Abstract
Although it is agreed that physicochemical features of molecules determine their perceived odor, the rules governing this relationship remain unknown. A significant obstacle to such understanding is the high dimensionality of features describing both percepts and molecules. We applied a statistical method to reduce dimensionality in both odor percepts and physicochemical descriptors for a large set of molecules. We found that the primary axis of perception was odor pleasantness, and critically, that the primary axis of physicochemical properties reflected the primary axis of olfactory perception. This allowed us to predict the pleasantness of novel molecules by their physicochemical properties alone. Olfactory perception is strongly shaped by experience and learning. However, our findings suggest that olfactory pleasantness is also partially innate, corresponding to a natural axis of maximal discriminability among biologically relevant molecules.
Keywords: odor, smell, olfaction, pleasantness, hedonics, valence
Introduction
The mapping of various physical stimulus attributes onto perception is well defined in vision and audition, but not in olfaction. Other than the mapping of molecular concentration onto perceived odor intensity (Cain, 1969), there is no known general systematic mapping of molecular properties onto the olfactory percept. In other words, there is no scientist or perfumer who can predict the smell of a novel molecule by its physicochemical structure, or the physicochemical structure of a novel smell.
Understanding this link between physicochemical structure and percept has been elusive because the percept is, in large part, plastic, dependent on experience and learning (Brennan and Keverne, 1997; Wilson and Stevenson, 2006). Nevertheless, part of the olfactory percept is innate and hard wired. For example, laboratory rodents that have never encountered a cat through generations of breeding still react fearfully to cat odor, but not other novel and noxious odors (Dielenberg and McGregor, 2001). Yet, the link between physicochemical structure and this hard-wired portion of the percept has proven equally elusive.
This may be attributable in part to the complexity of both the perceptual space and the stimulus space. The perceptual space is made of odors that can be described by a large number of verbal descriptors. The stimulus space is made of odorants, relatively small molecules (Ohloff, 1986) that can be described by a large number of physicochemical descriptors. Verbal descriptors for a large number of odorants have been reliably obtained, revealing considerable agreement in several aspects of olfactory perception (Dravnieks, 1982, 1985), and physicochemical descriptors can be mined and computed (Tetko et al., 2005) from structures available in chemical databases (e.g., PubChem). Thus, we sought to construct a perceptual space from verbal descriptors, an analogous physicochemical space from chemical descriptors, and then test for any systematic relation between these two spaces.
Materials and Methods
Subjects.
One hundred and eighty-five subjects (96 female) between the ages of 19 and 39 participated in the experiments after providing informed consent to procedures approved by the University of California, Berkeley, Committee for the Protection of Human Subjects. Subjects were generally healthy with no history of neurological disease, nasal passage disease, broken nose, or septoplasty.
Location and stimuli.
Experiments 1–6 were conducted in stainless-steel-coated rooms designed to minimize olfactory contamination. Experiment 7 was conducted in a well ventilated concrete room in the Arab village of Dir El Asad in the Northern Galilee part of Israel. All odorants were from Aldrich Chemicals (St. Louis, MO), of the highest purity available, and matched for perceived intensity by dilution with mineral oil or deionized distilled water as appropriate. The odorants used are detailed in supplemental Tables 5 and 6 (available at www.jneurosci.org as supplemental material), and referenced in the results section per experiment. Intensity-matching dilution procedures used a group of 10 experienced subjects used only for that purpose. Intensity matching was obtained by first identifying those odorants that were perceived as least intense in their undiluted form by two experimenters. Diluted samples were prepared for each more intense odor by the two experimenters and judged to be of the same intensity as the least intense odors. These prepared odors were then presented to each of the 10 rating subjects, who rated their intensity using a visual–analog scale (VAS). Pairwise differences between odors were tested using paired t tests. For any odor pair showing a significant difference in intensity, the more intense odor was diluted and was rerated by the group of 10 rating subjects until no significant differences in perceived intensities were evident.
Perceptual estimation studies.
Perceptual estimates were obtained by rating the applicability of the property of interest onto a given odorant using a VAS. All interactions with subjects during experiments were by computer, with an experimenter viewing behavior from a neighboring room via video monitor and one-way window. Odorants were sniffed from jars marked arbitrarily (e.g., jar A001). Experimental instructions were provided by computer using digitized voice, and perceptual estimates were obtained through mouse clicks on a 20-cm-long VAS. For example, in an experiment on odorant pleasantness, in a given trial, the subject would hear a digitized voice reading “Please sniff jar A001 at the tone, three, two, one, TONE,” and then the subject would hear “Please rate odorant pleasantness.” At this time, a 20-cm-long VAS line would appear on the monitor with the labels “extremely pleasant” at one end of the line and “extremely unpleasant” at the other. Subjects would initiate a mouse-click at a point along the line that reflected their perception. For the different experiments, scale extremes were “low similarity” versus “high similarity,” “extremely pleasant” versus “extremely unpleasant,” “edible (foods)” versus “inedible (toxic items)”, “not at all flowery” versus “extremely flowery,” and “not at all sweet” versus “extremely sweet.” In every experiment, subjects also rated intensity (“very low intensity” vs “extremely high intensity”), and in any case where, despite the initial intensity-matching procedure, an odorant was an outlier in terms of perceived intensity (>2 SD), it was deleted from additional analysis (this occurred for only four odorants of the 90 odorants tested across experiments). VAS values (in mm) were z-scored for every subject across odorants. All interstimulus intervals ranged from 30 to 45 s. Odorant presentation order was counterbalanced across subjects.
Reaction time study.
We used a 40-trial forced-choice same–different discrimination task (4 s intersniff interval) using five odorants. Stimuli were presented using an olfactometer that delivered odorants into a nasal mask with ∼2 ms temporal resolution (Johnson et al., 2003; Johnson and Sobel, 2007). On every trial, the subject heard a digitized voice instructing to sniff at the tone, followed by a second sniff instruction 4 s later. Subjects then had to respond within 2.5 s by pressing one of two buttons that denoted either “same” or “different.” The response interval ended with a buzzer if no response was recorded. The intertrial interval was set to 45 s so as to minimize adaptation. Each odorant was presented eight times, four times paired with itself, and once paired with each of the other four odorants. After pooling all of the same-same pairs as one condition (same), this matrix generated 11 possible pairings.
PC analysis for dimension reduction.
