Abstract
Recognition memory requires judgments of the previous occurrence of stimuli made on the basis of the relative familiarity of individual objects, or by integrating information concerning objects and location, or by using recency information. The present study examined the role of the medial prefrontal cortex (mPFC) and perirhinal cortex (PRH) in these distinct recognition memory processes using a series of behavioral tests: a novel object preference task, an object-in-place task, and a temporal order memory task. Also, a disconnection procedure was used to test whether these regions form components of an integrated system for recognition memory. Male DA rats received bilateral lesions in the PRH or mPFC or unilateral lesions placed in both cortices in either the same (PRH–mPFC IPSI) or contralateral (PRH–mPFC CONTRA) hemispheres. A fifth group underwent sham surgery (SHAM). In the object-in-place and temporal order memory tasks, the PRH, mPFC, and PRH–mPFC CONTRA groups were significantly impaired. However, performance in the novel object preference task was only impaired in the PRH group. No group was impaired in the object location task. These results demonstrate that the mPFC and PRH are crucial for object-in-place associational and recency discriminations, whereas the PRH but not the mPFC is important for the discrimination of novel and familiar individual objects. Importantly, these results provide direct support for the hypothesis that to make discriminations based on associational or recency information, both cortical regions operate within an integrated neural network for recognition memory.
Keywords: familiarity discrimination, associational memory, recency memory, rat, perirhinal cortex, medial prefrontal cortex
Introduction
Recognition memory requires judgments to be made about the previous occurrence of stimuli. Such judgments can be made on the basis of the relative familiarity of individual objects, by using recency information, or by integrating information concerning objects and location. Much evidence has indicated a critical role for the perirhinal cortex (PRH) in familiarity discrimination of objects (Brown et al., 1987; Gaffan and Murray, 1992; Fahy et al., 1993; Li et al., 1993; Meunier et al., 1993; Suzuki et al., 1993; Ennaceur et al., 1996; Ringo, 1996; Xiang and Brown, 1998; Murray and Bussey, 1999; Brown and Aggleton, 2001), in object location associations (Bussey et al., 2000), and in discriminations based on the relative recency of presented objects (Hannesson et al., 2004).
Electrophysiological and behavioral evidence suggests that the medial prefrontal cortex (mPFC) may also contribute to recognition memory. PFC neurons have been shown to carry information concerning the relative familiarity of individual stimuli (Miller et al., 1996; Xiang and Brown, 2004), and damage to this region has been shown to impair recognition memory tasks (Bachevalier and Mishkin, 1986; Kolb et al., 1994; Buckner and Peterson, 1996; Meunier et al., 1997). The mPFC, like the PRH, has also been shown to play an important role in recency discriminations for objects or spatial locations (Chiba et al., 1997; Mitchell and Laiacona, 1998; Hannesson et al., 2004), and, furthermore, the PFC may be concerned with the integration of object and place information necessary for object-in-place discriminations (Kesner and Ragozzino, 2003; Browning et al., 2005).
Thus, the PRH and mPFC appear to play a role in discriminations based on familiarity, recency, and object–place information, raising the important question of whether these regions cooperate in making such discriminations. Anatomical evidence supports this hypothesis, because the PRH is reciprocally interconnected with the ventromedial PFC (Beckstead et al., 1979; Deacon et al., 1983; Conde et al., 1995; Delatour and Witter, 2002).
The present study addressed the issue of whether the PRH and mPFC function as part of a neural network using a disconnection analysis in which animals were prepared with unilateral lesions of both the PRH and mPFC. In one group, the unilateral lesions were in contralateral hemispheres, and in the other group, the lesions were in the same hemisphere. If the two regions are functionally interdependent, then those animals with crossed lesions should be considerably more impaired.
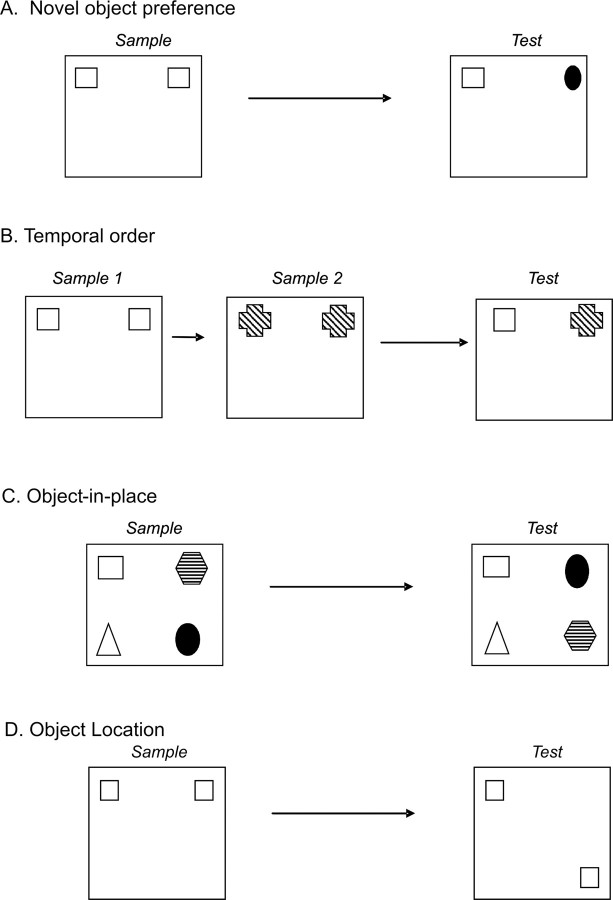
To assess the different components of recognition memory, a battery of tests were used: (1) novel object preference task, in which the rat's exploration of a novel object is compared with that of a familiar object; (2) recency recognition task, in which the animal's ability to differentiate between two familiar objects presented at different intervals is tested; (3) object-in-place task, in which the animal's ability to detect a particular object relative to its location and surrounding objects is examined; and (4) object location task, which tests the animal's ability to detect the movement of a familiar object to a novel location.
Materials and Methods
All experiments were conducted in male pigmented rats (DA strain; Bantin and Kingman, Hull, UK), weighing 200–250 g at the start of the experiments. The animals were housed under a 12 h light/dark cycle (light phase, 6:00 P.M. to 6:00 A.M.). Behavioral training and testing were conducted during the dark phase of the cycle. All animal procedures were performed in accordance with the United Kingdom Animals Scientific Procedures Act (1986) and associated guidelines. All efforts were made to minimize any suffering and the number of animals used.
All statistical analyses used a significance level of 0.05.
Surgery
Rats received either bilateral lesions of the PRH or mPFC or unilateral lesions of both cortices for the disconnection analyses. Rats in the disconnection groups received unilateral lesions of the PRH combined with a lesion of the mPFC. For approximately half of the animals, the contralateral group, the PRH and mPFC lesions were placed in opposite hemispheres (PRH–mPFC CONTRA). For the remaining animals, the ipsilateral group, unilateral PRH and mPFC lesions were made in the same hemisphere (PRH–mPFC IPSI).
Each rat was anesthetized with isoflurane (induction, 4%; maintenance, 2–3%). The rat was secured in a stereotaxic frame with the incisor bar set at +5 mm above the interaural line. The scalp was then cut and retracted to expose the skull. Craniotomies were then made directly above the target regions, and the dura was cut to expose the cortex.
