Abstract
Drugs activating group III metabotropic glutamate receptors (mGluRs) represent therapeutic alternatives to l-DOPA (l-3,4-dihydroxyphenylalanine) for the treatment of Parkinson's disease (PD). Their presynaptic location at GABAergic and glutamatergic synapses within basal ganglia nuclei provide a critical target to reduce abnormal activities associated with PD. The effects of selective group III mGluR agonists (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) and l-(+)-2-amino-4-phosphonobutyric acid (l-AP4) infused into the globus pallidus (GP) or the substantia nigra pars reticulata (SNr) were thus studied in rat models of PD. Bilateral infusions of ACPT-I (1, 2.5, and 5 nmol/μl) into the GP fully reverse the severe akinetic deficits produced by 6-hydroxydopamine nigrostriatal dopamine lesions in a reaction-time task without affecting the performance of controls. Similar results were observed after l-AP4 (1 nmol) or picrotoxin, a GABAA receptor antagonist, infused into the GP. In addition, intrapallidal ACPT-I counteracts haloperidol-induced catalepsy. This effect is reversed by concomitant administration of a selective group III receptor antagonist (RS)-α-cyclopropyl-4-phosphonophenylglycine. In contrast, ACPT-I (0.05, 0.1, and 0.25 nmol) infusions into the SNr enhance the lesion-induced akinetic deficits in control and lesioned rats and do not reverse haloperidol-induced catalepsy. l-AP4 (0.05 nmol) and picrotoxin in the SNr produce the same effects. Together, these results show that activation of group III mGluRs in the GP provides benefits in parkinsonian rats, presumably by modulating GABAergic neurotransmission. The opposite effects produced by group III mGluR activation in the SNr, also observed with a selective mGluR8 agonist, support the use of subtype-selective group III mGluR agonists as a potential antiparkinsonian strategy.
Keywords: metabotropic glutamate receptor, 6-hydroxydopamine, Parkinson's disease, GABAA receptor, substantia nigra, globus pallidus
Introduction
The progressive loss of midbrain dopamine (DA) neurons is a primary cause of the hypokinetic symptoms (akinesia and bradykinesia) of Parkinson's disease (PD). The DA denervation of the striatum leads to an imbalance between dopaminergic and glutamatergic transmission in the basal ganglia such that excessive activity in basal ganglia outflow disrupts motor control. The classical l-3,4-dihydroxyphenylalanine (l-DOPA) therapy of PD provides dramatic palliative benefit but leads to motor complication, particularly dyskinesia, after prolonged treatment (Jankovic, 2002). This led to the development of novel pharmacological therapeutic targets that bypass the DA system, among which glutamatergic drugs have previously been highlighted (Conn et al., 2005).
Because of their modulatory action on glutamate neurotransmission, the metabotropic glutamate receptors (mGluRs) previously received much attention as potential targets in the treatment of neurodegenerative and psychiatric disorders (Rouse et al., 2000; Conn et al., 2005). Based on primary sequence, second messenger coupling and pharmacological profiles, mGluRs are classified into three subgroups: group I (mGluR1, mGluR5), group II (mGluR2, mGluR3), and group III (mGluR4, mGluR6, mGluR7, and mGluR8) (Conn and Pin, 1997). In animal models of PD, blockade of group I or activation of group II mGluRs produces beneficial effects on parkinsonian symptoms by reducing the aberrant firing activity in the basal ganglia (BG) circuitry (Feeley Kearney and Albin, 2003; Senkowska and Ossowska, 2003; Conn et al., 2005). Comparatively, less is known about the therapeutic potential of group III mGluR agonists in animal models of the disease. Three of the four group III mGluRs (mGluR4, mGluR7, and mGluR8) are presynaptically localized on glutamatergic and GABAergic nerve terminals in key structures of the BG: striatum, globus pallidus (GP), or the substantia nigra pars reticulata (SNr) (Bradley et al., 1999b; Kosinski et al., 1999; Corti et al., 2002). Electrophysiological studies conducted in brain slices elegantly demonstrated that the group III mGluR-specific agonist l-(+)-2-amino-4-phosphonobutyric acid (l-AP4) modulates inhibitory and excitatory transmission in the GP and the SNr (Wittmann et al., 2001; Marino et al., 2003; Matsui and Kita, 2003; Valenti et al., 2003). Group III mGluR agonists have antiparkinsonian actions in reserpine or haloperidol-treated rats (Marino et al., 2003; Valenti et al., 2003; MacInnes et al., 2004; Konieczny et al., 2007) and in unilateral 6-hydroxydopamine (6-OHDA)-lesioned rats by improving forelimb asymmetry and producing contraversive rotation (Kearney et al., 1998; Zhang and Albin, 2000; Valenti et al., 2003). However, the ability of group III mGluR agonists to provide functional relief in the early symptomatic stage of PD remains to be investigated. We showed previously that 6-OHDA-induced lesions of the dorsolateral part of the striatum, reproducing the prominent denervation of the dorsal and caudal putamen in early PD, produce consistent deficits in a reaction-time (RT) task in rats that are related to the akinesia observed in PD patients (Amalric and Koob, 1987; Amalric et al., 1995). Blockade of mGluR5 with the selective antagonist 2-methyl-6-(phenylethynyl)-pyridine was found previously to reverse 6-OHDA-induced akinesia in the reaction-time task (Breysse et al., 2002). The effects of intracerebral administration into the GP or the SNr of group III mGluR agonists (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) (Acher et al., 1997; Schann et al., 2006) and l-AP4 were thus tested in the same model of early PD and against haloperidol-induced catalepsy.
Materials and Methods
Animals
Male Wistar rats (Charles River, L'Arbresle, France), weighing 175–185 g at the beginning of the experiment, were housed in groups of two per cage and maintained in temperature-controlled conditions with a 12 h light/dark cycle (lights off from 7:00 A.M. to 7:00 P.M.). Rats tested in the RT task were fed 15–17 g/d of laboratory chow, delivered 3 h after testing period, so as to maintain 85% ad libitum feeding body weight. Water was provided ad libitum. For the catalepsy experiment, water and food were provided ad libitum. All procedures were conducted in accordance with the requirements of the French Ministère de l'Agriculture et de la Pêche Décret No. 87–848 (October 19, 1987) and the European Communities council directive of November 24, 1986 (86/609/EEC).
Behavioral procedure
Catalepsy test.
Catalepsy was assessed using the bar test (a metal rod positioned 9 cm above the floor). The time elapsing before the rat climbed down from the bar was recorded in seconds (with a cutoff time of 120 s) each 10 min for the 1 h of testing.
Reaction-time task.
Eight operant boxes (Campden Instruments, Cambridge, UK) were used for the RT task. Each box was equipped with a retractable lever, a food magazine and a cue light (2.8 W bulb) located above the lever corresponding to the response signal. The lever required a force of 0.7 N for switch closure. A house light located on the ceiling was turned on at the beginning of the testing session. Each box was placed in a wooden sound-attenuating cabinet that was ventilated by a low-level noise fan to reduce outside noise. Food restriction was introduced at the beginning of training period. Rats were trained daily for 3 months to quickly release the lever after the visual cue onset presented after four randomly and equiprobably generated foreperiods (0.5, 0.75, 1.0, or 1.25 s). To be rewarded by a 45 mg food pellet (P. J. Noyes, Lancaster, NH), the rat was required to release the lever with RTs <600 ms. RTs were measured in milliseconds as the time elapsing from the response signal onset to lever release. Performance was measured by recording the number of correct and incorrect (nonrewarded) responses as either “premature,” corresponding to early withdrawal of the lever (before the onset of the response signal), or “delayed,” when the lever was released after the 600 ms time limit. Each daily session ended after 100 trials. Premature responses were recorded independently and were not limited. At the end of training period, rats were tested for 7 consecutive days in the RT task to measure preoperative baseline values before surgery. After a 7 d recovery period, postoperative performance was recorded daily until day 60 postsurgery.
Surgery
The animals were anesthetized by systemic injection of xylazine (15 mg/kg) and ketamine (100 mg/kg) and placed in a stereotaxic instrument (David Kopf, Tujunga, CA) with the incisor bar positioned −3.0 mm under the interaural line for surgical procedures based on the stereotaxic coordinates (Paxinos and Watson, 2005). Lesioned animals received bilateral injections of 6-hydroxydopamine hydrochloride (Sigma, Lyon, France) (4 μg/μl, 3 μl per side) in the dorsolateral striatum at the following coordinates: anteroposterior (AP), −0.2 mm; lateral (L), ±3.5 mm; and dorsoventral (DV), −4.8 mm according to bregma. The sham control group received the vehicle solution (ascorbic acid, 0.1% solution) at the same coordinates. Injections were performed with a micropump set at a flow rate of 0.33 μl/min, as described in the injection procedure section. In addition to 6-OHDA or vehicle injections, all animals were implanted with 10 mm bilateral stainless-steel guide cannulas (23 gauge) positioned 3 mm above the injection site in the GP or the SNr at the following coordinates: AP, −0.92 mm; L, ±3.0 mm; and DV, −4 mm from bregma for GP; and AP, −5.5 mm; L, ±2.2 mm; and DV, −5.4 mm from bregma for SNr. The guide cannulas were then anchored to the skull with four stainless-steel screws and dental cement. Stainless-steel wire inlet cannulas (10 mm) were placed inside to prevent occlusion.
