Abstract
Studies in laboratory rodents suggest that previously neutral stimuli repeatedly paired with the administration of drugs of abuse can acquire the ability to increase striatal dopamine release. This conditioned neurochemical response is believed to prompt drug seeking in animals and has been hypothesized to contribute to drug craving and relapse in substance abusers. In the present study, we used positron emission tomography and [11C]raclopride to investigate whether amphetamine-predictive stimuli can elicit striatal dopamine release in humans. Nine healthy male volunteers received a capsule containing amphetamine tablets (0.3 mg/kg) on three separate occasions approximately every other day (mean ± SD, 2.25 ± 1.13 d apart) in the same environment (scanner suite). At least 2 weeks later, the amphetamine was switched to a placebo of identical appearance and given in the same environmental context. [11C]Raclopride binding to dopamine D2/3 receptors was assessed after exposure to the first amphetamine-containing pill, after placebo administration, and during a control (no pill) scan. Relative to the control scan, amphetamine administration decreased [11C]raclopride binding potential by 22% in the ventral striatum and 11% in the putamen. Placebo also decreased [11C]raclopride binding potential in the ventral striatum and did so with the same amplitude as amphetamine (23%). These results suggest that cues associated with amphetamine increase dopamine transmission, providing evidence that this system is involved in reward prediction in humans.
Keywords: positron emission tomography, dopamine, amphetamine, conditioning, placebo, addiction
Introduction
Reexposure to stimuli that have been paired previously with the pharmacological effects of a drug, such as environmental context that surrounds drug administration or the drug vehicle (e.g., a syringe or a pill), is believed to increase the likelihood of relapse by eliciting drug-similar effects, craving, and drug-seeking behavior (Childress et al., 1988; Carter and Tiffany, 1999; Weiss, 2005). Indeed, in animals, exposure to predictive drug cues, even after prolonged drug-free periods, reliably elicits conditioned psychomotor activation and drug seeking (Weiss et al., 2001). In humans, exposure to drug paraphernalia provokes craving in drug-dependent subjects (Childress et al., 1993; Maas et al., 1998) and increases the self-reported positive response to drug (Volkow et al., 2003).
The best studied neurobiological substrate of cue-induced drug seeking is the midbrain dopamine (DA) pathway. Electrophysiological recordings in nonhuman primates have demonstrated that dopaminergic neurons (A10/A9) respond in an adaptive manner to events and cues that predict rewarding stimuli (primarily natural rewards) (Schultz et al., 1997). Although some in vivo studies in rodents have reported significant increases in extracellular DA in the nucleus accumbens after the presentation of drug-predictive discriminative stimuli (Gratton and Wise, 1994; Kiyatkin and Stein, 1996; Di Ciano et al., 1998a, b; Duvauchelle et al., 2000; Weiss et al., 2001), others have not (Brown et al., 1992; Neisewander et al., 1996), nor did a microdialysis study in four monkeys who had received chronic cocaine injections (Bradberry et al., 2000).
In humans, neuroimaging studies have demonstrated the involvement of DA-innervated brain regions during responses to reward-related cues. For example, functional magnetic resonance imaging (fMRI) and metabolic (18F-fluoro-2-deoxy-glucose) positron emission tomography (PET) studies have shown activation of DA-rich brain regions during exposure to drug-related stimuli or during drug anticipation (Grant et al., 1996; Maas et al., 1998; Wexler et al., 2001; McBride et al., 2006). More recently, alterations in synaptic DA levels have been measured by PET and changes in [11C]raclopride binding potential (BP) to D2/3 receptors (Laruelle, 2000). Using this method, studies are providing direct neurochemical evidence of changes in DA after the presentation of cues, such as a video that portrayed subjects smoking cocaine or the administration of a placebo, evocative of a reinforcing substance (cocaine, caffeine, or apomorphine) (de la Fuente-Fernandez et al., 2002; Kaasinen et al., 2004; Volkow et al., 2006). To date, the possibility that cues explicitly paired with drug might induce DA release has not been tested in humans and has not been supported by studies in nonhuman primates (Bradberry et al., 2000). The mechanisms regulating cue-induced behaviors and affective or motivational responses and the role of DA in the reactivity to the drug-paired stimuli [conditioned stimuli (CS)] are poorly understood.