Excitotoxic lesions of the PRH and mPFC were made by injecting 0.09 m NMDA (Sigma, Poole, UK) dissolved in phosphate buffer, pH 7.2, through a 1 μl Hamilton (Reno, NV) syringe into the appropriate sites in the hemisphere. For the PRH lesions, each injection was made gradually over a 3 min period, and the needle was left in situ for an additional 3 min before being withdrawn; for the mPFC lesions, each injection was made over a 4 min period, and the needle was left in situ for an additional 4 min (because of a greater volume of fluid). The anteroposterior and lateral stereotaxic coordinates relative to bregma, with the incisor bar set at +5.0 to the horizontal plane, are shown in Tables 1 and 2. The dorsoventral coordinates were calculated relative to bregma for the PRH lesions and relative to the top of the cortex for the mPFC lesions.
Table 1.
Lesion coordinates for the mPFC relative to bregma
| AP | LAT | DV | Vol. of 0.09 m NMDA | |
|---|---|---|---|---|
| 1 | +2.7 | ±0.7 | −4.5 | 0.28 μl |
| 2 | +2.7 | ±0.7 | −2.2 | 0.28 μl |
| 3 | +4.0 | ±0.7 | −3.5 | 0.28 μl |
| 4 | +4.0 | ±0.7 | −2.0 | 0.28 μl |
AP, Anteroposterior; LAT, lateral; DV, dorsoventral; Vol., volume.
Table 2.
Lesion coordinates for the PRH relative to bregma
| AP | LAT | DV | Vol. of 0.09 m NMDA | |
|---|---|---|---|---|
| 1 | −1.2 | ±5.8 | −9.3 | 0.18 μl |
| 2 | −3.2 | ±6.1 | −9.5 | 0.18 μl |
| 3 | −4.7 | ±6.2 | −9.1 | 0.18 μl |
AP, Anteroposterior; LAT, lateral; DV, dorsoventral; Vol., volume.
At the completion of surgery, the skin was sutured, and an antibiotic powder (Acramide; Dales Pharmaceuticals, Skipton, UK) was applied. All animals then received a single administration of 5 ml of glucose saline subcutaneously and systemic analgesia intramuscularly (0.05 ml of Temgesic; Reckett and Colman, Hull, UK). All animals were allowed to recover for at least 10 d before habituation to the testing arena began.
Histology
At the end of the experiment, each rat was anesthetized with Euthetal (Rhône Mérieux, Toulouse, France) and perfused transcardially with PBS, followed by 4% paraformaldehyde. The brain was postfixed in paraformaldehyde for a minimum of 2 h before being transferred to 30% sucrose in 0.2 m phosphate buffer and left for 48 h. Coronal sections were cut at 50 μm on a cryostat and stained with cresyl violet.
Apparatus
Exploration occurred in an open-topped arena (50 × 90 × 100 cm) made of wood. The walls inside the arena were surrounded with a black cloth to a height of 1.5 m so that no external stimuli could be seen during the experiment (the black cloth was removed for the object-in-place and object location tasks), and the floor of the arena was covered with sawdust. An overhead camera and a video recorder were used to monitor and record the animal's behavior for subsequent analysis. The stimuli presented were copies of objects composed of “Duplo” (Lego UK, Slough, UK) that varied in shape, color, and size (9 × 8 × 7 cm to 25 × 15 × 10 cm) and were too heavy for the animal to displace.
Behavioral testing
Pretraining.
After being handled for 1 week, the animals were habituated to the arena without stimuli for 10–15 min daily for 2 d before the commencement of the behavioral testing.
Novel object preference task.
The procedure comprised an acquisition or sample phase, followed by a preference test after a delay of either 5 min or 2 h (Fig. 1A). In the sample phase, duplicate copies (A1 and A2) of an object were placed near the two corners at either end of one side of the arena (15 cm from each adjacent wall). The animal was placed into the arena facing the center of the opposite wall and allowed a total of either 40 s of exploration of A1 and A2 or 4 min in the arena. At test (3 min duration), the animal was replaced in the arena, presented with two objects in the same positions: one object (A3) was a third copy of the set of the objects used in the sample phase, and the other object was a novel object (B). The positions of the objects in the test and the objects used as novel or familiar were counterbalanced between the animals.
Figure 1.
Diagram of the four object recognition memory tasks. A, Novel object preference task. B, Temporal order task. C, Object-in-place task. D, Object location task.
Object location task.
In this test, the rat's ability to recognize that an object that it had experienced before had changed location was assessed. In the sample phase, the rat was exposed to objects A1 and A2, which were placed in the far corners of the arena (as in the object recognition test) (Fig. 1D). The animal was allowed to explore both objects during a sample phase of 3 min, and the amount of exploration of each object was recorded by the experimenter. After a delay of 5 min, the test phase began. In the test phase, object A3 was placed in the same position as object A1 had occupied in the sample phase. Object A4 was placed in the corner adjacent to the original position of A2, so that the two objects A3 and A4 were in diagonal corners. Thus, both objects in the test phase were equally familiar, but one was in a new location. The position of the moved object was counterbalanced between rats.
Temporal order task.
This task comprised two sample phases and one test trial (Fig. 1B). In each sample phase, the subjects were allowed to explore two copies of an identical object for a total of 4 min. Different objects were used for sample phases 1 and 2, with a delay between the sample phases of 1 h. The test trial (3 min duration) was given 3 h after sample phase 2. During the test trial, a third copy of the objects from sample phase 1 and a third copy of the objects from sample phase 2 were used. The positions of the objects in the test and the objects used in sample phase 1 and sample phase 2 were counterbalanced between the animals. If temporal order memory is intact, the subjects will spend more time exploring the object from sample 1 (i.e., the object presented less recently) compared with the object from sample 2 (i.e., the “new” object).
Object-in-place task.
This task comprised a sample phase and a test phase separated by a 5 min delay (Fig. 1C). In the sample phase, the subjects were presented with four different objects (A, B, C, D). These objects were placed in the corners of the arena 15 cm from the walls. Each subject was placed in the center of the arena and allowed to explore the objects for 5 min. During the delay period, all of the objects were cleaned with alcohol to remove olfactory cues and any sawdust that had stuck to the object. In the test phase, two of the objects (e.g., B and D, which were both on the left or right of the arena) exchanged positions, and the subjects were allowed to explore the objects for 3 min. The time spent exploring the two objects that had changed position was compared with the time spent exploring the two objects that had remained in the same position. The objects moved (i.e., those on the left or right), and the position of the objects in the sample phase were counterbalanced between rats. If object-in-place memory is intact, the subject will spend more time exploring the two objects that are in different locations compared with the two objects that are in the same locations.
Behavioral measures and statistical analyses
All measures of exploration were made with the experimenter blind to the lesion status of each animal. Exploratory behavior was defined as the animal directing its nose toward the object at a distance of <2 cm. Any other behavior, such as looking around while sitting on or resting against the object, was not considered as exploration. Any subjects that failed to complete a minimum of 15 s exploration in the sample phase or 10 s of exploration in the test phase were excluded from the analysis. Discrimination between the objects was calculated using a discrimination ratio that takes into account individual differences in the total amount of exploration (Ennaceur and Delacour, 1988; Dix and Aggleton, 1999). Group comparisons used one-way ANOVA, followed by post hoc Newman–Keuls tests. Additional analyses examined whether individual groups had discriminated between the objects, using a within-subjects t test (two-tailed), and a Pearson correlation coefficient was used to examine the relationship between exploratory behavior and discriminative performance.