Injection procedure
The bilateral intrapallidal or intranigral injections were performed with stainless-steel injector needles (13 mm, 30 gauge) inserted inside the implanted guide cannulas and fitted so that they protruded 3 mm below, into the GP or the SNr area. The injectors were connected via a polyethylene catheter (Tygon; 0.25 mm, i.d.) to Hamilton microsyringes (10 μl) fitted to a micropump (CMA/100; CMA/Microdialysis, Stockholm, Sweden). The flow delivered by the pump was set at 0.166 μl/min for a volume of 0.5 μl/side. At the end of injection, injector needles were left in place for 3 more minutes to allow the diffusion of the solution. Immediately afterward, inlet cannulas were replaced and the performance in the RT task was recorded.
Drugs
6-Hydroxydopamine hydrochloride (Sigma) was dissolved in ascorbic acid solution (0.1 mg/ml in 0.9% saline) to prevent oxidation. The mixed D1/D2 dopaminergic receptor antagonist haloperidol (haldol injectable solution, 5 mg/ml; Janssen, Boulogne, France) was dissolved in physiological 0.9% saline solution and injected systemically at a dose of 1 mg/kg. ACPT-I (Acher et al., 1997; Schann et al., 2006) and l-AP4 (Ascent Scientific, Weston-Super-Mare, UK) were freshly dissolved in distilled water and artificial CSF, respectively. (S)-3,4-Dicarboxyphenylglycine (DCPG; Ascent Scientific) (Thomas et al., 2001) was dissolved in artificial CSF solution. (R,S)-α-Cyclopropyl-4-phosphonophenylglycine (CPPG; Tocris, Bristol, UK) (Toms et al., 1996) and picrotoxin (Sigma) were dissolved in 0.9% saline solution. All solutions were adjusted to pH 7 with NaOH 0.1N.
Experimental procedure
Catalepsy test.
Male Wistar rats (n = 94; 280–300 gm; Charles River) were implanted with guide cannulas bilaterally above the GP or SNr at the same coordinates used for the RT experiment. After a recovery period, haloperidol (1 mg/kg) was administered 85 min before ACPT-I (GP: 0, 1, 2.5 nmol/μl; SNr: 0, 0.25, 1 nmol/μl; n = 5–7 per group) and the animals were tested in the horizontal bar test. A control group (n = 9) received a combination of systemic and intracerebral injections of vehicle solutions. Catalepsy was measured immediately after ACPT-I (or vehicle) central administration and every 10 min for the 1 h test. Additional experiments were conducted to test the effect of ACPT-I in the SNr (0, 0.1, 0.25, 1 nmol/μl; n = 5–7 per group) in a control group with no haloperidol. To control the selectivity of ACPT-I, the group III mGluR antagonist CPPG or vehicle was coadministered 10 min before ACPT-I into the GP (n = 7 per group) or SNr (n = 5–7 per group). Doses of CPPG, 10-fold higher than ACPT-1, were selected on the basis of a previous study (Palucha et al., 2004). Considering the similar affinity of ACPT-I for mGluR4 and mGluR8 subtypes (supplemental Fig. 1, available at www.jneurosci.org as supplemental material), we further tested the behavioral effects of a highly potent mGluR8-selective agonist, DCPG, injected in the GP (n = 6) and SNr (n = 7 per group) in another group of rats.
Reaction-time task.
Sham-operated (n = 39) and dopamine-depleted rats (6-OHDA; n = 77) were tested in the RT task. To familiarize the animals to the intracerebral injection procedure, each of them received a vehicle injection into the GP (n = 62) or the SNr (n = 54) at day 18 after surgery. Sham and 6-OHDA animals were divided into subgroups receiving four doses of ACPT-I (0, 1, 2.5, 5 nmol/μl for GP and 0, 0.05, 0.1, 0.25 nmol/μl for SNr) in a pseudorandom order following a Latin-square design at postsurgical days 22, 26, 30, and 34. Lower doses of ACPT-I injected into the GP (0, 0.1, 0.2, and 0.3 nmol/μl) were tested in an additional group of rats. After a washout period of 2 weeks, at postoperative day 50, sham and lesioned animals selected from the above-mentioned groups were injected into the GP or the SNr with the GABAA receptor antagonist picrotoxin (0.165 or 0.033 nmol/μl for the GP and SNr, respectively). Another group of animals received picrotoxin at postoperative day 26 as a first injection to verify the effects of manipulating GABA transmission in sham and 6-OHDA-lesioned animals on RT performance. Because the main effects of picrotoxin were very similar in the two experimental conditions (e.g., first injection at day 26 or after ACPT-I treatment at day 50), the performance of the animals was pooled into a single experimental group. To compare the effects of ACPT-I to a classical group III mGluR agonist, additional experiments were conducted with l-AP4, which displays a similar profile of activity on all group III mGluRs as ACPT-I (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). l-AP4 was injected at day 26 after surgery at the dose of 1 and 0.05 nmol/μl for the GP and the SNr, respectively.
Data and statistical analysis
Catalepsy test.
Data were analyzed by using a multiple Kruskal–Wallis H test. The median latency was calculated for each dose and for each 10 min period. Individual comparisons were performed using the nonparametric Mann–Whitney U test.
Reaction-time task.
The effects of dopamine depletion and ACPT-I injections on RT performance were evaluated on each variable (i.e., number of correct, delayed, premature responses and mean RTs) averaged across each session. Because the premature responses were not limited in the RT task paradigm, few animals (n = 5) exhibiting >100 premature responses/session were considered as outliers and excluded from the analysis of this parameter. For preoperative and postoperative conditions, we used four consecutive sessions before surgery [preoperative (Pre)] and the four sessions corresponding to the performance measured on the days before injection [postoperative (Post); e.g., days 21, 25, 29, and 33]. Because there was no difference between the four sessions either in the Pre or the Post conditions for any variable, the performance averaged over four sessions was thus compared with that measured after ACPT-I or vehicle injection into the GP or the SNr. We first analyzed the effect of the order of injections between the different groups tested. The subjects that did not receive all of the injections within a given order according to the Latin-square design (loss of cannula for example) were excluded from statistical analysis. If no effect of order was found, the animals were pooled into one group. Data were then submitted to a mixed-design ANOVA with different group (sham vs 6-OHDA) as the between-subject factor and condition (Pre, Post, vehicle, dose 1, dose 2, and dose 3 of ACPT-I) as the within-subject factor, as appropriate. In a similar manner, the effects of picrotoxin and l-AP4 were compared with one preoperative (the day before surgery) and postoperative session. Post hoc multiple comparisons between groups were made using simple main effects analysis and Fisher's protected least significant difference (PLSD) test, as appropriate.
Histology
At completion of behavioral testing, rats were deeply anesthetized and killed by decapitation and brains were stored at −80°C until cryostat sectioning. Brains coronal 10 μm sections were collected (−20°C) at the level of the striatum, GP, and SNr using a cryostat apparatus (CM3050; Leica, Nussloch, Germany). GP and SNr sections were stained with cresyl violet to check the accuracy of injection sites. Only rats showing the appropriate injection sites were used for data analysis. The extent of 6-OHDA lesions was verified by autoradiographic labeling of DA uptake sites at striatal level using [3H]mazindol as a ligand in randomly selected subjects of each group. Binding of [3H]mazindol was measured according to the procedure described previously (Javitch et al., 1985). Briefly, sections were first air-dried and rinsed for 5 min at 4°C in 50 mm Tris buffer with 120 mm NaCl and 5 mm KCl. Then, the sections were incubated for 40 min with 15 nm [3H]mazindol (specific activity 17 Ci/mm; DuPont NEN, Boston, MA) in 50 mm Tris buffer containing 300 mm NaCl and 5 mm KCl added with 0.3 mm desipramine to block the noradrenaline transporter. The possible unspecific binding was determined by incubating some sections in the same solution plus 30 mm benztropine. Sections were rinsed twice for 3 min in the incubation medium without mazindol and for 10 s in distilled water and were air dried. Finally, the sections were left in contact for 21 d to a specific 3H-sensitive film screen (Raytest, Courbevoie, France) to generate autoradiographs.
