Abstract
Changes in synaptic strength at striatal synapses, such as long-term depression (LTD), may be involved in striatal-based learning and memory. Several molecular mechanisms have been implicated in striatal LTD, but it is not clear which mechanisms are crucial for LTD induction. We found that the activation of L-type calcium channels by 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methylester (FPL64176), combined with modest postsynaptic depolarization and synaptic activation, is sufficient to induce robust LTD (FPL–LTD). The L-channel activator 1,4-dihydro-2,6-dimethyl-5-nitro-4-[2(trifluoromethyl)phenyl]pyridine-3-carboxylic acid methyl ester (Bay K 8644) has a similar action. FPL–LTD occludes LTD induced by high-frequency stimulation (HFS–LTD) and requires elevated postsynaptic calcium and retrograde endocannabinoid signaling, properties similar to those of HFS–LTD. In contrast, FPL–LTD does not require the activation of metabotropic glutamate receptors (mGluRs), phospholipase C, or dopamine D2 receptors. FPL–LTD induction also requires afferent stimulation. These findings suggest a scenario in which L-type calcium channel activation is a crucial switch for LTD induction, and mGluRs and D2 receptors can be bypassed if this channel is activated.
Keywords: synaptic plasticity, basal ganglia, dopamine, glutamate, endocannabinoid, presynaptic mechanisms
Introduction
The basal ganglia constitute a complex brain circuit that is involved in sensorimotor learning and cognitive functions (Graybiel, 1995; Bolam et al., 2000). The main input nucleus, the striatum, integrates synaptic information from cortical regions, storing and transforming this input into signals that control goal-directed behaviors and habits (Yin and Knowlton, 2006). Activity-dependent changes in synapses on medium spiny neurons (MSNs), the major striatal projection neurons, are thought to underlie the mechanisms of information storage in this brain region (Bonsi et al., 2003; Lovinger et al., 2003; Fino et al., 2005).
Striatal synaptic plasticity appears to require interaction among multiple neurotransmitters (Lovinger et al., 2003). In the case of striatal long-term synaptic depression (LTD) the activation of D2dopamine receptors is implicated in the induction of plasticity (Kreitzer and Malenka, 2005). However, recent research suggests that the D2 receptor role in LTD induction is mainly modulatory and can be bypassed by the activation of metabotropic glutamate receptors (mGluRs) (Kreitzer and Malenka, 2005). More recently, it has been demonstrated that the activation of D2 receptors facilitates LTD by reducing muscarinic (M1) receptor tone at corticostriatal synapses, thereby facilitating the activation of L-type calcium channels (Wang et al., 2006). mGluRs also participate in LTD induction by stimulating increases in intracellular calcium or enhancing inositol phospholipid turnover in the MSNs themselves (Gubellini et al., 2001; Sung et al., 2001; Ronesi et al., 2004; Sugiura et al., 2006). At present it is not clear whether mGluR activation is necessary for LTD induction or has a modulatory role similar to that of the D2 receptor.
Endocannabinoids are known to have retrograde signaling roles at synapses throughout the brain (for review, see Chevaleyre et al., 2006) and participate in striatal LTD by linking postsynaptic activation to presynaptic expression. Endocannabinoids appear to be released postsynaptically from MSNs (Hillard et al., 1997; Ronesi et al., 2004) and act on presynaptic cannabinoid (CB1) receptors to initiate mechanisms involved in LTD (Gerdeman et al., 2002; Gerdeman and Lovinger, 2003). The signal transduction systems connecting activation of CB1 receptors with the reduction in glutamate release probability are not yet known in detail. Recent work indicates that protein translation is one mechanism linking CB1 activation to the expression of striatal LTD (Yin et al., 2006).
Induction of LTD requires a large increase in postsynaptic [Ca2+]i (Bonsi et al., 2003), possibly for stimulation of endocannabinoid synthesis and release (Gerdeman et al., 2002). Activation of L-type calcium channels has been shown to be pivotal for the induction of LTD (Choi et al., 1997b; Kreitzer and Malenka, 2005; Wang et al., 2006), and the Cav1.3 channel subtype has been implicated in this induction mechanism (Wang et al., 2006). Thus we hypothesized that strong activation of the L-type channel could be sufficient to induce LTD at the corticostriatal synapse. This hypothesis was tested by studying activity-dependent changes in synapses caused by L-type calcium channel agonists. EPSCs were measured in MSNs in the dorsolateral striatum in tissue slices.
Materials and Methods
Striatal slices were prepared from 15- to 19-d-old Sprague Dawley rats. In some experiments the slices were prepared from 16- to 40-d-old mice engineered with bacterial artificial chromosomes such that D1 or D2 dopamine receptor promoters drive enhanced green fluorescent protein (EGFP) expression (D1-GFP and D2-GFP mice, respectively). The animals were anesthetized deeply with halothane (Sigma, St. Louis, MO) and perfused transcardially with 15 ml of ice-cold modified artificial CSF (aCSF) containing the following (in mm): 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 dextrose, saturated with 95% O2/5% CO2. Animals were decapitated, and brains were placed in modified aCSF. Brain tissue was blocked at the anterior and posterior ends and attached with cyanoacrylate to a Teflon pad. The tissue was submerged completely into ice-cold modified aCSF, and 350-μm-thick coronal brain slices containing striatum and cortex were sectioned with a Vibratome series 1000 sectioning system (Technical Products, O'Fallon, MO). Brain slices were allowed to equilibrate for at least 1 h in normal aCSF containing the following (in mm): 124 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 d-glucose, continuously bubbled with a mixture of 95% O2/5% CO2 gas. After 20 min at 35–37°C the heater was turned off, allowing the bath temperature in the preincubation chamber to return slowly to room temperature.
