Abstract
Vestibular hair cells have a distinct planar cell polarity (PCP) manifest in the morphology of their stereocilia bundles and the asymmetric localization of their kinocilia. In the utricle and saccule the hair cells are arranged in an orderly array about an abrupt line of reversal that separates fields of cells with opposite polarity. We report that the putative PCP protein Prickle-like 2 (Pk2) is distributed in crescents on the medial sides of vestibular epithelial cells before the morphological polarization of hair cells. Despite the presence of a line of polarity reversal, crescent position is not altered between hair cells of opposite polarity. Frizzled 6 (Fz6), a second PCP protein, is distributed opposite Pk2 along the lateral side of vestibular support cells. Similar to Pk2, the subcellular localization of Fz6 does not differ between cells located on opposite sides of the line of reversal. In addition, in Looptail/Van Gogh-like2 mutant mice Pk2 is distributed asymmetrically at embryonic day 14.5 (E14.5), but this localization is not coordinated between adjacent cells, and the crescents subsequently are lost by E18.5. Together, these results support the idea that a conserved PCP complex acts before stereocilia bundle development to provide an underlying polarity to all cells in the vestibular epithelia and that cells on either side of the line of reversal are programmed to direct the kinocilium in opposite directions with respect to the polarity axis defined by PCP protein distribution.
Keywords: planar cell polarity, Prickle, hair cell, vestibular, Looptail, line of reversal
Introduction
In the utricle and saccule of the inner ear, stereocilia bundles of vestibular hair cells are oriented in an orderly array that enables faithful detection of linear movements in all directions. Each bundle consists of a staircase array of actin stereocilia, organized with the tallest adjacent to a tubulin-based kinocilium. Movements of the bundle toward the kinocilium generate an excitatory response. In contrast, deflections away from the kinocilium are inhibitory (Corey, 2003). Consequently, hair cells have a functional polarity determined by the polarized disposition of the kinocilium. This type of polarization, oriented parallel to the epithelium, is called planar cell polarity (PCP) and is coordinated between adjacent cells. However, in the utricle and saccule the hair cells are divided by a line of reversal into two groups of opposite orientation. These two populations generate complementary excitatory and inhibitory responses that likely enhance the perception of movement (Fig. 1). Thus patterning the utricle and saccule presents a unique challenge during development: adjacent hair cells share the polarity of their neighbors, but there is an abrupt change in orientation at the line of reversal.
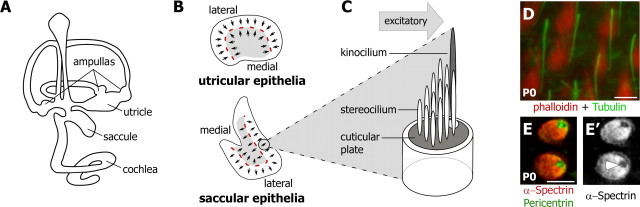
Figure 1.
Vestibular hair cells of the utricle and saccule are organized within the inner ear to optimize the detection of linear accelerations in all directions. A, The vestibular hair cells within the utricle and saccule facilitate the detection of horizontal (utricle) and gravitational accelerations (saccule). In addition, hair cells within the ampullas of the semicircular canals detect rotational movements. Auditory hair cells detect sound and are located within the cochlea. B, In the utricular and saccular epithelia the adjacent hair cells share a similar polarity (small arrows) and are organized about a line of reversal (red dashed line) that separates lateral and medial domains (light gray shading). Arrows indicate the functional and morphological polarity of the bundle and are drawn from the shortest stereocilia to the kinocilium. C, The stereocilia bundle of an individual hair cell is arranged in a staircase manner, with the tallest stereocilia adjacent to the kinocilium. Deflections of the bundle toward the kinocilium are excitatory. D, Polarity can be visualized by using phalloidin (red) and an antibody against acetylated tubulin (green) to label the stereocilia and kinocilia, respectively. E, Alternatively, polarity can be visualized by using α-spectrin antibodies (red) to label the cuticular plate. Pericentrin immunolabeling (green) corresponds to the basal body beneath the kinocilium. E′, Via visualization with α-spectrin alone, the insertion point of the kinocilium and corresponding orientation of a hair cell can be determined by the position of a void of immunofluorescent labeling (arrowhead). Scale bars, 5 μm.
Initiation and coordination of PCP in some tissues is regulated by a group of proteins that has been studied extensively in Drosophila (Klein and Mlodzik, 2005; Karner et al., 2006). These include Frizzled (Fz) (Vinson and Adler, 1987; Vinson et al., 1989), Dishevelled (Dsh) (Klingensmith et al., 1994; Theisen et al., 1994), Van Gogh (Vang) (Taylor et al., 1998; Wolff and Rubin, 1998), and Prickle (Pk) (Gubb et al., 1999). In flies PCP is evident in the organization of hairs emerging from epithelial cells and pointing toward the posterior body or distal wing. Before hair growth the PCP proteins assort to distinct domains reflecting the polarity of the cell, with Fz and Dsh accumulating along the distal edge and Vang and Pk on the proximal side (Adler, 2002; Tree et al., 2002; Klein and Mlodzik, 2005). These protein movements are biased by a directional cue mediated by the atypical cadherins Fat and Dachsous, and the Golgi protein Four-jointed (Yang et al., 2002; Ma et al., 2003; Simon, 2004). This polarizing signal is amplified and reinforced by an intercellular feedback loop that promotes asymmetric accumulation of PCP proteins and generates shared polarity across the field of cells (Strutt, 2001; Tree et al., 2002; Amonlirdviman et al., 2005).
Despite growing evidence that PCP molecules similarly regulate hair cell polarity (Montcouquiol et al., 2003; J. Wang et al., 2005; Y. Wang et al., 2006), it remains unclear when the PCP complex acts, how protein distribution correlates with bundle polarity, and whether the interrelationship of PCP proteins is conserved in vertebrates. Therefore, we assayed the distribution of the PCP molecule Pk2 before bundle morphogenesis and evaluated the subcellular localization of Pk2 and Fz6 in cells on opposite sides of the line of reversal. Our results demonstrate that an underlying molecular polarization is established throughout the entire sensory epithelium early in development and indicate that an additional patterning event determines the line of reversal by affecting how cells interpret this information.
