Abstract
Background
Studies investigating hippocampal volume changes after treatment with serotonergic antidepressants in patients with major depressive disorder yielded inconsistent results, and effects on hippocampal subfields are unclear.
Methods
To detail treatment effects on total hippocampal and subfield volumes, we conducted an open-label study with escitalopram followed by venlafaxine upon nonresponse in 20 unmedicated patients with major depressive disorder. Before and after 12 weeks treatment, we measured total hippocampal formation volumes and subfield volumes with ultra-high field (7 Tesla), T1-weighted, structural magnetic resonance imaging, and FreeSurfer. Twenty-eight remitted patients and 22 healthy subjects were included as controls. We hypothesized to detect increased volumes after treatment in major depressive disorder.
Results
We did not detect treatment-related changes of total hippocampal or subfield volumes in patients with major depressive disorder. Secondary results indicated that the control group of untreated, stable remitted patients, compared with healthy controls, had larger volumes of the right hippocampal-amygdaloid transition area and right fissure at both measurement time points. Depressed patients exhibited larger volumes of the right subiculum compared with healthy controls at MRI-2. Exploratory data analyses indicated lower baseline volumes in the subgroup of remitting (n = 10) vs nonremitting (n = 10) acute patients.
Conclusions
The results demonstrate that monoaminergic antidepressant treatment in major depressive disorder patients was not associated with volume changes in hippocampal subfields. Studies with larger sample sizes to detect smaller effects as well as other imaging modalities are needed to further assess the impact of antidepressant treatment on hippocampal subfields.
Keywords: depression, hippocampus, hippocampal subfields, ultra-high field MRI, antidepressant
Significance Statement.
Altered neuronal plasticity and volumetric changes in the hippocampus are correlates of major depression. Antidepressants were demonstrated to induce structural changes in the brain’s neuronal networks in rodent studies but results in depressed patients are ambiguous. This study investigated whether subfields—such as the dentate gyrus of the hippocampus—change in response to antidepressant treatment. Against our expectations, we did not find increased subfield volumes after 12 weeks of antidepressant therapy. These negative results provide a basis for further investigations with more refined imaging modalities and larger sample sizes.
Introduction
Hippocampal volume reductions and altered neuronal plasticity are pathophysiological substrates of major depressive disorder (MDD) and other psychiatric disorders (Pittenger and Duman, 2008; Macqueen and Frodl, 2011; Kuhn and Gallinat, 2013), while preclinical results suggest that antidepressants facilitate neuroplasticity (Castrén and Rantamäki, 2010; Duman et al., 2016). Studies in animals and humans indicate that stress and a history of adverse events—both important risk factors for MDD—impact hippocampal neuronal survival, glial cells, and neurogenesis (Pittenger and Duman, 2008; Serretti et al., 2013; Rabl et al., 2014; Saleh et al., 2017). On a hormonal level, stress hormones altered in MDD such as glucocorticoids affect spine synapses and dendrites in the cornu ammonis (CA1-3) (Pittenger and Duman, 2008; Hajszan et al., 2009). In addition, antidepressants such as selective serotonin reuptake inhibitors (SSRIs) were found to closely interact with neurotrophins such as the brain-derived neurotrophic factor (Castrén and Rantamäki, 2010). Treatment with SSRIs was demonstrated to oppose the effects of stress by stimulating hippocampal neurogenesis (Malberg and Duman, 2003; Anacker and Hen, 2017) and increasing synaptic spine volumes in the hippocampus, in particular in the dentate gyrus (Kasper and McEwen, 2008; Kitahara et al., 2016). However, this evidence mostly originated from rodent models and needs to be translated to patients with MDD.
Hippocampal formation volumes represent a surrogate of neuronal plasticity and can be measured in MDD patients in vivo with structural magnetic resonance imaging (MRI) and automatic or manual volume analysis. Several previous studies reported hippocampal formation volume increases after treatment. After 8 weeks of antidepressant treatment with citalopram, hippocampal formation volume increases were demonstrated in patients with MDD (Arnone et al., 2013). An earlier study reported volume increases after 12 weeks of treatment with paroxetine in patients with posttraumatic stress disorder (Vermetten et al., 2003). A third study with a naturalistic inpatient setting and a mixed antidepressant treatment regime detailed posterior hippocampal volume increases after approximately 23 weeks of treatment (Schermuly et al., 2011). Those studies highlight that pro-neuroplastic effects of monoaminergic antidepressants supported by animal literature (Pittenger and Duman, 2008; Malykhin and Coupland, 2015; Duman et al., 2016) could mediate hippocampal volume increases in humans. In contrast, several results found no volume increases after treatment (Frodl et al., 2004; Vythilingam et al., 2004; Phillips et al., 2015). Still, subgroup analyses in 2 of these studies found hippocampal volume increases in remitted and acute patients continuously taking antidepressants (Frodl et al., 2004; Phillips et al., 2015). While the reasons for discrepancies between these results remain unclear, further studies using refined methods are warranted.
Due to its multifaceted cortical structure, volumetric measurements of hippocampal subfields result in greater gains of information (Mueller et al., 2018). Therefore, automatic segmentations such as FreeSurfer’s (FS) hippocampal subfield analysis are advantageous, because they do not require (much) prior anatomical knowledge and are time efficient. A cross-sectional study with this algorithm (n = 270) reported larger tail volumes and smaller CA2/3, CA4 molecular layer, granule cell layer, and alveus in medication-free MDD patients compared with controls (Maller et al., 2018). Almost a dozen other cross-sectional studies reported altered subfield volumes in MDD patients; for an overview of these findings see (Maller et al., 2018).
Higher spatial MRI resolutions enabled increasingly detailed delineation of hippocampal subfields. Longitudinal measurements of hippocampal subfields with 7T MRI in MDD could provide further insights into pro-neuroplastic effects of monoaminergic antidepressants. In this study, we analyzed hippocampal subfield volumes in unmedicated patients with MDD and included remitted patients and healthy control subjects as control groups. Based on previous longitudinal results on hippocampal formation volumes, known pro-neuroplastic effects of SSRIs, and advantages of 7T MRI image details, we hypothesized: compared with baseline volumes and between-measurement changes in control groups, unmedicated patients with MDD will exhibit significantly increased volumes of hippocampal subfields and volumes of the hippocampal formation after treatment at MRI-2.
Methods
Participants and Study Design
The study sample consists of subjects previously reported (for detailed sample descriptions, please refer to Spies et al., 2017; Kraus et al., 2019). All participants consented to study participation and protocol procedures by oral and written consent. Subjects were financially compensated for study participation. The study protocol and all study-related procedures were approved prior to the start of the study by the Ethics Committee of the Medical University (EK 103/2011) of Vienna and was registered at clinicaltrials.gov (NCT01477203).