One hemisphere of a striatal brain slice was transferred to a recording chamber and superfused at a constant rate of 2 ml/min with aCSF bubbled with a mixture of 95% O2/5% CO2 gas. The brain slice was viewed with an upright Olympus (Tokyo, Japan) BX50WI microscope with a water-immersion objective (40×; numerical aperture, 0.80; LUMPlan Fl) and a CCD72 camera (MTI Technology, Tustin, CA). Patch pipettes made from thin-walled borosilicate glass (TW150F-4; World Precision Instruments, Sarasota, FL) were pulled to micrometer diameter tips (Model P-97; Sutter Instrument, Novato, CA) with a typical resistance of 2–5 MΩ. Currents were measured in conventional ruptured patch whole-cell mode by using pipettes filled with a solution that consisted of the following (in mm): 120 CsMeSO3, 5 NaCl, 10 TEA-Cl, 10 HEPES, 5 QX-314, 1.1 EGTA, 4 Mg-ATP, and 0.3 Na-GTP, with pH set at 7.2 with CsOH. In a subset of experiments the EGTA was replaced with 20 mm BAPTA. Intracellular solution was filtered, and osmolarity of the intracellular solution and aCSF were set with sucrose to 300 and 314 mmol/kg, respectively.
An Axopatch 200B amplifier (Molecular Devices, Foster City, CA) was used for signal amplification and as a low-pass filter (2 kHz, Bessel filter). The preamplifier head stage and pipette holder were mounted onto a Burleigh PCS-5000 micromanipulator (EXFO Life Sciences Group, Ontario, Canada). A Digidata 1322A (Molecular Devices) interfaced to a PC-compatible computer digitized the signal on line at 10 kHz. pClamp 8.2 software (Molecular Devices) was used for data acquisition.
MSNs were voltage clamped at −50 or −70 mV. To evoke baseline synaptic currents, we delivered paired stimuli with a 50 ms interpulse interval every 20 s via an electrode placed in the overlying white matter. Stimulation was delivered by a Master-8 stimulator and optical stimulus isolation unit (A.M.P.I., Jerusalem, Israel). Stimulus parameters were adjusted to elicit baseline EPSC amplitudes between 200 and 400 pA. Stimulus pulse duration ranged from 0.08 to 0.21 ms, and stimulus intensity ranged between 0.1 and 3 mA. EPSC amplitude was expressed as a percentage of the baseline value. A 10 mV, 5.6 ms pulse was delivered 126 ms after the second of the paired stimuli to monitor series resistance and measure input resistance.
In selected experiments we used a high-frequency stimulation (HFS) protocol, consisting of four 1 s 100 Hz trains delivered every 10 s, paired with depolarization of the postsynaptic cell to 0 mV. In a subset of experiments spontaneous EPSCs (sEPSCs) were measured. Baseline sEPSCs were recorded 5 min after the whole-cell recording configuration was established, after 10 min of 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methylester (FPL64176) application (500 nm; no presynaptic stimulation), and after 20 min of presynaptic stimulation in aCSF. sEPSCs were analyzed by the MiniAnalysis program, version 6.0.3 (Synaptosoft, Decatur, GA).
Most chemicals were purchased from Sigma or Tocris (Ellisville, MO). The L-channel activator FPL64176 was dissolved in ethanol to 25 mm and was diluted further in aCSF to final concentrations of 10, 50, 100, or 500 nm. 1,4-Dihydro-2,6-dimethyl-5-nitro-4-[2(trifluoromethyl)phenyl]pyridine-3-carboxylic acid methyl ester (Bay K 8644) was dissolved in aCSF. The calcium channel blocker nifedipine was dissolved in DMSO shortly before each experiment and used at a concentration of 20 μm. The CB1 receptor blocker N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) was dissolved in DMSO to 50 mm; stock solution was diluted in aCSF containing bovine serum albumin (0.5 g/l) and was used at 3 μm. The mGluR blockers 2-methyl-6-(phenylethynyl)pyridine (MPEP) and 7-(hydroxy-imino) cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) were dissolved in DMSO to 100 mm and were used at 40 μm (MPEP) and 80 μm (CPCCOEt). Sulpiride was dissolved in ethanol to 10 mm and was used at 5 μm. 1-(6-[(17β-Methoxyestra-1,3,5 [10]-trien-17-yl) amino] hexyl)-1H-pyrrole-2,5-dione (U73122) was dissolved to 5 mm in DMSO and was used at 5 μm. Experiments were performed at 30–33°C and were discontinued if the series resistance varied >20% or increased >30 MΩ.