Materials and Methods
Cloning of the mouse prickle-like and Fz6 genes.
Pk1, Pk2, and Fz6 cDNAs were cloned by reverse transcription-PCR from total RNA isolated from embryonic day 18.5 (E18.5) CD-1 mouse embryo, using gene-specific primers designed according to the genomic sequence retrieved by the BLAT (BLAST-like Alignment Tool) search program (University of California, Santa Cruz, Genome Bioinformatics; http://genome.ucsc.edu/). Full-length Pk2 cDNA was cloned into a eukaryotic expression vector and modified to contain an N-terminal enhanced green fluorescent protein (eGFP) tag, using the Invitrogen Gateway system (Carlsbad, CA). Full-length Fz6 cDNA was cloned into pEGFP-N2 (Invitrogen) to generate an expression vector containing C-terminal, eGFP-tagged Fz6.
Production and characterization of Pk2 antisera.
Extensive BLAST (Basic Local Alignment Search Tool) search revealed that a fragment of Pk2 cDNA encoding amino acids 344–526 is Pk2-specific. This fragment was subcloned into the pATH10 vector to generate transient receptor potential E (TrpE) fusion proteins for rabbit immunization. This region also was cloned into the pGEX6P1 vector to produce glutathione S-transferase-tagged Pk2 that was used for affinity purification of the resulting antiserum. Specificity of the Pk2 antibody was determined by Western blotting and immunocytochemistry. Human embryonic kidney 293T (HEK 293T) cells were transfected with the mammalian expression plasmid pDS (negative control) or with the pDS plasmid encoding the full-length Pk1 or Pk2 protein, using FuGENE (Roche, Indianapolis, IN). Protein lysates were made 24 h after transfection in a hypotonic buffer containing 10 mmfs Tris, pH 7.5, 50 mm KCl, 5 mm EDTA, 1% Triton X-100, and Roche complete protease inhibitors and were analyzed by Western blotting. For immunocytochemistry the HEK 293T cells were plated onto fibronectin-coated chamber slides and were fixed with 4% paraformaldehyde in PBS 24 h after transfection. Western blots and fixed cells were stained with the preimmune serum and with the antiserum to Pk2 protein. Affinity-purified antibody to Pk2 was tested against the total brain extract prepared from E18.5 mouse embryos with the hypotonic buffer.
Characterization of the Pk2-eGFP and Fz6-eGFP fusion proteins.
Madin-Darby canine kidney (MDCK) cells were transfected with the mammalian expression vectors encoding the N-terminal eGFP-Pk2 and C-terminal Fz6-eGFP fusion proteins with Lipofectamine (Invitrogen). Localization of fusion proteins was determined 24 h after transfection by confocal microscopy.
Immunofluorescent labeling of vestibular epithelia.
Embryonic tissues (all CD-1, stages E13.5–E18.5) or early postnatal tissues [postnatal days 0 and 5 (P0, P5)] were fixed for 2 h in a solution of 4% paraformaldehyde in PBS, pH 7.4. Utricles and saccules subsequently were removed, dissected to expose the surface of the hair cells, and permeabilized and blocked by using 5% goat or donkey serum, 1% bovine serum albumin (BSA), and 0.5% Triton X-100 in PBS. Primary antibodies and phalloidin were diluted in 5% serum, 1% BSA, and 0.1% Tween 20 or Triton X-100 in PBS and were incubated with the tissue at 4°C for 2 h to overnight. Tissue was washed thoroughly with PBS supplemented with 0.05% Tween 20, followed by incubation with species-specific Alexa Fluor-conjugated secondary antibodies (Invitrogen). Tissue subsequently was washed, mounted, and imaged via standard epifluorescence or confocal microscopy. For cryosections the fixed tissue was cryoprotected overnight in 30% sucrose, embedded in Neg-50 (Richard Allen Scientific, Kalamazoo, MI), frozen, and sectioned at 20 μm. Antibody labeling was performed as described, with detergents omitted from all solutions with the exception of 0.1% Tween 20 during the permeabilization step. The following primary antibodies were used in this study: mouse anti-acetylated tubulin (T6793; Sigma, St. Louis, MO), mouse anti-α-spectrin (MAB1622; Millipore, Bedford, MA), goat anti-eGFP (ab6658; Abcam, Cambridge, UK), guinea pig anti-MyosinVIIa (kindly provided by S. Heller, Stanford University, Stanford, CA), rabbit anti-pericentrin (PRB-432C; Covance, Princeton, NJ), and phalloidin Alexa 568 or Alexa 647 (Invitrogen).
Electroporations.
Utricles were prepared for electroporation from E15.5 to E17.5 embryos with HBSS (Invitrogen) and dissected to remove any visible otolithic membrane and to expose the surface of the hair cells. Then the tissue was transferred to a 7.5 μl drop of plasmid DNA (1–2 μg/μl in HBSS), suspended between two gold-plated electrodes (BTX Model 514, Harvard Apparatus, Holliston, MA), oriented with the hair cells facing the cathode, and electroporated. Electroporation consisted of eight consecutive square waves of 20 V, with 25–50 ms duration and 900 ms pauses between pulses, and was delivered by using a BEX model CUY21 electroporator (BEX, Tokyo, Japan). After electroporation the tissue was secured to noncoated glass coverslips with minutia pins (26002-10, Fine Science Tools, Foster City, CA) that were attached parallel to the glass surface with a small drop of Silicone RTV (Silpak, Pomona, CA). Generally, sufficient nonsensory epithelia or cristae ampullaris tissues were present after dissection so that the utricle could be secured beneath the pins without contacting the field of hair cells. Utricles were cultured at 37°C in a medium consisting of Opti-MEM I (Invitrogen) supplemented with 2% horse serum and 50 μg/ml carbenicillin for 48–72 h before fixation and immunolabeling, as described. Cells were analyzed that could be easily identified as support cells and that expressed asymmetrically localized eGFP-fusion protein. Cells expressing high levels of eGFP throughout the cytoplasm and cell surface were not evaluated. Hair cells proved resistant to electroporation with the use of these parameters.
Animals.