To reduce the dimensionality of perceptual descriptors obtained previously by Dravnieks (1982, 1985) and of physicochemical descriptors obtained using Dragon software (Talete, Milan, Italy), we used the principal component (PC) function in the Statistics Toolbox of Matlab (Mathworks, Natick, MA). In brief, principal component analysis (PCA) takes a data set consisting of N points in an M-dimensional space [for example, 160 odorants in the 146-dimensional odor descriptor space, in the case of the Dravnieks' (1982, 1985) data] and finds a rotation matrix which rotates the N points onto a new M-dimensional space with certain special properties. These are (1) that the new dimensions are orthogonal and (2) that the new dimensions, called principal components (e.g., PC1, PC2, …, PCM), are ordered so that each successive PC has the maximal possible variance. Thus PC[1] explains the most variance of any linear transform of the original data space, PC[2] explains the next biggest amount of variance, and so on. Because the PCs are orthogonal, they explain mutually exclusive sets of variance. The entire set of M PCs thus accounts for all the variance in the original data set. In practice, for data spaces in which the apparent dimensionality exceeds the intrinsic dimensionality (as is the case, for example, when many of the dimensions are intercorrelated or redundant), relatively few PCs will capture most of the variance in the original data space. A notable difference between PCA and other methods of multivariate analysis such as “factor analysis” is that PCA does not depend on the user to specify a particular data model, or to make a priori decisions regarding which data are to be included or excluded (supplemental Figs. 2, 3, available at www.jneurosci.org as supplemental material).
Because Dragon generated a very large number of physicochemical descriptors (1513) (supplemental Table 4, available at http://www.weizmann.ac.il/neurobiology/worg/materials.html as supplemental material), we did not apply PCA to only the 144 Dravnieks odorants, but rather to 1565 odorants commonly used in olfactory experiments and the fragrance industry (supplemental Table 8, available at www.jneurosci.org as supplemental material). This assured that all principal components could be estimated, and robustly. Each descriptor was normalized by z-scoring to prevent descriptors with larger ranges from artifactually dominating the dimensionality of the descriptor space.
After PCA, one can generate a subspace onto which one can project the data. For example, Figure 1b contains the 144 monomolecular odorants from Dravnieks projected onto a subspace made of the first four PCs (although only two are shown in the two-dimensional graph). In other words, each odorant is now described by four numbers rather than the original 146. Distances between odors using these four numbers were calculated using a Euclidean distance metric, in other words, D = sqrt(Σ (xi − yi)2), where x and y are the vectors for two different odors and i ranges over 1, 2,… 4.
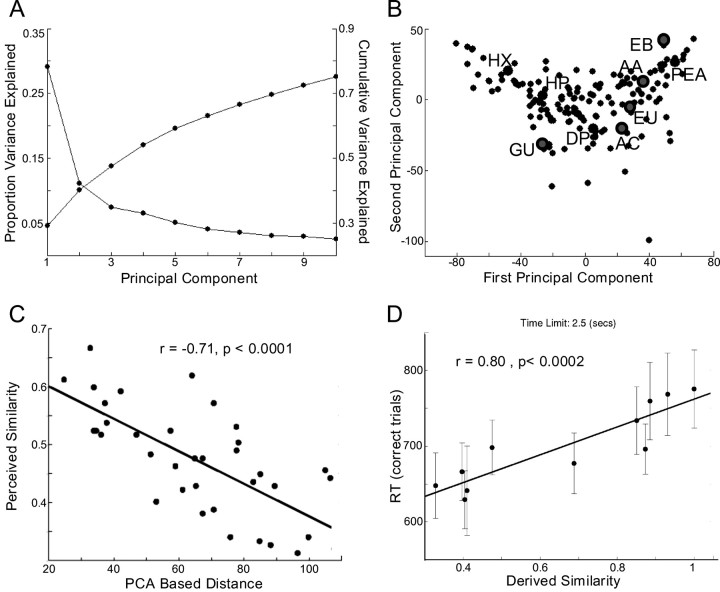
Figure 1.
Olfactory perceptual space. A, The proportion of variance in perceptual descriptions is explained by each of the PCs (starting at ∼0.3), and the cumulative variance is explained (starting at ∼0.05). B, The 144 odorants projected into a two-dimensional space made of the first and second PCs. The nine odorants used in experiment 1[acetophenone (AC), amyl acetate (AA), diphenyl oxide (DP), ethyl butyrate (EB), eugenol (EU), guaiacol (GU), heptanal (HP), hexanoic acid (HX), and phenyl ethanol (PEA)] are in enlarged circles, and the five odorants used in experiment 2 (acetophenone, amyl acetate, ethyl butyrate, eugenol, guaiacol) are in further enlarged circles. C, For the nine odorants, the correlation between explicit perceived similarity ratings and PCA-based distance for all pairwise comparisons. Odorants closer in the perceptual space were perceived as more similar. D, Reaction time for correct trials in a forced-choice same-different task using five of the nine odorants. Error bars reflect SE. The reaction time was longer for odorant pairs that were closer in PCA-based space, thus providing an implicit validation of the perceptual space.
Building a model from physicochemical onto perceptual olfactory space.
We modeled each perceptual PC independently as a linear combination of the physicochemical PCs plus an error term, Yi = Xbt + ei, where Yi is the vector of the ith perceptual descriptor (or ith PC of the perceptual space), X is a matrix of the predictive physicochemical descriptors, b is the weighting on X to fit Y, and ei is Gaussian noise. Fitting was done by least squares.
We used a stepwise cross-validation procedure to determine inclusion of physicochemical PCs. We increased the number of physicochemical PCs to predict perceptual PC1 until an increase resulted in no increase in predictive power. The predictive power of each model was tested as follows: we randomly divided the set of 144 odorants into two sets of 72, a model-building set and a test set. A model was built using the current number of physicochemical PCs and then this model was applied to the 72 odorants in the test set. The correlation between the predicted and actual values in the test set measures the power of the model. This cross-validation procedure was repeated1000 times for each size of model, and a distribution of each predictive measure was obtained. This procedure resulted in a model including the first seven physicochemical PCs.
Results
Constructing a perception-based space
We constructed a perceptual odor space using data from Dravnieks' Atlas of Odor Character Profiles, wherein ∼150 experts (perfumers and olfactory scientists) ranked (from 0 to 5, reflecting “absent” to “extremely” representative) 160 odorants (144 monomolecular species and 16 mixtures) against each of the 146 verbal descriptors (Dravnieks, 1985) (the lists of odorants and descriptors are provided in supplemental Tables 1, 2, available at www.jneurosci.org as supplemental material).
We applied PCA, a well established method for dimension reduction that generates a new set of dimensions (PCs) for the profile space in which (1) each successive dimension has the maximal possible variance and (2) all dimensions are uncorrelated. Figure 1A shows the percentage of the variance in the perceptual feature space explained by each of the first 10 PCs. As can be seen, the effective dimensionality of the odor profile space was much smaller than 146, with the first two PCs accounting for 40.1% of the total variance in the odor profiles, and the first four accounting for 54%. The full weighting of all 146 descriptors on the first four PCs is listed in supplemental Table 1 (available at www.jneurosci.org as supplemental material).