Results
Histology
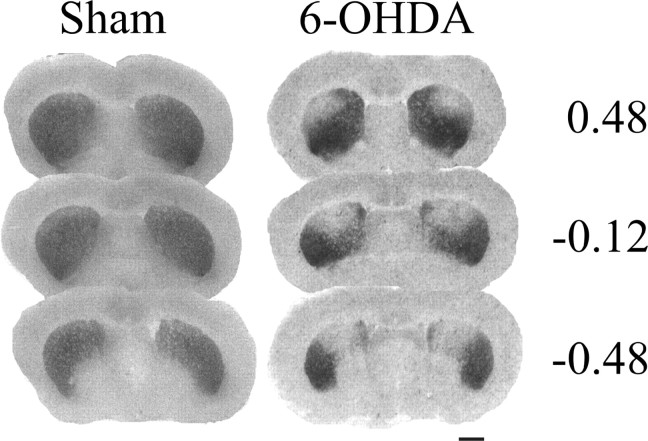
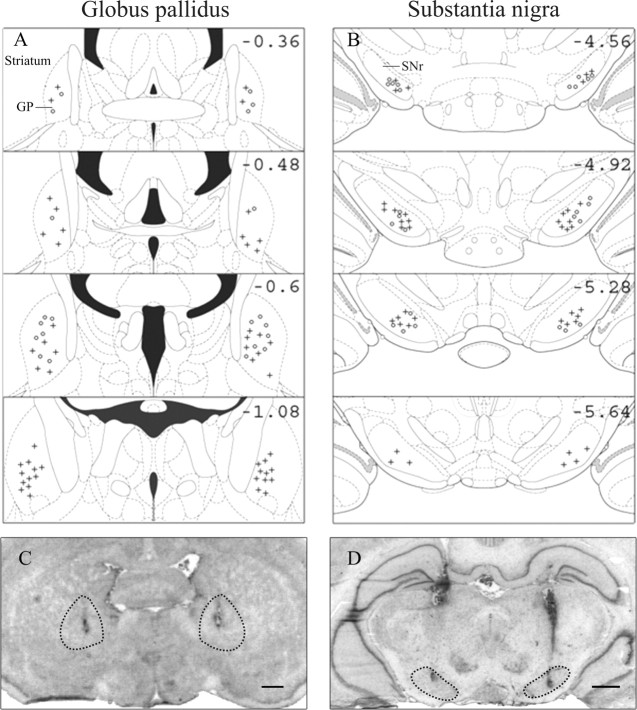
The binding of [3H]mazindol to dopamine uptake sites in the striatum as determined on coronal sections was used to delineate the extent of dopamine depletion induced by bilateral striatal 6-OHDA injections (Fig. 1). The dopamine lesions were consistently restricted to the dorsolateral part of the striatum at the rostral level and extended more ventrally at more caudal levels (end of the anterior commissure). As reported previously, striatal 6-OHDA infusions induce a 60% decrease in [3H]mazindol labeling in the dorsal area of the striatum compared with sham-operated animals (Turle-Lorenzo et al., 2006), which fits with the decrease of endogenous striatal DA contents assessed by HPLC (Amalric et al., 1995). Figure 2, A and B, shows schematic reconstructions of correct injection sites in the GP and the SNr, and C and D show the locations of injector needles on frontal sections of representative subjects. Subjects with incorrect injection sites located outside the GP (n = 10) or the SNr (n = 7) were excluded from statistical analysis.
Figure 1.
Binding of [3H]mazindol to dopamine uptake sites in the striatum. Photomicrographs comparing [3H]mazindol labeling in striatal sections from a sham-operated animal and a bilaterally 6-OHDA-lesioned animal are shown. The lack of mazindol binding shows the extent of dopamine depletion in the dorsal striatum measured at three different anteriority levels [AP, 0.48, −0.12, −0.48 mm related to bregma (Paxinos and Watson, 2005)]. Scale bar, 2 mm.
Figure 2.
A, B, Schematic reconstruction of the injection sites in the globus pallidus (A) and the substantia nigra pars reticulata (B) at different anteriority levels (AP, from −0.36 to −1.08 mm and from −4.56 to −5.64 mm related to bregma, for the GP and the SNr, respectively). C, D, Photomicrographs of Nissl-stained sections showing representative injector needle placement in the globus pallidus (C) and the substantia nigra pars reticulata (D). Scale bars: C, D, 1 mm. +Injection sites for the animals injected with ACPT-I; °injection sites for the animals injected with picrotoxin.
Effects of ACPT-I on haloperidol-induced catalepsy
Globus pallidus
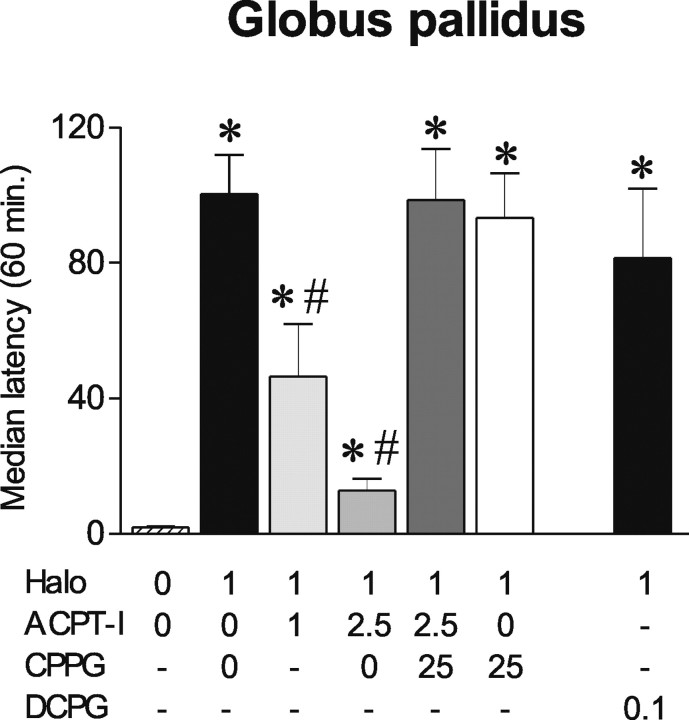
Haloperidol (1 mg/kg, i.p.) produced a profound cataleptic state as shown by increase in median latency to step down the rod compared with controls (p < 0.01; Mann–Whitney U test after a Kruskal–Wallis test; H = 132.26) (Fig. 3). Catalepsy induced by haloperidol was significantly antagonized by intrapallidal injection of ACPT-I at the two doses tested (p < 0.01; Mann–Whitney U test). Whereas ACPT-I 1 nmol/μl induced a transient reversal of catalepsy for the first 30 min of testing, 2.5 nmol/μl was effective for the total duration of the test and significantly different from 1 nmol (p < 0.01; Mann–Whitney U test). The selective group III mGluR antagonist CPPG (25 nmol/μl), which had no effect on its own, completely reversed the anticataleptic effect of ACPT-I 2.5 nmol/μl (p < 0.01; Mann–Whitney U test).
Figure 3.
Effects of ACPT-I, CPPG, and DCPG injections into the globus pallidus on haloperidol-induced catalepsy. Eighty-five minutes after haloperidol administration (1 mg/kg), animals (n = 5–7 per group) received intrapallidal injections of ACPT-I (0, 1, 2.5 nmol/μl) or the mGluR8-selective agonist DCPG (0.1 nmol/μl), and catalepsy was measured immediately and every 10 min for the 1 h testing. The group III mGluR antagonist CPPG (25 nmol/μl) was injected 10 min before ACPT-I or vehicle. The data are expressed as mean median latency ± SEM during the total duration of the test. *Significantly different from control group (p < 0.05; significant Mann–Whitney U test). #Significantly different from haloperidol group (p < 0.05; significant Mann–Whitney U test).
The classical group III mGluR agonists l-AP4 or ACPT-I show similar affinity for mGluR4 and mGluR8 [EC50 values (micromolar): mGluR4, 0.5–1 and 7.2, and mGluR8, 0.6 and 10.1 for l-AP4 and ACPT-I, respectively] with higher concentrations required to activate mGluR7 (160–800 and 1200 μm, respectively) (Acher et al., 1997; De Colle et al., 2000; Schann et al., 2006). We therefore examined whether DCPG, a selective mGluR8 agonist (Thomas et al., 2001), could modify haloperidol-induced catalepsy. Because DCPG is 100 times more potent than ACPT-I on mGluR8, but has similar affinity for mGluR4, we used a 10-fold lower concentration of DCPG (0.1 nmol/μl) compared with the effective doses of ACPT-I to ascertain specific activation of mGluR8 subtype. At that dose, DCPG did not reverse haloperidol-induced catalepsy (Fig. 3), suggesting that the antiparkinsonian effects of ACPT-I in the GP are not primarily mediated by mGluR8 subtypes.
Substantia nigra
In contrast to the effects observed in the GP, ACPT-I 1 nmol/μl only weakly reduced the haloperidol-induced catalepsy, whereas 0.25 nmol/μl was ineffective (p < 0.01; Mann–Whitney U test after Kruskal–Wallis test; H = 114.44) (Fig. 4A). The effect produced by ACPT-I 1 nmol/μl was reversed by CPPG 10 nmol/μl, which by itself did not modify haloperidol-induced catalepsy. The selective mGluR8 agonist DCPG 0.1 nmol/μl did not significantly modify haloperidol-induced catalepsy (Fig. 4A).
Figure 4.