Looptail mice were obtained from the The Jackson Laboratory (Bar Harbor, ME) and maintained by backcross to B6129PF1/J (The Jackson Laboratory) for no more than five generations. Heterozygous mice were selected for breeding based on tail phenotype. For the experiments those Looptail mutants with complete craniorachischisis were selected. Wild-type littermate controls had straight tails. Timed pregnant CD-1 females were purchased from Charles River (Wilmington, MA). All mice were maintained and bred as approved by the Institutional Animal Care and Use Committees at Harvard Medical School and Stanford University School of Medicine.
Results
Prickle-like 2 protein is localized asymmetrically in the vestibular sensory epithelia before development of the stereocilia bundle
The mouse genome contains four prickle-like genes based on the presence of sequences encoding one PET and three Lin1, Islet-1, Mec-3 (LIM) protein domains (Fig. 2A) (Katoh and Katoh, 2003). The PET domain is a motif that is conserved in Prickle, Epsinas, and Testin proteins, whereas LIM is a double zinc-finger domain that may facilitate protein dimerization (Feuerstein et al., 1994). This group of proteins includes Pk1 and Pk2, which share the greatest homology to Drosophila Prickle (64 and 67% identity, respectively, within the PET and LIM domains) as well as sharing a Prickle homology domain (PH), and also includes two shorter molecules called LIM domain-only 6 (LMO6) and Testin. Pk1, Pk2, and LMO6 are prenylated, which likely promotes association with the plasma membrane. Two additional genes have been reported as prickle orthologs, dyxin and the related gene zyxin (Bekman and Henrique, 2002); however, these lack the PET domain and have only two LIM domains (Katoh and Katoh, 2003). In Drosophila a single prickle gene is spliced alternatively to generate prickle, prickleM, and spiny legs isoforms. There is no evidence from Northern blot analysis (data not shown) or vertebrate expressed sequence tag (EST) databases that either Pk1 or Pk2 is spliced alternatively.
Figure 2.
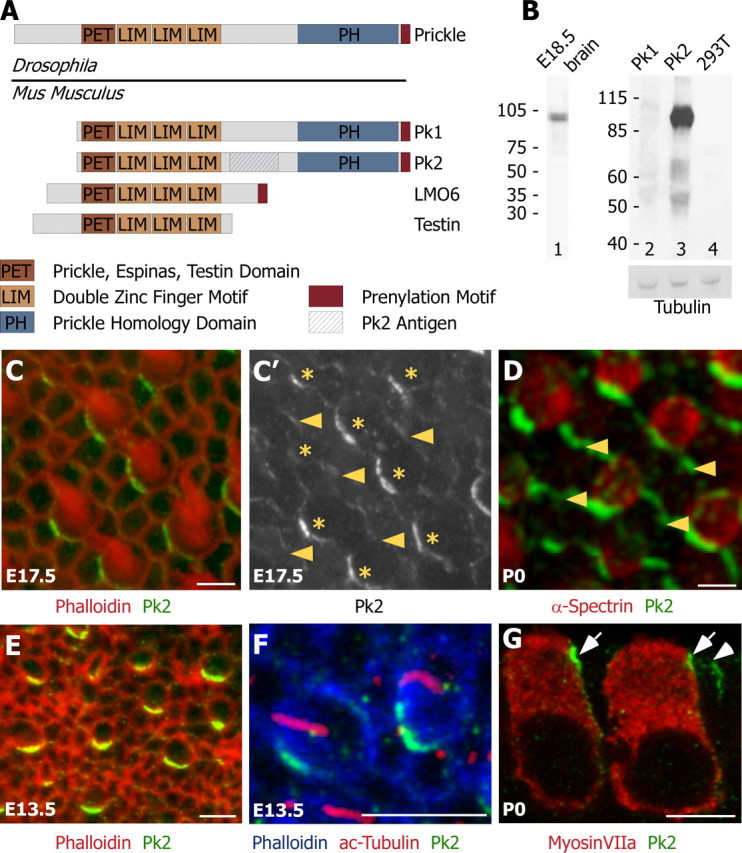
Pk2 is localized asymmetrically at cell boundaries throughout hair cell differentiation and polarization. A, Four Prickle-like proteins can be identified in mouse on the basis of the presence of a single PET and three LIM protein domains. Two of these, Pk1 and Pk2, also contain a Prickle homology domain and are the most similar to Drosophila Prickle. A region unique to Pk2 (hatched box) was used to generate Pk2-specific antiserum. B, On Western blots, the Pk2 antiserum recognizes a single band from E18.5 brain extract (lane 1) and HEK 293T cells expressing Pk2 (lane 3), but not Pk1 (lane 2) or nontransfected HEK 293T cell lysates (lane 4). Immunoblotting with a tubulin antibody was included as a loading control. C, In developing E17.5 utricles, Pk2 (green) is enriched at one edge of hair cells, labeled with phalloidin (red). C′, In the utricle, Pk2 is present at lower levels at boundaries between adjacent support cells than at boundaries between hair cells and support cells. Shown is a gray scale image of Pk2 labeling from C, with arrowheads indicating boundaries between adjacent supporting cells and asterisks marking the positions of hair cells. D, In the saccule, Pk2 labeling (green) is more prominent at boundaries between support cells (arrowheads) than in the utricle. Hair cells are labeled with α-spectrin (red). E, At E13.5, Pk2 (green) is enriched at one edge of hair cells that lack a stereocilia bundle but can be identified by their rounded shape and mosaic distribution via phalloidin stain (red). F, Pk2 accumulation (green) at E13.5 precedes the asymmetric localization of the kinocilium (red) to one edge of the cell (phalloidin; blue). G, In sections, Pk2 enrichment (green) is seen near the apical surface of the tissue at boundaries between supporting cells (arrowhead) and adjacent to hair cells (arrows), identified by the hair cell marker MyosinVIIa (red). Scale bars, 5 μm.
We have produced an antibody against Pk2 that recognizes a single band from E18.5 mouse brain lysate and does not cross-react with Pk1 produced in HEK 293T cells (Fig. 2B). Whole-mount utricles dissected from E17.5 mouse embryos and labeled with phalloidin to mark the stereocilia bundles have a marked, asymmetric localization of Pk2 at the cell boundary along one side of all hair cells (Fig. 2C). This subcellular localization is shared by adjacent hair cells and is reminiscent of the distribution of PCP molecules in Drosophila. Similar localization is seen at hair cell–support cell boundaries in the saccule (Fig. 2D) and the three cristas of the semicircular canals (data not shown).
