Abstract
Tonically active cholinergic interneurons in the striatum modulate activities of striatal outputs from medium spiny (MS) neurons and significantly influence overall functions of the basal ganglia. Cellular mechanisms of this modulation are not fully understood. Here we show that ambient acetylcholine (ACh) derived from tonically active cholinergic interneurons constitutively upregulates depolarization-induced release of endocannabinoids from MS neurons. The released endocannabinoids cause transient suppression of inhibitory synaptic inputs to MS neurons through acting retrogradely onto presynaptic CB1 cannabinoid receptors. The effects were mediated by postsynaptic M1 subtype of muscarinic ACh receptors, because the action of a muscarinic agonist to release endocannabinoids and the enhancement of depolarization-induced endocannabinoid release by ambient ACh were both deficient in M1 knock-out mice and were blocked by postsynaptic infusion of guanosine-5′-O-(2-thiodiphosphate). Suppression of spontaneous firings of cholinergic interneurons by inhibiting Ih current reduced the depolarization-induced release of endocannabinoids. Conversely, elevation of ambient ACh concentration by inhibiting choline esterase significantly enhanced the endocannabinoid release. Paired recording from a cholinergic interneuron and an MS neuron revealed that the activity of single cholinergic neuron could influence endocannabinoid-mediated signaling in neighboring MS neurons. These results clearly indicate that striatal endocannabinoid-mediated modulation is under the control of cholinergic interneuron activity. By immunofluorescent and immunoelectron microscopic examinations, we demonstrated that M1 receptor was densely distributed in perikarya and dendrites of dopamine D1 or D2 receptor-positive MS neurons. Thus, we have disclosed a novel mechanism by which the muscarinic system regulates striatal output and may contribute to motor control.
Keywords: CB1 receptor, acetylcholine, muscarinic receptor, DSI, medium spiny neuron, basal ganglia
Introduction
The striatum is the main input structure of the basal ganglia that subserves motor and cognitive functions (Wilson, 2004). The medium spiny (MS) neurons are the projection neurons from the striatum that constitute 95% of whole striatal neuronal population (Wilson, 2004). In contrast, cholinergic interneurons constitute only <2% of total striatal neurons, but they have widespread axonal projections and exhibit tonic firings and, therefore, can significantly influence activities of striatal neurons (Calabresi et al., 2000). Cholinergic system is known to contribute to various striatal functions, including control of motor activity (Kaneko et al., 2000), achievement of goal-directed behavior (Apicella, 2002), and synaptic plasticity (Calabresi et al., 2000; Wang et al., 2006). Effects of acetylcholine (ACh) are mediated by ionotropic and/or metabotropic (muscarinic) receptors. Muscarinic ACh receptors (mAChRs) comprise five subtypes, in which M1, M3, and M5 are coupled to Gq/11-protein, activate phospholipase Cβ (PLCβ), and produce IP3 and diacylglycerol (DAG), whereas M2 and M4 are coupled to Gi/o-proteins and inhibit cAMP production (Matsui et al., 2004). MS neurons were shown to possess M1 and M4 (Yan et al., 2001), and activation of mAChRs is known to modulate excitability of MS neurons. mAChR activation reduces K+ and/or Ca2+ conductance (Howe and Surmeier, 1995; Lin et al., 2004; Shen et al., 2005) and postsynaptically enhances NMDA receptor-mediated responses (Calabresi et al., 1998). Moreover, muscarinic agonists presynaptically inhibit both excitatory (Calabresi et al., 2000) and inhibitory (Koos and Tepper, 2002) synaptic inputs onto MS neurons. Thus, ACh can modulate striatal outputs through multiple mechanisms.
It has been demonstrated in the hippocampus that activation of postsynaptic M1 and/or M3 receptors triggers release of endocannabinoids from postsynaptic neurons and retrogradely suppresses inhibitory synaptic transmission by activating presynaptic cannabinoid CB1 receptor (CB1R) (Kim et al., 2002; Fukudome et al., 2004). Moreover, M1 and/or M3 activation significantly enhances depolarization-induced suppression of inhibition (DSI) (Kim et al., 2002; Ohno-Shosaku et al., 2003), a phenomenon known to be mediated by endocannabinoids (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). The striatum is a brain structure in which CB1R is abundantly expressed (Herkenham et al., 1991; Hohmann and Herkenham, 2000) and inhibitory synaptic responses on MS neurons are sensitive to cannabinoid agonists (Szabo et al., 1998). We recently reported that MS neurons undergo DSI (Narushima et al., 2006b). Because MS neurons are shown to possess M1 and M4 (Yan et al., 2001), these data led us to hypothesize that cholinergic action in the striatum may involve endocannabinoid-mediated retrograde signaling.
We show here that mAChR-dependent suppression of inhibitory transmission onto MS neuron is triggered by activation of postsynaptic M1 receptors expressed selectively on their somatodendritic compartments and mediated by retrogradely released endocannabinoids. Importantly, DSI of MS neurons is persistently upregulated by ambient ACh derived from tonically active cholinergic interneurons. By using paired recording techniques, we indicate that activity of single cholinergic interneuron significantly influences endocannabinoid signaling in neighboring MS neurons. These results indicate that striatal endocannabinoid-mediated modulation is under the bidirectional control of cholinergic interneuron activity.
Materials and Methods
Electrophysiology.
All experiments were performed according to the guidelines laid down by the animal welfare committee of Kanazawa University, Hokkaido University, and Osaka University. Coronal brain slices containing the cortex and the striatum (300 μm thick) were prepared from C57BL/6 mice or M1 receptor knock-out mice (Ohno-Shosaku et al., 2003) aged 15–22 d postnatally as described previously (Narushima et al., 2006a). In brief, mice were decapitated under deep halothane anesthesia, and the brains were cooled in ice-cold, modified external solution containing the following (in mm): 120 choline-Cl, 2 KCl, 8 MgCl2, 28 NaHCO3, 1.25 NaH2PO4, and 20 glucose (bubbling with 95% O2 and 5% CO2) (Narushima et al., 2006a). Slices were cut with a Leica (Wetzlar, Germany) VT1000S slicer. For recovery, slices were incubated at least 1 h in normal bathing solution composed of the following (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 20 glucose, pH 7.4 (bubbled continuously with a mixture of 95% O2 and 5% CO2 at room temperature).
Whole-cell recordings were made from MS neurons in the dorsolateral region of the striatum, using an upright microscope (BX50WI; Olympus Optical, Tokyo, Japan) equipped with an infrared CCD camera system (Hamamatsu Photonics, Hamamatsu, Japan). MS neurons were identified visually by their medium-sized, spherical somata as well as their electrophysiological properties (Kita et al., 1984). Resistance of the patch pipette was 3–5 MΩ when filled with the standard intracellular solution composed of the following (in mm): 50 KCl, 90 K-gluconate, 10 HEPES, 1 EGTA, 0.1 CaCl2, 4.6 MgCl2, 4 Na-ATP, and 0.4 Na-GTP, pH 7.2, adjusted with KOH. In experiments with ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride), we used a Cs+-based intracellular solution that was composed of the following (in mm): 50 CsCl, 90 Cs-gluconate, 10 HEPES, 1 EGTA, 0.1 CaCl2, 4.6 MgCl2, 4 Na-ATP, and 0.4 Na-GTP, pH 7.2, adjusted with CsOH. In experiments for recording spontaneous IPSCs (sIPSCs), we also used a Cs+-based intracellular solution that was composed of the following (in mm): 140 CsCl, 10 HEPES, 1 EGTA, 0.1 CaCl2, 4.6 MgCl2, 4 Na-ATP, and 0.4 Na-GTP, pH 7.2, adjusted with CsOH. The pipette access resistance was compensated by 20–50%. MS neurons were usually held at a membrane potential of −80 mV. The bath solution was supplemented with 10 μm 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo quinoxaline-7-sulfonamide and 5 μm (R)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic (Tocris Cookson, Bristol, UK). Membrane currents were recorded with an EPC8 or EPC9 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). The PULSE software (HEKA Elektronik) was used for stimulation and data acquisition. The signals were filtered at 3 kHz and digitized at 20 kHz. Glass pipettes filled with normal saline were used to stimulate putative GABAergic fibers. We also performed cell-attached and whole-cell recordings from cholinergic interneurons identified with their large somata and firing properties (Kawaguchi, 1993) with K+-based internal solution. In paired recording experiments, we made voltage-clamp recordings from an MS neuron adjacent to (<100 μm) the cholinergic interneuron.