All acute patients had to be within an episode of MDD (aMDD), 18 to 50 years old, and moderately to severely ill as assessed by clinical impression and corroborated by a Hamilton Depression Rating Scale (HAM-D24) score ≥17 at screening. Diagnosis was assured by an experienced psychiatrist conducting the structured clinical interview (SCID-I) for DSM-IV, and any comorbid personality disorders were excluded by SCID-II. All included patients were either medication-naïve or -free for at least 3 months prior to screening. No patient was left untreated to reach the 3 months inclusion limit, the average time between screening and MRI-1 was 7.9 ± 7 days, and treatment started on the morning after MRI-1. Any other current primary psychiatric disorder (including anxiety disorders or bipolar depression), substance abuse disorder within the last 12 months, or any major medical or neurologic illness were not permitted. Remitted MDD patients had the same inclusion criteria (rMDD), but they had to be in stable remission (HAM-D24 < 8) including free of medication for at least 3 months.
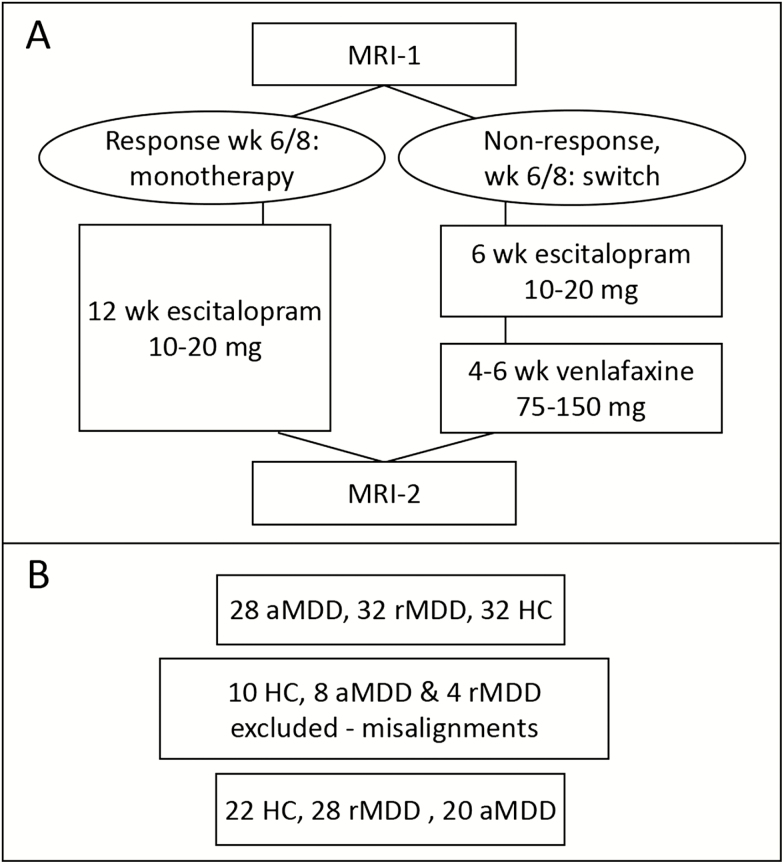
Healthy subjects had to be free of any psychiatric diagnosis their entire lifetime and absent of any significant illnesses, current medication intake, and current or past substance abuse disorder. During screening, all subjects underwent a medical and neurologic examination with medical history, blood and hormone (thyroid and sex hormones) analyses, urine drug screening, and pregnancy tests as well as an electrocardiogram. For this study, MRI scans from 32 HC, 28 aMDD, and 32 rMDD subjects were available (see Figure 1), and we included 22 HC, 28 rMDD, and 20 aMDD subjects in the final statistical analyses (for details, see next section).
Figure 1.
Study diagram outlining study design and patient numbers. (A) Study flow diagram. (B) Subject numbers. Note that 22 subjects had to be excluded due to misalignments (see “MRI Measurements and Hippocampal Subfield Analyses”). aMDD, acute depressed patients; HC, healthy control subjects; rMDD, remitted depressed patients (untreated); wk, week.
We treated all unmedicated aMDD patients with an open-label, flexible dose, standardized oral antidepressant for 12 weeks, with a mandatory switch of antidepressants on nonresponse to the first medication. The rationale for switching came to mimic a naturalistic treatment regime as performed in previous studies and recommended international guidelines at the time of study design (2009/2010) (Rush et al., 2006; Bauer et al., 2013). Neuropsychological testing and dosage adjustments were done every 2 weeks. Initially, all aMDD patients were treated with escitalopram oxalate (5–20 mg) for at least 6 weeks, with dosage adjustments according to clinical judgment and HAM-D curves by study psychiatrists. Down titration was allowed if any dose was not tolerated. Upon nonresponse to escitalopram after 6 weeks, defined by at least 50% HAM-D24 reduction compared with the first visit, a switch to venlafaxine extended release was conducted (allowed dosage range 75–150 mg). The second trial lasted for another 6 weeks. MRI measurements were at week 0 (MRI-1) and week 12 (MRI-2) of treatment. rMDD patients and HC were seen only at screening MRI-1 and MRI-2. None of the rMDD patients relapsed during study duration.
All study psychiatrists had extensive experience in clinical psychological testing. Rating scales administered at every visit were HAM-D24, Hamilton Anxiety Rating Scale, Beck Depression Index, and Clinical Global Impression Scale. Response to antidepressant treatment (−50% HAM-D24 compared with visit 1) was assessed at visits of week 6, 8, and 12 (=MRI-2). Remission was defined as <8 on the HAM-D24 scale, which was chosen as a conservative cutoff to minimize residual symptoms. Healthy controls and rMDD patients were tested with the same questionnaires at baseline visit, MRI-1, and MRI-2.