PCP can first be detected in the mouse vestibular epithelia by using scanning electron microscopy at E13.5 when the kinocilium migrates to one side of the earliest differentiating cells (Denman-Johnson and Forge, 1999). However, there are indications that in some contexts hair cell precursors may be polarized before kinocilium formation. For example, ciliated epithelia from the quail oviduct have a distinct PCP, and transplantation studies have revealed that the epithelial cell precursors are polarized before their differentiation and ciliogenesis (Boisvieux-Ulrich and Sandoz, 1991). Similarly, the polarity of zebrafish lateral line hair cells is determined by the direction of migration of the developing neuromast before differentiation (Lopez-Schier et al., 2004). In the mouse, vestibular hair cells are added continually to the epithelia between E12.5 and birth. At E13.5 the kinocilium appears centrally on the apical surface of the earliest born hair cells before migrating directly to its final polarized location (Denman-Johnson and Forge, 1999).
To determine when the PCP complex acts relative to these early morphological rearrangements, we evaluated the distribution of Pk2 in developing vestibular epithelia at E13.5. At this stage nascent hair cells lack visible stereocilia when viewed with phalloidin but can be distinguished from the surrounding epithelia by their rounded shape and regular distribution within the mosaic array of hair cells and support cells. In these cells Pk2 is enriched and localized asymmetrically along one edge, and this distribution is apparent soon after the hair cell can be distinguished from its neighbors (Fig. 2E). To determine whether the molecular polarization of Pk2 precedes morphological polarization of the hair cell, we visualized the position of the kinocilium by using antibodies against acetylated tubulin. At E13.5 prominent Pk2 crescents were present in developing hair cells that still had a centrally located kinocilium (Fig. 2F). Together with the work of Denman-Johnson and Forge (1999), these data suggest that the initial cohort of hair cells is born into a developing epithelium that contains polarity cues and that nascent hair cells are themselves molecularly polarized before becoming morphologically asymmetric. In addition, Pk2 crescents are still present at hair cell–support cell boundaries at P12 (data not shown), suggesting roles for the PCP complex in both the initiation and maintenance of hair cell polarity.
Although Pk2 levels are enriched at the hair cell–support cell boundary, protein is also detected at boundaries between adjacent support cells, which can be identified by the absence of the stereocilia bundle (Fig. 2C,D). This labeling is weak in utricular epithelia and more prominent in the saccule (Fig. 2D). The distribution of Pk2 in support cells is similar to reports of Fz3 and Fz6 localization at support cell boundaries although, unlike Pk2, an enrichment of Fz protein was not reported at hair cell–support cell boundaries (Y. Wang et al., 2006). When it is viewed in sections, Pk2 is localized to the apical junction of the hair cell–support cell and support cell–support cell boundaries (Fig. 2G). A similar apical localization occurs for other vertebrate PCP molecules (J. Wang et al., 2005; Montcouquiol et al., 2006; Y. Wang et al., 2006) and is consistent with the hypothesis that these molecules form a complex analogous to the PCP complex in Drosophila.
The high levels of Pk2 at the hair cell–support cell boundaries could be located either at the medial side of hair cells or the lateral side of adjacent support cells. Because of the close apposition of the hair cell and support cell membranes, the distribution in one cell type or the other could not be resolved by using standard immunofluorescence. Therefore, we assayed the distribution of exogenous Pk2 introduced by electroporation. Utricular epithelia were electroporated with N-terminal eGFP-tagged Pk2 and were labeled with antibodies against GFP, α-spectrin, and Pk2 to visualize both endogenous and exogenous Pk2. Hair cells were identified on the basis of α-spectrin expression, which is an actin cross-linking molecule enriched throughout the cuticular plate, a cytoskeletal structure that is unique to hair cells. The analysis was restricted to support cells, because hair cells did not take up plasmid DNA introduced by electroporation. Exogenous eGFP-Pk2 accumulated in crescents overlapping with endogenous Pk2, verifying that the fusion protein is a reliable indicator of Pk2 localization. In all of the cells that were assayed, the fusion protein was enriched at the medial edge of the cell, including boundaries between support cells and between support cells and hair cells (n = 26 cells) (Fig. 3A,B). Moreover, in electroporated support cells located adjacent to hair cells, eGFP-Pk2 formed a crescent on the medial side of the support cell that was distinct from the endogenous Pk2 present at the hair cell–support cell boundary (n = 5 cells) (Fig. 3A, i.e., S2). Therefore, we infer that the endogenous Pk2 enriched at the hair cell–support cell boundary is contributed by the hair cell and is localized to its medial edge rather than to the lateral edge of the adjacent support cell. In summary, Pk2 is distributed asymmetrically to the medial cell boundaries of hair cells and support cells and is enriched in hair cells.
Figure 3.
