Abstract
The primary action of several antidepressant treatments used in the clinic raises extracellular concentrations of serotonin (5-HT), which subsequently act on multiple 5-HT receptors. The present study examined whether 5-HT6 receptors might be involved in the antidepressant-like effects mediated by enhanced neurotransmission at 5-HT synapses. A selective 5-HT6 receptor antagonist, SB271046, was evaluated for its ability to counteract fluoxetine-induced biochemical and behavioral responses in mice. In addition, biochemical and behavioral effects of the 5-HT6 receptor agonist, 2-ethyl-5-methoxy-N,N-dimethyltryptamine (EMDT), were assessed in mice to ascertain whether enhancement of 5-HT6 receptor-mediated neurotransmission engenders antidepressant-like effects. SB271046 significantly counteracted the stimulatory actions of fluoxetine on cortical c-fos mRNA, phospho-Ser845-GluR1, and in the tail suspension antidepressant assay, whereas it had no effect on these parameters by itself. EMDT increased the phosphorylation states of Thr34-DARPP-32 and Ser845-GluR1, both in brain slices and in the intact brain, which were effects also seen with the antidepressant fluoxetine; as with fluoxetine, these effects were demonstrated to be independent of D1 receptor stimulation. Systemic administration of EMDT increased c-fos mRNA expression in the striatum and cerebral cortex and reduced immobility in the tail suspension test. The antidepressant-like effects of EMDT in the tail suspension test were prevented by SB271046. Our results indicate that 5-HT6 receptor stimulation may be a mechanism initiating some of the biochemical and behavioral outcomes of 5-HT reuptake inhibitors, such as fluoxetine. These findings also indicate that selective 5-HT6 receptor agonists may represent a novel antidepressant drug class.
Keywords: serotonin, antidepressants, fluoxetine, signal transduction, protein phosphorylation, tail suspension test
Introduction
The serotonin (5-hydroxytryptamine; 5-HT) neurotransmitter system regulates complex sensory, motor, affective, and cognitive functions. Many of the current treatments for depression and anxiety act by increasing serotonergic neurotransmission (Barnes and Sharp, 1999), and such data form the basis for the monoamine hypothesis of affective disorders (Iversen, 2005). However, a causative role of perturbed 5-HT function in depression has been difficult to prove (Heninger et al., 1996), and the specific serotonergic receptor targets responsible for antidepressant efficacy are poorly defined. Fourteen 5-HT receptor subtypes have been identified (Hoyer et al., 1994; Barnes and Sharp, 1999). They are divided into seven different subclasses: 5-HT1A–F, 5-HT2A–C, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors. These receptors act primarily through the following second messenger transduction systems: 5-HT1- and 5-HT5-class receptors decrease cAMP formation; 5-HT2-class receptors increase inositol triphosphate and diacylglycerol formation; 5-HT3 receptors increase Na+ and Ca2+ influx; and 5-HT4, 5-HT6, and 5-HT7 receptors increase cAMP formation.
Dopamine- and cAMP-regulated phosphoprotein (DARPP-32) plays an important role in integrating signaling via multiple neurotransmitters in several brain regions (Svenningsson et al., 2004). When phosphorylated at Thr34, DARPP-32 acts as an inhibitor of protein phosphatase-1 and thereby reduces the dephosphorylation and alters the function of multiple substrates, including glutamate receptor 1 (GluR1) subunits of AMPA receptors (Snyder et al., 2000). Systemic administration of fluoxetine increases the phosphorylation states of Thr34-DARPP-32 and of Ser845-GluR1 receptors, and DARPP-32 is involved in the fluoxetine-mediated decrease of immobility in the tail suspension test of antidepressant efficacy (Svenningsson et al., 2002a). Likewise, in brain slices, 5-HT activation of 5-HT4 and 5-HT6 receptors induces an increased phosphorylation state at Thr34-DARPP-32, the protein kinase A site, and a decreased phosphorylation state at Thr75-DARPP-32, the cyclin-dependent kinase 5 site (Svenningsson et al., 2002b). The ability of fluoxetine to modulate DARPP-32-mediated phosphorylation was, in turn, linked to phosphorylation of Ser831- and Ser845-GluR1 subunits of the AMPA receptor (Svenningsson et al., 2002a). Phosphorylation of these sites activates AMPA receptor conductance (Wang et al., 2005). Potentiation of AMPA receptors results in antidepressant-like effects in rodent models (Alt et al., 2006).
The present series of experiments was directed toward evaluating the role of the protein kinase A (PKA)-activating 5-HT6 receptor subtype (Monsma et al., 1993; Ruat et al., 1993) in the antidepressant-like effects of 5-HT reuptake inhibitors. Accordingly, the present study examined the role of 5-HT6 receptors in mediating biochemical and behavioral actions of fluoxetine indicative of its antidepressive properties. Specifically, we assessed the ability of the 5-HT6 receptor antagonist, SB271046 (Bromidge et al., 1999), to modify fluoxetine-induced c-fos mRNA, phospho-Ser845 GluR1, and antidepressant-like behavioral effects in mice. In addition, we examined the effects of the 5-HT6 receptor agonist 2-ethyl-5-methoxy-N,N-dimethyltryptamine (EMDT) (Glennon et al., 2000) on PKA-mediated signaling, c-fos mRNA expression, and antidepressant-like effects in mice. Collectively, the present biochemical and behavioral findings suggest that activation of 5-HT6 receptors initiates a cascade of events that may be involved in the antidepressant-like effects of 5-HT reuptake inhibitors. As such, selective targeting of this 5-HT receptor subtype may provide an improvement in the therapeutic outcome of 5-HT-based antidepressants.
Materials and Methods
Animals.
C57BL/6 male mice aged 2–4 months were used in all experiments in this study. Mice were bred at Rockefeller University or supplied by Harlan (Indianapolis, IN), Iffa Credo (Arbresle, France), or BK Universal (Sollentuna, Sweden) for United States and European locations, respectively, at 2–3 months of age. The mice were allowed to acclimatize to the colony for 2 weeks before they were used for experiments. All animals were group housed (four to six per cage).
[125I]-SB258585 autoradiography in mouse brain sections.
[125I]-SB258585, a selective antagonist radioligand at 5-HT6 receptors (Hirst et al., 2000), was used to determine the distribution of 5-HT6 receptors in the mouse forebrain. Coronal cryostat tissue sections (12 μm thick) from C57BL/6 mice were incubated in assay buffer consisting of 50 mm Tris-HCl (pH 7.4), 5 mm MgCl2, 10 μm pargyline, 0.1% ascorbic acid, and 0.5 mm EDTA. The sections were incubated in this solution containing 1 nm [125I]-SB258585 (specific activity, 2000 Ci mmol−1) (GE Healthcare, Uppsala, Sweden) for 45 min at 37°C. Slides were then washed three times in ice-cold (4°C) 50 mm Tris-HCl buffer (pH 7.4) for 30 min each, then dipped in ice-cold water to remove buffer salts. Nonspecific binding was generated on sections adjacent to those used for total binding by the addition of 10 μm 5-HT. Displacement experiments were performed with increasing concentrations (0.001–10 μm) of EMDT or SB271046 (synthetized at Eli Lilly and Company). Sections were dried in a stream of cool air and then exposed to autoradiographic film (Biomax MR; Kodak, Upplands Vasby, Sweden) for 4–7 d. Radioactive iodine standards (GE Healthcare) were coexposed with the sections on the same x-ray films. Autoradiograms were quantified by densitometry using NIH Image 1.61 software.