By selecting a number of PCs, we can define a perceptual space. Figure 1b contains a two-dimensional projection of the odorants onto the subspace formed by the first four PCs. In such a space, similar odorants are understood to be close to one another and dissimilar odorants are understood to be distant from one another (a navigable version of this space is available at http://www.weizmann.ac.il/neurobiology/worg/odorspace/Ptplot5.5/ptolemy/plot/demo/FourierSeries.htm).
Validating the perception-based space
To test our perceptual feature space, in experiment 1, we obtained pairwise perceived similarity ratings from 21 subjects for each of nine odorants that were pseudorandomly selected to span the space of the first four PCs (Fig. 1b). Pairwise similarities for each of the 36 possible odorant pairs were compared with pairwise Euclidean distances in our perceptual PC space using the first one, two, three, four, and five PCs. The addition of each successive component increased the correlation with the explicit pairwise similarity ratings until the fifth, which showed no statistically significant improvement in the correlation. Figure 1c shows the near linear relationship between the Euclidean distance over the first four PCs and the average pairwise similarity of our raters (r = −0.71; F(1,34) = 34.37; p < 0.0001). Pairwise similarity values for the odors can be found in supplemental Table 3 (available at www.jneurosci.org as supplemental material).
In experiment 2, we tested our perceptual space using an implicit similarity task. Using five of the nine previously used odorants (Fig. 1b), we presented 21 subjects with a forced-choice speeded reaction-time task in which subjects were presented with two odorants in succession and required to indicate as quickly as possible whether they were the same or different. As expected (Wise and Cain, 2000; Abraham et al., 2004; Rinberg et al., 2006), subjects took longer to make correct judgments for more similar odorant pairs than for dissimilar odorant pairs (Fig. 1d), where similarity was derived from our four-dimensional perceptual space (F(1,9) = 36.48; r = 0.80; p < 0.0002). Thus, in both explicit and implicit similarity tasks, our derived perceptual space corresponded to the subjects' judgments of similarity.
Identifying the primary dimension of the perception-based space: pleasantness
The descriptors that flank the first PC of perceptual space ranged from “sweet” and “floral” at one end, to “sickening” and “rancid” at the other (Fig. 2a, supplemental Table 1, available at www.jneurosci.org as supplemental material), suggesting that the first PC of perceptual space may be pleasantness (referring to the continuum from unpleasant to pleasant, also referred to as perceptual valence or hedonic tone).
Figure 2.
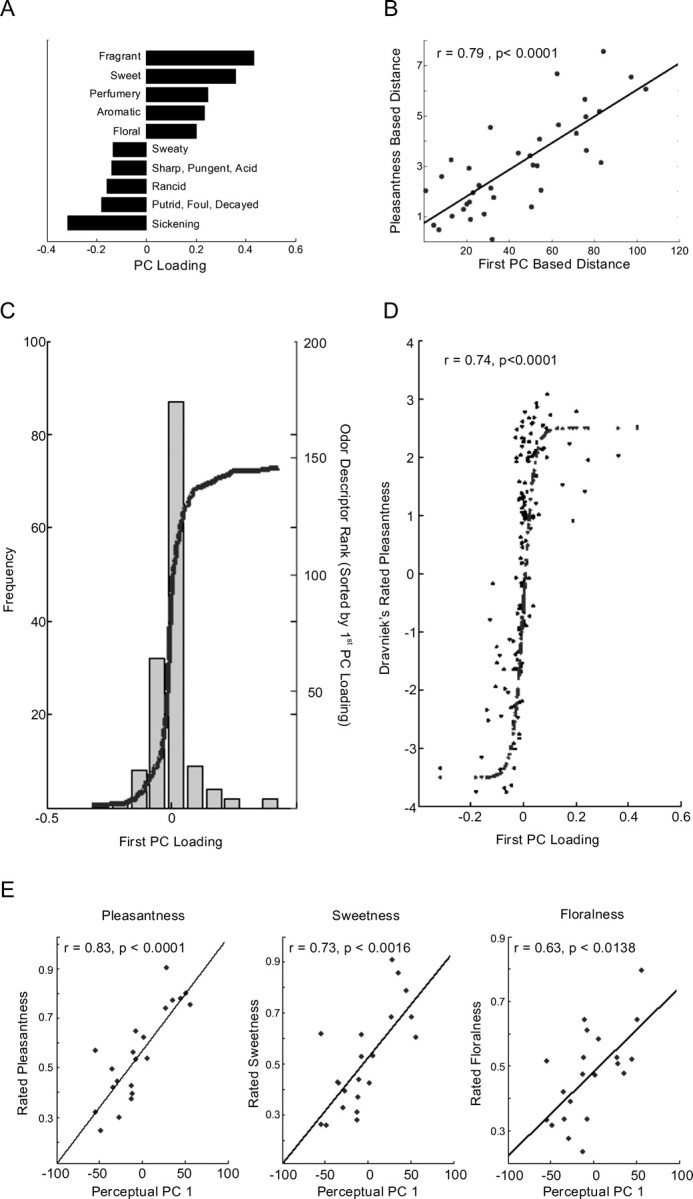
Identifying pleasantness as the first PC of perception. A, The five descriptors that flanked each end of PC1 of perception. We should stress that here, and in Figure 3B, we show the five extreme descriptors only to help give a sense of the PC. This does not reflect a cutoff in any stage of the analysis, but only an esthetic cutoff for the figure. B, For the nine odorants, the correlation between the pairwise difference in pleasantness and the pairwise distance along the first PC. Distance along the first PC was a strong predictor of difference in pleasantness. C, The 146 perceptual descriptors plotted as a function of their weighting on the first PC of perception. D, The previously published pleasantness associated with each one of the 146 perceptual descriptors. The descriptors clearly weighted on the first PC of perception in accordance with their pleasantness. E, We randomly selected 21 odorants tested previously by Dravnieks (1982, 1985) (acetyl pyridine, benzaldehyde, amyl acetate, camphor dl, celeriax, citral, dimethyl pyrrole2,5, eugenol, heptanal, hexanoic acid, hexanol1, hexanol3, indole, methyl-iso-borneol2, methyl quinolinepara, octanol1, octenol-1–3-OL, phenyl ethanol, skatole, vanillin) and had 22 subjects rate all odorants using three scales with VAS extremes of “not at all flowery” versus “extremely flowery,” “not at all sweet” versus “extremely sweet,” and “extremely unpleasant” versus “extremely pleasant.” The order of VAS scales was counterbalanced. Judgments were converted to z-scores for each subject, and scores for odorants averaged across subjects. We then regressed these normalized ratings against the PC1 values for these odorants.
