Abstract
Background
Increased pain sensitivity is observed following alcohol withdrawal, and attempts to alleviate this hyperalgesia can contribute to the cycle of addiction. The aim of this study was to determine if alcohol withdrawal-induced hyperalgesia was observed in a chronic ethanol exposure model and if this pain was affected by histone deacetylase inhibitors, thus revealing an epigenetic mechanism.
Methods
Adult male Sprague Dawley rats received Lieber-DeCarli liquid control or ethanol (9% v/v) diet for 15 days. Mechanical sensitivity was measured with von Frey hair stimulation of the hindpaw during ethanol administration and 24- and 72-hour withdrawal.
Results
Ethanol withdrawal produced severe and sustained mechanical hyperalgesia, an effect not observed in the control or ethanol-maintained groups. Furthermore, this hyperalgesia was attenuated by the histone deacetylase inhibitor, suberoylanilide hydroxamic acid treatment.
Conclusions
Heightened pain sensitivity was observed following withdrawal from chronic ethanol exposure, and histone deacetylase inhibitors could be novel treatments for this alcohol withdrawal-induced hyperalgesia.
Keywords: pain, SAHA, alcohol dependence
Introduction
There is a complex relationship between alcohol and pain processing. A recent meta-analysis confirmed that acute alcohol is analgesic and that this effect is dose dependent (Thompson et al., 2017). However, an intoxicating blood alcohol content of approximately 0.08% (3–4 drinks) was required before an analgesic response was observed. In contrast, chronic alcohol use can result in peripheral neuropathy (Ammendola et al., 2001), which may be due to the neurotoxic effects of alcohol and its metabolites (Zeng et al., 2017). Furthermore, withdrawal from chronic alcohol can also result in heightened pain sensitivity, which is resolved after several weeks to months of abstinence (Jochum et al., 2010). This alcohol withdrawal-induced hyperalgesia was also shown to correlate with negative emotional state (Jochum et al., 2010), and attempts to alleviate this heightened pain state could contribute to the cycle of addiction (Egli et al., 2012; Apkarian et al., 2013). Furthermore, there are a number of overlapping circuits that are altered during drug dependence and chronic pain (Elman and Borsook, 2016), and adaptations within these shared pathways could mediate pain hypersensitivity following alcohol exposure and withdrawal (Egli et al., 2012).
Chronic alcohol exposure can induce neuroplasticity that facilitates the development and maintenance of alcohol use disorders (Koob and Volkow, 2016). Epigenetic modifications are changes that occur at the structural level of DNA that alter the accessibility of the gene to the transcriptional machinery but do not alter the genetic code itself (Krishnan et al., 2014). Epigenetic modulation of gene expression has emerged as a key regulator of the alcohol withdrawal state (Berkel and Pandey, 2017). Alterations in the epigenome through DNA methylation or histone modifications are consistently observed following chronic alcohol exposure (Moonat et al., 2013; Sakharkar et al., 2014). For example, withdrawal from chronic alcohol has been shown to result in increased histone deacetylase (HDAC) activity in the amygdala and heightened anxiety-like behaviors, which are attenuated by the HDAC inhibitor trichostatin A (Pandey et al., 2008). Furthermore, deficits in brain-derived neurotrophic factor expression and dendritic spine density in amygdaloid circuits in response to chronic alcohol and withdrawal are also attenuated by trichostatin A (Pandey et al., 2008; You et al., 2014), which is mechanistically consistent with the role of this brain region in regulation of negative affect (Pandey et al., 2017). Pain represents another behavioral phenotype of negative affect, which develops during withdrawal (Edwards et al., 2012; Alongkronrusmee et al., 2016; Smith et al., 2016; Avegno et al., 2018; Kononoff et al., 2018) and can play a critical role in maintaining addictive behaviors (Egli et al., 2012; Apkarian et al., 2013). To the best of our knowledge, the effects of HDAC inhibitor treatment on ethanol withdrawal-induced hyperalgesia has not been investigated.
The aim of this study was to develop an alcohol withdrawal-induced hyperalgesia animal model using the Lieber-DeCarli ethanol diet drinking paradigm to mimic human alcoholics. An additional goal was to further examine if this hyperalgesia was affected by treatment with the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA).