Previous studies have shown that the most sensitive period of object recognition test phases depends on the type of task used (Dix and Aggleton, 1999; Bussey et al., 2000). A systematic study of discrimination performance in a range of object recognition tests demonstrated that in the novel object preference task, the data obtained from the first 2 min were the most sensitive measure of recognition memory, whereas in the object location and object-in-place tests, the most sensitive period of discrimination was in the first minute (Dix and Aggleton, 1999). For the purposes of completeness, we have analyzed and have presented the first minute and the first 2 min data for the novel object preference test. Differential exploration of objects across the test session in a temporal order recognition test has not been examined previously. However, preliminary investigations in our laboratory revealed that the strongest discrimination between the “old” object and the new object occurred within the first minute. Therefore, in the present study, we compared performance of the lesion groups over the first minute only.
Novel object preference task
The discrimination ratio was calculated as the difference in time spent by each animal exploring the novel compared with the familiar object divided by the total time spent exploring both objects. Data obtained in the first minute and in the first 2 min of the test period are presented.
Temporal order task
In this task, the discrimination ratio was calculated as the difference in time spent by each animal exploring the object from sample phase 1 compared with the object from sample phase 2 divided by the total time spent exploring both objects in the first minute of the test period.
Object-in-place task and object location task
In both of these tasks, the discrimination ratio was calculated as the difference in time spent by each animal an exploring object(s) that changed position compared with the object(s) that remained in the same position divided by the total time spent exploring all objects.
Results
Histology
Bilateral mPFC lesions
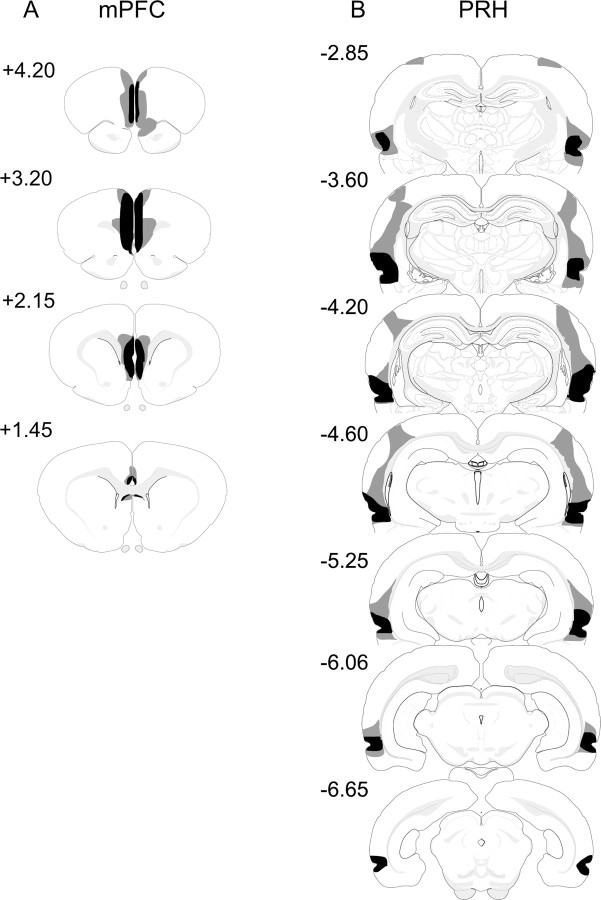
One animal was excluded from additional analysis because the lesion damage extended significantly into the medial and lateral septal nuclei. In the remaining eight cases, there was complete bilateral damage to the prelimbic cortex. The infralimbic cortex was also substantially damaged in all animals with only some limited sparing at the most caudal end. Seven animals sustained damage to the medial part of the orbital area, but in six of these animals, the damage was minor and restricted to the ventral posterior portion area. In all cases, the lesion extended into the dorsoventral anterior cingulate cortex, but the damage was minor. There was very minor damage in the secondary motor areas, which was also limited to the dorsal region. All animals also had a degree of restricted damage to the lateral septal nucleus. The cases with the largest and smallest lesions are shown in Figure 2A.
Figure 2.
Diagrammatic reconstructions showing the cases with the largest (gray) and smallest (black) lesions in the mPFC (A) and PRH (B) groups. The numbers correspond to the approximate position relative to bregma (Swanson, 1998).
Bilateral PRH lesions
The cases with the largest and smallest bilateral perirhinal lesions are shown in Figure 2B. In all nine cases, the perirhinal lesions were almost complete, although a small amount of tissue in the most posterior PRH was spared in two cases. In all cases, there was additional minor damage in the lateral entorhinal cortex. Dorsal to the PRH, there was significant damage to the anterior portion of area TE of the temporal lobe that extended into the ventral border of the auditory cortex. In five cases, there was minor unilateral damage to the somatosensory cortex, and in two cases, this damage was bilateral.
PRH–mPFC CONTRA
No animals showed any evidence of bilateral damage to the PFC. In all animals, there was extensive cellular loss in the prelimbic and infralimbic cortices, and the damage extended into the anterior cingulate cortex. In 9 of the 10 cases, there was minor damage to the secondary motor region.
In all animals, there was complete unilateral loss of tissue in the PRH. In addition, there was major damage to area TE, although this was restricted to the rostral portion. All animals sustained damage to the lateral entorhinal cortex and to the piriform cortex. Dorsal to the PRH, all animals had additional damage to the auditory cortex. Five animals had minor damage to the somatosensory cortex, and in four of these five cases, there was also restricted damage to the visual cortex. There was no damage to the hippocampal formation, although two animals had very minor damage to the basolateral amygdala and seven animals had minor damage to the lateral amygdala. The cases with the largest and smallest lesions are shown in Figure 3.
Figure 3.
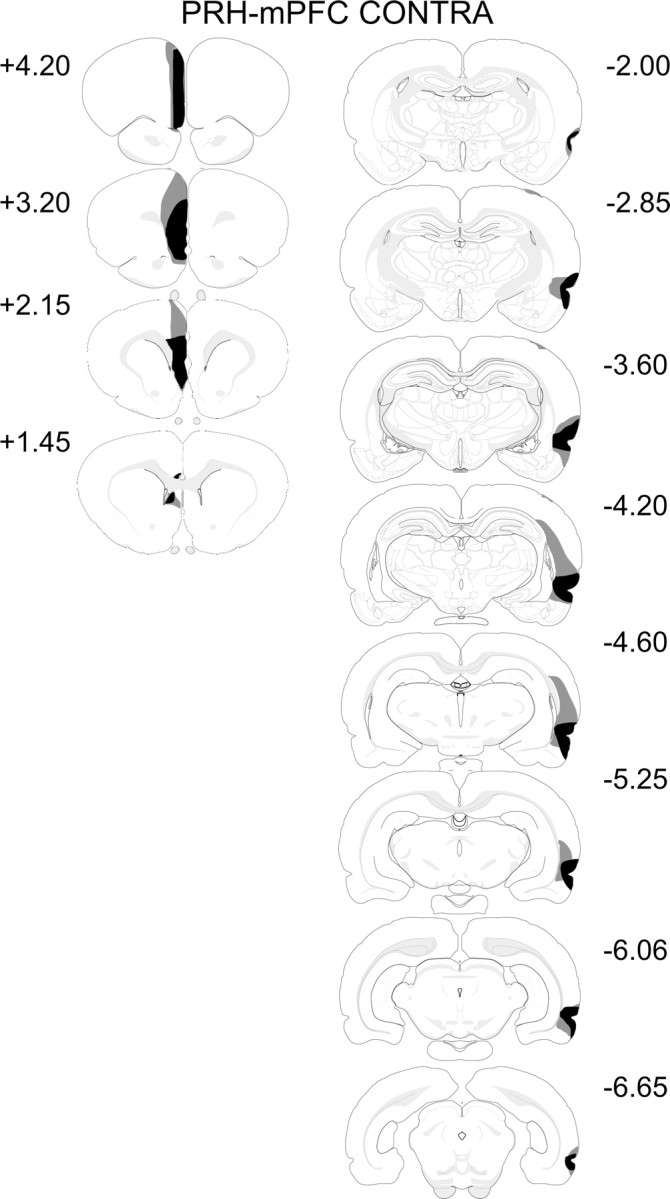
Diagrammatic reconstructions showing the cases with the largest (gray) and smallest (black) lesions in the PRH–mPFC CONTRA group. The numbers correspond to the approximate position from bregma (Swanson, 1998).