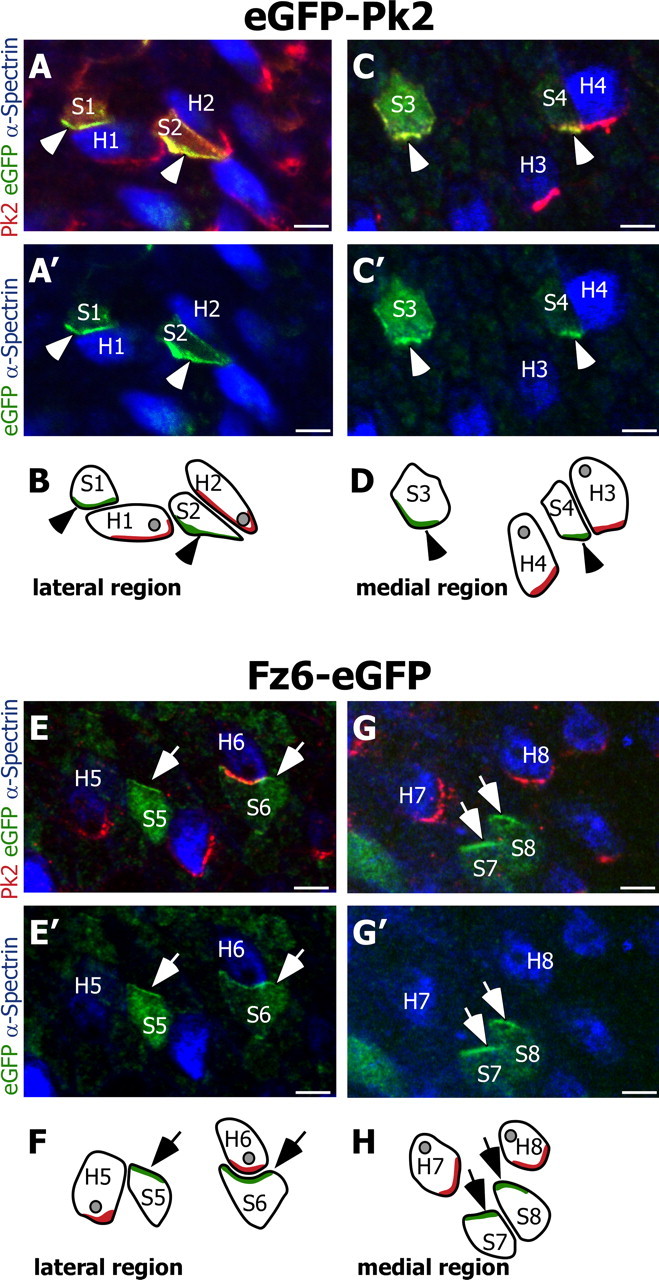
Pk2 and Fz6 are redistributed to opposite sides of utricular hair cells and support cells. A, Two support cells located within the lateral domain of the utricle (labeled S1 and S2) electroporated with eGFP-Pk2 (green) distribute eGFP-tagged Pk2 along their medial edges (arrowheads). Endogenous Pk2 (red) forms crescents along the medial cell boundary of two hair cells [identified by using α-spectrin (blue); labeled H1 and H2] and does not overlap with the distribution of eGFP-Pk2 in S1 or S2. The Pk2 antibody also labels exogenous eGFP-Pk2 in the support cells. Electroporated cells are located in the lateral domain of the utricle. B, Diagram illustrating the relative positions of support cells and hair cells from A and A′. In this and all subsequent diagrams the subcellular localizations of eGFP-tagged protein and endogenous Pk2 are indicated by green and red shading, respectively. The position of the hair cell kinocilia, based on α-spectrin labeling, is illustrated as a gray spot. C, C′, D, Support cells located on the opposite side of the line of reversal and within the medial region of the utricle (labeled S3 and S4) also distribute eGFP-tagged Pk2 (arrowheads) along their medial edges, similar to endogenous Pk2 in hair cells (labeled H3 and H4). E, E′, F, In contrast, support cells electroporated with Fz6-eGFP (labeled S5 and S6) redistribute Fz6-eGFP protein (green) to their lateral edge (arrows). In these cells Fz6-eGFP is enriched opposite of the endogenous Pk2 crescents (red) present in adjacent hair cells (H5 and H6). In E and F the electroporated cells are located in the lateral domain of the utricle. G, G′, H, Support cells located on the opposite side of the line of reversal and within the medial region of the utricle (labeled S7 and S8) also redistribute Fz6-eGFP to their lateral edge (arrows). This distribution is opposite from that of the endogenous Pk2 (red) in hair cells (labeled H7 and H8). For each utricle, the medial and lateral regions are identified by the position of the line of reversal, and images are oriented with the lateral edge of hair cells on top. Scale bars, 5 μm.
The subcellular distribution of PCP molecules does not change at the line of reversal
A unique feature of the vestibular epithelia is the presence of a line of polarity reversal contained within the striola, a specialized region containing hair cells of unique morphology and physiology (Desai et al., 2005). Cells on opposite sides of the boundary have stereocilia bundles oriented with opposite polarities (Fig. 1). The appearance of the line of reversal is correlated with the addition of a second group of hair cells of opposite polarity that emerges after the initial field of hair cells has differentiated. In the rat utricle the oldest hair cells are born in the medial epithelia, followed by the addition of cells of opposite polarity laterally. Similar events occur in the saccule, where hair cells differentiate first in the lateral regions and then are added, with opposite bundle polarities, to the center (Sans and Chat, 1982). In the mouse these two groups of cells can be distinguished from each other morphologically at E15.5, and additional hair cells are added continually to both regions throughout embryonic development (Denman-Johnson and Forge, 1999). Little is known about the molecular events that determine the position of the line of reversal; however, a similar patterning event occurs in the Drosophila eye, where ommatidia reverse polarity at the equator. Significantly, this change at the equator is correlated with a difference in distribution of the PCP molecule Fz (Zheng et al., 1995; Cooper and Bray, 1999; Tomlinson and Struhl, 1999; Yang et al., 2002). We therefore asked whether PCP protein localization changes across the line of reversal in the utricle and saccule.
The subcellular distribution of exogenous eGFP-Pk2 and Fz6-eGFP in electroporated support cells was compared between cells located on either side of the line of reversal. The polarity of hair cells was determined on the basis of the void of α-spectrin labeling of the cuticular plate that occurs at the position of the kinocilium basal body (Fig. 1D). We found that eGFP-Pk2 localizes to the medial edge of electroporated support cells in both the lateral (n = 11) (Fig. 3A,B) and medial regions (n = 15) (Fig. 3C,D) of cultured utricles. Hence the relative distribution of Pk2 is not altered between vestibular epithelial cells located on either side of the line of reversal. We also looked at the distribution of a second PCP molecule, Fz6, that together with Fz3 is necessary for the proper orientation of vestibular hair cells within the cristae ampullaris and auditory hair cells in the organ of Corti (Y. Wang et al., 2006). When electroporated into utricular support cells, Fz6-eGFP is localized to the lateral edge of cells located in both the lateral (n = 15) (Fig. 3E,F) and medial domains (n = 10) (Fig. 3G,H). Similar to eGFP-Pk2, the subcellular distribution of Fz6-eGFP is not altered between cells located on either side of the line of reversal. However, Fz6-eGFP is localized opposite to endogenous Pk2. Moreover, exogenous Fz6-eGFP located at the lateral edge of an electroporated support cell abuts endogenous Pk2 located at the medial edge of a hair cell (Fig. 3E, i.e., S6). Therefore, we infer that vertebrate PCP proteins are distributed throughout vestibular epithelia in a manner similar to the distribution of PCP proteins in Drosophila wing epithelia; Fz and presumably Dsh proteins are localized together at the lateral edge, and Pk2 and presumably Van Gogh-like 2 (Vangl2) are localized together at the medial edge of cells throughout the vestibular epithelia.