Materials and Methods
Chronic Ethanol Exposure Paradigm
All experiments were approved by the Institutional Animal Care and Use Committee of University of Illinois at Chicago and adhered to the NIH Guidelines for the Care and Use of Laboratory Animals. Animals were housed in a temperature-controlled room with a 12-hour-light/-dark cycle, with lights on at 7:00 am and off at 7:00 pm and food and water were provided ad libitum. Adult male Sprague-Dawley rats (postnatal days 75–87 at start of treatment) were initially group housed and then separated during alcohol or control diet feeding. As described previously (Pandey et al., 2008), rats were offered 80 mL/d of the nutritionally complete Lieber-DeCarli liquid control diet (Lieber-DeCarli Diet 82; Bio-Serv, Frenchtown, NJ) for 3 days. Control groups continued with the control diet for 16 days, while ethanol groups were gradually introduced to ethanol (1.8% through 8.1% within 7 days) and maintained on 9% v/v ethanol diet for 15 days. Rats were pair-fed and liquid diet intake and body weights were closely monitored. Fresh diet was provided daily between 17 and 18 hours before the beginning of the dark cycle. One group of ethanol-diet fed rats was withdrawn for 24 to 72 hours (withdrawal group) and given the control diet. Another group of ethanol-diet fed rats was maintained on the ethanol diet during this time. Blood alcohol levels were measured using an Analox Alcohol Analyzer (Analox Instruments, Lunenberg, MA).
Assessment of Sensory Sensitivity
Rats were habituated to the testing rack for 2 days for 20 minutes each prior to the first testing day. For all behavioral experiments, rats were counterbalanced into groups following the first test for mechanical sensitivity. The experimenter was blinded to the feeding paradigm and the drug condition tested. All rats were tested in a separate behavior room with low light (approximately 35–50 lux) and low noise, between 9 and 12 hours, roughly 15 to 18 hours after the placement of diet from the day before and 5 to 8 hours before the placement of fresh diet for that day. For hindpaw sensitivity, the threshold for responses to punctate mechanical stimuli (mechanical hyperalgesia) was tested according to the up-and-down method (Chaplan et al., 1994) in the left hindpaw. The plantar surface of the hindpaw was stimulated with a series of 8 von Frey hair filaments (0.4–15 g). A response was defined as lifting, shaking, or licking of the paw on stimulation. The first filament tested was 4 g. In the absence of a response, a heavier filament (up) was tried, and in the presence of a response, a lighter filament (down) was tested. This pattern was followed for a maximum of 4 filaments following the first response (Pradhan et al., 2010).
Experimental Outline
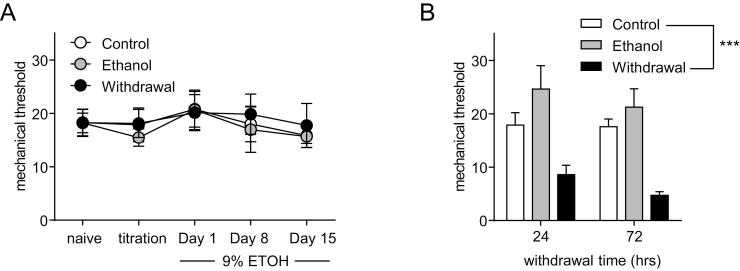
For all experiments rats were tested the day before start of the ethanol or control diet (naïve), on the first day of ethanol exposure (titration), on days 1 and 8, 14 or 15 of 9% ethanol, and 24 and 72 hours withdrawal (Figure 1).
Figure 1.
Withdrawal from chronic ethanol exposure produces hyperalgesia. (A) Male rats were tested when naïve and during ethanol or control diet feeding, and mechanical responses to von Frey hair stimulation remained stable regardless of group or time. (B) Ethanol withdrawal produced a severe and sustained mechanical hyperalgesia compared with control and ethanol-maintained groups. ***P < .001 compared with the control group (2-way repeated-measures ANOVA, P < .001 for group; n = 8/group). Alcohol withdrawal increases pain sensitivity.
For the SAHA experiment, rats were injected daily with SAHA or vehicle at 24 hours withdrawal until 72 hours withdrawal. On test days, basal responses were determined before the treatment, and posttreatment responses were determined 2 hours post-drug administration. SAHA (Vorinostat, Selleck Chemicals, Houston, TX) solution was prepared by dissolving 62.5 mg SAHA in 0.2 mL dimethylsulfoxide, 4 mL of PEG300, 0.5 mL propylene glycol, 0.1 mL Tween‐80, and 5.2 mL normal saline were added to the solution sequentially and vortexed before each addition. The final concentration of SAHA was 6.25 mg/mL, 2% dimethylsulfoxide, 40% PEG300, 5% propylene glycol, and 1% Tween‐80 in saline. Control vehicle solution was the same solvents in the same concentrations in saline. The dose of SAHA was 50 mg/kg i.p.