PRH–mPFC IPSI
No animals showed any evidence of bilateral damage to the PFC. In all cases, there was complete loss of neurons in the prelimbic and infralimbic cortices. In 8 of 10 cases, there was minor damage in the anterior cingulate cortex. In all cases, there was also minor damage to the secondary motor region.
In all animals, there was complete loss of tissue in the PRH. As with the PRH–mPFC CONTRA lesion group, the animals in the IPSI group also had extensive damage to the rostral area TE. All animals sustained some damage to lateral entorhinal cortex, and in nine cases, there was damage to the piriform cortex. Nine animals had additional damage to the auditory cortex, six animals had minor damage to the somatosensory cortex, and in five cases, there was also restricted damage to the visual cortex. There was no damage to the hippocampal formation, although three animals had very minor damage to the basolateral amygdala and eight animals had minor damage to the lateral amygdala. The extent of the largest and smallest lesions are shown in Figure 4.
Figure 4.
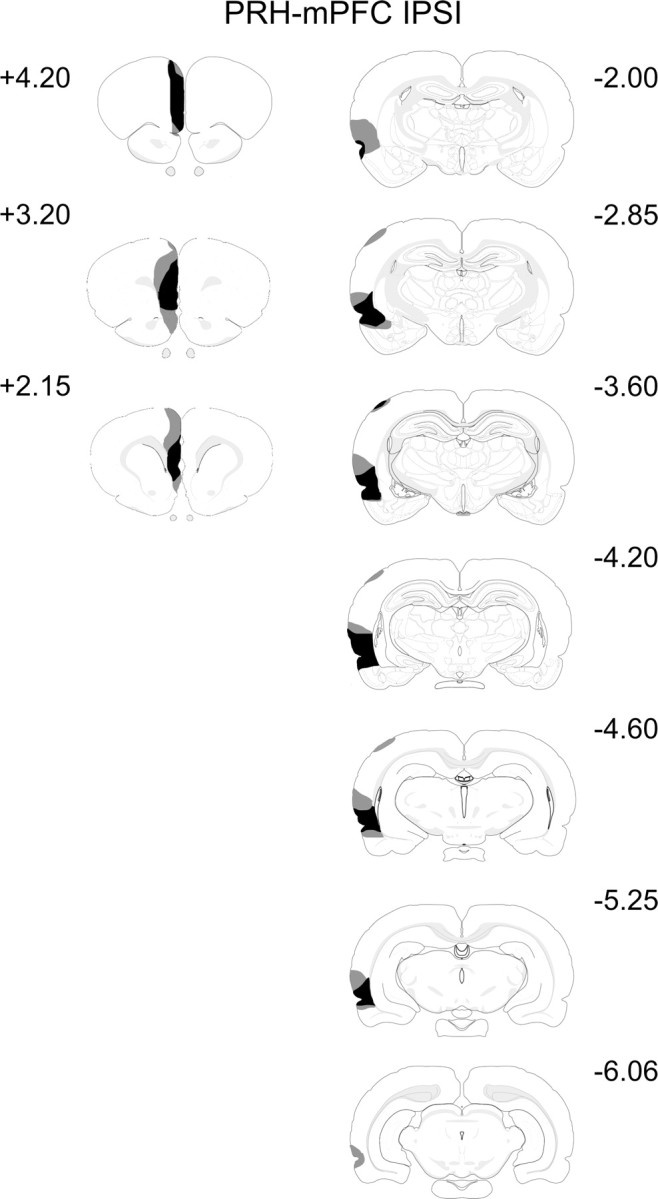
Diagrammatic reconstructions showing the cases with the largest (gray) and smallest (black) lesions in the PRH–mPFC IPSI group. The numbers correspond to the approximate position from bregma (Swanson, 1998).
Behavior
From the histological analysis, the final group numbers were as follows: SHAM, n = 20; PFC, n = 8; PRH, n = 9; PRH–mPFC CONTRA, n = 10; PRH–mPFC IPSI, n = 11. Two rats (one from the SHAM group and one from the PRH–mPFC IPSI group) that participated in the temporal order recognition memory test were excluded from any other behavioral testing because of ill health.
Novel object preference test (5 min delay): first minute of the test phase
Recognition during the test phase.
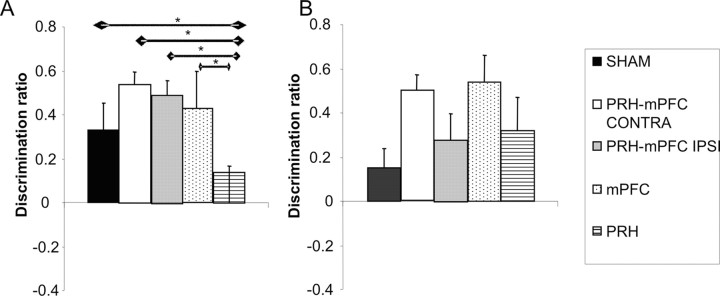
Figure 5A shows performance of the five groups of rats (SHAM, PRH, mPFC, PRH–mPFC IPSI, PRH–mPFC CONTRA) in the first minute of the test phase. Analysis revealed a significant main effect of lesion (F(4,51) = 2.91; p < 0.05). Post hoc analyses showed that the performance of the PRH group was significantly worse than the SHAM (p < 0.05), PRH–mPFC CONTRA (p < 0.05), PRH–mPFC IPSI (p < 0.05), and mPFC (p < 0.05) groups. There were no significant differences between any other groups. Additional analysis confirmed that the PRH group failed to show significant discrimination between the novel and familiar objects (t(8) = 0.78; p > 0.1).
Figure 5.
Performance of the five experimental groups in the novel object preference task tested with either a 5 min (A) or a 2 h (B) delay between the sample and test phases. Shown for each group is the mean (+SEM) discrimination ratio in the first minute of the test phase only. *p < 0.05.
Exploration during the sample and first minute of the test phase.
Analysis of the total amount of exploration completed in the sample phase revealed a significant main effect of lesion (F(4,51) = 2.73; p < 0.05). Additional analysis showed that the PRH group completed significantly less exploration than the PRH–mPFC IPSI (p < 0.05) and the mPFC (p < 0.01) groups; however, there were no significant differences between the PRH group and the SHAM and PRH–mPFC groups (Table 3). Additional analyses found that there was no significant correlation between the amount of exploration in the sample phase and the discrimination ratio in the test phase in the PRH group (correlation coefficient, 9 = 0.001; p > 0.1). In addition there were no differences between the groups in the time taken to complete the sample phase (F(4,51) < 1.0). Finally, analysis of the amount of exploration completed in the first minute of the test phase (F(4,51) = 2.07; p ≥ 0.1) showed no significant main effect of lesion.