To test this, in experiment 3, 10 subjects rated the pleasantness and intensity of the nine odorants used in experiment 1. To compare these results with the PCA results, for each pair of odorants we computed a pleasantness distance (the absolute difference between their ratings) and regressed this against their distance based on the first PC. Whereas these two measures were strongly correlated (Fig. 2b) (F(1,34) = 55.1; r = 0.79; p < 0.0001), a similar analysis on the intensity estimates revealed no significant correlation (F(1,34) = 0.46; r = −0.12; p > 0.5).
As an second test of whether perceptual PC1 reflects odor pleasantness, we compared previously published pleasantness ratings for each of the 146 perceptual descriptors used (Dravnieks et al., 1984) (note, these are pleasantness ratings associated with the descriptors, not the odorants) to the component weights on the first PC. Figure 2c shows the component weights, sorted by value for the first PC. Figure 2d shows the previously collected pleasantness ratings plotted against component weights. The relationship between rated pleasantness and component weights was clearly not linear, so to assess the relationship, we first transformed the component weights with a sigmoid transform, which we then regressed against the pleasantness ratings. The correspondence between descriptor pleasantness and the first PC weighting was quite strong (r = 0.74; F(1,144) = 178.4; p < 0.0001).
As a third test, we asked whether the term “pleasantness” is better at capturing the first PC of perception when compared with individual related descriptors such as “sweetness” or 'floweriness,“ both of which had the among the highest weight on perceptual PC1 (Fig. 2a). In experiment 4, 22 subjects rated the pleasantness, floweriness, and sweetness of 22 odorants randomly selected from the 144 odorants used by Dravnieks (1985). As predicted, ratings of all three labels were significantly correlated with PC1 values (pleasantness, r = 0.83, p < 0.0001; sweetness r = 0.73, p < 0.0016; floralness, r = 0.63, p < 0.0138), but the correlation of PC1 with pleasantness was significantly higher than the correlations for sweetness and floweriness (binomial sign test on difference in r scores across subjects: pleasantness vs sweetness, p < 0.0262; pleasantness vs floralness, p < 0.0001; sweetness vs floralness, p < 0.0022), suggesting that this label captures significantly more of the variance in PC1 than do even closely related terms (Fig. 2e).
Experiments 3 and 4, combined with several additional analyses detailed in supplemental Figure 1 (available at www.jneurosci.org as supplemental material), and an additional test of “edibility” as an alternative label to perceptual PC1 (to be described below), all supported our observation that the first PC reflected odor pleasantness.
Building a physicochemical molecular descriptor space
To relate this perceptual space to physicochemical properties of molecules, we used a similar procedure to reduce the dimensionality of the physicochemical space. We obtained 1514 physicochemical descriptors for each of 1565 odorants. These descriptors were of many types (e.g., atom counts, functional group counts, counts of types of bonds, molecular weights, topological descriptors, and so on). Applying PCA revealed that the effective dimensionality of the space of descriptors was much lower than the apparent dimensionality of 1514. Figure 3a shows the percentage variance explained by each of the first 10 PCs. The first PC accounted for ∼32% of the variance, and the first 10 accounted for ∼70% of the variance. Figure 3b shows the five descriptors that anchor the first PC of the space (the full names of these physicochemical descriptors are listed in the legend of Figure 3 and in supplemental Table 4 (available at http://www.weizmann.ac.il/neurobiology/worg/materials.html as supplemental material), which also lists the full weighting of all 1513 descriptors on the first five PCs.
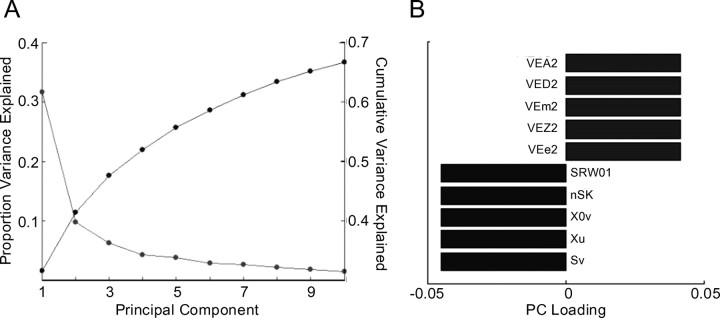
Figure 3.
Reducing dimensionality of physicochemical space. A, The proportion of variance in physicochemical descriptors is explained by each of the PCs (starting at ∼0.32), and the cumulative variance is explained (starting at ∼0.01). B, The five descriptors that weighted most heavily at the ends of PC1 of physicochemical space. The descriptors are as follows: sV, sum of atomic van der Waals volumes (scaled on carbon atom); Xu, Xu index; Xov, pleasantness connectivity index χ-0′; nSK, number of non-H atoms; SRW01, self-returning walk count of order 01 (number of non-H atoms, nSK); VEe2, average eigenvector coefficient sum from electronegativity weighted distance matrix; VEZ2, average eigenvector coefficient sum from z-weighted distance matrix (Barysz matrix); Vem2, average eigenvector coefficient sum from mass weighted distance matrix; VEA2, average eigenvector coefficient sum from adjacency matrix; VED2, average eigenvector coefficient sum from distance matrix.
Identifying the primary dimension of physicochemical space
Characterizing the primary dimension of the PCA space of the physicochemical descriptors is more complex than the task for the perceptual space, both because of the set size and the variety of descriptors involved. The first physicochemical PC was weighted at one end by factors that are reasonable proxies for molecular size or weight: the sum of the atomic van der Waals volumes is essentially a crude count of atoms, as is the count of the number of nonhydrogen atoms, and the self-returning walk count of order one for nonhydrogen atoms (which is actually identical to a count of the nonhydrogen atoms). The characterization of these descriptors as indices of “weight” is borne out by the very high weighting that “molecular weight” itself has on this side of the first PC: −0.044 (supplemental Table 4, available at http://www.weizmann.ac.il/neurobiology/worg/materials.html as supplemental material).
At the other end of the dimension is a series of topological descriptors that vary with the “extent” of a molecule. In fact, all five of the descriptors are average eigenvectors of distance or adjacency matrices, normalized in slightly different ways: average eigenvector coefficient sum from electronegativity-weighted distance matrix; average eigenvector coefficient sum from z-weighted distance matrix (Barysz matrix); average eigenvector coefficient sum from mass-weighted distance matrix; average eigenvector coefficient sum from distance matrix; average eigenvector coefficient sum from adjacency matrix. Each of these measures increases as the denseness of the atomic connections increases (i.e., as the number of atoms is packed more closely together). In combination then, these two extremes anchor a dimension that characterizes the amount and distribution of mass within a molecule. That said, unlike the case of the perceptual data where we also applied an intuitively simple label to PC1 (pleasantness), we have no equally valid label for PC1 of physicochemical organization, and characterization of the true nature of this dimension awaits detailed analysis of all of the relevant physicochemical features involved in its construction. Supplemental Figure 4 (available at www.jneurosci.org as supplemental material) shows some representative molecules arranged in the space of the first and second physicochemical PCs.