MRI Measurements and Hippocampal Subfield Analyses
Every study subject underwent 2 7T MRI scans with a Siemens Magnetom scanner and a 32-channel head coil. We applied a MP2RAGE sequence with TR = 4060 milliseconds, TE = 3.07 milliseconds, resulting in a total MRI scan time of 11:20 minutes in a field of view of 192 × 312 × 384 mm (x/y/z) and a voxel size of 0.74 × 0.68 × 0.68 mm (x/y/z). Functional sequences were conducted prior to structural MRI, which are not within the scope of this article and are reported elsewhere. The sequence was previously demonstrated to be appropriate for hippocampal longitudinal analyses, while other regions (e.g., middle and inferior temporal gyri) had worse test-retest values (Seiger et al., 2015). Hippocampal subfield segmentation was performed with the FS image analysis suite v6.0 beta-version for the following subfields (in alphabetical order): CA1, CA3, CA4, fimbria, fissure, granule cell layer of the dentate gyrus, hippocampus–amygdala transition area, molecular layer, parasubiculum, presubiculum, subiculum, and tail (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, http://surfer.nmr.mgh.harvard.edu/) (Iglesias et al., 2015). The whole brain segmentation for calculating total intracranial volume (TIV) and total brain volume were done with SPM-12 (Wellcome Centre for Human Neuroimaging, London, UK, https://www.fil.ion.ucl.ac.uk/spm/) and Matlab 8.3 scripts (The MathWorks, Inc. Natick, MA), while total hippocampal volume was done with FS 5.3, since at the time of analysis only a beta version of FS 6.0 was available online. We previously showed that hyperintensities occurred in our MP2RAGE images at 7T that could distort whole-brain within-subject registrations, especially near air-tissue borders (Seiger et al., 2015). Since this could result in failures of whole-brain registrations, we decided not to use the FS longitudinal pipeline. Instead, we implemented rigorous quality control as follows: (1) image quality was visually checked by R. S. and subfield segmentations were visually inspected after segmentation by R. S. and C. K. independently; (2) an automated quality control analysis used by the ENIGMA consortium (http://enigma.ini.usc.edu, kindly provided by Philipp Säman) was conducted; and (3) outliers in absolute subfield values (5 SDs) were excluded. We excluded 22 subjects (10 HC, 8 aMDD, and 4 rMDD) after step 1 for visible misalignments of the subfield segmentations with the underlying hippocampus, and none after steps 2 and 3. As a result, we included 22 HC, 28 rMDD, and 20 aMDD subjects in the final statistical analyses (see Figure 1 for an overview of the study and subject numbers). The excluded HC were significantly older than the analyzed HC (34 ± 6.9 years in excluded vs 25.9 ± 6.7 years in the analyzed HC, t = 3.1 P = .007). All other clinical characteristics of excluded subjects in aMDD and rMDD did not differ significantly from the analyzed sample (all P > .05). All data are available on request from the corresponding author.
Statistical Analyses
All subfield volumes were checked for normal distribution with histograms, q-q plots, and Shapiro-Wilk tests, and residual diagnostics were performed for statistical models. Based on these procedures, all analyses were done with log-transformed values (see supplemental Material, page 1; figures show untransformed values).
Since test-retest reliability of subfield segmentations with FS 6.0 has not been established, we initially conducted a reliability analysis in healthy subjects only. For this purpose, we calculated the intraclass correlation coefficient (2-way mixed, average measure, absolute agreement) with subfields’ volumes between both MRI measurements with SPSS v.23 (IBM Corporation, Armonk, NY). Moreover, we calculated Spearman’s correlations between MRI-1 and MRI-2 for volumes of the whole hippocampus as well as each subfield (see supplemental Figures 1 and 2).
According to our hypotheses, we compared total hippocampal volume changes between healthy controls, remitted, and acute patients by fitting a linear model with the “lm”-function in R (R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org). Logarithmically transformed volumes of both hippocampal formations were added as dependent variables and group × time × hemisphere including combinations of interactions as independent variables, controlling for TIV, sex, and age. Longitudinal changes (between MRI-1 and MRI-2) of hippocampal volumes in both models were investigated with repeated-measures ANOVAs (type-II).
The same procedures were applied for our main hypothesis analyzing subfield volumes. Again, logarithmized values of subfields were dependent variables and group × time × subfield (subfield = each subfield volume) including combinations of interactions as independent variables, controlling for TIV, sex and age. Of note, hemisphere is concatenated in “subfield,” which is why it was not necessary to include “hemisphere” as additional independent variable. Following previous literature (Maller et al., 2018), we also investigated the influence of correcting for total hippocampal volume and total brain volume (total gray and white matter). For both total hippocampal formation and subfield ANOVAs, we calculated estimated marginal means with R’s “emmeans” package and conducted t tests on contrasts within groups for each MRI time point as well as between MRI time points. All statistical tests assumed an alpha level of P < .05. We used Tukey’s method to adjust for multiple comparisons in post-hoc t tests; uncorrected P values are reported. Residual distribution indicated adequacy for both ANOVAs with log-transformed values.
Finally, we explored subfield values between remitting (aMDDrem, HAM-D24 < 8) after treatment and nonremitting patients (aMDDnon-rem, HAM-D24 ≥ 8). We computed a model identical to the but restricted analysis within the aMDD patients. Subfield volumes were dependent variables and remission × subfield independent variables, correcting for sex, age, and baseline HAM-D24. Group × time × subfield interactions were tested with type-II ANOVA, and post-hoc comparisons were calculated with the “emmeans” package and t tests on contrasts within groups for each MRI time point as well as between MRI time points. Note that results of comparisons between remitting and nonremitting patients have to be interpreted with caution, since a low n = 10 in each group enhances chances of false positives. For further exploratory analyses, we also correlated psychosocial variables (age at first episode, previous episodes, duration of last episode) in aMDD patients with baseline subfield values.
Results
Clinical Results and Test-Retest Analysis
Clinical results of the sample were previously published (Spies et al., 2017; Kraus et al., 2019). Briefly, 9 aMDD patients had psychopharmacological antidepressant treatment during a previous episode, while 9 were naïve and information on 2 patients was missing. For more detailed information, see our previous work. Hamilton Anxiety Rating Scale, HAM-D24, and Beck Depression Index values significantly decreased after 12 weeks of antidepressant treatment from MRI-1 to MRI-2 in aMDD patients (see Table 1, all P < .05). Ten of the 20 patients in the final analysis remitted, and 10 patients were considered as nonremitter (HAM-D24≥8). After 12 weeks of antidepressant treatment with a flexible dose regime at MRI-2, 11 patients were on escitalopram (16.5 ± 4.5 mg) and 9 patients were on venlafaxine (100 ± 37.5 mg). Of the 10 remitting aMDD patients 8 were on escitalopram and 2 on velanfaxine, while in the 10 nonremitting aMDDs 7 were on venlafaxine and 3 on escitalopram. Note that the study design demanded a switch after nonresponse (<50% HAM-D24 from visit one) at week 6 and/or 8. Notably, a patient could meet the criterion for response (50% HAM-D24 reduction) but not reach the study criterion for remission. Hence, several patients were taking escitalopram at MRI-2.
Table 1.