Table 3.
Time taken to complete the sample phase and total exploration levels in sample and test phases in the novel object preference test (5 min and 2 h delays)
| Lesion | 5 min delay |
2 h delay |
||||||
|---|---|---|---|---|---|---|---|---|
| Time taken to complete sample phase (s) | Expl. in sample phase (s) | Expl. in test phase (first min) (s) | Expl. in test phase (first 2 min) (s) | Time taken to complete sample phase (s) | Expl. in sample phase (s) | Expl. in test phase (first min) (s) | Expl. in test phase (first 2 min) (s) | |
| SHAM | 225 ± 8.0 | 33.1 ± 1.2 | 11.0 ± 0.9 | 17.3 ± 1.3 | 240 ± 0 | 29.1 ± 1.2 | 7.9 ± 0.7 | 11.7 ± 0.7 |
| PRH–mPFC CONTRA | 228 ± 9.2 | 33.6 ± 1.9 | 13.4 ± 1.0 | 17.3 ± 1.2 | 231 ± 8.3 | 27.2 ± 2.2 | 8.5 ± 1.0 | 12.3 ± 0.9 |
| PRH–mPFC IPSI | 226 ± 9.6 | 35.7 ± 1.5 | 12.3 ± 2.2 | 16.9 ± 2.7 | 239 ± 1.0 | 29.1 ± 2.2 | 6.0 ± 0.8 | 11.9 ± 1.3 |
| mPFC | 233 ± 4.1 | 37.3 ± 4.1 | 9.1 ± 1.2 | 13.9 ± 1.7 | 240 ± 0 | 28.0 ± 3.2 | 7.8 ± 1.1 | 12.3 ± 1.6 |
| PRH | 240 ± 0 | 29.3 ± 2.0 | 7.05 ± 0.9 | 12.3 ± 1.5 | 240 ± 0 | 25.3 ± 1.7 | 7.2 ± 1.0 | 11.3 ± 1.6 |
Expl., Exploration.
Novel object preference test (5 min delay): first 2 min of the test phase
Recognition during the test phase.
The performance of the five groups of rats (SHAM, PRH, mPFC, PRH–mPFC IPSI, PRH–mPFC CONTRA) in the first 2 min of the test phase is shown in Figure 6A. ANOVA revealed a significant main effect of lesion (F(4,51) = 7.95; p < 0.001). Post hoc analyses showed that the performance of the PRH group was significantly worse than the SHAM (p < 0.05), PRH–mPFC CONTRA (p < 0.05), PRH–mPFC IPSI (p < 0.01), and mPFC (p < 0.01) groups. There were no significant differences between any other groups. Additional analysis confirmed that the PRH group failed to show significant discrimination between the novel and familiar objects (t(8) = 0.42; p > 0.1), whereas the SHAM (t(18) = 7.46; p < 0.01), PRH–mPFC CONTRA (t(8) = 6.65; p < 0.01), PRH–mPFC IPSI (t(9) = 9.09; p < 0.01), and mPFC (t(7) = 8.35; p < 0.01) groups showed significant discrimination.
Figure 6.
Performance of the five experimental groups in the novel object preference task tested with either a 5 min (A) or a 2 h (B) delay between the sample and test phases. Shown for each group is the mean (+SEM) discrimination ratio in the first 2 min of the test phase. *p < 0.05; **p < 0.01.
Exploration during the first 2 min of the test phase.
Analysis of the amount of exploration completed in the first 2 min of the test phase (F(4,51) = 1.66; p > 0.1) showed no significant main effect of lesion.
Novel object preference test (2 h delay): first minute of the test phase
One rat from the PRH group was excluded because of low levels of exploration (<15 s).
Recognition during the test phase.
Analysis of the performance of the five groups of rats (SHAM, PRH, mPFC, PRH–mPFC IPSI, PRH–mPFC CONTRA) over the first minute of the test phase revealed no significant main effect of lesion (F(4,50) = 2.55; p < 0.05) (Fig. 5B).
Exploration during the sample and first minute of the test phase.
Analysis of the time taken to complete the sample phase (F(4,50) = 1.07; p > 0.1) and the amount of exploration completed in the sample phase (F(4,50) < 1.0) showed no significant main effect of lesion group. In addition, analysis of the amount of exploration completed in the first minute of the test phase (F(4,50) = 1.13; p > 0.1) showed no significant main effect of lesion.
Novel object preference test (2 h delay): first 2 min of the test phase
One rat from the PRH group was excluded because of low levels of exploration (<15 s).
Recognition during the test phase.
The performance of the five groups of rats in the first 2 min of the test phase is depicted in Figure 6B. ANOVA revealed a significant main effect of lesion (F(4,50) = 6.36; p < 0.001). Post hoc analyses showed that the performance of the PRH group was significantly worse than the SHAM (p < 0.01), PRH–mPFC CONTRA (p < 0.01), PRH–mPFC IPSI (p < 0.01), and mPFC (p < 0.01) groups. There were no significant differences between the other groups. Additional analysis found that the SHAM (t(18) = 4.63; p < 0.001), PRH–mPFC CONTRA (t(9) = 5.36; p < 0.001), PRH–mPFC IPSI (t(9) = 7.08; p < 0.001), and mPFC (t(7) = 4.85; p < 0.01) groups showed significant discrimination between the novel and familiar objects; however, the PRH group (t(7) = 1.24; p > 0.1) failed to show significant discrimination.
Exploration during the first 2 min of the test phase.
Analysis of the amount of exploration completed in the first 2 min of the test phase (F(4,50) < 1.0) showed no significant main effect of lesion group (Table 3).
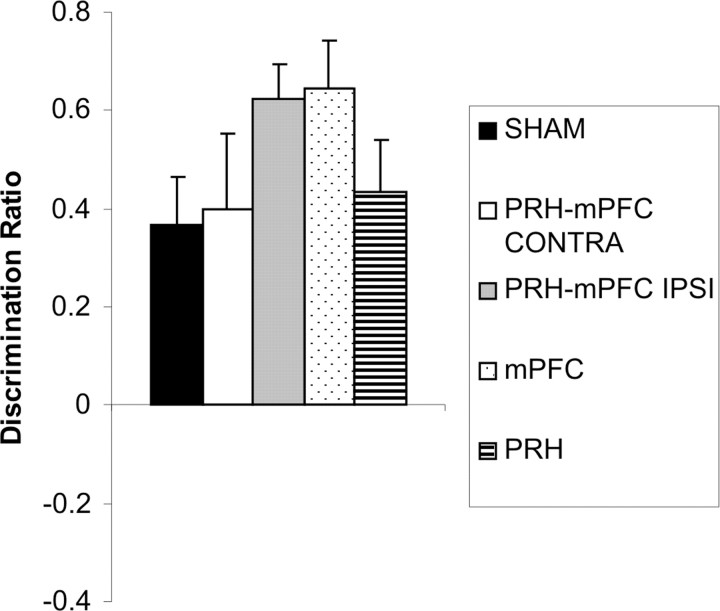
Object location task
Two rats from the PRH group were excluded because of low levels of exploration (<15 s).