Building a model from physical to perceptual space
We have used PCA to construct two spaces: a perceptual space and a physicochemical space. PCA generates ordered sets of orthogonal axes, constructed to maximize the variance they capture in the original feature space. Because the axes are uncorrelated (orthogonal), we can compare them independently.
For each of the four perceptual PCs, we asked whether they were correlated with any of the first few physicochemical PCs. Each of the four panels in Figure 4A corresponds to one of the first four perceptual PCs. Each panel shows the correlation of a single perceptual PC with the first seven physicochemical PCs. Error bars represent 1 SEM derived from 1000 bootstrap replicates. Strikingly, the strongest correlation was between the first perceptual PC and the first physicochemical PC [for 1000 bootstrap replicates, this correlation (r = 0.49; p < 0.001) was significantly stronger than correlations between the first physicochemical PC and the second (r = 0.11; p < 0.001), third (r = 0.12; p < 0.001), and fourth (r = 0.20; p < 0.001) PCs of perception; this correlation was also significantly stronger than the correlation between the first perceptual PC of perception and the second physicochemical PC (r = 0.11; p < 0.001)]. In other words, there was a privileged relationship between PC1 of perception and PC1 of physicochemical organization. The single best axis for explaining the variance in the physicochemical data was the best predictor of the single best axis for explaining the variance in the perceptual data. The higher-order and lower-variance dimensions of the perceptual space were more correlated with higher-order and lower-variance physicochemical dimensions.
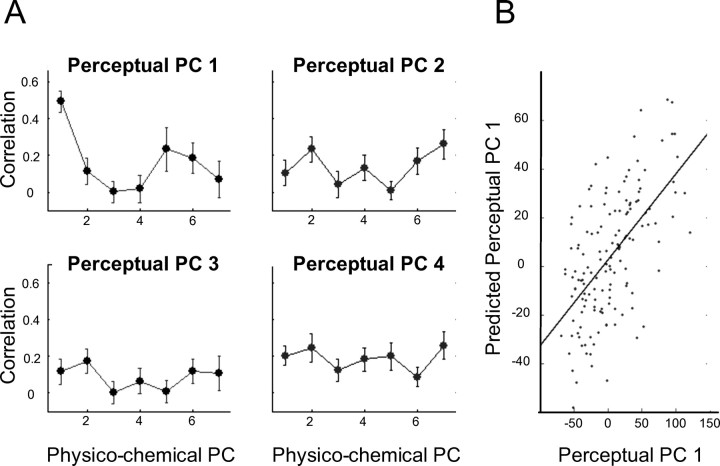
Figure 4.
Relating physicochemical space to perceptual space. A, The correlation between the first to fourth (descending in the figure) perceptual PC and each of the first seven physicochemical PCs for the 144 odorants. Error bars reflect the SE from 1000 bootstrap replicates. The best correlation was between the first PC of perception and the first PC of physicochemical space. This correlation was significantly larger than all other correlations. B, For the 144 odorants, the correlation between their actual first perceptual PC value and the value our model predicted from their physicochemical data.
Having established that the physicochemical space is related to the perceptual space, we next built linear predictive models through a cross-validation procedure. Figure 4B shows the correlation between the first perceptual PC and its predicted values resulting from the model based on the first seven physicochemical components (r = 0.59; F(1,136) = 10.62; p < 0.0001). In other words, we generated a model that predicted odor perception from odorant structure.
Experimental validation: predicting the perceptual qualities of novel molecules
To test the predictive power of our model, we obtained physicochemical parameters for 52 odorants commonly used in olfaction experiments, but not present in the set of 144 used in the foregoing experiments and model building (supplemental Table 5, available at www.jneurosci.org as supplemental material). We applied our model to the 52 new molecules so that for each we had predicted values for the first PC of perceptual space. The distribution of predicted first-PC values is shown in Figure 5a.
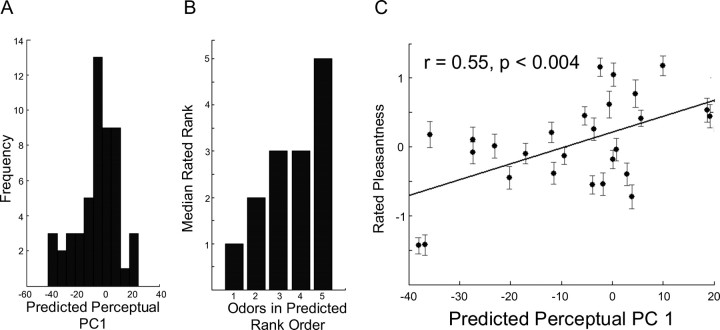
Figure 5.
Predicting perception of novel odorants. A, The distribution of predicted first PC values for 52 novel odorants. B, The median pleasantness ranking for each of five odorants that spanned the first predicted PC, sorted by expected ranking from our model. C, The correlation between the rated pleasantness of 27 of the 52 odorants and the first PC value as predicted by our model.
As an initial test, in experiment 5, we pseudorandomly selected a subset of five of the 52 odorants that spanned the range of first PC values and asked 14 subjects to rank these five odorants (equated for intensity) in order of pleasantness to unpleasantness. The median ranking for each of the five odorants sorted by expected ranking from our model is shown in Figure 5b. As can be seen, subjects' rankings of the five odorants matched the predicted ordering from our model (Spearman rank correlation, r = 0.72; p < 0.0004).
In experiment 6, we selected 27 of the 52 molecules at random and asked 20 subjects to rate the pleasantness or unpleasantness of each. Figure 5c shows a plot of the predicted first PC value against the average pleasantness rating. The two measures were significantly correlated (r = 0.55; F(1,25) = 10.64; p < 0.004). These results confirm those of the cross-validation procedure: a model based on physicochemical properties of molecules can provide a good prediction of the perceived pleasantness or unpleasantness of those molecules.
Cross-cultural validation
Judgments of pleasantness and other olfactory properties can vary across cultures (Wysocki et al., 1991; Ayabe-Kanamura et al., 1998; Hudson, 1999). Given that molecules are universal but that olfactory perceptions may be culturally specific, in experiment 7, we tested our predictive model in three different cultures: among Americans in California, Muslim-Arab Israelis, and Jewish Israelis. To test our model, we selected 27 new odorants [not in the Dravnieks (1985) set and not tested in the previous prediction experiments] that were (1) odorous and (2) were used very infrequently in human olfaction experiments (for details, see supplemental Table 7, available at www.jneurosci.org as supplemental material) so as to minimize any bias toward commonly used odors. Finally, we addressed the possibility that an alternative characterization of the first PC might be edibility, or food-like versus nonfood-like odors. In addition to rating pleasantness, subjects from all three cultures also rated edibility of odorants for which we predicted PC1 values.