Clinical Characteristics of the Sample
| aMDD | Subsample (aMDD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rMDD | HC | total | P | aMDDnon-rem | aMDDrem | P | |||||
| n | 28 | 22 | 20 | 10 | 10 | ||||||
| Age | 26.6 ± 5.7 | 25.9 ± 6.7 | 30.5 ± 9.6 | * | 30.5 ± 11.5 | 30.4 ± 6.6 | .49 | ||||
| Sex (f/m) | 16/12 | 12/10 | 14/6 | * | 7/3 | 7/3 | — | ||||
| TIV (MRI-1, cm3) | 1490.3 ± 133.4 | 1453 ± 133 | 1416.1 ± 139.1 | * | 1411.3 ± 123.7 | 1420.8 ± 159.7 | .45 | ||||
| Handedness (r/l) | 28/0 | 22/0 | (19/1) | ||||||||
| Previous medication (relation yes/no) | 1:1 | — | 1:1 | ||||||||
| Age at first episode (y) | 22 ± 5 | — | 22.8 ± 11.7 | 26 ± 12.2 | 20.1 ± 9.7 | .046 | |||||
| Previous episodes (n) | 1.6 ± 1.4 | — | 2.9 ± 1.5 | * | 2.2 ± 0.97 | 2 ± 0.82 | .12 | ||||
| Duration of last (rMDD)/ current (aMDD) episode (months) | 8.3 ± 5.4 | — | 10.1 ± 9 | * | 6.4 ± 6.3 | 9.6 ± 18 | .21 | ||||
| MRI-1 | MRI-2 | MRI-1 | MRI-2 | P | MRI-1 | MRI-2 | P | ||||
| HAM-D24 | 2.3 ± 2.8 | — | 27.2 ± 7.5 | 9 ± 6.9 | * | 27.1 ± 9.6 | 14.4 ± 5.7 | * | 27.3 ± 5 | 3.6 ± 1.8 | * |
| HAMA | 2.6 ± 2.7 | — | 21.3 ± 6.4 | 6.8 ± 5.3 | * | 21.7 ± 8.5 | 10.7 ± 4.8 | * | 20.8 ± 3.9 | 2.8 ± 1.5 | * |
| BDI | 4.2 ± 4.9 | — | 20.6 ± 8.1 | 8.1 ± 5.8 | * | 22.3 ± 9 | 11.5 ± 6.1 | * | 18.9 ± 7.2 | 4.7 ± 3 | * |
| CGI | 1.5 ± 0.5 | — | 5.1 ± 0.7 | 3.5 ± 1.2 | * | 5.2 ± 0.4 | 4 ± 0.5 | * | 5 ± 0.8 | 3 ± 1.4 | * |
Abbreviations: aMDD, acute depressed patients; aMDnon-rem; acute MDD patients nonremitting after treatment; aMDDrem, acute depressed patients remitting; BDI, Beck Depression Index; CGI, Clinical Global Impression Scale; HAM-D24, Hamilton Depression Rating Scale; HC, healthy controls; rMDD, remitted depressed subjects; TIV, total intracranial volume. *P < .001; P values from F-tests (ANOVA), chi-square test, or t test.
In the initial test-retest analysis, intraclass correlation coefficients ranged from 0.532 (left hippocampal-amygdaloid transition area [HATA]) to 0.945 (right dentate gyrus) at an intraclass correlation coefficient of 0.87 ± 0.11 (average ± SD) for left and 0.9 ± 0.06 for right subfields, leaving all regions but the left HATA with sufficiently high correlations between the measurements (see supplemental Material; supplemental Figure 2; supplemental Table 1). Test-retest analysis revealed positive correlations ranging within 0.59 < r < 0.93 of absolute volumes between both measurements in all regions (including total hippocampi) apart from the left HATA (r = 0.35, P = .12; see supplemental Figure 2). Therefore, we left out this region in a separate model in the subsequent analysis (see below).
Hippocampal Formation Volumes
We did not observe group × hemisphere (F2,265 = 0.35, P = .7), group × time (F2,265 = 0.72, P = .49), or group × time × hemisphere (F2,265 = 0.03, P = .96) interactions. Across all scans, we found a main effect of hemisphere (F2,265 = 14.32, P < .001), suggesting hippocampal formation volumes were different between sides. This was driven by larger left (3387.56 ± 274.72 mm3; real values, not estimates) compared with right hippocampal volumes (3289.23 ± 288.54 mm3, t = 3, P = .003). There was also a significant effect of group (F2,265 = 4.21, P = .015), whereby rMDD subjects (3420.05 ± 25.95 mm3) had significantly larger hippocampi (both sides averaged between measurements ± SD) compared with aMDD patients (3271.25 ± 27.76 mm3, t = 3.9, P < .001) and HC subjects (3295.48 ± 32.79 mm3, t = 3.1, P = .002), whereas hippocampi did not differ in volume between HC and aMDD (t = −0.4, P = .643). Post-hoc comparisons are shown in supplemental Table 2, and estimated means by region, measurements, and groups are shown in supplemental Table 3.
Hippocampal Subfield Volumes
We detected no significant effect for the interaction group × time (F2,3213 = 2.99, P = .05) and a significant effect of group on subfield values (F2,3213 = 17.15, P < .001), while other relevant interactions such as group × subfield, time × subfield, and group × time × subfield were not significant (all P > .05). To confirm that the influence of group was present at each measurement, we repeated the same linear model without the interaction term “time” at each time point separately. Indeed, at both MRIs, the effect of group on subfield values was significant (MRI-1, F2,1605 = 6.9, P = .001; MRI-2, F2,1605 = 12.28, P < .001), while group × subfield did not change considerably (all P > .9). For plots of the interaction group × time and the effect of group (across both MRIs) see supplemental Figure 5. Because of low test-retest correlations in the left HATA, we excluded this region in a separate model. However, the results did not change (i.e., group × time: F2,3213 = 2.95, P = .052). See Figure 2 and supplemental Figure 3 for boxplots of original values and supplemental Tables 4 and 5 and for statistical results of hippocampal subfields.
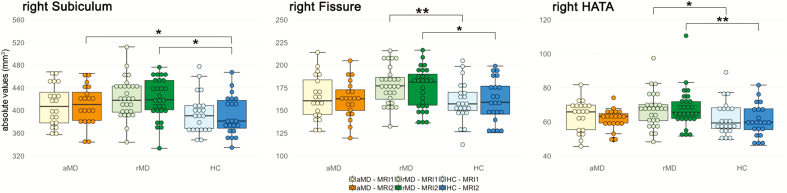
Figure 2.
Group differences in 3 hippocampal subfield volumes at MRI-1 and MRI-2. Untransformed hippocampal subfield volumes are plotted by groups and time points. Solely, group differences at each time point were obtained in the right hippocampus in the hippocampal fissure, subiculum, and HATA. All other nonsignificant subfields are shown in supplemental Figure 3. **P < .05, corrected with the Tukey method, *P < .05, uncorrected. aMDD, acute MDD patients received 12 weeks antidepressant treatment between MRI-1 and MRI-2; HATA, hippocampal-amygdaloid transition area; HC, healthy control subjects, both control groups did not take psychopharmaceuticals; rMDD, patients in stable remission before and during the study.