In situ hybridization. Adult male C57BL/6 mice were injected intraperitoneally with saline, EMDT (5 or 15 mg/kg), fluoxetine (10 or 20 mg/kg), SB271046 (1 or 10 mg/kg), or SB271046 (1 or 10 mg/kg) together with fluoxetine (10 or 20 mg/kg) and killed 20 min after the injection by decapitation. Brains were rapidly dissected out and frozen at −80°C. Cryostat sections (12 μm thick) were prepared and hybridized with [α-35S] UTP-labeled riboprobes prepared by in vitro transcription from a cDNA clone corresponding to c-fos mRNA as described previously (Svenningsson et al., 1997). After hybridization, the sections were exposed to Biomax MR film (Kodak) for 2–14 d and quantified by densitometry using NIH Image 1.61 software.
In vivo whole animal studies to measure protein phosphorylation.
Adult male C57BL/6 mice were given intraperitoneal injections of saline, fluoxetine (20 mg/kg), SB271046 (10 mg/kg), or SB271046 (10 mg/kg) together with fluoxetine (20 mg/kg) and killed 30 min after the injection by focused microwave irradiation (4.5–5 kW for 1.4 s) using a small animal microwave (Muromachi Kikai, Tokyo, Japan). In a separate experiment, adult male C57BL/6 mice were given intraperitoneal injections of saline or EMDT (5 mg/kg) and killed 15 min after the injection. Frontal cortices and striata were rapidly dissected out and stored at −80°C until assayed.
In vitro brain slice experiments to measure protein phosphorylation.
Striatal slices (300 μm) were prepared from adult male C57BL/6 wild-type or D1 knock-out mice. The slices were preincubated in Krebs buffer at 30°C under constant oxygenation (95% O2/5% CO2) for 60 min, with a change of buffer after 30 min. The slices were then treated with EMDT (3–100 μm) for 5 min. After drug treatment, the buffer was removed and the slices were rapidly frozen on dry ice and stored at −80°C until assayed.
Immunoblotting.
Frozen tissue samples from the in vitro and in vivo experiments were sonicated in 1% SDS and boiled for 10 min. Small aliquots of the homogenate were retained for protein determination by the bicinchoninic acid protein assay method (Pierce, Stockholm, Sweden). Equal amounts of protein were processed using 12% acrylamide gels as described previously (Svenningsson et al., 2003). Immunoblotting was performed with phosphorylation state-specific antibodies against phospho-Thr34-DARPP-32 (Snyder et al., 1992), phospho-Thr75-DARPP-32 (Bibb et al., 1999), phospho-Ser831-GluR1 (Millipore, Bedford, MA), phospho-Ser845-GluR1 (Millipore), or antibodies that are not phosphorylation state specific against total DARPP-32 (Hemmings and Greengard, 1986) or total GluR1 (Millipore). Antibody binding was detected by enhanced chemiluminescence (GE Healthcare) and quantified by densitometry using NIH Image 1.61 software. Data on protein phosphorylation are expressed as percentage of control.
Tail suspension test.
The day of the tail suspension test, experimental mice were transferred to the experiment room and allowed to acclimatize for 3–4 h. Mice were injected intraperitoneally with saline, EMDT (1, 2.5, 5, 10, or 15 mg/kg), fluoxetine (20 mg/kg), SB271046 (1, 5, or 10 mg/kg), or SB271046 (1, 5, or 10 mg/kg) combined with fluoxetine (20 mg/kg) 30 min before the tail suspension test trial. In the tail suspension test paradigm, each mouse was tested in an individual cubicle while suspended from a tail hanger with adhesive tape wrapped around its tail (1.5–2 cm from tip) 80 cm above the floor. The trial was conducted for a period of 5 min, during which the duration of immobility was measured with the Porsolt program (Infallible Software, Rockville, MD) and manually by a blinded observer. Mice were considered immobile when they hung passively and motionless. In our hands, an extremely small number of animals (∼1–2%) climbed their tails. These animals were removed from the study. Decreases in basal levels of immobility are highly predictive of antidepressant efficacy (Steru et al., 1985; Cryan et al., 2002).
Results
Autoradiographic determination of 5-HT6 receptors in the mouse brain
[125I]-SB258585 is an antagonist radioligand at 5-HT6 receptors (Hirst et al., 2000) that can detect these receptors in the rat brain (Roberts et al., 2002). The level of 5-HT6 receptors is lower and less concentrated in the forebrain of mice than rats (Hirst et al., 2003). Nevertheless, in an autoradiographic experiment, using [125I]-SB258585 as a radioligand, we demonstrated specific binding in the striatum, nucleus accumbens, and cortex of mice (Fig. 1). Binding of [125I]-SB258585 could be displaced by both the antagonist, SB271046, and the agonist, EMDT, with EC50 values of 4 and 15 nm, respectively (Fig. 1).
Figure 1.
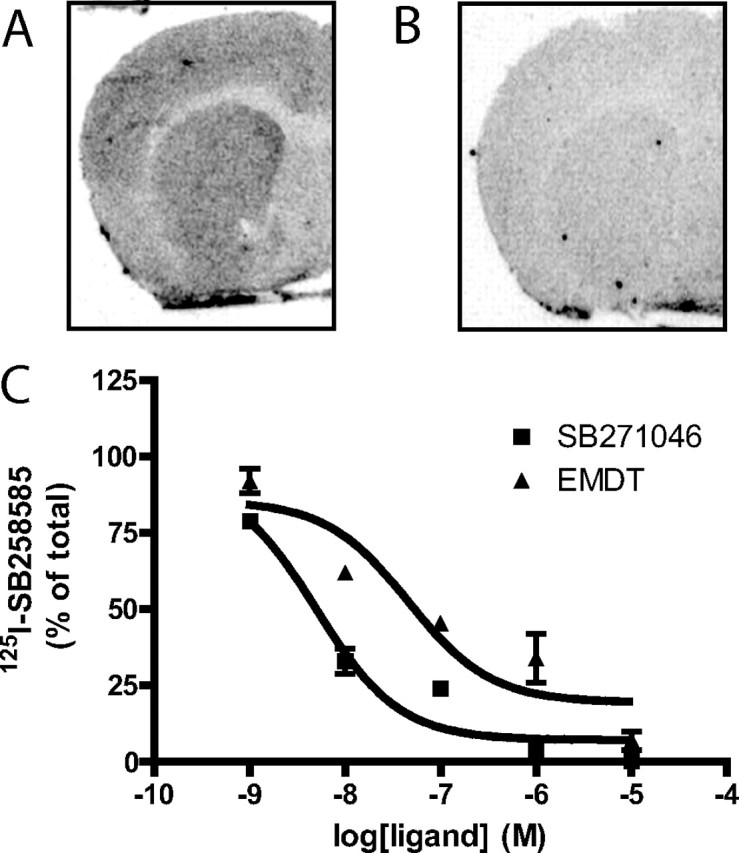
[125I]-SB258585 binding in the mouse brain. A, Autoradiogram showing total binding of [125I]-SB258585 on a coronal section of a mouse brain, through the rostral part of the corpus striatum. B, Autoradiogram generated over an adjacent section but incubated with [125I]-SB258585 in the presence of 10 μm unlabeled serotonin to define nonspecific binding. C, Displacement of [125I]-SB258585 by increasing concentrations of EMDT and SB271046. Error bars indicate SEM.