The amplitude of the baseline EPSC was compared with EPSC amplitude 20–25 min after the initiation of drug treatment and was presented as the mean value with 95% confidence interval (CI). EPSC data in time course figures are plotted as the mean EPSC amplitude compared with baseline, with SEM. The paired Student's t test was used for the majority of statistical analyses. Cumulative sEPSC distributions were compared with the Kolmogorov–Smirnov test.
Results
Induction of LTD by L-type channel activation
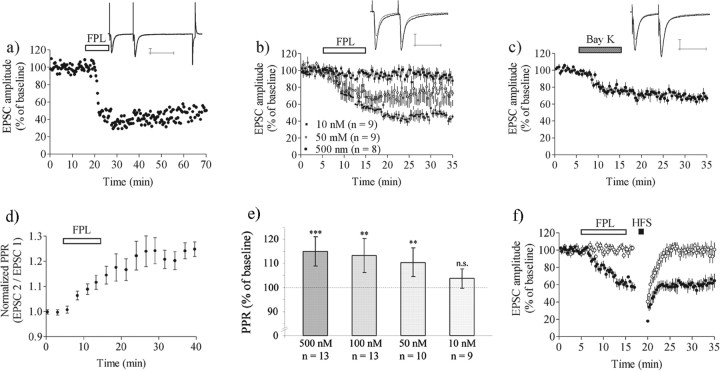
The L-type calcium channel activator FPL64176 was applied for 10 min, with the postsynaptic membrane potential held at −50 mV and afferent stimulation given with paired pulses (50 ms interpulse interval) delivered at 50 mHz (1/20 s). Application of FPL under this condition induced a decrease in EPSC amplitude recorded from striatal MSNs (Fig. 1). FPL concentrations of 500, 100, and 50 nm produced reductions in the EPSC to 44 ± 9.0% (n = 8) (percentage of baseline ± 95% CI), 64 ± 7.1% (n = 10), and 69 ± 9.8% (n = 9) of control level, respectively, whereas 10 nm FPL was without effect (EPSC amplitude, 95 ± 7.6%; n = 9). In previous experiments we have observed that EPSC amplitude is not altered by ethanol at the concentrations used as a vehicle for FPL in this study and thus LTD that is attributable to FPL rather than ethanol actions. A robust decrease (>20%) in EPSC amplitude that persisted for >20 min was detected in 70% (25 of 36) of the cells treated with 500 nm FPL with this protocol, and EPSC data are based on affected MSNs. An example of the long-lasting effect of 500 nm FPL in a single cell is shown in Figure 1a; averaged data from treatment with three different FPL concentrations are shown in Figure 1b. FPL (500 nm) did not affect input resistance as measured from the steady-state response to a 10 mV, 5.6 ms pulse delivered at the end of the stimulus protocol. Responses to hyperpolarization in 12 of 12 cells were indistinguishable under the two conditions. A significant decrease in EPSC amplitude was also observed during perfusion of 1 μm Bay K 8644, another potent L-channel activator (EPSC amplitude, 71 ± 4.7%; n = 9) (Fig. 1c).
Figure 1.
Activation of L-type calcium channels induces LTD in a concentration-dependent manner at the corticostriatal synapse. a–c, Data show EPSC amplitude in MSNs clamped at–50 mV and stimulated every 20 s, with paired pulse stimuli delivered with a 50 ms interpulse interval (50 μm AP-5 and 50 μm picrotoxin present in the bath solution). a, EPSC amplitude in a single-cell example experiment during treatment with 500 nm FPL. FPL–LTD typically was induced after 5 min of drug perfusion. These traces also include the response to the poststimulus hyperpolarizing step used to calculate the relative input resistance. b, Averaged data for slices treated with 500, 50, or 10 nm FPL. c, Averaged data for seven slices treated with 1 μm Bay K 8644. The PPR (EPSC 2/EPSC 1) was increased significantly after FPL treatment. d, PPR expressed as a percentage of pre-drug baseline values for six experiments with a concentration of 500 nm FPL. e, Graph shows PPR values 20–25 min after the onset of the recording. Data are the mean values with 95% CI. ** p < 0.01; *** p < 0.001. f, FPL–LTD occludes HFS–LTD. HFS did not induce LTD when given after the onset of FPL–LTD (n = 6; filled circles), and HFS was similarly ineffective in cells in which FPL did not induce LTD (n = 8; empty circles). EPSC values are the mean ± SEM from responding MSNs. Traces are EPSCs evoked by paired pulses before (black line) and after (gray line) LTD induction. Data are presented as the mean ± SEM; n.s., not significant. Calibration: 100 pA, 50 ms.
A gradual increase in the ratio of the amplitudes of two EPSCs evoked with a 50 ms interpulse interval (paired pulse ratio, PPR) also was observed during FPL application. PPR was significantly larger than control 5–10 min after FPL treatment in 50 nm (110 ± 6.1%; p < 0.01), 100 nm (113 ± 7.2%; p < 0.01), and 500 nm (115 ± 6%; p < 0.001), but not in 10 nm (104 ± 6.9%; n = 9; p > 0.05), FPL groups (Fig. 1e). The PPR also was increased after Bay K treatment (116 ± 5.2%; n = 9; p < 0.001). The time course of the increase in PPR induced by 500 nm FPL is shown in Figure 1d.