By testing post-hoc pairwise comparisons with the emmeans “pair” function between groups in all regions, we detected significant differences in subfields of the right hippocampus at single measurements only between groups, not between measurements. Specifically and only at baseline or follow-up scans, the right hippocampal fissure (MRI-1: t = 3, PTukey = .034, Cohen’s d = 0.11; MRI-2: t = 2.4, Puncorr = .016, d = 0.08) and right HATA (MRI-1: t = 2.13, Puncorr = .034, d = 0.07; MRI-2: t = 3.2, PTukey = .017, d = 0.11) exhibited larger values in rMDD compared with HC at both MRI-1 and MRI-2. Moreover, we detected significantly larger right subiculum values in aMDD patients (t = 2.02, Puncorr = .044, d = 0.07) and rMDD subjects (t = 2.14, Puncorr = .033, d = 0.08) compared with HC at MRI-2 only.
Of note, upon investigating results with alternative covariates, we obtained increased total gray matter volumes in rMDD vs aMDD at both time points (MRI-1: 78.4 ± 24.1, t = 3.3, PTukey = .004: MRI-2: −74.6 ± 24.1, t = 3.1, PTukey = .007; see supplemental Statistics and Figure 4). Main results remain unchanged on correcting with total gray matter or total brain volume (see Supplementary Material).
Exploratory Analyses: Associations With Response and Remission
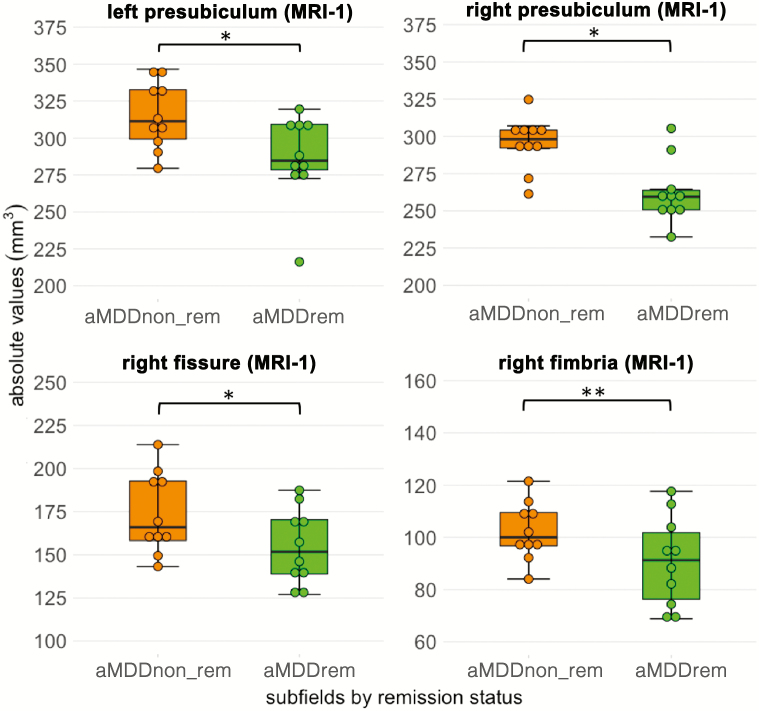
We explored differences of hippocampal formation volume and subfields according to remission status in acute patients. This was done by repeating the same linear models within aMDD patients stratified by acute patients remitting (aMDDrem, n = 10) and nonremitting (aMDDnon-rem, n = 10) after treatment. Of note, we did not design the study to investigate differences between remitter and nonremitter. Hence, based on the low number of subjects in these subgroups, results of these ANOVAs have to be interpreted with caution. In the total hippocampus, there was no effect of remission × time (F1,69 = 1.3, P = .26) or each factor alone. On hippocampal subfield values, we found a significant effect of remission × time (F1,860 = 8.14, P = .004) and remission status (F1,860 = 15.24, P < .001), yet no significant interaction between remission × time × subfield (F1,860 = 0.23, P = 1). Post-hoc testing was performed with the emmeans “pair” function between groups in all regions. We found significantly higher values in aMDDnon-rem patients compared with aMDDrem in the right fimbria (MRI-1: t = 2.8, PTukey = .027, d = 0.19), bilateral presubiculum (MRI-1, right: t = 2.55, Puncorr = .011, d = 0.17; MRI-1, left: t = 2.1, Puncorr = .036, d = 0.14), and right fissure (MRI-1: t = 2.51, Puncorr = .012, d = 0.17). There were no significant differences between aMDDrem and aMDDnon-rem between MRI-1 and MRI-2. For statistical results and means see supplemental Tables 6 and 7 as well as Figure 3. Moreover, we found a positive correlation between age at onset and baseline CA3 volume (r = 0.48, P = .035), duration of disease and right HATA volume (r = 0.46, P = .047), and (logarithmized) duration of the episode on left parasubicular volume (r = -0.5, P = .034; see supplemental Figure 6).
Figure 3.
Exploratory analysis of hippocampal subfield differences before treatment in acute depressed patients according to remitter status after 12 weeks of treatment (n = 10/10). Larger subfield volumes were found in the presubiculum, right fissure, and right fimbria in nonremitting depressed patients (aMDDnon_rem) compared with remitting patients (aMDDrem) before treatment. No significant changes were obtained between MRI-1 and MRI-2 or at MRI-2. **P < .05 corrected with Tukey’s method, *P < .05, uncorrected.
Discussion
In this 12-week antidepressant treatment study measuring hippocampal subfields in MDD with 7T MRI, we did not detect longitudinal changes of hippocampal formation volumes or hippocampal subfield volumes. Compared with healthy controls, we found hints for larger volumes of the hippocampal formation averaged over both time points in unmedicated, stable remitted patients. Analyses revealed that subfield volumes in remitted depressed patients serving as controls to depressed patients in acute episodes were larger in the subiculum, HATA, as well as the right hippocampal fissure.