Recognition during the test phase.
The performance of the five groups of rats in the first minute of the test phase is shown in Figure 7. ANOVA did not reveal any significant differences between the lesion groups (F(4,49) = 1.22; p > 0.1). Additional analysis showed that the SHAM (t(18) = 3.80; p < 0.001), mPFC (t(7) = 5.98; p < 0.01), PRH (t(6) = 3.73; p ≤ 0.01), PRH–mPFC CONTRA (t(9) = 2.38; p < 0.05), and PRH–mPFC IPSI (t(9) = 8.59; p < 0.001) groups showed significant discrimination between the object that had changed position than the object that had remained in a constant position.
Figure 7.
Performance of the five experimental groups in the object location task. Shown for each group is the mean (+SEM) discrimination ratio.
Exploration during the sample and test phases.
Analysis of the amount of time taken to complete the sample phase and the amount of exploration completed in the sample phase showed no significant main effect of lesion group (F(4,49) < 1.0 and F(4,49) = 1.11, respectively; p > 0.1). In addition, analysis of the amount of exploration completed in the first minute of the test phase showed no significant main effect of lesion group (F(4,49) = 1.03; p > 0.1) (Table 4).
Table 4.
Time taken to complete the sample phase and total exploration levels in sample and test phases in the object location recognition test
| Lesion | Time taken to complete sample phase (s) | Expl. in sample phase (s) | Expl. in test phase (s) |
|---|---|---|---|
| SHAM | 232 ± 5.5 | 33.5 ± 1.2 | 10.3 ± 1.0 |
| PRH–mPFC CONTRA | 231 ± 5.8 | 32.4 ± 1.9 | 9.7 ± 1.2 |
| PRH–mPFC IPSI | 237 ± 2.8 | 34.2 ± 1.6 | 10.2 ± 0.7 |
| mPFC | 240 ± 0 | 30.3 ± 2.0 | 8.8 ± 1.5 |
| PRH | 240 ± 0 | 31.1 ± 1.5 | 7.1 ± 0.9 |
Expl., Exploration.
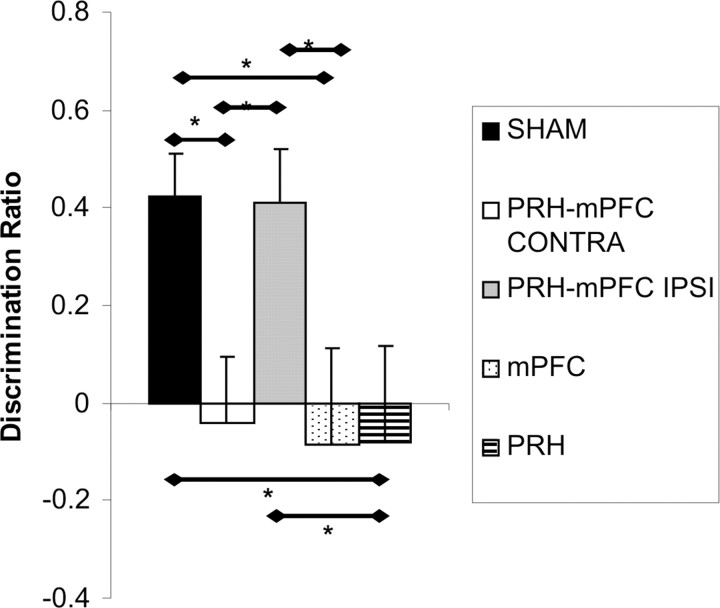
Temporal order memory task
One rat from the PRH group was excluded because of low levels of exploration (<15 s).
Recognition during the test phase.
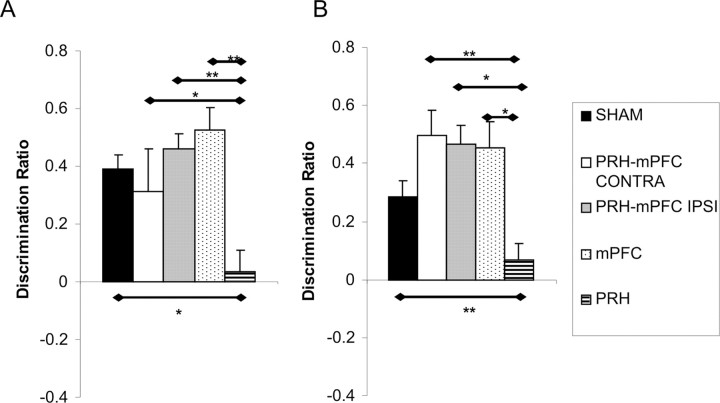
The performance of the five groups of rats in the first minute of the test phase is shown in Figure 8. ANOVA revealed a significant main effect of lesion (F(4,52) = 3.69; p ≤ 0.01). Post hoc analyses showed that the performance of the PRH, mPFC, and PRH–mPFC CONTRA groups was significantly worse than the performance of the SHAM and PRH–mPFC IPSI groups (p < 0.05 in all cases). There were no other significant group differences. Additional analysis found that the SHAM (t(19) = 4.75; p < 0.001) and PRH–mPFC IPSI (t(10) = 3.55; p < 0.01) groups showed significant discrimination between the object presented least recently (from sample phase 1) and the object presented most recently (from sample phase 2). In contrast, the PRH–mPFC CONTRA (t(9) = 0.28; p > 0.01), mPFC (t(7) = 0.36; p > 0.1), and PRH (t(7) = 0.38; p > 0.1) groups failed to show significant discrimination.
Figure 8.
Performance of the five experimental groups in the temporal order memory task. Shown for each group is the mean (+SEM) discrimination ratio. *p < 0.05.
Exploration during the sample and test phases.
Statistical comparisons of the amount of exploration completed in sample phase 1 and sample phase 2 found no significant effect of lesion (F(4,52) < 1.0), sample phase (F(4,52) < 1.0), or sample phase × lesion interaction (F(4,52) = 1.85; p > 0.1). In addition, there were no significant differences in the amount of exploration completed by any of the groups in the first minute of the test phase (F(4,52) < 1.0). The data are presented in Table 5.
Table 5.
Time taken to complete the sample phase and total exploration levels in sample and test phases in the temporal order recognition test
| Lesion | Expl. in sample phase 1 (s) | Expl. in sample phase 2 (s) | Expl. in test phase (s) |
|---|---|---|---|
| SHAM | 29.0 ± 1.5 | 29.5 ± 1.8 | 9.1 ± 0.8 |
| PRH–mPFC CONTRA | 28.8 ± 2.8 | 28.0 ± 1.9 | 10.4 ± 1.4 |
| PRH–mPFC IPSI | 28.5 ± 1.5 | 33.2 ± 1.8 | 10.6 ± 1.1 |
| mPFC | 30.5 ± 4.3 | 25.1 ± 3.6 | 7.7 ± 1.4 |
| PRH | 34.5 ± 3.1 | 31.4 ± 2.9 | 7.1 ± 1.2 |
Expl., Exploration.