The 5-HT6 receptor antagonist, SB271046, reverses biochemical and behavioral antidepressant-like effects of fluoxetine
Acute administration of antidepressant drugs increases expression of the immediate early gene c-fos mRNA in the brain (Beck 1995; Torres et al., 1998; Horowitz et al., 2003) and reduces immobility in behavioral tests of despair (see below). We examined the effects of the selective 5-HT6 receptor antagonist, SB271046, on fluoxetine-induced c-fos mRNA expression. In agreement with previous studies (Torres et al., 1998; Horowitz et al., 2003), fluoxetine (10 or 20 mg/kg) increased the expression of c-fos mRNA in certain limbic regions of the frontal cerebral cortex, including the cingulate cortex and the endopiriform cortex (Fig. 2). Treatment with SB271046 (1 or 10 mg/kg) alone had no effect on c-fos mRNA expression in these regions. However, 10 mg/kg of SB271046, administered before fluoxetine (10 mg/kg, data not shown; or 20 mg/kg) (Fig. 2), significantly counteracted fluoxetine-induced c-fos mRNA expression in both the cingulate and the endopiriform cortex (Fig. 2).
Figure 2.
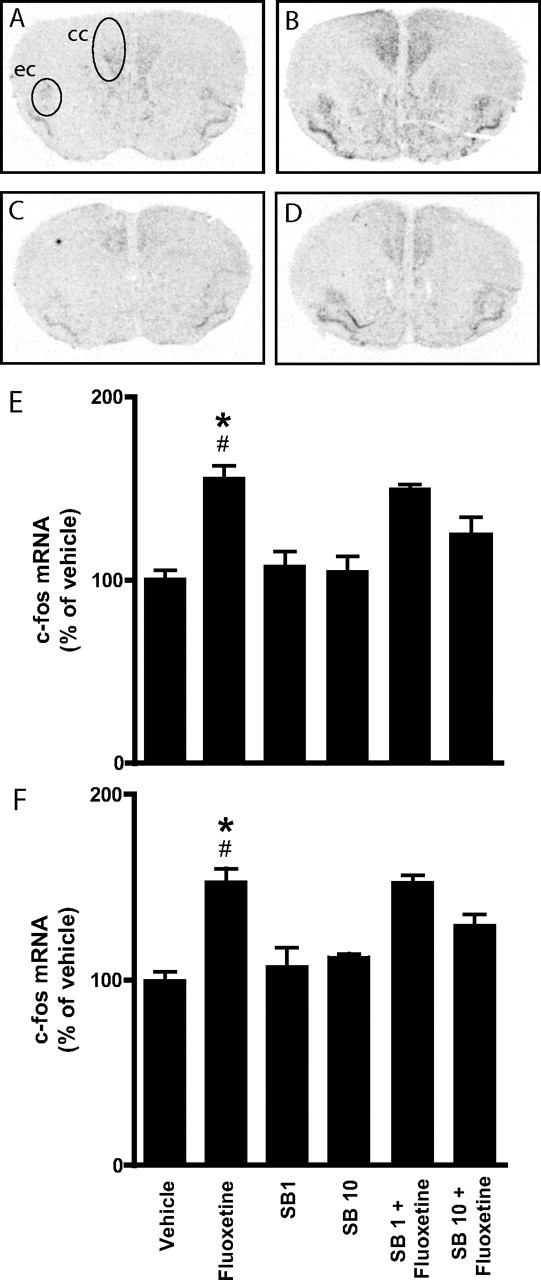
Regulation by fluoxetine and SB271046 of c-fos mRNA expression in the cerebral cortex of intact mice. A–D, Bright-field autoradiograms showing the expression of c-fos mRNA 20 min after intraperitoneal administration of saline (A), fluoxetine (20 mg/kg) (B), SB271046 (10 mg/kg) (C), or SB271046 (10 mg/kg) together with fluoxetine (20 mg/kg) (D) in mice (magnification, 5×). ec, Endopiriform cortex; cc, cingulate cortex. E, F, Histograms show quantification of the expression of c-fos mRNA in the cingulate cortex (E) and dorsal endopiriform cortex (F) after the indicated treatments. Data represent means ± SEM for four to six mice per group. *p < 0.05 compared with saline-treated mice; #p < 0.05 compared with SB271046 (10 mg/kg) plus fluoxetine-cotreated mice; one-way ANOVA followed by Newman–Keuls test.
In agreement with our previous study (Svenningsson et al., 2002), fluoxetine (20 mg/kg) increased the levels of phospho-Ser845-GluR1 in the frontal cortex (Fig. 3) and striatum (data not shown). Treatment with SB271046 (10 mg/kg) alone had no effect on phospho-Ser845-GluR1 in these regions but significantly counteracted fluoxetine-induced phospho-Ser845-GluR1 in the frontal cortex (Fig. 3).
Figure 3.
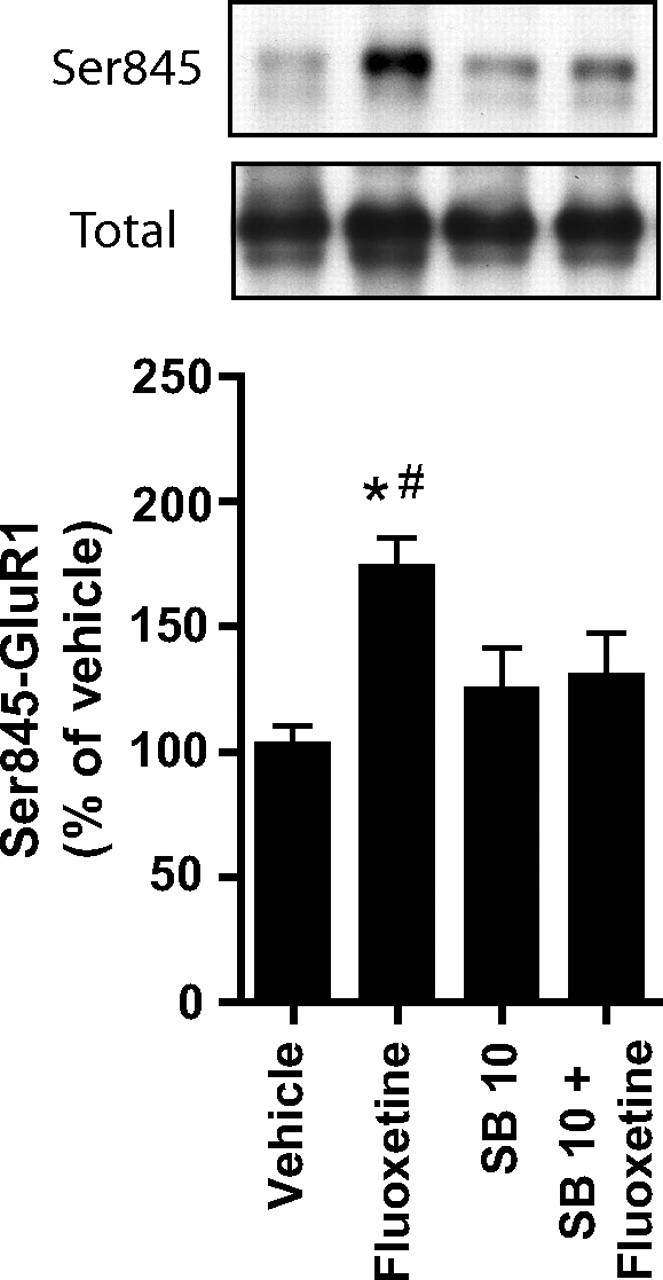
Regulation by fluoxetine and SB271046 of phospho-Ser845-GluR1 in the frontal cortex in intact mice. Top, Immunoblots showing the levels of phospho-Ser845-GluR1 and total GluR1 in the frontal cortex 30 min after intraperitoneal administration of saline, fluoxetine (20 mg/kg), SB271046 (10 mg/kg), or SB271046 (10 mg/kg) together with fluoxetine (20 mg/kg) in mice. Bottom, The histogram shows the quantification of phospho-Ser845-GluR1 in the frontal cortex after the indicated treatments. Data represent means ± SEM for five to six mice per group. *p < 0.05 compared with saline-treated mice; #p < 0.05 compared with SB271046 (10 mg/kg) plus fluoxetine-cotreated mice; one-way ANOVA followed by Newman–Keuls test for pairwise comparisons.