Our primary findings are absent hippocampal formation volume and subfield volume changes after antidepressant treatment in depressed patients. These results are in contrast to a study reporting increases of the total hippocampus after 8 weeks of treatment with citalopram (n = 32, 1.5 T; 6-week measurement interval) (Arnone et al., 2013). This study also found larger hippocampal volumes in patients who were remitted, although not in the same pattern as we found (aMDD < HC < rMDD in our study vs aMDD < rMDD < HC in Arnone et al.). A higher number of episodes in their remitted sample might explain this discrepancy, and this study was better matched for sex and age than in our sample. Hippocampal volume increases after paroxetine treatment were found earlier in posttraumatic stress disorder (n = 23, 1.5 T, 12 months (Vermetten et al., 2003). While the first study used a voxel-based morphometry approach with masks, the latter applied manual delineation, which are methodologically different to newer methods (Cao et al., 2017; Maller et al., 2018). Absent volume increases in the total hippocampus as well as absent total hippocampal differences between depressed patients and controls are in line with a study using FS 4.3 and a mixed antidepressant treatment paradigm (Phillips et al., 2015). Likewise, the authors did not find baseline hippocampal differences between depressed patients and healthy controls. Importantly, this was a more severe treatment-resistant sample undergoing antidepressant therapy with an average scan interval of 331 days in remitters and 420 in nonremitters. Moreover, Philipps et al. found that patients who did not remit over the course of the treatment period exhibited larger baseline volumes. Only patients who remitted exhibited volume increases according to treatment (n = 26, 1.5T, 12 months) (Phillips et al., 2015). Larger baseline volumes in patients who do not remit in are in concert with our results; however, we did not replicate their positive remission × time interaction in the whole hippocampus in our subsample. Increased baseline volumes, however, speak against a meta-analytic finding, indicating that smaller baseline volumes are associated with lower response/remission rates (Colle et al., 2018). At this stage, it is only possible to speculate about reasons for these findings. Studies incorporating disease-inherent heterogeneity with methodologically standardized measurement techniques at comparable time points of the disease phases are needed to better compare hippocampal volume studies.
There is more compelling evidence on cross-sectional hippocampal volume reductions in MDD. Our data indicate smaller volumes of the hippocampal formation of aMDD compared with rMDD (across both scans), but there was no statistically significant difference between aMDD and HC. Of note, hippocampal volume reductions in MDD at cross-sectional levels exhibit small effect sizes even at very large samples (e.g., d = −0.14) (Schmaal et al., 2016). In contrast, electroconvulsive therapy appears to have stronger effects on the hippocampus, since increases have been demonstrated with MRI now by several studies (Abbott et al., 2014; Gbyl and Videbech, 2018; Gryglewski et al., 2019).
Increased synaptic plasticity and stimulated neurogenesis is considered as one of several mechanisms of action of SSRIs (Castrén and Rantamäki, 2010; Duman et al., 2016). But the level of existence of neurogenesis in adult humans remains controversial (Boldrini et al., 2018; Sorrells et al., 2018). In that regard, reduced neurogenesis in adult age would fit our findings. Still, increased synaptic plasticity in the dentate gyrus after treatment with various classes of antidepressants was demonstrated (Seo et al., 2014; Patricio et al., 2015). Therefore, discrepancies in existing findings are unlikely to arise from mixed antidepressant paradigms in negative studies, including ours. Moreover, venlafaxine is engaging the serotonin transporter substantially more than the norepinephrine transporter at low doses. The time point of sampling might play an important role but has not been investigated systematically. Interestingly, we found very large variabilities of published hippocampal volumes. For example, total (right) hippocampal volumes of depressed, nondemented patients younger than 60 years in the MRI literature range from 2415 mm3 to 4363.4 mm3 (MacQueen et al., 2008; Phillips et al., 2015). Of note, most studies included in a meta-analysis reported bilateral hippocampal volumes between 4794 mm3 and 8298 mm3 (Colle et al., 2018). A similar variation exists for subfields, for example, right CA1 volumes in healthy subjects between 34 and 1635 mm3 are reported (Sone et al., 2016; Voets et al., 2017).
As secondary results of this study, we found a significant main effect of group (although no group × subfield interaction) and significant post-hoc tests in subfields’ volumes in remitted subjects in the right HATA, subiculum, and fissure. In the present study, rMDD exhibited larger hippocampi when values of both measurements where combined. In addition, rMDD exhibited larger total brain gray matter. While the reasons for these observations remain unclear, structural and functional hippocampal alterations in remitted depression have been reported (Neumeister et al., 2005). This study compared remitted patients with healthy controls and found decreased total volumes of the hippocampal formation compared with healthy controls. Interestingly and in contrast to this result, we found the same pattern of gray matter—aMDD < HC < rMDD—as another study with unmedicated acute depressed and remitted patients (Salvadore et al., 2011). The authors discussed that increases in gray matter in rMDD might be a subsample-specific trait in patients who are more likely to remit, but they also cannot rule out neurotrophic effects of previous antidepressant exposure. Similar to this study, our rMDD patients were exposed to the same amount of previous medication as the aMDD group but had significantly fewer episodes and therefore less sickness activity (see Table 1 and Kraus et al., 2019) for more details).
To describe our results, the HATA is located between the medial entorhinal cortex, the cortical nucleus of the amygdala, CA1, and the subiculum (DL Rosene, 1987) and shares close connections with amygdalar nuclei. A previous study detailed substantial amounts of intersubject variability in the HATA (Amunts et al., 2005). We also detected low test-retest reliability in the left HATA, while ICC in the right HATA was low as well (0.793). In addition, we found indications of increased HATA values in rMDD compared with HC at both time points, as we did for the right fissure, which exhibited sufficiently high enough ICCs. Enlargement of the hippocampal fissure was previously related to hippocampal atrophy in humans with Alzheimer’s (Bastos-Leite et al., 2006) and mice after chronic unpredictable stress (Li et al., 2018). Correlations with psychosocial variables such as onset of disease or duration of the disease constitute an approach to link disease parameters with biology, but these results are only hints for future studies. In addition, our secondary results hinting towards increased subfield volumes in rMDD have to be scrutinized given lower power and a lack of statistically significant interaction of group × subfield.
Mostly negative results in our study between aMDD and HC contradict previous a study reporting volume differences in MDD patients compared with healthy subjects in the tail, CA 2/3, CA 4, and molecular layer (Maller et al., 2018) and another study demonstrating volume reductions in CA1-4, the dentate gyrus, and subiculum (Roddy et al., 2019). Larger subiculum volumes in depressed patients and patients in stable remission in relation to healthy controls, as we found after treatment at MRI-2, contradict a series of studies (Cho et al., 2010; Cole et al., 2010; Wisse et al., 2015; Han et al., 2016), while others undermine cross-sectionally reduced dentate gyrus as well as CA1-4 volumes (Huang et al., 2013; Travis et al., 2015, 2016) or negative results (Cao et al., 2017). Again, interpretation of our findings should be under the premise that this study was designed to obtain longitudinal results. Interestingly, there is heterogeneity between findings comparing subfields in unipolar and bipolar depression as well. Cao et al. (2017) found reduced subfield volumes (CA4, molecular layer, granule cell layer, tail) in bipolar depression; they did not report reduced volumes in unipolar depression. In contrast, a study with FS 6.0 reported reduced volumes in unipolar depression (CA1-CA4, granule and molecular cell layers, tail) in MDD vs HC only and did not find alterations in bipolar depression vs HC (Han et al., 2019). Reasons for these discrepancies remain open so far.