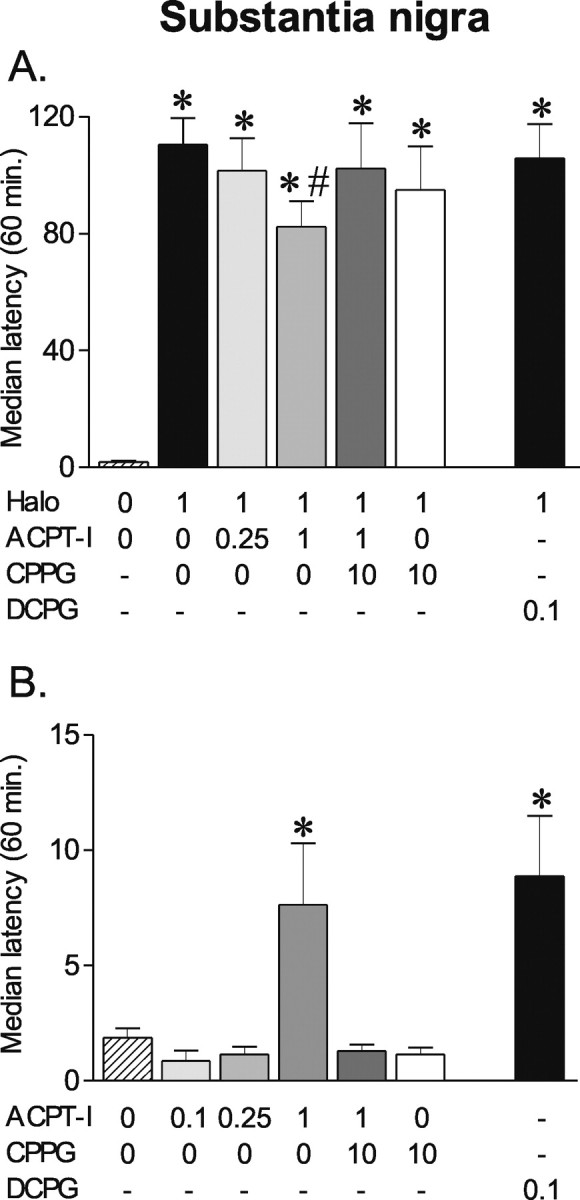
Effects of ACPT-I, DCPG, and CPPG injections into the substantia nigra pars reticulata on haloperidol-induced catalepsy. A, B, Animals (n = 5–7 per group) received intranigral injections of ACPT-I (0, 0.25, 1 nmol/μl) or mGluR8-selective agonist DCPG (0.1 nmol/μl) 85 min after haloperidol (1 mg/kg) administration (A) or after saline injection (B). Catalepsy was measured immediately after intranigral injections and every 10 min for the 1 h testing. The group III mGluR antagonist CPPG (10 nmol/μl) was injected 10 min before ACPT-I or vehicle. The data are expressed as mean median latency ± SEM during the total duration of the test. *Significantly different from control group (p < 0.05; significant Mann–Whitney U test). #Significantly different from haloperidol group (p < 0.05; significant Mann–Whitney U test).
In control animals, which received vehicle of haloperidol (Fig. 4B), ACPT-I 1nmol/μl was found to induce a weak but significant cataleptic state for the first 20 min (p < 0.01; Mann–Whitney U test after a Kruskal–Wallis test; H = 85.26). This effect was blocked by CPPG (p < 0.01; Mann–Whitney U test). Low doses of ACPT-I (0.1 and 0.25 nmol/μl) or CPPG alone did not produce any effect. DCPG 0.1 nmol/μl induced a similar level of catalepsy than that produced by ACPT-I 1 nmol/μl (p < 0.01; Mann–Whitney U test compared with vehicle).
6-OHDA lesion effects on RT performance
At completion of the training phase, all animals reached a preoperative level ranging from 80 to 90 correct responses/session (corresponding to 10–20 delayed responses/session), with mean RTs of 330–360 ms. Whereas performance of sham-operated animals was unchanged after surgery, 6-OHDA lesions impaired correct performance in the various lesion groups (Figs. 5, 6). These deficits were characterized by a significant increase of delayed responses relative to preoperative levels (ANOVA, GP, F(1,38) = 38.25; SNr, F(1,36) = 13.39; p < 0.01). Mean RTs were significantly lengthened (to 370–390 ms) after 6-OHDA lesions compared with preoperative levels (ANOVA, GP, F(1,38) = 16.29; SNr, F(1,36) = 7.97; p < 0.01). Moreover, performance of lesioned animals measured during the postoperative period was significantly different from performance of sham-operated animals (ANOVA, GP, F(1,28) = 15.71 and 15.36; SNr, F(1,28) = 8.56 and 5.25; p < 0.05 for delayed responses and RTs, respectively). The lesion-induced deficits were stable over time (up to postoperative day 60) (data not shown). No significant effect of 6-OHDA lesions was found on premature responding whatever the groups tested (Tables 1–3). Mean postoperative levels were similar to preoperative performance both in sham and lesioned animals.
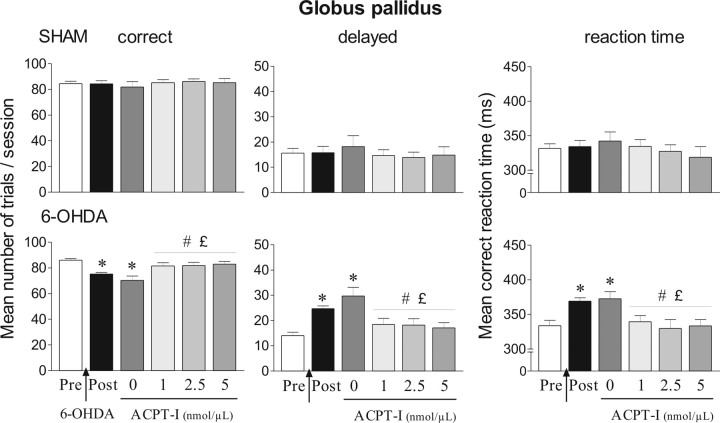
Figure 5.
Effects of ACPT-I injections into the globus pallidus in the reaction-time task for sham (n = 10) and 6-OHDA-lesioned rats (n = 20). Performance is expressed by the mean number of correct and delayed trials ± SEM and mean ± SEM reaction times in milliseconds. Performance is measured on different sessions: four preoperative sessions preceding the surgery (Pre) and four postoperative sessions corresponding to the days before injection of ACPT-I (Post). ACPT-I (0, 1, 2.5, and 5 nmol/μl) is injected at postoperative days 22, 26, 30, and 34 following a Latin-square design, and the effects induced by the different doses of ACPT-I are compared with the preoperative, postoperative, and control performance. *Significantly different from preoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). #Significantly different from postoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). £Significantly different from vehicle level (p < 0.05, Fisher's PLSD test after significant ANOVA).
Figure 6.
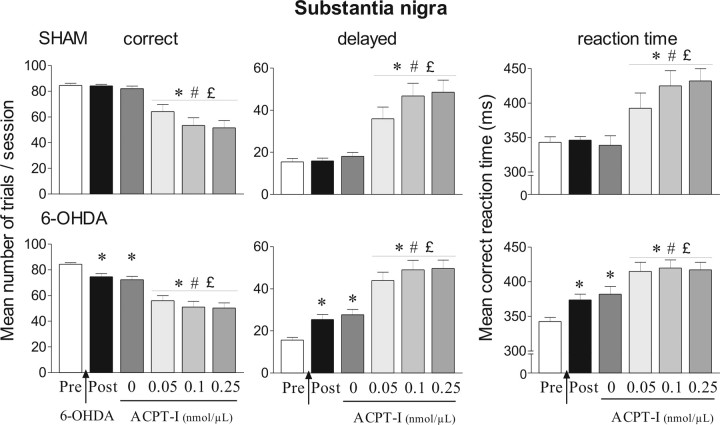
Effects of ACPT-I injection into the substantia nigra pars reticulata in the reaction-time task for sham (n = 11) and 6-OHDA-lesioned rats (n = 19). Performance is expressed by the mean number of correct and delayed trials ± SEM and by mean ± SEM reaction times in milliseconds. ACPT-I (0, 0.05, 0.1, and 0.25 nmol/μl) is injected at postoperative days 22, 26, 30, and 34 following a Latin-square design, and the effects induced by the different doses of ACPT-I are compared with the preoperative, postoperative, and control performance. *Significantly different from preoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). #Significantly different from postoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). £Significantly different from vehicle level (p < 0.05, Fisher's PLSD test after significant ANOVA).
Table 1.
Effects of ACPT-I injection into the globus pallidus on the number of premature responses
| Pre | Post | ACPT-I (nmol/μl) |
||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2.5 | 5 | |||
| Globus pallidus | ||||||
| Sham (n = 10) | 58.0 ± 5.9 | 64.4 ± 7.3 | 65.2 ± 15.7 | 104.4 ± 22.1 | 206.4 ± 29.3*,**,*** | 272.1 ± 71.4*,**,*** |
| 6-OHDA (n = 15) | 64.7 ± 15.6 | 64.6 ± 13.6 | 60.4 ± 10.6 | 122.8 ± 23.2 | 184.4 ± 32.8*,**,*** | 176.5 ± 38.1*,**,*** |
Values correspond to the mean number of premature responses ±SEM on different sessions: four sessions preceding the surgery (Pre) and four postoperative sessions (Post) corresponding to the days before infusion of ACPT-I at various doses (days 22, 26, 30, and 34).