For testing effects of chemicals, two successive test pulses with an interstimulus interval of 50 ms were applied every 20 s. All drugs except for guanosine-5′-O-(2-thiodiphosphate) (GDP-β-S) were bath applied. Effects of drugs were estimated as the percentage of the mean amplitudes of five consecutive IPSCs during drug application relative to that before application. Oxotremorine-M (oxo-M), AM281 [1-(2,4-dichloro-phenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide], WIN55,212-2 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate], and ZD7288 were purchased from Tocris Cookson, atropine was from Nacalai Tesque (Kyoto, Japan), and GDP-β-S was from Sigma (St. Louis, MO). SR141716A [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole carboxamide] was a generous gift from Sanofi Recherche (Libourne, France).
Induction and estimation of DSI.
In experiments for recording evoked IPSCs, two successive test pulses (duration, 0.1 ms; amplitude, 0–90 V) with an interstimulus interval of 50 ms were applied every 5 s for monitoring the paired-pulse ratio. To induce DSI, a depolarizing pulse (0.1, 1, or 5 s duration from −80 to 0 mV) was applied to MS neurons. The magnitude of suppression was calculated as the percentage of reduction in the mean of three consecutive response amplitudes after depolarization relative to that of 10 consecutive response amplitudes just before depolarization. sIPSCs were analyzed using Mini analysis program (Synaptosoft, Decatur, GA). Threshold for detecting sIPSC was set at the level three times larger than the noise level. To calculate suppression of sIPSCs, the membrane charges for detected sIPSCs were accumulated for 10 s before and after depolarization. Magnitude of DSI for sIPSC was calculated as the percentage of reduction in the accumulated membrane charge after depolarization relative to that before depolarization. To quantify enhancement of DSI by some experimental manipulation, ΔDSI was calculated by subtracting the DSI magnitude in the control condition from that after the experimental manipulation.
Statistics.
Averaged data from different experiments are presented as mean ± SEM. Statistical significance was assessed by Mann–Whitney U test (for comparison of two independent samples) or Wilcoxon's signed rank test (for paired comparison of the same sample).
Antibodies for immunohistochemistry and immunoblotting.
We used polyclonal antibodies to mouse CB1R (raised in the rabbit and guinea pig) (Fukudome et al., 2004; Kawamura et al., 2006), mouse dopamine D1 (guinea pig and goat) and D2 (rabbit and guinea pig) receptors (Narushima et al., 2006b), mouse parvalbumin (PV) (rabbit and goat) (Nakamura et al., 2004; Miura et al., 2006), mouse vesicular acetylcholine transporter VAChT (rabbit and goat) (Nakamura et al., 2004; Miura et al., 2006), and somatostatin (sc-13099; Santa Cruz Biotechnology, Santa Cruz, CA). In addition, we produced specific antibodies to mouse muscarinic M1 receptor (amino acid residues 247–345; GenBank accession number NM007698, rabbit), mouse high-affinity choline transporter CHT1 (amino acid residues 531–580; GenBank accession number BC022025, rabbit and goat), mouse neuronal nitric oxide synthase (nNOS) (amino acid residues 1415–1429; GenBank accession number NM008712, rabbit and guinea pig), and glutathione-S transferase (GST) fusion proteins were used as antigens and GST-free polypeptides were for affinity purification, as reported previously (Nakamura et al., 2004). The antigenic regions for M1 and nNOS were chosen by low-sequence homology to other subtypes, i.e., M3/M5 muscarinic receptors (see Fig. 7A) and inducible NOS/endothelial NOS (data not shown), respectively. Specificities of M1, CHT1, and nNOS antibodies were checked by immunoblot detection of single protein bands at 64, 65, and 160 kDa, respectively (see Fig. 7B) (supplemental Fig. 2A,E, available at www.jneurosci.org as supplemental material).
Figure 7.
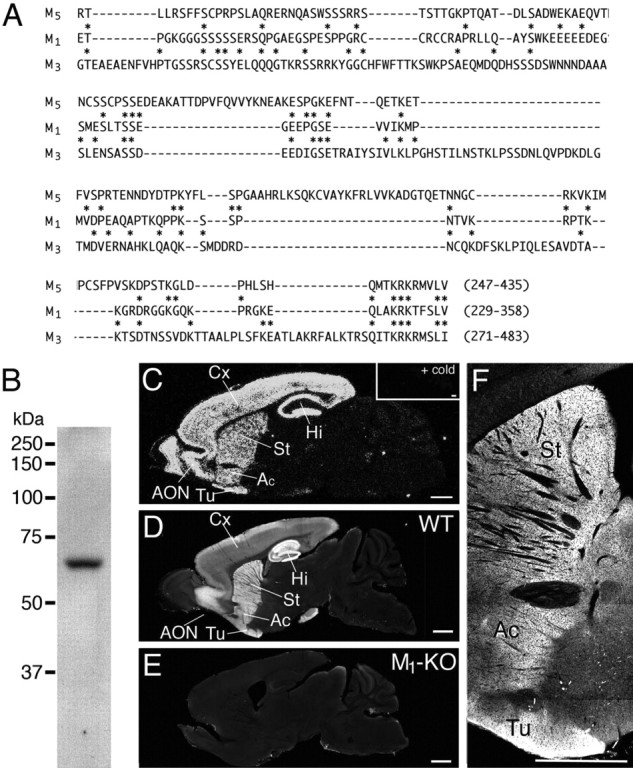
Production and characterization of antibodies against mouse M1 receptor. A, Low sequence homology of M1 antigen polypeptide to corresponding regions of mouse M3 and M5 receptors. Asterisks indicate identical amino acid residues between M1 and M3 or M1 and M5. B, Immunoblot with protein samples from telencephalon. Rabbit M1 antibody recognized single protein bands at 64 kDa. C, In situ hybridization showing preferential telencephalic expression of M1 mRNA in the adult mouse brain. An inset shows the specificity of hybridization by the virtual disappearance of signals when hybridization was performed in the presence of unlabeled oligonucleotides in excess. D, Immunofluorescence showing preferential telencephalic distribution of M1 protein in the adult mouse brain. E, The lack of M1 immunostaining in the M1 knock-out brain, indicating the specificity of M1 immunohistochemistry. F, An enlarged view of D around the striatum. Scale bars, 1 mm. Ac, Accumbens; AON, anterior olfactory nucleus; Cx, cortex; Hi, hippocampus; St, striatum; Tu, olfactory tubercle; WT, wild type.
Immunofluorescence.
The parasagittal sections (50 μm in thickness) were incubated at room temperature in the free-floating method using PBS/0.1% Triton X-100 for antibody diluent and washing buffer. After blocking with 10% normal donkey serum for 20 min, sections were incubated overnight with a mixture of primary antibodies at the concentration of 1 μg/ml for each. Then, they were incubated for 2 h with a mixture of indocarbocyanine-, indodicarbocyanine-, or Alexa fluor 488-labeled species-specific secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA; Invitrogen, Carlsbad, CA). Single optical sections were taken with a confocal laser scanning microscope (FV1000; Olympus Optical).