The following limitations that could potentially impact this study have to be reported. We had to exclude 22 subjects (23.9%) due to misalignments after segmentation. Others had to exclude 9.9% (Maller et al., 2018). A systematic comparison suggests T2-weighted images were better suited for subfield segmentations (Mueller et al., 2018). Not recording T2-weighted images might have been a main shortcoming leading to high failure rates of the subfield atlas in our study. Many MRI sequences and analysis approaches have been developed, but optimal methods for 7T have not yet been established (Wisse et al., 2017). An optimal longitudinal subfield’s sequence, also allowing application of FS’ longitudinal pipeline, is desirable for future studies. Moreover, it would have been optimal for test-retest reliability to conduct scans within several hours/days, since volumes could change in a 12-week period in HC. Still, we consider our test-retest results useful for future studies with 7T and to interpret our results. In addition, our groups were not matched according to age. Acute MDD patients had the highest mean age, suggesting that they would have the highest age-related atrophy, which we did not find in our results. Third, the study was powered for longitudinal effects; results at each separate time point have to be replicated by larger cross-sectional datasets in remitted subjects. Fourth, we did not collect data on years of education in this study, which could have confounded our results and should be addressed in future studies. Finally, there was not enough statistical power to test for group effects of medication. As outlined above, we consider it unlikely that the mixed drug-design obscures positive results. However, a lack of venlafaxine to facilitate neuroplasticity could still be possible.
To conclude, first, we found indications for increased volumes in stable remitted patients, hinting at hippocampal alterations in depression beyond acute episodes, but these results must be scrutinized. Second, we demonstrated with 7T MRI that SSRI and SNRI antidepressant treatment did not yield longitudinal changes in subfield or total hippocampal formation volumes.
Supplementary Material
Acknowledgments
This research was supported by the intramural grant “Multimodal Neuroimaging in Clinical Neurosciences—Assessment of Neurobiological Markers for Psychiatric Disorders” of the research cluster between the Medical University of Vienna and the University of Vienna, by a grant from the Austrian science Fund (FWF, grant number: KLI 551) to S.K. and by the grant “Interdisciplinary Translational Brain Research Cluster (ITHC) With Highfield MR” from the Federal Ministry of Science, Research, and Economy, Austria. We thank G. S. Kranz, S. Ganger, J. Losak, M. Küblböck, A. Hoffmann, A. Hummer, I. L. Stürkat, A. Wucherer, A. Grahl, C. Siegl, D. Fraissl, D. Willinger, M. Hubinger, and J. Hass for methodological or technical support; D. Winkler, M. Spies, P. Baldinger, A. Höflich, J. Unterholzner, and M. Godbersen for clinical support; L. Schwarz, L. Silberbauer, P. Köck, O. Mahlberg, C. Winkler, R. Hoffmann, M. Svagr, and V. Rotter for administrative support with the study; and J. W. Evans and D. Greenstein for helpful comments on the manuscript. Finally, we thank P. Sämann from the ENIGMA consortium for providing scripts and methodology for quality control.
Statement of Interest
S. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, Celegne GmbH, Eli Lilly, Janssen-Cilag Pharma GmbH, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd., and Takeda. R. Lanzenberger received travel grants and/or conference speaker honoraria from Shire, AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. G. Gryglewski is the recipient of a DOC fellowship from the Austrian Academy of Sciences. R. Seiger received funding from the Hochschuljubilaeumsstiftung of the City of Vienna. T. Vanicek received travel grants and compensation for workshop participation from Pfizer and Eli Lilly and speaker honoraria from Eli Lilly. C. Kraus has received travel grants from Roche Austria GmbH and AOP Orphan. The other authors report no conflicts of interest.
References
- Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, Bustillo J, Calhoun VD (2014) Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry 4:e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. [DOI] [PubMed] [Google Scholar]
- Anacker C, Hen R (2017) Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci 18:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, Williams S, Deakin JF, Anderson IM (2013) State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry 18:1265–1272. [DOI] [PubMed] [Google Scholar]
- Bastos-Leite AJ, van Waesberghe JH, Oen AL, van der Flier WM, Scheltens P, Barkhof F (2006) Hippocampal sulcus width and cavities: comparison between patients with Alzheimer disease and nondemented elderly subjects. AJNR Am J Neuroradiol 27:2141–2145. [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ; World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders (2013) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 14:334–385. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22:589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Passos IC, Mwangi B, Amaral-Silva H, Tannous J, Wu MJ, Zunta-Soares GB, Soares JC (2017) Hippocampal subfield volumes in mood disorders. Mol Psychiatry 22:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70:289–297. [DOI] [PubMed] [Google Scholar]
- Cho ZH, Kim YB, Han JY, Kim NB, Hwang SI, Kim SJ, Cho SJ (2010) Altered T2* relaxation time of the hippocampus in major depressive disorder: implications of ultra-high field magnetic resonance imaging. J Psychiatr Res 44:881–886. [DOI] [PubMed] [Google Scholar]
- Cole J, Toga AW, Hojatkashani C, Thompson P, Costafreda SG, Cleare AJ, Williams SC, Bullmore ET, Scott JL, Mitterschiffthaler MT, Walsh ND, Donaldson C, Mirza M, Marquand A, Nosarti C, McGuffin P, Fu CH (2010) Subregional hippocampal deformations in major depressive disorder. J Affect Disord 126:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle R, Dupong I, Colliot O, Deflesselle E, Hardy P, Falissard B, Ducreux D, Chupin M, Corruble E (2018) Smaller hippocampal volumes predict lower antidepressant response/remission rates in depressed patients: a meta-analysis. World J Biol Psychiatry 19:360–367. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Höhne T, Banac S, Schorr C, Jäger M, Leinsinger G, Bottlender R, Reiser M, Möller HJ (2004) Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry 65:492–499. [DOI] [PubMed] [Google Scholar]
- Gbyl K, Videbech P (2018) Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatr Scand 138:180–195. [DOI] [PubMed] [Google Scholar]
- Giuliano A, Donatelli G, Cosottini M, Tosetti M, Retico A, Fantacci ME (2017) Hippocampal subfields at ultra high field MRI: an overview of segmentation and measurement methods. Hippocampus 27:481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski G, Baldinger-Melich P, Seiger R, Godbersen GM, Michenthaler P, Klöbl M, Spurny B, Kautzky A, Vanicek T, Kasper S, Frey R, Lanzenberger R (2019) Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br J Psychiatry 214:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS (2009) Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry 65:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KM, Won E, Sim Y, Tae WS (2016) Hippocampal subfield analysis in medication-naïve female patients with major depressive disorder. J Affect Disord 194:21–29. [DOI] [PubMed] [Google Scholar]