*p< 0.05, Fisher's PLSD test, compared with preoperative performance after significant ANOVA.
**p< 0.05, Fisher's PLSD test, compared with postoperative performance after significant ANOVA.
***p< 0.05, Fisher's PLSD test, compared with vehicle infusion after significant ANOVA.
Table 2.
Effects of ACPT-I injection into the substantia nigra pars reticulata on the number of premature responses
| Pre | Post | ACPT-I (nmol/μl) |
||||
|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.25 | |||
| Substantia nigra | ||||||
| Sham (n = 11) | 57.4 ± 14.9 | 77.9 ± 15.0 | 65.4 ± 15.0 | 54.0 ± 9.9 | 63.4 ± 17.3 | 61.4 ± 14.8 |
| 6-OHDA (n = 19) | 60.2 ± 11.3 | 70.7 ± 8.8 | 57.6 ± 11.0 | 72.1 ± 14.8 | 73.0 ± 10.3 | 85.2 ± 15.7 |
Values correspond to the mean number of premature responses ± SEM on different sessions: four sessions preceding the surgery (Pre) and four postoperative sessions (Post) corresponding to the days before infusion of ACPT-I at various doses (days 22, 26, 30, and 34).
Table 3.
Effects of picrotoxin injection into the globus pallidus or the substantia nigra pars reticulata on the number of premature responses
| Pre | Post | Picrotoxin | |
|---|---|---|---|
| Globus pallidus | |||
| Sham (n = 6) | 38.2 ± 12.1 | 49 ± 12.4 | 221.5 ± 104.8*,** |
| 6-OHDA (n = 6) | 45 ± 12 | 33.7 ± 5.1 | 172.3 ± 49.3*,** |
| Substantia nigra | |||
| Sham (n = 8) | 38.6 ± 7.2 | 36.7 ± 6.5 | 31.13 ± 5.6 |
| 6-OHDA (n = 8) | 38.6 ± 4.9 | 45 ± 8.6 | 51.63 ± 9.3 |
The values correspond to the mean number of premature responses ±SEM recorded on different sessions of the preoperative (Pre) or postoperative (Post) period. Postoperative days correspond to the sessions preceding the day of picrotoxin injection into the GP or the SNr (0.165 and 0.033 nmol/μl, respectively).
*p< 0.05, Fisher's PLSD test, compared with preoperative performance after significant ANOVA.
**p< 0.05, Fisher's PLSD test, compared with postoperative performance after significant ANOVA.
Effects of ACPT-I injection on RT performance
Globus pallidus
No significant effect of injection order for the various ACPT-I doses tested according to a Latin-square design was found for any variable at any time postlesion, showing the stability of the behavioral effects over time. Low doses of ACPT-I (0.1, 0.2, 0.3 nmol/μl) failed to attenuate the deficits induced by bilateral 6-OHDA lesions and did not modify performance of sham-operated animals (data not shown). In contrast, the overall ANOVA revealed that highest doses of ACPT-I significantly modified the number of correct, delayed responses (ANOVA, F(5,140) = 4.55, p < 0.01) and mean RTs (ANOVA, F(5,140) = 3.66, p < 0.01) in the 6-OHDA group, although it did not affect these parameters in the sham group (Fig. 5). ACPT-I at 1, 2.5, and 5 nmol/μl completely reversed the increase of both delayed responses and RTs compared with postoperative performance and after vehicle injection (p < 0.05, Fisher's PLSD test after significant ANOVA, F(5,114) = 6.61 and 4.55, respectively). Moreover, ACPT-I induced a total recovery of preoperative performance level, which returned to control group level. As shown in Table 1, ACPT-I significantly modified the number of premature responses in sham-operated and 6-OHDA-lesioned rats (overall main treatment effect ANOVA, F(5,115) = 16.22, p < 0.01). Whereas ACPT-I at 1 nmol/μl had no effect, 2.5 and 5 nmol/μl induced a marked increase in premature responses in the two groups compared with preoperative and postoperative periods and to vehicle injections (p < 0.01, Fisher's PLSD test after a significant ANOVA, F(5,54) = 7.94; F(5,84) = 5.93 for sham and lesioned animals, respectively). This increase of premature responding could be related to a nonspecific enhancement of motor reactivity or attentional deficits.
Substantia nigra
In a preliminary experiment, we first tested the effects of ACPT-I at a dose of 1 nmol/μl. This dose produced a profound akinesia, which prevented the animals from performing the task. We therefore completed another series of experiments using lower doses. Figure 6 illustrates the effects of ACPT-I (0.05, 0.1, and 0.25 nmol/μl) injection into the SNr in sham and lesioned animals. There was no significant effect of injection order for any variable whatever the postoperative day. The overall ANOVA performed on the number of delayed responses and mean RTs revealed a significant main “treatment” effect (ANOVA, F(5,140) = 43.82 and 20.59, respectively; p < 0.01). In both sham-operated and 6-OHDA-lesioned groups, injection of vehicle into the SNr did not significantly modify the performance compared with the postoperative period. At all doses tested, ACPT-I induced a dramatic increase in the number of delayed responses in the sham and lesion groups compared with preoperative and postoperative periods and after vehicle injection (p < 0.01, Fisher's PLSD test after a significant ANOVA; F(5,60) = 14.69 and F(5,108) = 19.49 for sham and lesioned animals, respectively). In line with this, ACPT-I produced similar lengthening of RTs in the two groups at all doses (p < 0.01, Fisher's PLSD test after a significant ANOVA; F(5,60) = 6.88 and F(5,108) = 8.73 for sham and lesioned animals, respectively). As shown in Table 2, ACPT-I did not significantly modify the number of premature responses in the two groups compared with preoperative and postoperative periods and after vehicle injection.
Effects of l-AP4 injection on RT performance
To compare the effects of ACPT-I with those of a classical group III mGluR agonist, an additional group of 6-OHDA-lesioned rats was injected with l-AP4 either into the GP or the SNr (Fig. 7). As found previously, 6-OHDA-induced lesions increased the number of delayed responses and lengthened RTs compared with preoperative period (ANOVA, GP, F(2,15) = 7.11 and 3.78; SNr, F(2,21) = 16.71 and 14.04, p < 0.01, respectively).
Figure 7.
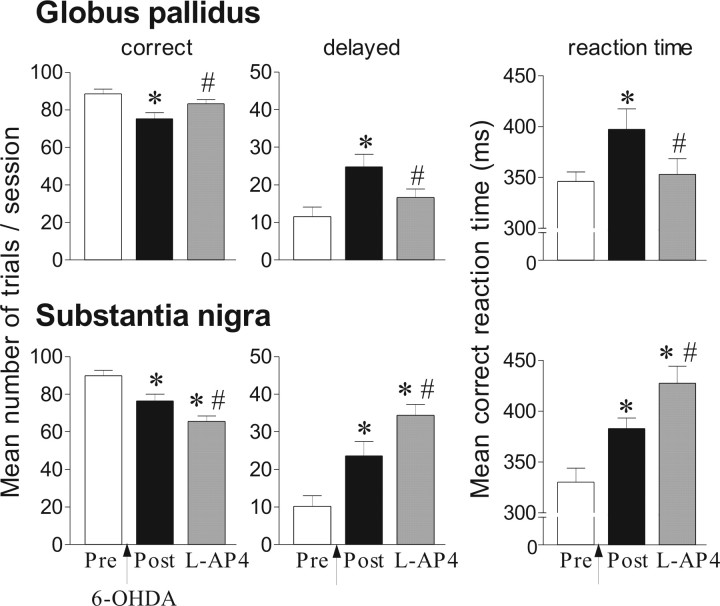
Effects of l-AP4 injection into the globus pallidus (n = 6) or the substantia nigra pars reticulata (n = 8) in the reaction-time task for 6-OHDA-lesioned rats. Performance is expressed by the mean number of correct and delayed trials ± SEM and by mean ± SEM reaction times in milliseconds. Performance is measured on one preoperative session (Pre), one postoperative session corresponding to the day before injection of l-AP4 (Post). l-AP4 (1 and 0.05 nmol/μl for the GP and SNr, respectively) is injected at postoperative day 26. *Significantly different from preoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). #Significantly different from postoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA).
Globus pallidus
As shown in Figure 7, l-AP4 1 nmol/μl reversed the lesion-induced deficits by reducing the number of delayed responses and RTs compared with the postoperative period (p < 0.05, Fisher's PLSD). In addition, l-AP4 induced a nonsignificant increase of premature responses (data not shown).
Substantia nigra
l-AP4 0.05 nmol/μl induced a marked increase of delayed responses and RTs compared with preoperative and postoperative periods (p < 0.05, Fisher's PLSD) with no significant change of premature responses.
The effects induced by l-AP4 in the GP or the SNr were similar to those produced by the same doses of ACPT-I in the same site, confirming the selectivity of ACPT-1 on group III mGluRs.