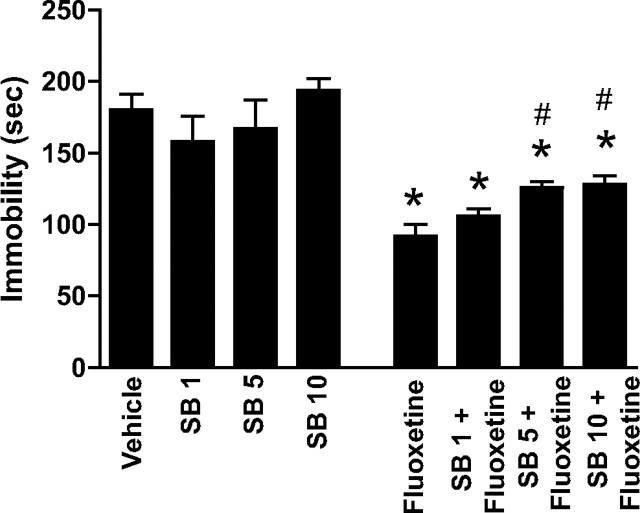
SB271046 was also tested for its activity in the mouse tail suspension test. Learned-helplessness models, such as the tail suspension test, in which experimental animals are exposed to inescapable aversive situations, are of utility for predicting antidepressant efficacy. During these tests, mice show alternate periods of agitation and immobility (Steru et al., 1985). It is well established that acute treatment with various antidepressant drugs increases active attempts to escape and, thus, reduces immobility in these tests. In agreement with the biochemical data, SB271046 (1, 5, or 10 mg/kg) had no effect in the tail suspension test when administered alone. When SB271046 (5 or 10 mg/kg) was given in conjunction with an antidepressant-like dose of fluoxetine (20 mg/kg), however, there was a partial reversal of its anti-immobility effect in this test (Fig. 4). It can be concluded from these studies that specific biochemical and behavioral actions of fluoxetine that are associated with its antidepressant effects may involve activation of 5-HT6 receptors.
Figure 4.
Effects of SB271046 on antidepressant-like effects of fluoxetine in the tail suspension test. Saline, fluoxetine (20 mg/kg), SB271046 (1,5, or 10 mg/kg), or SB271046 (1,5, or 10 mg/kg) combined with fluoxetine (20 mg/kg), 30 min before the tail-suspension test trial. The trial was conducted for a period of 5 min, during which the duration of immobility was recorded. Data represent means ± SEM for eight mice per group. *p < 0.05 compared with saline; #p < 0.05 compared with fluoxetine; one-way ANOVA followed by Duncan's test.
Antidepressant effects of the 5-HT6 receptor agonist EMDT
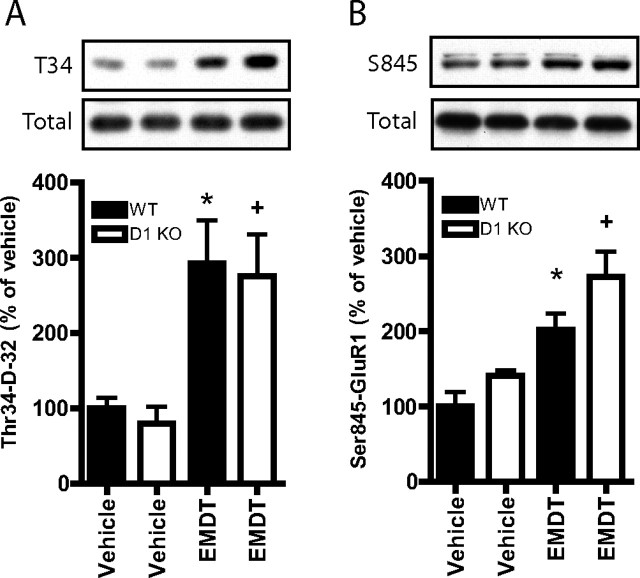
We next assessed the ability of the 5-HT6 receptor agonist EMDT to mimic some of the antidepressant-like biochemical and behavioral effects of fluoxetine. First, we measured its ability to regulate the phosphorylation state of two PKA phosphosubstrates, Thr34-DARPP-32 and Ser845-GluR1, in striatal slices. EMDT increased the phosphorylation states of Thr34-DARPP-32 and Ser845-GluR1 in a dose-dependent manner (Fig. 5). The phosphorylation of Thr34-DARPP-32 and Ser845-GluR1 in striatum is potently regulated by D1 receptor stimulation (Snyder et al. 2000). To determine whether the effect of EMDT on phospho-Thr34-DARPP-32 and phospho-Ser845-GluR1 involved D1 receptor activation, we compared the effect of EMDT (100 μm) on these phosphosubstrates in wild-type and D1 receptor knock-out mice. As shown in Figure 6, EMDT significantly increased phospho-Thr34-DARPP-32 and phospho-Ser845-GluR1 not only in slices from wild-type mice but also in slices from D1 receptor knock-out mice. It can be concluded that the stimulatory effect of EMDT on phospho-Thr34-DARPP-32 and phospho-Ser845-GluR1 is independent of D1 receptor activation.
Figure 5.
Regulation by EMDT of the phosphorylation states of DARPP-32 and GluR1 in slices of neostriatum. Dose-response experiments of in vitro regulation by EMDT of phosphorylation of Thr34-DARPP-32 (A) and Ser845-GluR1 (B) in striatal slices. Slices were incubated with EMDT (3, 10, 30, and 100 μm) for 5 min. Data represent means ± SEM (n = 6–10). *p < 0.05 compared with vehicle; one-way ANOVA followed by Newman–Keuls test.
Figure 6.
Comparison of the regulation by EMDT of the phosphorylation states of DARPP-32 and GluR1 in striatal slices from wild-type (WT) and D1 receptor knock-out (D1 KO) mice. In vitro regulation of Thr34-DARPP-32 (A) and Ser845-GluR1 (B) phosphorylation by EMDT (100 μm) in slices of neostriatum from wild-type and D1 knock-out mice. The amounts of phospho-Thr34-DARPP-32 and phospho-Ser845-GluR1 in extracts of slices were quantified by densitometry. Data represent means ± SEM (n = 6–12). *p < 0.05 compared with wild-type control; +p < 0.05 compared with D1 knock-out control; unpaired two-tailed Student's t test.
We next examined the effect of systemic administration of EMDT on the PKA sites, phospho-Thr34-DARPP-32 and phospho-Ser845-GluR1. It was found that 5 mg/kg of EMDT increased the phosphorylation states of both Thr34-DARPP-32 and Ser845-GluR1 in striatal extracts (Fig. 7). No significant alterations of phospho-Thr75-DARPP-32 or phospho-Ser831-GluR1 were found in the same extracts. Treatment with EMDT also increased phospho-Ser845-GluR1, but not phospho-Ser831-GluR1, in the frontal cortex (Fig. 7). These data indicate that the effects of EMDT on phosphorylation of PKA phosphosubstrates in brain slices can be reproduced by its systemic administration to intact animals.