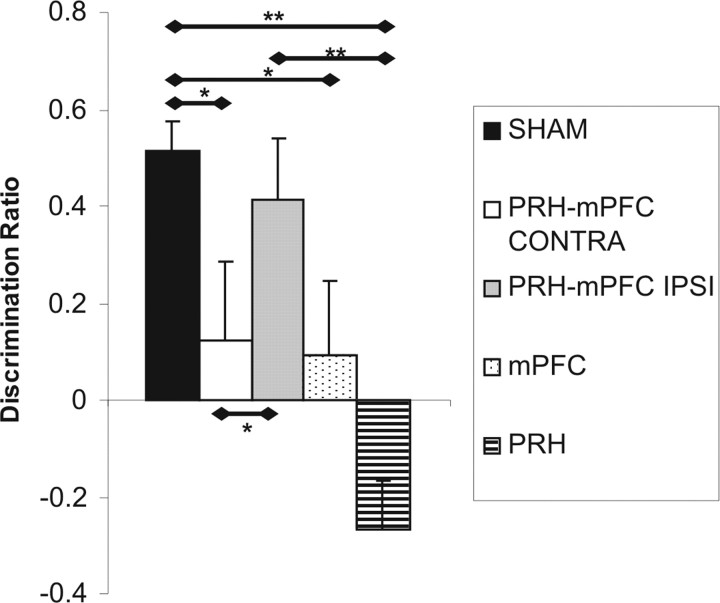
Object- in-place task
Recognition during the test phase.
The performance of the five groups of rats in the first minute of the test phase is shown in Figure 9. ANOVA revealed a significant main effect of lesion (F(4,51) = 6.39; p < 0.001). Post hoc analyses showed that the performance of the PRH (p < 0.01), mPFC (p < 0.05), and PRH–mPFC CONTRA (p < 0.05) groups was significantly worse than the SHAM and PRH–mPFC IPSI groups. There were no other significant group differences. Additional analysis found that the SHAM (t(18) = 7.50; p < 0.001) and PRH–mPFC IPSI (t(9) = 2.92; p < 0.05) groups showed a significant preference for the two objects that had changed position compared with objects that had remained in the same position. The mPFC (t(7) = 0.54; p > 0.1) and PRH–mPFC CONTRA (t(9) = 0.69; p > 0.1) groups failed to show significant discrimination between the objects, whereas the PRH lesion group explored the two objects that had remained in the same position significantly more than the objects that had moved (t(8) = 2.52; p < 0.05).
Figure 9.
Performance of the five experimental groups in the object-in-place task. Shown for each group is the mean (+SEM) discrimination ratio. *p < 0.05; **p < 0.01.
Exploration during the sample and test phases.
Analysis of the amount of exploration completed in the sample phase showed no lesion group differences (F(4,51) = 1.96; p > 0.1). However, analysis of the amount of exploration completed in the first minute of the test phase did show a significant main effect of lesion (F(4,51) = 2.93; p < 0.05), and post hoc analyses revealed that the mPFC groups completed significantly less object exploration during test compared with the SHAM and PRH–mPFC IPSI groups (for all, p < 0.05). There were no other statistically significant differences (Table 6).
Table 6.
Total exploration levels in sample and test phases in the object-in-place recognition test
| Lesion | Expl. in sample phase (s) | Expl. in test phase (s) |
|---|---|---|
| SHAM | 44.3 ± 2.2 | 11.8 ± 0.8 |
| PRH–mPFC CONTRA | 40.2 ± 2.6 | 8.4 ± 1.3 |
| PRH–mPFC IPSI | 47.1 ± 1.7 | 12.5 ± 1.5 |
| mPFC | 48.4 ± 6.0 | 7.4 ± 1.3 |
| PRH | 38.9 ± 2.7 | 9.0 ± 1.6 |
Expl., Exploration.
Discussion
The present study examined the role of the PRH and mPFC in different components of recognition memory [i.e., familiarity discriminations of (1) individual objects, (2) object–location associations, and (3) the recency of presented objects]. Furthermore, the extent to which these two cortical regions function within a neural network was investigated. Thus, the behavioral consequences of a combined unilateral PRH lesion and prefrontal lesion placed in the contralateral or same hemisphere were examined in a series of object discrimination tasks designed to assess these distinct recognition memory processes.
In the object-in-place and temporal order memory tasks, the PRH, mPFC, and PRH–mPFC lesion groups showed significant memory impairments compared with the SHAM and PRH–mPFC IPSI groups. In contrast, only the PRH lesion group was impaired in the novel object preference task. Object location memory was not impaired.
Previous studies show that the best measure for novel object discrimination is taken in the first 2 min of the test phase (Dix and Aggleton, 1999), and the present results are mostly consistent with that report. Thus, the SHAM group showed significant discrimination at both delays when the data from the first 2 min are combined, whereas this group did not discriminate in the first minute after the 2 h delay. Therefore, the interpretation of the results from the lesion groups is based on the data from the first 2 min.
That the mPFC plays little, if any, role in novel–familiar object discriminations agrees with previous reports. One study previously concentrated on the dorsal PFC (i.e., anterior cingulate) (Ennaceur et al., 1997; Mitchell and Laiacona, 1998), whereas another used post-sample lidocaine infusions into the prelimbic cortex (Hannesson et al., 2004) and therefore did not examine the role of the PFC during acquisition of familiarity. The present study addressed these gaps in knowledge demonstrating that the ventromedial PFC is not required for object familiarity acquisition.
Recognition memory deficits after PFC damage have been reported. However, in one study, nonselective lesion techniques were used; therefore, damage to fibers of passage may have accounted for the deficits (Kesner et al., 1996). In another study, the task included an appetitive component (Ragozzino et al., 2002), thus other learning processes may have been compromised by the lesion.
In the present study, the mPFC and PRH were clearly shown to be required for object–place associative discriminations. Because mPFC damage had no effect in the novel object preference or object location tasks, the deficits observed in the object-in-place task could not be a consequence of disrupted attentional processing, because such a disruption would compromise performance in all the tasks. Rather, the results obtained suggest both cortical regions are crucial for associative recognition memory.
To solve the object-in-place task, familiarity–novelty detection alone cannot be used because the objects and the spatial locations used in the test phase of the object-in-place task are equally familiar. The subject must therefore combine visual object and spatial information and recognize the topographical relationship between the objects (Goodrich-Hunsaker, 2005), raising the question of where these processes occur. The PRH has been shown to be important for object identification (Murray and Richmond, 2001; Buckley, 2005; Bussey and Saksida, 2005) and object–object associational learning (Murray et al., 1993; Buckley and Gaffan, 1998); thus, the object-in-place deficits produced by perirhinal lesions may simply reflect the loss of perceptual information or, more intriguingly, may suggest that the PRH is the site in which object and location information is integrated. Whereas in the present study the PRH lesion group spent significantly more time exploring the objects that remained in the familiar location, compared with the objects in the novel location, this pattern of performance was not found in a previous study using an identical task (Bussey et al., 2000); therefore, additional experiments are required to investigate the potential significance of these results.
In contrast to the PRH, the mPFC has not been shown to be important for object perception or object identification per se, and thus its role in object–place associational learning may be distinct to that of the PRH. Although the mPFC lesion group was impaired the object-in-place task, this group performed normally in the novel object preference and object location tasks. Because all of the animals were run in the behavioral tasks in the same order and the mPFC lesion group was impaired in the last two tests run, it is possible that the testing order may have contributed to the results obtained. However, this explanation seems unlikely because additional results from our laboratory have confirmed the importance of the mPFC in object-in-place and temporal order discrimination memory (G. R. I. Barker and E. C. Warburton, unpublished observations). The results therefore suggest that the mPFC may be involved in integrating object and spatial location information received from other neural regions.