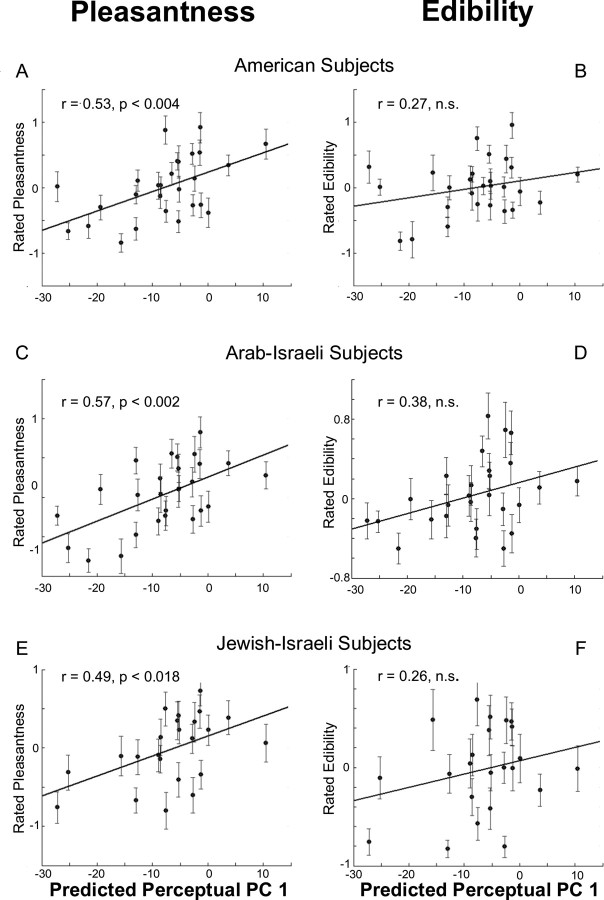
Figure 6a–c shows plots of the mean ratings of pleasantness and Figure 6d–f show mean ratings of edibility from each of the three cultural groups plotted against predicted PC 1 values from our physicochemical model. In all cases, the model predictions were better correlated with the pleasantness than the edibility judgments (Americans: pleasantness, r = 0.53, F(1,25) = 9.99, p < 0.004; edibility, r = 0.27, F(1,25) = 1.9, p > 0.05; Muslim Arab-Israelis: pleasantness, r = 0.57, F(1,25) = 12.14, p < 0.002; edibility, r = 0.38, F(1,25) = 4.32, p < 0.048; Jewish-Israelis: pleasantness, r = 0.49, F(1,21) = 6.65, p < 0.018; edibility, r = 0.26, F(1,21) = 1.47, p > 0.05) indicating that our predictions were valid across cultures, and that perceptual PC1 better reflects pleasantness than edibility. In addition, judgments of pleasantness were more consistent across cultures than judgments of edibility: Americans and Arab-Israeli judgments of pleasantness were correlated (r = 0.70; F(1,25) = 24.17; p < 0.0001) whereas judgments of edibility were correlated (r = 0.46; F(1,25) = 6.70; p < 0.02). Similarly, Americans and Jewish-Israeli judgments of pleasantness were correlated (r = 0.71; F(1,21) = 21.24; p < 0.0002). The correlation in judgments of edibility was higher than that between Americans and Arab-Israelis (r = 0.69; F(1,21) = 19.41; p < 0.0002) possibly reflecting the more European eating habits of Jewish Israelis compared with Arab Israelis.
Figure 6.
A–C, Cross-cultural validation. Twenty-seven odorous molecules not commonly used in olfactory studies, and not previously tested by us were presented to three cultural groups of naive subjects: Americans (23 subjects), Arab-Israelis (22 subjects), and Jewish-Israelis (20 subjects). In all cases, our predictions of odorant pleasantness were in fact better than in the test data (Fig. 4B). D–F, Ratings of edibility for the same odorants and groups. Across all cultures, our predicted PC1 values were significantly better correlated with judgments of pleasantness than judgments of edibility.
Discussion
We first reduced the dimensionality of olfactory perception and observed that pleasantness is the principal perceptual aspect of olfaction. This characterization of the first PC as pleasantness is in agreement with previous research (Richardson and Zucco, 1989). Pleasantness is the primary perceptual aspect humans use to discriminate odorants (Schiffman, 1974; Godinot and Sicard, 1995) or combine them into groups (Berglund et al., 1973; Schiffman et al., 1977). Pleasant and unpleasant odorants are evaluated at different speeds (Bensafi et al., 2002) and by dissociable neural substrates, as evidenced in both electrophysiological recordings (Kobal et al., 1992; Pause and Krauel, 2000; Masago et al., 2001) and functional neuroimaging studies (Zald and Pardo, 1997; Royet et al., 2000; Gottfried et al., 2002; Anderson et al., 2003; Rolls et al., 2003). Finally, studies with newborns suggest that at least some aspects of olfactory pleasantness may be innate (Steiner, 1979; Soussignan et al., 1997). Thus, our initial finding here is consistent with the view that “it is clearly the hedonic meaning of odor that dominates odor perception” (Engen, 1982).
We next reduced the dimensionality of physicochemical properties and identified a primary axis of physicochemical space. This axis was weighted at one end by measures reflecting molecular weight, and at the other end by measures reflecting molecular extent. Although it is tempting to label such a continuum, for the main finding of this manuscript, the importance lies in identifying a first PC of physicochemical space (i.e., an ordering of 1513 molecular descriptors) and not in its label.
The key finding of this manuscript was that 144 molecules were similarly ordered by these two independently obtained principal axes, one for perception and one for physicochemical structure. In other words, when physicochemical measures with no a priori connection to any particular percepts were analyzed, those physicochemical measures that were best at discriminating a set of molecules were found to be those that were most correlated with the perception of olfactory pleasantness. In other words, when one orders a set of odorants based on the variance in their physicochemical properties alone, they end up roughly ordered by perceptual pleasantness as well. This phenomenon allowed us to predict (r = ∼0.5; p < ∼0.004) odorant pleasantness of >50 molecules that we did not smell previously (and that were not part of our model building set), that were here tested in >80 subjects spanning three cultures. This ability to predict perceptual properties of novel odorants was a critical aspect of this manuscript. One may argue that the odorants selected by Dravnieks (1985) were somehow skewed, that our choice of method for dimension reduction (PCA) has limitations, or that our model building was somehow erroneous. Such concerns would gain increased weight if all we did was predict within the test set, that is, from one portion of the modeled data onto another portion of the modeled data (as in Fig. 4B). However, considering that we made predictions on novel odorants outside of the test set (Figs. 5B,C, 6A,C,E) suggests that we have revealed a genuine mechanism that is independent of the odorants used to build the model or the particular methods of dimension reduction and model building that we applied.