These results were validated by our visualizing the distribution of endogenous Pk2 in cells on either side of the line of reversal, using α-spectrin distribution to define hair cell orientation unambiguously. We found that, in both the utricle and saccule, Pk2 was present on the medial edge of hair cells on either side of the line of reversal, regardless of bundle orientation and kinocilium placement (Fig. 4A–D). As a result, in saccular hair cells located lateral to the line of reversal, Pk2 protein is present in a crescent opposite the kinocilium, whereas in hair cells located medial to the line of reversal Pk2 and the kinocilium are adjacent (Fig. 4A,B). By comparison, in the utricle, where hair cells point toward each other across the line of reversal, Pk2 and the kinocilia are opposite to each other in medial regions and adjacent laterally (Fig. 4C,D). Furthermore, the uniform distribution of Pk2 is apparent at E13.5 (Fig. 2F) when the initial cohort of hair cells is differentiating and persists beyond E15.5 when the line of reversal first can be visualized (Fig. 4E). We observe no evidence of cellular rearrangements or rotation of polarizing hair cells on either side of the line of reversal, suggesting that newly differentiating cells directly adopt and then retain their morphological polarity after acquiring asymmetric localization of PCP proteins. These findings show that the asymmetric distribution of the PCP proteins is uniform across the entire epithelium throughout development, with all cells localizing Pk2 to their medial side, but suggest that cells differentially interpret polarization information on the two sides of the line of reversal. Consequently, there must be an additional patterning event that determines the final position of the kinocilium and hence the line of reversal, relative to the polarity axis established by the PCP complex.
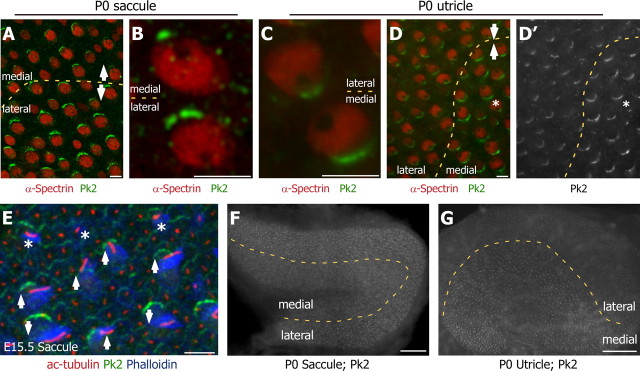
Figure 4.
The relative distribution of Pk2 does not change across the line of reversal. A, D, The line of reversal (dashed yellow line) in P0 saccule A and utricle D is visualized easily by the position of the kinocilium void within the cuticular plate of hair cells that have been labeled with an antibody against α-spectrin (red). The polarities of pairs of cells on opposite sides of the line of reversal from both epithelia are indicated by arrows. Despite the change in hair cell polarity, the relative distribution of Pk2 (green) remains constant. B, C, Higher-magnification image containing two hair cells from saccule B and utricle C located on opposite sides of the line of reversal. In each the Pk2 (green) is located on the medial edge of the cell; however, there is a relative change in the amount of Pk2 protein between the two cells. D′, Gray scale image of Pk2 across the line of reversal in utricle reveals a correlation between the position of the kinocilium and relative levels of Pk2. An asterisk (D, D′) indicates a hair cell that does not share the polarity of its neighbors and has a corresponding decrease in Pk2 protein. E, The line of reversal is first apparent at E15.5 and can be visualized by the position of the kinocilium (acetylated tubulin; red) relative to the stereocilia bundle (phalloidin; blue). At this time, Pk2 (green) already is forming crescents along the medial edges of both mature and newly differentiated hair cells (asterisks). The polarity of developing hair cells is indicated by arrows. F, G, Low-magnification images of Pk2 immunofluorescence across the entire saccular (F) and utricular (G) epithelia emphasize the correlation between levels of Pk2 protein and the location of the line of reversal (dashed yellow line). Scale bars: A–E, 5 μm; F–G, 100 μm.
Although the orientation of the Pk2 crescent does not determine bundle placement and orientation directly, we observed that the amount of Pk2 present at an individual hair cell boundary correlates with the final orientation of the stereocilia bundle so that Pk2 labeling is more intense when it is opposite to the kinocilium than when it is adjacent (Fig. 4D′). Even the occasional cell near the line of reversal that points in the “wrong” direction shows a Pk2 level appropriate for its orientation and does not match its neighbors (Fig. 4D,D′, asterisk). The change in Pk2 levels about the line of reversal is prominent in utricles and saccules viewed at lower magnification and distinguishes medial and lateral domains of the epithelia (Fig. 4F,G). Overall, we observed that in both the utricle and saccule the kinocilia are located opposite large Pk2 crescents and adjacent to small Pk2 crescents. Thus Pk2 protein levels can be used reliably to identify the line of reversal in both epithelia.
Vangl2 acts early to coordinate hair cell polarity and is necessary for the maintenance of Pk2
In Drosophila an initial polarity cue is amplified by interactions between PCP proteins, which results in the localization of Pk and Vang at one end of a cell and Fz and Dsh at the opposite side of that cell. The loss of one protein results in the mislocalization of the others (Axelrod, 2001; Shimada et al., 2001; Strutt, 2001; Tree et al., 2002; Bastock et al., 2003). Similar protein interactions are likely to occur in the vertebrate ear. For example, a murine ortholog of vang, called vangl2, is mutated in Looptail mice, resulting in individual cells that remain polarized but are misoriented relative to each other (Montcouquiol et al., 2003). The orientations of subsets of hair cells also are disorganized in Fz3–Fz6 double knock-outs (Y. Wang et al., 2006) and Dsh1–Dsh2 double knock-outs (J. Wang et al., 2005). In addition, the asymmetric localization of some of these proteins suggests a mechanism of action that is similar to that proposed in Drosophila, although differences also have been proposed (Murdoch et al., 2003; Lu et al., 2004; Montcouquiol et al., 2006).