In the present study, we investigated the dopaminergic and subjective responses to conditioned drug cues in non-drug-using individuals. The primary aim of this PET experiment was to determine whether the CS, which consisted of the administration of an (amphetamine) placebo in the PET environment, would acquire the ability to elicit DA release and conditioned affective and motivational states after three sessions of associative pairing.
Materials and Methods
Subjects.
Nine men (age, 26.5 ± 3.2 years) recruited through on-campus advertisement provided written informed consent to be enrolled in the study. One subject's data were removed from the analysis because of excessive head movement during scans. Subjects were nonsmokers, nondrug users, and had no current or previous personal history of significant medical illness or personal or family history of psychiatric disorders (as per Structured Clinical Interview for Diagnostic Statistical Manual of Mental Disorders IV psychiatric diagnosis).
Associative drug pairing.
To achieve associative learning between the subjective effects of amphetamine and environmental cues, subjects received a capsule containing amphetamine (dextroamphetamine sulfate, 0.3 mg/kg, p.o.) on three consecutive occasions approximately every other day (mean ± SD, 2.25 ± 1.13 d apart). The choice of dose and route of administration (per mouth) was based on previous studies conducted in this laboratory (Leyton et al., 2002; Boileau et al., 2006). Subjects were told that each capsule might contain amphetamine or sugar. Response to the CS was assessed at least 2 weeks (16.3 ± 2 d for six of the eight subjects) after the administration of the triple-dose amphetamine regimen to allow withdrawal effects to dissipate. Because of PET scanner maintenance, the latency period between the amphetamine regimen and the CS session was longer for two of the eight subjects (70 and 105 d). During this session, the amphetamine-containing capsule was switched to a placebo-containing capsule of identical appearance also administered by mouth (amphetamine placebo).
PET acquisition protocol.
All subjects underwent PET scans on a CTI/Siemens (Knoxville, TN) ECAT-HR+ PET camera with lead septa removed (63 slice coverage, with a maximum resolution of 4.2 mm full-width half-maximum in the center of the field of view). A catheter was inserted into the subject's antecubital vein for tracer injection and blood drawing. Attenuation correction was performed via a 10 min 68Ga transmission scan. A negative toxicology screen for illicit drugs by urine analyses was confirmed on every scan session (Triage TM-Panel; Biosite Diagnostics, San Diego, CA). Amphetamine or placebo was administered by mouth 60 min before the intravenous bolus injection of [11C]raclopride (7 mCi). Emission data were collected over 60 min in time frames of progressively longer duration. Measurements of [11C]raclopride BP were obtained at the same time of day on three separate occasions, 1 h after amphetamine, 1 h after placebo, and during a control scan when subjects received no pill. This study design was counterbalanced such that four of eight subjects underwent the control scan before their first amphetamine session (30, 85, 85, and 7 d before; mean ± SD, 51.7 ± 39 d), whereas four received it afterward (24, 28, 21, and 137 d after; mean ± SD, 47.8 ± 49 d) (Table 1). Amphetamine doses 2 and 3 were administered during sham scans, in the course of which subjects underwent all aspects of the PET procedure, except for tracer injection.
Table 1.
Experimental design
| Day 0 or >21 | Day 1 | Day 3 | Day 5 | More than day 21 | |
|---|---|---|---|---|---|
| 11C scan | 11C scan | Sham | Sham | >2 weeks | 11C scan |
| Control | AMPH | AMPH | AMPH | Latency | Placebo |
AMPH, Amphetamine.
Behavioral and physiological assessment.
Mood and alertness were assessed at baseline and at regular intervals, throughout each experimental session using both visual analog scales and the Bipolar Profile of Mood States (McNair et al., 1992). The Addiction Research Center Inventory (ARCI) Benzedrine scale (Haertzen et al., 1963) was administered at the end of every experimental session.