The specificity was confirmed by distribution pattern of M1 immunostaining identical to that of M1 mRNA in the adult mouse brain and by the lack of immunostaining in the M1 knock-out (M1-KO) brain (see Fig. 7C–E). The specificity of CHT1 and nNOS immunohistochemistry was confirmed by the lack of characteristic staining with use of antibodies preabsorbed with antigens (supplemental Fig. 2C,G, available at www.jneurosci.org as supplemental material) and also by selective coexpressions of CHT1 with VAChT in striatal cholinergic interneurons (Nakamura et al., 2004) and of nNOS in somatostatin-containing striatal neurons (supplemental Fig. 2D,H, available at www.jneurosci.org as supplemental material). The specificity was further ascertained by obtaining the same results with the use of primary antibodies raised for the same antigens in different species (data not shown).
Immunoelectron microscopy.
The microslicer sections were permeabilized with 0.02% saponin for 30 min, and 0.004% saponin was added to diluent of blocking serum to facilitate tissue penetration of primary and secondary antibodies. Sections were incubated overnight with M1 antibody (1 μg/ml) and then with anti-rabbit IgG conjugated with colloidal gold for 2 h (1.4 nm in diameter, Nanogold; Nanoprobes, Stony Brook, NY). After silver enhancement (HQ silver; Nanoprobes), sections were treated with 0.5% osmium tetroxide, stained in block with 2% uranyl acetate, dehydrated, and embedded in Epon 812. Ultrathin sections were prepared from <1.5 μm from the section surface, mounted on nickel grids, and stained with 2% uranyl acetate for 10 min. Electron micrographs were taken randomly by an H7100 electron microscope (Hitachi, Tokyo, Japan). The number of metal particles and the length of plasma membrane were measured on electron micrographs, using an IPLab software (Nippon Roper, Tokyo, Japan). Statistical significance was assessed by Student's t test.
Immunoblot.
Under pentobarbital anesthesia (100 mg/kg of body weight, i.p.), brains were freshly removed from the skull, and the forebrain was quickly dissected and homogenized using a Potter homogenizer in 10 vol of ice-cold buffer containing 320 mm sucrose, 1 mm EDTA, 20 mm KCl, 10 mm Tris-HCl, pH 7.0, and an appropriate amount of protease inhibitor cocktail (Sigma). Homogenates were centrifuged for 10 min at 1000 × g, and the supernatant (i.e., the post-nuclear or S1 fraction) was used for specificity test of antibody. Proteins were separated by 10% SDS-PAGE and then electroblotted onto nitrocellulose membranes (Schleicher and Schell, Dassel, Germany). After blocking with 5% skimmed milk for 2 h, membranes were incubated with primary antibody (1 μg/ml) diluted with Tris-buffered saline, pH 7.5, containing 0.1% Tween 20 and 5% skimmed milk for 2 h and then with peroxidase-linked secondary antibody (1:15000; Amersham Biosciences, Tokyo, Japan) for 1 h. Immunoreaction was visualized with the ECL chemiluminescence detection system (Amersham Biosciences).
In situ hybridization.
Under deep pentobarbital anesthesia, the brains were freshly obtained from C57BL/6J mice at 2–3 months of age. Fresh frozen sections (20 μm thickness) were cut with a cryostat (CM1900; Leica, Nussloch, Germany) and mounted on glass slides precoated with 3-aminopropyltriethoxysilane. Oligonucleotide probes for M1 mRNA (5′-gcctgtcactgtagccagagacaggaggcctgtggttgatccgat-3′, antisense to nucleotide residues 112–156) were synthesized and radiolabeled for in situ hybridization. Sections were treated at room temperature with the following incubation steps: fixation with 4% paraformaldehyde–0.1 m sodium phosphate buffer, pH 7.2, for 10 min, 2 mg/ml glycine–PBS, pH 7.2, for 10 min, acetylation with 0.25% acetic anhydride in 0.1 m triethanolamine-HCl, pH 8.0, for 10 min, and prehybridization for 1 h in a buffer containing 50% formamide, 50 mm Tris-HCl, pH 7.5, 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin, 0.6 m NaCl, 0.25% SDS, 200 μg/ml tRNA, 1 mm EDTA, and 10% dextran sulfate. Hybridization was performed at 42°C for 12 h in the prehybridization buffer supplemented with 10,000 cpm/μl [33P]dATP-labeled oligonucleotides. Slides were washed twice at 55°C for 40 min in 0.1× SSC containing 0.1% sarcosyl. Sections were exposed for 3 weeks to BioMax x-ray films (Eastman Kodak, Rochester, NY).
Results
Postsynaptic M1 receptor activation induces endocannabinoid-mediated suppression of inhibition to MS neurons
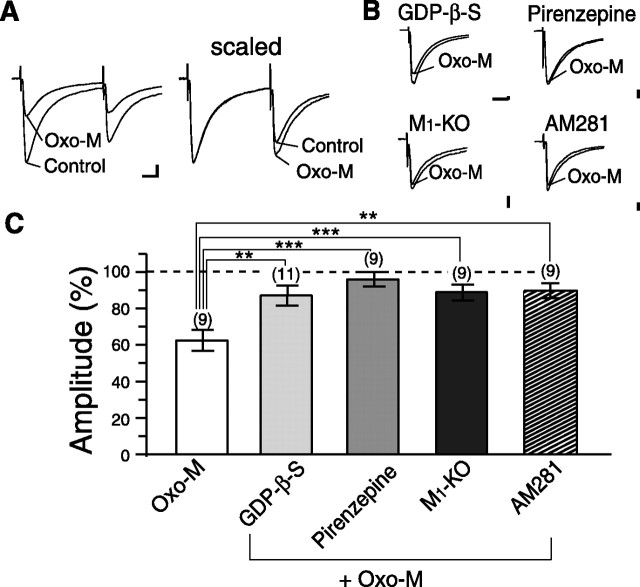
We began by examining mAChR subtype(s) involved in muscarinic suppression of inhibitory synaptic transmission onto MS neurons. We stimulated inhibitory synaptic inputs with a glass microelectrode placed near the MS neuron under recording (<50 μm). Bath application of an mAChR agonist, oxo-M (1 μm), reduced the amplitudes of IPSCs (63.8 ± 4.8% of control; n = 9) (Fig. 1A,C). This suppression accompanied a significant change in the paired-pulse ratio (0.70 ± 0.03 to 0.81 ± 0.03; n = 9; p = 0.02) (Fig. 1A), indicating that the suppression was of presynaptic origin. This effect of oxo-M on IPSCs was blocked by an mAChR antagonist, atropine (10 μm, IPSC amplitude; 94.6 ± 3.1% of control; p = 0.0007; n = 9; data not shown).
Figure 1.
Muscarinic suppression of IPSCs of MS neurons requires postsynaptic M1 receptor and endocannabinoid signaling. A, Representative result showing the effect of bath applied oxo-M (1 μm) on IPSCs of an MS neuron. Left, Sample traces of IPSCs (average of 3 traces) in response to paired stimuli. Traces recorded before and during bath application of 1 μm oxo-M are superimposed. Calibration: 100 pA, 10 ms. Right, The amplitude of the first IPSC in oxo-M was scaled to that of control. B, Sample data showing the effect of oxo-M on IPSCs of MS neurons with intracellular GDP-β-S in the presence of pirenzepine from M1-KO and with preincubation of AM281. IPSC traces recorded before and during bath application of 1 μm oxo-M are superimposed. Calibration: 100 pA, 5 ms. C, Summary bar graph showing the effect of 1 μm oxo-M alone or with intracellular GDP-β-S in the presence of pirenzepine from M1-KO and with preincubation of AM281 on IPSCs. Data are expressed as percentage of IPSC amplitudes in oxo-M relative to the values in the standard extracellular solution. Numbers of tested cells are indicated in parentheses for this and subsequent figures. **p < 0.01; ***p < 0.001.