The PRH–mPFC CONTRA group was significantly impaired compared with the PRH–mPFC IPSI group, indicating that the mPFC must function closely with the PRH for the processing of object-in-place information. One may speculate further by suggesting that the mPFC receives object information from the PRH that is integrated with spatial information received from the hippocampus, because transection of the fimbria-fornix impairs object-in-place discrimination performance (Bussey et al., 2000). However, it is also possible that the object and spatial information is integrated downstream of the mPFC; hence additional studies are required to establish the transmission routes within the neural network.
There are direct reciprocal connections between the PRH and mPFC (Beckstead, 1979; Deacon et al., 1983; Conde et al., 1995; Delatour and Witter, 2002), and electrophysiological studies indicate that the firing latencies of repetition-sensitive neurons in the PRH are shorter than those in the mPFC, suggesting that information flows from the PRH to the PFC (Xiang and Brown, 2004). However, this statement has yet to be confirmed, and it is not possible to rule out the contribution of the loss of input from the PFC to the PRH to the observed deficits in the disconnection study.
Few studies have investigated the potential importance of the mPFC in object–place associations (Kesner and Ragozzino, 2003; Browning et al., 2005), whereas only one has indicated that there is a functional interaction between the frontal and inferotemporal cortices in object–place learning in monkeys (Browning et al., 2005). Hence, the present results are the first demonstrating that object–location associative memory in the rat requires an interaction with regions outside either the PFC or the PRH.
Our results also provide a clear demonstration of the importance of the PRH and mPFC and their interconnections in recency discriminations. One explanation for the dissociation between the effect of mPFC lesions in the recency discrimination and familiarity discrimination tasks could be the longer delay in the recency experiment (3 h) compared with the familiarity discrimination experiment (2 h). However, this possibility seems unlikely because a comparison of the performances of the SHAM group in both tasks revealed higher levels of discrimination in the recency task. Second, intracerebral infusions of the AMPA receptor antagonist CNQX into the mPFC has no effect on familiarity discrimination after a 3 h delay but impairs recency discriminations (Barker and Warburton, unpublished observations). Thus, the temporal order task was not simply more demanding than the object preference task, but instead requires different recognition memory processes.
One previous study examined the roles of the mPFC and PRH in recency memory using infusions of lidocaine to disrupt cortical function (Hannesson et al., 2004). Lidocane, a local anesthetic, will disrupt both cells within a structure and fibers of passage. Thus, the results obtained in the present study are the first demonstration that the mPFC and PRH are crucial for recency discriminations and that these regions are functionally dependent on one another for processing this type of information. In support of this assertion, electrophysiological recording studies show that both regions contain neurons that signal information concerning the relative recency as well as the relative familiarity of visual stimuli (Brown and Xiang, 1998; Xiang and Brown, 2004).
In the present study, the infralimbic and prelimbic regions of the PFC were targeted although the ventral anterior cingulate cortex was also damaged. Because the dorsomedial PFC has been implicated in the temporal processing of information (Dalley et al., 2004), an involvement of the anterior cingulate in temporal order recognition memory cannot be excluded.
Several conclusions may be drawn concerning the distinct contributions of the PRH and mPFC to recognition memory. The PRH but not the mPFC is crucial for novel–familiar object discriminations. Both regions, however, are important for object-in-place and recency discriminations. The results also establish that the mPFC and PRH form a functional relationship to process associational and recency recognition memory information. The present study did not extend to an investigation of the parietal cortex, a region also implicated in the formation of representations of space based on arrangements of proximal cues (Save et al., 1992; Save and Poucet, 2000) and the detection of novelty (Montag-Sallaz et al., 1999; Yamaguchi et al., 2004) and also interconnected with the PFC [albeit with the orbital PFC (Reep et al., 1994)] and PRH (Burwell and Amaral, 1998). Therefore, what remains to be established are the contributions of brain regions interconnected with the PRH such as the parietal cortex, the lateral-orbital PFC (Delatour and Witter, 2002), and the hippocampus to information processing within this neural network.
Footnotes
This work was supported by a project grant from the Biotechnology and Biological Sciences Research Council. We thank Jane Robbins and Katherine Narduzzo for assistance with histology.
References
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Beckstead R. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:43–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- Brown M, Aggleton J. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown M, Xiang J. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Brown M, Wilson F, Riches I. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- Browning P, Easton A, Buckley M, Gaffan D. The role of prefrontal cortex in object-in-place learning in monkeys. Eur J Neurosci. 2005;22:3281–3291. doi: 10.1111/j.1460-9568.2005.04477.x. [DOI] [PubMed] [Google Scholar]
- Buckley MJ. The role of the perirhinal cortex and hippocampus in learning, memory and perception. Q J Exp Psychol. 2005;58B:246–268. doi: 10.1080/02724990444000186. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36:535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE. What does neuroimaging tell us about the role of prefrontal cortex in memory retrieval? Semin Neurosci. 1996;8:47–55. [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal and entorhinal cortces of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bussey T, Duck J, Muir J, Aggleton J. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: an alternative approach. Curr Opin Neurobiol. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kesner R, Gibson C. Memory for temporal order of new and familiar spatial location sequences: role of the medial prefrontal cortex. Learn Mem. 1997;4:311–317. doi: 10.1101/lm.4.4.311. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Deacon T, Eichenbaum H, Rosenberg P, Eckmann K. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol. 1983;220:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Delatour B, Witter M. Projections from the parahippocampal region to the prefrontal cortex in the rat evidence of multiple pathways. Eur J Neurosci. 2002;15:1400–1407. doi: 10.1046/j.1460-9568.2002.01973.x. [DOI] [PubMed] [Google Scholar]
- Dix S, Aggleton J. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton J. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fahy F, Riches I, Brown M. Neuronal activity related to visual recognition memory long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray E. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Beh Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci. 2004;24:4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner R, Ragozzino M. The role of the prefrontal cortex in object-place learning: a test of the attribute specificity model. Behav Brain Res. 2003;146:159–165. doi: 10.1016/j.bbr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb Cortex. 1994;4:664–680. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray E. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Laiacona J. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behav Brain Res. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg3.1 in the mouse brain. J Neurobiol. 1999;38:234–246. [PubMed] [Google Scholar]
- Murray E, Bussey T. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus–stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M, Detrick S, Kesner R. The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol Learn Mem. 2002;77:29–43. doi: 10.1006/nlme.2001.4003. [DOI] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Ringo J. Stimulus specific adaptation in inferior temporal and medial temporal cortex of the monkey. Behav Brain Res. 1996;76:191–197. doi: 10.1016/0166-4328(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B. Involvement of the hippocampus and associative parietal cortex in the use of proximal and distal landmarks for navigation. Behav Brain Res. 2000;109:195–206. doi: 10.1016/s0166-4328(99)00173-4. [DOI] [PubMed] [Google Scholar]
- Save E, Buhot MC, Foreman N, Thinus-Blanc C. Exploratory activity and response to a spatial change in rats with hippocampal or posterior parietal cortical lesions. Behav Brain Res. 1992;47:113–127. doi: 10.1016/s0166-4328(05)80118-4. [DOI] [PubMed] [Google Scholar]
- Suzuki W, Zola-Morgan S, Squire L, Amaral D. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- Xiang J, Brown M. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Xiang J, Brown M. Neuronal responses related to long-term recognition memory processes in prefrontal cortex. Neuron. 2004;42:817–829. doi: 10.1016/j.neuron.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]