Image analysis.
PET images were reconstructed using a 6 mm full-width half-maximum Hanning filter. Each individual's weighted radioactivity PET images were coregistered to average MRI (Evans et al., 1992) derived from each subjects' high-resolution T1-weighted MRI. PET scans corrupted by movement were corrected by applying an algorithm that compensates for between-frame misalignment (Perruchot et al., 2004). Parametric images were generated by computing [11C]raclopride BP at each voxel using a simplified compartmental model with cerebellar activity as a reference (Gunn et al., 1997). Separate t maps illustrating the significance of effects of amphetamine and placebo relative to control BP were generated using in-house software that applies the statistical method described by Aston et al. (2000). This technique uses the residuals of the fit of the compartment model to estimate the variance of the BP at each voxel. Peak height significance was t ≥ 4.2, corresponding to p < 0.05, corrected for multiple comparisons based on random field theory (Worsley et al., 1996), as described by Aston et al. (2000).
Region of interest analysis.
Six regions of interest (ROIs) were selected bilaterally on each individual's MRI using a combination of manual and automatic approaches. These regions included the ventral striatum (VS), pre-commissural (DC) and post-commissural (PDC) dorsal caudate, pre-commissural dorsal putamen (DP), post-commissural putamen (PDP), and the cerebellum (cerebellar cortex), which served as the reference region for compartmental modeling. For better consistency, ROI delineation into gross anatomical brain structures was first obtained by applying a tissue classification (gray matter, white matter, and CSF) and an automatic segmentation (Collins et al., 1995). Each subject's output ROI map was then manually edited to fit the criteria described by Martinez et al. (2003), using the coronal slice as default. The corresponding VS (3370 ± 484.243 mm3), DC (7424 ± 1079.2 mm3), and DP (8933 ± 1530 mm3) extended rostrally from planes x = 4, x = 0, and x = 0 respectively, to the anterior boundary of the striatum; the PDC (3049 ± 494.5 mm3) and PDP (4908 ± 636.5 mm3) extended caudally from the anterior commissure to the posterior edge of the striatum. The cerebellum (11000 ± 2079 mm3) was drawn on five consecutive slices of cerebellar cortex. To minimize the regional point-spread and tissue-fraction effects resulting from the limited resolution of the PET camera, a partial volume effect correction was performed (Aston et al., 2002).
Statistical analysis.
Partial volume-corrected BP values extracted from ROI (two-way, ROI × session) and changes in mood and alertness/energy (two-way, session × time) during the drug-free control, amphetamine, and placebo scans were statistically examined using repeated-measures ANOVA. A one-tailed analysis for planned comparison was chosen based on the known pharmacology of amphetamine and on results from our previous PET studies and also on the direction of the voxelwise analysis. Pearson's product moment correlation coefficients were used to examine the putative relationships between response to CS and response to amphetamine and between BP reduction and changes in mood. For all analyses, data from left-sided and right-sided ROIs were averaged.
Results
Effect of amphetamine and amphetamine-predictive cues on [11C]raclopride BP
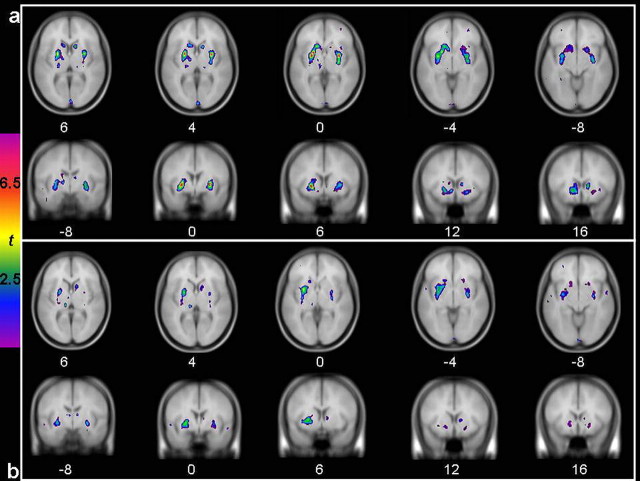
Voxelwise analysis over the whole brain revealed significant (t ≥4.2; p < 0.05) bilateral clusters of decreased [11C]raclopride BP in response to amphetamine relative to the control scan (Fig. 1a). Clusters of decreased [11C]raclopride BP were also observed when comparing the placebo session with the control condition (Fig. 1b). Those clusters were similar although they had lower peak t values and smaller spatial extent.