To determine whether presynaptic or postsynaptic mAChRs are responsible for this inhibition, we inactivated postsynaptic mAChR signaling cascade by loading GDP-β-S (2 mm) into postsynaptic MS neurons. Infusion of GDP-β-S effectively reduced the oxo-M-induced suppression of IPSCs (87.9 ± 5.5% of control; p = 0.009 compared with the oxo-M effect in normal pipette solution; n = 11) (Fig. 1B,C), indicating that postsynaptic mAChRs are important for the presynaptic suppression of IPSCs. Application of the M1 receptor-preferring antagonist pirenzepine (1 μm) effectively blocked the oxo-M-induced suppression of IPSCs (96.8 ± 3.9% of control; p = 0.0005; n = 9) (Fig. 1B,C). Because pirenzepine also blocks M4 receptors at higher concentrations, we examined M1-KO mice (Ohno-Shosaku et al., 2003). Basic properties of inhibitory synaptic transmission recorded in MS neurons were normal in M1-KO mice (IPSC amplitude, 829.8 ± 36.7 pA in wild-type, n = 65 vs 909.3 ± 63.5 pA in M1-KO, n = 39, p = 0.1; paired-pulse ratio, 0.70 ± 0.01 in wild-type vs 0.69 ± 0.01 in M1-KO, p = 0.3 by Student's t test). oxo-M (1 μm)-induced suppression of IPSC in M1-KO mice was much smaller than that in wild-type mice (89.7 ± 4.1% of control; p = 0.0009; n = 9) (Fig. 1B,C). These results indicate that the postsynaptic M1 receptor is responsible for cholinergic modulation of inhibitory transmission in MS neurons.
This oxo-M-induced suppression of IPSC was markedly reduced by AM281 (3 μm), a cannabinoid receptor antagonist (89.9 ± 4.0% of control; p = 0.009; n = 9) (Fig. 1B). This result clearly indicates that oxo-M-induced suppression of inhibitory synaptic transmission involves endocannabinoid signaling.
It should be noted that a small suppression of IPSCs remained under the blockade of postsynaptic G-protein cascade (by GDP-β-S) or CB1 receptors (by AM281) (Fig. 1B,C). The magnitude of this residual suppression was similar to that of the oxo-M-induced suppression of IPSCs in M1-KO mice (Fig. 1B,C). We assume that the residual suppression was caused by direct activation of presynaptic mAChR, presumably M4 receptors reported to be expressed in GABAergic interneurons (Hersch et al., 1994). Nevertheless, the present results clearly indicate that the major component of the muscarinic suppression of IPSCs is mediated by postsynaptic M1 receptors and involves retrograde endocannabinoid signaling.
DSI is constitutively upregulated by ambient ACh through the M1 receptor
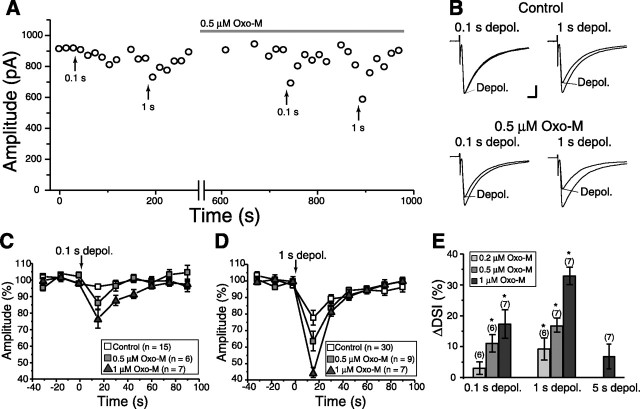
It is now well established that endocannabinoids act as a retrograde messenger at excitatory and inhibitory synapses in various regions of the brain (Piomelli, 2003). It has also been reported that coincidence of mAChR activation and postsynaptic Ca2+ elevation supralinearly facilitates endocannabinoid production in the hippocampus (Kim et al., 2002; Ohno-Shosaku et al., 2003). We tested whether similar cooperativity was observed in MS neurons of the striatum. As shown in Figure 2A, 0.1 s depolarization of an MS neuron in standard extracellular solution caused no perceptible depression of IPSCs, whereas 1 s depolarization caused a small suppression. With a low concentration of oxo-M (0.5 μm), which by itself did not affect basal synaptic transmission, 0.1 s depolarization now induced clear suppression of IPSCs, and 1 s depolarization induced even more pronounced suppression (Fig. 2A,B). For 0.1 s depolarization, the suppression was 4.9 ± 1.7% in control and 14.0 ± 3.5% in 0.5 μm oxo-M (n = 6). For 1 s depolarization, the suppression was 20.7 ± 4.2% in control and 35.6 ± 5.9% in oxo-M at 0.5 μm (n = 7). We then investigated dose dependency of oxo-M on DSI. As summarized in Figure 2, C and D, oxo-M enhanced suppression induced by 0.1 s (Fig. 2C) and 1 s (Fig. 2D) depolarization in a dose-dependent manner. Threshold dose of significant enhancement of DSI was 0.5 μm for 0.1 s depolarization and 0.2 μm for 1 s depolarization (Fig. 2E). For 5 s depolarization, 1 μm oxo-M did not induce significant enhancement of DSI (ΔDSI, 6.5 ± 4.1%; n = 7; p = 0.17) (Fig. 2E), suggesting that 5 s or longer depolarization causes maximum activation of endocannabinoid-producing machinery in MS neurons. These results indicate that mAChR activation and postsynaptic depolarization supralinearly facilitated endocannabinoid production in striatal MS neurons as in the hippocampus.
Figure 2.
Enhancement of DSI by muscarinic receptor activation. A, Representative data demonstrating enhancement of DSI by oxo-M. Each point represents the average of three consecutive traces. Depolarizing pulses with duration of 0.1 or 1 s were applied before and during oxo-M (0.5 μm) application at the time points indicated with upward arrows. B, Examples of DSI recorded in the normal external solution (top) and in the presence of 0.5 μm oxo-M (bottom). IPSCs were recorded at the indicated time points in A. Calibration: 200 pA, 5 ms. C, D, Summary data showing dose-dependent enhancement by oxo-M of DSI induced by 0.1 s (C) or 1 s (D) depolarization. E, Summary bar graphs showing the enhancement of DSI induced by 0.1 s (left), 1 s (middle), or 5 s (right) depolarization by oxo-M at indicated concentrations (in micromolar). *p < 0.05. depol., Depolarization.
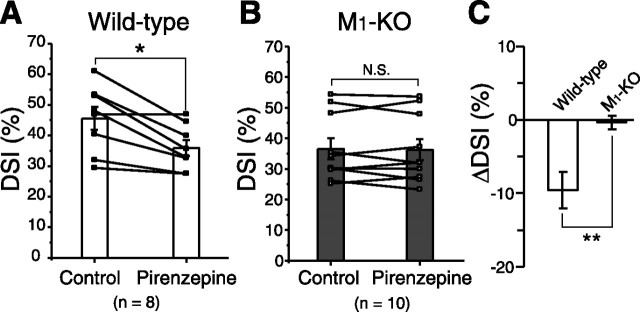
In the striatum, cholinergic interneurons exhibit tonic spontaneous firing in intact animals (Wilson et al., 1990), and similar activity was shown in striatal slices (Bennett and Wilson, 1999). We examined whether intrinsic cholinergic system can modulate endocannabinoid-induced signaling in MS neurons. First, we found that inhibition of postsynaptic G-proteins by applying GDP-β-S from the recording pipette significantly reduced the magnitude of DSI. DSI amplitude induced by 5 s depolarization was 44.2 ± 2.7% (n = 36) with normal intracellular solution but 35.5 ± 2.2% with GDP-β-S-containing solution (n = 7; p = 0.04; data not shown). This result suggests that postsynaptic G-protein cascade contributes to tonic enhancement of DSI. Next, we measured the magnitudes of DSI before and after pirenzepine application in the same neurons. As summarized in Figure 3A, suppression induced by 5 s depolarization became significantly smaller after pirenzepine application in wild-type mice (p = 0.02; n = 8). Reduction in the magnitude of DSI (ΔDSI) was −9.5 ± 2.4% for 5 s depolarization (n = 8) (Fig. 3A,C). We then examined DSI by 5 s depolarization in M1-KO mice. In contrast to wild-type mice, pirenzepine had no effect on the magnitude of DSI in M1-KO mice (ΔDSI, −0.3 ± 1.0%; p = 0.6; n = 10) (Fig. 3B,C). Pirenzepine alone had no effect on IPSC amplitude (wild-type, 105.4 ± 2.6%, n = 7; M1-KO, 99.7 ± 3.7%, n = 10; data not illustrated), indicating no tonic suppression of inhibitory transmission. Moreover, we found that the magnitude of basal DSI in M1-KO mice (34.8 ± 2.4%; n = 40) was significantly (p = 0.02) smaller than that in wild-type mice (44.1 ± 2.3%; n = 41). These results suggest that the endocannabinoid release mechanism is constitutively upregulated through M1 receptor activation by ambient ACh, although the concentration of ambient ACh is not high enough to trigger endocannabinoid synthesis by itself.
