neuron was treated first with ZD7288 (30 μm) and then with dopamine (50 μm). Dopamine was unable to further prolong sAHP when Ih was mostly blocked (arrows). E, Histogram showing the changes of sAHP in the presence of ZD7288 alone or ZD7288 plus dopamine. The effects of dopamine on sAHP duration were completely occluded by ZD7288. *p < 0.01.
To further determine the role of Ih, we tested whether Ih channel blocker can occlude the effects of dopamine on sAHP. Cholinergic interneurons were first exposed to ZD7288 (30 μm; ≥15 min), and then dopamine (50 μm) was applied in the presence of ZD7288. Whereas the amplitude and time-to-peak were not changed, the time course of sAHP was significantly increased by ZD7288; importantly, dopamine had no additional effect on sAHP duration (ZD7288 alone, 77.2 ± 7.6%, n = 10; ZD7288 plus dopamine, 74.7 ± 10.4%, n = 6; p > 0.1) (Fig. 6D,E). These results lead to the conclusion that inhibition of Ih contributes to the dopamine-induced prolongation of sAHP in cholinergic interneurons.
Discussion
Our findings demonstrate that dopamine increases the time course of depolarization-induced sAHP. This effect results from a partial inhibition of Ih through D2 receptor activation. Because sAHP accounts for the transient suppression of tonic firing, dopamine-dependent Ih inhibition may be one of the mechanisms for modulating the pause response in cholinergic interneurons that relates to associative learning.
Properties and functional implications of Ih in cholinergic interneurons
Striatal cholinergic interneurons exhibit tonic firing both in vivo and in vitro, which is dependent on intrinsic membrane conductances, but not synaptic inputs (Bennett and Wilson, 1999; Bennett et al., 2000). The results reported here support the conclusion that Ih is essential to the spontaneous activity by providing depolarizing currents at subthreshold membrane potentials (Bennett et al., 2000; Wilson, 2005). These currents are active at membrane potentials below −50 mV, with a half-activation voltage of −88 mV. Thus, Ih channels can be activated during AHP, depolarizing the membrane potential to a voltage range that is enough to activate subthreshold sodium currents that drive the neuron to the spike threshold.
The properties of Ih and its functional roles are primarily determined by channel composition (Ludwig et al., 1998; Santoro et al., 1998; Monteggia et al., 2000). Previous studies have shown that cholinergic interneurons express Ih channel subtypes (HCN), such as HCN2 and HCN4 (Santoro et al., 2000), and HCN3 (Notomi and Shigemoto, 2004). Consistent with morphological observations, Ih in these neurons displayed a slow activating and deactivating kinetics. The absence of fast component (usually with activating time constant <100 ms at −90 mV in native neurons) suggests that cholinergic interneurons do not express HCN1 subtypes (Ludwig et al., 1998; Santoro et al., 1998; Chen et al., 2001; Wilson, 2005). Based on its slow kinetics of both activation and deactivation, Ih may play a major role in determining firing rate in cholinergic interneurons that spontaneously discharge at a mean rate of 2–5 Hz (Bennett and Wilson, 1999; Bennett et al., 2000; Maurice et al., 2004). Our data show that partial blockade of Ih (30–40%) is sufficient to induce a decrease of firing rate and an increase of CV in the interspike interval. However, Ih may not be the only current responsible for controlling firing rate and regularity, because a reduction of sodium currents can produce similar changes in tonic firing (Maurice et al., 2004).
Dopaminergic modulation of Ih in cholinergic interneurons
One of the characteristics of Ih is its sensitivity to cyclic nucleotides (e.g., cAMP). Whereas HCN1 subunits composed channels are weakly sensitive to cAMP, HCN2- to HCN4-mediated currents are highly sensitive to cAMP (Santoro et al., 1998; Chen et al., 2001; Wang et al., 2002; Ulens and Siegelbaum, 2003). The present study demonstrated that Ih in cholinergic interneurons was significantly enhanced by exogenous application of 8-bromo-cAMP. Furthermore, inhibition of cAMP pathway with Rp-cAMP reduced Ih, indicating a constitutional regulation of Ih channels by intracellular cAMP in these neurons.