Effects of the GABAA antagonist picrotoxin injection on RT performance
To determine whether the behavioral effects produced by group III mGluR activation in the GP or SNr could be related to a modulation of GABA transmission, we tested the effects produced by picrotoxin, a selective GABAA antagonist, in the same locations. As previously found, 6-OHDA lesions increased the mean number of delayed responses and lengthened RTs (GP, ANOVA, F(1,10) = 11.94 and 10.75; SNr, F(1,14) = 9.78 and 10.41, p < 0.01, for delayed responses and mean RTs, respectively) (Figs. 8, 9).
Figure 8.
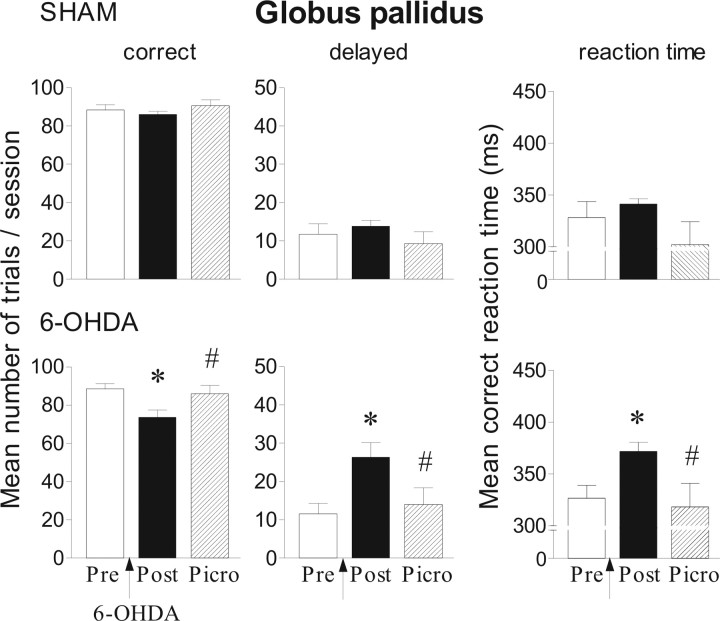
Effects of picrotoxin (0.165 nmol/μl) injection into the globus pallidus in the reaction-time task for sham (n = 6) and 6-OHDA-lesioned rats (n = 6). Performance is expressed by the mean number of correct and delayed trials ± SEM and by the mean ± SEM reaction times in milliseconds. Performance is measured on one preoperative session (Pre), one postoperative session (Post) corresponding to the day before picrotoxin injection (day 26). *Significantly different from preoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). #Significantly different from postoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA).
Figure 9.
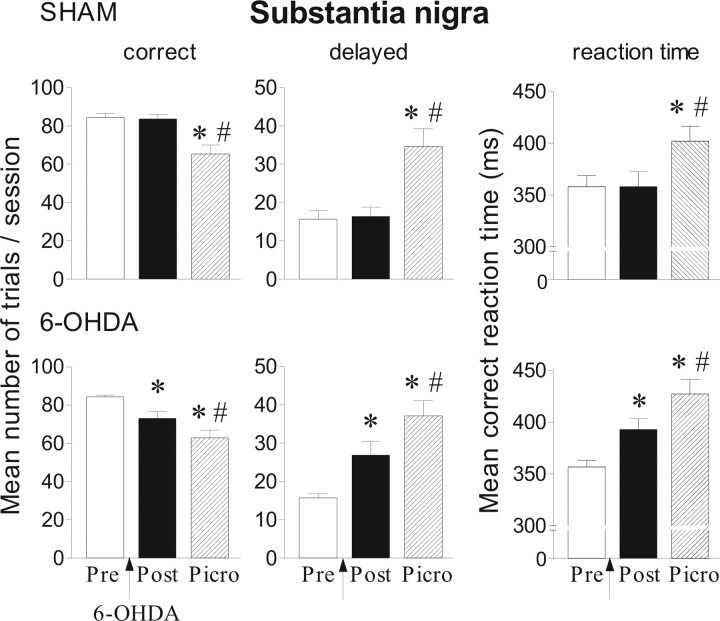
Effects of picrotoxin (0.033 nmol/μl) injection into the SNr in the reaction-time task for sham (n = 9) and 6-OHDA-lesioned rats (n = 8). Performance is expressed by the mean number of correct and delayed trials ± SEM and by the mean ± SEM reaction times in milliseconds. Performance is measured on preoperative and postoperative sessions and after picrotoxin injection. *Significantly different from preoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA). #Significantly different from postoperative performance (p < 0.05, Fisher's PLSD test after significant ANOVA).
Globus pallidus
As shown in Figure 8, 0.165 nmol/μl picrotoxin reversed the lesion-induced deficits by decreasing the number of delayed responses and RTs compared with postoperative period (p < 0.05, Fisher's PLSD test after a significant ANOVA; F(2,15) = 5.58, F(2,15) = 3.97, respectively). As observed previously with ACPT-I, picrotoxin injection significantly increased the number of premature responses in lesioned animals compared with preoperative and postoperative conditions (Table 3) (p < 0.01, Fisher's PLSD test after a significant ANOVA; F(2,15) = 8.2). A similar tendency was observed in sham-operated animals (Table 3) (p = 0.06).
Substantia nigra
Picrotoxin at 0.033 nmol/μl induced a dramatic increase of delayed responses both in sham-operated and 6-OHDA lesioned animals compared with preoperative and postoperative periods (Fig. 9) (p < 0.01, Fisher's PLSD test after a significant ANOVA; F(2,21) = 12.1 and 12.9 for sham and lesioned groups, respectively). In addition, RTs were significantly lengthened after picrotoxin injection (p < 0.05, compared with preoperative and postoperative levels, Fisher's PLSD test after a significant ANOVA; F(2,21) = 4.05, F(2,21) = 12,59). As mentioned in Table 3, picrotoxin had no significant effect on premature responses either in sham or lesioned animals.
Discussion
The present study reveals that activation of group III mGluRs in the GP or SNr with agonists such as ACPT-I and l-AP4 produces opposite behavioral effects in rat models of PD. Although the pallidal injection of ACPT-I or l-AP4 has antiparkinsonian actions both in acute and progressive rat models of the disease, the same compounds injected at a 10-fold lower concentration in the SNr potentiate the motor impairment in lesioned and control animals. In addition, the GABAA antagonist picrotoxin injected into the GP or SNr produces behavioral effects similar to those induced by group III mGluRs agonists injected in the same locations.
The different potency of ACPT-I at group I, II, and III mGluRs shows a profile of activity strikingly similar to the classical agonist, l-AP4, with a slightly weaker affinity (Acher et al., 1997; Moldrich et al., 2003; Schann et al., 2006) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). l-AP4 and ACPT-I exhibit similar potencies on mGluR4 and mGluR8 with lower affinity for mGluR7 and no consistent effects on other mGluRs (Acher et al., 1997; De Colle et al., 2000; Schann et al., 2006). Our results show that equipotent doses of ACPT-I or l-AP4 produced similar effects on RT performance and that ACPT-I effects on catalepsy were fully reversed by CPPG, a selective group III mGluRs antagonist. Last, ACPT-I produces anticonvulsant actions, anxiolytic effects, and analgesia, which are thought to be mediated by group III mGluRs (Chapman et al., 2001; Moldrich et al., 2003; Palucha et al., 2004; Goudet et al., 2006). All this converges to show selective activation of group III mGluRs by ACPT-I.
Group III mGluRs activation in the globus pallidus produces antiparkinsonian action
Our findings that ACPT-I reverses haloperidol- and 6-OHDA-induced akinesia are in agreement with the antiparkinsonian actions of group III mGluR agonists in pharmacological and lesion models of PD (Marino et al., 2003; Valenti et al., 2003; MacInnes et al., 2004; Konieczny et al., 2007). Our results extend these findings by demonstrating that dysfunction of glutamate activity at group III mGluRs probably occurs early in the progression of the disease when DA depletion is restricted to the dorsal striatum. According to the specific expression pattern of group III mGluRs in the BG (Testa et al., 1994; Bradley et al., 1999a,b; Kosinski et al., 1999; Corti et al., 2002; Messenger et al., 2002), ACPT-I and l-AP4 at low concentrations may act primarily at mGluR4 and/or mGluR8 to produce their behavioral effects. Previous in vitro and ex vivo studies reported that a selective mGluR4 agonist induces neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity (Battaglia et al., 2006) and potentiates l-AP4-induced inhibition at striatopallidal synapse, whereas the mGluR8 agonist DCPG had no effect (Marino et al., 2003; Valenti et al., 2003). In line with this, our results favor mGluR4-mediated effects at GP level because DCPG did not reverse haloperidol-induced catalepsy.