Figure 3.
Ambient ACh tonically enhances DSI. A, B, Summary bar graphs and data from individual cells showing the magnitudes of DSI induced by 5 s depolarization with or without pirenzepine (1 μm) for wild-type (A) and M1 knock-out (B) mice. Data from the same cells are connected. *p < 0.05. C, Summary bar graph showing the pirenzepine-induced change in DSI magnitude (ΔDSI) for wild-type and M1 knock-out mice. **p < 0.01.
Firing of cholinergic interneuron influences endocannabinoid-mediated suppression
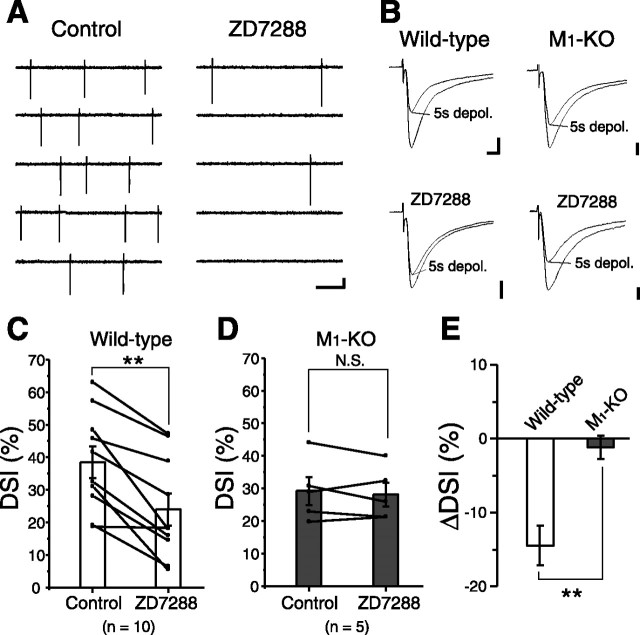
Suppression of cholinergic interneuron activity is expected to lower ambient ACh concentration and affect endocannabinoid signaling in MS neurons. In the striatum, only cholinergic interneurons possess Ih current (Kawaguchi, 1993). Blockade of Ih current hyperpolarizes cholinergic interneurons and blocks their spontaneous tonic firings (Bennett et al., 2000). Therefore, we applied an Ih channel blocker, ZD7288 (20 μm), to suppress firing of cholinergic interneurons. We monitored their activity with cell-attached recording mode and found that their firing rates were greatly reduced from 2.7 ± 0.6 to 0.3 ± 0.1 Hz (p = 0.03; n = 6) after 15 min of ZD7288 application (Fig. 4A). Then we performed whole-cell recordings from MS neurons and measured the magnitude of DSI before and during ZD7288 treatment. Suppression of IPSCs induced by 5 s depolarization was greatly attenuated after ZD7288 application (n = 10) (Fig. 4B,D). In M1-KO mice, ZD7288 had no effect on the amplitude of suppression (p = 0.3) (Fig. 4C,D). These results support that endogenous ACh in the striatum is derived from cholinergic interneurons and indicate that suppression of their spontaneous firings lowers ambient ACh level and blocks M1-mediated tonic enhancement of endocannabinoid release mechanism.
Figure 4.
Ambient ACh concentration regulates the magnitude of DSI. A, Sample records showing the effect of ZD7288 (20 μm) on spontaneous firing of a cholinergic interneuron. Extracellular spikes were recorded in cell-attached configuration. Calibration: 20 pA, 200 ms. B, Examples of the suppression induced by 5 s depolarization (depol.) with (bottom) or without (top) ZD7288 in wild-type (left column) and M1 knock-out (right column) MS neurons. Calibration: 200 pA, 5 ms. C, D, Summary bar graphs and data from individual cells showing the magnitudes of DSI induced by 5 s depolarization with or without ZD7288 for wild-type (C) and M1 knock-out (D) mice. Data from the same cells are connected. **p < 0.01. E, Summary bar graph showing the ZD7288-induced change in DSI magnitude (ΔDSI) for wild-type and M1 knock-out mice. **p < 0.01.
We then tried to elevate ambient ACh concentration in slices by applying choline esterase inhibitor, eserine, to the external solution. Eserine (10 μm) significantly reduced IPSC amplitude (n = 6) (Fig. 5A,B). This suppression was totally blocked by the CB1R antagonist SR141716A (3 μm; n = 6) or pirenzepine (1 μm; n = 6) (Fig. 5A,B). Moreover, eserine significantly enhanced suppression of IPSCs induced by 0.1 s (n = 6) or 1 s (n = 6) depolarization (Fig. 5C,D). This enhancement was completely blocked by pirenzepine (n = 5) (Fig. 5C,D). These results indicate that constitutive upregulation of endocannabinoid signaling (Fig. 3) is enhanced by increasing ambient ACh. Together, these lines of evidence suggest that cholinergic activity can regulate striatal outputs by modulating endocannabinoid-mediated retrograde suppression of inhibition on MS neurons.
Figure 5.
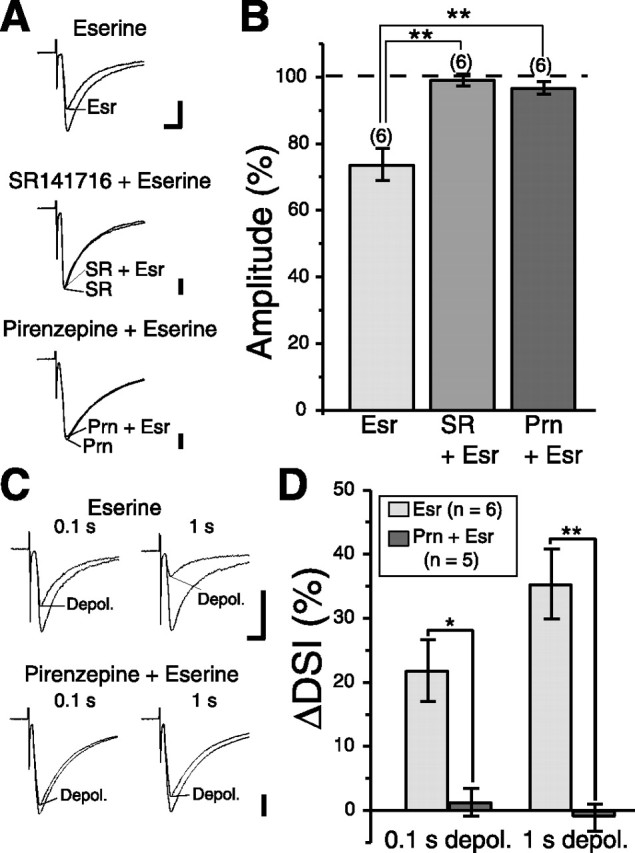
Elevation of ambient ACh concentration enhances DSI. A, Sample traces before and during bath application of a choline esterase inhibitor, eserine, in the standard extracellular solution (top), in the presence of SR141716A (middle), and in the presence of pirenzepine (bottom). Calibration: 200 pA, 5 ms. B, Summary bar graph showing the effect of eserine (10 μm) application on IPSC amplitude for the following experimental conditions: Esr, eserine alone; SR + Esr, eserine in the presence of SR141716A (3 μm); Prn + Esr, eserine in the presence of pirenzepine (1 μm). **p < 0.01. C, Sample IPSC traces showing eserine-induced enhancement of suppression after 0.1 s (top left) and 1 s (top right) depolarizing pulses. The enhancement was reduced by pirenzepine application (bottom row). Calibration: 100 pA, 5 ms. D, Summary bar graphs showing eserine-induced enhancement of suppression after 0.1 and 1 s depolarizing pulses in the standard extracellular solution (left) and in the presence of pirenzepine (right). *p < 0.05; **p < 0.01.