Figure 1.
t statistical maps of [11C]raclopride BP change illustrating decreased [11C]raclopride BP after the single-dose amphetamine administration (A) (0.3 mg/kg, p.o.) and the placebo administration (B) relative to the control condition. Colored t maps are overlaid on an average T1 MRI of all participants.
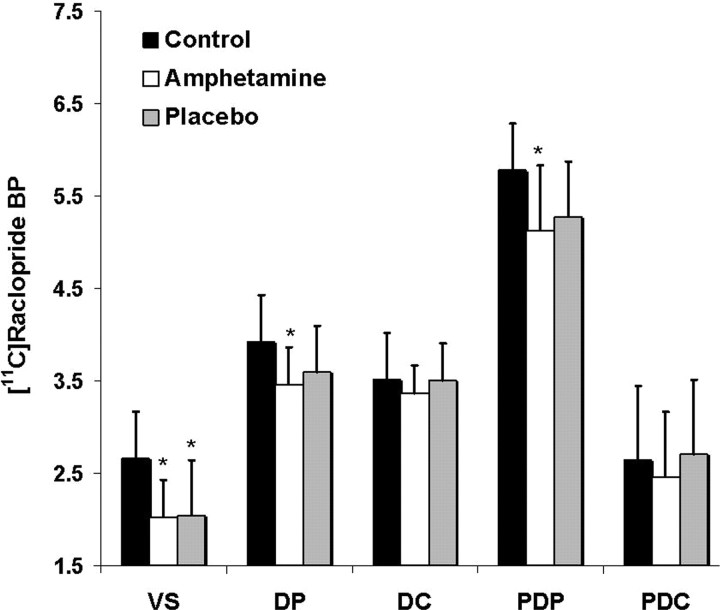
ROI analyses confirmed the results from the voxelwise analysis (Fig. 2). The single-dose administration of amphetamine was associated with a significant decrease of [11C]raclopride BP, relative to the control baseline, in subcompartments of the striatum (ROI × session interaction; F(8,56) = 2.54; p = 0.02). This effect corresponded to a mean decrease of [11C]raclopride BP of, respectively, −22.24 ± 18% in the VS (Bonferroni's corrected for one-tailed planned comparison; p = 0.01), −11.48 ± 10% in the PDP (p = 0.01), and −11.42 ± 7% in the DP (p = 0.008). Placebo also significantly decreased [11C]raclopride BP in the VS; this decrease, corresponding to −23.25 ± 39%, was significantly different from the control condition (p = 0.02) but not from amphetamine. Smaller decreases after placebo were also noted in the DP (−7.8 ± 12%; p = 0.12) and PDP (−8.3 ± 13%; p = 0.1), but they were not statistically significant. Neither amphetamine nor placebo significantly reduced [11C]raclopride BP in the DC (amphetamine, −3.6 ± 8%, p = 0.21; placebo, 0.6 ± 13%, p = 0.94) or PDC (amphetamine, −3.9 ± 16%, p = 0.23; placebo, 5.1 ± 17%, p = 0.64). There were no significant differences between baseline [11C]raclopride BP measured in the VS or in the rest of the striatum before (n = 4; VS, 2.9 ± 0.4; DC, 3.4 ± 0.6; DP, 3.8 ± 0.6) or after (n = 4; VS, 2.4 ± 07; DC, 3.6 ± 0.7; DP, 3.9 ± 0.6) the amphetamine regimen.
Figure 2.
Mean (and SD) [11C]raclopride BP in five subcompartments of the striatum during the control, amphetamine, and placebo scans. *p < 0.05, significantly different from the control scan.