Figure 7.
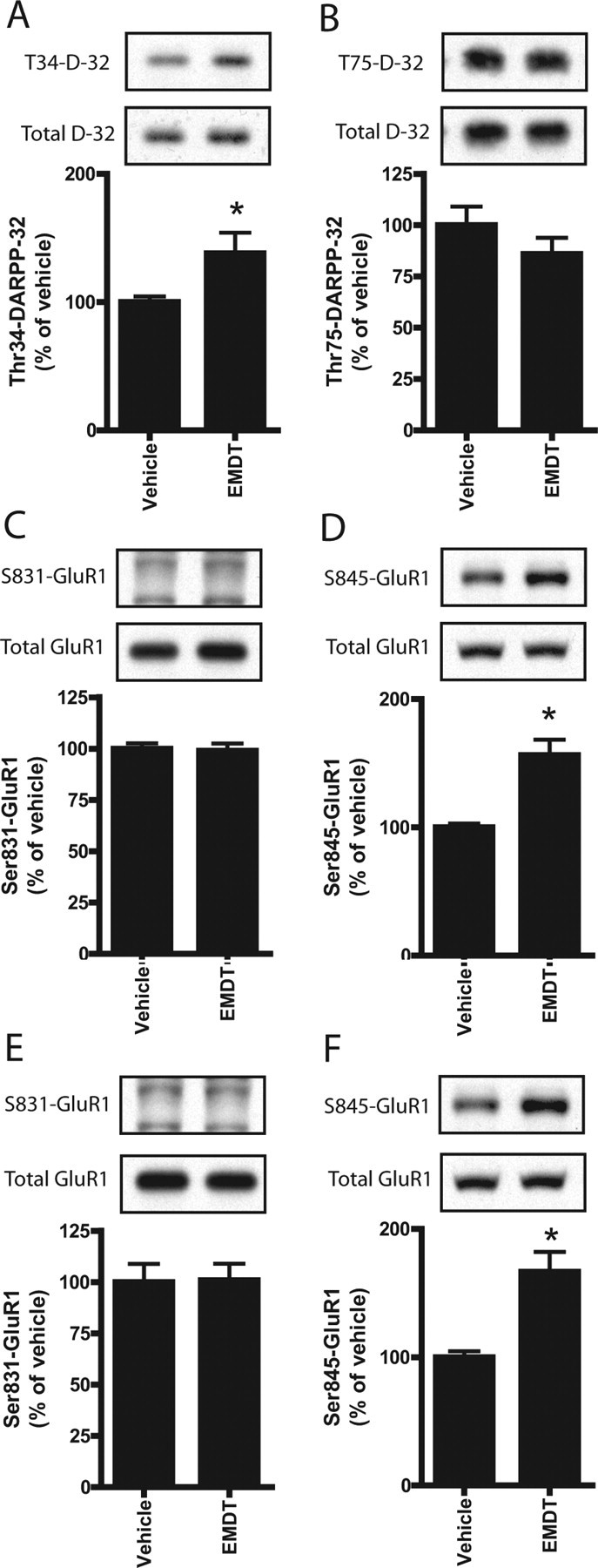
Regulation by EMDT of the phosphorylation states of DARPP-32 and GluR1 in striatal and cortical extracts from intact mice. Regulation of Thr34- and Thr75-DARPP-32 and Ser831- and Ser845-GluR1 phosphorylation in vivo in the striatum (A–D) and frontal cortex (E, F) by EMDT. Mice were injected intraperitoneally with saline or EMDT (5 mg/kg). Fifteen minutes later, mice were killed by focused microwave irradiation. Data represent means ± SE for 5–10 mice per group. *p < 0.05 compared with saline-treated mice; one-way ANOVA followed by Newman–Keuls test.
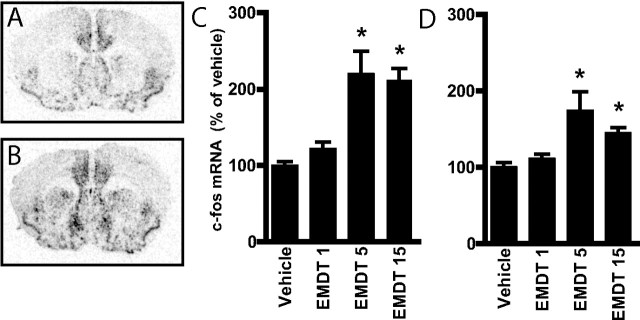
To further examine the ability of EMDT to regulate signal transduction in the intact brain, we studied its effect on c-fos mRNA expression. EMDT was found to significantly induce c-fos mRNA expression in striatum as well as subregions of the cerebral cortex, including the cingulate cortex (Fig. 8). This effect of EMDT was observed after its systemic administration at 5 and 15 mg/kg but not at 1 mg/kg. To investigate the involvement of 5-HT6 receptors in engendering antidepressant-like activity, we assessed the effects of acute EMDT administration in the tail suspension test in mice. It was found that EMDT dose-dependently decreased immobility in the tail suspension test (Fig. 9). This effect was abolished by pretreatment with the 5-HT6 receptor antagonist SB271046 (Fig. 9), demonstrating the specificity of the antidepressant-like profile of this compound for 5-HT6 receptors.
Figure 8.
Regulation by EMDT of c-fos mRNA expression in the striatum and cerebral cortex in intact mice. A, B, Bright-field autoradiograms showing the expression of c-fos mRNA 20 min after treatment with saline (A) or EMDT (5 mg/kg) (B) in mice (magnification, 5×). C, D, Histograms show quantification of the expression of c-fos mRNA in the periventricular area of the striatum (C) and cingulate cortex (D) after each treatment. Data represent means ± SEM for four to six mice per group. *p < 0.05 compared with saline-treated mice; one-way ANOVA followed by Newman–Keuls test.
Figure 9.

Antidepressant-like effects of EMDT in the tail suspension test. A, Mice were injected with EMDT (1, 2.5, 5, 10, or 15 mg/kg) 30 min before the trial. B, Saline, SB271046 (10 mg/kg), EMDT (10 mg/kg), or SB271046 (10 mg/kg) combined with EMDT (10 mg/kg), 30 min before the tail suspension test trial. The trial was conducted for a period of 5 min, during which the duration of immobility was recorded. Data represent means ± SEM for eight mice per group. *p < 0.05 compared with saline; #p < 0.05 compared with SB271046 (10 mg/kg) plus EMDT; one-way ANOVA followed by Duncan's test.
Discussion
We have previously reported that fluoxetine increases the phosphorylation states of Thr34-DARPP-32 and of Ser845-GluR1 receptors and that DARPP-32 is involved in the fluoxetine-mediated decrease of immobility in the tail-suspension test of antidepressant efficacy (Svenningsson et al., 2002b), in agreement with the idea that activation of cAMP/PKA signaling is associated with antidepressant effects (Duman et al., 1997). These findings indicated that at least one of the 5-HT receptors that stimulate PKA activity (i.e., 5-HT4, 5-HT6, and/or 5-HT7 receptors) is involved in mediating the actions of fluoxetine.