Single cholinergic interneurons regulate endocannabinoid-mediated signaling in neighboring MS neurons
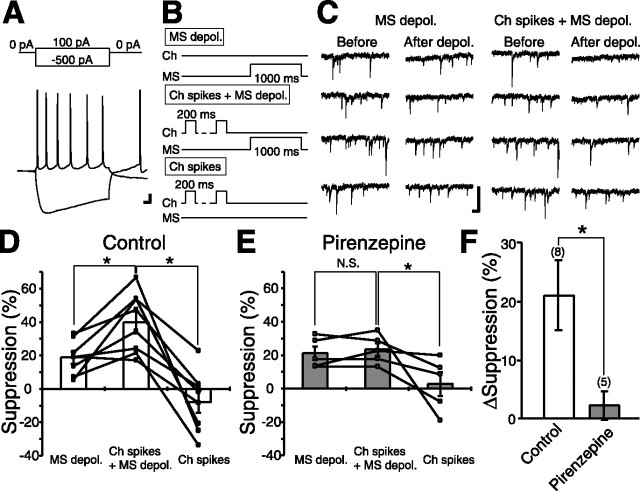
To further investigate physiological relevance of endocannabinoid-mediated modulation on inhibitory transmission, we checked whether DSI and its modulation by the cholinergic system are also observed in sIPSCs. We found that 1 or 5 s depolarization induced clear suppression of charge transfer mediated by sIPSCs and that this DSI was significantly enhanced by 0.5 μm oxo-M (supplemental Fig. 1, available at www.jneurosci.org as supplemental material), which was essentially the same as DSI for evoked IPSCs. We then examined whether alteration of single cholinergic neuron activity could modulate DSI magnitude in neighboring MS neurons. For this purpose, we performed paired recording from a cholinergic interneuron (under current-clamp mode) and an MS neuron (under voltage-clamp mode). Cholinergic interneurons were characterized by prominent hyperpolarization followed by a sag in membrane potential in response to negative current injection (Fig. 6A). Conversely, these neurons exhibit tonic firings with positive current injection (Bennett et al., 2000). We found that DSI for sIPSC by 1 s depolarization (Fig. 6B, top, “MS depol.” protocol; C, left; D) was significantly enhanced (n = 8; p = 0.02) by preceding repetitive firing of the cholinergic interneuron (Fig. 6B, middle, “Ch spikes + MS depol.” protocol; C, right; D). The repetitive firing of the cholinergic interneuron alone (Fig. 6B, bottom, “Ch spikes” protocol) had no effect on sIPSCs (n = 8) (Fig. 6D). The enhancement was completely blocked by pirenzepine (p = 0.5; n = 5) (Fig. 6E). Increase in the magnitude of suppression (Δsuppression) was 21.0 ± 5.9% in normal solution (n = 8) but 2.2 ± 2.3% in the presence of pirenzepine (n = 5) (Fig. 6F). These results indicate that ACh released from cholinergic interneurons modulates the magnitudes of DSI through activation of M1 receptors in MS neurons. Because the activity of only one cholinergic interneuron in the slice could be controlled in this experiment, the results indicate that single cholinergic interneurons can regulate endocannabinoid-mediated signaling in neighboring MS neurons.
Figure 6.
Paired recording from cholinergic interneurons and MS neurons. A, Representative voltage traces from a cholinergic interneuron (recorded in current-clamp configuration) in response to current pulses indicated in the top. The resting membrane potential was −55 mV. Calibration: 10 mV, 10 ms. B, Protocols for inducing suppression of spontaneous IPSCs in an MS neuron (voltage-clamp mode) under paired recording with a cholinergic interneuron (current-clamp mode). MS depol., Depolarization of the MS neuron alone (to 0 mV for 1 s). Ch spikes + MS depol., Repetitive depolarization of the cholinergic interneuron [with 5 or 10 positive current pulses (200 ms) applied every 1 s] followed by the depolarization of the MS neuron (to 0 mV for 1 s) 500 ms after the end of the cholinergic interneuron depolarization. Ch spikes, Repetitive depolarization of the cholinergic interneuron alone. C, Sample traces of spontaneous IPSCs before and after MS depol. (left) and Ch spikes + MS depol. protocols (right). Calibration: 100 pA, 100 ms. D, E, Summary bar graphs and data from individual cells showing the magnitudes of suppression in standard extracellular solution (D) and in the presence of 1 μm pirenzepine (E). Data from the same cells are connected. *p < 0.05. F, Summary data showing the enhancement of DSI for spontaneous IPSCs by the firings of a cholinergic interneuron in standard extracellular solution and with pirenzepine. ΔSuppression was calculated by subtracting the magnitude of DSI for MS depol. protocol from that for Ch spikes + MS depol. protocol in the same MS neuron. *p < 0.05.
M1 is selectively expressed at somatodendritic domains of MS neurons in the striatum
To obtain the morphological basis, we produced a specific antibody against mouse muscarinic M1 receptor (Fig. 7A,B) and examined its expression in the striatum (Fig. 8). We also raised antibodies against mouse high-affinity CHT1 and mouse nNOS to differentially label striatal interneurons (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). We found that M1 was highly expressed in the striatum, accumbens, olfactory tubercle, anterior olfactory nucleus, and hippocampus (Fig. 7C–F). In the striatum, M1 was densely distributed in perikarya and dendrites of D1 (D1R) or D2 (D2R) receptor-positive MS neurons (Fig. 8A), whereas it was hardly detectable in perikarya and processes of parvalbumin-positive, nNOS-positive, or CHT1-positive interneurons (Fig. 8B–D).
Figure 8.