Statistical Analysis
Data are expressed as mean + SEM. All rats tested were included in the analysis. All statistical analyses were performed by SigmaStat, and graphs were generated using GraphPad Prism. Basal responses over time were analyzed using 2-way repeated-measures (RM) ANOVA with time and treatment as factors. For withdrawal responses, data were analyzed using 2-way RM ANOVA with Holm-Sidak post-hoc analysis.
Results
Alcohol Withdrawal Produces Severe Hyperalgesia
The mean ± SEM ethanol intake did not differ between ethanol and withdrawal groups (14.43 ± 0.3 and 14.75 ± 0.23 g/kg/d, respectively). The blood ethanol level in ethanol diet-maintained rats was 183 ± 22 mg%. These are similar levels to our previous publications (Pandey et al., 2008). In addition, there were no significant differences in the body weights (mean ± SEM) among the various groups (control, 305 ± 4.5 g; ethanol, 306 ± 5.6 g; withdrawal, 295 ± 3.9 g).
Mechanical responses were determined using von Frey hair stimulation of the plantar surface of the hindpaw. Naïve mechanical responses were taken when rats were still group housed and on the standard laboratory chow diet. In addition, measurements were taken during ethanol titration, and on days 1, 8, and 15 of 9% ethanol exposure. Ethanol diet did not significantly alter mechanical response to von Frey hair stimulation relative to liquid diet controls (Figure 1A). Data were analyzed using a 2-way RM ANOVA with group and time as factors. Further, there was no difference in responses in any group relative to naïve baselines; thus these results also show that singly housing the rats and switching to liquid diet did not significantly affect nociceptive responding. In contrast, ethanol withdrawal resulted in a significant decrease in mechanical thresholds, indicative of hyperalgesia, and this effect was observed at both 24 and 72 hours of withdrawal (Figure 1B). Data were analyzed using a 2-way RM ANOVA with group and time as factors, with a significant effect of group (F (2,21) = 12.357, P < .001).
SAHA Treatment Attenuates Alcohol Withdrawal-Induced Hyperalgesia
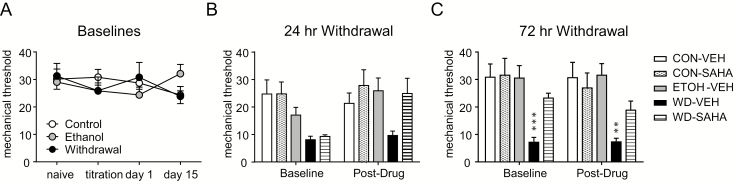
To determine the effect of HDAC inhibitors on hyperalgesia induced by alcohol withdrawal, we tested the pan-HDAC inhibitor SAHA in a separate group of animals. The mean ± SEM ethanol intake between the ethanol, withdrawal-vehicle, and withdrawal-SAHA groups did not significantly differ from each other (ethanol = 11.89 ± 0.24; withdrawal-vehicle = 12.46 ± 0.73; withdrawal-SAHA = 11 ± 0.29 g/kg/d). As above, control or ethanol liquid diet did not affect baseline mechanical responses (Figure 2A). For 24 and 72 hours withdrawal, baseline and post-drug responses were analyzed using 2-way RM ANOVA with Holm-Sidak post-hoc analysis. Twenty-four hours after withdrawal, the withdrawn rats were significantly different from the ethanol control group [Figure 2B,F (2,21) = 3.917, P = .036]. Animals were then injected with vehicle or SAHA (50 mg/kg i.p.) and tested 2 hours later (Figure 2B, post-drug). At this time point, a comparison between control-VEH/SAHA and withdrawal-VEH/SAHA groups revealed a significant group difference [F(3, 28) = 4.063, P = .016]. Rats were given a second injection of VEH/SAHA at 48 hours withdrawal, but they were not tested on this day. However, rats were tested prior to the 3rd injection at 72 hours withdrawal (Figure 2C, baseline). After 2 injections, SAHA significantly inhibited hyperalgesia (Figure 2C, baseline), an effect that was maintained after the third and final SAHA injection [Figure 2C, post-drug; control-VEH/SAHA vs withdrawal-VEH/SAHA group effect F(3,27) = 6.769, P = .002]. These data clearly suggest that SAHA is an effective treatment for preventing development of pain during ethanol withdrawal after chronic ethanol exposure in rats.