Accumulating evidence shows that the activity of the Ih channel is regulated by a variety of neuromodulators (Pape, 1996; Frere et al., 2004). Such regulation of Ih is of fundamental importance for the neuronal activity. Cholinergic interneurons receive innervation of several neuromodulators such as dopamine (Yan et al., 1997; Aosaki et al., 1998; Bennett and Wilson, 1998), noradrenaline (Pisani et al., 2003), and 5-HT (Ward and Dorsa, 1996; Blomeley and Bracci, 2005). Among these neuromodulators, 5-HT has no effect on Ih (Blomeley and Bracci, 2005), whereas activation of β1 noradrenergic receptor enhances Ih through a cAMP-dependent mechanism without PKA activation (Pisani et al., 2003). Because neuromodulators can regulate Ih by either increasing or decreasing intracellular cAMP levels (Robinson and Siegelbaum, 2003; Frere et al., 2004), the dopaminergic modulation of Ih may depend on receptor subtypes (i.e., D1- or D2-like receptors). Previous studies have shown that dopamine inhibits Ih via activation of D2-like receptors in ventral tegmental neurons (Jiang et al., 1993) and rod photoreceptors (Akopian and Witkovsky, 1996). Conversely, in neocortex layer I interneurons, dopamine enhances Ih via a synergistic activation of D1- and D2-like receptors, which involves cAMP-dependent PKA activation (Wu and Hablitz, 2005). Those observations suggest that dopamine may differentially modulate Ih in different types of neuron. In the present study, we have shown a dose-dependent inhibition of Ih by exogenous dopamine. Cholinergic interneurons express both D2 and D5 dopamine receptors (Yan et al., 1997). The modulatory effects of D5 receptor activation on Ih are inconsistent (Aosaki et al., 1998; Pisani et al., 2003) as D1-like receptor agonist SKF38393 slightly affects Ih in about half of the neurons. Our data suggest that D5 receptors may not be involved in the dopaminergic modulation of Ih. Instead, D2 receptor activation is responsible for the dopaminergic inhibition of Ih in cholinergic interneurons. The most convincing evidence comes from the data that D2-like receptor agonist quinpirole mimicked the inhibitory effects of dopamine, and that the effects of dopamine could be blocked by D2-like receptor antagonist sulpiride, but not by D1-like receptor antagonist SCH23390. The finding that cAMP pathway inhibitor Rp-cAMP occluded the effects of quinpirole strongly suggests that the dopaminergic modulation of Ih involves a cAMP-dependent mechanism. In addition, the PKA inhibitor H-89 failed to block the effects of dopamine, indicating that cAMP-dependent PKA activation is not required. These results are consistent with that the efforts of neuromodulator are most likely mediated by direct binding of cAMP to Ih channels (Pedarzani and Storm, 1995; Robinson and Siegelbaum, 2003). Notably, dopamine resulted in a hyperpolarizing shift of the voltage-dependent activation of Ih, which may contribute to the reduction of this current.
Implications of sAHP modulation in pause response in TANs
In cholinergic interneurons, sAHP can occur after driven firing or prolonged subthreshold depolarizations (Bennett et al., 2000; Reynolds et al., 2004; Wilson, 2005). The amplitude and duration of sAHP are primarily controlled by complex interactions of ion currents, among which Ih is critical to the recovery from hyperpolarization and thus determines the duration of sAHP (Wilson, 2005; Wilson and Goldberg, 2006). Indeed, our data have shown that partial blockade of Ih results in an increase of sAHP duration. Previous studies have indicated that sAHP may underlie the pause response in cholinergic interneurons (Reynolds et al., 2004; Wilson and Goldberg, 2006). If so, modulation of Ih may affect the time course of pause in tonic firing. It is known that the pause response in TANs is dependent on dopaminergic activity (Aosaki et al., 1994a; Watanabe and Kimura, 1998). Our findings, together with previous observations, suggest that at least three mechanisms may be responsible for the time course of firing pause. Firstly, dopamine facilitates sAHP (both amplitude and duration) by enhancing excitatory synaptic inputs, probably through D5 receptor activation (Suzuki et al., 2001; Reynolds et al., 2004). Secondly, via activating D2 receptors, dopamine reduces subthreshold sodium currents that may contribute to the generation of spikes after sAHP (Maurice et al., 2004). Finally, as reported in the present study, dopamine causes an increase in sAHP duration, which results from D2 receptor-mediated inhibition of Ih. In our experiments, activation of D2 receptor had no effect on sAHP amplitude. The most compelling explanation is that the calcium channels coupled to IsAHP are insensitive to D2 receptor activation (Yan et al., 1997; Goldberg and Wilson, 2005). It is plausible that, although dopamine-induced potentiation of excitatory inputs triggers the occurrence of sAHP and, thus, the generation of pause in tonic firing in single-spiking neurons, inhibition of Ih influences the time course of pause response. This is consistent with reports that the pause response in TANs is mainly dependent on D2 receptors (Watanabe and Kimura, 1998). Although it is notable that the time course of sAHP (and pause) of cholinergic interneurons in vitro differs from that of