The FPL-induced decrease in EPSC amplitude did not require the activation of NMDA receptors or GABAA receptors as antagonists for these receptors [50 μm dl-2-amino-5-phosphonopentanoic acid (AP-5) and 50 μm picrotoxin were present while collecting the data presented in Figure 1a–d]. From these findings it appears that FPL can induce a lasting decrease in excitatory transmission that resembles striatal LTD, which we term FPL–LTD. All subsequent experiments were performed with 500 nm FPL and at a holding potential of −50 mV, unless otherwise stated.
Induction of FPL–LTD occluded subsequent induction of LTD by HFS (EPSC amplitude at t = 25–30, 57 ± 8.7%; n = 6; p < 0.001) (Fig. 1f), and also HFS–LTD was not observed in slices in which FPL failed to induce LTD (EPSC amplitude at t = 25–30, 100 ± 9.7%; n = 8; p > 0.05) (Fig. 1f). In the absence of FPL, HFS–LTD was induced in six of nine cells clamped at −50 mV (EPSC amplitude at t = 25–30, 69 ± 12%; n = 7; p < 0.01).
Molecular mechanisms of FPL–LTD induction
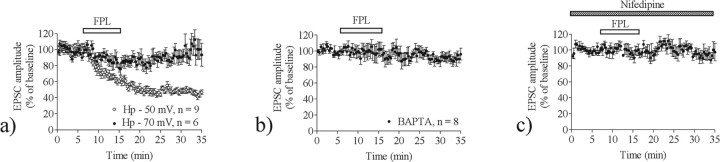
To compare further both FPL–LTD and HFS–LTD, we examined treatments known to block HFS–LTD for their impact on the effect of FPL. We began by examining the involvement of depolarization and calcium channel activation in FPL–LTD. LTD was not induced by FPL when the MSN membrane potential was held at −70 mV. No significant decrease in EPSC amplitude was observed in the presence of FPL even during the drug application period (EPSC amplitude after FPL treatment, 90 ± 11%; n = 7; p > 0.05) (Hp −50 mV vs Hp −70 mV; p < 0.001) (Fig. 2a). Furthermore, FPL was unable to induce LTD when the postsynaptic cell was loaded with 20 mm BAPTA (EPSC amplitude, 95 ± 5.2%; n = 8; p > 0.05) (Fig. 2b). No LTD was detected in slices perfused with nifedipine (20 μm) during FPL treatment (EPSC amplitude, 101 ± 11%; n = 6; p > 0.05) (Fig. 2c). These findings generally support a role for activation of voltage-dependent L-type calcium channels and increased postsynaptic calcium in FPL–LTD.
Figure 2.
FPL–LTD is dependent on increased postsynaptic [Ca2+]i. a, LTD was not induced in cells held at–70 mV. b, No change in synaptic strength was detected during chelation of postsynaptic intracellular calcium with 20 mm BAPTA. c, FPL–LTD was inhibited during incubation with the calcium channel blocker nifedipine (20 μm) (n = 6). EPSC values are the mean ± SEM.
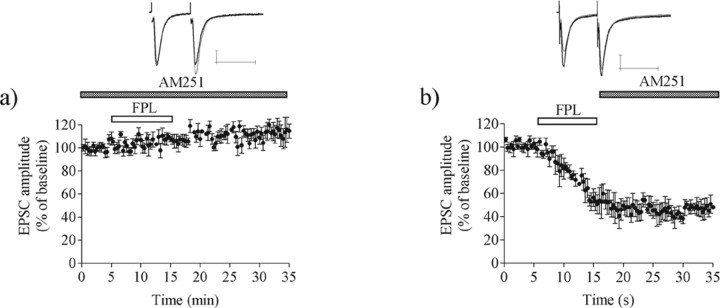
The FPL-induced decrease in EPSC amplitude required the activation of CB1 receptors, because no reduction in transmission was observed in slices perfused with the CB1 receptor antagonist AM251 (3 μm). Indeed, the synaptic response was increased significantly under this condition (EPSC amplitude, 111 ± 6.2%; n = 7; p < 0.001) (Fig. 3a). AM251 did not reverse established LTD when it was applied beginning 10 min after the onset of FPL application (EPSC amplitude, 47 ± 9.2%; n = 5; p < 0.001) (Fig. 3b).
Figure 3.
FPL–LTD induction requires CB1 receptor activation and protein translation. a, LTD was blocked by the CB1 receptor antagonist AM251 (3 μm), suggesting that FPL perfusion induces endocannabinoid release (n = 7). b, Established FPL–LTD was not reversed by blockade with AM251, which is in line with studies from LTD induced by HFS (n = 5). EPSC values are the mean ± SEM. Traces are EPSCs evoked by paired pulses before (black line) and after (gray line) LTD induction. Calibration: 100 pA, 50 ms.