Effect of amphetamine and placebo on drug-related mood ratings
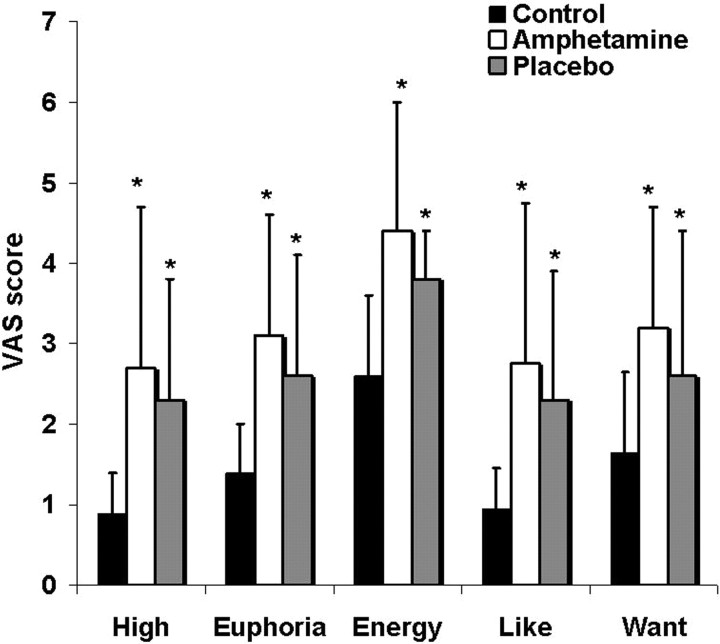
The administration of amphetamine led to significant increases in self-reported high (p = 0.01), euphoria (p = 0.01), energy level (p < 0.001; amphetamine vs control; p = 0.001), drug liking (p = 0.03), and a trend toward an increase in drug wanting (p = 0.09). Placebo administration resulted in similar increases in positive mood relative to the control condition, which correlated with the changes in mood secondary to amphetamine (correlation between amphetamine and placebo sessions: high, r = 0.88, p = 0.04; euphoria, r = 0.80, p = 0.02; energy, r = 0.78, p = 0.02). Specifically, the placebo response included increased high (p = 0.007), euphoria (p = 0.02), energy (p = 0.002), drug liking (p = 0.002), and drug wanting (p = 0.05) (Fig. 3). The magnitude of the conditioned and unconditioned responses were not significantly different; however, the conditioned response appeared to be more transient, with maximum scores occurring early after placebo administration (60 min). Scoring of the ARCI 120 min after drug or placebo indicated that the amphetamine condition but not placebo was associated with increased drug-related ratings (p = 0.03).
Figure 3.
Visual analog scale scores (mean ± SD) recorded after 0.3 mg/kg of oral amphetamine after placebo and during the control condition. * indicates significantly increased compared with control. *p < 0.05.
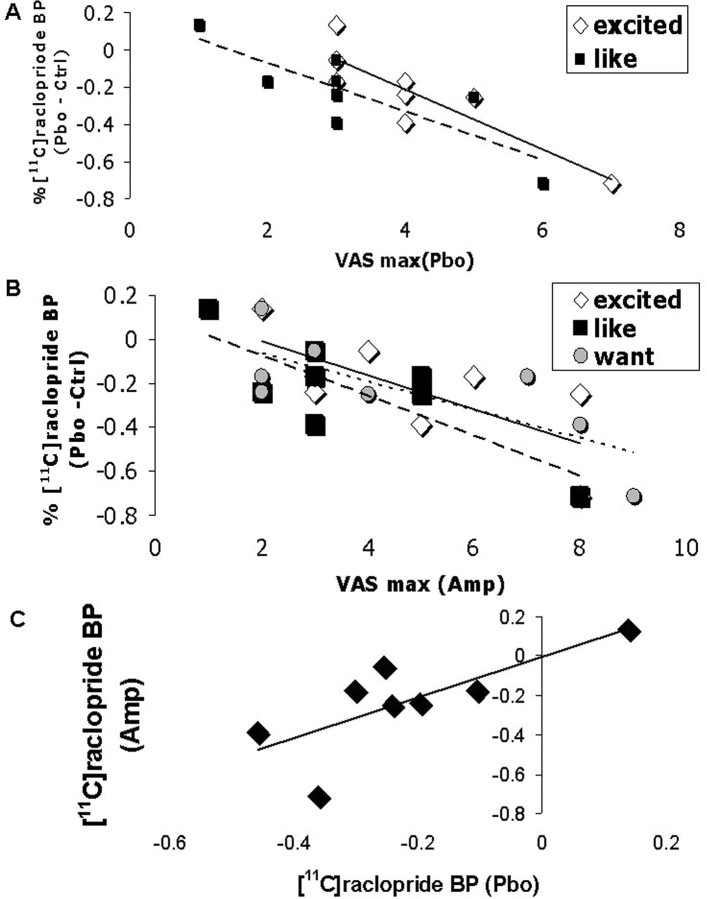
Correlation analyses revealed that drug liking (r = −0.83; p = 0.01) and feeling excited (r = −0.88; p = 0.004) during the placebo session correlated with cue (or placebo)-induced decreased [11C]raclopride BP. Moreover, positive ratings during amphetamine exposure (excited, r = −0.71, p = 0.05; drug liking, r = −0.80, p = 0.016; drug wanting, r = −0.76, p = 0.02) and amphetamine-induced changes in [11C]raclopride BP (r = 0.74; p = 0.03) both predicted changes in [11C]raclopride BP during response to placebo (Fig. 4a–c).
Figure 4.
Predictors of conditioned DA response. A, Relationship between mood during the placebo session (maximum) and dopaminergic response during the placebo session ([11C]raclopride BP percentage compared with control). B, Relationship between mood during the amphetamine session (maximum all sessions) and dopaminergic response during the placebo session ([11C]raclopride BP percentage change from control). C, Relationship between conditioned (placebo session) and unconditioned (amphetamine session) dopaminergic responses ([11C]raclopride BP percentage change from control).
Discussion
DA release in the VS in response to amphetamine-predictive cues
In the present study, we used PET and [11C]raclopride in humans to investigate whether repeated pairing of amphetamine with specific cues would lead to conditioned DA release in the striatum during reexposure to the cues in the absence of amphetamine. Consistent with previous reports, acute amphetamine administration decreased [11C]raclopride BP in the limbic VS and putamen (Martinez et al., 2003; Boileau et al., 2006). A CS consisting of a placebo pill identical in appearance to the amphetamine pill promoted a change in [11C]raclopride BP within the VS (−23%) and did so to the same extent as amphetamine (−22%). This effect of the CS is in keeping with in vivo voltammetry results in animals, which suggest that a CS can be as effective as amphetamine in raising the DA signal (Di Ciano et al., 1998b).