TANs in vivo, our observation may provide some explanation for the changes in pause response in TANs during behavioral learning. For example, in addition to an increase in the number of responsive TANs, the pauses become coordinated and tend to last longer after conditioning stimuli (Aosaki et al., 1995). It is possible that the dopaminergic prolongation of sAHP via Ih inhibition may be one of the mechanisms for the longer pause responses. It has been speculated that the pause periods of most responsive TANs are likely overlapping (Aosaki et al., 1995). If this is true, prolongation of sAHP, which increases pause duration, may contribute to the coordination of pause responses. Furthermore, D2 receptor-mediated Ih inhibition is sufficient to cause a suppression of tonic firing, which may account for the pause response in TANs without preceding excitation. In behaving animals, midbrain dopamine neurons and striatal TANs show coincident responses to rewards and signals predicting rewards, with burst firing and firing pause, respectively (Morris et al., 2004). It is suggested that the pause response in TANs may provide a time window for dopamine to modify the efficacy of corticostriatal synapses. Therefore, dopamine-dependent modulation of pause duration may affect the synaptic plasticity in corticostriatal circuit, which is crucial to behavioral learning.
Footnotes
This work was supported by National Institutes of Health Grant NS38053 to Z.C.X. P.D. is a recipient of American Heart Association (AHA) Postdoctoral Fellowships 0225549Z and 0425689Z, and AHA Scientist Development Grant 0630172N.
References
- Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. J Neurophysiol. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994a;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994b;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci. 1998;18:5180–5190. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci. 2002;16:2017–2026. doi: 10.1046/j.1460-9568.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J Neurosci. 1998;18:8539–8549. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeley C, Bracci E. Excitatory effects of serotonin on rat striatal cholinergic interneurones. J Physiol (Lond) 2005;569:715–721. doi: 10.1113/jphysiol.2005.098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Pang ZP, Zhang Y, Xu ZC. Increase of delayed rectifier potassium currents in large aspiny neurons in the neostriatum following transient forebrain ischemia. Neuroscience. 2005;131:135–146. doi: 10.1016/j.neuroscience.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Frere SG, Kuisle M, Luthi A. Regulation of recombinant and native hyperpolarization-activated cation channels. Mol Neurobiol. 2004;30:279–305. doi: 10.1385/MN:30:3:279. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci. 2005;25:10230–10238. doi: 10.1523/JNEUROSCI.2734-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol (Lond) 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Pessia M, North RA. Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J Physiol (Lond) 1993;462:753–764. doi: 10.1113/jphysiol.1993.sp019580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Deng P, Ruan YW, Xu ZC. Depression of fast excitatory synaptic transmission in large aspiny neurons of the neostriatum after transient forebrain ischemia. J Neurosci. 2002;22:10948–10957. doi: 10.1523/JNEUROSCI.22-24-10948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Protein kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proc Natl Acad Sci USA. 1995;92:11716–11720. doi: 10.1073/pnas.92.25.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Martorana A, Fusco F, Sancesario G, De Persis C, Bernardi G, Calabresi P. Activation of β1-adrenoceptors excites striatal cholinergic interneurons through a cAMP-dependent, protein kinase-independent pathway. J Neurosci. 2003;23:5272–5282. doi: 10.1523/JNEUROSCI.23-12-05272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Miura M, Nishimura K, Aosaki T. Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J Neurosci. 2001;21:6492–6501. doi: 10.1523/JNEUROSCI.21-17-06492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Siegelbaum SA. Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry. Neuron. 2003;40:959–970. doi: 10.1016/s0896-6273(03)00753-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen S, Nolan MF, Siegelbaum SA. Activity-dependent regulation of HCN pacemaker channels by cyclic AMP: signaling through dynamic allosteric coupling. Neuron. 2002;36:451–461. doi: 10.1016/s0896-6273(02)00968-6. [DOI] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol. 1996;370:405–414. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kimura M. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol. 1998;79:2568–2580. doi: 10.1152/jn.1998.79.5.2568. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron. 2005;45:575–585. doi: 10.1016/j.neuron.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Goldberg JA. Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol. 2006;95:196–204. doi: 10.1152/jn.00630.2005. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hablitz JJ. Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J Neurosci. 2005;25:6322–6328. doi: 10.1523/JNEUROSCI.1405-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]