These observations indicate that L-channel activation precedes and stimulates endocannabinoid release and CB1 receptor activation in the course of LTD induction. As is the case for HFS–LTD (Ronesi et al., 2004), FPL–LTD becomes independent of CB1 activation shortly after induction.
Activation of mGluRs, D2Rs, or phospholipase C is not needed for FPL–LTD
Postsynaptic G-protein-coupled receptors implicated in the induction of HFS–LTD do not play a role in FPL–LTD. Activation of mGluRs has been shown to be involved in the induction of LTD at the corticostriatal synapse (Gubellini et al., 2001; Sung et al., 2001), which might be connected to dependence on phospholipase C (PLC) activation and/or IP3 release (Sugiura et al., 2006). Blockade of mGluR group I receptors by MPEP (40 μm) plus CPCCOEt (80 μm) did not inhibit LTD induced by FPL (EPSC amplitude, 55 ± 1.9%; n = 6; p < 0.001) (Fig. 4a). Furthermore, FPL–LTD remained when PLC was blocked by intracellular loading of 5 μm U73122 (EPSC amplitude, 67 ± 6.2%; n = 9; p < 0.001) (Fig. 4b). This concentration of U73122 previously was shown to prevent short-term mGluR and endocannabinoid-dependent synaptic depression induced by D2 receptor (D2R) activation (Yin et al., 2006), indicating that the drug is effective when applied intracellularly to MSNs. FPL also induced LTD in slices treated with the D2R antagonist sulpiride (5 μm) (EPSC amplitude, 54 ± 9.1%; n = 6; p < 0.001) (Fig. 4c), and LTD could be induced in both D1- and D2-expressing mouse MSNs (EPSC amplitude D1-GFP mice, 60 ± 9.7%; n = 7; p < 0.001; EPSC amplitude D2-GFP mice, 63 ± 12%; n = 7; p < 0.001; D1-GFP mice vs D2-GFP mice, p > 0.05) (Fig. 4d). The success rate for FPL–LTD induction did not differ between mouse and rat neurons (14 of 23 vs 25 of 36; χ2 test; p > 0.05). Thus group I mGluRs and D2Rs do not play a role in this form of LTD.
Figure 4.
FPL–LTD does not require activation of group I mGluRs or D2Rs. a, FPL induces LTD in the combined presence of the group I mGluR antagonists MPEP (40 μm) and CPCCOEt (80 μm) (n = 6). b, FPL–LTD remained during intracellular loading with the PLC inhibitor U73122 (5 μm). c, The D2R antagonist sulpiride (5 μm) also did not alter LTD (n = 6). d, FPL–LTD could be induced in both D1-GFP and D2-GFP mice. EPSC values are the mean ± SEM from responding MSNs. Traces are EPSCs evoked by paired pulses before (black line) and after (gray line) LTD induction. Calibration: 100 pA, 50 ms.
FPL–LTD induction requires synaptic activation
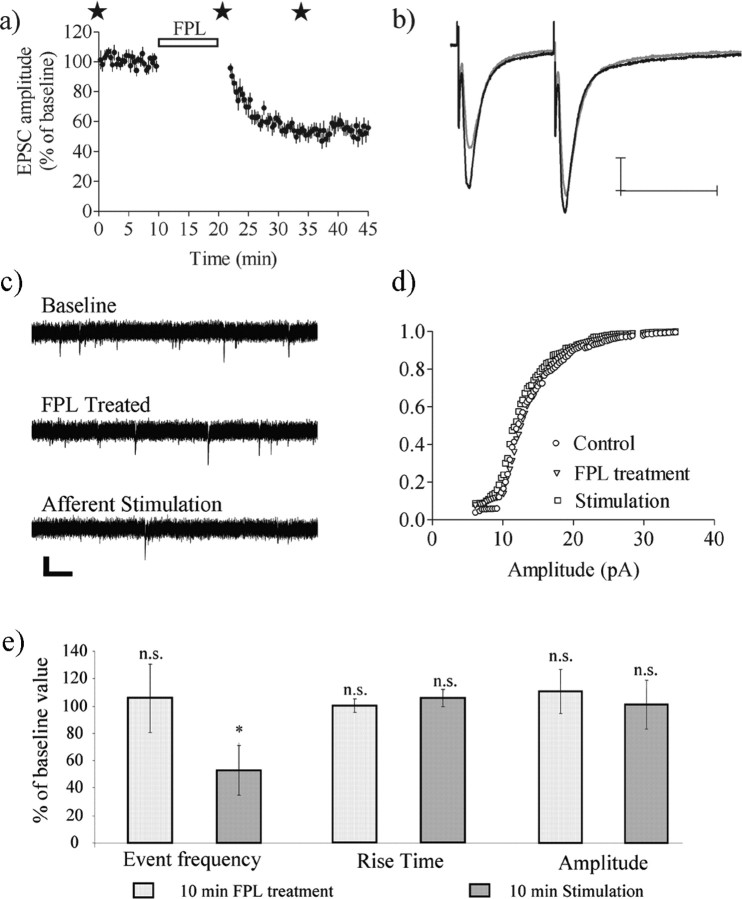
We next tested whether FPL–LTD was dependent on coincident synaptic activation by completely interrupting afferent stimulation during FPL treatment. After a stable baseline EPSC amplitude was achieved, stimulation was stopped during the 10 min FPL application and was resumed 2 min after the wash was begun with drug-free aCSF. The EPSC amplitude was not affected significantly by this treatment just after presynaptic stimulation was resumed (96 ± 5.7%; n = 6; p > 0.05), but a large depression was established within the first 10 min after the resumption of stimulation (EPSC amplitude, 60 ± 9.3%; n = 6; p < 0.001) (Fig. 5a). In the absence of FPL the EPSC amplitude was not affected significantly by the pause in stimulation (EPSC amplitude just after stimulation resumed, 113 ± 20%; n = 6; p > 0.05; EPSC amplitude after 20–25 min stimulation, 98 ± 12%; n = 6; p > 0.05).