In the present study, we examined biochemical and behavioral effects exerted via 5-HT6 receptors with a special emphasis on the potential role of this receptor subtype in antidepressant actions. For this purpose, we used selective pharmacological tools, namely the selective 5-HT6 receptor antagonist, SB271046 (Bromidge et al., 1999), and the 5-HT6 receptor agonist, EMDT (Glennon et al., 2000). We confirmed that both ligands have high affinities for 5-HT6 receptors in the mouse brain by showing that they displace specific [125I]-SB258585 binding at nanomolar concentrations in the forebrain. Our results are consistent with previous studies showing that 5-HT6 receptors appear restricted to the brain with high levels in the caudate–putamen, nucleus accumbens, and olfactory tubercle and moderate levels in the cerebral cortex hippocampus and amygdala (Monsma et al., 1993; Ruat et al., 1993; Ward et al., 1995; Gerard et al., 1997; Hamon et al., 1999; Roberts et al., 2002).
Despite their well characterized anatomical distribution, the functional importance of 5-HT6 receptors in brain pathophysiology is only emerging. In accordance with a predominant corticolimbic localization of 5-HT6 receptors, their blockade modulates responses to the psychostimulant amphetamine (Frantz et al., 2002), as well as motor, emotional, and cognitive functions. Thus, administration of either 5-HT6 receptor antagonists or antisense oligonucleotides toward 5-HT6 receptors decreases locomotion, induces chewing, yawning, and stretching, and improves performance in learning and memory tasks (Bourson et al., 1995; Sleight et al., 1996; Sleight et al., 1998; Yoshioka et al., 1998; Bentley et al., 1999; Rogers and Hagan, 2001; Woolley et al., 2001; Lindner et al., 2003; Riemer et al., 2003; Hatcher et al., 2005), while increasing anxiety (Hamon et al., 1999; Otano et al., 1999). 5-HT6 receptor knock-out mice, however, perform normally in a wide variety of behavioral assays that assess cognition and anxiety (Bonasera et al., 2006). To our knowledge, the present study is the first to examine 5-HT6 receptor function in relation to antidepressant-like activity and to indicate that 5-HT6 receptor stimulation causes antidepressant-like behavioral and biochemical alterations.
Indeed, we show that blockade of the 5-HT6 receptor with the antagonist SB271046 counteracts the stimulatory actions of fluoxetine on cortical c-fos mRNA and phospho-Ser845-GluR1 and reduces the antidepressant-like action of fluoxetine in the tail suspension test. Previous work demonstrated that several classes of atypical antipsychotics and tricyclic antidepressants, such as clozapine, mianserin, and amytryptiline, bind with high affinity to 5-HT6 receptors (Monsma et al., 1993). However, fluoxetine has only low-to-moderate affinity for 5-HT6 receptors (Monsma et al., 1993). It is therefore unlikely that the inhibitory action of SB271046 on fluoxetine-mediated actions depends on direct competition at 5-HT6 receptors, but rather involves blockade of 5-HT6 receptor activation elicited by the fluoxetine-induced elevations of extracellular 5-HT levels.
Furthermore, we show that the 5-HT6 receptor agonist EMDT mimics antidepressant-like behavioral and biochemical effects of fluoxetine. Like fluoxetine and 5-HT (Svenningsson et al., 2002a,b), EMDT increases the phosphorylation state of Thr34-DARPP-32 both in brain slices and in the intact brain. The fact that micromolar concentrations of EMDT are needed to cause a significant increase in P-Thr34-DARPP-32, despite the fact that this compound has nanomolar affinity for 5-HT6 receptors, is consistent with previous studies using other ligands at monoamine receptors (Svenningsson et al., 2002b). This discrepancy between the binding affinity and functional protein phosphorylation response may be because of the dilution of the compound when diffusing throughout the slices and to difficulties in accessing receptors in the synaptic cleft. Acute systemic administration of EMDT also increases phospho-Ser845-GluR1 but not phospho-Ser831-GluR1. Systemic administration of EMDT also leads to an increase in the expression of c-fos mRNA expression throughout the striatum and cerebral cortex, similar to that observed with fluoxetine. In the same dose range, EMDT leads to a significant antidepressant-like effect in the tail suspension test. These data indicate that stimulation of the positively cAMP/PKA coupled 5-HT6 receptors may be involved in antidepressant-like actions of 5-HT reuptake inhibitors. This is in accordance with our previous findings that 5-HT-induced phosphorylation of DARPP-32 at Thr34, a key molecular element in antidepressant action, is mediated through activation of the cAMP pathway and primarily depends on 5-HT receptors positively coupled to adenylyl cyclase (Svenningsson et al., 2002b). The framework that our previous and present results provide agrees with the evidence for the participation of the cAMP cascade, particularly in the frontal cortex, in short- and long-term effects of antidepressants. However, it remains to be established which neuronal populations mediate the antidepressant-like actions of 5-HT6 receptor stimulation. In this context, it should be noted that activation of cAMP/PKA/P-Ser133-calcium/cAMP response element-binding protein (CREB) signaling in the nucleus accumbens and striatum actually appears to counteract antidepressant actions. Indeed, previous studies have demonstrated that overexpression of CREB in the nucleus accumbens increases, and expression of a dominant negative mutant of CREB decreases, immobility in the forced swim test (Carlezon et al., 2005). It is possible that additional signaling pathways mediate important actions of 5-HT6 receptors. It has, for example, recently been demonstrated that 5-HT6 receptors stimulate ERK1/2 (extracellular signal-regulated kinase 1/2) signaling via a Fyn tyrosine kinase-dependent mechanism (Yun et al., 2007).
Reports on the role of the other two cAMP/PKA coupled 5-HT receptors, 5-HT4 and 5-HT7 receptors, in relation to depression and to antidepressant-like activity are scarce. Overall, there is little evidence for an involvement of 5-HT4 receptors in mediating antidepressant effects. Thus, stimulation of 5-HT4 receptors does not mediate behavioral antidepressant-like actions of tricyclic antidepressants or fluoxetine (Cryan and Lucki, 2000). However, stimulation of 5-HT4 receptors exerts a facilitatory response on dorsal raphe 5-HT neurons (Lucas et al., 2005) and might be implicated in some behavioral effects of chronically administered fluoxetine (Holick et al., 2005). Surprisingly, it was shown that 5-HT7 receptor antagonists could have antidepressant properties and that 5-HT7 receptor knock-out mice exhibited an antidepressant-like phenotype (Guscott et al., 2005; Hedlund et al., 2005). This suggests that 5-HT4, 5-HT6, and 5-HT7 receptors, despite their common effects on signal transduction, could have markedly different, and even opposing, effects in tests of antidepressant-like activity. It should, however, be noted that the antidepressant-like effects of 5-HT7 receptor antagonists could be linked to the 5-HT7 receptor-dependent regulation of the light/dark cycle (Guscott et al., 2005). An alternative possible explanation for the discrepancies in the actions of 5-HT4, 5-HT6, and 5-HT7 receptors in antidepressant effects may reside in their different anatomical localization, in which case activation of each of these receptors would stimulate distinct and independent neuronal circuitries. In accordance with the latter possibility, a review of the literature on the effects of selective 5-HT receptor ligands on behavioral despair in mice (Table 1) indicates that selective 5-HT receptor stimulation can yield stimulatory or inhibitory behavioral responses depending on the receptor subtype. Similar results on behavioral despair have been described previously in rats (Cryan et al., 2005).
Table 1.