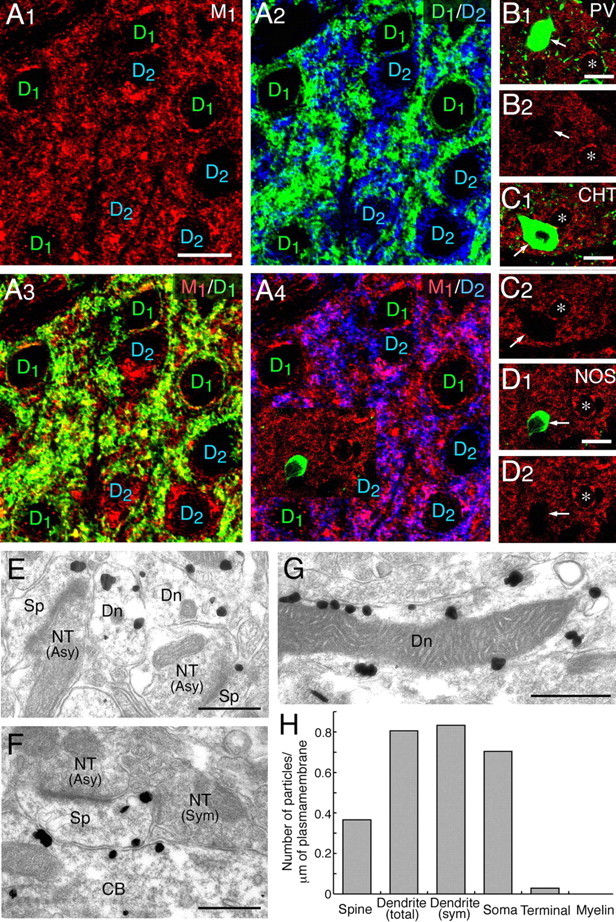
Muscarinic M1 receptor is preferentially expressed in the somatodendritic compartments of D1- and D2-positive MS neurons. A, Triple labeling for M1 (red), D1 (green), and D2 (blue) receptors. Note colabeling of M1 with D1 or D2 receptors in thin perikaryal rims and neuropils. Scale bar, 10 μm. B–D, Arrows indicate interneurons expressing PV (B), high-affinity CHT (C), or neuronal NOS (D). Note vacant M1 staining in these interneurons in contrast to abundant M1 expression in perikarya of putative MS neurons (asterisks). Scale bars, 10 μm. E–G, Silver-enhanced immunogold microscopy for M1 receptor. Note preferential M1 labeling in spines (Sp), dendritic shafts (Dn), and cell bodies (CB) of putative MS neurons, in contrast to negative labeling in nerve terminals (NT) forming asymmetrical (Asy) and symmetrical (Sym) synapses. Scale bars, 1 μm. H, The mean number of metal particles per 1 μm of the cell membrane on spines (measured length, 177.5 μm), total dendrites (173.9 μm), dendrites within 500 nm from the edge of symmetrical synapses (12.0 μm), soma (29.8 μm), nerve terminals (71.5 μm), and myelin sheath (33.4 μm).
Subcellular localization of M1 in MS neurons was assessed by the preembedding silver-enhanced immunogold method (Fig. 8E–G). Metal particles representing M1 were detected in association with the cytoplasmic side of the cell membrane and membranous organelles, such as the smooth endoplasmic reticulum. They were distributed in spines, shafts of spiny dendrites, and thin perikaryal rims, all being suggestive of MS neurons. In particular, cell membrane-attached metal particles were detected on the extrasynaptic surface but not on the synaptic surface. When counting cell membrane-attached metal particles, the number per 1 μm of the cell membrane was twice as high as on the somatic (0.70) and dendritic (0.81) surface than on the spine surface (0.37) (Fig. 8H). These densities were much higher than those on nerve terminals (0.03) or myelin sheath (0.00). On dendritic shafts, no particular accumulation of M1 around symmetrical synapses was discerned, because labeling density within 500 nm from the edge of symmetrical synapses (0.83) was similar to that on the total dendritic surface (0.81). In the striatum, M1 is thus preferentially expressed in the somatodendritic compartments of MS neurons and particularly enriched on the surface of perikarya and dendritic shafts.
Discussion
We presented evidence that indicates ambient ACh derived from tonically active cholinergic interneuron cannot trigger endocannabinoid release by itself but is sufficient to enhance depolarization-induced endocannabinoid signaling. Magnitude of DSI was decreased by suppression of cholinergic interneuron firing, whereas it was increased by inhibiting cholinesterase. This bidirectional modulation indicates that endocannabinoid release from MS neurons is strongly dependent on ambient ACh concentration. Furthermore, we demonstrated that activity of a single cholinergic neuron regulates endocannabinoid-mediated signaling in neighboring MS neurons. Because MS neurons send major outputs from the striatum, the present results collectively suggest a novel mechanism by which the cholinergic system can regulate striatal output and contribute to motor control.
Cholinergic modulation of inhibitory synaptic transmission
Inhibitory synapses in the striatum arise from local GABAergic interneurons and the recurrent collaterals of the MS neurons. The PV-positive and the somatostatin-positive interneurons form GABAergic inhibitory synapses onto MS neurons (Koos and Tepper, 1999; Kubota and Kawaguchi, 2000). Because these two types of interneurons receive excitatory inputs from the cortex, they constitute feedforward inhibitory circuits in the striatum (Plenz, 2003; Tepper et al., 2004). In contrast, connections between MS neurons through their recurrent collaterals are observed much less frequently (Tepper et al., 2004), but they constitute feedback inhibitory circuits that are known to be modulated in an activity-dependent manner (Czubayko and Plenz, 2002). It has been reported recently that inhibitory inputs from PV-positive interneurons, which served strong feedforward inhibition to MS neurons, were suppressed by endocannabinoids after depolarization of MS neurons (Freiman et al., 2006; Narushima et al., 2006b). Together with this observation, our data suggest that the cholinergic system can modulate at least feedforward inhibition of MS neurons through endocannabinoid-mediated pathways. It remains to be determined, however, whether inhibitory transmission originated from other subtype(s) of interneurons or MS neurons also regulated by endocannabinoids and/or the cholinergic system.
It has been suggested that pirenzepine-sensitive presumably M1 receptors are involved in presynaptic inhibition of GABAergic inputs to MS neurons (Koos and Tepper, 2002). However, we demonstrated that M1 receptor-mediated suppression of IPSCs required activation of postsynaptic G-protein cascade and presynaptic cannabinoid receptors. These results indicate that cholinergic inputs activate postsynaptic M1 receptors and produce endocannabinoids through postsynaptic G-protein cascade and then released endocannabinoids suppress GABA release through presynaptic CB1 receptors.
Excitatory synaptic transmission to MS neurons is also presynaptically suppressed by cholinergic agonist (Calabresi et al., 2000) by direct activation of presynaptic mAChRs (Narushima et al., 2006a). Ambient ACh seemed to tonically inhibit excitatory inputs because atropine application itself increased amplitude of evoked EPSPs (Dodt and Misgeld, 1986). Thus, cortical excitatory inputs that overcome cholinergic tonic inhibition may activate MS neurons, and then the firing of MS neurons may trigger depolarization-induced, M1-mediated endocannabinoid release and suppress local inhibitory transmission. It is possible that the cholinergic system highlights activated corticostriatal inputs but suppresses background excitation and thus improves the signal-to-noise ratio of corticostriatal information flow.
Although cholinergic suppression of excitatory transmission to MS neurons is independent of endocannabinoid signaling (Narushima et al., 2006a), corticostriatal excitatory synaptic terminals are sensitive to cannabinoids (Gerdeman et al., 2002). In MS neurons, excitatory synaptic terminals are located exclusively on dendritic spines, whereas inhibitory synaptic terminals are on dendritic shafts and somata (Kubota and Kawaguchi, 2000; Wilson, 2004). Although subcellular distributions of enzymes for endocannabinoid synthesis are not precisely known in MS neurons, the preferential somatodendritic localization of M1 receptors as shown in the present study may explain the differential actions of endocannabinoids on excitatory and inhibitory synapses.
It has been reported recently that M1 activation tonically inhibits endocannabinoid release at glutamatergic synapses through suppression of CaV1.3 channel-mediated Ca2+ currents (Wang et al., 2006). Thus, M1 activation exerts opposite effects on endocannabinoid production at excitatory and inhibitory synapses. This might be attributable to the difference in subcellular localization of M1 and related signaling molecules between excitatory synapses on dendritic spines and inhibitory synapses on dendritic shafts and somata. The M1-mediated CaV1.3 modulation was reported to require functional interaction among CaV1.3, Shank, and Homer that form a protein complex with postsynaptic density-95 in dendritic spines (Olson et al., 2005). Because these signaling molecules are localized in dendritic spines of MS neurons (Olson et al., 2005; Day et al., 2006), M1 activation in the dendritic shaft and soma may not cause tonic suppression of endocannabinoid production at inhibitory synapses but rather enhance endocannabinoid synthesis through PLCβ as reported in the hippocampus (Kim et al., 2002; Ohno-Shosaku et al., 2003; Hashimotodani et al., 2005) and the cerebellum (Maejima et al., 2005).