Figure 5.
FPL–LTD requires afferent stimulation. a, EPSC amplitude was at control level when afferent activation was stopped during the 10 min FPL treatment but rapidly decreased as stimulation continued (n = 6). Stars mark the time points at which sEPSCs were sampled. EPSC amplitude values are the mean ± SEM. b, Traces are EPSCs evoked by paired pulses at baseline (black line) and after 15 min of afferent activation (gray line). Calibration: 100 pA, 50 ms. c, Representative traces showing sEPSCs before FPL treatment, just after treatment but before afferent stimulation, and after a subsequent 10 min period of afferent stimulation. sEPSCs were not altered after a 10 min application of FPL, but after a subsequent 10 min presynaptic stimulation period the frequency of events was reduced significantly (c, e). e, sEPSC rise time was unaffected by FPL, and the sEPSC amplitude assessed by mean amplitude and cumulative probability distribution analyses also was not altered by FPL (d, e). Graphs show the mean values with 95% CI. Data are based on seven recordings and are compared with a paired Student's t test; *p < 0.05. n.s., Not significant.
The dependence on synaptic activation was supported additionally by the examination of sEPSCs measured at the time points marked with stars in Figure 5a. Event frequency was not altered significantly just after the 10 min FPL application (99 ± 27%; n = 8; p > 0.05) but was reduced significantly after the subsequent 20 min of afferent stimulation (51 ± 17%; n = 8; p < 0.05) (Fig. 5c,e). The sEPSC amplitude was not altered by FPL treatment plus synaptic activation as assessed by the lack of change in mean amplitude and the lack of change in the cumulative amplitude distribution (p > 0.05; Kolmogorov–Smirnov test) (Fig. 5d,e). In addition, the rise time and half-decay time of sEPSCs were not altered at any time point during the experiment (Fig. 5e). These findings support the idea that FPL cannot induce LTD in the absence of afferent stimulation/synaptic activation.
Discussion
LTD induced by FPL64176 treatment at the corticostriatal synapse
Our findings support the idea that activation of L-type calcium channels is an important switch for the induction of LTD. Strong activation of these channels in combination with afferent stimulation is sufficient to produce a long-lasting decrease in synaptic strength at striatal excitatory synapses. FPL treatment did not affect EPSC amplitude during the application of nifedipine or when MSNs were clamped at −70 mV or were loaded with 20 mm BAPTA, clearly showing the dependence on increased postsynaptic [Ca2+]i. The need for an increase in postsynaptic [Ca2+]i also has been described for HFS–LTD (Calabresi et al., 1992b; Gerdeman et al., 2002). Furthermore, both FPL–LTD and HFS–LTD are blocked by CB1 antagonists, but only if perfusion of the blocker is initiated before LTD onset. FPL–LTD occluded HFS–LTD, and in slices in which FPL failed to induce LTD the HFS was also ineffective. Analysis of paired pulse responses and sEPSC frequency both indicate that FPL–LTD expression involves a decrease in neurotransmitter release, as previously shown for HFS–LTD in striatum (Choi and Lovinger, 1997a,b). Thus the mechanisms for endocannabinoid-mediated induction and maintained expression of these two types of LTD are likely the same (Gerdeman et al., 2002; Gerdeman and Lovinger, 2003).
The Cav1.3 channel is most likely the L-channel subtype that participates in striatal LTD induction. Previous results indicate that blockade of striatal LTD requires high concentrations of dihydropyridine antagonists, which is consistent with the known pharmacological profile of this receptor (Surmeier et al., 1994). Induction of LTD by mGluR agonists requires depolarization to membrane potentials in which Cav1.3 is active, but Cav1.2, the other major L-type channel in MSNs, is not activated (Kreitzer and Malenka, 2005; Yin et al., 2006). Likewise, the FPL-induced LTD shown at present requires only modest postsynaptic depolarization. Finally, Wang and colleagues (2006) have shown that striatal LTD is lost in Cav1.3 knock-out mice. Thus a major role for this L-channel subtype in striatal LTD is indicated.