A main consequence of these results is that they provide a perceptually validated and physicochemically constrained metric for probing the olfactory system. For example, this result suggests a framework for selecting candidate ligands to test for affinity to particular olfactory receptors, and for axes of spatial encoding in the olfactory bulb and cortex. One can simply select odorants from the olfactory perceptual space (a navigable version of this space is available at http://www.weizmann.ac.il/neurobiology/worg/odorspace/Ptplot5.5/ptolemy/plot/demo/FourierSeries.htm), and test the hypothesis that the extent of distance within the space reflects the extent of difference in neural response, whether for amplitude, rate, or location.
All that said, it is important to stress the limitations of our claim. First, one qualifier to our predictive abilities is that they relate to odorants that have been equated for perceived intensity. Intensity and pleasantness interact in complex ways (Henion, 1971; Doty, 1975; Moskowitz et al., 1976) and our model requires additional refinement to reflect this complexity. Furthermore, as in other senses, the perception of odor and of pleasantness is a complex process involving both innately tuned and learned components. We do not suggest that there is a rigid transform from odorant structure to odor perception that will ultimately explain all of perception. Olfactory perception and subsequent neural representations are significantly influenced by several aspects clearly unrelated to physicochemical structure, such as context (Schoenbaum and Eichenbaum, 1995; Kay and Laurent, 1999; Herz and von Clef, 2001), expectation (de Araujo et al., 2005; Zelano et al., 2005), multisensory convergence (Haberly, 2001; Gottfried and Dolan, 2003; Rolls, 2004) and various top-down state-dependent modulatory influences (Pager, 1983; Critchley and Rolls, 1996; Kay and Freeman, 1998; Murakami et al., 2005). Furthermore, olfactory perception is a heavily learned process (Brennan and Keverne, 1997; Wilson and Stevenson, 2006) that critically depends on past and ongoing experience and exposure (Keverne, 1995; Wilson, 2003; Davis, 2004). The dynamics of this dominant aspect of olfactory perception will obviously not be reflected in physicochemical structure. However, a portion of olfactory perception is innate and hard-wired (Dielenberg and McGregor, 2001), and our results concern this part.
A final important caveat about our results is the relatively simple nature of our predictive model. We were able to predict the pleasantness of new odors using a simple linear model on physicochemical descriptors. Although a linear model is a useful simplification of a complex problem and was able to account for ∼30% of the variance in the data, it is unlikely that real olfactory systems will be linear. No doubt, more complex nonlinear models of the mapping from physicochemical to perceptual space, which will require much larger datasets to develop and independently test, will have significantly more explanatory power.
That perceptual pleasantness is a reflection of optimal physicochemical discrimination may at first appear at odds with the notion that olfactory pleasantness is both variable across individuals and cultures (Wysocki et al., 1991; Ayabe-Kanamura et al., 1998), and also malleable within individuals over time (Cain and Johnson, 1978; Hudson, 1999; Stevenson and Repacholi, 2003). Thus, given our result, how does one explain existing variance in perceived pleasantness? Even when the pleasantness scale reflects an organizational aspect of the physical world, an initial rigid encoding of odor pleasantness may be recoded at later stages of processing to reflect changing contingencies obtained through experience and learning (Brennan and Keverne, 1997; Wilson and Stevenson, 2006). Indeed, the olfactory system is known for plasticity at multiple levels (Graziadei et al., 1979; Wilson et al., 2004; Barkai, 2005; Mandairon et al., 2006), which reflects an advantageous evolutionary mechanism. To automatically reject food that smells fermented is generally a safe bet. However, if through experience one learns that exceptions exist, and for example fermented fish can be both tasty and healthy, than its pleasantness representation may shift. This is what allows Swedes to enjoy their Surströmming Herring (a dish not for the faint of heart), although even they will not say they like the odor per se. Finally in this respect, it is notable that although our predictive power for pleasantness was significant (p < 0.004), we explained only a portion of the variance. This leaves open the possibility that individual differences and plasticity in olfactory hedonics make important contributions to olfactory perception.
In this study, we probed the link between physicochemical properties and odor perception. Past studies have approached this by varying stimuli along a single and simple physicochemical dimension such as carbon chain length (Laska and Teubner, 1999), or a single and simple perceptual descriptor (Rossiter, 1996) such as “bitter almond-like” (Zakarya et al., 1993). Here, rather than moving from chemistry to perception, we went from perception to chemistry and probed a large set of physicochemical descriptors using an unbiased statistical approach. In this, we have followed in the footsteps of Schiffman (1974, 1977), Amoore (1963), Dravnieks (1982, 1984, 1985), and others, that together laid the groundwork for this approach between the early 1950s to late 1970s (Amoore, 1963; Laffort and Dravnieks, 1973; Schiffman, 1974). In this respect, the contribution of the current work is in observing that the perception of pleasantness corresponded to a physicochemical axis that was the best single discriminator of molecules that have a smell. This enabled a critical component of this manuscript, namely, predicting perceptual aspects of a novel odorants that were not in the original test set.
In conclusion, we generated an olfactory perceptual space and a molecular physicochemical space. Relating these spaces to each other allowed us to predict a moderate portion of the variance in olfactory perception, but more critically, led us to conclude that the major axis of perception reflects the major axis of physicochemical organization. Whereas the particular labels applied to the primary axes of perception and physicochemical organization may be modified through future research or interpreted differently by different researchers, we suggest that the privileged link between PC1 of perception and PC1 of physicochemical organization reflects an organizational property of the sense of smell.