In Drosophila Prickle and Vang form a functional complex on one side of the cell, with Frizzled on the other. However, recent studies of Vangl2 function raise questions as to whether these relationships are maintained in vertebrates (Montcouquiol et al., 2006). To test whether Vangl2 likewise is required for the asymmetric localization of Pk2, we examined the distribution of Pk2 protein in Looptail mutants beginning at E14.5, because the retarded growth and small inner ear bone of Looptail mutants precluded dissections at earlier stages. We found that at E14.5 Pk2 crescents are present within individual cells but that the location of the crescents is not coordinated between adjacent cells (Fig. 5A,B), revealing a disorganized pattern of cell polarities reminiscent of the partially randomized final polarity of hair cells in Looptail mutants (Fig. 5D,F). Pk2 distribution 2 d later is symmetrical and completely surrounds Looptail mutant cells (Fig. 5C,D). This is most prominent in the medial utricle and lateral saccule where Pk2 immunolabeling also is strongest in wild-type tissues. By E18.5 Pk2 levels are dramatically lower, and very little Pk2 accumulation at the hair cell–support cell boundary is seen (Fig. 5E,F). This developmental series reveals the dynamic nature of Pk2 localization in Looptail mutants. Indeed, at E16.5 there is a mixture of mature and newborn hair cells, and individual cells can be identified with Pk2 distributed in each of three states: asymmetric but uncoordinated, symmetric, and degraded. The observed asymmetric distribution of Pk2 in E14.5 Looptail mutant hair cells is striking; however, we do not know whether this initial localization reflects residual function of Vangl2 or potential compensation by Vangl1. It seems likely that Vangl2 and Pk2 colocalize at the medial edge of all cells in vestibular epithelia because Vangl2 is also asymmetrically localized in vestibular epithelia, the Looptail mutation disrupts Pk2 localization, and because Vang binds Pk in flies (Jenny et al., 2003). Together, these results demonstrate that Vangl2 functions before E14.5 to coordinate the planar polarization of differentiating hair cells and that Vangl2 is required for the maintenance of Pk2 asymmetric localization at later stages.
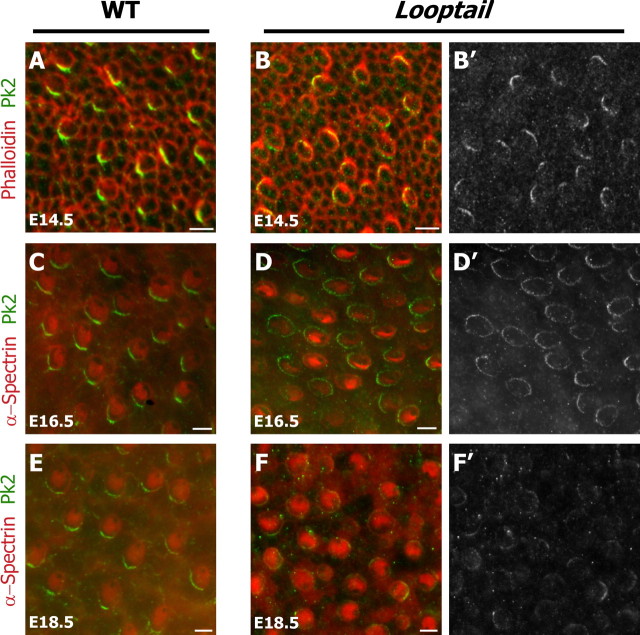
Figure 5.
The Looptail mutation disrupts the maintenance, but not the early asymmetric localization, of Pk2. A, B, At E14.5, before maturation of the stereocilia bundle is evident with phalloidin (red), Pk2 (green) is localized to one edge of developing hair cells in wild-type and Looptail mutants. However, this localization is not coordinated between adjacent cells in mutant tissue (B). C, D, Similarly, at E16.5 Pk2 localization differs between wild-type (C) and Looptail mutant (D) hair cells labeled with α-spectrin (red). In Looptail, the distribution is symmetric and appears to surround individual hair cells. E, F, At E18.5 the Pk2 (green) is enriched at the medial edge of wild-type utricular hair cells (E). In contrast, the Pk2 crescents are disrupted in Looptail mutant hair cells (F), which also are misoriented relative to each other. B′, D′, F′, Gray scale images of the distribution of Pk2 corresponding to B, D, F. Scale bars, 5 μm.
Discussion
We have used an antibody against Pk2 to visualize the earliest stages of vestibular hair cell polarization and to assess the organization of PCP proteins on both sides of the line of reversal. We found that the PCP complex functions early, before the time when stereocilia bundles can be visualized with phalloidin and before the polarized migration of the kinocilia to one edge of the cell (Denman-Johnson and Forge, 1999). Hence the distribution of PCP molecules reflects the polarization of the developing epithelia and could define a polarity axis that guides the initial movements of the kinocilia. In the developing organ of Corti Vangl2 is redistributed from a uniform distribution to one edge of differentiating auditory hair cells (Montcouquiol et al., 2006). By comparison, Pk2 appears to be localized asymmetrically shortly after the protein is apparent and before hair cells begin to become morphologically asymmetric. Although it is not known yet whether Fat–Dachsous interactions set up the initial polarity of the PCP complex as in flies, our results indicate that a polarizing cue is active before E13.5 and is likely to regulate the distribution of Pk2.
The persistence of Pk2, Fz3/6, and Vangl2 proteins that follow the morphological polarization of vestibular and auditory hair cells suggests that the PCP complex also may operate after polarity is first established (Montcouquiol et al., 2006; Y. Wang et al., 2006). For auditory hair cells one of these functions is the reorientation that fine tunes the final position of the kinocilium during early postnatal maturation. This process may involve Wnt extracellular ligands that bind to Fz and influence PCP signaling (Dabdoub et al., 2003), because Fz3/6, Vangl2, and Dsh1/2 are present in auditory hair cells at this time (J. Wang et al., 2005; Montcouquiol et al., 2006; Y. Wang et al., 2006). Similar reorientation events have not been described in the vestibular system; however, these may be difficult to discern because the organization of hair cells is not as stereotypic as in the organ of Corti. Together, these studies support the idea that vertebrate PCP proteins are involved in the initiation, reinforcement, and maintenance of hair cell polarity in all sensory epithelia of the ear.