In contrast to L-type channels, the activation of other molecules that increase intracellular calcium does not induce LTD. Activation of NMDA receptors produces increased intracellular calcium in dendritic spines (Carter and Sabatini, 2004). However, this receptor participates in the induction of striatal long-term potentiation (LTP) (Calabresi et al., 1992a; Partridge et al., 2000; Kerr and Wickens, 2001), and the majority of evidence indicates that NMDAR activation is not necessary for LTD induction in striatum. Calcium-permeable AMPA receptors appear to be present on MSN dendritic spines (Carter and Sabatini, 2004), but it is unlikely that the activation of these receptors provides a large source of calcium for LTD induction, because LTD is not induced readily at membrane potentials near the resting potential (Calabresi et al., 1992a; Choi et al., 1997b) despite the fact that calcium influx through AMPA receptors would be strong at these potentials. Stimulation of postsynaptic intracellular calcium rises by mGluR activation of calcium store-mediated release also appears to be insufficient for LTD induction, because LTD induced by group I mGluR activation requires membrane depolarization and L-type calcium channel activation (Kreitzer and Malenka, 2005).
It is not yet clear what accounts for the specificity with which L-type channels provide the calcium influx needed for LTD induction. The Cav1.3 channel is presented in dendritic spines (Day et al., 2006) along with other calcium-permeable channels such as AMPA and NMDA receptors (Carter and Sabatini, 2004). Thus localization in the spine is not sufficient to explain the specific L-channel role. One interesting clue comes from studies indicating that the C-terminal region of Cav1.3a binds to Shank proteins (Zhang et al., 2005). Shank, in turn, binds to Homer isoforms known to be linked to mGluRs (Tu et al., 1999) and could bring IP3-regulated intracellular calcium stores in close proximity to the Cav1.3 channels (Xiao et al., 2000; Olson et al., 2005). The strategic position of Cav1.3 thus may be crucial for efficient activation of Ca2+-dependent modulatory cascades involved in LTD induction (Olson et al., 2005). The 2-arachidonoylglycerol (2-AG) synthetic enzyme diacylglycerol lipase also is located in the perisynaptic region of MSN dendritic spines near mGluR5 (Uchigashima et al., 2007), although it must be noted that the role of 2-AG in LTD is still unclear (Ade and Lovinger, 2007; Kreitzer and Malenka, 2007). Thus Cav1.3 may be part of a signaling complex within the dendritic spine that links mGluRs and other molecules to endocannabinoid production. The relative timing and magnitude of the dendritic spine calcium transients and the interactions with membrane potential also may determine whether LTD is induced. L-type calcium channels appear to have a strong role in depolarization-induced calcium transients in dendritic MSNs evoked at −50 mV, and these transients are relatively rapid (Carter and Sabatini, 2004). Synaptic activation elicits calcium transients mediated by both AMPA and NMDA receptors, and those mediated by NMDARs can be large and prolonged when evoked at depolarized potentials (Carter and Sabatini, 2004). It is likely that the relative contributions of voltage-gated and ligand-activated channels and the magnitude and time course of the resultant calcium transients determine whether LTD or LTP will be elicited at a given MSN excitatory synapse.
FPL was able to induce LTD in slices perfused with MPEP and CPCCOEt, demonstrating that group I mGluR activation is not necessary for LTD during strong activation of L-type calcium channels. It is clear that HFS–LTD involves group I mGluR activation and that activation of these mGluRs can induce LTD when paired with slight postsynaptic depolarization (Gubellini et al., 2001; Sung et al., 2001; Kreitzer and Malenka, 2005; Yin et al., 2006). However, it appears that activation of L-type calcium channels is necessary for LTD induced by mGluR agonists (Kreitzer and Malenka, 2005). Thus mGluRs act either upstream of or in conjunction with L-channels in LTD induction. In this context it also should be noted that striatal LTD induced by sustained 10 Hz stimulation does not involve mGluR activation, but it is reduced in magnitude by L-channel blockade (Ronesi and Lovinger, 2005). One possible role for group I mGluRs may be stimulation of PLC activity that contributes to endocannabinoid production (Hashimotodani et al., 2005). Because FPL–LTD was induced during blockade of PLC with U73122, our data suggest that the dependence of PLC activation becomes redundant during periods of high postsynaptic [Ca2+]i, because endocannabinoid production may be stimulated sufficiently by calcium alone (Chevaleyre et al., 2006). Recent studies have suggested that the endocannabinoid anandamide participates in the induction of striatal LTD (Ade and Lovinger, 2007; Kreitzer and Malenka, 2007). Anandamide synthesis can be stimulated by increased intracellular calcium via molecular steps that do not involve PLC (Di Marzo et al., 1994). Thus calcium stimulation of anandamide production is one mechanism that could underlie FPL–LTD induction. However, alternative pathways for 2-AG synthesis that do not involve PLC also should be considered in this context (Sugiura et al., 2006).
LTD also was induced by FPL in slices perfused with sulpiride, indicating that the activation of D2 receptors can be bypassed during direct L-channel activation. This finding supports data from Kreitzer and Malenka (2005) and Wang and colleagues (2006), suggesting that D2 receptor activation plays a modulatory role in LTD induction. We observed robust FPL–LTD in presumed striatonigral (D1-expressing) and presumed striatopallidal (D2-expressing) neurons examined in the D1-GFP and D2-GFP mice. This finding indicates that the presence of postsynaptic D2 receptors is not required for this form of LTD. A D2 receptor-mediated decrease in cholinergic inhibitory tone appears to facilitate L-type calcium channel activation during induction of HFS–LTD (Wang et al., 2006), although postsynaptic D2 receptors also may play a role in HFS–LTD (Kreitzer and Malenka, 2007).