Figure 2.
Alcohol withdrawal-induced hyperalgesia is blocked by suberoylanilide hydroxamic acid (SAHA). (A) Mechanical responses remained stable throughout ethanol or control treatment. During the withdrawal period, control diet and withdrawal groups were injected daily with SAHA (50 mg/kg i.p.) or vehicle following ethanol withdrawal, while the ethanol-maintained group was concurrently injected with vehicle. (B) At 24-hour withdrawal, there was a significant difference between withdrawn animals and ethanol (P < .05) and control groups (P < .05). (C) Repeated injection of SAHA significantly reduced this hyperalgesia by 72-hour withdrawal; n = 8/group, **P < .01, ***P < .001 compared with the control-VEH group (2-way repeated-measures ANOVA with Holm Sidak post-hoc analysis).
Discussion
In this study we found that withdrawal from chronic ethanol exposure resulted in severe mechanical hyperalgesia, an effect that was maintained for at least 72 hours post-withdrawal. We also observed that this hyperalgesia was prevented by treatment with an HDAC inhibitor, suggesting that this heightened pain sensitivity is regulated epigenetically.
Increased pain sensitivity has been observed in alcoholics undergoing alcohol withdrawal or abstinence (Jochum et al., 2010). In addition, several studies have observed alcohol withdrawal-induced hyperalgesia in different animal models of alcohol exposure. Withdrawal following chronic intermittent exposure to ethanol vapor was shown by 2 separate groups to induce increased pain sensitivity during withdrawal periods (Roltsch Hellard et al., 2017; Kononoff et al., 2018). Additionally, repeated cycles of exposure and withdrawal from the Lieber-DeCarli diet was also found to increase mechanical hyperalgesia (Dina et al., 2006). In mice, withdrawal from voluntary alcohol consumption (Alongkronrusmee et al., 2016; Smith et al., 2016), or forced exposure through oral gavage (Alongkronrusmee et al., 2016) can also reliably produce a robust hyperalgesic response. In our study, rats were gradually introduced to ethanol over 7 days and then maintained at 9% for 15 days. Withdrawal from this paradigm resulted in severe mechanical hyperalgesia that was maintained for at least 72 hours post-withdrawal. Increased pain sensitivity during withdrawal could be a factor to the “dark side” of addiction, and a study in patients confirmed that alcohol withdrawal-induced hyperalgesia correlated with increased depression scores (Jochum et al., 2010).
In the paradigm used in this study, rats maintained mechanical responses similar to controls during ethanol exposure, and hyperalgesia was only observed in the withdrawal state. However, a previous study using the Lieber-DeCarli diet showed that chronic alcohol alone could result in painful peripheral neuropathy (Dina et al., 2000). However in this latter study, rats were maintained on a liquid diet for 12 weeks, and significant hyperalgesia was only observed 4 weeks into the diet. Interestingly, in this model, females showed a similar time course to develop hyperalgesia but had increased pain sensitivity relative to males (Dina et al., 2007), an effect that was estrogen dependent. In an ethanol vapor exposure model, decreased pain thresholds were observed only after 8 weeks of alcohol exposure (Edwards et al., 2012). Furthermore, peripheral neuropathy has been demonstrated in chronic alcoholics (Ammendola et al., 2001). In our study, rats were maintained at 9.0% ethanol for only 15 days, and perhaps a longer duration of exposure would result in alcohol-induced hyperalgesia or neuropathy.
Epigenetic modifications mediated by acetylation or methylation of histones and DNA methylation have been identified as important factors regulating chronic alcohol use and withdrawal (Krishnan et al., 2014; Berkel and Pandey, 2017; Pandey et al., 2017). In our study, alcohol withdrawal-induced hyperalgesia was blocked by the pan-HDAC inhibitor SAHA. We have previously observed that alcohol withdrawal also results in increased anxiety-related behaviors (Pandey et al., 2008; Sakharkar et al., 2014) and that these effects are blocked by HDAC inhibition (Pandey et al., 2008; You et al., 2014). Furthermore, withdrawal from chronic alcohol is associated with decreased expression of BDNF, Arc, and NPY in the central nucleus of amygdala, and this process is correspondingly reversed by HDAC inhibition (Pandey et al., 2008; You et al., 2014). Alcohol withdrawal-induced hyperalgesia could be the result of similar changes in brain circuitry regulating pain processing and may be related to increased HDAC activity, changes in histone acetylation or methylation, and subsequent neuroplasticity. A recent study demonstrated that alcohol withdrawal-induced hyperalgesia could be regulated by disruption to circuits projecting from the central amygdala to the periaqueductal grey (Avegno et al., 2018). In addition, chronic alcohol exposure and withdrawal could also be due to alterations occurring in the dorsal root ganglia or lumbar spinal cord. Future studies will focus on identifying the epigenetic mechanisms that mediate ethanol withdrawal-induced hyperalgesia and where these changes occur. In addition, SAHA is a pan-HDAC inhibitor and can affect acetylation of both histones and other targets, such as tubulin (Hubbert et al., 2002), which will also be explored in future studies.