- Han KM, Kim A, Kang W, Kang Y, Kang J, Won E, Tae WS, Ham BJ (2019) Hippocampal subfield volumes in major depressive disorder and bipolar disorder. Eur Psychiatry 57:70–77. [DOI] [PubMed] [Google Scholar]
- Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, Malykhin NV (2013) Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry 74:62–68. [DOI] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, Fischl B, Van Leemput K; Alzheimer’s Disease Neuroimaging Initiative (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, McEwen BS (2008) Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs 22:15–26. [DOI] [PubMed] [Google Scholar]
- Kitahara Y, Ohta K, Hasuo H, Shuto T, Kuroiwa M, Sotogaku N, Togo A, Nakamura K, Nishi A (2016) Chronic fluoxetine induces the enlargement of perforant path-granule cell synapses in the mouse dentate gyrus. PLOS One 11:e0147307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Klöbl M, Tik M, Auer B, Vanicek T, Geissberger N, Pfabigan DM, Hahn A, Woletz M, Paul K, Komorowski A, Kasper S, Windischberger C, Lamm C, Lanzenberger R (2019) The pulvinar nucleus and antidepressant treatment: dynamic modeling of antidepressant response and remission with ultra-high field functional MRI. Mol Psychiatry 24:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J (2013) Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry 73:70–74. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan J, Zhu X, Zhu Y, Qin J, Zhang N, Ju S (2018) Increased hippocampal fissure width is a sensitive indicator of rat hippocampal atrophy. Brain Res Bull 137:91–97. [DOI] [PubMed] [Google Scholar]
- MacQueen G, Frodl T (2011) The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16:252–264. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R (2008) Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry 64:880–883. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS (2003) Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28:1562–1571. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Broadhouse K, Rush AJ, Gordon E, Koslow S, Grieve SM (2018) Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry 23:1737–1744. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Coupland NJ (2015) Hippocampal neuroplasticity in major depressive disorder. Neuroscience 309:200–213. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Yushkevich PA, Das S, Wang L, Van Leemput K, Iglesias JE, Alpert K, Mezher A, Ng P, Paz K, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative (2018) Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. Neuroimage Clin 17:1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC (2005) Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry 57:935–937. [DOI] [PubMed] [Google Scholar]
- Patrício P, Mateus-Pinheiro A, Irmler M, Alves ND, Machado-Santos AR, Morais M, Correia JS, Korostynski M, Piechota M, Stoffel R, Beckers J, Bessa JM, Almeida OF, Sousa N, Pinto L (2015) Differential and converging molecular mechanisms of antidepressants’ action in the hippocampal dentate gyrus. Neuropsychopharmacology 40:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P (2015) A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Neuropsychopharmacologicum 18 (8):Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109. [DOI] [PubMed] [Google Scholar]
- Rabl U, et al. (2014) Additive gene-environment effects on hippocampal structure in healthy humans. J Neurosci 34:9917–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, O’Keane V, O’Hanlon E (2019) The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry 85:487–497. [DOI] [PubMed] [Google Scholar]
- Rosene DL.(1987) The hippocampal formation of the primate brain. A review of some comparative aspects of cytoarchitecture and connections. In: Further aspects of cortical function, including hippocampus, pp. 345–456. New York: Barnes & Noble. [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M; STAR*D Study Team (2006) Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 354:1231–1242. [DOI] [PubMed] [Google Scholar]
- Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, Taylor WD (2017) Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med 47:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Neumeister A, Zarate CA Jr, Drevets WC (2011) Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage 54:2643–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A (2011) State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord 135:405–409. [DOI] [PubMed] [Google Scholar]
- Schmaal L, et al. (2016) Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol Psychiatry 21:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiger R, Hahn A, Hummer A, Kranz GS, Ganger S, Küblböck M, Kraus C, Sladky R, Kasper S, Windischberger C, Lanzenberger R (2015) Voxel-based morphometry at ultra-high fields. A comparison of 7T and 3T MRI data. Neuroimage 113:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MK, Lee CH, Cho HY, Lee JG, Lee BJ, Kim JE, Seol W, Kim YH, Park SW (2014) Effects of antidepressant drugs on synaptic protein levels and dendritic outgrowth in hippocampal neuronal cultures. Neuropharmacology 79:222–233. [DOI] [PubMed] [Google Scholar]
- Serretti A, Souery D, Antypa N, Calati R, Sentissi O, Amital D, Moser U, Kasper S, Zohar J, Mendlewicz J (2013) The impact of adverse life events on clinical features and interaction with gene variants in mood disorder patients. Psychopathology 46:384–389. [DOI] [PubMed] [Google Scholar]
- Sone D, Sato N, Maikusa N, Ota M, Sumida K, Yokoyama K, Kimura Y, Imabayashi E, Watanabe Y, Watanabe M, Okazaki M, Onuma T, Matsuda H (2016) Automated subfield volumetric analysis of hippocampus in temporal lobe epilepsy using high-resolution T2-weighed MR imaging. Neuroimage Clin 12:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A (2018) Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M, Kraus C, Geissberger N, Auer B, Klöbl M, Tik M, Stürkat IL, Hahn A, Woletz M, Pfabigan DM, Kasper S, Lamm C, Windischberger C, Lanzenberger R (2017) Default mode network deactivation during emotion processing predicts early antidepressant response. Transl Psychiatry 7:e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S, Coupland NJ, Silversone PH, Huang Y, Fujiwara E, Carter R, Seres P, Malykhin NV (2015) Dentate gyrus volume and memory performance in major depressive disorder. J Affect Disord 172:159–164. [DOI] [PubMed] [Google Scholar]
- Travis SG, Coupland NJ, Hegadoren K, Silverstone PH, Huang Y, Carter R, Fujiwara E, Seres P, Malykhin NV (2016) Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J Affect Disord 201:34–41. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD (2003) Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 54:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets NL, Hodgetts CJ, Sen A, Adcock JE, Emir U (2017) Hippocampal MRS and subfield volumetry at 7T detects dysfunction not specific to seizure focus. Sci Rep 7:16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD (2004) Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry 56:101–112. [DOI] [PubMed] [Google Scholar]
- Wisse LE, Biessels GJ, Stegenga BT, Kooistra M, van der Veen PH, Zwanenburg JJ, van der Graaf Y, Geerlings MI (2015) Major depressive episodes over the course of 7 years and hippocampal subfield volumes at 7 tesla MRI: the PREDICT-MR study. J Affect Disord 175:1–7. [DOI] [PubMed] [Google Scholar]
- Wisse LEM, et al. ; Hippocampal Subfields Group (2017) A harmonized segmentation protocol for hippocampal and parahippocampal subregions: why do we need one and what are the key goals? Hippocampus 27:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.