Footnotes
This work was supported by Army Research Office Grant 46666-LS and National Institutes of Health–National Institute on Deafness and Other Communication Disorders Grants DC006915 and DC005958. We thank Dr. Jamal Asadi and his family for their help and hospitality. We are thankful for helpful comments on drafts of this manuscript provided by Tom Young and Lubert Stryer. We especially thank Arak Elite. A previous version of this manuscript received a critical and helpful review from the late Pierre-Gilles de Gennes, and this manuscript is dedicated to his memory.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Amoore JE. Stereochemical theory of olfaction. Nature. 1963;198:271–272. doi: 10.1038/198271a0. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Ayabe-Kanamura S, Schicker I, Laska M, Hudson R, Distel H, Kobayakawa T, Saito S. Differences in perception of everyday odors: a Japanese-German cross-cultural study. Chem Senses. 1998;23:31–38. doi: 10.1093/chemse/23.1.31. [DOI] [PubMed] [Google Scholar]
- Barkai E. Dynamics of learning-induced cellular modifications in the cortex. Biol Cybern. 2005;92:360–366. doi: 10.1007/s00422-005-0564-0. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Pierson A, Rouby C, Farget V, Bertrand B, Vigouroux M, Jouvent R, Holley A. Modulation of visual event-related potentials by emotional olfactory stimuli. Neurophysiol Clin. 2002;32:335–342. doi: 10.1016/s0987-7053(02)00337-4. [DOI] [PubMed] [Google Scholar]
- Berglund B, Berglund U, Engen T, Ekman G. Multidimensional analysis of 21 odors. Scandinavian J Psychol. 1973;14:131–137. doi: 10.1111/j.1467-9450.1973.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Cain WS. Odor intensity. Differences in exponent of psychophysical function. Percept Psychophys. 1969;6:349–354. [Google Scholar]
- Cain WS, Johnson F., Jr Lability of odor pleasantness: influence of mere exposure. Perception. 1978;7:459–465. doi: 10.1068/p070459. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Doty RL. An examination of relationships between the pleasantness, intensity and concentration of 10 odorous stimuli. Percept Psychophysiol. 1975:492–496.. [Google Scholar]
- Dravnieks A. Odor quality: semantically generated multi-dimensional profiles are stable. Science. 1982;218:799–801. doi: 10.1126/science.7134974. [DOI] [PubMed] [Google Scholar]
- Dravnieks A, editor. Atlas of odor character profiles. Philadelphia, PA: ASTM; 1985. [Google Scholar]
- Dravnieks A, Masurat T, Lamm RA. Hedonics of odors and odor descriptors. J Air Pollut Control Assoc. 1984;34:752–776. [Google Scholar]
- Engen T, editor. The perception of odors. New York: Academic; 1982. [Google Scholar]
- Godinot N, Sicard G. Odor categorization by human-subjects: an experimental approach. Chem Senses. 1995;20:101. [Google Scholar]
- Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PP, Levine RR, Monti Graziadei GA. Plasticity of connections of the olfactory sensory neuron: regeneration into the forebrain following bulbectomy in the neonatal mouse. Neuroscience. 1979;4:713–727. doi: 10.1016/0306-4522(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Henion KE. Odor pleasantness and intensity: a single dimension? J Exp Psychol. 1971;90:275–279. doi: 10.1037/h0031549. [DOI] [PubMed] [Google Scholar]
- Herz RS, von Clef J. The influence of verbal labeling on the perception of odors: evidence for olfactory illusions? Perception. 2001;30:381–391. doi: 10.1068/p3179. [DOI] [PubMed] [Google Scholar]
- Hudson R. From molecule to mind: the role of experience in shaping olfactory function. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Sobel N. Methods for building an olfactometer with known concentration outcomes. J Neurosci Methods. 2007;160:231–245. doi: 10.1016/j.jneumeth.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90:1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 1998;112:541–553. doi: 10.1037//0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Olfactory learning. Curr Opin Neurobiol. 1995;5:482–488. doi: 10.1016/0959-4388(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Vantoller S. Differences in human chemosensory evoked-potentials to olfactory and somatosensory chemical stimuli presented to left and right nostrils. Chem Senses. 1992;17:233–244. [Google Scholar]
- Laffort P, Dravnieks A. An approach to a physico-chemical model of olfactory stimulation in vertebrates by single compounds. J Theor Biol. 1973;38:335–345. doi: 10.1016/0022-5193(73)90178-1. [DOI] [PubMed] [Google Scholar]
- Laska M, Teubner P. Olfactory discrimination ability for homologous series of aliphatic alcohols and aldehydes. Chem Senses. 1999;24:263–270. doi: 10.1093/chemse/24.3.263. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci USA. 2006;103:13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masago R, Shimomura Y, Iwanaga K, Katsuura T. The effects of hedonic properties of odors and attentional modulation on the olfactory event-related potentials. J Physiol Anthropol Appl Human Sci. 2001;20:7–13. doi: 10.2114/jpa.20.7. [DOI] [PubMed] [Google Scholar]
- Moskowitz HR, Dravnieks A, Klarman LA. Odor intensity and pleasantness for a diverse set of odorants. Percept Psychophysiol. 1976;19:122–128. [Google Scholar]
- Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Ohloff G. Chemistry of odor stimuli. Experientia. 1986;42:271–279. doi: 10.1007/BF01942507. [DOI] [PubMed] [Google Scholar]
- Pager J. Unit responses changing with behavioral outcome in the olfactory bulb of unrestrained rats. Brain Res. 1983;289:87–98. doi: 10.1016/0006-8993(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Pause BM, Krauel K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int J Psychophysiol. 2000;36:105–122. doi: 10.1016/s0167-8760(99)00105-1. [DOI] [PubMed] [Google Scholar]
- Richardson JT, Zucco GM. Cognition and olfaction: a review. Psychol Bull. 1989;105:352–360. doi: 10.1037/0033-2909.105.3.352. [DOI] [PubMed] [Google Scholar]
- Rinberg d, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;3:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Rossiter KJ. Structureminus signOdor relationships. Chem Rev. 1996;96:3201–3240. doi: 10.1021/cr950068a. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20:7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S, Robinson DE, Erickson RP. Multidimensional-scaling of odorants: examination of psychological and physiochemical dimensions. Chem Senses Flav. 1977;2:375–390. [Google Scholar]
- Schiffman SS. Physicochemical correlates of olfactory quality. Science. 1974;185:112–117. doi: 10.1126/science.185.4146.112. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Soussignan R, Schaal B, Marlier L, Jiang T. Facial and autonomic responses to biological and artificial olfactory stimuli in human neonates: Re-examining early hedonic discrimination of odors. Physiol Behav. 1997;62:745–758. doi: 10.1016/s0031-9384(97)00187-x. [DOI] [PubMed] [Google Scholar]
- Steiner JE. Human facial expressions in response to taste and smell stimulation. Adv Child Dev Behav. 1979;13:257–295. doi: 10.1016/s0065-2407(08)60349-3. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Repacholi BM. Age-related changes in children's hedonic response to male body odor. Dev Psychol. 2003;39:670–679. doi: 10.1037/0012-1649.39.4.670. [DOI] [PubMed] [Google Scholar]
- Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. Virtual computational chemistry laboratory—design and description. J Comput Aided Mol Des. 2005;19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ, editors. Learning to smell. Baltimore: Johns Hopkins UP; 2006. [Google Scholar]
- Wilson DA, Best AR, Sullivan RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist. 2004;10:513–524. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Cain WS. Latency and accuracy of discriminations of odor quality between binary mixtures and their components. Chem Senses. 2000;25:247–265. doi: 10.1093/chemse/25.3.247. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Pierce JD, Gilbert AN. Geographic, cross-cultural, and individual variation in human olfaction. In: Getchell TV, editor. Smell and taste in health and disease. New York: Raven; 1991. pp. 287–314. [Google Scholar]
- Zakarya D, Yahiaoui M, Fkih-Tetouani S. Structure-odour relations for bitter almond odorants. J Phys Organic Chem. 1993;6:627–633. [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]