Although the polarity of hair cells on either side of the line of reversal is likely to be directed by the core PCP complex, an additional patterning event also is required to determine the final orientation of hair cells and hence the line of reversal. This is in contrast to the PCP patterning that occurs at the equator of the fly eye, where the change in ommatidia orientation is correlated with a change in the relative distribution of PCP molecules. By comparison, individual vestibular hair cells appear to make a position-specific polarity decision that is guided by the distribution of PCP molecules in combination with other information. It is not clear whether the differences in Pk2 levels on either side of the line of reversal are causative, perhaps by changing the balance of competitive interactions with other PCP molecules such as Diego (Jenny et al., 2005). Alternatively, the change in levels could reflect other differences in cells on either side of the line of reversal and raises the possibility that additional cell-intrinsic mechanisms determine the change in orientation. This result fits with the observation from Y. Wang et al. (2006) that the location of Fz relative to the kinocilium is different in auditory versus vestibular epithelia. The authors suggested that this was attributable to differences between vestibular and auditory hair cells. More likely, the observation reflects the cell-intrinsic mechanism that we infer operates at the line of reversal.
In Drosophila the core PCP molecules are responsible for both the generation and coordination of polarity, with hairs growing from the centers of mutant cells and often pointing in directions different from their neighbors (Wong and Adler, 1993). If general PCP mechanisms are conserved between these species, then a similar linkage should be present in the mouse. The transient localization of Pk2 in Looptail mutant cells reveals a random organization of hair cells as early as E14.5, indicating that Vangl2 must play an early role in coordinating polarity. However, in Looptail mutants individual hair cells have asymmetrically localized bundles despite being misoriented relative to their neighbors (Montcouquiol et al., 2003), and a similar phenotype is present in other vertebrate PCP mutants (Curtin et al., 2003; Lu et al., 2004; J. Wang et al., 2005; Y. Wang et al., 2006). The vangl2 mutation in the Looptail mouse disrupts protein localization (Montcouquiol et al., 2006) and binding to Dsh (Torban et al., 2004), but it is not a null allele. It is possible that residual Vangl2 function or compensation from another vang ortholog or other PCP molecules facilitates polarization of individual cells but is insufficient to coordinate the polarity of adjacent cells. Indeed, there is likely to be significant redundancy in the vertebrate system, because single Fz3 or Fz6 mutants and Dsh1 or Dsh2 mutants lack the hair cell PCP phenotype that appears in Fz3/6 and Dsh1/2 double mutants (J. Wang et al., 2005; Y. Wang et al., 2006). Alternatively, vertebrate PCP molecules may function primarily to coordinate polarity between neighbors. In this scenario it is unclear what directs movement of the kinocilium to one side of the cell. One possibility is that kinocilium movement is a default event and that hair cells acquire polarity even in the absence of a directive cue. This would be analogous to yeast, Dictyostelium cells, and neutrophils that spontaneously polarize even in uniform chemoattractant environments (Weiner et al., 2002; Devreotes and Janetopoulos, 2003; Wedlich-Soldner et al., 2003).
At multiple levels our data are consistent with the idea that the PCP complex acts in vertebrates as it does in flies. First, polarization is evident in both hair cells and support cells, so cell–cell communication can occur as predicted, i.e., in a bucket brigade-like cascade of cell–cell interactions. Second, Fz6 and Pk2 are localized to the opposite sides of individual cells, similar to the distribution of PCP molecules in Drosophila wing epithelia. Third, Pk2 becomes localized asymmetrically in the vestibular system at a time that is consistent with an involvement in the initiation and coordination of polarization. This result, combined with our observation that Vangl2 is required before E14.5, predicts that any polarizing cue must be active before hair cell differentiation. Finally, the maintenance of Pk2 at cell–cell boundaries requires fully functional Vangl2, similar to what is observed for Vang and Pk in Drosophila. Furthermore, we report the unexpected finding that an additional patterning mechanism is required to generate discontinuity at the line of reversal. This mechanism does not require a change in the distribution of PCP molecules and likely involves region-specific information that specifies how the axis of PCP protein asymmetry is interpreted.
Footnotes
This work was supported by the Alfred P. Sloan Foundation and a Basil O' Connor Starter Scholar Award (to L.V.G.), by a Deafness Research Foundation research grant (to M.R.D.), by Grant RSG-03-239-01 from the American Cancer Society (to J.D.A.), and by National Institutes of Health Grant CA088060 (to M.P.S.). M.P.S. is an investigator of the Howard Hughes Medical Institute.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localized and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bekman E, Henrique D. Embryonic expression of three mouse genes with homology to the Drosophila melanogaster prickle gene. Mech Dev. 2002;119(Suppl 1):S77–S81. doi: 10.1016/s0925-4773(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol Cell. 1991;72:3–14. doi: 10.1016/0248-4900(91)90072-u. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signaling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- Corey D. Sensory transduction in the ear. J Cell Sci. 2003;116:1–3. doi: 10.1242/jcs.00101. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- Denman-Johnson K, Forge A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol. 1999;28:821–835. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005;93:251–266. doi: 10.1152/jn.00746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- Feuerstein R, Wang X, Song D, Cooke NE, Liebhaber SA. The LIM/double zinc-finger motif functions as a protein dimerization domain. Proc Natl Acad Sci USA. 1994;91:10655–10659. doi: 10.1073/pnas.91.22.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Green C, Huen D, Coulson D, Johnson G, Tree D, Collier S, Roote J. The balance between isoforms of the Prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signaling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Identification and characterization of human PRICKLE1 and PRICKLE2 genes as well as mouse Prickle1 and Prickle2 genes homologous to Drosophila tissue polarity gene prickle. Int J Mol Med. 2003;11:249–256. [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Dev Cell. 2004;7:401–412. doi: 10.1016/j.devcel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signaling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Sans A, Chat M. Analysis of temporal and spatial patterns of rat vestibular hair cell differentiation by tritiated thymidine radioautography. J Comp Neurol. 1982;206:1–8. doi: 10.1002/cne.902060102. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Struhl G. Decoding vectorial information from a gradient: sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development. 1999;126:5725–5738. doi: 10.1242/dev.126.24.5725. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in Looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP3- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]