Although a conditioned increase in synaptic DA levels was also noted in the putamen (7–8% decrease in the ROI analysis, nonsignificant), increased DA was preferential and statistically significant only in the VS. Although the involvement of the VS is in agreement with reports of increased DA release in the nucleus accumbens of rats reexposed to stimulant drug-paired cues (Gratton and Wise, 1994; Kiyatkin and Stein, 1996; Di Ciano et al., 1998a, b; Duvauchelle et al., 2000; Weiss et al., 2001), it contrasts with the results of a study in rhesus monkeys (n = 4) in which no conditioned DA response in the VS was reported (Bradberry et al., 2000). This negative finding in monkeys might be accounted for by the small sample size, drug withdrawal effects after over 3 months of twice weekly cocaine administration, differences in pharmacokinetics and pharmacodynamic between drugs, differences between cues that are paired with contingent versus noncontingent drug administration, the likely ready ability of the monkeys to identify the absence of interoceptive changes after the saline infusion, and other methodological differences in the cues and dependent measures; for example, PET is believed to primarily measure synaptic DA flux, which might be of equal magnitude after CS and amphetamine, whereas microdialysis records extracellular changes in DA concentrations, which might be less pronounced after CS. Findings are also seemingly at odds with results from a recently published PET study investigating the effects of cocaine-related videotapes on DA release in dependent cocaine users (Volkow et al., 2006). Similar to some cue reactivity paradigms in rodents (Ito et al., 2002), the PET study with cocaine evocative imagery implicated DA release in the DC but not the VS (Volkow et al., 2006). This discrepancy might reflect differences in subject populations (cocaine-dependent subjects with a history of habitual drug use vs healthy non-users), the difference between a cue that elicits memories of drug use versus CS that have been explicitly and systematically paired with the drug, or, as Volkow et al. (2006) suggested, a difference between a cue that, in drug abusers, would prime drug-seeking behavior (e.g., drug-associated visual imagery) versus one that signals reward regardless of action (e.g., pill ingestion), i.e., the difference between drug reward that is contingent versus noncontingent on behavior. Indeed, animal studies designed to separate these different modalities suggest that contingent cues elicit drug-seeking behavior and DA release in the DC (Ito et al., 2002), whereas noncontingent cues induce DA release in the nucleus accumbens (Neisewander et al., 1996).