Effects of selective 5-HT receptor ligands alone or in combination with an SSRI on immobility in the tail suspension test or forced swimming test in mice
| Class of 5-HT receptor | Compound | Alone | Plus SSRI |
|---|---|---|---|
| 5-HT1A/B agonist | |||
| RU24969a,b | ↓ | ↓ | |
| 5-HT1A agonists | |||
| 8-OH-DPATc,d,e | ↓ | ||
| LB50016f | ↓ | ||
| MKC-242g | ↓ | ||
| 5-HT1A antagonists | |||
| WAY-100635a,d,h | ⇆ | ↑d,h; ⇆a | |
| NAN190e | ⇆ | ||
| 5-HT1B agonists | |||
| Anpirtolineb,i | ↓ | ||
| GP94253j | ↓ | ||
| 5-HT1B antagonist | |||
| GR125743a,h,k | ⇆ | ↑a,k; ↓h | |
| 5-HT2A/C agonist | |||
| DOIl | ⇆ | ||
| 5-HT2A/C antagonists | |||
| LY53857d | ↑ | ||
| Ritanserinl | ↓ | ||
| 5-HT2A antagonist/SSRI | |||
| YM992m | ↓ | ||
| 5-HT2C agonists | |||
| WAY 161503n | ↓ | ||
| RO 60-0175n | ↓ | ||
| RO 60-0332n | ↓ | ||
| 5-HT2C antagonist | |||
| SB206553n,o | ⇆ | ↓o; ↑n | |
| 5-HT3 antagonists | |||
| Ondansetronl | ↓ | ||
| MDL72222p | ↓ | ||
| 5-HT4 antagonist | |||
| SB 204070Aq | ⇆ | ⇆ | |
| 5-HT6 agonist | |||
| EMDT | ↓ | ||
| 5-HT6 antagonist | |||
| SB271046 | ↑ | ||
| 5-HT7 antagonists | |||
| SB-258719r | ↓ | ||
| SB-269970s | ↓ |
↓, Decreased immobility; ↑, increased/potentiated immobility; ⇆, no effect on immobility. References:
n, q Studies were performed on rats.
Our data are consistent with the notion that fluoxetine exerts its antidepressant actions by stimulating multiple 5-HT receptors. The distribution of 5-HT6 receptors in the mouse brain is uniform throughout the cortex and striatum. However, it appears that certain regions of the cortex, including the cingulate cortex, are particularly sensitive in terms of fluoxetine-mediated c-fos mRNA induction. Because SB271046 only partially blocks the actions of fluoxetine on c-fos mRNA and phospho-Ser845-GluR1, it is very likely that concomitant activation of several 5-HT receptors is required for the biochemical actions of fluoxetine in the cortex. Similarly, in the tail suspension test, the maximal antidepressant-like effects of EMDT are less pronounced than those of fluoxetine. It is likely that, in addition to 5-HT6 receptors, other 5-HT receptors also contribute to the effects of fluoxetine, as evidenced by the fact that the 5-HT6 antagonist SB271046 only partially blocks the antidepressant-like effects of fluoxetine in the tail suspension test when administered at 10 mg/kg, whereas at the same dose it completely blocks the antidepressant-like effects of the selective 5-HT6 agonist EMDT.
In conclusion, the results from the present study suggest that stimulation of 5-HT6 receptors causes antidepressant-like behavioral and biochemical effects and provide additional support to the idea that 5-HT6 receptors may contribute to serotonergic modulation of clinically relevant psychopharmacological processes.
Footnotes
This work was supported by National Institutes of Health Grants MH074899 and DA10044 (P.G.) and Vetenskapsrådet, Torsten och Ragnar Söderbergs stiftelse and Hjärnfonden (P.S.).
References
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71:1273–1288. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beck CH. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci. 1995;20:25–32. [PMC free article] [PubMed] [Google Scholar]
- Bentley JC, Bourson A, Boess FG, Fone KC, Marsden CA, Petit N, Sleight AJ. Investigation of stretching behaviour induced by the selective 5-HT6 receptor antagonist, Ro 04–6790, in rats. Br J Pharmacol. 1999;126:1537–1542. doi: 10.1038/sj.bjp.0702445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G. Antidepressant-like properties of some serotonin receptor ligands and calcium channel antagonists measured with the forced swimming test in mice. Pol J Pharmacol. 1998;50:117–124. [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Chu HM, Brennan TJ, Tecott LH. A Null Mutation of the Serotonin 6 Receptor Alters Acute Responses to Ethanol. Neuropsychopharmacol. 2006;31:1801–1813. doi: 10.1038/sj.npp.1301030. [DOI] [PubMed] [Google Scholar]
- Bourson A, Borroni E, Austin RH, Monsma FJ, Jr, Sleight AJ. Determination of the role of the 5-HT6 receptor in the rat brain: a study using antisense oligonucleotides. J Pharmacol Exp Ther. 1995;274:173–1-1780. [PubMed] [Google Scholar]
- Bromidge SM, Brown AM, Clarke SE, Dodgson K, Gager T, Grassam HL, Jeffrey PM, Joiner GF, King FD, Middlemiss DN, Moss SF, Newman H, Riley G, Routledge C, Wyman P. 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem. 1999;42:202–205. doi: 10.1021/jm980532e. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cremers TIFH, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, Honig G, Bogeso KP, Westerink BH, den Boer H, Wikstrom HV, Tecott LH. Induction of 5-HT2C receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacol. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000a;295:1120–1126. [PubMed] [Google Scholar]
- Cryan JF, Lucki I. 5-HT4 receptors do not mediate the antidepressant-like behavioral effects of fluoxetine in a modified forced swim test. Eur J Pharmacol. 2000b;409:295–299. doi: 10.1016/s0014-2999(00)00858-x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assesing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, Hansson KJ, Stouffer DG, Parsons LH. 5-HT6 receptor antagonism potentiates the behavioral and neurochemical effects of amphetamine but not cocaine. Neuropharmacology. 2002;44:170–180. doi: 10.1016/s0028-3908(01)00165-4. [DOI] [PubMed] [Google Scholar]
- Gardier AM, Trillat AC, Malagie I, David D, Hascoet M, Colombel MC, Jolliet P, Jacquot c, Hen R, Bourin M. 5-HT1B serotonin receptors and antidepressant effects of selective serotonin reuptake inhibitors. C R Acad Sci III. 2001;324:433–441. doi: 10.1016/s0764-4469(01)01332-4. [DOI] [PubMed] [Google Scholar]
- Gerard C, Martres MP, Lefèvre K, Miquel MC, Verge D, Lanfumey L, Doucet E, Hamon M, el Mestikawy S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–219. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Lee M, Rangisetty JB, Dukat M, Roth BL, Savage JE, McBride A, Rauser L, Hufeisen S, Lee DK. 2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors. J Med Chem. 2000;43:1011–1018. doi: 10.1021/jm990550b. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacol. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Verge D. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology. 1999;21:68S–76S. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DN. 5-HT6R antagonists improve performance in an attentional set shifting task in rats. Psychopharmacol. 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressant-like behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Minton JA, Bromidge SM, Moss SF, Latter AJ, Riley G, Routledge C, Middlemiss DN, Price GW. Characterization of [(125)I]-SB-258585 binding to human recombinant and native 5-HT6 receptors in rat, pig and human brain tissue. Br J Pharmacol. 2000;130:1597–1605. doi: 10.1038/sj.bjp.0703458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Holick KA, Dulawa SC, Hen R. The contribution of 5-HT4 receptors to anti-depressant activity in mouse. Soc Neurosci Abstr. 2005;31:567–14. [Google Scholar]