At the pallidal level, electrophysiological in vitro studies demonstrate that l-AP4 decreases GABAergic and glutamatergic transmission at striatopallidal and subthalamopallidal synapses, respectively (Matsui and Kita, 2003; Valenti et al., 2003). At a behavioral level, ACPT-I, LAP-4, or picrotoxin injected into the GP produce similar effects on RT performance. Although this comparison only provides indirect evidence, these results suggest that the functional effects of ACPT-I and l-AP4 may be related to a modulation of GABA activity in the GP. In line with this suggestion, intrapallidal injections of picrotoxin or bicuculline induce anti-akinetic effects in various models of PD in rats (Ossowska et al., 1984; Scheel-Kruger, 1984; Herrera-Marschitz and Ungerstedt, 1987; Maneuf et al., 1994; Wisniecki et al., 2003). In contrast, intrapallidal injections of muscimol induce catalepsy (Scheel-Kruger, 1984) and akinesia in the RT task (Amalric and Koob, 1989). This hypothesis, which implies hypoactivity of the GP in PD state (Albin et al., 1989; DeLong, 1990), does not fit however with recent reconsideration of GP function in the BG circuitry (Levy et al., 1997). The expression of the GABA-synthesizing enzyme GAD67 is enhanced in the GP rather than decreased after extensive unilateral DA denervation (Kincaid et al., 1992; Soghomonian and Chesselet, 1992; Soghomonian et al., 1994; Nielsen and Soghomonian, 2003). In contrast, pallidal expression of cytochrome oxidase subunit I (COI), a metabolic index of neuronal activity, was not enhanced after partial DA denervation (Oueslati et al., 2005). These discrepancies might be explained by extent of DA denervation in the different PD models and by the different markers of neuronal metabolic activity. Nevertheless, whatever the basal level of GP activity, reduction of GABA transmission should increase its activity (Matsumura et al., 1995) and ultimately influence the overall functional output (Obeso et al., 2006).
Alternatively, group III mGluR agonists may also reduce glutamatergic activity by presynaptic activation of autoreceptors on subthalamopallidal synapses (Matsui and Kita, 2003), which should ultimately produce opposite behavioral effect (e.g., enhancement of akinesia). Indirect functional evidence after pallidal NMDA receptors blockade argue in favor of this hypothesis. Indeed, selective NMDA antagonists produce rigidity, catalepsy (Turski et al., 1990), and akinesia in the RT task (Baunez and Amalric, 1996).
Paradoxical effects of group III mGluR activation into the substantia nigra pars reticulata
Group III mGluR activation in the SNr induces weak catalepsy in control animals and further exacerbates 6-OHDA-induced deficits. The doses of ACPT-I or l-AP4 required to produce behavioral effects on RT were 10-fold lower than in the GP. This could be attributable to either a different level of expression of mGluR subtypes or a different involvement of the various subtypes to produce these behavioral effects. Our results support the later hypothesis, because both ACPT-I at 1 nmol and the selective mGluR8 agonist DCPG at 0.1 nmol produce a similar cataleptic level in control animals. Moreover, at a same concentration, ACPT-I has no effect whereas DCPG still produces catalepsy, suggesting that mGluR8 rather than mGluR4 in the SNr are mediating these parkinsonian symptoms. However, characterization of the receptor subtypes involved await for development of selective compounds appropriate for in vivo studies.
In haloperidol-treated rats, the same dose of ACPT-I slightly reversed akinesia in agreement with a recent finding published during the course of the present study (Konieczny et al., 2007). Similar results were found in reserpine model of PD with l-SOP (l-serine-O-phosphate), another group III agonist (MacInnes et al., 2004). The differential effects produced by ACPT-I in control animals or after haloperidol treatment in the present study may be related to recent evidence demonstrating that dopamine modulates group III mGluR function in the SNr (Wittmann et al., 2002). Indeed, in vitro experiments conducted in reserpine or haloperidol model of PD demonstrate that whereas action of group III mGluR agonist on autoreceptors located on subthalamonigral nerve terminals remains functional, their action on heteroreceptors at the striatonigral synapse is dramatically reduced (Wittmann et al., 2002). This suggests that, in the acute model of PD, the prominent action of group III mGluR agonists in the SNr is to primarily decrease overactive subthalamonigral glutamate activity, ultimately leading to reversal of akinesia. In contrast, in the 6-OHDA-lesion model of PD and in basal condition, group III mGluR agonists could preferentially act on inhibitory striatonigral synapses. In line with this, GABAA receptors blockade in the SNr produces akinesia in the RT task. Accordingly, the increase of COI expression in the SNr was positively correlated with the magnitude of akinesia (Breysse et al., 2002; Oueslati et al., 2005), suggesting a specific involvement of this structure in the expression of akinetic deficits. Any additional reduction of GABA release in the SNr would thus enhance its activity and contribute to impair motor function as predicted by the canonical model of BG circuitry in PD (Albin et al., 1989; DeLong, 1990). Consistent with this, intranigral injections of GABAA receptor antagonists produce motor impairment in a lever-pressing task (Correa et al., 2003) and catalepsy (Scheel-Kruger, 1984), suggesting that group III agonists could produce their paradoxical effects by reducing GABA activity. In contrast, NMDA receptor blockade in the SNr increases locomotor activity (Klockgether and Turski, 1990; Koch et al., 2000) and facilitates movement initiation by decreasing RTs (Baunez and Amalric, 1996). The akinetic deficits induced by activation of group III mGluRs in the SNr in the partial 6-OHDA model of PD is likely to be caused by an additional decrease of GABAergic neurotransmission. However, a decrease of glutamate transmission cannot be ruled out in other models of extensive DA denervation.
Conclusion
Together, our results emphasize the role of group III mGluRs in the GP and SNr in modulating motor processes in both control and parkinsonian rats, presumably by an indirect action on GABAergic neurotransmission. The opposite behavioral effects produced by activation of group III mGluR in the GP and the SNr may be mediated by different receptor subtypes. In agreement with previous studies (Marino et al., 2003; Valenti et al., 2003), our results suggest a role for mGluR4 rather than mGluR8 in normalizing BG activity and question therefore the efficacy of non-subtype-selective group III agonists as an antiparkinsonian strategy in the early stage of PD.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, University of Aix-Marseille I and research grants of the Fondation de France, French Ministry of Education and Research (ACI n° 04 2 91), National Research Agency (ANR-05-NEUR-021-01), and French–Italian Galilée program between French Foreign Ministry and Conferenza dei Rettori delle Università Italiane (M.A., A.M.). S.L. was supported by a grant from the Ministry of Education and Research.
References
- Acher FC, Tellier FJ, Azerad R, Brabet IN, Fagni L, Pin JP. Synthesis and pharmacological characterization of aminocyclopentanetricarboxylic acids: new tools to discriminate between metabotropic glutamate receptor subtypes. J Med Chem. 1997;40:3119–3129. doi: 10.1021/jm970207b. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Depletion of dopamine in the caudate nucleus but not in nucleus accumbens impairs reaction-time performance in rats. J Neurosci. 1987;7:2129–2134. doi: 10.1523/JNEUROSCI.07-07-02129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Dorsal pallidum as a functional motor output of the corpus striatum. Brain Res. 1989;483:389–394. doi: 10.1016/0006-8993(89)90186-8. [DOI] [PubMed] [Google Scholar]
- Amalric M, Moukhles H, Nieoullon A, Daszuta A. Complex deficits on reaction time performance following bilateral intrastriatal 6-OHDA infusion in the rat. Eur J Neurosci. 1995;7:972–980. doi: 10.1111/j.1460-9568.1995.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, Nicoletti F, Bruno V. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci. 2006;26:7222–7229. doi: 10.1523/JNEUROSCI.1595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Amalric M. Evidence for functional differences between entopeduncular nucleus and substantia nigra: effects of APV (DL-2-amino-5-phosphonovaleric acid) microinfusion on reaction time performance in the rat. Eur J Neurosci. 1996;8:1972–1982. doi: 10.1111/j.1460-9568.1996.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Levey AI, Conn PJ. Distribution of group III mGluRs in rat basal ganglia with subtype-specific antibodies. Ann NY Acad Sci. 1999a;868:531–534. doi: 10.1111/j.1749-6632.1999.tb11322.x. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999b;407:33–46. [PubMed] [Google Scholar]
- Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22:5669–5678. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AG, Talebi A, Yip PK, Meldrum BS. Anticonvulsant activity of a mGlu(4alpha) receptor selective agonist, (1S,3R,4S)-1-aminocyclopentane-1,2,4-tricarboxylic acid. Eur J Pharmacol. 2001;424:107–113. doi: 10.1016/s0014-2999(01)01013-5. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Correa M, Mingote S, Betz A, Wisniecki A, Salamone JD. Substantia nigra pars reticulata GABA is involved in the regulation of operant lever pressing: pharmacological and microdialysis studies. Neuroscience. 2003;119:759–766. doi: 10.1016/s0306-4522(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- De Colle C, Bessis AS, Bockaert J, Acher F, Pin JP. Pharmacological characterization of the rat metabotropic glutamate receptor type 8a revealed strong similarities and slight differences with the type 4a receptor. Eur J Pharmacol. 2000;394:17–26. doi: 10.1016/s0014-2999(00)00113-8. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Feeley Kearney JA, Albin RL. mGluRs: a target for pharmacotherapy in Parkinson disease. Exp Neurol. 2003;184(Suppl 1):S30–S36. doi: 10.1016/s0014-4886(03)00391-1. [DOI] [PubMed] [Google Scholar]
- Goudet C, Chapuy E, Alloui A, Acher F, Pin J, Eschalier A. Modulation of inflammatory and neuropathic pain by group III metabotropic glutamate receptors. FENS. 2006 doi: 10.1016/j.pain.2007.08.020. Abstr A110.6. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M, Ungerstedt U. The dopamine-gamma aminobutyric acid interaction in the striatum of the rat is differently regulated by dopamine D-1 and D-2 types of receptor: evidence obtained with rotational behavioural experiments. Acta Physiol Scand. 1987;129:371–380. doi: 10.1111/j.1748-1716.1987.tb08080.x. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Levodopa strengths and weaknesses. Neurology. 2002;58:S19–S32. doi: 10.1212/wnl.58.suppl_1.s19. [DOI] [PubMed] [Google Scholar]
- Javitch JA, Strittmatter SM, Snyder SH. Differential visualization of dopamine and norepinephrine uptake sites in rat brain using [3H]mazindol autoradiography. J Neurosci. 1985;5:1513–1521. doi: 10.1523/JNEUROSCI.05-06-01513.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JA, Becker JB, Frey KA, Albin RL. The role of nigrostriatal dopamine in metabotropic glutamate agonist-induced rotation. Neuroscience. 1998;87:881–891. doi: 10.1016/s0306-4522(98)00193-6. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Albin RL, Newman SW, Penney JB, Young AB. 6-Hydroxydopamine lesions of the nigrostriatal pathway alter the expression of glutamate decarboxylase messenger RNA in rat globus pallidus projection neurons. Neuroscience. 1992;51:705–718. doi: 10.1016/0306-4522(92)90309-p. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Turski L. NMDA antagonists potentiate antiparkinsonian actions of L-dopa in monoamine-depleted rats. Ann Neurol. 1990;28:539–546. doi: 10.1002/ana.410280411. [DOI] [PubMed] [Google Scholar]
- Koch M, Fendt M, Kretschmer BD. Role of the substantia nigra pars reticulata in sensorimotor gating, measured by prepulse inhibition of startle in rats. Behav Brain Res. 2000;117:153–162. doi: 10.1016/s0166-4328(00)00299-0. [DOI] [PubMed] [Google Scholar]
- Konieczny J, Wardas J, Kuter K, Pilc A, Ossowska K. The influence of group III metabotropic glutamate receptor stimulation by (1S,3R,4S)-1-aminocyclo-pentane-1,3,4-tricarboxylic acid on the parkinsonian-like akinesia and striatal proenkephalin and prodynorphin mRNA expression in rats. Neuroscience. 2007;145:611–620. doi: 10.1016/j.neuroscience.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Kosinski CM, Risso Bradley S, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Jr, Young AB, Standaert DG. Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J Comp Neurol. 1999;415:266–284. [PubMed] [Google Scholar]
- Levy R, Hazrati LN, Herrero MT, Vila M, Hassani OK, Mouroux M, Ruberg M, Asensi H, Agid Y, Feger J, Obeso JA, Parent A, Hirsch EC. Re-evaluation of the functional anatomy of the basal ganglia in normal and Parkinsonian states. Neuroscience. 1997;76:335–343. doi: 10.1016/s0306-4522(96)00409-5. [DOI] [PubMed] [Google Scholar]
- MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br J Pharmacol. 2004;141:15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Mitchell IJ, Crossman AR, Brotchie JM. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp Neurol. 1994;125:65–71. doi: 10.1006/exnr.1994.1007. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci USA. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122:727–737. doi: 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Tremblay L, Richard H, Filion M. Activity of pallidal neurons in the monkey during dyskinesia induced by injection of bicuculline in the external pallidum. Neuroscience. 1995;65:59–70. doi: 10.1016/0306-4522(94)00484-m. [DOI] [PubMed] [Google Scholar]
- Messenger MJ, Dawson LG, Duty S. Changes in metabotropic glutamate receptor 1–8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology. 2002;43:261–271. doi: 10.1016/s0028-3908(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Chapman AG, De Sarro G, Meldrum BS. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur J Pharmacol. 2003;476:3–16. doi: 10.1016/s0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- Nielsen KM, Soghomonian JJ. Dual effects of intermittent or continuous L-DOPA administration on gene expression in the globus pallidus and subthalamic nucleus of adult rats with a unilateral 6-OHDA lesion. Synapse. 2003;49:246–260. doi: 10.1002/syn.10234. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Javier Blesa F, Guridi J. The globus pallidus pars externa and Parkinson's disease. Ready for prime time? Exp Neurol. 2006;202:1–7. doi: 10.1016/j.expneurol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Wedzony K, Wolfarth S. The role of the GABA mechanisms of the globus pallidus in mediating catalepsy, stereotypy and locomotor activity. Pharmacol Biochem Behav. 1984;21:825–831. doi: 10.1016/s0091-3057(84)80060-x. [DOI] [PubMed] [Google Scholar]
- Oueslati A, Breysse N, Amalric M, Kerkerian-Le Goff L, Salin P. Dysfunction of the cortico-basal ganglia-cortical loop in a rat model of early parkinsonism is reversed by metabotropic glutamate receptor 5 antagonism. Eur J Neurosci. 2005;22:2765–2774. doi: 10.1111/j.1460-9568.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- Palucha A, Tatarczynska E, Branski P, Szewczyk B, Wieronska JM, Klak K, Chojnacka-Wojcik E, Nowak G, Pilc A. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology. 2004;46:151–159. doi: 10.1016/j.neuropharm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Ed 5. Sydney: Academic; 2005. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson's disease and related disorders. Pharmacol Ther. 2000;88:427–435. doi: 10.1016/s0163-7258(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Schann S, Menet C, Arvault P, Mercier G, Frauli M, Mayer S, Hubert N, Triballeau N, Bertrand HO, Acher F, Neuville P. Design and synthesis of APTCs (aminopyrrolidinetricarboxylic acids): identification of a new group III metabotropic glutamate receptor selective agonist. Bioorg Med Chem Lett. 2006;16:4856–4860. doi: 10.1016/j.bmcl.2006.06.062. [DOI] [PubMed] [Google Scholar]
- Scheel-Kruger J. GABA in the striatonigral and striatopallidal systems as moderator and mediator of striatal functions. Adv Neurol. 1984;40:85–90. [PubMed] [Google Scholar]
- Senkowska A, Ossowska K. Role of metabotropic glutamate receptors in animal models of Parkinson's disease. Pol J Pharmacol. 2003;55:935–950. [PubMed] [Google Scholar]
- Soghomonian JJ, Chesselet MF. Effects of nigrostriatal lesions on the levels of messenger RNAs encoding two isoforms of glutamate decarboxylase in the globus pallidus and entopeduncular nucleus of the rat. Synapse. 1992;11:124–133. doi: 10.1002/syn.890110205. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Pedneault S, Audet G, Parent A. Increased glutamate decarboxylase mRNA levels in the striatum and pallidum of MPTP-treated primates. J Neurosci. 1994;14:6256–6265. doi: 10.1523/JNEUROSCI.14-10-06256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE. (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology. 2001;40:311–318. doi: 10.1016/s0028-3908(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Toms NJ, Jane DE, Kemp MC, Bedingfield JS, Roberts PJ. The effects of (RS)-alpha-cyclopropyl-4-phosphonophenylglycine ((RS)-CPPG), a potent and selective metabotropic glutamate receptor antagonist. Br J Pharmacol. 1996;119:851–854. doi: 10.1111/j.1476-5381.1996.tb15750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turle-Lorenzo N, Maurin B, Puma C, Chezaubernard C, Morain P, Baunez C, Nieoullon A, Amalric M. The dopamine agonist piribedil with L-Dopa improves attentional dysfunction: relevance for Parkinson's disease. J Pharmacol Exp Ther. 2006;319:914–923. doi: 10.1124/jpet.106.109207. [DOI] [PubMed] [Google Scholar]
- Turski L, Klockgether T, Turski WA, Schwarz M, Sontag KH. Blockade of excitatory neurotransmission in the globus pallidus induces rigidity and akinesia in the rat: implications for excitatory neurotransmission in pathogenesis of Parkinson's diseases. Brain Res. 1990;512:125–131. doi: 10.1016/0006-8993(90)91180-o. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniecki A, Correa M, Arizzi MN, Ishiwari K, Salamone JD. Motor effects of GABA(A) antagonism in globus pallidus: studies of locomotion and tremulous jaw movements in rats. Psychopharmacology (Berl) 2003;170:140–149. doi: 10.1007/s00213-003-1521-z. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85:1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Conn PJ. Dopamine modulates the function of group II and group III metabotropic glutamate receptors in the substantia nigra pars reticulata. J Pharmacol Exp Ther. 2002;302:433–441. doi: 10.1124/jpet.102.033266. [DOI] [PubMed] [Google Scholar]
- Zhang C, Albin RL. Increased response to intrastriatal L(+)-2-amino-4-phosphonobutyrate (L-AP4) in unilateral 6-hydroxydopamine-lesioned rats. Exp Neurol. 2000;165:278–284. doi: 10.1006/exnr.2000.7452. [DOI] [PubMed] [Google Scholar]