Expectancy or conditioning?
One consideration relevant to the interpretation of the present results resides in whether DA changes secondary to placebo can be attributed to conscious expectation of reward or to classical conditioning per se. In humans, it has been shown that at least some effects of associative pairing on the placebo response are dependant on expectancy, such that despite repeated pairing, verbal information regarding pill content drives the behavioral response (Montgomery and Kirsch, 1997). Expectation of rewarding clinical benefits has been used to explain activation of the VS (DA release in PET) after placebo apomorphine administration in patients with Parkinson's disease (de la Fuente-Fernandez et al., 2002). Relative to the DC, which has been implicated in action preparation and the instrumental component of reward once a contingency is established, the action of the VS has chiefly been reported during the tracking of reward predictions (O'Doherty, 2004). These findings could explain why we found changes in the VS but not in the DC. Despite the fact that subjects in the present study were blind to pill content, the effects of anticipation on placebo-induced DA release in the VS, independent of conditioned effects, cannot be ruled out.
Sensitization to repeated amphetamine
To achieve an association between drug effect and context, repeated amphetamine administration was necessary. Preexposure to stimulants can affect the DA system, making it more responsive to drugs, stress, or cues associated with reward (Vezina, 2004). Indeed, studies in animals and in humans have shown that repeated exposure to amphetamine produces sensitization to the dopaminergic and psychomotor-activating effects of the drug during delayed reexposure (Stewart and Badiani, 1993; Boileau et al., 2006). Furthermore, sensitization-inducing drug regimens increase cue-induced instrumental responding for natural rewards (Wyvell and Berridge, 2001) and stimulant drugs (Robinson and Berridge, 1993). We reported previously that the present amphetamine regimen produces sensitization (increased DA release) in healthy volunteers (Boileau et al., 2006); therefore, nonspecific potentiation of the conditioned response through sensitization of the DA system may have contributed to our findings.
Changes in baseline [11C]raclopride
Repeated amphetamine exposure leads to numerous adaptive changes including some that could affect the stability of [11C]raclopride BP (Pierce and Kalivas, 1997). To preclude the possibility that changes in receptor density or affinity, resulting from amphetamine preexposure might account for the lower [11C]raclopride BP on the placebo test, half of the subjects completed their control scans last. Consistent with previous observations (Boileau et al., 2006), baseline [11C]raclopride BP, determined in the VS or in the rest of the striatum, did not differ, whether measured previous, or after, the amphetamine regimen, thereby suggesting that D2/3 receptor density remained unaffected by the amphetamine regimen.