- Horowitz JM, Goyal A, Ramdeen N, Hallas BH, Horowitz AT, Torres G. Characterization of fluoxetine plus olanzapine treatment in rats: a behavior, endocrine, and immediate-early gene expression analysis. Synapse. 2003;50:353–364. doi: 10.1002/syn.10276. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Iversen L. The monoamine hypothesis of depression. In: Licinio J, Wong ML, editors. Biology of depression. Weinheim, Germany: Wiley-VCH; 2005. pp. 71–86. [Google Scholar]
- Kos T, Popik P, Pietraszek M, Schafer D, Danysz W, Dravolina O, Blokhina E, Galankin T, Bespalov AY. Effect of 5-HT(3) receptor antagonist MDL 72222 on behaviors induced by ketamine in rats and mice. Eur Neuropsychopharmacol. 2006;16:297–310. doi: 10.1016/j.euroneuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lee CH, Oh JI, Park HD, Kim HJ, Park TK, Kim JS, Hong CY, Lee SJ, Ahn KH, Kim YZ. Pharmacological characterization of LB50016, N-(4-amino)butyl 3-phenylpyrrolidine derivative, as a new 5-HT1A receptor agonist. Arch Pharm Res. 1999;22:157–164. doi: 10.1007/BF02976540. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Hodges DB, Jr, Hogan JB, Orie AF, Corsa JA, Barten DM, Polson C, Robertson BJ, Guss VL, Gillman KW, Starrett JE, Jr, Gribkoff VK. An assessment of the effects of serotonin 6 (5-HT6) receptor antagonists in rodent models of learning. J Pharmacol Exp Ther. 2003;307:682–691. doi: 10.1124/jpet.103.056002. [DOI] [PubMed] [Google Scholar]
- Lucas G, Compan V, Charnay Y, Neve RL, Nestler EJ, Bockaert J, Barrot M, Debonnel G. Frontocortical 5-HT4 receptors exert positive feedback on serotonergic activity: viral transfections, subacute and chronic treatments with 5-HT4 agonists. Biol Psychiatry. 2005;57:918–925. doi: 10.1016/j.biopsych.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Somboonthum P, Suzuki M, Asano S, Baba A. Antidepressant-like effect by postsynaptic 5-HT1A receptor activation in mice. Eur J Pharmacol. 1995;280:235–238. doi: 10.1016/0014-2999(95)00254-i. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levison S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–1107. [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J. Diabetes attenuates the antidepressant-like effect mediated by the activation of 5-HT1A receptor in the mouse tail suspension test. Neuropsychopharmacol. 2004;29:461–469. doi: 10.1038/sj.npp.1300354. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- Otano A, Frechilla D, Cobreros A, Cruz-Orive LM, Insausti A, Insausti R, Hamon M, Del Rio J. Anxiogenic-like effects and reduced stereological counting of immunolabelled 5 hydroxytryptamine 6 receptors in rat nucleus accumbens by antisense oligonucleotides. Neuroscience. 1999;92:1001–1009. doi: 10.1016/s0306-4522(99)00066-4. [DOI] [PubMed] [Google Scholar]
- O'Neill MF, Conway MW. Role of 5-HT1A and 5-HT1B receptors in the mediation of behaviour in the forced swim test in mice. Neuropsychopharmacol. 2001;24:391–398. doi: 10.1016/S0893-133X(00)00196-2. [DOI] [PubMed] [Google Scholar]
- O'Neill MF, Fernandez AG, Palacios JM. GR 127935 blocks the locomotor and antidepressant-like effects of RU 24969 and the action of antidepressants in the mouse tail suspension test. Pharmacol Biochem Behav. 1996;53:535–539. doi: 10.1016/0091-3057(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol. 1997;325:129–135. doi: 10.1016/s0014-2999(97)00115-5. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Evidence of the activity of lithium on 5-HT1B receptors in the mouse forced swimming test: comparison with carbamazepine and sodium valproate. Psychopharmacol. 1999;141:370–377. doi: 10.1007/s002130050846. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, MacSweeney CP, Bourin M. The role of 5-HT1A and 5-HT1B receptors with antidepressant drugs in the mouse forced swimming test. Eur J Pharmacol. 1996;318:213–217. doi: 10.1016/s0014-2999(96)00772-8. [DOI] [PubMed] [Google Scholar]
- Riemer C, Borroni E, Levet-Trafit B, Martin JR, Poli S, Porter RH, Bos M. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-bromo-6-pyrrolidin-1-ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem. 2003;46:1273–1276. doi: 10.1021/jm021085c. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Reavill C, East SZ, Harrison PJ, Patel S, Routledge C, Leslie RA. The distribution of 5-HT(6) receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT(6) receptor antagonist [(125)I]SB-258585. Brain Res. 2002;934:49–57. doi: 10.1016/s0006-8993(02)02360-0. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Hagan JJ. 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacology (Berl) 2001;158:114–119. doi: 10.1007/s002130100840. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Monsma FJ, Jr, Borroni E, Austin RH, Bourson A. Effects of altered 5-ht6 expression in the rat: functional studies using antisense oligonucleotides. Behav Brain Res. 1996;73:245–248. doi: 10.1016/0166-4328(96)00105-2. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Boess FG, Bos M, Levet-Trafit B, Riemer C, Bourson A. Characterization of Ro 04–6790 and Ro 63–0563: potent and selective antagonists at human and rat 5-HT6 receptors. Br J Pharmacol. 1998;124:556–562. doi: 10.1038/sj.bjp.0701851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Girault JA, Chen JYC, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc Natl Acad Sci USA. 2002a;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P. DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci USA. 2002b;99:3188–3193. doi: 10.1073/pnas.052712699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yatsugi S, Hatanaka K, Nakato K, Hattori H, Sonoda R, Koshiya K, Fujii M, Yamaguchi T. Pharmacological studies on YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT2A receptor antagonistic activity. Eur J Pharmacol. 1997;329:27–35. doi: 10.1016/s0014-2999(97)10108-x. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E, Antkiewicz-Michaluk L, Klodzinska A, Stachowisz K, Chojnacka-Wojcik E. Antidepressant-like effects of the selective 5-HT1B receptor agonist CP 94253: a possible mechanism of action. Eur J Pharmacol. 2005;516:46–50. doi: 10.1016/j.ejphar.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Torres G, Horowitz JM, Laflamme N, Rivest S. Fluoxetine induces the transcription of genes encoding c-fos, corticotropin-releasing factor and its type 1 receptor in rat brain. Neuroscience. 1998;87:463–477. doi: 10.1016/s0306-4522(98)00147-x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe ES, Mao L. Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol. 2005;32:237–249. doi: 10.1385/MN:32:3:237. [DOI] [PubMed] [Google Scholar]
- Ward RP, Hamblin MW, Lachowicz JE, Hoffman BJ, Sibley DR, Dorsa DM. Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience. 1995;64:1105–1111. doi: 10.1016/0306-4522(94)00439-c. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC. A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology. 2001;41:210–219. doi: 10.1016/s0028-3908(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, Mori K, Saito H. Central distribution and function of 5-HT6 receptor subtype in the rat brain. Life Sci. 1998;62:1473–1477. doi: 10.1016/s0024-3205(98)00092-7. [DOI] [PubMed] [Google Scholar]
- Yun HM, Kim S, Kim HJ, Kostenis E, Kim JI, Seong JY, Baik JH, Rhim H. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem. 2007;282:5496–5505. doi: 10.1074/jbc.M606215200. [DOI] [PubMed] [Google Scholar]