Two endocannabinoid systems in the striatum
The striatum contains a moderate level of CB1R as investigated with 3H-labeled CB1R agonist binding (Herkenham et al., 1991). In rodents, cannabinoid administration produces impairments of basal ganglia-related behaviors, including decrease in spontaneous activity, induction of catalepsy, and increase in circling behavior (Romero et al., 2002). CB1R knock-out mice have altered gene expression of various neuropeptides and transmitter-related enzymes in the striatum (Steiner et al., 1999) and exhibit motor deficits related to the basal ganglia (Zimmer et al., 1999). Endocannabinoid signaling has been reported to be necessary for induction of corticostriatal long-term depression (LTD) (Gerdeman et al., 2002). These lines of evidence suggest that endocannabinoid signaling through CB1R in the striatum contributes to motor control.
Recent electrophysiological results using a DAG lipase inhibitor suggest that a candidate endocannabinoid, 2-arachidonoylglycerol (2-AG), can be released after postsynaptic depolarization and/or Gq/11-coupled receptor activation and act as a retrograde messenger (Melis et al., 2004; Maejima et al., 2005). In the hippocampus, activation of Gq/11-coupled mAChRs or group I mGluRs drives PLCβ1, produces DAG, and induces endocannabinoid-mediated retrograde suppression of inhibition (Hashimotodani et al., 2005). Because DAG is the precursor for 2-AG, these data suggest that 2-AG is released by Gq/11-coupled receptor activation. Together with the observation that M1 receptor activation can drive PLCs in MS neurons (Lin et al., 2004), our results suggest the possibility that 2-AG is synthesized by M1 receptor activation in MS neurons.
Another candidate endocannabinoid, anandamide, is considered to be synthesized from phosphatidylethanolamine by N-acyltransferase and phospholipase D (Piomelli, 2003). It is reported that D2 but not D1 receptor activation increases striatal anandamide content in vivo (Giuffrida et al., 1999), suggesting that the striatal anandamide level is controlled by dopaminergic inputs specifically through D2 receptors. Moreover, anandamide appears to be important for LTD at the corticostriatal inputs (Gerdeman et al., 2002). However, mechanisms of anandamide production after D2 receptor activation or during LTD induction remain to be elucidated.
Functional implications of muscarinic modulation of striatal endocannabinoid system
Striatal cholinergic system is crucial for proper functions of the basal ganglia. Mice ablated with unilateral cholinergic interneurons exhibit abnormal turning behavior to the contralateral side relative to the lesioned side (Kaneko et al., 2000). Moreover, several studies demonstrate that cholinergic interneuron activity can be changed dynamically in the striatum. For example, during sensorimotor learning in the primates, tonically active neurons (TANs), which correspond to cholinergic interneurons in rodents, change their activity in response to reward-related or aversive stimuli (Apicella, 2002). The firing rate of TANs, normally at ∼2–10 Hz, exhibits a transient depression followed by a rebound excitation, which may influence ambient ACh concentration in the striatum (Aosaki et al., 1994). Other studies showed that striatal ACh content can be modulated depending on excitatory/inhibitory balance (Bennett and Wilson, 1998) or by dopaminergic inputs (Reynolds et al., 2004).
In the present study, we disclosed for the first time a crosslink between muscarinic and endocannabinoid systems in the striatum. Importantly, tonic firing of cholinergic interneurons persistently activates M1 receptors on MS neurons. This tonic M1 activation by itself does not produce endocannabinoid, but it provides a state in which a relatively small amount of depolarization-induced Ca2+ influx can readily release endocannabinoids. In addition, our paired-recording study has revealed that the firing of single cholinergic interneuron is sufficient to activate M1 receptor and to enhance endocannabinoid signaling in neighboring MS neurons. Cholinergic interneurons spontaneously fire at ∼3 Hz, but they can fire at higher frequencies in vivo (Wilson et al., 1990; Aosaki et al., 1994). Therefore, the M1-mediated enhancement of the striatal endocannabinoid system appears to be physiologically relevant.
We recently reported that CB1R is expressed at GABAergic terminals that synapse on both D1R-positive and D2R-positive MS neurons that constitute the direct and the indirect pathways, respectively (Narushima et al., 2006b). We demonstrate that M1 receptors are expressed in both D1R-positive and D2R-positive MS neurons. These results suggest that the cholinergic enhancement of endocannabinoid production can affect both the direct and indirect pathways. The released endocannabinoids retrogradely suppress inhibitory transmission and transiently enhance striatal outputs. This is in contrast to the dopaminergic modulation of anandamide production, which involves D2 receptors (Giuffrida et al., 1999) and therefore presumably occurs only in MS neurons for the indirect pathway. Thus, the cholinergic and dopaminergic systems, which are well known to be crucial for proper functions of the basal ganglia, appear to exert their actions through endocannabinoid systems. We disclosed a novel mechanism by which the muscarinic system regulates striatal output through modulating endocannabinoid signaling.
Footnotes
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grants-in Aid for Scientific Research 17023021 and 17100004 (M.K.), 16067101 (M.M.), 17023001 (M.W.), and 17023011 (T.M.) and the Toyota Riken Foundation (M.K.). M.N. was a recipient of Japan Society for the Promotion of Science Research Fellowship for Young Scientists Grant 16-54131. We thank T. Ohno-Shosaku and T. Tabata for valuable comments and S. Kusakawa for technical assistance.
References
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci. 2002;16:2017–2026. doi: 10.1046/j.1460-9568.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J Neurosci. 1998;18:8539–8549. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci. 1998;10:2887–2895. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Czubayko U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci USA. 2002;99:15764–15769. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Misgeld U. Muscarinic slow excitation and muscarinic inhibition of synaptic transmission in the rat neostriatum. J Physiol (Lond) 1986;380:593–608. doi: 10.1113/jphysiol.1986.sp016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol (Lond) 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of cannabinoid CB1 receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H, Kitai ST. Passive electrical membrane properties of rat neostriatal neurons in an in vitro slice preparation. Brain Res. 1984;300:129–139. doi: 10.1016/0006-8993(84)91347-7. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Chung KK, de Castro D, Funk GD, Lipski J. Effects of muscarinic acetylcholine receptor activation on membrane currents and intracellular messengers in medium spiny neurones of the rat striatum. Eur J Neurosci. 2004;20:1219–1230. doi: 10.1111/j.1460-9568.2004.03576.x. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Yamada S, Oki T, Manabe T, Taketo MM, Ehlert FJ. Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci. 2004;75:2971–2981. doi: 10.1016/j.lfs.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura E, Fukaya M, Sato T, Sugihara K, Asano M, Yoshioka K, Watanabe M. Expression and distribution of JNK/SAPK-associated scaffold protein JSAP1 in developing and adult mouse brain. J Neurochem. 2006;97:1431–1446. doi: 10.1111/j.1471-4159.2006.03835.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cβ4 with metabotropic glutamate receptor type 1a and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Hashimoto K, Kano M. Endocannabinoid-mediated short-term suppression of excitatory synaptic transmission to medium spiny neurons in the striatum. Neurosci Res. 2006a;54:159–164. doi: 10.1016/j.neures.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006b;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J, Lastres-Becker I, de Miguel R, Berrendero F, Ramos JA, Fernandez-Ruiz J. The endogenous cannabinoid system and the basal ganglia. Biochemical, pharmacological, and therapeutic aspects. Pharmacol Ther. 2002;95:137–152. doi: 10.1016/s0163-7258(02)00253-x. [DOI] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Bonner TI, Zimmer AM, Kitai ST, Zimmer A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. Ed 5. Oxford: Oxford UP; 2004. pp. 361–413. [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]