Relationship between conditioned and unconditioned responses to amphetamine
In animal studies, reexposure to drug paired stimuli elicits a range of behavioral and neurobiological effects that can resemble those produced by the drug itself (Stewart et al., 1984). Recent work suggests that the same effects might occur in humans. For example, in functional neuroimaging studies, exposure to drug-themed cues that elicit craving increases fMRI blood oxygenation level-dependent signal (Garavan et al., 2000) and cerebral blood flow or glucose metabolism measured with PET (Volkow et al., 1999; Wang et al., 1999) in DA-innervated limbic brain regions. More recently, direct dopaminergic involvement in cue reactivity has been described with [11C]raclopride PET (Volkow et al., 2006). Decreasing DA transmission, in comparison, reduces drug cue-induced craving and physiological arousal (Berger et al., 1996; Leyton et al., 2005; Mahler and de Wit, 2005). In the present study, we report that cues alone elicited a synaptic dopaminergic response and a transient behavioral state similar but not as long-lasting as that induced by amphetamine. It is plausible that, similar to the behavioral pattern, DA efflux after exposure to cues is also more transient than the response observed after amphetamine; however, PET lacks the temporal resolution to detect this difference if it exists.
Conclusion
Much of the interest about the effects of drug-paired cues stems from the observation that exposure to drug cues in human drug addicts may trigger intense feelings of craving and susceptibility to relapse. Animal studies, in comparison, suggest that both drug- and cue-induced reinstatement of drug-seeking behavior are associated with increased DA neurotransmission. It has been proposed that conditioned DA release increases the motivational salience of the drug-paired cues and in this way could contribute to the re-initiation of drug-seeking behavior and craving states (Robinson and Berridge, 1993; Berridge and Robinson, 1998). The results presented in this report suggest that such a mechanism, conditioned dopamine release, may also be at play in humans.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (MOP 64412) (C.B., A.D.). We thank Rick Fukusawa, Gary Sauchuk, Dean Jolly, Dr. Shadreck Mzengeza, and Mirjana Kovacevic for excellent technical assistance and Dr. Jean Paul Soucy (Chief of Nuclear Medicine at the Montreal Neurological Institute) for valuable help during the PET scans.
References
- Aston JA, Gunn RN, Worsley KJ, Ma Y, Evans AC, Dagher A. A statistical method for the analysis of positron emission tomography neuroreceptor ligand data. NeuroImage. 2000;12:245–256. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Aston JA, Cunningham VJ, Asselin MC, Hammers A, Evans AC, Gunn RN. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab. 2002;22:1019–1034. doi: 10.1097/00004647-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, Carr K. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn R, Baker G, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: a [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Collins DLHC, Peters TM, Evans AC. Automatic 3D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- de la Fuente-Fernandez R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ, Stoessl AJ. Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res. 2002;136:359–363. doi: 10.1016/s0166-4328(02)00130-4. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. The relation between dopamine oxidation currents in the nucleus accumbens and conditioned increases in motor activity in rats following repeated administration of d-amphetamine or cocaine. Eur J Neurosci. 1998a;10:1113–1120. doi: 10.1046/j.1460-9568.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci. 1998b;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Castaneda E. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav Neurosci. 2000;114:1156–1166. doi: 10.1037//0735-7044.114.6.1156. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. NeuroImage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci. 1994;14:4130–4146. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Hill HE, Belleville RE. Development of the addiction research center inventory (ARCI): selection of items that are sensitive to the effects of various drugs. Psychopharmacologia. 1963;70:155–166. doi: 10.1007/BF02584088. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Nagren K, Rinne JO. Expectation of caffeine induces dopaminergic responses in humans. Eur J Neurosci. 2004;19:2352–2356. doi: 10.1111/j.1460-9568.2004.03310.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin E, Stein E. Conditioned changes in nucleus accumbens dopamine signal established by intravenous cocaine in rats. J Neurochem. 1996;211:73–76. doi: 10.1016/0304-3940(96)12731-2. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119:1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Effects of haloperidol on reactions to smoking cues in humans. Behav Pharmacol. 2005;16:123–126. doi: 10.1097/00008877-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. II. Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. San Diego: Educational and Industrial Testing Service; 1992. EITS manual for profile of mood states. [Google Scholar]
- Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, O'Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Perruchot F, Reilhac A, Grova C, Evans AC, Dagher A. Motion correction of multi-frame PET data. IEEE Trans Nucl Sci Conference Record. 2004;5:3186–3190. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psych Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]