Alcohol use disorder is a chronic relapsing condition characterized by cycles of alcohol use and withdrawal. A major characteristic of alcohol dependence is the need to consume alcohol not just for its rewarding effects but also to alleviate negative states associated with withdrawal (Koob and Volkow, 2016). The alcohol withdrawal-induced hyperalgesia we observed reflects the increased pain sensitivity observed in patients undergoing withdrawal (Jochum et al., 2010). HDAC inhibitors have been proposed as therapeutic targets for the treatment of emotional dysregulation associated with withdrawal (Pandey et al., 2017). Our behavioral and pharmacological findings indicate that hyperalgesia could contribute to the cycle of addiction and that HDAC inhibitors may also be effective at managing this component of the “dark side” of addiction.
Acknowledgments
This work was supported by NIAAA P50AA-022538 (Center for Alcohol Research in Epigenetics), RO1AA-010005; and by the Department of Veterans Affairs (Senior Research Career Scientist award) to S.C.P. A.P. is funded by a pilot project within P50AA-022538, and by NIDA RO1DA-040688.
Statement of Interest
None.
References
- Alongkronrusmee D, Chiang T, van Rijn RM (2016) Involvement of delta opioid receptors in alcohol withdrawal-induced mechanical allodynia in male C57BL/6 mice. Drug Alcohol Depend 167:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola A, Tata MR, Aurilio C, Ciccone G, Gemini D, Ammendola E, Ugolini G, Argenzio F (2001) Peripheral neuropathy in chronic alcoholism: a retrospective cross-sectional study in 76 subjects. Alcohol 36:271–275. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S (2013) Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav 112:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S, Middleton JW, Gilpin NW (2018) Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J Neurosci 38:7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel TD, Pandey SC (2017) Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res 41:666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD (2000) Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci 20:8614–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD (2006) Ethanol withdrawal induces hyperalgesia mediated by pkcepsilon. Eur J Neurosci 24:197–204. [DOI] [PubMed] [Google Scholar]
- Dina OA, Gear RW, Messing RO, Levine JD (2007) Severity of alcohol-induced painful peripheral neuropathy in female rats: role of estrogen and protein kinase (A and cepsilon). Neuroscience 145:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF (2012) Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology 62:1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36:2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D (2016) Common brain mechanisms of chronic pain and addiction. Neuron 89:11–36. [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458. [DOI] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bär KJ (2010) Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain 14:713–718. [DOI] [PubMed] [Google Scholar]
- Kononoff J, Kallupi M, Kimbrough A, Conlisk D, de Guglielmo G, George O (2018) Systemic and intra-habenular activation of the orphan G protein-coupled receptor GPR139 decreases compulsive-like alcohol drinking and hyperalgesia in alcohol-dependent rats. eNeuro 5. pii: ENEURO.0153-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC (2014) The epigenetic landscape of alcoholism. Int Rev Neurobiol 115:75–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC (2013) Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry 73:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A (2008) Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28:3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Kyzar EJ, Zhang H (2017) Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology 122:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Yu XH, Laird JM (2010) Modality of hyperalgesia tested, not type of nerve damage, predicts pharmacological sensitivity in rat models of neuropathic pain. Eur J Pain 14:503–509. [DOI] [PubMed] [Google Scholar]
- Roltsch Hellard EA, Impastato RA, Gilpin NW (2017) Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict Biol 22:692–701. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC (2014) Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol 17:1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE (2016) Social transfer of pain in mice. Sci Adv 2:e1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B (2017) Analgesic effects of alcohol: a systematic review and meta-analysis of controlled experimental studies in healthy participants. J Pain 18:499–510. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC (2014) Reversal of deficits in dendritic spines, BDNF and arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol 17:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Alongkronrusmee D, van Rijn RM (2017) An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 10:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]