LTD induction resulted in a significant change in the PPR, which supports presynaptic expression and a role for decreased release probability in LTD expression. The magnitude of the change in PPR during LTD expression was similar to what previously was reported from our laboratory (Gerdeman et al., 2002; Ronesi et al., 2004). Other groups have reported that only a trend in PPR change can be detected (Kreitzer and Malenka, 2005), which might be connected to the baseline PPR. If the initial release probability is sufficiently high, small changes in the probability of release might not be detected as a change in PPR (Atzori et al., 2001). We also observed decreased sEPSC frequency, but not amplitude, associated with the expression of FPL–LTD, which supports a presynaptic locus for LTD expression.
LTD induction requires synaptic activation
FPL did not induce LTD if presynaptic afferent stimulation was stopped during drug application. In addition, monitoring of spontaneous activity confirmed that afferent activation was required for the FPL-induced reduction in sEPSC frequency. Afferent stimulation is also necessary for synaptic depression induced by the muscarinic receptor antagonist pirenzepine (Wang et al., 2006). Similar to data presented here, EPSC amplitude in the Wang and colleagues (2006) study was at the control level when stimulation resumed and then decreased with subsequent afferent activation. A decreased muscarinic tone facilitates L-type calcium channel activation; thus it is possible that similar mechanisms are engaged by synaptic activation for both treatments.
At present there are two prominent potential roles for synaptic activation. First, synaptic activation might be required for some postsynaptic process, such as endocannabinoid production or release. Second, synaptic activation might produce a presynaptic effect that synergizes with CB1 activation to produce LTD. At present we cannot distinguish fully between these two possibilities. Afferent stimulation might be required to increase postsynaptic calcium levels and enable endocannabinoid production. Synaptic activation also may have some role in activating the endocannabinoid transporter or otherwise “mobilizing” endocannabinoids in a manner similar to that suggested by Alger and coworkers (Edwards et al., 2006). Evidence from synapses elsewhere in the brain suggests that presynaptic CB1 activation synergizes with other presynaptic mechanisms in LTD induction (Sjostrom et al., 2003). Thus another role for afferent stimulation might be to provide such a synergistic presynaptic signal, as we have suggested previously (Ronesi et al., 2004). In line with this hypothesis is a new study by Malenka and coworkers (Singla et al., 2007), showing that afferent activation is required for striatal LTD induced by the CB1 agonist R-(+)-(2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrol[1,2,3-de]-1,4-benzoxazin-6-yl)(1-naphthalenyl) methanone mesylate (WIN 55,212-2). This study suggests that the need for presynaptic activation is related to a requirement for increased calcium levels in the presynaptic terminal. Additional studies will be needed to determine what other factors induced by afferent activation are necessary for endocannabinoid production and release and for LTD induction.
Conclusion
The observations described at present show that the activation of L-type calcium channels and the subsequent postsynaptic [Ca2+]i increase likely serve as a “molecular switch” in the induction of striatal LTD. Other mechanisms implicated in LTD induction, such as D2 and mGluR activation, may serve to modulate or synergize with L-channel function. Pharmacological or molecular manipulations aimed at altering the function of L-type channels, the Cav1.3 channel in particular, are thus attractive candidates for manipulation of striatum-based synaptic plasticity, learning, and memory, as well as neurological disorders that involve an LTD-like process (Calabresi et al., 1992b; Ding et al., 2006).
Footnotes
This work was supported by the Division of Intramural Clinical and Basic Research, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. We acknowledge Dr. Henry Yin for providing training and help in initiating the first set of experiments and Dr. Sukwoo Choi for stimulating ideas about L-channels in LTP induction.
References
- Ade K, Lovinger D. Anandamide regulates developmental changes in long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci. 2001;4:1230–1237. doi: 10.1038/nn760. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Pisani A, Bernardi G, Calabresi P. Stimulus frequency, calcium levels and striatal synaptic plasticity. NeuroReport. 2003;14:419–422. doi: 10.1097/00001756-200303030-00024. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci. 1992a;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992b;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA. 1997a;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. J Neurosci. 1997b;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, Surmeier DJ. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, Conquet F, Calabresi P. Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology. 2001;40:839–846. doi: 10.1016/s0028-3908(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann NY Acad Sci. 2003;1003:226–240. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. J Physiol. 2005;562:245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology, and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol. 2001;86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Seno N, Kitai ST. Acutely isolated neurons of the rat globus pallidus exhibit four types of high-voltage-activated Ca2+ current. J Neurophysiol. 1994;71:1272–1280. doi: 10.1152/jn.1994.71.3.1272. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Xu F, Fang X, Tang T-S, Tkatch T, Surmeier DJ, Bezprozvanny I. Association of Cav1.3 L-type calcium channels with Shank. J Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]